Abstract
Blood glucose monitoring is essential for the treatment of diabetes, a chronic disease that affects millions of people worldwide. Non-electrochemical blood glucose sensors often lack sensitivity and selectivity, especially in complex biological fluids, and are not suitable for wearable point-of-care devices. Electrochemical blood glucose sensors, on the other hand, are easy to handle, inexpensive, and offer high sensitivity and selectivity even in the presence of interfering molecules. They can also be seamlessly integrated into wearable devices. This review explores the key blood glucose technologies, emphasizing the operating principle and classification of electrochemical glucose sensors. It also highlights the role of functional solid–liquid interfaces in optimizing sensor performance. Recent developments in solid–liquid interfacial materials, including metal-based, metal oxide-based, carbon-based, nanoparticle-based, conductive polymer, and graphene-based interfaces, are systematically analyzed for their sensing potential. Furthermore, this review highlights existing patents, the evolving market landscape, and data from clinical studies that bridge the gap between laboratory research and commercial application. Finally, we present future perspectives and highlight the need for next-generation wearable and enzyme-free glucose sensors for continuous and non-invasive glucose monitoring.
1. Introduction
Glucose sensing plays a critical role in various healthcare applications, particularly in the timely detection and monitoring of chronic diseases such as diabetes, which, according to current statistics, is the leading cause of death and disability worldwide [1]. According to the International Diabetes Federation (IDF) and the World Health Organization (WHO), the number of diabetics is expected to increase significantly in the coming decades, from 422 million in 2023 to an estimated 700 million in 2030 [2]. Moreover, elevated blood glucose levels have been reported to have adverse effects on the kidneys, heart, and vision [3]. If the muscles and liver fail to store postprandial glucose as glycogen, hyperglycemia (>120 mg/dL) can easily result [4]. Conversely, hypoglycemia resulting from a marked drop in blood glucose level (<70 mg/dL) impairs brain functions and leads to symptoms such as seizures, loss of consciousness, and cardiovascular risks. Therefore, it is important to regulate the amount of glucose in body fluids by appropriate glucose measurement [5].
Glucose sensors were first developed in 1962, when Clark and Lyons developed the first enzyme biosensor that exclusively measured glucose [6]. In 1975, Yellow Springs Instruments (YSI) launched the first commercial blood glucose meter, and in 1987, Medisense launched the first glucose meter [7]. Since then, interest in the development of glucose biosensors and their impact on the biosensor market has grown enormously (85% of the market consists of glucose sensors or glucose meters) [8]. This trend continues to be strong, as glucose biosensors are among the best point-of-care devices in the biosensor industry [9].
Numerous techniques have been developed for the determination of serum glucose, including enzyme electrodes, the hexokinase method, spectrophotometry, high-performance liquid chromatography, and glucose test strips [10]. Colorimetry, surface plasmon resonance, electrochemistry, chemiluminescence, and fluorescence are among the intensively researched techniques for glucose determination in the laboratory [11]. Electrochemical sensors function by monitoring changes in electrical properties such as potential and current induced by chemical reactions between the analyte and the electrodes and are used to identify and measure target analytes [12]. They are considered superior to all other glucose determination techniques due to their high sensitivity in determining glucose levels [13], fast response times [14], low cost [15], and small size [16], as well as their high selectivity for glucose, which reduces interference from other substances [17].
The WHO has established a set of standards for evaluating point-of-care (POC) tests and emphasized the importance of developing POC tests. Electrochemical sensors meet these standards and are well-suited for POC applications [18]. However, it should be noted that there is a distinct difference between blood-based glucose monitoring and interstitial fluid (ISF) glucose monitoring. Blood-based electrochemical sensors measure glucose concentration directly in capillary or venous blood [19] and provide immediate and highly accurate readings based on enzymatic reactions, usually mediated by glucose oxidase or dehydrogenase, at the electrode surfaces [20]. In contrast, ISF-based electrochemical sensors measure glucose levels in the interstitium of the subcutaneous tissue with a physiological time delay of approximately 5–10 min compared to blood glucose levels [21].
Continuous long-term electrochemical measurement of glucose directly in blood, while conceptually attractive, is practically unachieved in human clinical settings due to ongoing biophysical, biochemical, and material-related challenges [22]. Compared to interstitial fluid, whole blood presents more challenges such as thrombosis, clot formation, protein adsorption [23], electrode fouling by proteins, cells, or reactive species [24], degradation of enzymatic recognition elements [25], passivation of non-enzymatic catalysts [26], and mechanical shear forces [23]. Commercial CGMs such as Dexcom and FreeStyle Libre use minimally invasive ISF sensors under the skin, where biofouling, clotting, and shear forces are lower, allowing stability for several days to weeks [27]. Consequently, it has been argued that no clinically viable blood-based CGM system currently exists and the concept of clinically practical long-term continuous glucose monitoring must remain limited to ISF-based devices rather than blood-based electrochemical sensors unless significant breakthroughs in antifouling strategies and sensor designs are achieved [28].
Furthermore, metal oxides and metal sulfides are widely used as catalysts in non-enzymatic electrochemical glucose sensors due to their lower band gap, higher conductivity, improved stability, different chemical states, and effective ability of glucose oxidation [11]. The exceptional electrical conductivity, hydrophilicity, tunable structure, biocompatibility, and high surface area of MXene make it a promising material for electrochemical sensing applications [29]. Over 40 different types of MXene materials have been synthesized in the last 15 years since Gogotsi et al. prepared the first type of MXene (Ti3C2Tx) in 2011 [30]. Organic ligands and metal ions or clusters self-assemble to form metal–organic frameworks (MOFs), extremely porous materials for electrochemical sensing applications [31]. MOFs exhibit exposed active sites that produce remarkable catalytic properties, including the lowest LOD and exceptional sensing efficiency [32].
The concept of electrochemical blood glucose monitoring and the design of functional solid–liquid interfaces have evolved over the years. Early clinical intravascular work (e.g., GlySure) demonstrated short-term accuracy in intensive care units, but the fundamental problems of invasiveness, limited lifetime, and signal drift under real blood flow led to a transition to subcutaneous approaches for chronic monitoring [33]. Recent studies using nanomaterials and non-enzymatic electrodes have demonstrated improved sensitivity and limit of detection (LOD) under in vitro conditions, although these gains have not yet translated to in vivo or clinical performance. Despite improved in vitro performance, active sites in blood are rapidly passivated due to protein and cell deposition and shear forces, limiting their effectiveness in practice [34]. Therefore, there have been parallel advances in antifouling chemistry, demonstrating reproducible reductions in protein adsorption and platelet activation [35]. Complementary studies on biological strategies have demonstrated combined active antithrombotic release and permselective membrane activity, but release-based strategies are inherently time-limited [36]. A critical synthesis highlights that the future development of blood-based electrochemical CGMs remains an unmet engineering and clinical challenge. Future research should focus on integrated sensor designs; combining nanostructured electrodes, ultrathin antifouling coatings and permselective layers to balance transport-reaction-fouling dynamics; combining non-enzymatic catalysts with robust antifouling strategies rather than solely increasing catalytic activity; and establishing standardized in vivo protocols for measuring protein adsorption, thrombogenicity, and sensor performance under dynamic flow conditions [37].
This review highlights the advances in electrochemical blood glucose sensors based on solid–liquid interfaces, focusing on research findings from the past five years, shown in Figure 1. Emerging technologies for blood glucose sensing and the functionalization of solid–liquid interfaces for electrochemical glucose sensing have been discussed. Through a thorough analysis of various types of electrochemical sensors and solid–liquid interfaces for electrochemical blood glucose sensing, the associated challenges and potential solutions are highlighted. This review aims to provide a comprehensive assessment that will guide further studies and developments.
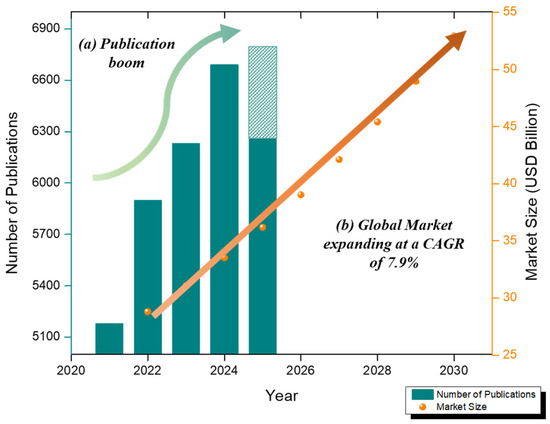
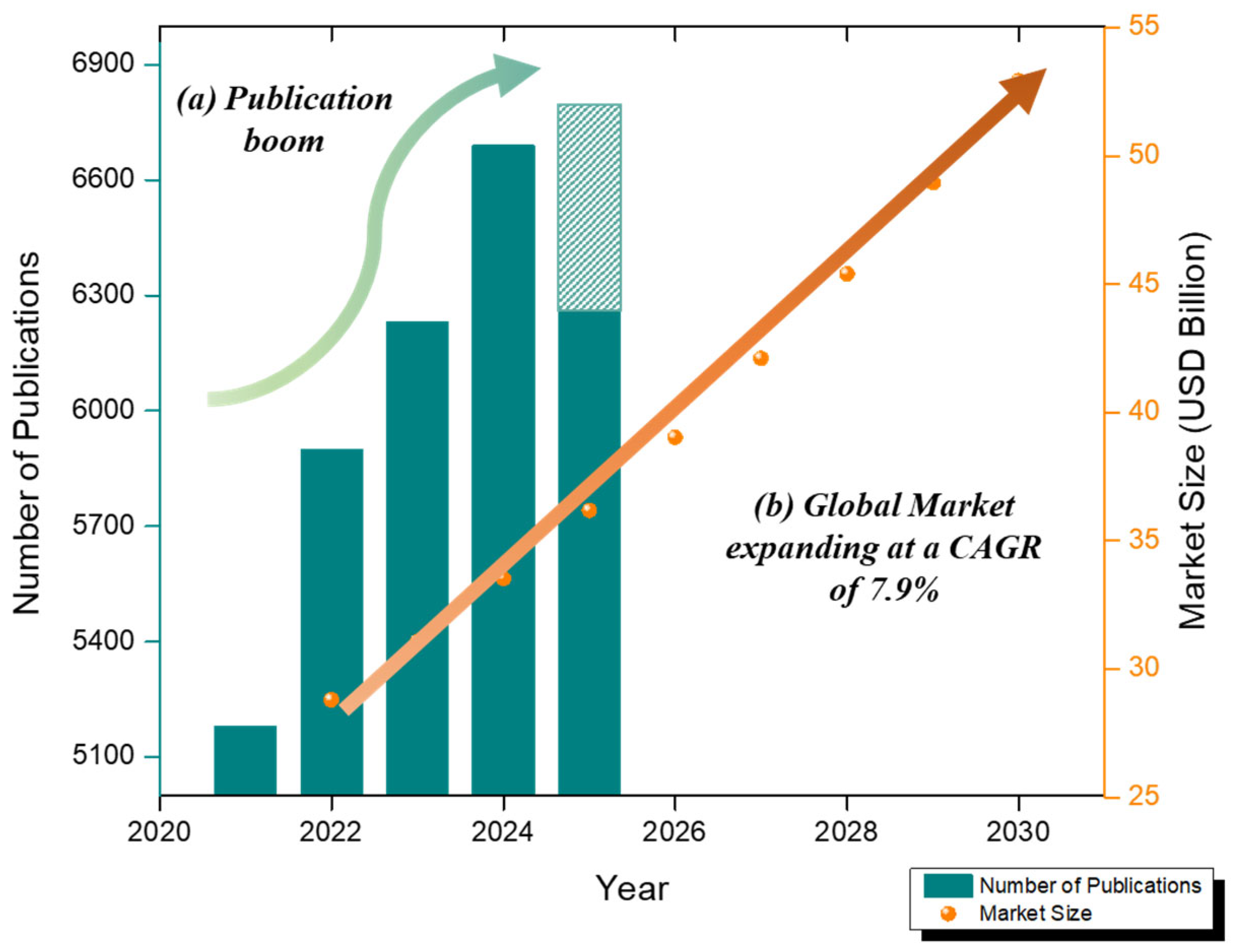
Figure 1.
(a) The number of articles published on electrochemical ‘blood glucose’ sensors in the last 5 years (data collected from database Google Scholar as of 11 August 2024)—crayon line. (b) Global biosensor market growth (2022–2030) at CAGR 7.9%—orange line.
2. Market Landscape of Electrochemical Glucose Sensors
Electrochemical glucose sensors are experiencing rapid growth due to the increasing demand for 2D nanomaterial-based sensors and wearable patch-based innovations. The biosensor market was valued at USD 28.80 billion in 2022 and is expected to grow at a compound annual growth rate of 7.9% between 2023 and 2030, reaching USD 52.93 billion in 2030 [38]. A Scopus analysis (2013–2023) recorded 2529 publications on electrochemical glucose sensors, with increasing annual production indicating accelerated scientific progress [39]. Commercial CGMs (Dexcom G6/G7, Abbott FreeStyle Libre) are enzymatic and invasive, but wearable and non-invasive technologies are constantly evolving [40]. Figure 1 summarizes the number of articles published on electrochemical blood glucose sensors in the last 5 years (data from the Google Scholar database as of 11 August 2024) and the growth of the global biosensor market (2022–2030).
The market for biosensors made of graphene and 2D materials is reported to grow from approximately USD 100 million in 2020 to an expected USD 700 million by 2031, primarily driven by the development of glucose sensors [41]. Two-dimensional nanomaterials (graphene, transition metal dichalcogenides, BP/g-CN composites) have revolutionized sensor sensitivity, selectivity, and stability in both enzymatic and non-enzymatic sensors. New wearable patches (e.g., BP/g CN-based) are in the proof-of-concept phase, while field tests have not yet been widely reported. A novel patch with a BP/g CN heterostructure demonstrates a sensitivity of approximately 1.1 µA mM−1 cm−2 for sweat glucose measurement and features NFC readout capabilities [42]. Platforms integrating smartphones and NFC potentiostats with AuNP/PEDOT-PSS Prussian blue/carbon electrodes report detection limits close to 0.15 µM and linear ranges of 0.5–500 µM [43]. Despite these advances, addressing stability issues, biofouling, and interference from other electroactive species remains a crucial aspect of implementing electrochemical blood glucose sensors in clinical practice and real-world applications. Researchers have shown increasing interest in the development of solid–liquid interfaces for electrochemical blood glucose detection in recent years.
3. Methods of Blood Glucose Sensing
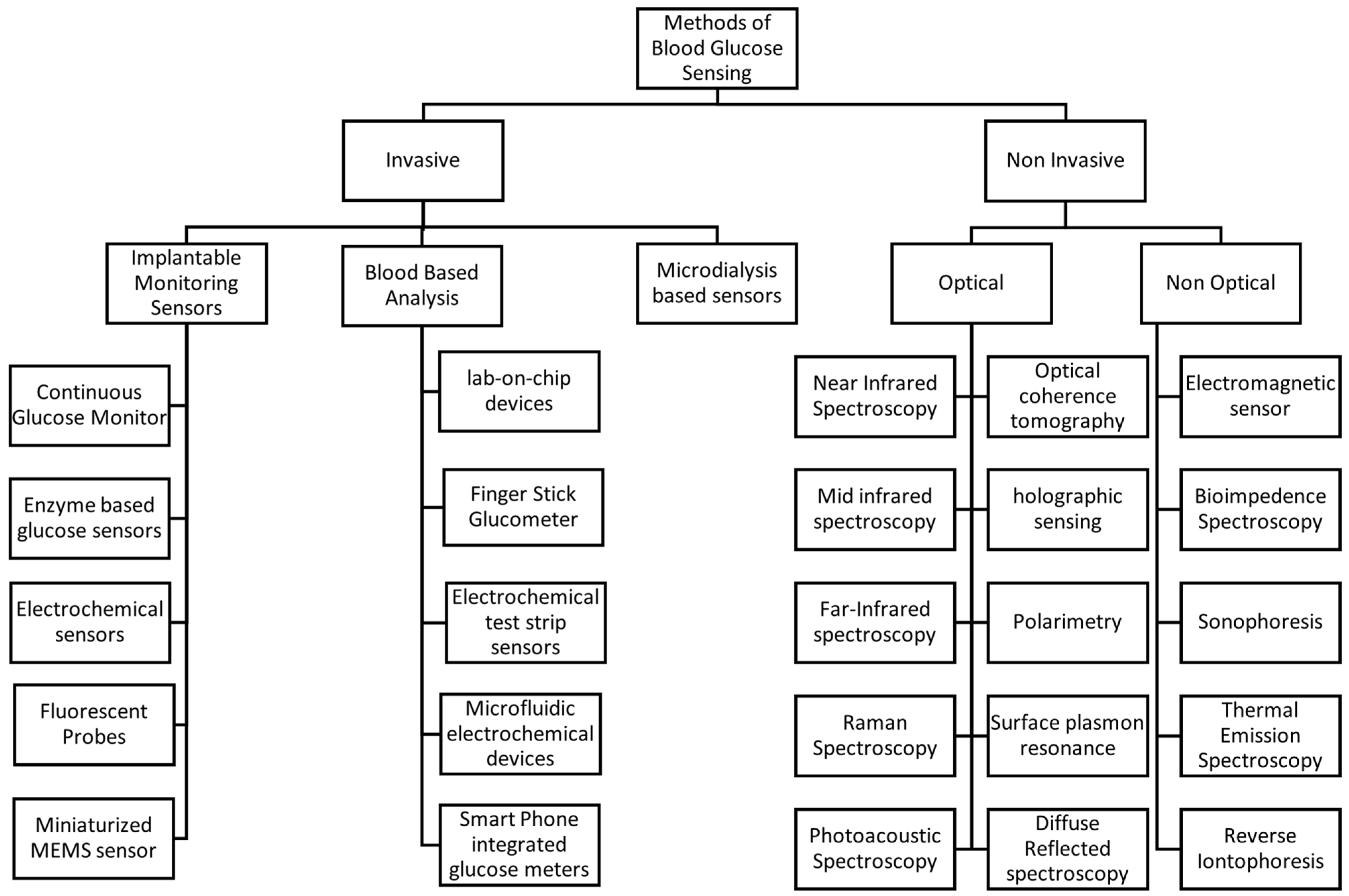
Monitoring blood glucose levels is essential for the treatment of diabetes. Various methods have been developed, which can be broadly divided into invasive and non-invasive techniques [44]. Figure 2 shows the selective methods commonly used for blood glucose measurement.
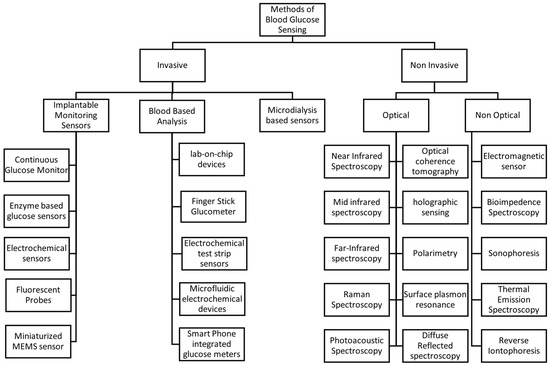
Figure 2.
Methods of blood glucose sensing.
3.1. Invasive Methods
Invasive sensors include implantable glucose sensors, which are inserted under the skin for glucose monitoring [45]. These devices often use electrochemical sensors that detect glucose levels via enzymatic reactions, which generate an electrical signal proportional to the glucose concentration [46].
Conventional glucose meters use an invasive approach; they require a small blood sample obtained by a finger prick. The sample is applied to a test strip containing enzymes that react with glucose, producing a measurable electrical signal. Although this method is generally accurate, it can be inconvenient and uncomfortable for frequent measurements [47]. Fluorescent probes represent another approach in implantable glucose sensors, where glucose binding induces a change in fluorescence intensity, enabling quantification of glucose levels [44]. Microdialysis involves inserting a probe into subcutaneous tissue to collect interstitial fluid for glucose analysis [48].
3.2. Non-Invasive Methods
Non-invasive methods include both optical and non-optical methods. Optical methods aim to measure glucose levels without penetrating the skin, using various spectroscopic techniques including Near-Infrared (NIR) Spectroscopy [49], Mid-Infrared (MIR) Spectroscopy [50], Far-Infrared (FIR) Spectroscopy [50], Raman Spectroscopy [51], Photoacoustic Spectroscopy [52], Optical Coherence Tomography (OCT) [53], Holographic Sensing [54], surface plasmon resonance (SPR) and Diffuse Reflectance Spectroscopy [28]. Non-optical methods employ different physical principles for glucose detection; for example, Electromagnetic Sensors detect changes in the dielectric properties of tissues caused by fluctuations in glucose levels, utilizing microwave or radiofrequency waves [55]. Bioimpedance Spectroscopy measures the electrical impedance of biological tissues, which varies with glucose concentration due to changes in cellular and extracellular fluid composition [56]. Sonophoresis employs ultrasound to enhance skin permeability, facilitating the extraction and measurement of glucose [57]. Thermal Emission Spectroscopy detects infrared radiation emitted by the body, which varies with glucose-induced changes in metabolic heat production [58], and reverse iontophoresis applies a mild electric current to extract the fluid through the skin, enabling glucose measurement without the need for blood sampling [59].
These methods represent the diverse approaches being explored and implemented for glucose monitoring, each with its advantages and limitations. Ongoing research aims to improve the accuracy, convenience, and affordability of these technologies to enhance diabetes management.
3.3. Challenges Associated with Blood Glucose Sensing
Optical techniques, including Raman Spectroscopy and Near-Infrared Spectroscopy, are among the most extensively studied non-invasive approaches to glucose monitoring [60]. Despite their promise, these methods face challenges such as low signal intensity and interference from biological tissues. Factors like skin tone, hydration levels, and temperature variations can significantly impact measurement accuracy, necessitating advanced signal processing and frequent calibration [49]. Colorimetric detection is based on glucose oxidase-mediated reactions that produce a color change in the presence of glucose [61]. Although this method is user-friendly, it is limited by subjective interpretation of color intensity, short reagent shelf life, and susceptibility to environmental factors such as humidity and temperature [62]. Fluorescent and luminescent sensors operate based on fluorescence quenching or enhancement mechanisms in the presence of glucose [63]. While they offer high sensitivity, their performance can be compromised by photobleaching and sensitivity to pH variations [64]. Moreover, miniaturizing these sensors and integrating them into compact, wearable devices remains a significant technical challenge [65].
Electrochemical sensing, especially using enzyme-based ampere-metric sensors, has become the gold standard for glucose detection, owing to several intrinsic advantages. The incorporation of specific enzymes, such as glucose oxidase, ensures high selectivity for glucose over other biomolecules [19]. The electron transfer reaction produces a measurable current that is linearly proportional to glucose concentration, enabling highly sensitive detection even at low glucose levels [66]. Electrochemical sensors are cost-effective to manufacture and can be mass-produced using techniques such as screen printing and microfabrication [67]. This makes them suitable for integration into portable glucometers and wearable CGMs [68]. Electrochemical sensors deliver near-instant results typically within 5 to 10 s, making them well-suited for real-time monitoring [69]. Their robust design, incorporating protective membranes and stabilizing agents, helps minimize sensitivity to variations in temperature, pH, and humidity [70]. Modern electrochemical sensors incorporate selectively permeable membranes or redox mediators to reduce interference from common blood constituents such as ascorbic acid, uric acid, and acetaminophen [71].
Moreover, the development of continuous glucose monitoring (CGM) systems based on electrochemical sensors remains a major unfulfilled challenge. Continuous glucose monitoring (CGM) systems face several challenges that limit their long-term accuracy and stability. A major limitation arises from signal drift, often caused by enzyme degradation or cofactor leaching under physiological conditions [72]. Biofouling further exacerbates this problem through the nonspecific adsorption of proteins, platelets, and cells to sensor surfaces. This creates a diffusion barrier that reduces electron transfer, particularly in blood-based sensors, whereas ISF-based devices exhibit less fouling [73]. Calibration dependence and a physiological delay of 5–15 min between blood and ISF glucose further complicate reliable measurements [74]. Biocompatibility, temperature, pH, and shear stress further influence sensor performance. Furthermore, there is a fundamental trade-off between protective coatings, which extend lifetime, and analyte diffusion, which regulates response time [75]. Overcoming these limitations requires integrative approaches combining materials science and surface engineering to achieve continuous, real-time glucose monitoring suitable for both ISF-based and intravascular applications.
Notably, signal drift continues to be a primary technical challenge for long-term sensor operation. Commercial CGMs integrate various calibration and drift-compensation methods to mitigate signal instability over time [76]. Although traditional single-point calibrations serve as an initial baseline, modern CGMs utilize adaptive algorithms to estimate sensor gain and offset drift in real time [77] and estimation techniques like RLS, Kalman filtering [78] and ML regression [79] to minimize MARD throughout device use [80]. Adaptive calibration algorithms dynamically adjust the sensor response according to time-dependent variations, compensating for gradual signal drift and minimizing the need for user recalibration [81]. A complementary dual-electrode scheme designed to mitigate signal drift incorporates a secondary reference electrode, allowing real-time baseline subtraction and isolation of glucose-specific signals [82]. Real-time baseline stabilization techniques such as adaptive filtering and moving-average correction effectively minimize transient potential shifts arising from biofouling or temperature fluctuations [83]. Collectively, these techniques improve sensor stability under physiological conditions, extending operational life and reducing the frequency of manual recalibration.
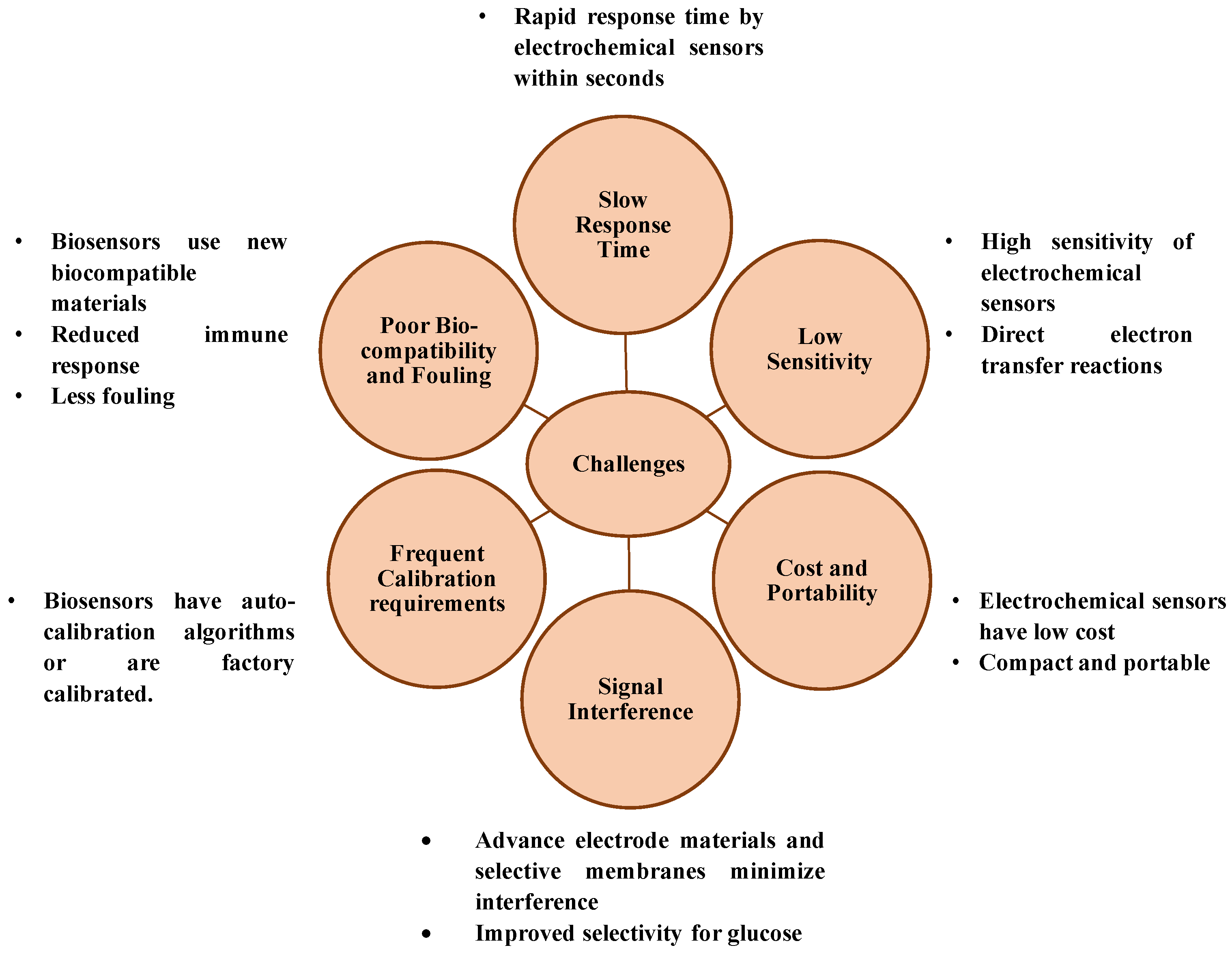
While innovative glucose sensing techniques continue to emerge, most face critical challenges that hinder their clinical adoption. Optical and colorimetric methods struggle with environmental sensitivity and interpretation errors, while non-invasive techniques face issues of biofluid correlation and contamination. In contrast, electrochemical sensing offers a balanced solution combining sensitivity, selectivity, affordability, and adaptability. As such, it remains the backbone of current and next-generation glucose monitoring technologies. Figure 3 describes the challenges of blood glucose sensing and role of electrochemical blood glucose sensors in overcoming it.
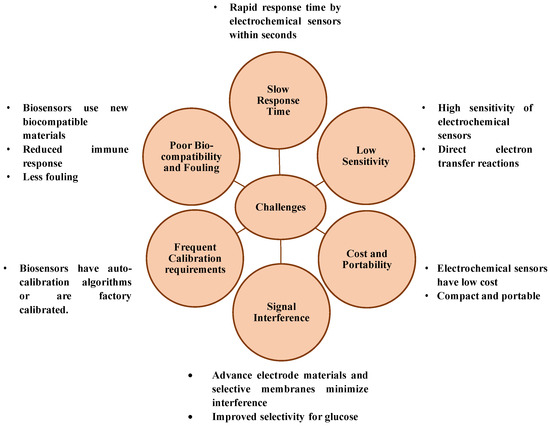
Figure 3.
Challenges of blood glucose sensing and role of electrochemical blood glucose sensors in overcoming it.
4. Electrochemical Sensing of Glucose in Blood
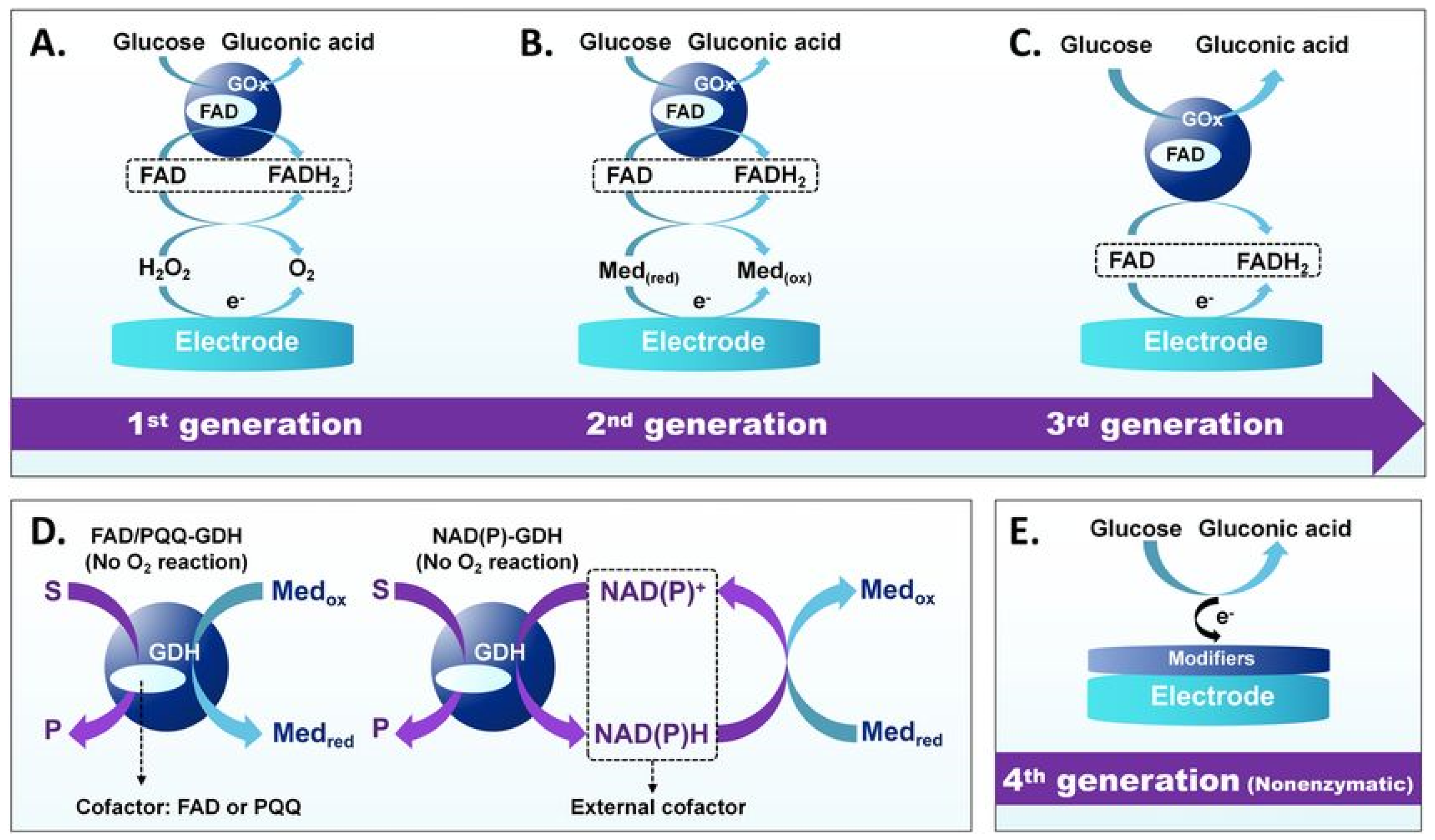
Electrochemical sensors offer a cost-effective and convenient solution for biochemical assays, and more importantly, they provide reliable detection across a wide range of analytes [84]. There are two main parts of electrochemical sensors. One is the receptor that recognizes the reacting substance and other is the transducer that converts the recognition into a measurable signal for detecting pollutants, medical tests and industrial monitoring [85]. The commercial use of electrochemical sensors started in the 1950s for monitoring industrial oxygen. Currently, many types of electrochemical sensors are available to improve detection sensitivity, selectivity and response time [86]. Moreover, the progression of electrochemical sensor technologies can be understood by the evolution of electron transfer pathways at solid–liquid interfaces, from direct enzymatic mechanisms in first-generation sensors to mediator-assisted and nanomaterial-facilitated electron transport in later generations, as shown in Figure 4.
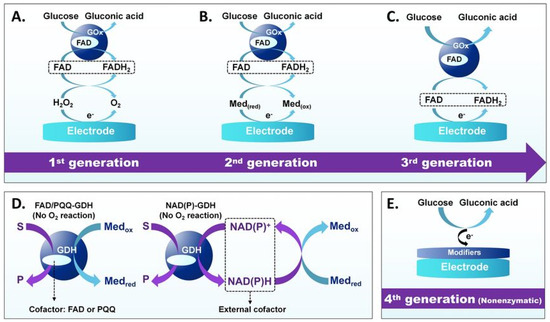
Figure 4.
Evolution of electrochemical glucose sensors’ operating principles. (A) First-generation GOx-based sensors, (B) second-generation sensors use artificial mediators, (C) third-generation sensors enable direct electron transfer, (D) GDH-based sensors operate at lower potentials, and (E) Fourth-generation nonenzymatic sensors. Adapted with permission from ref. [87]. Copyright 2023, Springer Nature.
4.1. Principle of Electrochemical Sensors
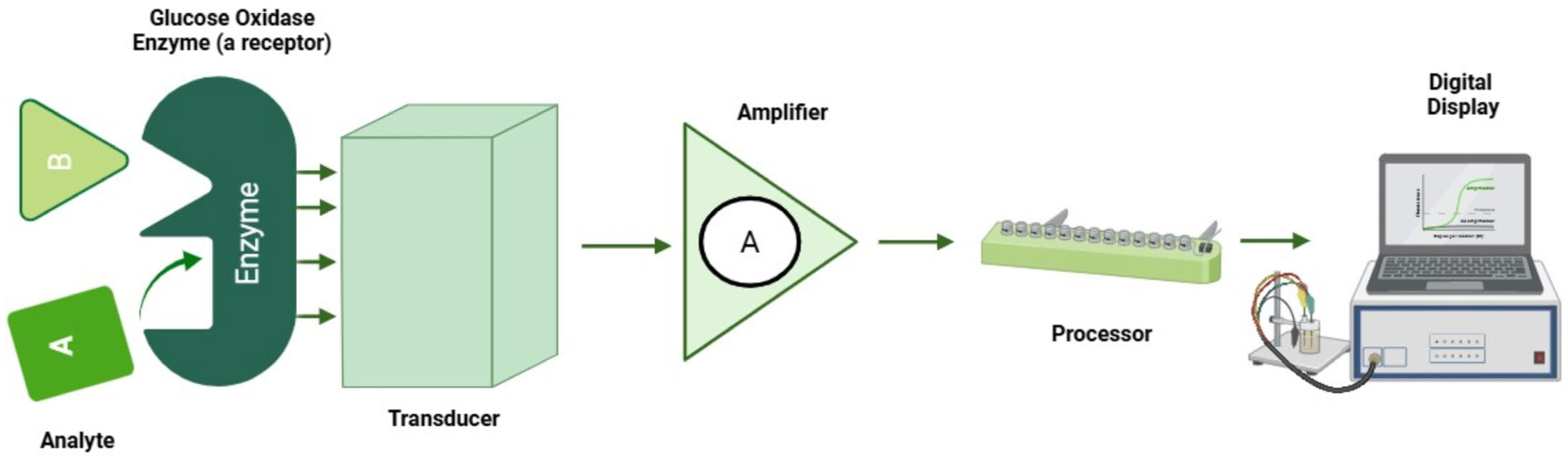
Electrochemical sensors function by detecting changes in electrical properties such as current, potential or conductivity resulting from chemical reactions occurring at the electrode surface. These reactions involve the analyte of interest, which undergoes either oxidation or reduction at the electrode, and the resulting electrical signal is directly correlated with the analyte concentration [12,88]. Figure 5 describes an electrochemical setup for glucose sensing and measurements.
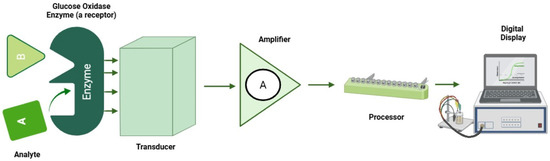
Figure 5.
Electrochemical setup for glucose sensing.
4.2. Types of Electrochemical Sensors
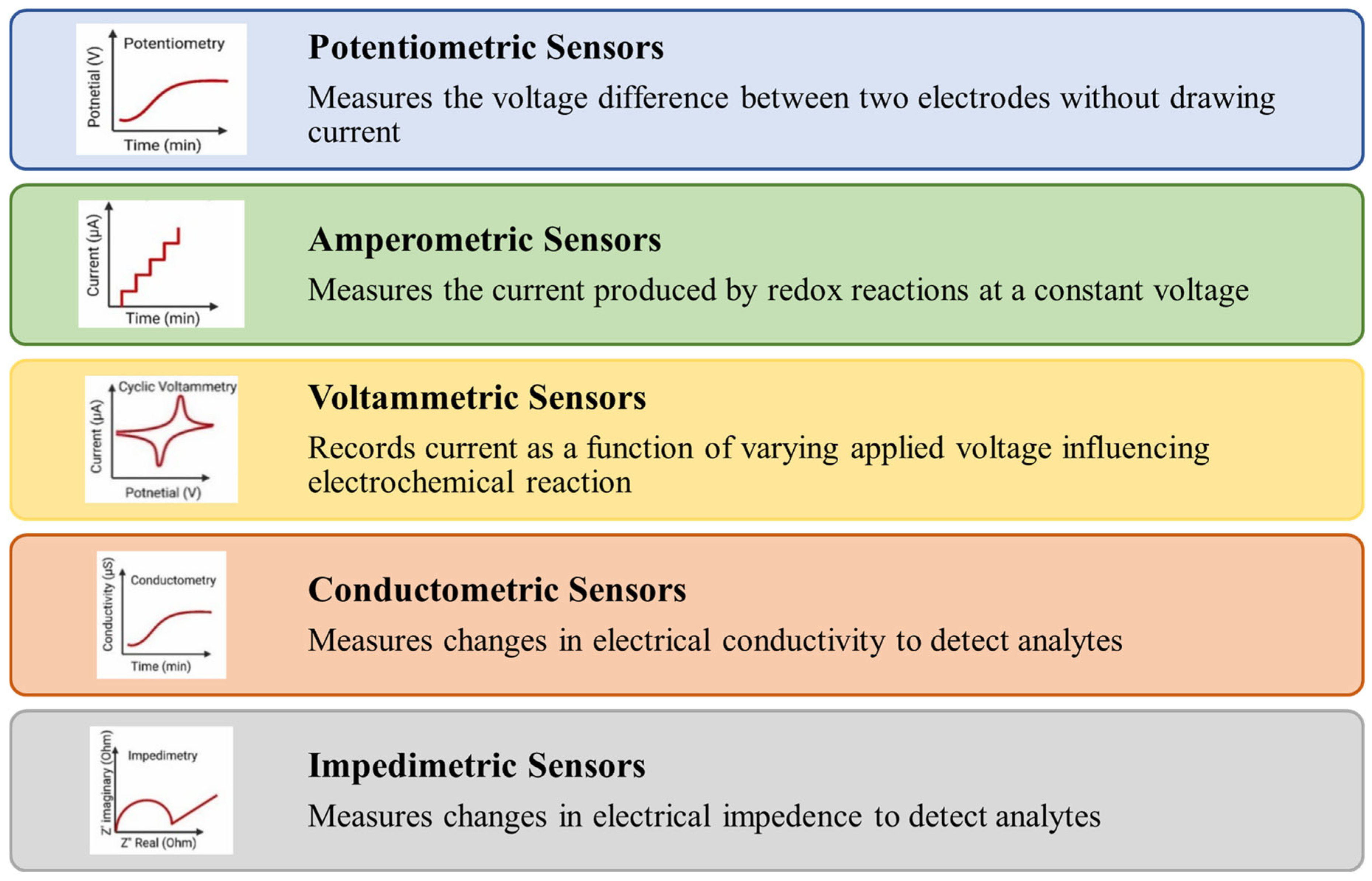
Electrochemical sensors are broadly classified into different types of sensors as shown in Figure 6. Potentiometric sensors based on ion-selective electrodes are used to detect the analyte concentration by determining potential changes between the working (ion-selective electrode) and reference electrodes [89].
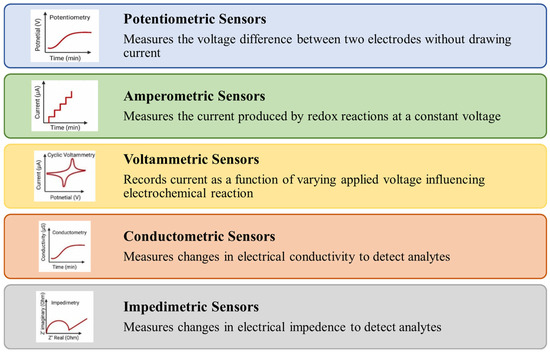
Figure 6.
Types of electrochemical sensors.
Amperometric sensors work by measuring changes in current resulting from redox reactions occurring at the electrode surface under a constant applied potential [90]. The magnitude of the current response is directly related to the concentration of the analyte, making this method highly effective for quantitative analysis at a fixed working potential [91]. Voltammetric methods measure the current in an electrochemical cell by applying a varying potential to an electrode and the resulting current is monitored over time. Voltammetry is capable of detecting multiple analytes simultaneously and is widely regarded as a versatile biosensing technique due to its high sensitivity and rapid detection [92]. A conductometric sensor operates by passing an electrical current through the sample and measuring the resulting resistance or conductance, which is then correlated to the concentration of the target substances [93], while impedimetric sensors measure changes in electrical impedance, which includes both resistance and capacitance [92].
5. Types of Interfaces in Blood Glucose Detection
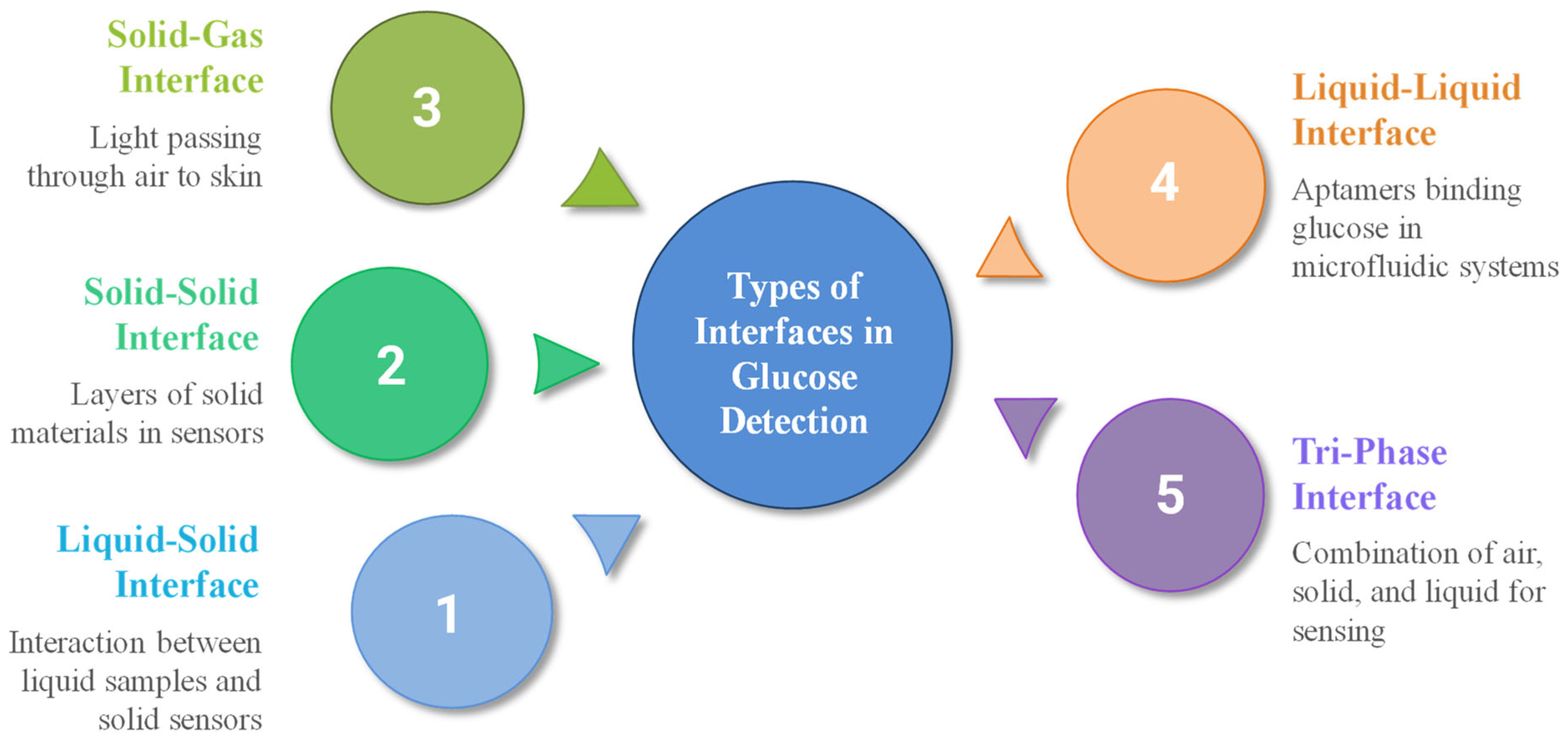
Various types of interfaces are employed in blood glucose detection, particularly at the junction between a solid sensor surface and a liquid sample, where glucose interacts with enzymes or electrochemical transducers. Figure 7 illustrates the types of interfaces for blood glucose detection and measurements.
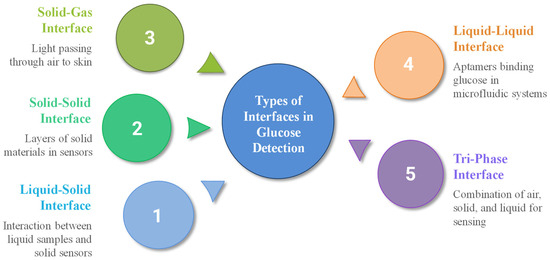
Figure 7.
Types of interfaces for blood glucose detection.
One example is the liquid–solid interface triboelectric sensor (LS-TES), which has shown potential for non-invasive disease screening and glucose sensing [94]. Solid–solid interfaces, commonly found in multi-layer sensors, are also utilized, such as electrode layers composed of gold deposited on polymer substrates [95], core–shell structures where Ni-MOF is deposited on a UiO-67 core [96], or modified glassy carbon electrodes with Au-CuO nanocomposites [97]. Ion-selective membranes can be layered onto solid substrates, as seen in the design of sodium ion-selective electrodes, where Na0.33MnO2 is used as the inner contact layer and DD16C5, a crown ether with high Na+/K+ selectivity, serves as the ionophore, whereas another sodium ISE sensor design employs a sodium ion conductor (NASICON) as the solid electrolyte, coupled with a platinum layer immobilized with glucose oxidase (GOD) serving as the sensing electrode [98]. Enzyme-immobilized membranes on solid electrodes can be achieved by using various techniques, such as entrapment within polyacrylamide gels or crosslinking agents on modified electrodes [99].
Solid–gas interfaces are less common in glucose sensing, such as in non-invasive optical sensors, where light passes through air to skin [100]; our body emits various gases, including acetone, which can serve as a biomarker for blood glucose levels, particularly in individuals with diabetes [101]. The use of a liquid–liquid interface is rare in direct glucose sensing but can occur in lab-on-a-chip or microfluidic systems [102]. At these interfaces, aptamers (single-stranded DNA or RNA molecules) can be used to selectively bind glucose, triggering changes in liquid crystal (LC) orientation [103]. Moreover, emulsion-based biosensors such as those utilizing two nicotinic acid amphiphiles as glucose sensors or compartmentalized fluidics have also been explored for glucose detection [104]. A non-enzymatic sensor featuring an air–solid–liquid tri-phase interface has also been developed, synergistically combining local alkalinity generation with electrocatalytic glucose oxidation to enhance sensing performance [105].
Functionalization of Solid–Liquid Interfaces for Blood Glucose Sensing
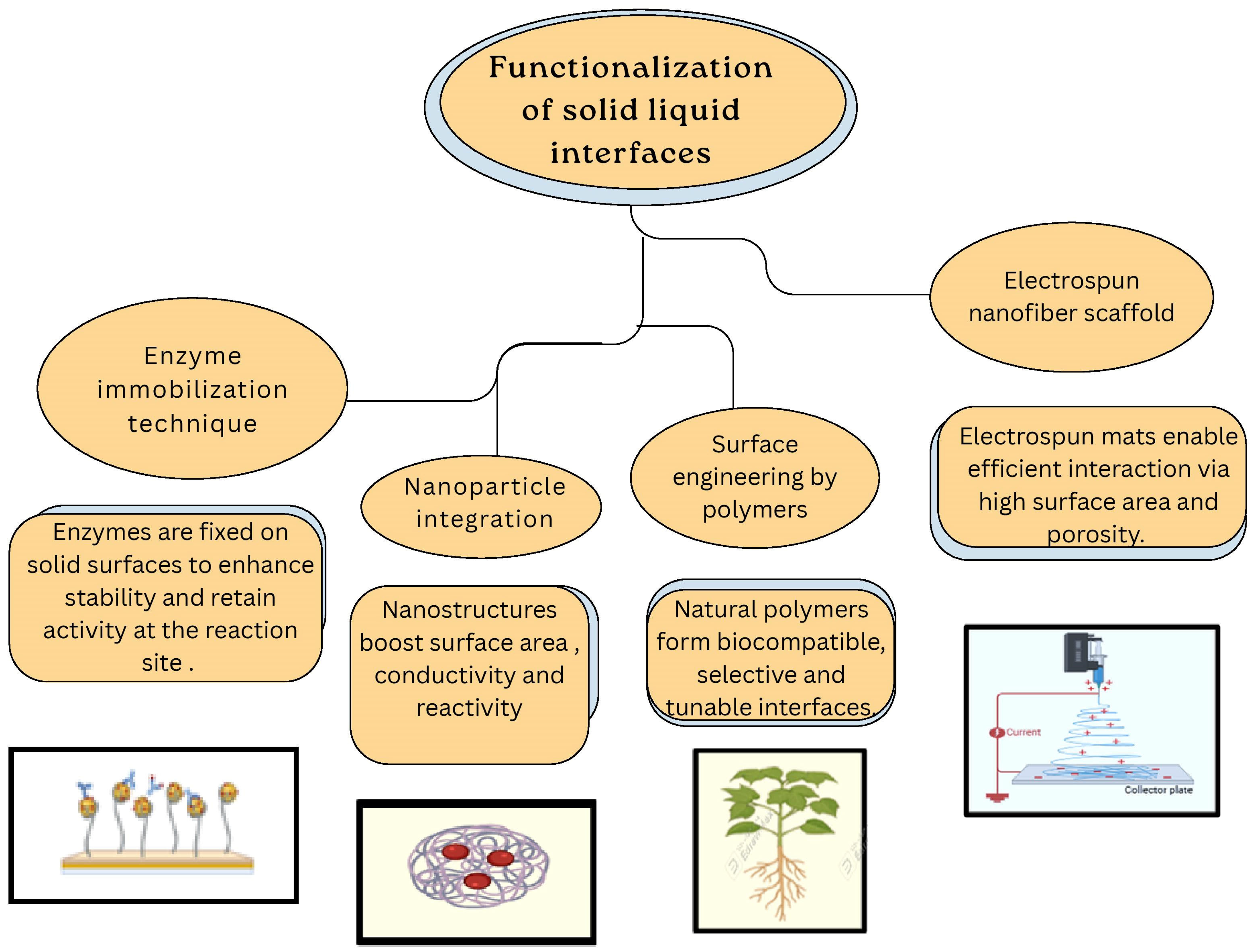
Functionalization of solid–liquid interfaces is pivotal in enhancing the performance of blood glucose biosensors. Various strategies have been employed to enhance enzyme immobilization, facilitate electron transfer and improve overall sensor stability.
Figure 8 describes functionalization approaches of solid–liquid interfaces for electrochemical glucose sensing and measurements.
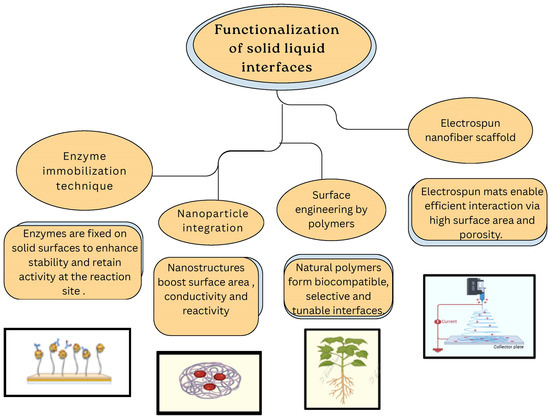
Figure 8.
Functionalization of solid–liquid interfaces for electrochemical glucose sensing.
One such approach involves the covalent attachment of glucose oxidase (GOx) to electrode surfaces, ensuring stable binding and efficient electron transfer [106]. For instance, glucose oxidase has been functionalized with redox-active ferrocene groups in organic solvents [107] and by covalent attachment to gold nanoparticle monolayer-modified Au electrodes to improve stability and electron transfer [108]. Incorporating nanomaterials such as carbon nanotubes (CNTs) and metal oxides at interfaces significantly increases surface area and conductivity [109]; e.g., Co(OH)2-functionalized CNTs have been used to create highly sensitive non-enzymatic electrochemical glucose sensors. Electrodeposition of chitosan films on electrode surfaces provides a biocompatible matrix that enhances enzyme retention and sensor stability [110]. Electrospinning techniques produce nanofiber mats with a high surface area, making them well-suited for enzyme immobilization [111]. Modifying substrates such as cellulosic paper with functional groups can enhance hydrophilicity and promote effective enzyme binding. For instance, a study demonstrated the use of phosphorylated microfibrillated cellulose to develop a biobased sensor for non-invasive glucose monitoring [112]; embedding redox mediators within the sensor interface facilitates efficient electron transfer between the enzyme and the electrode, thereby improving sensor responsiveness and accuracy [113]. Horseradish peroxidase (HRP) functionalizes interfaces by detecting hydrogen peroxide, which produces a visible color change in a chromogenic substrate [114].
Several strategies have been designed for increasing performance of solid–liquid interfaces. These include anti-biofouling and biocompatible coatings, nanomaterial and conductive polymer interfaces, permselective and diffusion controlling barriers, and redox mediator and enzyme immobilization layers. Although the details are summarized in Table 1, it is important to emphasize that the performance of these solid–liquid interface designs differs significantly under dynamic blood flow versus in static and in vitro conditions. Hydrophilic zwitterionic coatings and PEG-based hydrogels effectively resist protein adsorption in static environments but undergo gradual dehydration and mechanical erosion under shear stress, compromising their long-term stability in blood [35]. Similarly, nanomaterial–polymer composites can enhance charge transfer efficiency but are prone to surface restructuring or nanoparticle detachment under physiological flow [115]. Moreover, permselective membranes such as Nafion and polyurethane, while chemically robust and mechanically stable, introduce additional diffusion barriers that slow glucose transport and extend sensor response time [116]. Consequently, the in-flow behavior of these coatings often deviates from their static performance.

Table 1.
Classification of solid–liquid interfacial strategies.
6. Recent Patents on Solid–Liquid Interface-Based Electrochemical Blood Glucose Sensors
Electrochemical sensors have sparked a rise in patent applications, validating their growing significance in glucose sensing innovations. The patents mainly focus on novel solid–liquid interface-based electrochemical glucose sensors and advanced methods of analyte detection, leading to improved and targeted sensing of blood glucose levels. The literature on such patents is presented in Table 2.

Table 2.
Summary of recent patents on solid–liquid interface-based electrochemical sensors.
7. Clinical Potential of Solid–Liquid Interfaces for Electrochemical Glucose Sensing Applications
Solid–liquid interfaces act as dynamic junctions where molecular recognition meets charge transport, opening novel pathways for glucose detection. By engineering interfacial microenvironments, these platforms unlock precision sensing through synergistic bio-catalysis and electroactivity. Advanced materials (e.g., hydrogels, nanostructured electrodes, permselective membranes) enhance sensitivity, selectivity, and biocompatibility, making sensors more reliable in complex biological environments. The interface design supports continuous, non-invasive or minimally invasive monitoring, which is especially critical for patients requiring tight glycemic control, such as those with Type 1 diabetes. Future innovations in solid–liquid interfaces may lead to fully implantable, longer-lasting, and calibration-free glucose sensors with even higher clinical accuracy. Some clinical trials have been performed using solid–liquid interface-based electrochemical glucose biosensors, as discussed in Table 3.

Table 3.
Clinical trials of solid–liquid interface-based electrochemical glucose sensor technologies based on PubMed, ClinicalTrials.gov and related sources.
8. Applications of Solid–Liquid Interfaces for Electrochemical Blood Glucose Sensing
Solid–liquid interfaces have numerous applications in electrochemical blood glucose sensing due to their well-engineered interfaces that enhance both sensitivity and selectivity. These optimized interfaces pave the way for next-generation wearable electrochemical sensors for personalized healthcare. Various solid–liquid interfaces have been explored for this purpose, as illustrated in Figure 9.
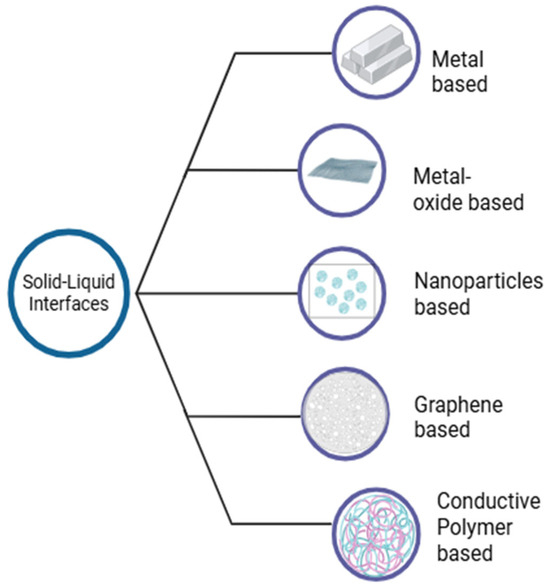
Figure 9.
Overview of solid–liquid interfaces in blood glucose sensing.
8.1. Metal-Based Interfaces as Glucose Sensors
Metal-based solid–liquid interfaces have attracted significant attention in the development of electrochemical glucose sensors, owing to their high catalytic activity, stability, and cost-effectiveness [11]. Recent advancements have focused on improving sensor performance through innovative material design and surface engineering. In particular, transition metal oxides (TMOs) and transition metal sulfides (TMSs) have been extensively investigated for non-enzymatic glucose sensing due to their excellent electrocatalytic properties [130]. Noble metals like gold (Au) and platinum (Pt) exhibit superior catalytic activities and biocompatibility [131]. The fabrication of nanostructured noble metal electrodes such as mushroom-like gold nanowires has resulted in substantial improvements in sensor sensitivity and mechanical flexibility [132]. Additionally, the integration of conductive metal–organic frameworks (MOFs) with metal hydroxides has emerged as a promising strategy to further enhance the performance of glucose sensors by combining high conductivity with excellent catalytic activity [133]. Surface functionalization techniques like modifying titanium dioxide nanotubes with Prussian blue and anodization of copper films have played a crucial role in advancing glucose sensor performance [134]. By optimizing parameters, researchers have achieved significant enhancements in glucose oxidation efficiency, demonstrating the potential of microfabricated platforms for high-performance glucose sensing. The literature on some potential metal-based interfaces as blood glucose sensors is listed in Table 4.

Table 4.
Metal-based interfaces as blood glucose sensors.
8.2. Metal Oxide-Based Interfaces as Glucose Sensors
Metal oxides have been widely studied for non-enzymatic glucose sensing, particularly in alkaline environments, where they exhibit enhanced sensitivity and selectivity. Notably, certain metal oxides such as ferric oxide have demonstrated the ability to catalyze glucose oxidation under neutral pH conditions. Additionally, ultraviolet (UV) light activation has been employed to enable metal oxides like zinc oxide to mimic enzymatic activity for glucose oxidation in neutral pH, further expanding their potential for biocompatible sensing applications [141]. The literature on some potential metal oxide-based interfaces as blood glucose sensors is listed in Table 5.

Table 5.
Metal oxide-based interfaces as blood glucose sensors.
8.3. Carbon-Based Interfaces as Glucose Sensors
Carbon-based solid–liquid interfaces have become a cornerstone in the advancement of electrochemical glucose sensors, owing to their ability to facilitate efficient electron transfer and enhance both sensitivity and selectivity. These properties make them highly suitable for both enzymatic and non-enzymatic sensing applications. Recent studies have highlighted the efficacy of various carbon nanostructures in glucose detection. In particular, graphene and carbon nanotubes (CNTs) have been widely employed due to their exceptional electrochemical conductivity, large surface area, and strong mechanical stability [148,149]. The literature on some potential carbon-based interfaces as blood glucose sensors is listed in Table 6.

Table 6.
Carbon-based interfaces as blood glucose sensors.
8.4. Nanoparticle-Based Interfaces as Glucose Sensors
Through strategic selection of nanoparticle materials and interface architectures, non-enzymatic glucose sensors have achieved rapid response times (sub-seconds), broad linear ranges (μM to mM), sub-μM detection limits and high selectivity in complex biofluids [159]. Gold and platinum nanoparticles enhance electron transfer for enzymatic and non-enzymatic systems alike [160]; bimetallic alloys modulate electronic structure for optimized catalysis [161]; and metal-doped nanocomposites provide a sustainable option for point-of-care platforms [162]. Continued innovation in nanoparticle synthesis, interface functionalization, and device integration promises further improvements toward wearable, real-time glucose monitoring technologies. The literature on some potential nanoparticles-based interfaces as blood glucose sensors is listed in Table 7.

Table 7.
Nanoparticle-based interfaces as blood glucose sensors.
8.5. Graphene-Based Interfaces as Glucose Sensors
Recent studies have explored various graphene derivatives and composites to enhance sensor performance [171,172]. For instance, Gričar et al. developed a highly sensitive enzymatic glucose sensor utilizing graphene nanoribbons on screen-printed electrodes, achieving superior selectivity and stability [173]. Similarly, Pilo et al. reported on poly(thiophene)/graphene oxide-modified electrodes, demonstrating improved amperometric response for glucose detection [174]. According to the Graphene Market and 2D Materials Assessment Report (2021–2031), graphene biosensors will move from the lab to the commercial market in the next several decades, with 18 main application areas anticipated. This assessment analysis projects that the graphene market will grow from USD 100 million in 2020 to USD 700 million by 2031. They have been employed as efficient glucose-sensing electrode materials. Recently, scientists from the University of Bath worked with integrated graphene to create an electrochemical glucose sensor that is insensitive to temperature or pH variations. The literature on some potential graphene-based interfaces as blood glucose sensors is listed in Table 8.

Table 8.
Graphene-based interfaces as blood glucose sensors.
8.6. Conductive Polymer-Based Interfaces as Glucose Sensors
Conductive polymer-based solid–liquid interfaces have garnered significant attention in the development of electrochemical glucose sensors due to their unique combination of electrical conductivity, mechanical flexibility, and biocompatibility. Recent advancements have focused on enhancing sensor performance through innovative material design and fabrication techniques. For instance, Ozlu and Shim (2024) synthesized highly conductive melanin-like polymers (eMLPs) via electrochemical deposition, achieving a sensitivity of 752.5 μA mM−1 cm−2 and a wide linear detection range up to 70 mM, demonstrating the potential of eMLPs in non-enzymatic glucose sensing [183,184]. Similarly, Kousseff et al. (2024) developed a single-component electroactive polymer PEDOT-PBA, which integrates conducting and receptor moieties, enabling efficient glucose binding and signal transduction [185].
In addition to material innovations, structural engineering of conductive polymers has led to significant improvements in sensor performance. Himori and Sakata (2022) introduced a free-standing conductive hydrogel electrode composed of polyaniline and phenyl boronic acid, eliminating the need for supporting substrates and enhancing mechanical toughness for potentiometric glucose sensing [186]. Furthermore, Phasuksom and Sirivat (2022) developed a portable enzymatic glucose sensor by modifying screen-printed carbon electrodes with doped polyindole and multi-walled carbon nanotubes, achieving a sensitivity of 182.9 μA mM−1 cm−2 and a detection limit of 0.01 mM [187]. These advancements underscore the versatility and efficacy of conductive polymers in enhancing the performance of electrochemical glucose sensors. The literature on some potential conductive polymer-based interfaces as blood glucose sensors is listed in Table 9.

Table 9.
Conductive polymer-based interfaces as blood glucose sensors.
9. Conclusions
The continuous development of glucose sensing technology underscores its critical role in improving the management of diabetes and related chronic diseases. Among the various methods developed over the decades—from invasive to non-invasive approaches—electrochemical sensors have emerged as the most promising due to their high sensitivity, rapid response, low cost, and easy miniaturization for point-of-care (POC) use. The development of solid–liquid interfaces has significantly enhanced the performance of these sensors through improved enzyme immobilization, electron transfer, and overall sensor stability. Functionalization techniques such as the incorporation of nanomaterials, covalent enzyme binding, and the embedding of redox mediators have been critical for achieving higher selectivity, sensitivity, and operating time. Advances in materials science have led to the development of diverse interfacial platforms, ranging from metals and metal oxides to carbon-based nanomaterials, conductive polymers, and graphene derivatives, offering unique electrochemical properties specifically tailored for glucose sensing. Functional solid–liquid interfaces therefore represent a promising element in electrochemical blood glucose measurement and have enormous potential to advance personalized medicine worldwide.
10. Future Perspectives
Future prospects point to the integration of advanced materials with smart electronics to develop wearable, minimally or non-invasive, accurate, and user-friendly real-time glucose monitoring systems. Research should continue to focus on improving the stability and biocompatibility of novel sensor materials, as well as their miniaturization and integration with wireless data transmission for continuous glucose monitoring (CGM). Furthermore, a deeper understanding of interfacial phenomena at the molecular level, supported by computational modeling and advanced characterization techniques, will open up new possibilities in sensor design. By leveraging interdisciplinary approaches that combine materials science, nanotechnology, bioengineering, and data analytics, future glucose sensors can become more efficient, accessible, and personalized, ultimately transforming diabetes treatment and chronic disease management.
Author Contributions
Data curation: Z.M.; methodology: Z.M.; software: Z.M.; writing—original draft: Z.M. and A.H.; funding acquisition: N.R.; resources: N.R.; visualization: N.R., A.H. and M.E.S.; writing—review and editing: N.R. and M.F.N.; validation: A.H. and M.E.S.; supervision: M.F.N.; project administration: M.F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Being a review, no new data were created.
Acknowledgments
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tonelli, D.; Gualandi, I.; Scavetta, E.; Mariani, F. Focus Review on Nanomaterial-Based Electrochemical Sensing of Glucose for Health Applications. Nanomaterials 2023, 13, 1883. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Maurya, K.K.; Malviya, M. Recent progress on nanomaterial-based electrochemical sensors for glucose detection in human body fluids. Microchim. Acta 2025, 192, 110. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen metabolism in humans. BBA Clin. 2016, 5, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wan, C.; Wei, S.; Chai, H.; Tao, T. Research Progress on the Application of Nanocellulose in Glucose Sensing. Curr. Org. Synth. 2025, 22, 24–35. [Google Scholar] [CrossRef]
- Karuppaiah, G.; Lee, M.-H.; Bhansali, S.; Manickam, P. Electrochemical sensors for cortisol detection: Principles, designs, fabrication, and characterisation. Biosens. Bioelectron. 2023, 239, 115600. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Lisi, F.; Peterson, J.R.; Gooding, J.J. The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens. Bioelectron. 2020, 148, 111835. [Google Scholar] [CrossRef] [PubMed]
- Amor-Gutiérrez, O.; Costa-Rama, E.; Fernández-Abedul, M.T. Paper-Based Enzymatic Electrochemical Sensors for Glucose Determination. Sensors 2022, 22, 6232. [Google Scholar] [CrossRef]
- Fiedorova, K.; Augustynek, M.; Kubicek, J.; Kudrna, P.; Bibbo, D. Review of present method of glucose from human blood and body fluids assessment. Biosens. Bioelectron. 2022, 211, 114348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shi, F.; Peng, M.; Zhang, Y.; Long, S.; Liu, R.; Li, J.; Yang, Z. Non-Enzymatic Electrochemical Glucose Sensors Based on Metal Oxides and Sulfides: Recent Progress and Perspectives. Chemosensors 2025, 13, 19. [Google Scholar] [CrossRef]
- Muthukumaran, M.K.; Govindaraj, M.; Kogularasu, S.; Sriram, B.; Raja, B.K.; Wang, S.-F.; Chang-Chien, G.-P.; Selvi J, A. Recent advances in metal-organic frameworks for electrochemical sensing applications. Talanta Open 2025, 11, 100396. [Google Scholar] [CrossRef]
- Hussein, B.A.; Tsegaye, A.A.; Shifera, G.; Taddesse, A.M. A sensitive non-enzymatic electrochemical glucose sensor based on a ZnO/Co3O4/reduced graphene oxide nanocomposite. Sens. Diagn. 2023, 2, 347–360. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, J.; Wang, C.; Feng, L.; Song, Z.; Zhao, W.; Wang, Q.; Liu, C. The Application of Wearable Glucose Sensors in Point-of-Care Testing. Front. Bioeng. Biotechnol. 2021, 9, 774210. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Lai, T.; Shu, H.; Yao, B.; Lai, S.; Chen, T.; Xiao, X.; Wang, Y. A Highly Selective Electrochemical Sensor Based on Molecularly Imprinted Copolymer Functionalized with Arginine for the Detection of Chloramphenicol in Honey. Biosensors 2023, 13, 505. [Google Scholar] [CrossRef]
- Sajeevan, A.; Sukumaran, R.A.; Panicker, L.R.; Kotagiri, Y.G. Trends in ready-to-use portable electrochemical sensing devices for healthcare diagnosis. Microchim. Acta 2025, 192, 80. [Google Scholar] [CrossRef]
- Bruckschlegel, C.; Fleischmann, V.; Gajovic-Eichelmann, N.; Wongkaew, N. Non-enzymatic electrochemical sensors for point-of-care testing: Current status, challenges, and future prospects. Talanta 2025, 291, 127850. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Soranzo, T.; Ben Tahar, A.; Chmayssem, A.; Zelsmann, M.; Vadgama, P.; Lenormand, J.-L.; Cinquin, P.; Martin, D.K.; Zebda, A. Electrochemical Biosensing of Glucose Based on the Enzymatic Reduction of Glucose. Sensors 2022, 22, 7105. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Dube, S.; Slama, M.; Errazuriz, I.; Amezcua, J.C.; Kudva, Y.C.; Peyser, T.; Carter, R.E.; Cobelli, C.; Basu, R. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 2013, 62, 4083–4087. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, J.; Zhang, G.; Lu, S.; Zhou, J. Wearable Electrochemical Glucose Sensors for Fluid Monitoring: Advances and Challenges in Non-Invasive and Minimally Invasive Technologies. Biosensors 2025, 15, 309. [Google Scholar] [CrossRef]
- Hong, J.K.; Gao, L.; Singh, J.; Goh, T.; Ruhoff, A.M.; Neto, C.; Waterhouse, A. Evaluating medical device and material thrombosis under flow: Current and emerging technologies. Biomater. Sci. 2020, 8, 5824–5845. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.T.; Yuan, F.; Reichert, W.M. Predicting glucose sensor behavior in blood using transport modeling: Relative impacts of protein biofouling and cellular metabolic effects. J. Diabetes Sci. Technol. 2013, 7, 1547–1560. [Google Scholar] [CrossRef]
- Arshad, F.; Hassan, I.U.; AlGhamadi, J.M.; Naikoo, G.A. Biofouling-resistant nanomaterials for non-enzymatic glucose sensors: A critical review. Mater. Today Bio 2025, 32, 101746. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: Opportunities and challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Tsoukas, M.; Rutkowski, J.; El-Fathi, A.; Yale, J.F.; Bernier-Twardy, S.; Bossy, A.; Pytka, E.; Legault, L.; Haidar, A. Accuracy of FreeStyle Libre in Adults with Type 1 Diabetes: The Effect of Sensor Age. Diabetes Technol. Ther. 2020, 22, 203–207. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sun, X.; Attique, F.; Saleh, M.T.; Ahmad, N.; Atiq, K.; Shafi, M.; Ahmed, I.A.; Barsoum, I.; Rafique, M.S.; et al. Unveiling the future: Breakthroughs and innovations in MXene-based electrochemical sensors. Chem. Eng. J. 2025, 507, 160392. [Google Scholar] [CrossRef]
- Rana, I.; Malakar, V.K.; Ranjan, K.R.; Verma, C.; AlFantazi, A.; Singh, P.; Kumari, K. MXenes and their composites for high-performance detection of pharmaceuticals and pesticides: A comprehensive review. Compos. Part B Eng. 2025, 302, 112521. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef]
- Shi, W.; Li, W.; Nguyen, W.; Chen, W.; Wang, J.; Chen, M. Advances of metal organic frameworks in analytical applications. Mater. Today Adv. 2022, 15, 100273. [Google Scholar] [CrossRef]
- Crane, B.C.; Barwell, N.P.; Gopal, P.; Gopichand, M.; Higgs, T.; James, T.D.; Jones, C.M.; Mackenzie, A.; Mulavisala, K.P.; Paterson, W. The Development of a Continuous Intravascular Glucose Monitoring Sensor. J. Diabetes Sci. Technol. 2015, 9, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Y.; Che, S.; Wang, K. Recent Advances in Non-Enzymatic Glucose Sensors Based on Nanomaterials. Coatings 2025, 15, 892. [Google Scholar] [CrossRef]
- Amoako, K.; Ukita, R.; Cook, K.E. Antifouling Zwitterionic Polymer Coatings for Blood-Bearing Medical Devices. Langmuir 2025, 41, 2994–3006. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Merricks, E.P.; Wimsey, L.E.; Nichols, T.C.; Schoenfisch, M.H. Long-Term Accurate Continuous Glucose Biosensors via Extended Nitric Oxide Release. ACS Sens. 2019, 4, 3257–3264. [Google Scholar] [CrossRef]
- Tong, X.; Jiang, T.; Yang, J.; Song, Y.; Ao, Q.; Tang, J.; Zhang, L. Continuous glucose monitoring (CGM) system based on protein hydrogel anti-biofouling coating for long-term accurate and point-of-care glucose monitoring. Biosens. Bioelectron. 2025, 277, 117307. [Google Scholar] [CrossRef] [PubMed]
- Abbott Laboratories; Bayer; Ag Azur; Environmental Biosensor; Bv Dupont; Biosensor Materials; Wearable Biosensors; Piezoelectric Biosensors; Thermal; Electrochemical Biosensors; et al. Biosensors Market Demand & Analysis 2023–2030. 2023. Available online: https://www.researchgate.net/publication/368879985_Biosensors_Market_Demand_Analysis_2023-2030 (accessed on 28 October 2025).
- Ambaye, A.D.; Mamo, M.D.; Zigyalew, Y.; Mengistu, W.M.; Fito Nure, J.; Mokrani, T.; Ntsendwana, B. The development of carbon nanostructured biosensors for glucose detection to enhance healthcare services: A review. Front. Sens. 2024, 5, 2024. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Aktar, M.N.; Preda, V.; Nasiri, N. Advances in electrochemical sensors for real-time glucose monitoring. Sens. Diagn. 2024, 3, 893–913. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Lakshmy, S.; Santhosh, S.; Kalarikkal, N.; Chakraborty, B.; Rout, C.S. Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials. Biosensors 2022, 12, 467. [Google Scholar] [CrossRef]
- Özkahraman, E.E.; Eroğlu, Z.; Efremov, V.; Maryyam, A.; Abbasiasl, T.; Das, R.; Mirzajani, H.; Akgenc Hanedar, B.; Beker, L.; Metin, O. High-Performance Black Phosphorus/Graphitic Carbon Nitride Heterostructure-Based Wearable Sensor for Real-time Sweat Glucose Monitoring. Adv. Mater. Technol. 2024, 10, 00106. [Google Scholar] [CrossRef]
- Promsuwan, K.; Soleh, A.; Samoson, K.; Saisahas, K.; Wangchuk, S.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Novel biosensor platform for glucose monitoring via smartphone based on battery-less NFC potentiostat. Talanta 2023, 256, 124266. [Google Scholar] [CrossRef]
- Dey, K.; Santra, T.S.; Tseng, F.G. Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors. Appl. Sci. 2025, 15, 2523. [Google Scholar] [CrossRef]
- Saur, N.M.; England, M.R.; Menzie, W.; Melanson, A.M.; Trieu, M.Q.; Berlin, J.; Hurley, J.; Krystyniak, K.; Kongable, G.L.; Nasraway, S.A., Jr. Accuracy of a novel noninvasive transdermal continuous glucose monitor in critically ill patients. J. Diabetes Sci. Technol. 2014, 8, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Haider, A.J.; Nafil, R.Q.; Benzerroug, N.; Al-Muntaser, A.A. Studying the effect of pH optimization on the performance of copper oxide electrodes for glucose sensor applications. Eur. Phys. J. B 2025, 98, 55. [Google Scholar] [CrossRef]
- Ghosh, M.; Bora, V.R. Evolution in blood glucose monitoring: A comprehensive review of invasive to non-invasive devices and sensors. Discov. Med. 2025, 2, 74. [Google Scholar] [CrossRef]
- Heinemann, L. Continuous glucose monitoring by means of the microdialysis technique: Underlying fundamental aspects. Diabetes Technol. Ther. 2003, 5, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Kandwal, A.; Sharma, Y.D.; Jasrotia, R.; Kit, C.C.; Lakshmaiya, N.; Sillanpää, M.; Liu, L.W.Y.; Igbe, T.; Kumari, A.; Sharma, R.; et al. A comprehensive review on electromagnetic wave based non-invasive glucose monitoring in microwave frequencies. Heliyon 2024, 10, e37825. [Google Scholar] [CrossRef]
- Li, T.; Wang, Q.; An, Y.; Guo, L.; Ren, L.; Lei, L.; Chen, X. Infrared absorption spectroscopy-based non-invasive blood glucose monitoring technology: A comprehensive review. Biomed. Signal Process. Control 2025, 106, 107750. [Google Scholar] [CrossRef]
- Azimzadeh Andarabi, E.; Norouzian-Alam, S.; Shayganmanesh, M.; Haji Abdolvahab, M. Analysis of glucose concentrations in blood solutions using FTIR and Raman spectroscopy methods. Biomed. Opt. Express 2025, 16, 2631–2662. [Google Scholar] [CrossRef] [PubMed]
- Kaysir, M.R.; Zaman, T.M.; Rassel, S.; Wang, J.; Ban, D. Photoacoustic Resonators for Non-Invasive Blood Glucose Detection Through Photoacoustic Spectroscopy: A Systematic Review. Sensors 2024, 24, 6963. [Google Scholar] [CrossRef]
- Naresh, M.; Nagaraju, V.S.; Kollem, S.; Kumar, J.; Peddakrishna, S. Non-invasive glucose prediction and classification using NIR technology with machine learning. Heliyon 2024, 10, e28720. [Google Scholar] [CrossRef] [PubMed]
- Bachache, L.N.; Hasan, J.A.; Al-Neam, A.Q. A Review: Non Invasive Sensing System for Detection Glucose Level. J. Phys. Conf. Ser. 2021, 1963, 012125. [Google Scholar] [CrossRef]
- Shi, J.; Fernández-García, R.; Gil, I. Sensor Technologies for Non-Invasive Blood Glucose Monitoring. Sensors 2025, 25, 3591. [Google Scholar] [CrossRef]
- Abasi, S.; Aggas, J.R.; Garayar-Leyva, G.G.; Walther, B.K.; Guiseppi-Elie, A. Bioelectrical Impedance Spectroscopy for Monitoring Mammalian Cells and Tissues under Different Frequency Domains: A Review. ACS Meas. Sci. Au 2022, 2, 495–516. [Google Scholar] [CrossRef]
- Herwadkar, A.; Banga, A.K. Chapter 4—Transdermal Delivery of Peptides and Proteins. In Peptide and Protein Delivery; Van Der Walle, C., Ed.; Academic Press: Boston, MA, USA, 2011; pp. 69–86. [Google Scholar]
- Buchert, M. Thermal emission spectroscopy as a tool for non-invasive wood glucose measurements. Proc. SPIE 2004, 5566, 100–111. [Google Scholar] [CrossRef]
- Wang, G.; Moriyama, N.; Tottori, S.; Nishizawa, M. Recent advances in iontophoresis-assisted microneedle devices for transdermal biosensing and drug delivery. Mater. Today Bio 2025, 31, 101504. [Google Scholar] [CrossRef] [PubMed]
- Al-Jammas, M.H.; Iobaid, A.S.; Al-Deen, M.M.N.; Aziz, Y.W. A non-invasive blood glucose monitoring system. Comput. Biol. Med. 2025, 191, 110133. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, Y.; Di, J. Colorimetric detection of glucose based on gold nanoparticles coupled with silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 207–212. [Google Scholar] [CrossRef]
- Li, T.; Bu, J.; Yang, Y.; Zhong, S. A smartphone-assisted one-step bicolor colorimetric detection of glucose in neutral environment based on molecularly imprinted polymer nanozymes. Talanta 2024, 267, 125256. [Google Scholar] [CrossRef] [PubMed]
- The Huy, B.; Thangadurai, D.T.; Sharipov, M.; Ngoc Nghia, N.; Van Cuong, N.; Lee, Y.-I. Recent advances in turn off-on fluorescence sensing strategies for sensitive biochemical analysis—A mechanistic approach. Microchem. J. 2022, 179, 107511. [Google Scholar] [CrossRef]
- Mai, H.H.; Tran, D.H.; Janssens, E. Non-enzymatic fluorescent glucose sensor using vertically aligned ZnO nanotubes grown by a one-step, seedless hydrothermal method. Microchim. Acta 2019, 186, 245. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, X.; Yang, C.; Yang, S.; Liu, C.; Cao, Y. Wearable Sensors Based on Miniaturized High-Performance Hybrid Nanogenerator for Medical Health Monitoring. Biosensors 2024, 14, 361. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Govedarica, M.; Milosevic, I.; Jankovic, V.; Mitrovic, R.; Kundacina, I.; Nastasijevic, I.; Radonic, V. A Cost-Effective and Rapid Manufacturing Approach for Electrochemical Transducers with Magnetic Beads for Biosensing. Micromachines 2025, 16, 343. [Google Scholar] [CrossRef]
- Mansour, M.; Saeed Darweesh, M.; Soltan, A. Wearable devices for glucose monitoring: A review of state-of-the-art technologies and emerging trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Dube, A.; Malode, S.J.; Alodhayb, A.N.; Mondal, K.; Shetti, N.P. Conducting polymer-based electrochemical sensors: Progress, challenges, and future perspectives. Talanta Open 2025, 11, 100395. [Google Scholar] [CrossRef]
- Bao, M.-H.; Lv, Q.-L.; Li, H.-G.; Zhang, Y.-W.; Xu, B.-F.; He, B.-S. A novel putative role of tnk1 in atherosclerotic inflammation implicating the tyk2/stat1 pathway. Mediat. Inflamm. 2020, 2020, 6268514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Q.; Cao, B.-Z.; Cao, Q.-T.; Hun, M.; Cao, L.; Zhao, M.-Y. An analysis of reported cases of hemophagocytic lymphohistiocytosis (hlh) after covid-19 vaccination. Hum. Vaccines Immunother. 2023, 19, 2263229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, X.; Zhao, C.; Wang, S.; Tang, X. Recent advance in biological responsive nanomaterials for biosensing and molecular imaging application. Int. J. Mol. Sci. 2022, 23, 1923. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Antifouling (Bio)materials for Electrochemical (Bio)sensing. Int. J. Mol. Sci. 2019, 20, 423. [Google Scholar] [CrossRef]
- Færch, K.; Amadid, H.; Bruhn, L.; Clemmensen, K.K.B.; Hulman, A.; Ried-Larsen, M.; Blond, M.B.; Jørgensen, M.E.; Vistisen, D. Discordance Between Glucose Levels Measured in Interstitial Fluid vs in Venous Plasma After Oral Glucose Administration: A Post-Hoc Analysis From the Randomised Controlled PRE-D Trial. Front. Endocrinol. 2021, 12, 753810. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Xia, Y.; Bian, Y. The influence of ICAM1 rs5498 on diabetes mellitus risk: Evidence from a meta-analysis. Inflamm. Res. 2019, 68, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Calibration of Minimally Invasive Continuous Glucose Monitoring Sensors: State-of-The-Art and Current Perspectives. Biosensors 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Barcelo-Rico, F.; Diez, J.L.; Rossetti, P.; Vehi, J.; Bondia, J. Adaptive calibration algorithm for plasma glucose estimation in continuous glucose monitoring. IEEE J. Biomed. Health Inf. 2013, 17, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Fayaz, M.; Kim, D. Improving Accuracy of the Kalman Filter Algorithm in Dynamic Conditions Using ANN-Based Learning Module. Symmetry 2019, 11, 94. [Google Scholar] [CrossRef]
- Bequette, B.W. Continuous glucose monitoring: Real-time algorithms for calibration, filtering, and alarms. J. Diabetes Sci. Technol. 2010, 4, 404–418. [Google Scholar] [CrossRef]
- Facchinetti, A.; Sparacino, G.; Guerra, S.; Luijf, Y.M.; DeVries, J.H.; Mader, J.K.; Ellmerer, M.; Benesch, C.; Heinemann, L.; Bruttomesso, D.; et al. Real-time improvement of continuous glucose monitoring accuracy: The smart sensor concept. Diabetes Care 2013, 36, 793–800. [Google Scholar] [CrossRef]
- Sun, T.; Liu, J.; Chen, C.J. Calibration algorithms for continuous glucose monitoring systems based on interstitial fluid sensing. Biosens. Bioelectron. 2024, 260, 116450. [Google Scholar] [CrossRef]
- McGrath, M.J.; Iwuoha, E.I.; Diamond, D.; Smyth, M.R. The use of differential measurements with a glucose biosensor for interference compensation during glucose determinations by flow injection analysis. Biosens. Bioelectron. 1995, 10, 937–943. [Google Scholar] [CrossRef]
- Jia, Z.; Huang, L.; Liu, H.; Huang, Y.; Li, W.; Pi, X.; Zheng, X. Design of a Real-time Self-adjusting Calibration Algorithm to Improve the Accuracy of Continuous Blood Glucose Monitoring. Appl. Biochem. Biotechnol. 2020, 190, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncová, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Barhoum, A.; Hamimed, S.; Slimi, H.; Othmani, A.; Abdel-Haleem, F.M.; Bechelany, M. Modern designs of electrochemical sensor platforms for environmental analyses: Principles, nanofabrication opportunities, and challenges. Trends Environ. Anal. Chem. 2023, 38, e00199. [Google Scholar] [CrossRef]
- Yuwen, T.; Shu, D.; Zou, H.; Yang, X.; Wang, S.; Zhang, S.; Liu, Q.; Wang, X.; Wang, G.; Zhang, Y.; et al. Carbon nanotubes: A powerful bridge for conductivity and flexibility in electrochemical glucose sensors. J. Nanobiotechnol. 2023, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, A.R.; Karami, M.M. Roles of nanotechnology in electrochemical sensors for medical diagnostic purposes: A review. Sens. Bio-Sens. Res. 2025, 47, 100733. [Google Scholar] [CrossRef]
- Bi, H.; Han, X. 10-Chemical sensors for environmental pollutant determination. In Chemical, Gas, and Biosensors for Internet of Things and Related Applications; Mitsubayashi, K., Niwa, O., Ueno, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–160. [Google Scholar]
- Peng, H.; Zhang, L.; Soeller, C.; Travas-Sejdic, J. Conducting polymers for electrochemical DNA sensing. Biomaterials 2009, 30, 2132–2148. [Google Scholar] [CrossRef]
- Ghanam, A.; Mohammadi, H.; Amine, A.; Haddour, N.; Buret, F. Chemical Sensors: Voltammetric and Amperometric Electrochemical Sensors. In Encyclopedia of Sensors and Biosensors, 1st ed.; Narayan, R., Ed.; Elsevier: Oxford, UK, 2023; pp. 161–177. [Google Scholar]
- Madadelahi, M.; Romero-Soto, F.O.; Kumar, R.; Tlaxcala, U.B.; Madou, M.J. Electrochemical sensors: Types, applications, and the novel impacts of vibration and fluid flow for microfluidic integration. Biosens. Bioelectron. 2025, 272, 117099. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, M.L.; Noordin, R.; Razak, K.A. Chapter 1—Advanced Nanoparticle-Based Biosensors for Diagnosing Foodborne Pathogens. In Advanced Biosensors for Health Care Applications; Inamuddin, R., Khan, A.M., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–43. [Google Scholar]
- Pharino, U.; Chaithaweep, K.; Pongampai, S.; Chanlek, N.; Kothan, S.; Kaewkhao, J.; Hajra, S.; Kim, H.J.; Vittayakorn, W.; Sriphan, S.; et al. A highly sensitive disease pre-screening approach for glycosuria: Triboelectric sensing at the liquid-solid interface. Chem. Eng. J. 2025, 508, 160901. [Google Scholar] [CrossRef]
- Chien, M.-N.; Chen, Y.-J.; Bai, C.-H.; Huang, J.-T. Continuous Glucose Monitoring System Based on Percutaneous Microneedle Array. Micromachines 2022, 13, 478. [Google Scholar] [CrossRef]
- Dai, S.; Tissot, A.; Serre, C. Recent progresses in metal–organic frameworks based core–shell composites. Adv. Energy Mater. 2022, 12, 2100061. [Google Scholar] [CrossRef]
- Felix, S.; Grace, A.N.; Jayavel, R. Sensitive electrochemical detection of glucose based on Au-CuO nanocomposites. J. Phys. Chem. Solids 2018, 122, 255–260. [Google Scholar] [CrossRef]
- Kojima, J.; Hosoya, S.; Suminaka, C.; Hori, N.; Sato, T. An Integrated Glucose Sensor with an All-Solid-State Sodium Ion-Selective Electrode for a Minimally Invasive Glucose Monitoring System. Micromachines 2015, 6, 831–841. [Google Scholar] [CrossRef]
- Robescu, M.S.; Bavaro, T. A Comprehensive Guide to Enzyme Immobilization: All You Need to Know. Molecules 2025, 30, 939. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, D.; Sunstrum, F.N.; Khan, J.U.; Welsh, A.W. Non-Invasive Glucose Sensing Technologies and Products: A Comprehensive Review for Researchers and Clinicians. Sensors 2023, 23, 9130. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, K.; Thaneerat, S.; Kongsawang, N.; Pathak, A.K.; Viphavakit, C. Highly sensitive and real-time detection of acetone biomarker for diabetes using a ZnO-coated optical fiber sensor. Biosens. Bioelectron. 2025, 271, 117061. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guan, X.; Zhang, P.; Tan, Q.; Li, T.; Jin, X.; Xu, H.; Li, C.; Zhao, J. A Fully Integrated Wearable Microfluidic Electrochemical Sensor with Ultrasonic Connecting and Hot-Pressing Bonded Multilayer Structure for Sweat Biomarker Analysis. Anal. Chem. 2025, 97, 22858–22870. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Luan, H.; Luo, D. Application and Technique of Liquid Crystal-Based Biosensors. Micromachines 2020, 11, 176. [Google Scholar] [CrossRef]
- Ming, T.; Lan, T.; Yu, M.; Duan, X.; Cheng, S.; Wang, H.; Deng, J.; Kong, D.; Yang, S.; Shen, Z. A novel electrochemical microneedle sensor for highly sensitive real time monitoring of glucose. Microchem. J. 2024, 207, 112021. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ding, Z.; Chen, L.; Wang, H.; Zhang, M.; Feng, X. Three-Phases Interface Induced Local Alkalinity Generation Enables Electrocatalytic Glucose Oxidation in Neutral Electrolyte. Front. Bioeng. Biotechnol. 2022, 10, 909187. [Google Scholar] [CrossRef]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor nanoengineering: Design, operation, and implementation for biomolecular analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Dudkaitė, V.; Bagdžiūnas, G. Functionalization of Glucose Oxidase in Organic Solvent: Towards Direct Electrical Communication across Enzyme-Electrode Interface. Biosensors 2022, 12, 335. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, N.; Yu, H.; Niu, Y.; Sun, C. Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 2005, 67, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, P.K.; Ganesan, V. Chapter Eight - Metal oxide-carbon nanotubes nanocomposite-modified electrochemical sensors for toxic chemicals. In Metal Oxides in Nanocomposite-Based Electrochemical Sensors for Toxic Chemicals; Pandikumar, A., Rameshkumar, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–261. [Google Scholar]
- Bolaños-Mendez, D.; Fernández, L.; Uribe, R.; Cunalata-Castro, A.; González, G.; Rojas, I.; Chico-Proano, A.; Debut, A.; Celi, L.A.; Espinoza-Montero, P. Evaluation of a Non-Enzymatic Electrochemical Sensor Based on Co(OH)2-Functionalized Carbon Nanotubes for Glucose Detection. Sensors 2024, 24, 7707. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.-G.; Ge, R. Electrospun nanofiber-based glucose sensors for glucose detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Khan, M.J.; Hussain, A.; Ahmed, F.; Khan, Z.H. Multilayer patch functionalized microfibrillated cellulosic paper sensor for sweat glucose monitoring. Sci. Rep. 2024, 14, 23434. [Google Scholar] [CrossRef]
- Lee, H.; Reginald, S.S.; Sravan, J.S.; Lee, M.; Chang, I.S. Advanced strategies for enzyme–electrode interfacing in bioelectrocatalytic systems. Trends Biotechnol. 2025, 43, 1328–1355. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Yang, B.; Liu, Z.; Liu, Q.; Zhang, X. Colorimetric and ultrasensitive detection of H2O2 based on Au/Co3O4-CeOx nanocomposites with enhanced peroxidase-like performance. Sens. Actuators B Chem. 2018, 271, 336–345. [Google Scholar] [CrossRef]
- Janardhanan, J.A.; Yu, H.-h. Recent advances in PEDOT/PProDOT-derived nano biosensors: Engineering nano assemblies for fostering advanced detection platforms for biomolecule detection. Nanoscale 2024, 16, 17202–17229. [Google Scholar] [CrossRef]
- Cordeiro, C.A.; Vries, M.G.; Cremers, T.I.F.H.; Westerink, B.H.C. The role of surface availability in membrane-induced selectivity for amperometric enzyme-based biosensors. Sens. Actuators B Chem. 2016, 223, 679–688. [Google Scholar] [CrossRef]
- Yao, M.; Wei, Z.; Li, J.; Guo, Z.; Yan, Z.; Sun, X.; Yu, Q.; Wu, X.; Yu, C.; Yao, F.; et al. Microgel reinforced zwitterionic hydrogel coating for blood-contacting biomedical devices. Nat. Commun. 2022, 13, 5339. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, M.; Mateev, V.; Iliev, I. Behavior of Polymer Electrode PEDOT:PSS/Graphene on Flexible Substrate for Wearable Biosensor at Different Loading Modes. Nanomaterials 2024, 14, 1357. [Google Scholar] [CrossRef]
- Myndrul, V.; Iatsunskyi, I.; Babayevska, N.; Jarek, M.; Jesionowski, T. Effect of Electrode Modification with Chitosan and Nafion® on the Efficiency of Real-Time Enzyme Glucose Biosensors Based on ZnO Tetrapods. Materials 2022, 15, 4672. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Yuan, C.Y.; Halim, B.; Kong, Y.W.; Lu, J.; Dutt-Ballerstadt, R.; Eckenberg, P.; Hillen, K.; Koski, A.; Milenkowic, V.; Netzer, E.; et al. Combining an Electrochemical Continuous Glucose Sensor with an Insulin Delivery Cannula: A Feasibility Study. J. Diabetes Sci. Technol. 2024, 18, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring. Biosens. Bioelectron. 2022, 203, 114026. [Google Scholar] [CrossRef]
- Ju, J.; Li, L.; Regmi, S.; Zhang, X.; Tang, S. Microneedle-Based Glucose Sensor Platform: From Vitro to Wearable Point-of-Care Testing Systems. Biosensors 2022, 12, 606. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.; Tian, X.; Wang, Y.; Cui, B.; Yang, Y.; Dai, S.; Lin, W.; Zhu, J.; Wang, J.; et al. Coin-sized, fully integrated, and minimally invasive continuous glucose monitoring system based on organic electrochemical transistors. Sci. Adv. 2024, 10, eadl1856. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, L.; Xu, S.; Peng, L.; Gao, Z.; Liu, S.; Xi, S.; Ma, S.; Cai, W. A high-performance wearable microneedle sensor based on a prussian blue-carbon nanotube composite electrode for the detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2024, 419, 136436. [Google Scholar] [CrossRef]
- Hossain, G.M.M.; Jalal, A.; Huq, H.; Islam, N.; Lozano, K.; Pala, N.; Alam, F. Sonochemically Synthesized ZnO Nanowires (NWs)-Based Sensor for Non-Invasive Glucose Detection in Sweat; SPIE: Bremen, Germany, 2025; Volume 13481. [Google Scholar]
- Davis, G.M.; Spanakis, E.K.; Migdal, A.L.; Singh, L.G.; Albury, B.; Urrutia, M.A.; Zamudio-Coronado, K.W.; Scott, W.H.; Doerfler, R.; Lizama, S.; et al. Accuracy of Dexcom G6 Continuous Glucose Monitoring in Non–Critically Ill Hospitalized Patients With Diabetes. Diabetes Care 2021, 44, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, C.A.; Boucsein, A.; Styles, S.E.; Chamberlain, B.; Michaels, V.R.; Crockett, H.R.; De Lange, M.; Lala, A.; Cunningham, V.; Wiltshire, E.J.; et al. Effects of 12-Week Freestyle Libre 2.0 in Children with Type 1 Diabetes and Elevated HbA1c: A Multicenter Randomized Controlled Trial. Diabetes Technol. Ther. 2023, 25, 827–835. [Google Scholar] [CrossRef]
- Mahieddine, A.; Adnane-Amara, L. A novel non-enzymatic electrochemical sensor based on NiS/Co3S4@h-Ni NWs core-shell electrode for glucose detection in human serum. Mater. Chem. Phys. 2023, 302, 127730. [Google Scholar] [CrossRef]
- Basova, T.; Vikulova, E.; Dorovskikh, S.; Hassan, A.K.; Morozova, N. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Zhai, Q.; Gong, S.; Wang, Y.; Lyu, Q.; Liu, Y.; Ling, Y.; Wang, J.; Simon, G.; Cheng, W. Enokitake Mushroom-like Standing Gold Nanowires toward Wearable Noninvasive Bimodal Glucose and Strain Sensing. ACS Appl. Mater. Interfaces 2019, 11. [Google Scholar] [CrossRef]
- Meskher, H.; Belhaouari, S.B.; Sharifianjazi, F. Mini review about metal organic framework (MOF)-based wearable sensors: Challenges and prospects. Heliyon 2023, 9, e21621. [Google Scholar] [CrossRef]
- Urgunde, A.B. Nanomaterials-Modified Electrodes for Glucose Sensing. In Nanomaterial-Modified Electrodes: Design and Applications; Khan, A.A.P., Kulkarni, R.M., Ansari, M.O., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 203–220. [Google Scholar]
- Wang, C.; Gao, N.; Gao, Z.; Li, R.; Wang, Y.; Gong, W. An Effective Non-Enzymatic Glucose Biosensor Based on Nanostructured CuxO/Cu Electrodes Synthesized in Situ by Copper Anodization. Eur. J. Inorg. Chem. 2023, 26, e202200640. [Google Scholar] [CrossRef]
- Farajpour, N.; Deivanayagam, R.; Phakatkar, A.; Narayanan, S.; Shahbazian-Yassar, R.; Shokuhfar, T. A novel antimicrobial electrochemical glucose biosensor based on silver–Prussian blue-modified TiO2 nanotube arrays. Med. Devices Sens. 2020, 3, e10061. [Google Scholar] [CrossRef]
- Lakhdari, D.; Guittoum, A.; Benbrahim, N.; Belgherbi, O.; Berkani, M.; Vasseghian, Y.; Lakhdari, N. A novel non-enzymatic glucose sensor based on NiFe(NPs)–polyaniline hybrid materials. Food Chem. Toxicol. 2021, 151, 112099. [Google Scholar] [CrossRef]
- Song, S.; Wu, J. Motion State Estimation of Target Vehicle under Unknown Time-Varying Noises Based on Improved Square-Root Cubature Kalman Filter. Sensors 2020, 20, 2620. [Google Scholar] [CrossRef]
- Safadi, B.N.; Gonçalves, J.M.; Castaldelli, E.; Matias, T.A.; Rossini, P.O.; Nakamura, M.; Angnes, L.; Araki, K. Lamellar FeOcPc-Ni/GO Composite-Based Enzymeless Glucose Sensor. ChemElectroChem 2020, 7, 2553–2563. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zhang, Q.; Li, M.; Zhao, Z.; Lin, B.; Peng, J.; Shen, H.; He, Q. Fenton-like system of UV/Glucose-oxidase@Kaolin coupled with organic green rust: UV-enhanced enzyme activity and the mechanism of UV synergistic degradation of photosensitive pollutants. Environ. Res. 2024, 247, 118257. [Google Scholar] [CrossRef]
- Ali, M.; Mir, S.; Ahmed, S. Non-enzymatic amperometric glucose sensing on CuO/mesoporous TiO2 modified glassy carbon electrode. RSC Adv. 2023, 13, 26275–26286. [Google Scholar] [CrossRef]
- Arif, D.; Hussain, Z.; Abbasi, A.D.; Sohail, M. Ag Functionalized In2O3 Derived From MIL-68(In) as an Efficient Electrochemical Glucose Sensor. Front. Chem. 2022, 10, 906031. [Google Scholar] [CrossRef]
- Franceschini, F.; Fernandes, C.; Schouteden, K.; Ustarroz, J.; Locquet, J.P.; Taurino, I. Tailoring the Glucose Oxidation Activity of Anodized Copper Films on Microfabricated Platforms. arXiv 2025, arXiv:2501.05953. [Google Scholar] [CrossRef]
- Sedaghat, S.; Piepenburg, C.R.; Zareei, A.; Qi, Z.; Peana, S.; Wang, H.; Rahimi, R. Laser-Induced Mesoporous Nickel Oxide as a Highly Sensitive Nonenzymatic Glucose Sensor. ACS Appl. Nano Mater. 2020, 3, 5260–5270. [Google Scholar] [CrossRef]
- Scandurra, A.; Censabella, M.; Boscarino, S.; Grimaldi, M.G.; Ruffino, F.; Condorelli, G.G.; Malandrino, G. Solid-State Fabrication of Cu2O/CuO Hydroxide Nanoelectrode Array onto Graphene Paper by Thermal Dewetting for High-Sensitive Detection of Glucose. Phys. Status Solidi A 2021, 218, 2100389. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, H.; Wang, L.; Zhao, H.; Gong, J.; Wu, S.; Nie, Q. Electrodeposition of Au@NiO Nanotube Arrays for Highly Sensitive Non-enzymatic Glucose Sensing. J. Electron. Mater. 2021, 50, 6392–6402. [Google Scholar] [CrossRef]
- Lin, M.-H.; Gupta, S.; Chang, C.; Lee, C.-Y.; Tai, N.-H. Carbon nanotubes/polyethylenimine/glucose oxidase as a non-invasive electrochemical biosensor performs high sensitivity for detecting glucose in saliva. Microchem. J. 2022, 180, 107547. [Google Scholar] [CrossRef]
- Ullah, Z.; Mustafa, G.; Raza, A.; Khalil, A.; Bahajjaj, A.A.; Batool, R.; Sonil, N.; Ali, I.; Nazar, M. Facile assembly of flexible humidity sensors based on nanostructured graphite/zinc oxide-coated cellulose fibrous frameworks for human healthcare. RSC Adv. 2024, 14, 37570–37579. [Google Scholar]
- Ali, A.; Shoukat, R.; Ashraf, A.R.; Rasheed, Z.; Muqaddas, S.; Iqbal, M.; Khalid, M.; Mnif, W.; Mohamed, I.E.S. Cobalt-Based Ferrite Modified Carbon Nanotubes Fibers for Flexible and Disposable Microelectrode Toward Electrochemical Glucose Sensing. Anal. Sci. Adv. 2024, 5, e202400032. [Google Scholar] [CrossRef]
- Melios, N.; Tsouti, V.; Chatzandroulis, S.; Tsekenis, G. Development of an All-Carbon Electrochemical Biosensor on a Flexible Substrate for the Sensitive Detection of Glucose. Eng. Proc. 2022, 16, 17. [Google Scholar]
- Pandey, R.R.; Liang, J.; Cakiroglu, D.; Charlot, B.; Todri-Sanial, A. Electrochemical glucose sensor using single-wall carbon nanotube field effect transistor. arXiv 2020, arXiv:2006.12973. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Kose, D.A.; Gokmese, E.; Gokmese, F. Electrochemical Glucose Detection Using PdAg Nanoparticles Anchored on rGO/MWCNT Nanohybrids. J. Clust. Sci. 2020, 31, 231–239. [Google Scholar] [CrossRef]
- Kim, S.e.; Muthurasu, A. Highly Oriented Nitrogen-doped Carbon Nanotube Integrated Bimetallic Cobalt Copper Organic Framework for Non-enzymatic Electrochemical Glucose and Hydrogen Peroxide Sensor. Electroanalysis 2021, 33, 1333–1345. [Google Scholar] [CrossRef]
- Rębiś, T.; Kuznowicz, M.; Jędrzak, A.; Milczarek, G.; Jesionowski, T. Design and fabrication of low potential NADH-sensor based on poly(caffeic acid)@multi-walled carbon nanotubes. Electrochim. Acta 2021, 386, 138384. [Google Scholar] [CrossRef]
- Muthusankar, E.; Ragupathy, D. Graphene/Poly(aniline-co-diphenylamine) nanohybrid for ultrasensitive electrochemical glucose sensor. Nano-Struct. Nano-Objects 2019, 20, 100390. [Google Scholar] [CrossRef]
- Eryiğit, M.; Çepni, E.; Kurt Urhan, B.; Öztürk Doğan, H.; Öznülüer Özer, T. Nonenzymatic glucose sensor based on poly(3,4-ethylene dioxythiophene)/electroreduced graphene oxide modified gold electrode. Synth. Met. 2020, 268, 116488. [Google Scholar] [CrossRef]
- Kuznowicz, M.; Rębiś, T.; Jędrzak, A.; Nowaczyk, G.; Szybowicz, M.; Jesionowski, T. Glucose determination using amperometric non-enzymatic sensor based on electroactive poly(caffeic acid)@MWCNT decorated with CuO nanoparticles. Microchim. Acta 2022, 189, 159. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.-Y.; Kwon, D.-W.; Yang, H.-Y.; Hahn, Y.-B. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, W.; Yan, B.; Wu, B.; Ma, J.; Wang, X.; Qiao, B.; Tu, J.; Pei, H.; Chen, D.; et al. Gold and Platinum Nanoparticle-Functionalized TiO2 Nanotubes for Photoelectrochemical Glucose Sensing. ACS Omega 2022, 7, 2474–2483. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Zhao, P.; Xie, Y. Ultrasensitive electrochemical sensor for fenitrothion based on mil-125 derived iron/titanium bimetallic oxides doped porous carbon composite. Microchem. J. 2024, 200, 110426. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Huang, Y.; Zhao, P.; Zhao, J.; Fei, J.; Xie, Y. An ultrasensitive 4-aminophenol electrochemical sensors based on zinc and nitrogen-doped γ-cyclodextrin composites. Microchem. J. 2024, 197, 109905. [Google Scholar] [CrossRef]
- Waqas, M.; Lan, J.; Zhang, X.; Fan, Y.; Zhang, P.; Liu, C.; Jiang, Z.; Wang, X.; Zeng, J.; Chen, W. Fabrication of Non-enzymatic Electrochemical Glucose Sensor Based on Pd−Mn Alloy Nanoparticles Supported on Reduced Graphene Oxide. Electroanalysis 2020, 32, 1226–1236. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Kang, B.-C.; Ha, T.-J. Non-enzymatic electrochemical glucose sensors based on polyaniline/reduced-graphene-oxide nanocomposites functionalized with silver nanoparticles. J. Mater. Chem. C 2020, 8, 5112–5123. [Google Scholar] [CrossRef]
- Rattanawarinchai, P.; Khemasiri, N.; Soyeux, N.; Jessadaluk, S.; Klamchuen, A.; Wirunchit, S.; Rangkasikorn, A.; Kayunkid, N.; Phromyothin, D.; Rahong, S.; et al. Gold nanoparticles decorated zinc oxide nanorods as electrodes for a highly sensitive non-enzymatic electrochemical glucose detection. Jpn. J. Appl. Phys. 2019, 58, SDDE04. [Google Scholar] [CrossRef]
- Lv, X.; Tan, R.; Xu, X.; Li, Y.; Geng, C.; Fang, Y.; Tan, C.; Cui, B.; Wang, L. A highly sensitive non-enzymatic glucose sensor based on CuNi nanoalloys through one-step electrodeposition strategy. J. Appl. Electrochem. 2022, 52, 895–905. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, N.; Yang, C.; Hao, W.; Du, Y. Two-dimensional ZIF-L nanosheets as high performance non-enzymatic glucose sensor. arXiv 2021, arXiv:2110.04552. [Google Scholar]
- Mai, H.H.; Janssens, E. Au nanoparticle–decorated ZnO nanorods as fluorescent non-enzymatic glucose probe. Microchim. Acta 2020, 187, 577. [Google Scholar] [CrossRef]
- Sharma, A.; Agrawal, A.; Pandey, G.; Kumar, S.; Awasthi, K.; Awasthi, A. Carbon Nano-Onion-Decorated ZnO Composite-Based Enzyme-Less Electrochemical Biosensing Approach for Glucose. ACS Omega 2022, 7, 37748–37756. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Shit, A.; Jhon, H.S.; Park, S.Y. Highly sensitive non-enzymatic wireless glucose sensor based on Ni–Co oxide nanoneedle-anchored polymer dots. J. Ind. Eng. Chem. 2020, 89, 485–493. [Google Scholar] [CrossRef]
- Hasnain, M.; Ullah, Z.; Sonil, N.I.; Ahmad, W.; Khalil, A.; Ali, S.M.; Mustafa, G.M.; Nazar, M.F.; Rouf, S.A.; Shamain, N. Ultrasensitive strain sensor based on graphite coated fibrous frameworks for security applications. Mater. Today Commun. 2023, 37, 106859. [Google Scholar] [CrossRef]
- Raza, A.; Ullah, Z.; Khalil, A.; Batool, R.; Haider, S.; Alam, K.; Sonil, N.I.; Rouf, A.M.; Nazar, M.F. Facile fabrication of a graphene-based chemical sensor with ultrasensitivity for nitrobenzene. RSC Adv. 2024, 14, 9799–9804. [Google Scholar] [CrossRef] [PubMed]
- Gričar, E.; Radić, J.; Genorio, B.; Kolar, M. Highly Sensitive and Selective Graphene Nanoribbon Based Enzymatic Glucose Screen-Printed Electrochemical Sensor. Sensor 2022, 22, 9590. [Google Scholar] [CrossRef]
- Pilo, M.I.; Baluta, S.; Loria, A.C.; Sanna, G.; Spano, N. Poly(Thiophene)/Graphene Oxide-Modified Electrodes for Amperometric Glucose Biosensing. Nanomaterials 2022, 12, 2840. [Google Scholar] [CrossRef]
- Gao, J.; He, S.; Nag, A. Electrochemical Detection of Glucose Molecules Using Laser-Induced Graphene Sensors: A Review. Sensors 2021, 21, 2818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jing, Q.; Ao, S.; Schneider, G.F.; Kireev, D.; Zhang, Z.; Fu, W. Ultrasensitive field-effect biosensors enabled by the unique electronic properties of graphene. Small 2020, 16, 1902820. [Google Scholar] [CrossRef]
- Poletti, F.; Favaretto, L.; Kovtun, A.; Treossi, E.; Corticelli, F.; Gazzano, M.; Palermo, V.; Zanardi, C.; Melucci, M. Electrochemical sensing of glucose by chitosan modified graphene oxide. J. Phys. Mater. 2020, 3, 014011. [Google Scholar] [CrossRef]
- Özbek, M.A.; Yaşar, A.; Çete, S.; Er, E.; Erk, N. A novel biosensor based on graphene/platinum nanoparticles/Nafion composites for determination of glucose. J. Solid State Electrochem. 2021, 25, 1601–1610. [Google Scholar] [CrossRef]
- Xu, B.; Xiang, X.; Ding, L.; Luo, Z.; Zhao, J.; Huang, J.; Li, H.; Jiang, X. A Novel Large-Diameter Optical Fiber Glucose Biosensor Based on Glucose Oxidase– Graphene Oxide–Gold Nanoparticles. IEEE Sens. J. 2023, 23, 6832–6839. [Google Scholar] [CrossRef]
- Hossain, M.F.; Slaughter, G. Flexible electrochemical uric acid and glucose biosensor. Bioelectrochemistry 2021, 141, 107870. [Google Scholar] [CrossRef]
- Altuntaş, D.B.; Kuralay, F. MoS2/Chitosan/GOx-Gelatin modified graphite surface: Preparation, characterization and its use for glucose determination. Mater. Sci. Eng. B 2021, 270, 115215. [Google Scholar] [CrossRef]
- Matiyani, M.; Rana, A.; Karki, N.; Garwal, K.; Pal, M.; Sahoo, N.G. Development of multi-functionalized graphene oxide based nanocarrier for the delivery of poorly water soluble anticancer drugs. J. Drug Deliv. Sci. Technol. 2023, 83, 104412. [Google Scholar] [CrossRef]
- Ozlu, B.; Ahmed, M.B.; Muthoka, R.M.; Wen, Z.; Bea, Y.; Youk, J.H.; Lee, Y.; Yoon, M.H.; Shim, B.S. Naturally derived electrically active materials for eco-friendly electronics. Mater. Today Adv. 2024, 21, 100470. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Guo, X.; Li, Y.; Li, L.; Pan, L. Recent Advances in Conductive Polymers-Based Electrochemical Sensors for Biomedical and Environmental Applications. Polymers 2024, 16, 1597. [Google Scholar] [CrossRef] [PubMed]
- Kousseff, C.J.; Wustoni, S.; Silva, R.K.S.; Lifer, A.; Savva, A.; Frey, G.L.; Inal, S.; Nielsen, C.B. Single-Component Electroactive Polymer Architectures for Non-Enzymatic Glucose Sensing. Adv. Sci. 2024, 11, 2308281. [Google Scholar] [CrossRef] [PubMed]
- Himori, S.; Sakata, T. Free-standing conductive hydrogel electrode for potentiometric glucose sensing. RSC Adv. 2022, 12, 5369–5373. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Chronoampermetric detection of enzymatic glucose sensor based on doped polyindole/MWCNT composites modified onto screen-printed carbon electrode as portable sensing device for diabetes. RSC Adv. 2022, 12, 28505–28518. [Google Scholar] [CrossRef]
- Ragauskaitė, E.; Marčiukaitis, S.; Radveikienė, I.; Bagdžiūnas, G. An electrografted monolayer of polyaniline as a tuneable platform for a glucose biosensor. Nanoscale 2024, 16, 4647–4655. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, L.; Xie, H.; Liu, C.; Jin, W. An aggregation interpenetrating induced poly(dopamine) conductive hydrogel grid for efficient glucose detection. Biosens. Bioelectron. 2025, 290, 117951. [Google Scholar] [CrossRef] [PubMed]
- Stephy, J.; Das, S.; Vakamalla, T.R.; Sen, D. Electrochemical Glucose Sensing Using Molecularly Imprinted Polyaniline–Copper Oxide Coated Electrode. Surf. Eng. Appl. Electrochem. 2022, 58, 260–268. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Lamiri, L.; Belgherbi, O.; Dehchar, C.; Laidoudi, S.; Tounsi, A.; Nessark, B.; Habelhames, F.; Hamam, A.; Gourari, B. Performance of polybithiophene-palladium particles modified electrode for non-enzymatic glucose detection. Synth. Met. 2020, 266, 116437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).