MOF-Derived Catalytic Interfaces for Low-Temperature Chemiresistive VOC Sensing in Complex Backgrounds
Abstract
1. Introduction
2. Synthesis and Engineering of MOF-Derived Catalytic Interfaces
2.1. Pyrolytic Transformation: From MOF to Functional Oxide/Carbon
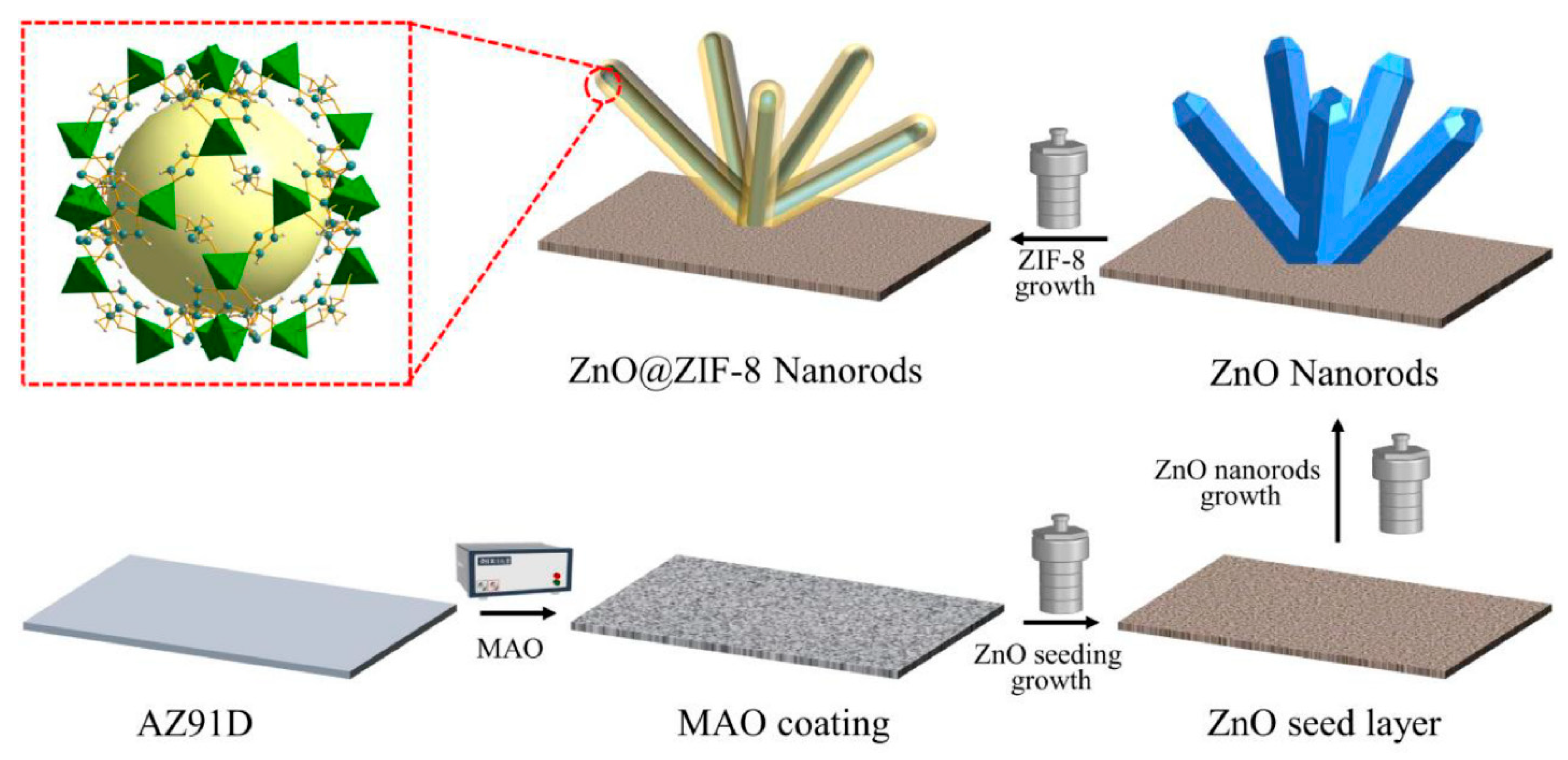
2.2. Engineering Heterostructured and Bimetallic Interfaces
2.3. Templating Hierarchical and Hollow Architectures
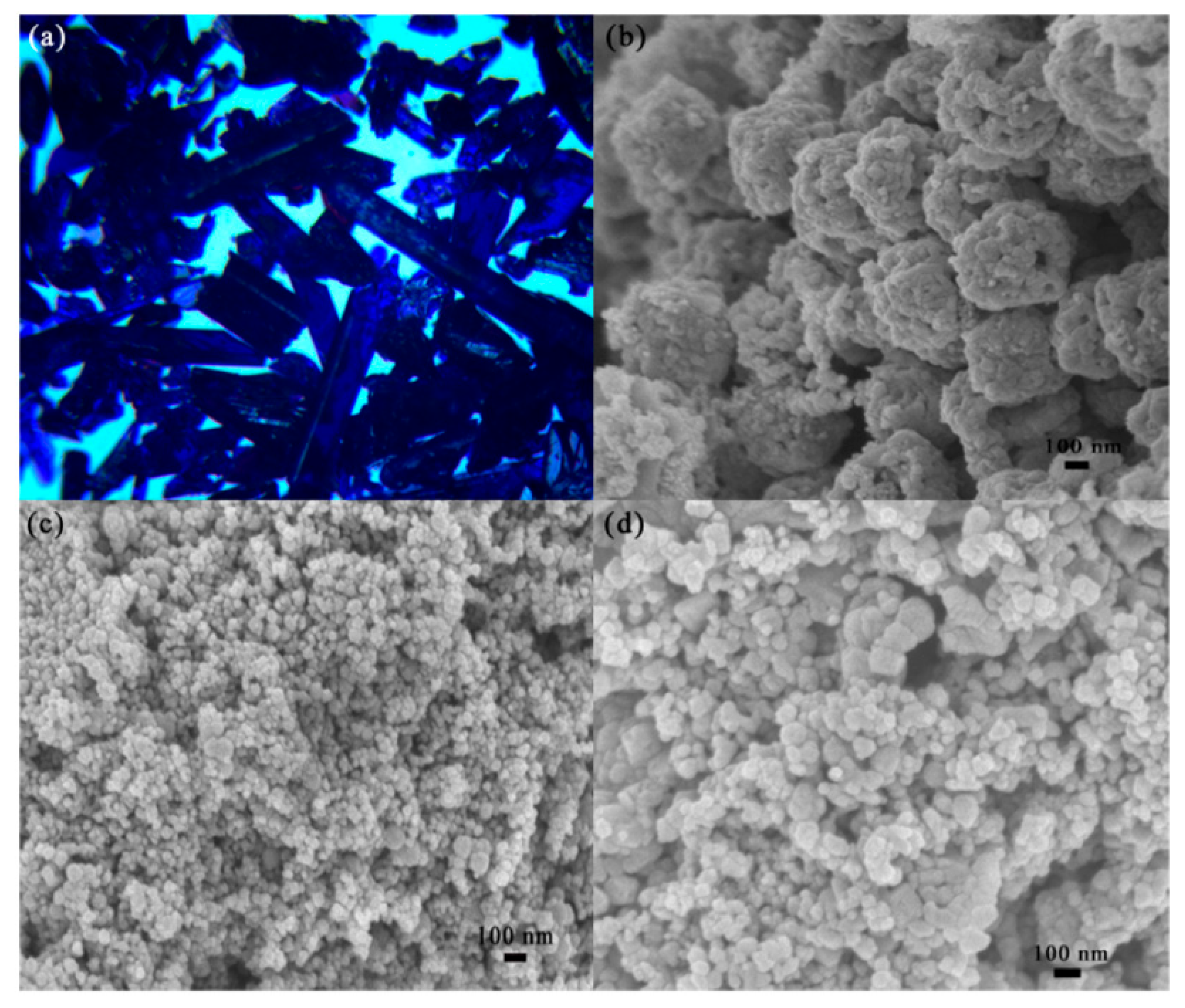
3. Electrochemical Sensing Mechanisms and Performance Analysis
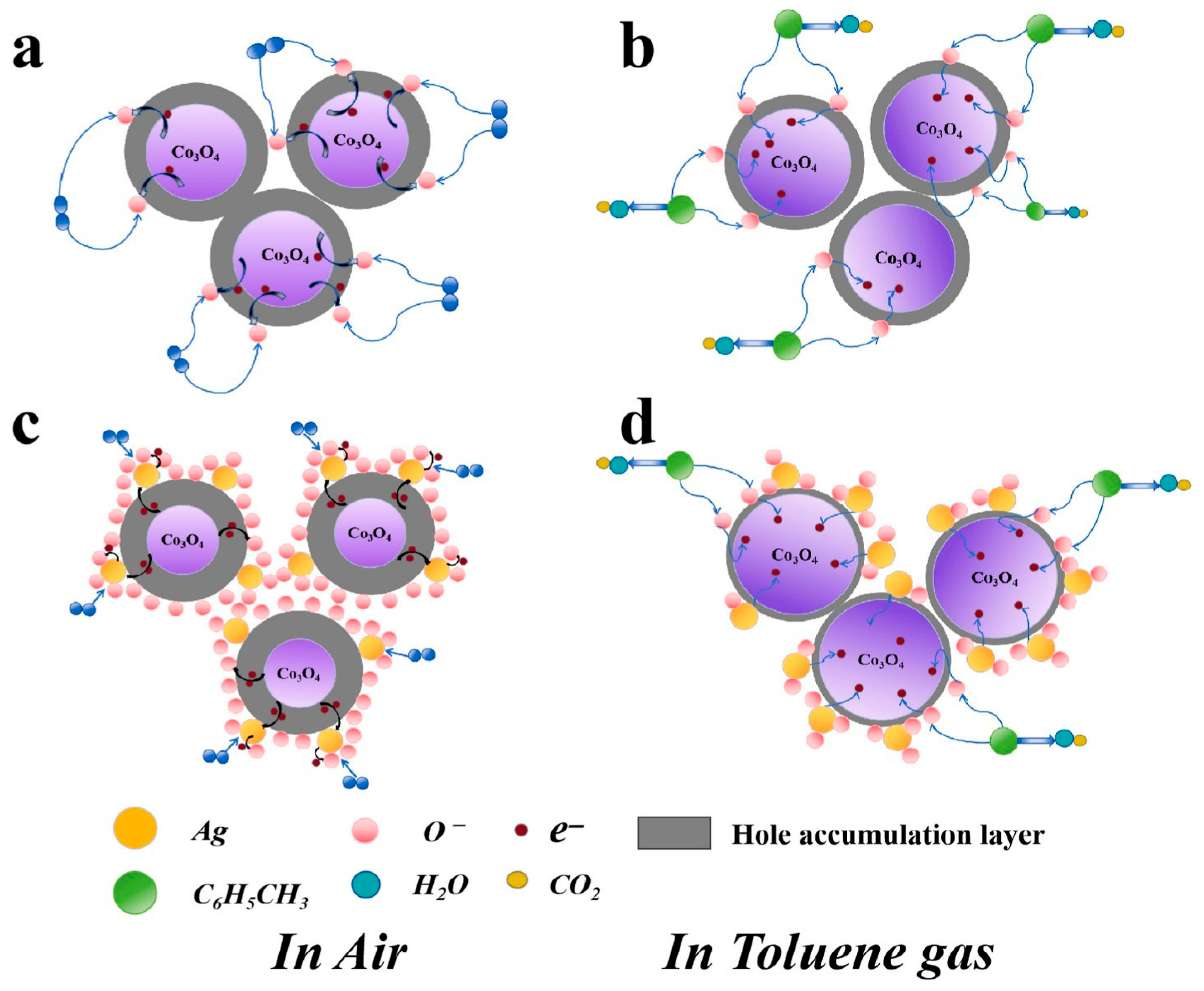
3.1. Low-Temperature Catalytic Sensing Mechanisms
3.2. Performance Benchmarking for Key VOCs
3.2.1. Acetone Sensing
3.2.2. Formaldehyde Sensing
3.2.3. Benzene and Aromatic VOCs Sensing
3.2.4. Ethanol Sensing
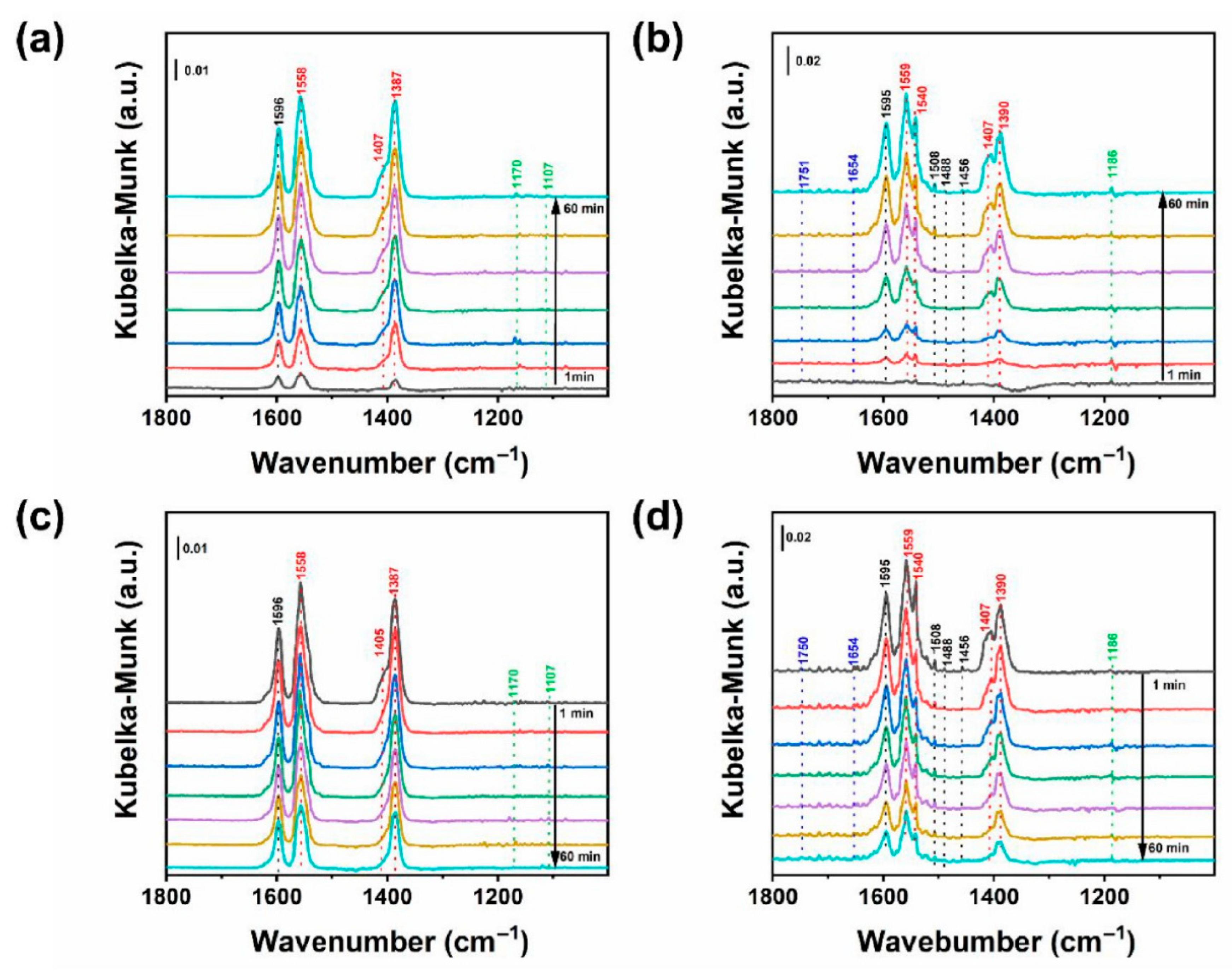
3.3. Advanced Mechanistic Investigations
4. Critical Challenges and Future Perspectives in Complex Environments
4.1. The Selectivity and Anti-Interference Conundrum
| Strategy | Mechanism | Key Advantages | Major Limitations/Challenges | Representative Examples/Refs. |
|---|---|---|---|---|
| Intrinsic Selectivity | ||||
| Pore Engineering/Molecular Sieving | MOF precursor pores are sized to physically exclude larger interferents while allowing smaller target molecules to access active sites. | True physical selectivity based on size; can be highly effective for disparate molecules. | Difficult to discriminate between molecules of similar size; pore structure may change during pyrolysis. | [69] |
| Surface Functionalization/Doping | Active sites are chemically modified to have a specific affinity (e.g., Lewis acid/base interaction) for the target VOC. | Can provide selectivity based on chemical properties, not just size. Doping can enhance catalytic activity for a specific reaction. | Functional groups may not survive pyrolysis; achieving high specificity is challenging. | [106] |
| Extrinsic Selectivity | ||||
| Sensor Array + Machine Learning | An array of cross-sensitive sensors generates a unique response pattern (“fingerprint”) for each analyte, which is classified by an ML algorithm. | Highly effective for discriminating components in known mixtures (>95% accuracy); pragmatic approach. | Requires a training dataset; performance may degrade with novel, untrained interferents; adds system complexity. | [113] |
4.2. Mitigating Humidity-Induced Performance Degradation
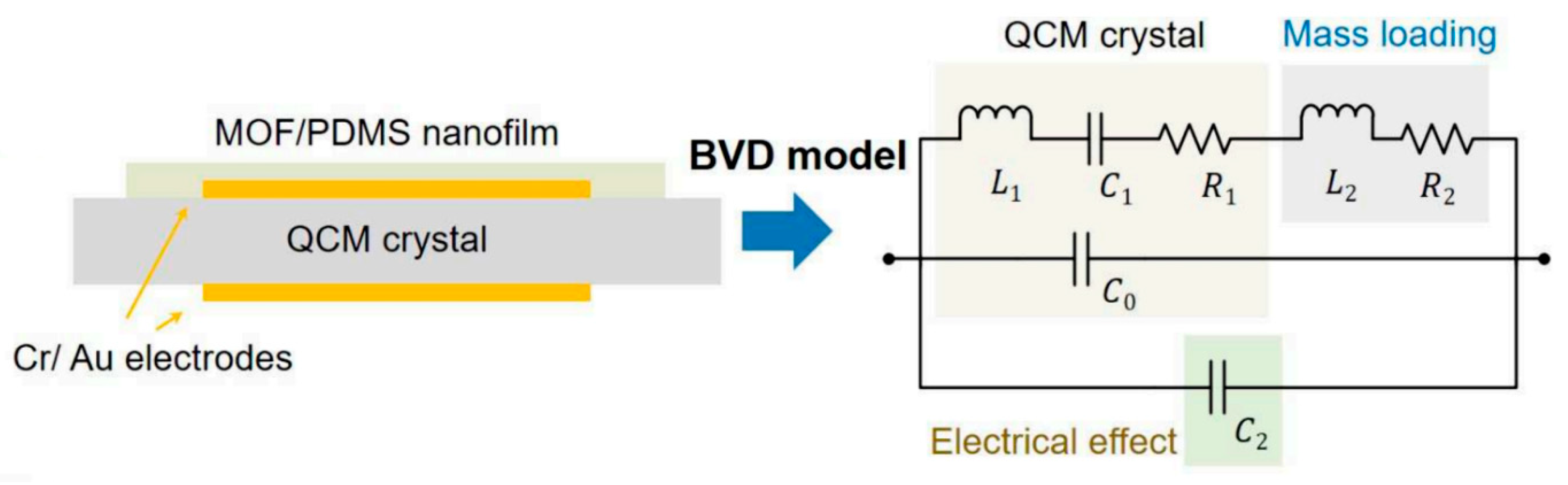
| Mitigation Strategy | Working Principle | Effectiveness (RH Range) | Pros | Cons | Key References |
|---|---|---|---|---|---|
| Hydrophobic MOF Linkers | Intrinsic hydrophobicity of the material repels water molecules from active sites. | Varies; can be effective up to moderate RH. | No additional layers; does not impede gas diffusion. | Difficult to synthesize stable, highly porous hydrophobic MOFs; may reduce affinity for polar VOCs. | [116] |
| Hydrophobic Polymer Coating (e.g., PDMS) | A gas-permeable, water-repellent physical barrier is coated on the sensor. | Highly effective, up to 90–100% RH. | Can be applied to a wide range of sensing materials; excellent water resistance. | May increase response/recovery time; potential for pore blocking or swelling; may reduce sensitivity to large VOCs. | [117] |
| UV-Assisted Desorption | UV light provides energy to desorb water molecules from the sensor surface at RT. | Effective for improving RT performance under humidity. | Enables low-power, RT operation. | Requires an external UV source, increasing system complexity and power consumption. | [7] |
| Algorithmic Compensation | A separate humidity sensor is used, and an algorithm corrects the VOC sensor’s output based on the measured RH. | Can be effective if the relationship between humidity and response is well-characterized. | Can be implemented in software; does not modify the sensor material. | Requires dual sensors; complex calibration; may fail under rapid humidity changes. | [113] |
4.3. Bridging the Gap to Application: Stability, Reproducibility, and Scalability
4.4. Future Outlook and Emerging Frontiers
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Epping, R.; Koch, M. On-Site Detection of Volatile Organic Compounds (VOCs). Molecules 2023, 28, 1598. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, Y.; Song, X.; Liu, Y.; Xie, Y.; Xu, L.; Yu, J.; Qiu, L.; Wang, X.; Lin, J. Application and Development of SERS Technology in Detection of VOC Gases. Mater. Chem. Front. 2025, 9, 349–366. [Google Scholar] [CrossRef]
- Meng, F.; Yuan, Z.; Meng, D. Chemical Sensors for Volatile Organic Compound Detection. Chemosensors 2023, 11, 553. [Google Scholar] [CrossRef]
- Li, X.-B.; Yuan, B.; Huangfu, Y.; Yang, S.; Song, X.; Qi, J.; He, X.; Wang, S.; Chen, Y.; Yang, Q.; et al. Vertical Changes in Volatile Organic Compounds (VOCs) and Impacts on Photochemical Ozone Formation. Atmos. Chem. Phys. 2025, 25, 2459–2472. [Google Scholar] [CrossRef]
- Pathak, A.K.; Swargiary, K.; Kongsawang, N.; Jitpratak, P.; Ajchareeyasoontorn, N.; Udomkittivorakul, J.; Viphavakit, C. Recent Advances in Sensing Materials Targeting Clinical Volatile Organic Compound (VOC) Biomarkers: A Review. Biosensors 2023, 13, 114. [Google Scholar] [CrossRef]
- Chen, X.; Behboodian, R.; Bagnall, D.; Taheri, M.; Nasiri, N. Metal-Organic-Frameworks: Low Temperature Gas Sensing and Air Quality Monitoring. Chemosensors 2021, 9, 316. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Mao, S.; Chen, S.; Guo, Z. Challenges and Applications of Bio-Sniffers for Monitoring Volatile Organic Compounds in Medical Diagnostics. Chemosensors 2025, 13, 127. [Google Scholar] [CrossRef]
- Zhang, C.; Qian, L.; Zeng, W. MOS Based Gas Sensor in Detection of Volatile Organic Compounds: A Review. Sens. Actuators Phys. 2025, 393, 116818. [Google Scholar] [CrossRef]
- Kim, T.; Kim, Y.; Cho, W.; Kwak, J.-H.; Cho, J.; Pyeon, Y.; Kim, J.J.; Shin, H. Ultralow-Power Single-Sensor-Based E-Nose System Powered by Duty Cycling and Deep Learning for Real-Time Gas Identification. ACS Sens. 2024, 9, 3557–3572. [Google Scholar] [CrossRef]
- Ochoa-Muñoz, Y.H.; Mejía de Gutiérrez, R.; Rodríguez-Páez, J.E. Metal Oxide Gas Sensors to Study Acetone Detection Considering Their Potential in the Diagnosis of Diabetes: A Review. Molecules 2023, 28, 1150. [Google Scholar] [CrossRef]
- Khandelwal, G.; Deswal, S.; Dahiya, R. Triboelectric Nanogenerators as Power Sources for Chemical Sensors and Biosensors. ACS Omega 2022, 7, 44573–44590. [Google Scholar] [CrossRef]
- So, S.H.; Lee, S.Y.; Kang, H.; Min, H.; Jung, H.-T.; Lee, K.H.; Kim, D. Metal-Organic Frameworks for Gas Sensors: Comprehensive Review from Principal, Fabrication to Application. Int. J. Extrem. Manuf. 2025, 8, 012001. [Google Scholar] [CrossRef]
- Ma, T.; Ma, J.-G.; Cheng, P. Metal-Organic Frameworks as Electrochemical Sensors. In Metal-Organic Frameworks in Analytical Sample Preparation and Sensing; Elsevier: Amsterdam, The Netherlands, 2024; pp. 305–342. [Google Scholar]
- Abid, H.R.; Azhar, M.R.; Iglauer, S.; Rada, Z.H.; Al-Yaseri, A.; Keshavarz, A. Physicochemical Characterization of Metal Organic Framework Materials: A Mini Review. Heliyon 2024, 10, e23840. [Google Scholar] [CrossRef]
- Rasheed, T.; Anwar, M.T. Metal Organic Frameworks as Self-Sacrificing Modalities for Potential Environmental Catalysis and Energy Applications: Challenges and Perspectives. Coord. Chem. Rev. 2023, 480, 215011. [Google Scholar] [CrossRef]
- Huang, B.; Li, Y.; Zeng, W. Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance. Chemosensors 2021, 9, 226. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Song, B.; Yuan, K.; Xiao, H.; Cao, Y.; Cao, Q. Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review. Int. J. Environ. Res. Public Health 2023, 20, 4388. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-N.; Li, H.-Q.; Yan, M.-W.; Yuan, C.-F.; Zhan, W.-W.; Jiang, Y.-Q.; Xie, Z.-X.; Kuang, Q.; Zheng, L.-S. Ternary Alloys Encapsulated within Different MOFs via a Self-Sacrificing Template Process: A Potential Platform for the Investigation of Size-Selective Catalytic Performances. Small 2017, 13, 1700683. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, N.; Fan, W.; Cai, H.; Zhao, D. Metal-Organic Framework Based Gas Sensors. Adv. Sci. 2022, 9, 2104374. [Google Scholar] [CrossRef]
- Majhi, S.M.; Ali, A.; Rai, P.; Greish, Y.E.; Alzamly, A.; Surya, S.G.; Qamhieh, N.; Mahmoud, S.T. Metal–Organic Frameworks for Advanced Transducer Based Gas Sensors: Review and Perspectives. Nanoscale Adv. 2022, 4, 697–732. [Google Scholar] [CrossRef]
- Feng, H.; Guo, S.; Guo, Y.; Zhao, Q.; Xia, Y.; Duan, Z.; Hou, M.; Yang, L.; Gao, L.; Tai, H. Advances in Metal-Organic Framework-Based Hydrogen Sulfide Gas Sensors. Coord. Chem. Rev. 2026, 546, 217087. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; Ly, A.; Gili, A.; Murphy, E.; Asset, T.; Liu, Y.; De Andrade, V.; Segre, C.U.; et al. Catalysts by Pyrolysis: Transforming Metal-Organic Frameworks (MOFs) Precursors into Metal-Nitrogen-Carbon (MNC) Materials. Mater. Today 2023, 69, 66–78. [Google Scholar] [CrossRef]
- Wang, X.-F.; Song, X.-Z.; Sun, K.-M.; Cheng, L.; Ma, W. MOFs-Derived Porous Nanomaterials for Gas Sensing. Polyhedron 2018, 152, 155–163. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Long, S.; Peh, S.B.; Dong, J.; Wang, Y.; Karmakar, A.; Yuan, Y.D.; Cheng, Y.; Zhao, D. Luminescent Metal–Organic Frameworks for the Detection and Discrimination of o-Xylene from Xylene Isomers. Inorg. Chem. 2018, 57, 13631–13639. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, R.; Jiao, L.; Jiang, H.-L. Metal–Organic Framework-Derived Porous Materials for Catalysis. Coord. Chem. Rev. 2018, 362, 1–23. [Google Scholar] [CrossRef]
- Chen, K.; Bai, S.; Li, H.; Xue, Y.; Zhang, X.; Liu, M.; Jia, J. The Co3O4 Catalyst Derived from ZIF-67 and Their Catalytic Performance of Toluene. Appl. Catal. Gen. 2020, 599, 117614. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, S.; Du, Q.; Bi, F.; Xie, K.; Wang, L. Effect of Calcination Temperature on the Structure and Performance of Rod-like MnCeOx Derived from MOFs Catalysts. Mol. Catal. 2022, 522, 112226. [Google Scholar] [CrossRef]
- da Silva Gropelo, H.; dos Santos Theodoro, R.; dos Santos, G.S.M.; Perfecto, T.M.; Volanti, D.P. Crystallite Size Control in MOF-5-Derived Nanostructured ZnO Sensors for Detecting 1-Pentanol. J. Alloys Compd. 2025, 1036, 181745. [Google Scholar] [CrossRef]
- Shen, B.; Yuan, T.; Zhang, W.; Chen, Y.; Xu, J. Complex Shell Fe-ZnO Derived from ZIF-8 as High-Quality Acetone MEMS Sensor. Chin. Chem. Lett. 2024, 35, 109490. [Google Scholar] [CrossRef]
- Xian, J.; Li, J.; Wang, W.; Zhu, J.; Li, P.; Leung, C.M.; Zeng, M.; Lu, X.; Gao, X.; Liu, J.-M. Enhanced Specific Surface Area of ZIF-8 Derived ZnO Induced by Sulfuric Acid Modification for High-Performance Acetone Gas Sensor. Appl. Surf. Sci. 2023, 614, 156175. [Google Scholar] [CrossRef]
- Guo, R.; Hou, X.; Shi, C.; Zhang, W.; Zhou, Y. MOF-Derived Co3O4 Hierarchical Porous Structure for Enhanced Acetone Sensing Performance with High Sensitivity and Low Detection Limit. Sens. Actuators B Chem. 2023, 376, 132973. [Google Scholar] [CrossRef]
- Li, C.; Zhou, N.; Xia, Z.; Yan, C. UiO-66 for Composite Template Derived Cu/Zr-O with Dodecahedral Structure for Efficient Asymmetry Supercapacitor. J. Power Sources 2024, 623, 235506. [Google Scholar] [CrossRef]
- Hua, M.; Wang, S.; Cheng, M.; Liang, G.; Xu, L.; Zhou, Y. The Application of TiO2@ UiO-66-(OH) 2 Composites for the Photocatalytic Degradation of Gaseous Formaldehyde under Visible Light. J. Photochem. Photobiol. Chem. 2025, 464, 116328. [Google Scholar] [CrossRef]
- Guo, W.; Niu, J.; Hong, B.; Xu, J.; Han, Y.; Peng, X.; Ge, H.; Li, J.; Zeng, Y.; Wang, X. Mesoporous Co3O4/In2O3 Nanocomposites for Formaldehyde Gas Sensors: Synthesis from ZIF-67 and Gas-Sensing Behavior. Mater. Res. Bull. 2023, 164, 112264. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Godino-Ojer, M.; Matos, I.; Bernardo, M. Opportunities from Metal Organic Frameworks to Develop Porous Carbons Catalysts Involved in Fine Chemical Synthesis. Catalysts 2023, 13, 541. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, W.; Xu, J.; Luo, Y.; Pan, J.; Liao, C.; Yang, L.; Tan, W.; Huang, X. ZIF-8 Derived Hierarchical Hollow ZnO Nanocages with Quantum Dots for Sensitive Ethanol Gas Detection. Sens. Actuators B Chem. 2019, 289, 144–152. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Guan, K.; Liu, Y.; Li, Y.; Sun, F.; Wang, X.; Zhang, C.; Feng, S.; Zhang, T. Influence of Oxygen Vacancies on the Performance of SnO2 Gas Sensing by Near-Ambient Pressure XPS Studies. Sens. Actuators B Chem. 2023, 393, 134252. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-Ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z.X. Tuning of ZIF-Derived Carbon with High Activity, Nitrogen Functionality, and Yield—A Case for Superior CO2 Capture. ChemSusChem 2015, 8, 2123–2132. [Google Scholar] [CrossRef]
- Jo, Y.-M.; Kim, T.-H.; Lee, C.-S.; Lim, K.; Na, C.W.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.-H. Metal–Organic Framework-Derived Hollow Hierarchical Co3O4 Nanocages with Tunable Size and Morphology: Ultrasensitive and Highly Selective Detection of Methylbenzenes. ACS Appl. Mater. Interfaces 2018, 10, 8860–8868. [Google Scholar] [CrossRef]
- Jiang, S.; Li, W.; Liu, J.; Jiang, J.; Zhang, Z.; Shang, W.; Peng, N.; Wen, Y. ZnO@ZIF-8 Core-Shell Structure Nanorods Superhydrophobic Coating on Magnesium Alloy with Corrosion Resistance and Self-Cleaning. J. Magnes. Alloys 2023, 11, 3287–3301. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, Z.; Xu, X.; Xiao, Y.; Meng, F. Bimetallic Organic Framework-Derived Porous Co3O4/Fe2O3 Nanosheets for Acetone Sensing. Sci. Rep. 2025, 15, 20912. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Tang, W.; Long, M.; Zhang, Z.; Liu, H.; An, D. Highly Sensitive Triethylamine Sensor Based on MOF-Derived Bimetallic NiO-SnO2 Nanomaterials. Ceram. Int. 2025, 51, 9329–9342. [Google Scholar] [CrossRef]
- Li, X.-B.; Sun, S.; Hu, X.; Zhang, Q.-Q.; Gao, C.; Zhou, H.; Wu, B.-X.; Wang, A.-Q.; Hu, W.-Y.; Wang, Y.-J.; et al. Fabrication and Performance Enhancement of an In2O3/BiVO4 Heterojunction for N-Butanol Gas Sensing Applications. RSC Adv. 2024, 14, 39715–39726. [Google Scholar] [CrossRef]
- Fan, X.; Yang, S.; Huang, C.; Lu, Y.; Dai, P. Preparation and Enhanced Acetone-Sensing Properties of ZIF-8-Derived Co3O4@ ZnO Microspheres. Chemosensors 2023, 11, 376. [Google Scholar] [CrossRef]
- Song, P.; Sun, F.; Luan, T.; Meng, Q.; Geng, W. MOF-Derived In2O3/BiVO4 Composites for Sensitive and Trace Detection of n-C4H9OH. ACS Sens. 2025, 10, 5589–5599. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Shawky, A. Visible-Light-Driven Hydrogen Production over ZIF-8 Derived Co3O4/ZnO S-Scheme Based p-n Heterojunctions. Opt. Mater. 2022, 124, 112012. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Cao, L.; Jin, P.; Zeng, X.; Zhang, X.; Wei, J. Engineering Mesoporous Semiconducting Metal Oxides from Metal-Organic Frameworks for Gas Sensing. Coord. Chem. Rev. 2021, 445, 214086. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, W.; Zhu, Z.; Xue, Q.; Lu, W.; Ding, D.; Zhu, L. ZIF-Derived Porous ZnO-Co3O4 Hollow Polyhedrons Heterostructure with Highly Enhanced Ethanol Detection Performance. Sens. Actuators B Chem. 2017, 253, 523–532. [Google Scholar] [CrossRef]
- Zayeri, S.; Asl, S.; Mehrasa, S.; Saboor, F.; Safajoo-Jahankhanemlou, M. Inhibition Semiconductor Gas Sensors Based on Hollow Metal Oxides: Focusing on MOF-Derived Structures. Prog. Chem. Biochem. Res. 2025, 8, 304–329. [Google Scholar]
- Zhou, W.; Wu, Y.-P.; Zhao, J.; Dong, W.-W.; Qiao, X.-Q.; Hou, D.-F.; Bu, X.; Li, D.-S. Efficient Gas-Sensing for Formaldehyde with 3D Hierarchical Co3O4 Derived from Co5-Based MOF Microcrystals. Inorg. Chem. 2017, 56, 14111–14117. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Gong, Y.; Han, N.; Liu, H.; Chen, Y. MOF-Derived Hierarchical ZnO/ZnFe2O4 Hollow Cubes for Enhanced Acetone Gas-Sensing Performance. RSC Adv. 2017, 7, 34609–34617. [Google Scholar] [CrossRef]
- Duan, J.; He, X.; Fang, X.; Yue, J.; Chen, G.; Wang, W. The Composite of UiO-66 Derived ZrO2 and g-C3N4 for Oxidative Removal of Formaldehyde at the Room Temperature. Diam. Relat. Mater. 2022, 129, 109365. [Google Scholar] [CrossRef]
- Liu, Z.; He, W.; Zhang, Q.; Shapour, H.; Bakhtari, M.F. Preparation of a GO/MIL-101(Fe) Composite for the Removal of Methyl Orange from Aqueous Solution. ACS Omega 2021, 6, 4597–4608. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, T.; Xu, X.; Bing, Y.; Sui, N.; Wang, J.; Li, J.; Zhang, T. Metal-Organic Frameworks Derived Inverse/Normal Bimetallic Spinel Oxides toward the Selective VOCs and H2S Sensing. J. Hazard. Mater. 2023, 457, 131734. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Zhang, Q.; Xi, X.; Cai, J.; Qi, H.; Shi, S.; Wang, J.; Yuan, D.; Fang, M. Synthesis and Characterization of the Interpenetrated MOF-5. J. Mater. Chem. 2010, 20, 3758–3767. [Google Scholar] [CrossRef]
- Liang, M.; Yan, Y.; Yang, J.; Liu, X.; Jia, R.; Ge, Y.; Li, Z.; Huang, L. In Situ-Derived N-Doped ZnO from ZIF-8 for Enhanced Ethanol Sensing in ZnO/MEMS Devices. Molecules 2024, 29, 1703. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, H.; Shang, Y.; Lei, L.; Zhu, S.; Wang, H.; Dong, W.; Al-Bahrani, M.; Wang, W.; Ma, L. MOF-5 Derived 3D ZnO/Ag Micro-Octahedra for Ultrahigh Response and Selective Triethylamine Detection at Low Temperature. Sens. Actuators B Chem. 2023, 390, 133975. [Google Scholar] [CrossRef]
- Gao, X.; Hou, X.; Ma, Z.; Xiao, C.; Jia, L. Highly Dispersed Ag Nanocrystals Functionalized ZIF-8 Derived ZnO Hollow Structures for Superior Sensitive and Selective Detection of Nitric Oxide. Sens. Actuators B Chem. 2025, 422, 136646. [Google Scholar] [CrossRef]
- Tan, J.; Hussain, S.; Ge, C.; Wang, M.; Shah, S.; Liu, G.; Qiao, G. ZIF-67 MOF-Derived Unique Double-Shelled Co3O4/NiCo2O4 Nanocages for Superior Gas-Sensing Performances. Sens. Actuators B Chem. 2020, 303, 127251. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Liu, Y. Co3O4/ZnO Nanoheterostructure Derived from Core–Shell ZIF-8@ZIF-67 for Supercapacitors. RSC Adv. 2016, 6, 52137–52142. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, Y.; Zhang, D.; Liu, Y.; Wang, H.; Li, Y.; Wei, H.; Wang, S.; Sun, M.; Sun, M. CuO/ZnO Hollow Nanocages Derived from Metal−organic Frameworks for Ultra-High and Rapid Response H2S Gas Sensor. Ceram. Int. 2024, 50, 15767–15779. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Liu, W.; Tian, H.; Du, Y.; Wei, H.; Tang, L. UiO-66 Derived ZrO2@C Catalysts for the Double-Bond Isomerization Reaction of 2-Butene. RSC Adv. 2023, 13, 15934–15941. [Google Scholar] [CrossRef]
- Li, S.; Zhao, L.; Zhang, Y.; Wan, Y.; Wang, H.; Zhang, Q.; Tang, Y. A Robust ZrO2 Rectifier Layer Derived from UiO-66 Enable Ultra-High Nickel Cathodes with High Stability and Ion Transport Kinetics for Lithium-Ion Batteries. J. Power Sources 2024, 608, 234533. [Google Scholar] [CrossRef]
- Huang, H.; Cheng, M.; Yin, J.; Zhang, J.; Kong, L.; Bu, X.-H. MIL-101(Fe)-Derived Iron Oxide/Carbon Anode for Lithium-Ion Batteries: Derivation Process Study and Performance Optimization. Electrochim. Acta 2022, 426, 140794. [Google Scholar] [CrossRef]
- Chen, J.; Dong, J.; Yang, J.; Chen, Y. CoFe2O4 Nanocubes Derived by Prussian Blue Analogs for Detecting Dopamine. Microchem. J. 2024, 199, 109999. [Google Scholar] [CrossRef]
- Khan, F.U.; Mehmood, S.; Liu, S.; Xu, W.; Shah, M.N.; Zhao, X.; Ma, J.; Yang, Y.; Pan, X. A P-n Heterojunction Based Pd/PdO@ZnO Organic Frameworks for High-Sensitivity Room-Temperature Formaldehyde Gas Sensor. Front. Chem. 2021, 9, 742488. [Google Scholar] [CrossRef]
- Li, J.; He, X.-Y.; Wang, X.; Lv, Y.; Ma, J.-G.; Li, B.; Cheng, P. Metal−Organic Framework-Derived Cu NWs@ZrO2 as a Highly Selective Catalyst for Methanol Synthesis from CO2 Hydrogenation. Inorg. Chem. 2025, 64, 8448–8454. [Google Scholar] [CrossRef]
- Shen, Y.; Tissot, A.; Serre, C. Recent Progress on MOF-Based Optical Sensors for VOC Sensing. Chem. Sci. 2022, 13, 13978–14007. [Google Scholar] [CrossRef]
- Saruhan, B.; Lontio Fomekong, R.; Nahirniak, S. Influences of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Liu, F.; Chen, X.; Jie, W.; Liu, Y.; Li, C.; Song, G.; Gong, X.; Liu, Q.; Qiu, M.; Ding, S.; et al. MOF-Derived High Oxygen Vacancies CuO/CeO2 Catalysts for Low-Temperature CO Preferential Oxidation. J. Colloid Interface Sci. 2024, 674, 778–790. [Google Scholar] [CrossRef]
- Sun, S.; Xie, D.; Zhang, F.; Guo, W.; Qu, F. MOF-Derived NiO/γ-Fe2O3 p–n Heterojunctions for Ethylene Glycol Sensing. J. Mater. Chem. C 2025. [Google Scholar] [CrossRef]
- Chu, N.; Wang, Z.; Gu, F. Oxygen Vacancies Enabled MOF-Derived Tb–SnO2 Compound for a High-Response, Low Detection Limit, and Humidity-Tolerant Chemiresistive Gas Sensor of Formaldehyde. ACS Appl. Electron. Mater. 2025, 7, 3041–3054. [Google Scholar] [CrossRef]
- Han, J.; Kong, D.; Zhou, W.; Gao, Y.; Gao, Y.; Liu, G.; Lu, G. Interface-Engineering in MOF-Derived In2O3 for Highly Sensitive and Dual-Functional Gas Sensor towards NO2 and Triethylamine. Sens. Actuators B Chem. 2023, 395, 134491. [Google Scholar] [CrossRef]
- Jossou, E.; Malakkal, L.; Dzade, N.Y.; Claisse, A.; Szpunar, B.; Szpunar, J. DFT + U Study of the Adsorption and Dissociation of Water on Clean, Defective, and Oxygen-Covered U3Si2{001}, {110}, and {111} Surfaces. J. Phys. Chem. C Nanomater. Interfaces 2019, 123, 19453–19467. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xiong, X.; Guan, W.; Chen, Y.; Long, H. Regulation of O-Vacancy and Heterojunction Structure in MOF-Derived Fe2O3-Co3O4 Enhancing Acetone Sensing Performance. Sens. Actuators B Chem. 2024, 401, 135082. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Y.; Xu, D.; Zi, B.; Zeng, J.; Lu, Q.; Xiong, K.; Zhang, J.; Zhao, J.; Liu, Q. Ultrasensitive Formaldehyde Sensor Based on SnO2 with Rich Adsorbed Oxygen Derived from a Metal Organic Framework. ACS Sens. 2022, 7, 2577–2588. [Google Scholar] [CrossRef]
- Guo, R.; Deng, Y.; Jia, Y.; Shi, C.; Zhang, W.; Zhou, Y.; Hou, X. Gallium Ions Induced In-Situ MOF-Derived Hierarchical Porous Co3O4 for Ultra-High Acetone Response. Sens. Actuators B Chem. 2024, 399, 134832. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, N.; Jia, M.; Wang, J.; Yang, S.; Liu, Y.; Chen, W. Dual Functionalized Co3O4 Porous Cages with Pd and Co-MOF for Acetone Gas Sensing under High Humidity. Mater. Today Commun. 2024, 40, 109582. [Google Scholar] [CrossRef]
- Ali, A.; Greish, Y.E.; Alzard, R.H.; Siddig, L.A.; Alzamly, A.; Qamhieh, N.; Mahmoud, S.T. Bismuth-Based Metal–Organic Framework as a Chemiresistive Sensor for Acetone Gas Detection. Nanomaterials 2023, 13, 3041. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Cheong, J.Y. MOF-Derived SnO2 Hollow Spheres for Acetone Gas Sensing. J. Mater. Sci. Mater. Electron. 2023, 34, 1060. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Liu, J.; Nasir, M.S.; Zhu, J.; Li, S.; Liang, J.; Yan, W. Smart Formaldehyde Detection Enabled by Metal Organic Framework-Derived Doped Electrospun Hollow Nanofibers. Sens. Actuators B Chem. 2021, 326, 128819. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiang, N.; Wen, S.; Huo, H.; Li, Q. ZIF-67 Derived Cu-Co Mixed Oxides for Efficient Catalytic Oxidation of Formaldehyde at Low-Temperature. Catalysts 2023, 13, 117. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Chang, X.; Li, J.; Pan, L.; Jiang, Y.; Gao, W.; Gao, C.; Sun, S. Metal-Organic Framework-Derived Porous SnO2 Nanosheets with Grain Sizes Comparable to Debye Length for Formaldehyde Detection with High Response and Low Detection Limit. Sens. Actuators B Chem. 2021, 347, 130599. [Google Scholar] [CrossRef]
- Ling, W.; Zhu, D.; Pu, Y.; Li, H. The Ppb-Level Formaldehyde Detection with UV Excitation for Yolk-Shell MOF-Derived ZnO at Room Temperature. Sens. Actuators B Chem. 2022, 355, 131294. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Wang, J.; Liu, J.; Zhang, J.; Yan, W. Pt-Embedded Metal–Organic Frameworks Deriving Pt/ZnO-In2O3 Electrospun Hollow Nanofibers for Enhanced Formaldehyde Gas Sensing. Chemosensors 2024, 12, 93. [Google Scholar] [CrossRef]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101 (Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef]
- Nikhar, S.; Chakraborty, M. Assessing the Photodegradation Efficiency of Benzene, Toluene, and Xylene (BTX): A Comparative Investigation Using Activated Charcoal (AC), Zeolitic Imidazolate Framework-8 (ZIF-8), and Zirconium Metal–Organic Framework (Zr-MOF). Water Sci. Technol. 2024, 90, 3193–3209. [Google Scholar] [CrossRef]
- Theka, T.J.; Thamaga, B.R.J.; Tshabalala, Z.P.; Motsoeneng, R.G.; Swart, H.C.; Motaung, D.E. Fabrication of Metal-Organic Frameworks Derived Co3O4 Loaded on TiO2: Influence of Fe Loading on the Co3O4/TiO2 Heterostructure for Low-Ppm Benzene Detection. Appl. Surf. Sci. 2024, 644, 158789. [Google Scholar] [CrossRef]
- Wu, W.J.; Hong, B.; Xu, J.; Peng, X.; Li, J.; Chen, H.; Qiu, S.; Zhang, N.; Wang, X. Ag/Co3O4 Nanocomposites from ZIF-67 MOF for Enhanced Low-Temperature Toluene Gas Sensing. Phys. E Low-Dimens. Syst. Nanostructures 2025, 167, 116174. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, L.; Wang, W.; Yu, F. Ethanol Sensing Properties and First Principles Study of Au Supported on Mesoporous ZnO Derived from Metal Organic Framework ZIF-8. Sensors 2021, 21, 4352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tang, X.; Zhang, J.; Zhou, T.; Wang, H.; Wu, C.; Xia, X.; Xie, C.; Zeng, D. Metal–Organic Framework-Assisted Construction of TiO2/Co3O4 Highly Ordered Necklace-like Heterostructures for Enhanced Ethanol Vapor Sensing Performance. Langmuir 2018, 34, 14577–14585. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Ning, P.; Song, Z.; Liu, X.; Duan, Y. In Situ DRIFTS Studies on CuO-Fe2O3 Catalysts for Low Temperature Selective Catalytic Oxidation of Ammonia to Nitrogen. Appl. Surf. Sci. 2017, 419, 733–743. [Google Scholar] [CrossRef]
- Fan, J.; Ren, Q.; Mo, S.; Sun, Y.; Fu, M.; Wu, J.; Chen, L.; Chen, P.; Ye, D. Transient In-Situ DRIFTS Investigation of Catalytic Oxidation of Toluene over α-, γ-and β-MnO2. ChemCatChem 2020, 12, 1046–1054. [Google Scholar] [CrossRef]
- Gu, H.; Yokoya, T.; Kang, L.; Marlow, S.; Su, X.; Gong, M.; Yan, J.; Ren, Y.; Wang, Z.; Guan, X.; et al. Oxygen Vacancy Formation as the Rate-Determining Step in the Mars-van Krevelen Mechanism. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, P.; Wang, W.; Lv, L.; Zhang, W.; Pan, B. In Situ Fabrication of Highly Dispersed Co–Fe-Doped-δ-MnO2 Catalyst by a Facile Redox-Driving MOFs-Derived Method for Low-Temperature Oxidation of Toluene. ACS Appl. Mater. Interfaces 2022, 14, 53872–53883. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-Based Gas Sensors for Detection of Benzene, Toluene and Xylene (BTX) Gases: A Review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Xie, Y.; Lyu, S.; Zhang, Y.; Cai, C. Adsorption and Degradation of Volatile Organic Compounds by Metal–Organic Frameworks (MOFs): A Review. Materials 2022, 15, 7727. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Luo, A.; Xing, X. A DFT Study of Volatile Organic Compounds Detection on Pristine and Pt-Decorated SnS Monolayers. Sensors 2023, 23, 7319. [Google Scholar] [CrossRef]
- Ftahi, W.; Nusaibah, A.-S.; Yang, Y.; Tang, Y.; Liu, Q.; Ni, Y. ReaxFF Molecular Dynamics Study of Acetone and Ethanol Adsorption on ZnO Nanowires for Enhanced Gas Sensor Applications. Appl. Surf. Sci. 2025, 716, 164654. [Google Scholar] [CrossRef]
- Zhan, M.; Xu, M.; Lin, W.; He, H.; He, C. Graphene Oxide Research: Current Developments and Future Directions. Nanomaterials 2025, 15, 507. [Google Scholar] [CrossRef]
- Chang, X.; Li, K.; Qiao, X.; Xiong, Y.; Xia, F.; Xue, Q. ZIF-8 Derived ZnO Polyhedrons Decorated with Biomass Derived Nitrogen-Doped Porous Carbon for Enhanced Acetone Sensing. Sens. Actuators B Chem. 2021, 330, 129366. [Google Scholar] [CrossRef]
- Ma, R.; Lei, H.; Han, M.; Hao, J. Recent Progress with Bismuth Sulfide for Room-Temperature Gas Sensing. Chemosensors 2025, 13, 120. [Google Scholar] [CrossRef]
- Matavž, A.; Verstreken, M.F.; Boullart, L.; Tietze, M.L.; Sugihara, M.; Heinke, L.; Ameloot, R. Kinetic Selectivity in Metal-Organic Framework Chemical Sensors. Nat. Commun. 2025, 16, 8347. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.F.; Fernández, E.; Valverde, A.; Gaboardi, M.; Salazar, H.; Petrenko, V.; Porro, J.M.; Cavalcanti, L.P.; Urtiaga, K.; Esperança, J.M.; et al. Exploring the Compositional Space of a Metal–Organic Framework with Ionic Liquids to Develop Porous Ionic Conductors for Enhanced Signal and Selectivity in VOC Capacitive Sensors. J. Mater. Chem. A 2024, 12, 14595–14607. [Google Scholar] [CrossRef]
- Thuy Nguyen, L.H.; Mirzaei, A.; Kim, J.-Y.; Bach Phan, T.; Dai Tran, L.; Wu, K.C.-W.; Woo Kim, H.; Sub Kim, S.; Hoang Doan, T.L. Advancements in MOF-Based Resistive Gas Sensors: Synthesis Methods and Applications for Toxic Gas Detection. Nanoscale Horiz. 2025, 10, 1025–1053. [Google Scholar] [CrossRef]
- Okur, S.; Hashem, T.; Bogdanova, E.; Hodapp, P.; Heinke, L.; Bräse, S.; Wöll, C. Optimized Detection of Volatile Organic Compounds Utilizing Durable and Selective Arrays of Tailored UiO-66-X SURMOF Sensors. ACS Sens. 2024, 9, 622–630. [Google Scholar] [CrossRef]
- Okur, S.; Zhang, Z.; Sarheed, M.; Nick, P.; Lemmer, U.; Heinke, L. Towards a MOF E-Nose: A SURMOF Sensor Array for Detection and Discrimination of Plant Oil Scents and Their Mixtures. Sens. Actuators B Chem. 2020, 306, 127502. [Google Scholar] [CrossRef]
- Sun, H.; Tian, F.; Liang, Z.; Sun, T.; Yu, B.; Yang, S.X.; He, Q.; Zhang, L.; Liu, X. Sensor Array Optimization of Electronic Nose for Detection of Bacteria in Wound Infection. IEEE Trans. Ind. Electron. 2017, 64, 7350–7358. [Google Scholar] [CrossRef]
- Qin, P.; Day, B.A.; Okur, S.; Li, C.; Chandresh, A.; Wilmer, C.E.; Heinke, L. VOC Mixture Sensing with a MOF Film Sensor Array: Detection and Discrimination of Xylene Isomers and Their Ternary Blends. ACS Sens. 2022, 7, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, H.; Cheng, J.; Ma, X.; Fu, Y. Advances in Metal Oxide Semiconductor Gas Sensor Arrays Based on Machine Learning Algorithms. J. Mater. Chem. C 2025, 13, 4285–4303. [Google Scholar] [CrossRef]
- Zong, S.; Zhang, Y.; Qin, C.; Bala, H.; Cao, J.; Wang, Y. Lanthanum Doped SnO/SnO2 Rod-like Structure Sensors with High Sensitivity and Selectivity toward HCHO Detection. J. Alloys Compd. 2025, 1010, 177829. [Google Scholar] [CrossRef]
- Benedetto, G.; Damacet, P.; Shehayeb, E.O.; Fabusola, G.; Simon, C.M.; Mirica, K.A. Metal–Organic Framework-Based Chemiresistive Array Paired with Machine Learning Algorithms for the Detection and Differentiation of Toxic Gases. ACS Sens. 2025, 10, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Zhou, Q.; Zheng, C.; Wang, J. Challenges and Applications of Volatile Organic Compounds Monitoring Technology in Plant Disease Diagnosis. Biosens. Bioelectron. 2023, 237, 115540. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y. Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors. Materials 2022, 15, 8728. [Google Scholar] [CrossRef]
- Jasuja, H.; Huang, Y.; Walton, K.S. Adjusting the Stability of Metal–Organic Frameworks under Humid Conditions by Ligand Functionalization. Langmuir 2012, 28, 16874–16880. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, M.; Fan, S.; Gao, C.; Ma, Z.; Hou, D. Hydrophobic MOF/PDMS-Based QCM Sensors for VOCs Identification and Quantitative Detection in High-Humidity Environments. ACS Appl. Mater. Interfaces 2024, 16, 7721–7731. [Google Scholar] [CrossRef]
- Singh, A.; Jhao, W.-C.; Chaudhary, P.; Lin, M.-F. UV-Enhanced, Humidity-Tolerant Formaldehyde Chemiresistor Based on Ce-MOF-Derived CeO2 Nanospheres Decorated 1D/2D Polyaniline Nanohybrid. Adv. Mater. Technol. 2025, e01495. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, L.; Chang, Y.; Liu, M. Gas Sensing Based on Metal-Organic Frameworks: Concepts, Functions, and Developments. J. Hazard. Mater. 2022, 429, 128321. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z. Machine-Learning-Assisted High-Throughput Screening of High-Performance MOFs for Multicomponent Gas Separation. Ind. Eng. Chem. Res. 2025, 64, 2926–2936. [Google Scholar] [CrossRef]
- Lalawmpuia, R.; Lalhruaitluangi, M.; Lalhmunsiama; Tiwari, D. Metal Organic Framework (MOF): Synthesis and Fabrication for the Application of Electrochemical Sensing. Environ. Eng. Res. 2024, 29, 230636. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Hadley, T.D.; Batten, M.P.; Constanti-Carey, K.; Barton, T.; Marley, D.; Mönch, A.; Lim, K.-S.; Hill, M.R. Scalability of Continuous Flow Production of Metal–Organic Frameworks. ChemSusChem 2016, 9, 938–941. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, S.; Tang, Y.; Yu, F.; Li, Z.; Pang, H. Recent Progress of MOF/MXene-Based Composites: Synthesis, Functionality and Application. Coord. Chem. Rev. 2023, 490, 215208. [Google Scholar] [CrossRef]
- Baba, T.; Janairo, L.G.; Maging, N.; Tañedo, H.S.; Concepcion, R.; Magdaong, J.J.; Bantang, J.P.; Del-amen, J.; Culaba, A. Advancements in Chemiresistive and Electrochemical Sensing Materials for Detecting Volatile Organic Compounds in Potato and Tomato Plants. AgriEngineering 2025, 7, 166. [Google Scholar] [CrossRef]
- Tomić, M.; Šetka, M.; Vojk\uuvka, L.; Vallejos, S. VOCs Sensing by Metal Oxides, Conductive Polymers, and Carbon-Based Materials. Nanomaterials 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Sung, I.-T.; Cheng, Y.-H.; Hsieh, C.-M.; Lin, L.-C. Machine Learning for Gas Adsorption in Metal–Organic Frameworks: A Review on Predictive Descriptors. Ind. Eng. Chem. Res. 2025, 64, 1859–1875. [Google Scholar] [CrossRef]
- Deeraj, B.; Jayan, J.S.; Raman, A.; Asok, A.; Paul, R.; Saritha, A.; Joseph, K. A Comprehensive Review of Recent Developments in Metal-Organic Framework/Polymer Composites and Their Applications. Surf. Interfaces 2023, 43, 103574. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Bo, G.; Xu, X.; See, K.W.; Johannessen, B.; Pang, W.K. Operando Synchrotron X-Ray Absorption Spectroscopy: A Key Tool for Cathode Material Studies in Next-Generation Batteries. Adv. Sci. 2025, 12, 2414480. [Google Scholar] [CrossRef] [PubMed]
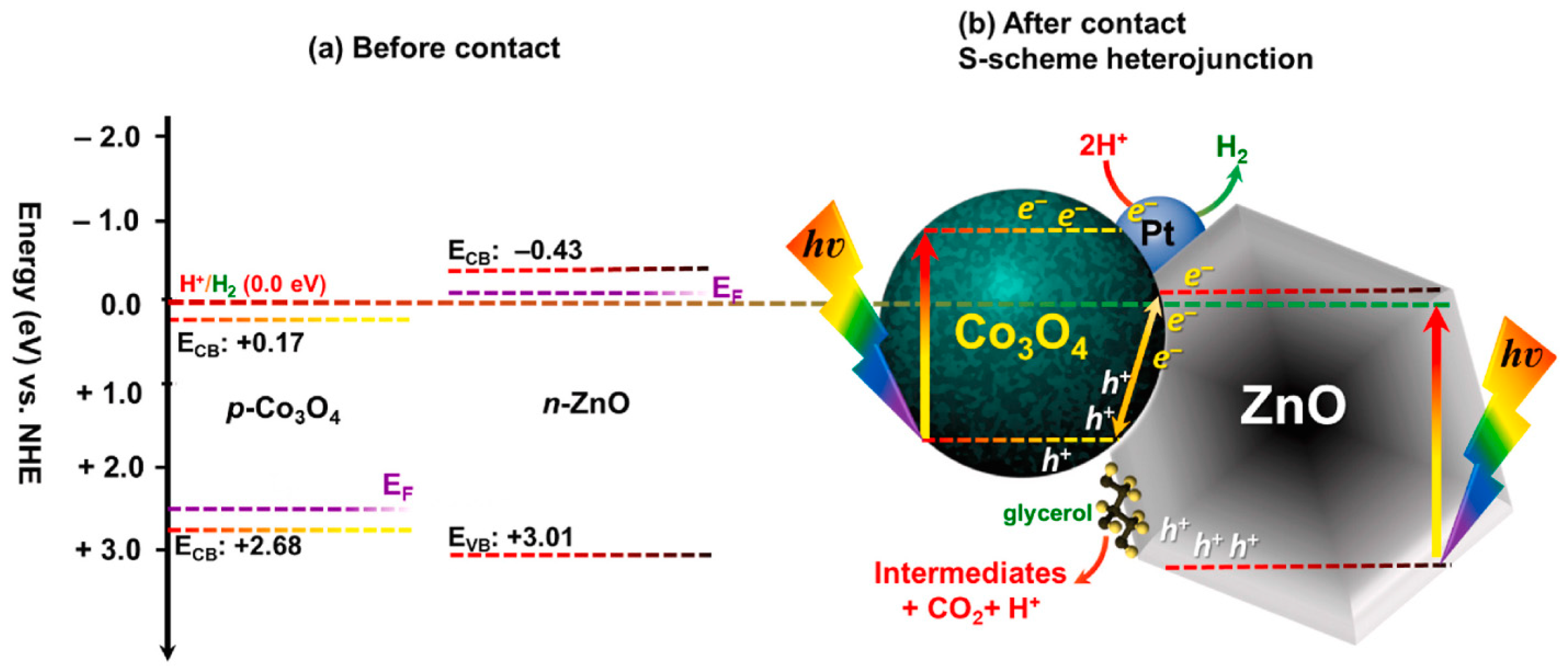

| MOF Precursor | Synthesis Method | Key Parameters | Derived Material | Key Structural Features | References |
|---|---|---|---|---|---|
| ZIF-8 | Pyrolysis in air | 400–600 °C | Porous ZnO | Hollow nanocages, polyhedra, high surface area | [30] |
| ZIF-67 | Pyrolysis in air | 350–500 °C | Porous Co3O4 | Hierarchical porous structure, hollow nanocubes | [31] |
| Bimetallic ZIF (Zn/Co) | Pyrolysis in air | 500 °C | Co3O4@ZnO | p-n heterojunction, porous microspheres | [45] |
| UiO-66 | Calcination | 500–600 °C | Porous ZrO2 | Retained crystal morphology, high thermal stability | [53] |
| MIL-101(Fe) | Pyrolysis | >330 °C | Fe2O3/Carbon | Porous iron oxide with carbon matrix | [54] |
| Bimetallic PBA (M/Fe) | Calcination | 400–600 °C | MFe2O4 Spinel Oxides | Inverse/normal spinel structures, nanocubes | [55] |
| MOF-5 (Zn) | Calcination | 450–800 °C | ZnO nanoparticles | Controllable crystallite size | [56] |
| ZIF-8 | Calcination/Pyrolysis | 300–600 °C | N-doped ZnO | Porous/hollow ZnO with N-defects, increased surface O− | [57] |
| ZIF-8 | Calcination + Ag loading | 450–600 °C | ZnO/Ag micro-octahedra | Plasmonic sites, abundant grain boundaries | [58] |
| ZIF-8 | Pyrolysis; Ag nanocrystal decoration | 450–600 °C | Ag–ZnO hollow structures | Hollow cages, highly dispersed Ag, boosted TEA sensing | [59] |
| ZIF-67 | Pyrolysis | 350–500 °C | Co3O4 hollow nanocages | Double-shelled/hollow dodecahedra, rich lattice O | [60] |
| ZIF-67 → ZIF-67@ZIF-8 (core–shell) | MOF@MOF then calcination | 400–600 °C | Co3O4/ZnO nano-heterostructure | Core–shell-derived p–n junction, mesoporous | [61] |
| ZIF-8 (with Cu modification) | Ion-exchange + calcination | 450–600 °C | CuO/ZnO hollow nanocages | Hollow polyhedra, multiple redox sites | [62] |
| UiO-66 | Calcination | 500–700 °C | ZrO2@C | Mesoporous ZrO2 with carbon residue; temperature-tunable pores | [63] |
| UiO-66 | Derived coating via calcination | — | ZrO2 protective layer | Conformal, porous ZrO2 film; high thermal stability | [64] |
| MIL-101(Fe) | Pyrolysis | 700–800 °C | Fe2O3/C composite | Porous Fe2O3 embedded in conductive carbon | [65] |
| Prussian Blue Analogue (Co/Fe) | Annealing | 350–500 °C | CoFe2O4 nanocubes | Spinel nanocubes from PBA template | [66] |
| MOF-5 (Zn) | Calcination of Pd@MOF-5 | 400–600 °C | Pd/PdO@ZnO | Noble-metal-sensitized ZnO, abundant oxygen vacancies | [67] |
| UiO-66 (Zr) + Cu source | Calcination | — | Cu NWs@ZrO2 | Metal/ZrO2 composite with uniform MOF-derived ZrO2 | [68] |
| Sensing Material | Operating Temp. (°C) | Target Conc. (ppm) | Response (Ra/Rg or S%) | Response/Recovery Time (s) | LOD (ppb/ppm) | References |
|---|---|---|---|---|---|---|
| MOF-derived Co3O4 | 140 | 50 | 27.6 | -/- | 0.1 ppm | [31] |
| ZIF-8 derived Co3O4@ZnO | 280 | 50 | ~18 | 15/19 | 0.5 ppm | [45] |
| MOF-derived NiFe2O4 | 240 | 100 | ~15 | 13/8 | 1 ppm | [55] |
| Bi-gallate MOF/CS/IL | 60 | 10 | ~1.5 | 15/3 | <10 ppm | [80] |
| Sensing Material | Operating Temp. (°C) | Target Conc. (ppm) | Response (Ra/Rg or S%) | Response/Recovery Time (s) | LOD (ppb/ppm) | References |
|---|---|---|---|---|---|---|
| Sn-MOF derived SnO2 | 120 | 10 | 10,000 | 33/142 | <10 ppb | [77] |
| Co5-MOF derived Co3O4 | 170 | 200 | ~14 | -/- | 10 ppm | [51] |
| ZIF-67 derived Cu-Co Oxides | 108 | 50 | 90% conversion | -/- | - | [83] |
| MOF-derived ZZS HNFs | 220 | 100 | 25.7 | 12/18 | - | [82] |
| MOF-derived porous SnO2 prisms | 120 | 2 | 882 | 19/– | – | [84] |
| MOF-derived ZnO (UV-assisted) | ~25 (UV on) | – | – | – | 100 ppb | [85] |
| Pt/ZnO–In2O3 hollow nanofibers (derived from Pt@ZIF-8) | 180 | 100 | 48.3 | 5/22 | 74.6 ppb | [86] |
| Co-MOF-derived Co3O4 (3D) | 170 | 100 | – | – | 10 ppm | [51] |
| ZIF-67-assisted Co3O4/In2O3 composite (MOF-templated) | – | 10 | (improved vs. In2O3) | – | – | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, S.; Zhu, J.; Fu, L. MOF-Derived Catalytic Interfaces for Low-Temperature Chemiresistive VOC Sensing in Complex Backgrounds. Chemosensors 2025, 13, 386. https://doi.org/10.3390/chemosensors13110386
Zhang L, Zhao S, Zhu J, Fu L. MOF-Derived Catalytic Interfaces for Low-Temperature Chemiresistive VOC Sensing in Complex Backgrounds. Chemosensors. 2025; 13(11):386. https://doi.org/10.3390/chemosensors13110386
Chicago/Turabian StyleZhang, Lu, Shichao Zhao, Jiangwei Zhu, and Li Fu. 2025. "MOF-Derived Catalytic Interfaces for Low-Temperature Chemiresistive VOC Sensing in Complex Backgrounds" Chemosensors 13, no. 11: 386. https://doi.org/10.3390/chemosensors13110386
APA StyleZhang, L., Zhao, S., Zhu, J., & Fu, L. (2025). MOF-Derived Catalytic Interfaces for Low-Temperature Chemiresistive VOC Sensing in Complex Backgrounds. Chemosensors, 13(11), 386. https://doi.org/10.3390/chemosensors13110386






