Abstract
Acute Myocardial Infarction (AMI) is a critical cardiac condition that poses a substantial threat to myocardial function. Expedient diagnosis of AMI is paramount and relies on serological assays for rapid and accurate quantification of relevant biomarkers. Electrochemical sensors have emerged as promising candidates for this application, owing to their accessibility, operational simplicity, and high specificity. In this study, we developed a paper-based electrochemical immunosensor to detect cardiac troponin I in serum and saliva specimens. The electrode was fabricated using screen-printing technology with photographic paper as the substrate, employing graphite-based ink, nail polish, and acetone as the solvent. A quasi-reference electrode was constructed using silver powder-based ink, nail polish, and acetone. The immunosensor was prepared by modifying the working electrode with gold nanoparticles (AuNP) functionalized with cardiac troponin I antibodies (anti-cTnI) and bovine serum albumin (BSA). This modified electrode was subsequently used to detect the troponin I antigen. The analyses were performed in 0.1 mol L−1 phosphate buffer medium, pH 7.00, in the presence of 5.0 mmol L−1 of the potassium ferrocyanide probe. The immunosensor exhibited a sensitivity of 0.006 µA/fg mL−1, a limit of detection of 9.83 fg mL−1, and a limit of quantification of 32.79 fg mL−1. Specificity studies conducted in the presence of other macromolecules demonstrated minimal interference, with relative standard deviations (RSD) below 5.00%, indicating a specific interaction with troponin I. Furthermore, the immunosensor demonstrated excellent reproducibility and stability. Upon application to serum and saliva samples, the immunosensor presented recoveries of approximately 99–105%, suggesting its potential applicability in clinical analyses.
1. Introduction
Cardiovascular Diseases (CVD) encompass all types of illnesses related to heart malfunction and disturbances in the cardiovascular system. Common examples include cardiomyopathies, strokes, heart failure, and Acute Myocardial Infarction (AMI) [1]. Projections estimate approximately 23.3 million deaths attributable to cardiovascular diseases by 2030 [1,2,3]. Among these conditions, AMI is particularly significant due to its elevated mortality rate and substantial post-event consequences, necessitating intensive medical treatment and monitoring. Notably, timely diagnosis during the initial symptomatic phase could potentially prevent cardiac events [4,5,6,7].
The identification of myocardial infarction typically follows established medical protocols: (i) initial observation of symptoms, (ii) subsequent evaluation of electrocardiogram findings, and (iii) performance of serological assays quantifying cardiac biomarkers associated with AMI. Serological testing has gained widespread adoption because it can yield precise and rapid diagnostic results, thereby facilitating disease management and reducing mortality rates [8,9,10,11,12]. Several cardiac biomarkers are well-established in the literature, including myoglobin, B-type Natriuretic Peptide (BNP), Creatine Kinase MB (CK-MB) protein, and the troponin complex [13,14]. Numerous studies have highlighted the troponin complex as the “gold standard” in AMI diagnosis, particularly focusing on Troponin T (TnT) and Troponin I (TnI), which play direct roles in cardiac muscle function [8,9,10,11,12,13,14].
In healthy individuals, serum cTnI levels are generally very low, typically below 0.04 ng mL−1 (upper reference limit). In many clinical protocols, cTnI values above this threshold are interpreted as indicative of possible cardiac damage, especially when these levels fluctuate over time. In myocardial infarction, cTnI levels can exceed several nanograms per milliliter, which is proportional to the severity of myocardial injury [9,12,13,14].
The detection of troponin predominantly involves immunological reactions, employing techniques such as Surface-enhanced Raman Scattering (SERS) [15,16], Enzyme-Linked Immunosorbent Assay ELISA [17], chromatography [18], electrochemiluminescence assays [19], and lateral flow assays (LFAs) [20,21,22]. The portability of analytical systems, the relatively low cost of instrumentation, and the favourable sensitivity and selectivity of electroanalytical methodologies represent key attributes that have driven the advancement and implementation of electrochemical biosensors in clinical diagnostics [14,15,16,17,18,19,20,21,22,23,24].
Electrochemical biosensors are distinguished by their high sensitivity and specificity in the detection of biomolecules. In this context, immunosensors, which are based on the immobilization of antigens or antibodies onto the surface of electrochemical transducers, are widely utilized in bioanalytical assays. The presence of these biological components directly influences their interaction with target molecules, leading to the formation of immunocomplexes. Moreover, these devices provide significant advantages, including low cost, potential for miniaturization, and rapid analytical performance. Nanomaterials, including metallic nanoparticles, carbon-based materials (such as graphene, graphite, and carbon nanotubes), conductive polymers, and quantum dots, have been integrated into electrochemical systems to enhance electron transfer processes and improve signal transduction efficiency. Moreover, recent investigations of electrochemical sensors have increasingly emphasized the use of printed electrodes [24,25,26,27,28,29,30]. Fabricated in various configurations, these devices integrate an electrode set (auxiliary, working, and reference) onto a single substrate. Additionally, printed electrodes significantly reduce the costs associated with electrochemical methodologies while fostering innovation in portable devices that are ideally suited for routine analytical applications [30,31,32,33,34,35,36,37,38,39].
Electrochemical immunoassays encompass two primary categories of immunosensors: sandwich-type and label-free. Both offer advantages such as good sensitivity, operational simplicity, and ease of use. Label-free detection utilizing electrodes is an efficient biosensor platform that has garnered increasing attention due to its simplicity and rapidity, demonstrating potential for point-of-care (POC) devices. In the construction of both labelled and label-free biosensors, the utilization of gold (Au) metallic nanoparticles and carbon-based nanostructures, such as graphene and carbon nanotubes (CNTs), for electrode modification is common [31,32,33,34,35,36,37,38,39].
Mansuriya and Altintas [40] developed an immunosensor based on a screen-printed gold electrode to detect Troponin I (cTnI). The sensor was modified using graphene quantum dots and gold nanoparticles (AuNP). Biomarkers were quantified using square wave voltammetry (SWV), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and amperometry. The limit of detection was calculated as 0.1 and 0.5 pg mL−1 for buffer and serum, respectively. This promising analytical platform has the potential to enable rapid and accurate AMI diagnosis in various settings [40]. Gupta et al. [41] described a novel electrochemical immunosensor based on functionalized graphene for high-sensitivity detection of cTnI. The sensor employed screen-printed electrodes coated with graphene oxide functionalized with aminotriimesic acid, providing an efficient and selective platform for cTnI detection. The detection limit was 0.08 ng mL−1, with a detection range of 0.1 to 100 ng mL−1, indicating the method’s efficiency in determining troponin [41]. Eshlaghi et al. [42] developed an aptasensor for cTnI detection using a carbon-printed electrode modified with polypyrrole-gold nanoparticles. Analyses were performed using square wave voltammetry, yielding a detection limit of 25 pg mL−1 with a linear detection range of 50 to 500 pg mL−1. The proposed aptasensor exhibited good selectivity and appropriate sensitivity for clinical diagnostics, enabling rapid, quantitative, and label-free cardiac biomarker detection [42].
In the present study, we developed an electrochemical immunosensor screen-printed with graphite and nail polish-based conductive ink, modified with gold nanoparticles, antibodies, and BSA, to enhance the specificity, detection, and quantification of Troponin I. Furthermore, we aimed to establish a simplified, low-cost, highly stable, and reproducible methodology applicable to clinical analyses of interest.
2. Materials and Methods
2.1. Reagents
Bibasic sodium phosphate heptahydrate (Na2HPO4.7H2O) was purchased from Synth® (Diadema, SP, Brazil), monobasic sodium phosphate monohydrate (NaH2PO4.H2O) was purchased from Synth® (Diadema, SP, Brazil), Potassium Ferrocyanide (K4[Fe(CN)6]) was purchased from Dinâmica Química (Indaiatuba, SP, Brazil), Bovine Serum Albumin produced by New England Biolabs (São Paulo, SP, Brazil), and imported and distributed by Uniscience do Brasil Ind. Com. And Repres. LTDA (São Paulo, SP, Brazil), Troponin I, anti-TnI, and human serum from AB plasma (sterilized via filtration) were purchased from Sigma-Aldrich ® (St. Louis, MO, USA). Normal saliva from a female human donor was obtained from Lee Biosolutions (Maryland Heights, MO, USA). Tris (hydroxymethyl) aminomethane (hydrochloride) (C4H11NO3.HCl) was purchased from Dinâmica Química Contemporânea Ltd.a. (Indaiatuba, SP, Brazil), Sodium Chloride (NaCl) was purchased from Vetec Química Fina Ltd.a. (Duque de Caxias, RJ, Brazil). All the chemical reagents used were of analytical grade. All solutions were prepared in distilled deionized water (resistivity >18 MΩ cm−1, 25 °C; Millipore® Milli-Q® purification system, Bedford, MA, USA).
2.2. Immunosensor Development
First, the electrodes printed on paper were produced following the methodology of previously published studies [43]. Graphite ink was prepared by mixing 1.250 g of graphite, 1.250 g of nail polish, and 1500 µL of acetone with a glass rod until homogeneous. These quantities were optimized in previous studies. The ink was subsequently deposited onto an adhesive mold secured to a photo paper matte, previously sanded with a piece of waterproof sandpaper, and uniformly distributed using a spatula. Graphite ink was used to print the working, auxiliary, and reference electrodes [43]. The quasi-reference electrode was prepared by adding a layer of silver ink on the graphite ink, following the methodology described in our previous work [44]. A layer of mineral spirit is used to define the electrical contact area. The dimensions of the electrode are 1.4 cm in width and 3.5 cm in length, with an inter-electrode distance of 0.25 cm and a working electrode geometric area of 0.6 cm2.
After printing, the working electrode was modified using a drop-casting method. Initially, AuNP were synthesized using the Turkevich method. Stock solutions for sodium citrate (10.0 mg mL−1) and sodium tetrachloroaurate (0.125 mol L−1) were prepared. A specific volume of the gold salt solution was heated to 90 °C, and the sodium citrate solution was quickly added upon reaching this temperature. The colour changes of the solution from yellow to gray and red indicated the formation of AuNPs. The suspension was cooled and stored at 4 °C [45]. AuNP exhibited an average size of 3.3 nm, as discussed in previous studies [45]. Then, 30 μL of the AuNP dispersion was deposited, and the electrodes were placed in an oven at 60 °C for approximately 30 min until the nanoparticles were dried on the electrode surface. The dispersion volume of AuNP in the SPE modification was then optimized (Supplementary Material Figure S1). A solution of Tris-HCl (10.0 mmol L−1) and sodium chloride (NaCl) (150.0 mmol L−1), pH 7.00, was used as a solvent to prepare antigen, antibody, and BSA solutions. To immobilize the antibody on the AuNP of the working electrode, an anti-cTnI solution with a concentration of 2.0 μg mL−1 was used. The anti-cTnI stock solution was sonicated in an ice bath before and after the dilution of the Tris-HCl/NaCl solution. The electrode was incubated with a 30 µL aliquot of anti-cTnI solution for 6 h at approximately 4 °C under a nitrogen atmosphere. Subsequently, the electrode was washed with 0.10 mol L−1 phosphate-buffered saline (PBS, pH 7.5) and dried under nitrogen before being stored in a refrigerator. A BSA solution (1.0 mg mL−1) was prepared, and 30 µL was added to the working electrode surface. After incubation for 1 h, the electrode was washed as described for antibody incubation. Finally, a 100.0 ng mL−1 Troponin I solution was prepared, and 30 µL was added to the working electrode surface. The immunosensor was incubated for 1 h at approximately 4 °C, followed by washing and drying, as was performed for BSA and the antibody. After completion of these steps, the immunosensor was used for electrochemical measurements in 0.1 mol L−1 phosphate buffer (pH 7.0) in the presence of 1.0 mmol L−1 potassium ferrocyanide.
2.3. Atomic Force Microscopy (AFM)
For morphological characterization, working electrodes with an area of 1 cm in diameter were prepared using one electrode for each modification. Therefore, the electrode configurations were evaluated using AFM: (i) SPE, (ii) SPE/AuNP, (iii) SPE/AuNP/Ab, (iv) SPE/AuNP/Ab/BSA, and (v) SPE/AuNP/Ab/BSA/cTnI. Atomic Force Microscopy was performed using a Bruker Multimode Microscope 8 (MM8) (Portland, OR, USA). A scan size of 5.0 µm and an amplitude of 5000 mV were used for the measurements. The samples were placed on a circular AFM holder using silver tape. The AFM probe was composed of silicon and features a sharp tip that scans across the sample surface.
2.4. Optimization Studies
The optimized parameters for the development of the immunosensor were the incubation time for the anti-cTnI (4, 6, and 8 h), the incubation time for cTnI (30, 60, and 90 min), the time of BSA (30, 60, and 90 min), the concentration of BSA (0.5, 1.0, and 1.5 mg mL−1), and PBS pH (6.5–8.5). One parameter was selectively varied during each optimization step, whereas all other parameters remained fixed. The sensors were incubated with 30 μL anti-cTnI (2.0 μg mL−1) and cTnI (100.0 ng mL−1). The electrochemical behavior of the immunosensor was evaluated by considering the modifications made to the surface of the working electrode. Electrochemical measurements were performed using conventional amperometry with an applied potential of +0.4 V for 120 s. All electrochemical measurements were conducted in a reaction medium containing 20 mL of 0.10 mol L−1 phosphate buffer at 7.00 pH and 1.0 mmol L−1 K4[Fe(CN)6].
2.5. Analytical Curve
After optimizing the electrochemical behavior of the immunosensor, electrochemical measurements were performed by varying the concentration of the immobilized troponin I on the electrode surface to construct an analytical curve. Immunosensors were prepared in duplicate with troponin I immobilization concentrations of 10, 20, 30, 40, 50, 60, 70, and 80 fg mL−1. Each electrode was subjected to electrochemical measurements under previously optimized conditions with a constant potassium ferrocyanide concentration. An analytical curve of the current versus cTnI concentration was generated from the obtained data. The sensitivity parameters, detection and quantification limits, and linear working range were calculated based on this curve.
2.6. Selectivity, Reproducibility, and Stability of the Immunosensor
To evaluate the selectivity, an interference study was conducted using solutions of BSA (1.0 mg mL−1), IgG (1.0 mg mL−1), PSA (4.0 ng mL−1), and CA 15-3 (20.0 U mL−1). The concentrations were chosen according to the reference normal concentrations of these interfering substances founded in the human serum and saliva, except the BSA. Each solution was prepared to contain a specific interferent and a 10.0 fg mL−1 concentration of cTnI. A 30 µL aliquot of each solution was immobilized on the surface of the working electrode and incubated according to the procedure outlined in Figure 1. Next, to assess the reproducibility, multiple identical electrodes were fabricated under varying conditions, and their electrochemical responses were compared. Finally, a stability study was performed by storing the immunosensors for 21 days and conducting the analysis throughout this period. Selectivity, reproducibility, and stability measurements were carried out using conventional amperometry in 0.10 mol L−1 phosphate buffer (pH 7.00) containing 1.0 mmol L−1 K4[Fe(CN)6]. Each electrode was incubated with 30 µL of a 10.0 fg mL−1 Troponin I solution.
2.7. Application of the Immunosensor in Blood Serum and Saliva Samples
Serum and saliva samples were obtained directly from the same laboratories that provided antigens and antibodies (Sigma Aldrich® and Lee Biosolutions); therefore, ethics committee approval was not required for this study. An immunosensor was used to determine troponin I levels in these samples. The samples were spiked with troponin I and incubated on the immunosensor surface before electrochemical analysis. Three electrodes were prepared for incubation with saliva samples containing different concentrations of cTnI (0.2 fg mL−1, 100.0 fg mL−1, and 1000.0 fg mL−1). Serum samples were analyzed for each concentration (10 fg mL−1, 30 fg mL−1, and 50 fg mL−1). The analyses were performed in the presence of 1.0 mmol L−1 K4[Fe(CN)6] in 0.10 mol L−1 phosphate buffer, pH 7.00, applying the amperometry technique (+0.4 V, 120 s).
3. Results
3.1. Electrochemical Behavior of the Immunosensor
After the immunosensor was prepared, electrochemical measurements were performed by amperometry. It is important to emphasize that most biological molecules, such as proteins or antibodies used in immunosensor development, do not have electro-active structural characteristics. In other words, these components do not undergo oxidation-reduction when subjected to potential applications. Therefore, electrochemical probes can be used to obtain electrochemical signals from an immunosensor that presents these molecules in its structure. In this study, potassium ferrocyanide was used as a redox probe, which was present at the same concentration in all the analyses. The amperometry technique was optimized by studying three different potentials (−0.2, +0.2, and +0.4 V). The best electrochemical response was evaluated based on the obtained signal variations. The sensor exhibited better current signal results when a potential of +0.4 V. In the presence of large structures, such as antibodies and antigens, well adsorbed on the electrode surface and covering practically all the free spaces to obtain the signal corresponding to the redox reaction, there is difficulty in the transfer of charge between the electrode and the solution. Therefore, the potential with the highest value (+0.4 V) facilitates this process and was chosen as the potential to be fixed in the subsequent measurements. Electrochemical measurements were obtained at each stage of immunosensor modification (Figure 1).
Figure 1A shows the electrochemical response of the unmodified electrode, in which the current related to the redox process of potassium ferrocyanide was observed. When AuNP were added (Figure 1B), an increase in current was observed owing to the conductivity of the nanoparticles. Furthermore, AuNPs serve as a biocompatible platform for the subsequent immobilization of biological materials. Figure 1C shows the results of antibody immobilization on the electrode, indicating a decrease in response. This reduction was attributed to the large, non-electroactive antibody molecules that occupied a significant portion of the electrode surface, hindering the electron transfer between the electrode and solution. BSA is used to increase device specificity. BSA molecules bind to unoccupied sites on the electrode surface, preventing non-specific analyte binding. Another decrease in signal was observed owing to the increased mass and insulation of BSA (Figure 1D). Finally, the target antigen, cTnI, was immobilized on the antibody-modified electrode. This additional layer of biomolecules further reduces the active surface area of the electrode, leading to a further decrease in the current signal (Figure 1E). This decrease in the current was directly correlated with the amount of cTnI bound to the electrode surface. Therefore, the immunosensor relies on a decrease in the current signal as a function of increased biomolecule immobilization. This principle allows for the detection of cTnI, as demonstrated in the subsequent analytical curve. The data presented in Figure 1 validates the feasibility of the immunosensor for cTnI detection.
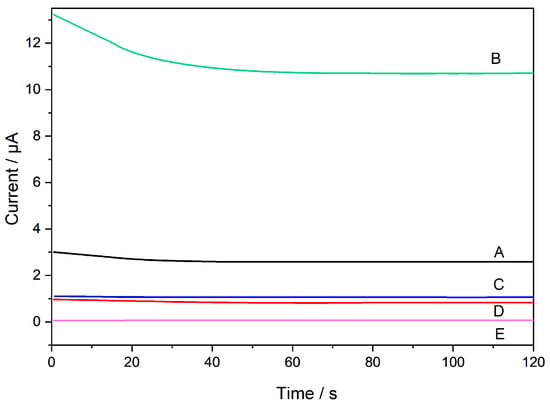

Figure 1.
Electrochemical behavior of immunosensor development: (A) bare sensor, (B) SPE/AuNP, (C) SPE/AuNP/Ab, (D) SPE/AuNP/Ab/BSA, and (E) SPE/AuNP/Ab/BSA/cTnI. Reaction medium: phosphate buffer (0.10 mol L−1, pH 7.00) in 1.0 mmol L−1 K4[Fe(CN)6]. (Concentrations immobilized on the sensor: Anti-cTnI 1.0 mg mL−1; BSA 1.0 mg mL−1; cTnI 100.0 ng mL−1).
The electrochemical behavior was also monitored by electrochemical impedance spectroscopy (EIS), in the presence of 0.1 mol L−1 KCl and the 5.0 mmol L−1 potassium ferrocyanide probe. Nyquist plots are presented in Supplementary Material Figure S2. The charge transfer resistance (Rct) of the SPE was 518.5 Ω. For SPE/AuNP, Rct decreases to 35.5 Ω, indicating a lower resistance to charge transfer at the electrode/solution interface. For the SPE/AuNP/ab, SPE/AuNP/ab/BSA, and SPE/AuNP/ab/BSA/cTnI configurations, Rct increased to 335.2, 797.9, and 928.7 Ω, respectively. This gradual increase in Rct reflects the formation of insulating biological layers that hinder charge transfer, demonstrating the stepwise assembly of the immunosensor. These EIS results not only corroborate the amperometric data but also confirm the high electrochemical performance and sensitivity of the AuNP-modified electrode, highlighting its strong potential for efficient and reliable cTnI detection.
Optimization Studies
To optimize the immunosensor performance, several parameters, such as incubation times for anti-cTnI, BSA, and cTnI, the concentration of BSA, and the pH of the phosphate buffer, were investigated. The ideal performance of the immunosensor is associated with the formation of a stable antigen–antibody immunocomplex. This complex increases the mass of the electrode surface, hindering the diffusion of the redox species and consequently reducing the current signal. Therefore, in this study, we monitored the decrease in the analytical response as an indication of successful immunocomplex formation. The optimized conditions are presented in Table 1. The analytical responses are provided in Supplementary Material Figure S3.

Table 1.
Summary of optimization steps in the construction of the immunosensor for cTnI.
3.2. Atomic Force Microscopy of the Immunosensor
Atomic Force Microscopy (AFM) images were obtained in the tapping mode in order to study the surface morphology. Comparing. AFM analysis was employed to confirm the presence of the immunosensor’s constituent components through observed surface structural changes. A clear distinction between the images lies in the surface roughness, which indicates the presence of immobilized structures. AFM images of the working electrodes for SPE, AuNP, AuNP/Ab, AuNP/Ab/BSA, and AuNP/Ab/BSA/cTnI are shown in Figure 2.
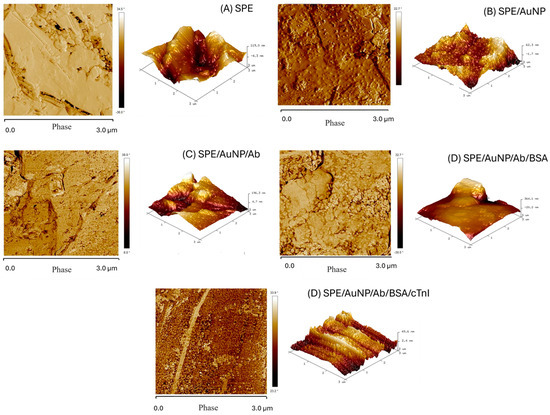
Figure 2.
Atomic Force Microscopy for: (A) EI-GR; (B) EI-GR/AuNP; (C) EI-GR/AuNP/Anti-cTnI; (D) EI-GR/AuNP/Anti-cTnI/BSA; (E) EI-GR/AuNP/Anti-cTnI/BSA/cTnI.
In Figure 2A, for SPE, structures consisting of graphite sheets can be observed, with a flaky appearance, but with smaller particles attached to the surface. The AFM images of SPE/AuNPs (Figure 2B) show a less homogeneous surface, resembling a film with immobilized nanospheres, indicating the presence of AuNPs [46,47]. The graphite flakes were visible beneath the AuNP layer, implying a thin nanoparticle layer. After antibody immobilization of the SPE/AuNP/Ab (Figure 2C), a striking change in the phase element and height was observed. The topography reveals the material covering the sensor structure. Furthermore, slightly wavy structures suggest helical amino acid chains, which are characteristic of antibodies [47,48]. The increase in surface roughness is credited to the conjugation of antibodies. The electrode was almost entirely covered with Anti-cTnI, indicating effective immobilization.
For the SPE/AuNP/Ab/BSA electrode (Figure 2D), the addition of BSA resulted in almost complete loss of the wavy structure of the antibody, producing a denser and more homogeneous appearance. This change indicates the presence of BSA, as confirmed by AFM height and phase element data, revealing the larger globular nature of the molecule [48]. The addition of the SPE/AuNP/Ab/BSA/cTnI antigen (Figure 2E) produced a significantly more porous surface with small globular structures covering the entire area, suggesting the presence of the cTnI antigen. The known globular structure of troponin supports antigen immobilization. The topography differentiates the denser material (Figure 2D) from the more porous structure in Figure 2E [46,47,48]. Therefore, AFM analysis corroborates the anticipated material changes and structurally confirms the immobilization suggested by the electrochemical data.
3.3. Analytical Curve
To obtain the analytical curve for the immunosensor, electrodes were constructed with different concentrations of cTnI (10–80 fg mL−1) and evaluated under the same conditions as the electrochemical system. The amperograms obtained are presented in Figure 3, accompanied by the respective analytical curve constructed using the current concentration data.
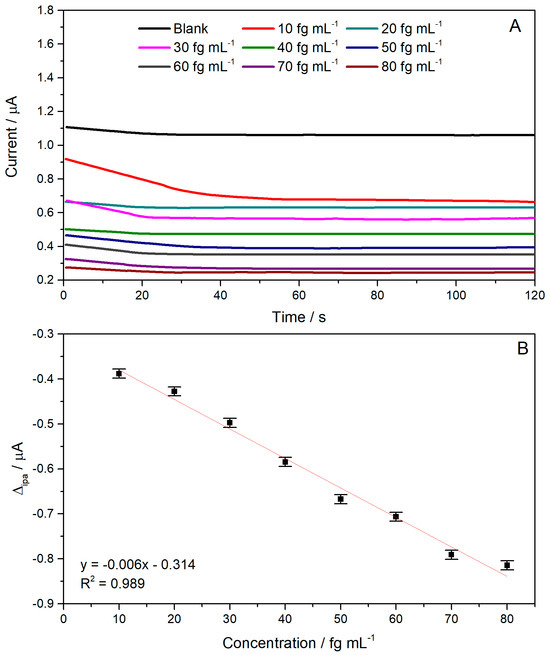
Figure 3.
(A) Chronoamperogram obtained at different cTnI concentrations (10 to 80 fg mL−1) using 0.50 mM K4[Fe(CN)6] in phosphate buffer. (B) Calibration curve showing the relationship between cTnI concentration and current values.
From the analytical curve obtained, it was possible to observe a decrease in current with increasing cTnI concentration. This behavior is directly related to the increase in antigen structures covering the free binding sites of specific antibodies, consequently blocking the empty spaces for charge transfer between the solution and the working electrode surface. These results indicate that the immunosensor could detect the antigen of interest at different cTnI concentrations. The current decrease was correlated with the cTnI concentration to obtain an analytical curve (Figure 4B). A linear relation between cTnI concentration and the electrochemical response was obtained. The curve showed a linear regression equation of ip[μA] = −0.006 [fg mL−1] − 0.314, with a linear range of 10 to 80 fg mL−1 (R2 = 0.989) and a sensitivity of 0.006 μA/fg mL−1. At higher concentrations the amperometric response is no longer linear due to the saturation of the antibody active site. The LOD and LOQ were obtained by dividing the standard deviation of the blank solution (n = 10) by the slope of the analytical curve three and ten times, respectively. The LOD and LOQ values obtained were 9.83 fg mL−1 and 32.79 fg mL−1, respectively.
The developed immunosensor demonstrated a limit of detection (LOD) of 9.83 fg mL−1, which demonstrates high analytical sensitivity. This performance was significantly lower than the serum troponin I levels observed under physiological and pathological conditions. In healthy individuals, cTnI concentrations are generally below 0.01 ng mL−1, while values above 0.04 ng mL−1 are considered indicative of possible acute myocardial infarction (AMI). Thus, the LOD obtained was approximately 1000 times lower than the lower limit of the clinically relevant range, demonstrating the potential of the biosensor for the early detection of cTnI, even in the early stages of myocardial injury. Furthermore, the wide linear response range observed reinforces the applicability of the proposed system for the quantitative monitoring of biological samples.
Table 2 presents a compilation of literature on cTnI determination using electrochemical sensors. These studies were used as benchmarks for comparing the analytical data obtained from the sensors developed in this study. The studies generally found in the literature for Troponin I determination are presented using conventional working electrodes or, in printed form, sensors that employ very complex molecular modifying structures or structure the sensor in a sandwich model that requires more time to prepare these sensors.

Table 2.
Comparative study of some electrochemical sensors and immunosensors for cTnI determination.
By comparing the data presented in Table 2, the advantages of the proposed device are evident in terms of the simplicity of preparation and high specificity. The use of paper as a support not only simplifies the manufacturing process and reduces costs and time but also offers significant potential for the creation of disposable and portable devices. While literature frequently describes electrochemical immunosensors based on conventional electrodes or printed versions that require complex molecular modifications, this study demonstrated the feasibility of an effective and low-cost immunosensor using paper. This innovative approach allows for the creation of a sensor applicable in real systems, offering a promising alternative for the rapid and accurate detection of troponin I, with the potential to significantly impact the diagnosis of acute cardiac conditions.
3.4. Specificity, Reproducibility, and Stability of the Immunosensor
The specificity of the immunosensor was investigated in the presence of molecules that can be excreted in the serum and saliva, such as IgG, as well as biomarkers CA 15-3 and PSA, except for BSA. Although BSA is not naturally found in human serum, it is frequently employed as a cost-effective substitute for human serum albumin, the predominant protein in blood. Each interfering molecule was evaluated at the concentrations found in the matrices, as described in Section 2.6. The main interference caused by these structures is directly related to their association with selective sites intended for the target antigen. In addition, the absence of cTnI in ten blank serum samples was evaluated using a sensor with and without the target analyte (reference sensor) to assess possible matrix effects.
The results of the electrochemical measurements are shown in Figure 4A, concerning the blank (sensor SPE/AuNP/Anti-cTnI/BSA with no matrix interference), the serum samples (in the absence of cTnI), and spiked serum samples (in the presence of cTnI). The variation in the current response between the blank and the serum samples was less than 0.05%, indicating that cTnI was not present in blank serum samples. About the spiked serum samples, it is possible to observe a decrease in the analytical response in the presence of cTnI compared to the response of the reference sensor in the absence of cTnI due to the formation of an immune complex on the immunosensor surface, resulting from the antigen–antibody interaction.
When the interfering molecules were inserted (Figure 4B), the variation in the current response was less than 5.00% of that without any interfering substances. The interference values slightly exceeded 5.00% for IgG and BSA, suggesting that these components may have interfered with the specific antigen–antibody interaction on the immunosensor surface, thus influencing the measured current. Despite these values, following the guidelines of the “M10 Bioanalytical Method Validation and Study Sample Analysis” document [51], the immunosensor can be applied to saliva and serum samples for cTnI detection. These findings demonstrate that the proposed immunosensor demonstrated high selectivity and specificity toward cTnI detection, supporting its applicability for analyses in biological matrices such as serum.
The reproducibility of the immunosensor was evaluated, as shown in Figure 4C, by analyzing Troponin I at a concentration of 10 fg mL−1 using six different immunosensors. The repeatability of each electrode was assessed using an immunosensor to detect cTnI three times. Figure 4C shows the current values for different immunosensors manufactured using the proposed method. The relative standard deviation of the different immunosensors was 1.68%, suggesting that the manufactured immunosensor had excellent reproducibility.
To verify the stability of the manufactured immunosensor, a series of immunosensors was stored at 4◦C for 21 days. After 21 days, the current response changed to 99.8% of the original current response for Troponin I, with a relative standard deviation of 2.80%, suggesting that the immunosensor stability was acceptable (as shown in Figure 4D).
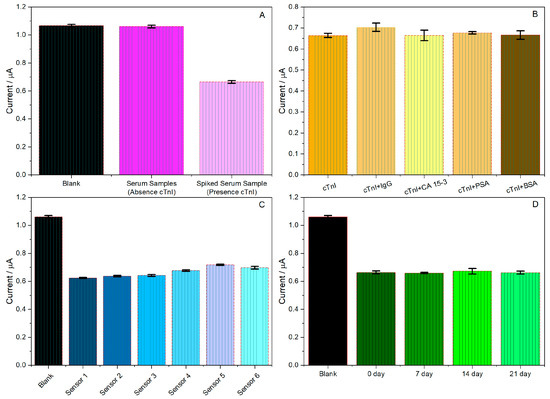

Figure 4.
Analytical responses to studies: (A) absence and presence of troponin, (B) specificity of the immunosensor in the presence of cTnI and interferers; (C) immunosensor reproducibility for four different devices; (D) immunosensor stability over 21 days. Experimental conditions 1.0 mmol L−1 K4[Fe(CN)6] in phosphate buffer (0.10 mol L−1; pH 7.00), cTnI concentration 10 fg mL−1, (n = 3).
3.5. Application in Blood and Saliva Samples
The immunosensor was evaluated for its application to saliva and blood serum samples. The resulting analytical curve demonstrated a limit of quantification of 32.79 fg mL−1 and a linear response range from 10 to 80 fg mL−1 Considering that the normal concentration of cTnI in blood can reach up to 0.04 ng mL−1, it was necessary to dilute the samples at a 1:2000 ratio to enable the detection of troponin I using the proposed sensor [51,52,53,54,55,56]. Following dilution, the resulting cTnI concentrations were 10, 30, and 50 fg mL−1. The enriched samples were incubated with the sensors. Table 3 presents the values obtained in this study using the blood serum samples. The results showed recovery rates ranging from 102.8% to 105.9%. Recovery values exceeding 100% may be attributed to matrix effects in the samples, which, despite being of synthetic origin, with a simplified composition and absence of troponin I, may still slightly affect the immunosensor selectivity.

Table 3.
Recovery study for serum samples.
For saliva samples, cTnI concentrations do not have a well-defined diagnostic pattern, mainly because this type of analysis is still a relatively recent study for infarction diagnosis. Among the main studies that have sought the use of saliva for cTnI detection, values of up to 1.0 pg mL−1 are found in patients still in normal health, but concentrations close to or above 675.0 pg mL−1 are indicative of a heart attack (myocardial infarction). As these concentrations are significantly lower, a distinct dilution factor compared with serum samples was necessary. Consequently, a 1:5000 dilution was applied to these samples [38,51,52,53,54,55,56]. The concentrations used for the saliva samples were 0.2 fg mL−1, 100.0 fg mL−1, and 1000.0 fg mL−1. Table 4 presents the values obtained in this study. As you can see from the data presented, only one of the samples exhibited satisfactory recovery results, close to 99.7% (100.0 fg mL−1). The results for the other two evaluated concentrations (0.2 fg mL−1 and 1000.0 fg mL−1) were inadequate. The results do not show good recovery values and accuracy. Problems may occur in specific interactions between the antigen and the antibody due to the complexity of the saliva sample, which directly influences the current signal obtained for these samples. Saliva is a complex fluid produced and secreted from salivary glands. Although mostly composed of water, saliva also contains electrolytes and an incredible variety of proteins and peptides. The inherent viscosity variability in human samples are the most common disadvantages of using saliva to detect biomarkers.

Table 4.
Recovery study for saliva samples.
In general, the immunosensor proved to be applicable for determining troponin in blood serum samples. However, it is not suitable to determine troponin I in saliva samples.
4. Conclusions
Developing low-cost and user-friendly electrochemical immunosensors for Troponin I detection offers significant potential for revolutionizing clinical analysis. These devices are attractive alternatives to traditional methods, eliminating the need for complex and expensive instrumentation. Determining Troponin I antigen levels in bodily fluids is instrumental in monitoring patients with acute myocardial infarction. The developed immunosensor demonstrated promising results for Troponin I detection, exhibiting a linear range of 10 to 80 fg mL−1, a limit of detection (LOD) of 9.83 fg mL−1, and a limit of quantification (LOQ) of 32.79 fg mL−1. The immunosensor was fabricated by modifying the working electrode with AuNP, anti-cTnI antibodies, and BSA. Interference studies confirmed the selectivity of the immunosensor for cTnI and demonstrated its reproducibility and stability. The applicability of the immunosensor was further evaluated using serum samples, which achieved recovery values ranging from 102.8 to 105.9%. For saliva samples, the recovery values ranged from 32 to 99%, and the lower recovery values may be attributed to sample complexity. These results highlight the efficient performance of the proposed immunosensor in detecting target analytes in real samples. Overall, the immunosensor demonstrated applicability for the determination of troponin in blood serum samples; however, it was not suitable for the detection of troponin I in saliva samples. In conclusion, the results underscore the potential of printed electrochemical immunosensors as a viable alternative to conventional methods for Troponin I analysis in human serum samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13110383/s1, Figure S1: Optimization of the amount of AuNP in the SPE modification: (A) cyclic voltammograms for SPE without AuNP and with different amounts of AuNP, and (B) Bar graph (n = 3). Reaction medium 1.0 mmol L−1 of K4[Fe(CN)6] in phosphate buffer (0.10 mol L−1; pH 7.0); Figure S2: Nyquist diagrams of SPE, SPE/AuNP, SPE/AuNP/ab, SPE/AuNP/ab/BSA, and SPE/AuNP/ab/BSA/cTnI. Reaction medium 5.0 mmol L−1 of K4[Fe(CN)6] in KCl 0.1 mol L−1; Figure S3: Bar graph for optimizations of (A) anti-cTnI incubation time, (B) cTnI antigen incubation time, (C) BSA incubation time, (D) BSA concentration, and (E) variation in electrolyte pH. Reaction medium 1.0 mmol L−1 of K4[Fe(CN)6] in phosphate buffer (0.10 mol L−1; pH 7.0).
Author Contributions
Conceptualization: M.A.C.d.R. and A.E.F.O.; methodology: M.A.C.d.R. and A.E.F.O.; study design: M.A.C.d.R. and A.E.F.O.; software: M.A.C.d.R.; validation: M.A.C.d.R.; formal analysis: M.A.C.d.R.; investigation: M.A.C.d.R.; data curation: M.A.C.d.R. and T.C.d.O.C.; writing—original draft: M.A.C.d.R., T.C.d.O.C., D.N.d.S. and S.O.D.d.T.; writing—review and editing: M.A.C.d.R., T.C.d.O.C., D.N.d.S., S.O.D.d.T. and L.F.F.; visualization: A.C.P. and L.F.F.; supervision: A.C.P.; project administration: A.C.P.; funding acquisition: A.C.P.; resources: L.F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding related to the processes: CEX—APQ-00679-22 FAPEMIG, 468 305360/2022-1 CNPq, and 465571/2014-0 INCT-DATREM.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank UFSJ, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) e Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and INCT-DATREM. Thalita Chiaramonte and student Filipe Santos Souza are responsible for the AFM characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CDV | Cardiovascular Diseases |
| AMI | Acute Myocardial Infarction |
| BNP | B-type Natriuretic Peptide |
| CK-MB | Creatine Kinase MB |
| SERS | Surface-enhanced Raman Scattering |
| LFAS | Lateral Flow Assays |
| cTnI | Troponin I |
| AuNP | Gold Nanoparticles |
| NaCl | Sodium Chloride |
| BSA | Bovine Serum Albumin |
| Ab | Antibody |
| SPE | Screen Printed Electrode |
| PBS | Phosphate Buffer |
| AFM | Atomic Force Microscopy |
| LOD | Limits of Detection |
| LOQ | Limits of Quantification |
References
- Negahdary, M. Aptamers in nanostructure-based electrochemical biosensors for cardiac biomarkers and cancer biomarkers: A review. Biosens. Bioelectron. 2020, 152, 112018. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.G.; Ahmed, S.R.; Keshavarz-Motamed, Z.; Srinivasan, S.; Rajabzadeh, A.R. Recent advancements of nanomodified electrodes-towards Point-of-Care detection of cardiac biomarkers. Bioelectrochemistry 2023, 152, 108440. [Google Scholar] [CrossRef]
- Han, K.; Li, G.; Tian, L.; Li, L.; Shi, Y.; Huang, T.; Li, Y.; Xu, Q. Multifunctional peptide-oligonucleotide conjugate promoted sensitive electrochemical biosensing of cardiac troponin I. Biochem. Eng. J. 2021, 174, 108104. [Google Scholar] [CrossRef]
- Minopoli, A.; Della Ventura, B.; Lenyk, B.; Gentile, F.; Tanner, J.A.; Offenhäusser, A.; Mayer, D.; Velotta, R. Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nat. Commun. 2020, 11, 6134. [Google Scholar] [CrossRef]
- Lim, S.H.; Sung, Y.J.; Jo, N.; Lee, N.Y.; Kim, K.S.; Lee, D.Y.; Kim, N.S.; Lee, J.; Byun, J.Y.; Shin, Y.B.; et al. Nanoplasmonic immunosensor for the detection of SCG2, a candidate serum biomarker for the early diagnosis of neurodevelopmental disorder. Sci. Rep. 2021, 11, 22764. [Google Scholar] [CrossRef]
- Lin, L.P.; Tham, S.Y.; Loh, H.S.; Tan, M.T.T. Biocompatible graphene-zirconia nanocomposite as a cyto-safe immunosensor for the rapid detection of carcinoembryonic antigen. Sci. Rep. 2021, 11, 22536. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Iemmo, L.; Urban, F.; Palomba, M.; Carotenuto, G.; Longo, A.; Sorrentino, A.; Giubileo, F.; Barucca, G.; Rovere, M.; et al. Graphite platelet films deposited by spray technique on low density polyethylene substrates. Mater. Today Proc. 2020, 20, 87–90. [Google Scholar] [CrossRef]
- Alam, M.A. Graphene based electrochemical biosensors for the detection of cardiac biomarkers. Biosens. Bioelectron. X 2024, 20, 100515. [Google Scholar] [CrossRef]
- Tang, L.; Yang, J.; Wang, Y.; Deng, R. Recent Advances in Cardiovascular Disease Biosensors and Monitoring Technologies. ACS Sens. 2023, 8, 956–973. [Google Scholar] [CrossRef]
- Negahdary, M.; Sharma, A.; Anthopoulos, T.D.; Angnes, L. Recent advances in electrochemical nanobiosensors for cardiac biomarkers. TrAC Trends Anal. Chem. 2023, 164, 117104. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Review on electrochemical sensing strategies for C-reactive protein and cardiac troponin I detection. Microchem. J. 2020, 156, 104857. [Google Scholar] [CrossRef]
- Arumugasamy, S.K.; Chellasamy, G.; Yun, K.; Hyun, J. Bio-quantum dots for electrochemical sensing of cardiac biomarkers of acute myocardial infarction. J. Ind. Eng. Chem. 2024, 129, 488–498. [Google Scholar] [CrossRef]
- Asl, S.K.; Rahimzadegan, M. The recent progress in the early diagnosis of acute myocardial infarction based on myoglobin biomarker: Nano-aptasensors approaches. J. Pharm. Biomed. Anal. 2022, 211, 114624. [Google Scholar] [CrossRef]
- Qin, X.; Li, D.; Qin, X.; Chen, F.; Guo, H.; Gui, Y.; Zhao, J.; Jiang, L.; Luo, D. Electrochemical detection of the cardiac biomarker cardiac troponin I. View 2024, 5, 20240025. [Google Scholar] [CrossRef]
- Wei, J.; Mu, Y.; Song, D.; Fang, X.; Liu, X.; Bu, L.; Zhang, H.; Zhang, G.; Ding, J.; Wang, W.; et al. A novel sandwich immunosensing method for measuring cardiac troponin I in sera. Anal. Biochem. 2003, 321, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xu, S.; Zhang, J.; Chen, X.; Jiang, L.-P.; Zheng, T.; Zhu, J.-J. Plasmon Near-Field Coupling of Bimetallic Nanostars and a Hierarchical Bimetallic SERS “Hot Field”: Toward Ultrasensitive Simultaneous Detection of Multiple Cardiorenal Syndrome Biomarkers. Anal. Chem. 2018, 19, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Bian, Z.-P.; Wang, W.; Zhu, J.-J. PDMS gold nanoparticle composite film-based silver enhanced colorimetric detection of cardiac troponin I. Sens. Actuators B Chem. 2010, 147, 298–303. [Google Scholar] [CrossRef]
- Cho, I.-H.; Paek, E.-H.; Kim, Y.-K.; Kim, J.-H.; Paek, S.-H. Chemiluminometric enzyme-linked immunosorbent assays (ELISA)-on-a-chip biosensor based on cross-flow chromatography. Anal. Chim. Acta 2009, 632, 247–255. [Google Scholar] [CrossRef]
- Li, F.; Guo, L.; Hu, Y.; Li, Z.; Liu, J.; He, J.; Cui, H. Multiplexed chemiluminescence determination of three acute myocardial infarction biomarkers based on microfluidic paper-based immunodevice dual amplified by multifunctionalized gold nanoparticles. Talanta 2020, 207, 120346. [Google Scholar] [CrossRef]
- Han, G.-R.; Gancharov, A.; Eryilmaz, M.; Joung, H.-A.; Ghosh, R.; Yim, G.; Chang, N.; Kim, M.; Ngo, K.; Veszpremi, M.; et al. Deep Learning-Enhanced Chemiluminescence Vertical Flow Assay for High-Sensitivity Cardiac Troponin I Testing. Small 2025, 21, 2411585. [Google Scholar] [CrossRef]
- Han, G.-R.; Ki, H.; Kim, M.-G. Automated, Universal, and Mass-Producible Paper-Based Lateral Flow Biosensing Platform for High-Performance Point-of-Care Testing. ACS Appl. Mater. Interfaces 2020, 8, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Jayaraj, J.; Prazeres, D.M.F. A Cellulose Paper-Based Fluorescent Lateral Flow Immunoassay for the Quantitative Detection of Cardiac Troponin I. Biosensors 2021, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Dudala, S.; Dubey, S.K.; Javed, A.; Ganguly, A.; Goel, S.J. Electromicrofluidic device with integrated PDMS microchannel and laser-induced graphene electrochemical detection of cardiac biomarker in a point-of-care platform. Micromech. Microeng 2022, 32, 104001. [Google Scholar] [CrossRef]
- Gerdan, Z.; Saylan, Y.; Denizli, A. Biosensing Platforms for Cardiac Biomarker Detection. ACS Omega 2024, 9, 9946–9960. [Google Scholar] [CrossRef]
- Vasantham, S.V.; Alhans, R.; Singhal, C.; Nagabooshanam, S.; Nissar, S.; Basu, T.; Ray, S.C.; Wadhwa, S.; Narang, J.; Mathur, A. Paper based point of care immunosensor for the impedimetric detection of cardiac troponin I biomarker. Biomed. Microdevices 2020, 22, 6. [Google Scholar] [CrossRef]
- Fu, H.; Qin, Z.; Li, X.; Pan, Y.; Xu, H.; Pan, P.; Song, P.; Liu, X. Paper-Based All-in-One Origami Nanobiosensor for Point-of-Care Detection of Cardiac Protein Markers in Whole Blood. ACS Sens. 2023, 8, 3574–3584. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Wang, H.; Han, Y.; Liu, J.; Lu, G.; Yu, H. MXene-functionalized paper-based electrochemical immunosensor for label-free detection of cardiac troponin I. J. Semicond. 2021, 42, 092601. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, L.; Shi, X.; Chen, G.; Sun, D.; Zhang, L. Recent advances in electrochemical aptasensors for detecting cardiac biomarkers: A review. Microchem. J. 2023, 193, 109063. [Google Scholar] [CrossRef]
- Vasudevan, M.; Tai, M.J.Y.; Perumal, V.; Gopinath, S.C.B.; Murthe, S.S.; Ovinis, M.; Mohamed, N.M.; Joshi, N. Highly sensitive and selective acute myocardial infarction detection using aptamer-tethered MoS2 nanoflower and screen-printed electrodes. Biotechnol. Appl. Biochem. 2021, 68, 1386–1395. [Google Scholar] [CrossRef]
- Carrasco, I.L.; Cuniberti, G.; Opitz, J.; Beshchasna, N. Evaluation of Transducer Elements Based on Different Material Configurations for Aptamer-Based Electrochemical Biosensors. Biosensors 2024, 14, 341. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khoshfetrat, S.M.; Mirzaeizadeh, Z.; Kabiri, S.; Rezaie, J.; Omidfar, K. Electrochemical immunosensor for determination of cardiac troponin I using two-dimensional metal-organic framework/Fe3O4-COOH nanosheet composites loaded with thionine and pCTAB/DES modified electrode. Talanta 2022, 237, 122911. [Google Scholar] [CrossRef]
- Ma, J.; Feng, L.; Li, J.; Zhu, D.; Wang, L.; Su, S. Biological Recognition-Based Electrochemical Aptasensor for Point-of-Care Detection of cTnI. Biosensors 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, R.; Loganathan, A.K.; Krishnamoorthy, L. Development of an aptasensor for highly sensitive detection of cardiac troponin I using cobalt–nickel metal-organic framework (CoNi-MOF). Heliyon 2024, 10, e33238. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, M.; Tai, M.J.Y.; Perumal, V.; Gopinath, S.C.B.; Murthe, S.S.; Ovinis, M.; Muti Mohamed, N.; Joshi, N. Cellulose acetate-MoS2 nanopetal hybrid: A highly sensitive and selective electrochemical aptasensor of Troponin I for the early diagnosis of Acute Myocardial Infarction. J. Taiwan Inst. Chem. Eng. 2021, 118, 245–253. [Google Scholar] [CrossRef]
- Sun, B.; Bao, L.; Sun, Y.; Liu, J.; Wu, Y.; Li, H.; Yu, S.; Liu, Y.; Dang, Q.; Yang, L. Electrochemical immunosensor based on ferrocene derivatives amplified signal for detection of acute myocardial infarction warning biomarker-cTnI. Microchem. J. 2024, 199, 110057. [Google Scholar] [CrossRef]
- Pourali, A.; Rashidi, M.R.; Barar, J.; Pavon-Djavid, G.; Omidi, Y. Voltammetric biosensors for analytical detection of cardiac troponin biomarkers in acute myocardial infarction. TrAC Trends Anal. Chem. 2021, 134, 116123. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, F.; Zhang, C.; Zheng, L.R.; Yang, J. The Biomarkers for Acute Myocardial Infarction and Heart Failure. Biomed Res. Int. 2020, 2020, 2018035. [Google Scholar] [CrossRef]
- Marston, S.; Zamora, J.E. Troponin structure and function: A view of recent progress. J. Muscle Res. Cell Motil. 2020, 41, 71–89. [Google Scholar] [CrossRef]
- Reyes-Retana, J.A.; Duque-Ossa, L.C. Acute myocardial infarction biosensor: A review from bottom up. Curr. Probl. Cardiol. 2021, 46, 100739. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Enzyme-Free Electrochemical Nano-Immunosensor Based on Graphene Quantum Dots and Gold Nanoparticles for Cardiac Biomarker Determination. Nanomaterials 2021, 11, 578. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.K.; Sharma, A.L.; Deep, A. 2-Aminotrimesic Acid-Functionalized Graphene Oxide-Modified Screen-Printed Electrodes for Sensitive Electrochemical Detection of Cardiac Marker Troponin I. Phys. Status Solidi A 2021, 218, 2000700. [Google Scholar] [CrossRef]
- Eshlaghi, S.N.; Syedmoradi, L.; Amini, A.; Omidfar, K. A Label-Free Electrochemical Aptasensor Based on Screen Printed Carbon Electrodes With Gold Nanoparticles-Polypyrrole Composite for Detection of Cardiac Troponin I. IEEE Sens. J. 2023, 23, 3439–3445. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C. Development of a Simple and Cheap Conductive Graphite Ink. J. Electrochem. Soc. 2021, 168, 087508. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; de Resende, M.A.C.; Ferreira, L.F. Fabrication of a Simple and Cheap Screen-printedSilver/Silver Chloride (Ag/AgCl) Quasi-reference Electrode. Electroanalysis 2022, 34, 809–819. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; de Resende, M.A.C.; Ferreira, L.F. Gold Nanoparticles: A Didactic Step-by-Step of the Synthesis Using the Turkevich Method, Mechanisms, and Characterizations. Analytica 2023, 4, 250–263. [Google Scholar] [CrossRef]
- Gholami, M.D.; O’Mullane, A.P.; Sonar, P.; Ayoko, G.A.; Izake, E.L. Antibody coated conductive polymer for the electrochemical immunosensing of Human Cardiac Troponin I in blood plasma. Anal. Chim. Acta 2021, 1185, 339082. [Google Scholar] [CrossRef]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef]
- Al-Shami, A.; Oweis, R.J.; Al-Fandi, M.G. Developing an electrochemical immunosensor for early diagnosis of hepatocellular carcinoma. Sens. Rev. 2021, 41, 125–134. [Google Scholar] [CrossRef]
- Anuthum, S.; Wiratchan, S.; Semakul, N.; Jakmunee, J.; Ounnunkad, K. Signaling redox probe/DNA aptamer complexes on a new POP/2D WSe2 composite-based immunosensor towards the simultaneous detection of three-protein overexpression as an alternative severe SARS-COV-2 infection diagnosis. Sens. Actuators B Chem. 2024, 404, 135196. [Google Scholar] [CrossRef]
- Jo, H.; Her, J.; Lee, H.; Shim, Y.B.; Ban, C. Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 2017, 165, 442–448. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). M10 Bioanalytical Method Validation and Study Sample Analysis: Guidance for Industry. Available online: https://www.fda.gov/media/162903/download (accessed on 14 March 2025).
- Miller, C.S.; Foley, J.D.; Floriano, P.N.; Christodoulides, N.; Ebersole, J.L.; Campbell, C.L.; Bailey, A.L.; Rose, B.G.; Kinane, D.F.; Novak, M.J.; et al. Utility of salivary biomarkers for demonstrating acute myocardial infarction. J. Dent. Res. 2014, 93, 72S–79S. [Google Scholar] [CrossRef]
- Chekin, F.; Vasilescu, A.; Jijie, R.; Singh, S.K.; Kurungot, S.; Iancu, M.; Badea, G.; Boukherroub, R.; Szunerits, S. Sensitive electrochemical detection of cardiac troponin I in serum and saliva by nitrogen-doped porous reduced graphene oxide electrode. Sens. Actuators B Chem. 2018, 262, 180–187. [Google Scholar] [CrossRef]
- Kirbas, Z.O.; Bayraktar, B.; Aktas, E.O. Investigation of the relationship of cardiac troponin I and cortisol hormone levels with some variables in children: Relational screening model. Med. Sci. 2024, 13, 310–314. [Google Scholar] [CrossRef]
- Roia, W.; Gala, T.; Yoavb, N.; Amjada, A.S.; Omria, B.; Danaa, B.; Talia, Z.; Feigaa, N.Z.; Omerb, D.; Aaronb, P.; et al. Development of saliva-based cardiac troponin I point-of-care test using alpha-amylase depletion: A feasibility study. Coron. Artery Dis. 2023, 34, 351–355. [Google Scholar] [CrossRef]
- Westreich, R.; Neumann, Y.; Deutsch, O.; Krief, G.; Stiubea-Choen, R.; Zager, D. Development of saliva-based cTnI point-of-care test: A feasibility study. Eur. Heart J. 2020, 41, ehaa946-1693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).