Abstract
This work presents a new optical dual sensor based on PtTFPP-containing electrospun fibers and CsPbBr3 perovskite quantum dots (PQDs) for simultaneous detection of oxygen (O2) and nitric oxide (NO) gases, wherein PtTFPP-containing electrospun fibers for O2 sensing was based on electrospinning process fabricated by platinum(II) meso-tetrakis (pentafluorophenyl) porphyrin (PtTFPP) complex immobilized in cellulose acetate (CA) matrix. CsPbBr3 PQDs were used as NO-sensitive material and coated on the surface of PtTFPP-containing electrospun fibers. Both materials were excited by a UV LED with a central wavelength of 380 nm, and the fluorescence intensities of sensing materials were recorded and analyzed with a spectrometer. The experiment results show that the optical NO and O2 sensors have linear Stern–Volmer plots, and the sensitivities are around 2.7 and 10.7, respectively. The response and recovery times of the optical NO sensor are 71 and 109 s, respectively. For optical O2, response and recovery times are 60 and 65 s, respectively. The optical dual sensor with a new method based on fluorescent dye containing electrospun fibers and coated with CsPbBr3 PQDs has been successfully developed to detect NO and O2 gases simultaneously. The optical dual gas sensor provides great potential for practical applications with low cost and ease of fabrication.
1. Introduction
Optical sensing research has grown significantly in recent years, with practical applications spanning various domains. Optical sensors offer multiplexing capabilities and remote, real-time monitoring. Due to their non-electric nature, it is intrinsically safe in explosive environments and harsh conditions, eliminating the risk of electric sparks. These sensors play a vital role in detecting various harmful gases, including colorless and odorless gases [1]. While the human olfactory system can sometimes identify gases in very low or high quantities, a more sensitive and high-performance device is needed to detect poisonous vapors and dangerous gases [2] reliably. Early identification of hazardous gases and vapors has become increasingly crucial in numerous fields, including environmental contamination monitoring [3], industrial emission control [4], the automotive industry [5], industrial process control [6], medical diagnosis [7], indoor air quality control [8], and food quality control [9]. The main principle underlying gas sensors is their ability to adapt to environmental changes, making flexible and portable gas sensors with high selective sensitivity a growing necessity [10].
Nitric oxide is one gas of particular interest, known for its diverse physiological roles, including regulation of inflammatory and immune responses, wound healing, and circulation in the human body [11]. Nevertheless, measuring restrictions are minimal due to its low concentration and brief half-life. The signaling molecule nitric oxide (NO), produced in biological systems, involves several physiological and pathological pathways [12], like inflammatory responses and vascular and neurological regulatory processes [13]. Defects in the nitric oxide pathway can lead to adverse pathophysiological conditions, such as hypertension, heart disease, kidney disease, diabetic nephropathy, stroke, and central nervous system conditions [14]. It is crucial to thoroughly examine how nitric oxide is produced and spreads in biological systems to grasp its physiological importance and develop innovative techniques for therapeutic purposes. However, detecting nitric oxide is challenging due to its short half-life (6–50 s) and low concentration (nanomolar scale) in biological systems [15]. Despite these constraints, nitric oxide is a vital messenger in biological systems and plays a significant role in various physiological and pathological pathways [16]. Table 1 shows the performance characteristics of the existing optical nitric oxide sensor.
On the other hand, oxygen (O2), the most prevalent gas in the Earth’s atmosphere, is essential for all life forms and respiration. Monitoring oxygen content is crucial in various applications, such as food packaging [17] and clinical [18], chemical [19], and environmental monitoring [20]. Increasingly complex problems involve toxic and dangerous gases that can harm health and even cause death, emphasizing the need for gas sensor devices to detect such hazards. Additionally, the development of oxygen sensors has garnered significant interest in various industries, such as the medical and environmental sectors. Over the past 40 years, researchers have explored multiple techniques to create reliable and durable oxygen sensors. Fluorescence-based oxygen sensors, in particular, require extended photostability, electrical interference resistance, increased gas selectivity, higher sensitivity, and faster response recovery times. At this time, it is necessary to select oxygen-sensitive materials with good material support, where pyrene-based transition metal complex dyes exhibit long-lived (microsecond) emission (phosphorescence) from excited states with triplet-like character. Pyrene-based phosphorescence molecules are PtTFPP [21,22,23,24,25,26], [Ru(dpp)3]2+ [27,28], Ir(ppy)3 [29], Os(dpp)3(PF6)2 [30], PdTFPP [31], and PtOEP [32]. In contrast, PtTFPP and [Ru(dpp)3]2+ showed good photostability. In this study, the emission peak wavelength of [Ru(dpp)3]2+ was 618 nm indicating spectral overlap with the NO-sensitive dye CsPbBr3 (518 nm). Thus, PtTFPP is the best molecule for oxygen-sensing signals in this study. Table 2 shows the material fluorescence properties of the optical oxygen sensor progress.
Gas sensing technology has gained significant attention due to its broad applications in various fields, as the increasing complexity of toxic and dangerous gases poses health risks and threatens living organisms. This motivates the authors to research optical dual gas sensing of nitric oxide (NO) and oxygen (O2). The development of a single fiber dual sensor for simultaneous NO and O2 detection is essential for several reasons. For instance, it enables monitoring of NO and O2 on electrospun polystyrene nanofiber material to produce two antibacterial species, addressing medical, biological, and environmental challenges [33]. Additionally, the research explores applications like measuring high-speed flows containing NO or O2 using a CW laser strategy [34]. Simultaneous detection of NO and O2 in gas mixtures, such as flue gas [35], and its relevance to health, specifically in cases involving high glucose and endothelial GTPCH1 and NADPH oxidase, further emphasize the significance of this study [36].
As mentioned above, there are several optical sensing approaches for dual sensors. First, in a single layer, each material sensing dye is embedded in a single matrix, while in the second method, it is located in a double layer, where each sensing material is located in a different layer. On the other hand, several researchers have presented fluorescence intensity and lifetime based on single/double-layer methods. The dual sensor design also relies on the gas permeability of the polymer buffer matrix used. For example, oxygen may be permeable to some polymers. Therefore, a suitable permeable matrix is needed to design multiple sensors. The fluorophore signal can be shared spectrally using fluorescence-based sensing based on different emission wavelengths or luminescence masses. Different wavelengths are mainly branched using optical filters or excitation sources. After conducting a literature survey, no optical dual gas sensor was found based on the fluorescence intensity of perovskite QDs for gas detection, thus prompting this research to develop a new optical gas sensor based on perovskite quantum dots. This work presents the creation of a new optical dual sensor based on PtTFPP-containing electrospun fibers for oxygen sensing using a fluorescence intensity-based electrospinning technique, and then CsPbBr3 perovskite quantum dots coated on the surface of PtTFPP-containing electrospun fibers for NO sensing, an approach to a new optical dual sensor for detecting NO and O2 simultaneously. Sensitivity, response, recovery, dynamic response, cross-sensitivity, and selectivity of optical dual gas sensors were observed. Therefore, the new optical dual sensor developed in this study can detect the concentrations of NO and O2 simultaneously, and it will be beneficial in various application fields.

Table 1.
Properties of typical optical NO sensors.
Table 1.
Properties of typical optical NO sensors.
| NO Probe | λex (nm)/λem (nm) | Range | Response | Sensing Type | Reference |
|---|---|---|---|---|---|
| Cu(II) complex | None | 0–1000 ppm | 300 s/600 s | Absorbance | [37] |
| Cu(dmp)22+ | None | 0–3 ppm | 100 s/600 s | Absorbance | [38] |
| Cu(ECR)2 | None | 0–6 ppm | 300 s/none | Absorbance | [39] |
| CdSe/ZnS QDs | 372/530 | 0–105 ppm | None | Intensity | [40] |
| Co-TPP | 415/435 | 10 ppm | None | Intensity | [41] |
| (Cu2+) PANI/WO3 | None | 1–116 ppb | 59 s/17 s | Frequency shift | [42] |
| CsPbBr3@ZnO | 900/518 | 0–200 ppm | 36.5 s/5.3 s | Intensity | [43] |
| CsPbBr3 perovskite QDs | 380/515 | 0–1000 ppm | 36 s/117 s | Intensity | [44] |
| CsPbBr3 perovskite QDs | 380/515 | 0–1000 ppm | 71 s/109 s | Intensity | This work |

Table 2.
Properties of typical optical O2 sensors.
Table 2.
Properties of typical optical O2 sensors.
| O2 Probe | Support Matrix | λex (nm)/λem (nm) | IN2/IO2 | Ref. |
|---|---|---|---|---|
| PtTFPP | Fluoro-polymer | 544/648 | 15.4 | [21] |
| PtTFPP | TFP-TriMOS/TEOS/Octyl-triEOS | 405/650 | 101 | [22] |
| PtTFPP | n-propyl-TriMos/TEOS/Octyl-triEOS | 405/650 | 155 | [22] |
| PtTFPP | Polystyrene polymer | 508, 541/650 | >3 | [23] |
| PtTFPP | TEOS/Octyl-triEOS | 380/650 | 22 | [24] |
| PtTFPP | n-propyl-TriMos/TFP-TriMOS | 400/650 | 68.7 | [25] |
| PtTFPP | TEOS/Octyl-triEOS | 405/650 | 166 | [26] |
| Ru(dpp)3 | DMOS ormosil | 467/592 | 14 | [27] |
| Ru(dpp)3 | Silicon rubber | 406/610 | 1.5 | [28] |
| Ir(ppy)3 | nBuPTP | 370/510 | 3.4 | [29] |
| Os(dpp)3(PF6)2 | PDMS rubber | 480, 502/729 | 1.7 | [30] |
| PdTFPP | Silica gel beads in silicone | 405/680 | 5 | [31] |
| PtOEP | Poly-IBM | 535/646 | 69 | [32] |
| PtTFPP | Cellulose Acetate electrospun fibers | 380/650 | 10.7 | This work |
2. Experiments
2.1. Materials
(Frontier Scientific, Logan, UT, USA) provided the Platinum(II) meso-tetrakis (pentafluorophenyl)-porphyrin, which is utilized as a sensitive dye oxygen. CsPbBr3 perovskite quantum dots (PQDs) were made using a reference-based synthetic material used as nitric oxide sensitive dye. An optical dual sensor that can simultaneously detect NO and O2 concentrations was created. n-octyltriethoxysilane (OctyltriEOS, 97.5%) and cellulose acetate (CA) powder were purchased from (Showa chemicals, Tokyo, Japan). (Arison Co., Hsinchu, Taiwan) supplied the chemicals acetone (99%) and dimethylacetamide (DMAC).
2.2. Synthesis of CsPbBr3 Perovskite Quantum Dots
Octadecent (9 mL, 90%), oleic acid (0.75 mL, 90%), and cesium carbonate (200 mg, 99.99%) were added to a 25 mL three-neck flask and agitated at 150 °C on a hot plate for 30 min until the solution turned clear. The procedure described in the reference [45] was employed to create CsPbBr3. To produce the reaction solution, 30 mL of ODE, 3 mL of OA, 3 mL of octylamine, and 0.09 M of PbBr2 were dissolved. Thirty minutes of stirring at 120 °C on a hot plate produced another transparent solution. The impure CsPbBr3 QDs solution was then created by injecting 0.5 mL of the precursor solution into the reaction solution and pouring the mixture into a glass bottle for an ice water bath.
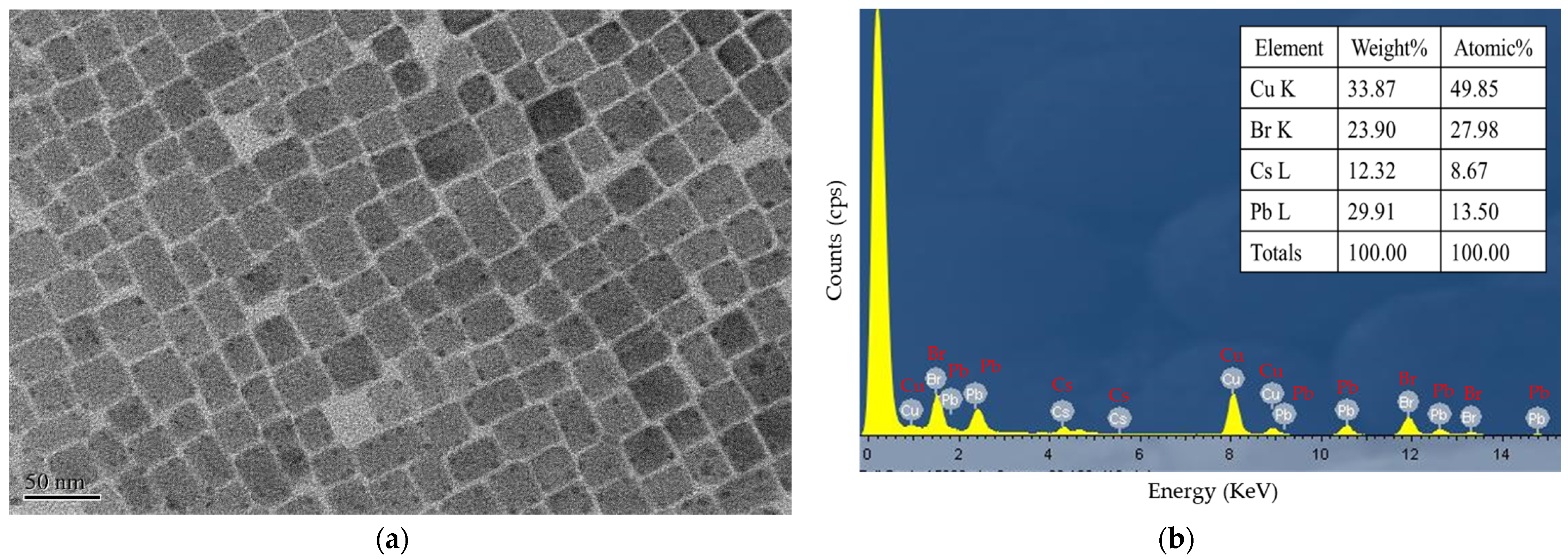
Figure 1a shows a TEM image of the CsPbBr3 at a resolution of 50 nm, and Figure 1b presents the energy-dispersive X-ray spectroscopy (EDX) result for the CsPbBr3 perovskite QDs composed of atomic Cs, Pb, Br, and Cu. Accordingly, the y-axis measures count per second per electron (essentially, X-ray intensity), and the x-axis measures energy (KeV). The Cu content originates from the copper grid.
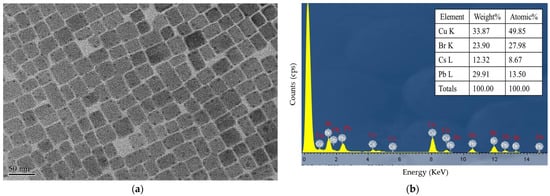
Figure 1.
(a) TEM image of the CsPbBr3 at a resolution of 50 nm and (b) EDX analysis result for CsPbBr3 PQDs.
2.3. Preparation of PtTFPP-Containing Electrospun Fibers Membrane
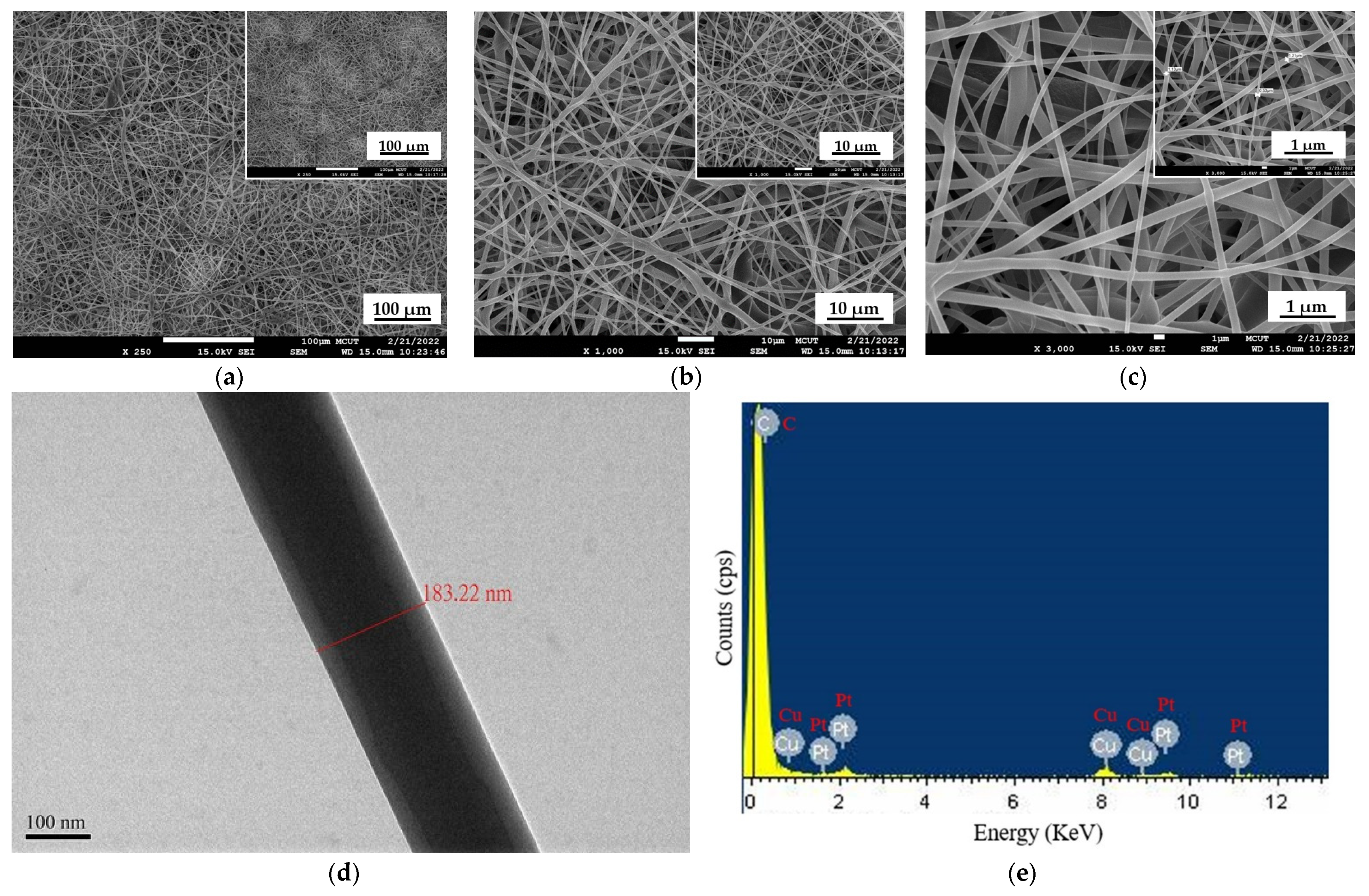
Morphological characteristics of PtTFPP-containing electrospun fibers membrane were obtained from a scanning electron microscope (SEM) and energy-dispersive X-ray (EDX) analysis data to determine the sample’s chemical composition. In this work, electrospun fibers used polymer cellulose acetate (CA) as a solvent and a combination of solvent acetone and DMAc in a 2:1 ratio. Then, PtTFPP was added as a dye sensitive to oxygen sensors. Figure 2a–c show the SEM results at resolutions of ×250, ×1000, and ×3000, respectively. The PtTFPP-containing electrospun fibers produced are good; no electrospun bubble fibers were found. The surface of the PtTFPP-containing electrospun fibers sample is completely covered in homogenous, equally spaced fibers. Figure 2d shows the TEM image morphology of a single electrospun fiber. Figure 2e shows the EDX analysis; the chemical composition of the detected atoms consists of Cu and Pt, where Cu comes from the copper mesh grid sample, and Pt comes from the sensing material used to detect oxygen gas.
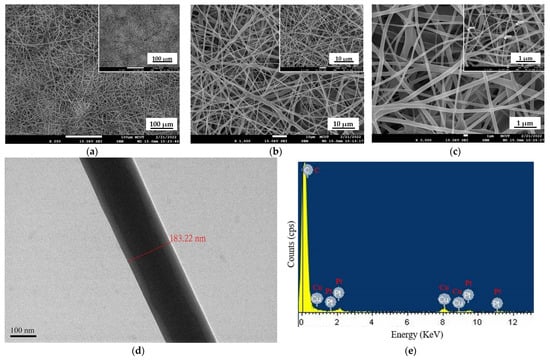
Figure 2.
SEM images of PtTFPP-containing electrospun fibers at (a) ×250, (b) ×1000, and (c) ×3000, (d) TEM image, and (e) EDX analysis result of a single fiber membrane.
2.4. Preparation of Optical NO and O2 Sensing Materials
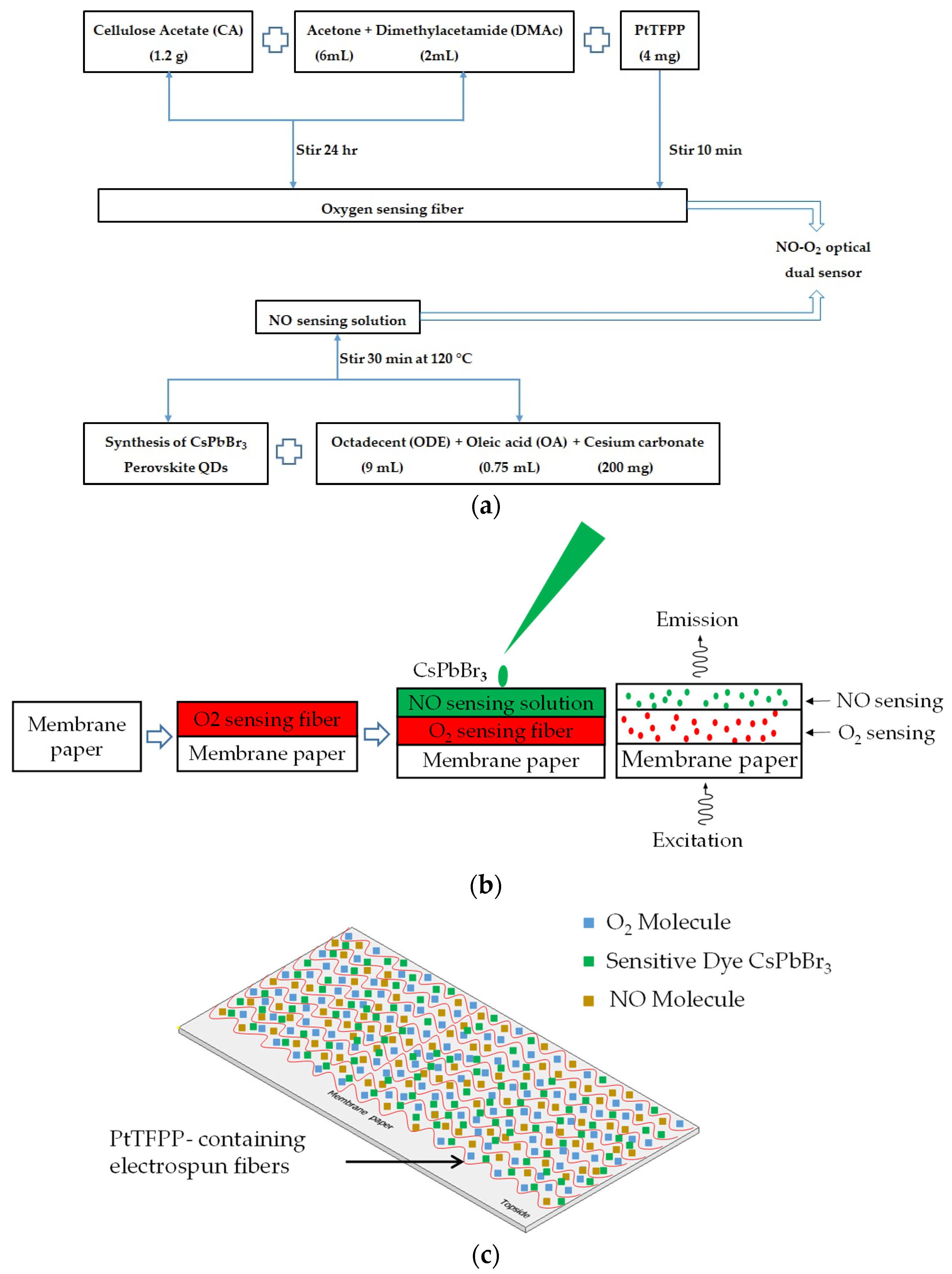
Initially, sensing materials were prepared for the optical dual sensor fabrication process. A total of 1.2 g of cellulose acetate (CA) powder was used as a support matrix dissolved in 6 mL acetone solution and 2 mL dimethylacetamide solution in a ratio of 2:1 and stirred for 24 h at room temperature. An amount of 4 mg of Pt(II) complex was added to the solution and stirred for 10 min to obtain a homogeneous solution to increase oxygen sensitivity. Finally, O2-sensitive dye PtTFPP-containing electrospun fibers were produced using electrospinning machines as oxygen-sensing material. The electrospinning parameters used were 3 mL/Hour (flow rate), 21 KV (supply voltage), and 15 cm (working distance). The flowchart in Figure 3a schematically shows the procedures to fabricate the optical NO and O2 dual sensing materials. Finally, the PtTFPP-containing electrospun fibers were created by this electrospinning method and then coated with 0.5 μL of the synthesized CsPbBr3 perovskite QDs solution, dried for 10–15 min at room temperature, and employed as optical dual sensing materials for nitric oxide and oxygen. The optical dual gas sensor for detecting nitric oxide and oxygen was coated with CsPbBr3 on the surface of PtTFPP-containing electrospun fibers by the NO and O2 optical dual sensor fabrication steps in Figure 3b. The structural detail process of the sensing membrane for the optical dual sensor is schematically displayed in Figure 3c.
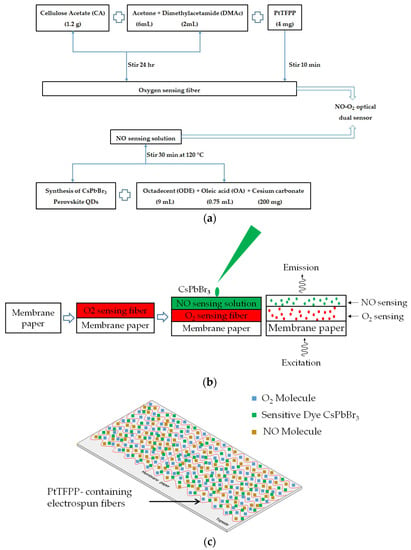
Figure 3.
(a) A flow chart showing the synthesis process of optical NO and O2 dual sensing materials and (b) fabrication steps of optical NO and O2 dual sensor (c) schematic structure of the sensing membrane.
2.5. Instrumentation
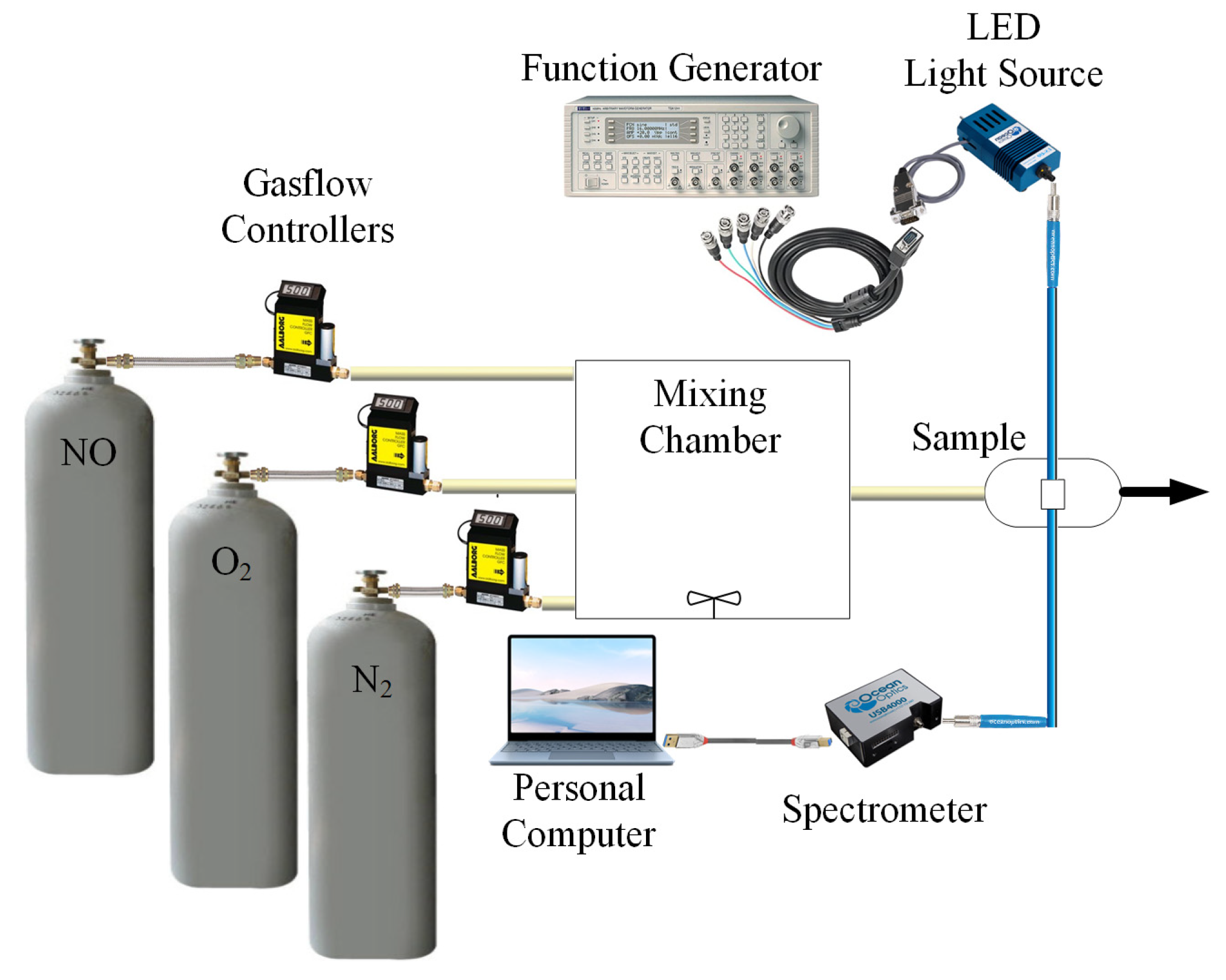
An absorption spectrophotometer was used to detect the absorption spectrum of the fluorescent materials in the optical dual sensor based on the perovskite QDs design. Energy-Dispersive X-ray Spectroscopy (JEM-2100) and Transmission Electron Microscopy (TEM) were used for materials analysis. The optical dual sensor equipment used for the sensing studies is shown in Figure 4. The fluorescence of the optical dual sensor is produced by the excitation of a UV LED light source with a peak wavelength of 380 nm, driven by a function generator with a pulse signal at a frequency of 10 kHz. Mass flow controllers control the NO, O2,, and N2 gas concentrations in the gas flow (Aalborg Instruments and Controls Inc., New York, NY, USA, Model GFC 17). The different gases are in a mixing chamber and blended before being sent to the sample holder. A USB 4000 fiber optic spectrometer was attached via the holder to one side of the sample, which measured the fluorescence intensities given off by various fluorophores. The spectra suite software, which has a 10 ms integration time, showed the detected intensity data on a computer. The intensity data were copied into the M.S. Excel program and then moved to the original program for graphing.
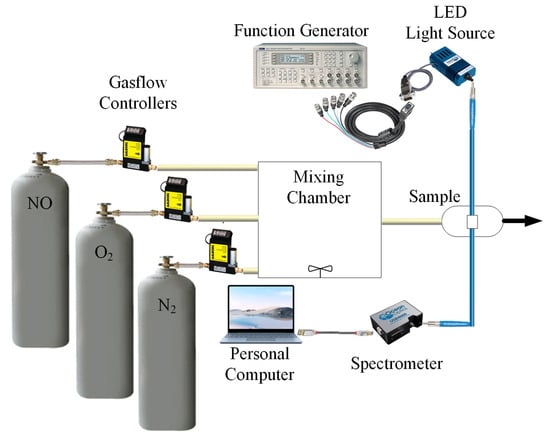
Figure 4.
Schematic diagram showing experimental arrangement used for characterization.
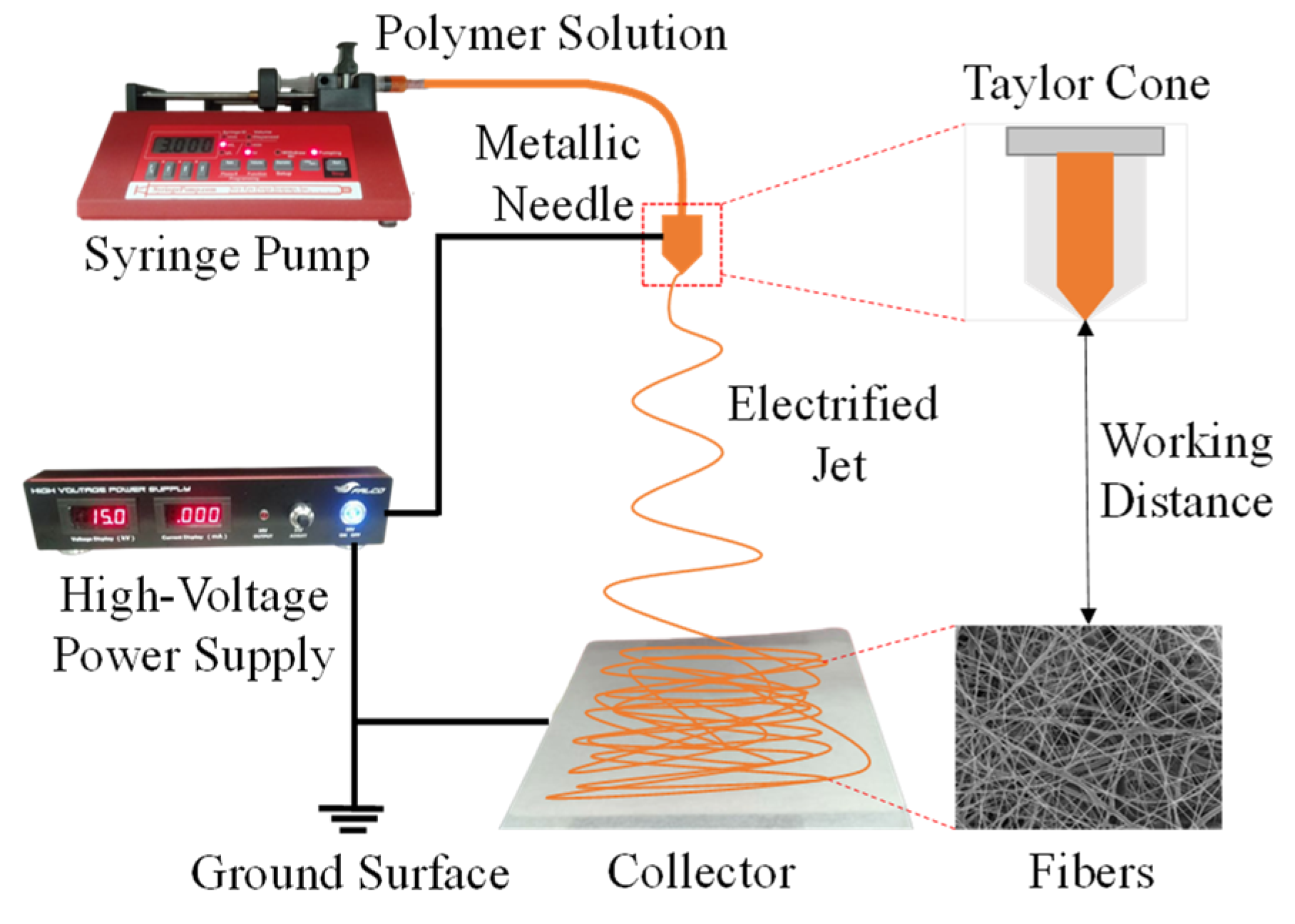
Electrospun nanofibers offer several advantages, such as low cost, simple product process, large specific surface area, and small pore size. A schematic representation of the basic setup for the electrospinning process is shown in Figure 5. The polymer solution or composite mixture is placed in a syringe during the electrospinning process. The volume and resolution of the syringe used were 10 mL and 2 mL, respectively. High voltage is applied to the syringe tip and collector during electrospinning to produce electrospun nanofiber (Falco tech enterprise Co., Ltd., New Taipei City, Taiwan). The needle tip is connected to the positive pole of the voltage source, which is connected to the negative pole of the voltage source collector. This condition causes the cellulose acetate solution on the needle to be polarized and generate an electric field towards the collector. The syringe pump is pressed, so the solution enters the syringe as a droplet. Droplets will experience electrostatic forces and electric fields. With the existence of these two forces, the droplets are attracted toward the collector to form a Taylor cone and then discharged from the needle’s point in the direction of the collector to create a polymer jet. The jets form very small non-woven fibers called electrospun nanofibers. Droplet deformation is due to the repelling of charges from the polymer solution and is balanced by the viscosity of the solution. The electrospun nanofibers formed result from thinning and evaporation of the solution. The solution that comes out of the tip of the needle is in the form of small droplets. The volume of this solution is controlled by a syringe pump in very slow motion. It includes several components, such as the high-power voltage supply as a voltage regulator that produces fiber. This syringe pump can be programmed as a polymer flow rate setting, a syringe as a polymer holder, and a nozzle where the fiber exits from the power voltage supply and flow rate setup process. Furthermore, the results of the nanofiber fibers are collected on the collector plate.
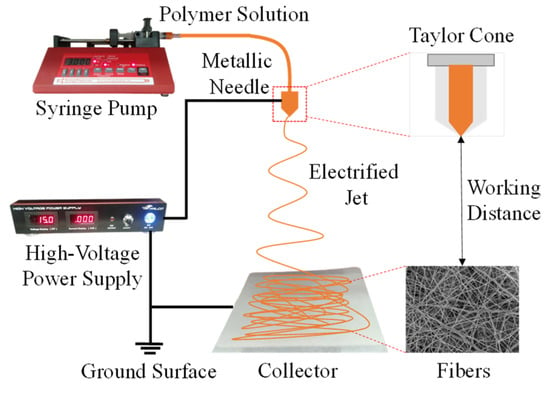
Figure 5.
Schematic diagram of the system setup for the electrospinning process.
3. Results and Discussions
3.1. Optical Properties of Optical Dual Sensor
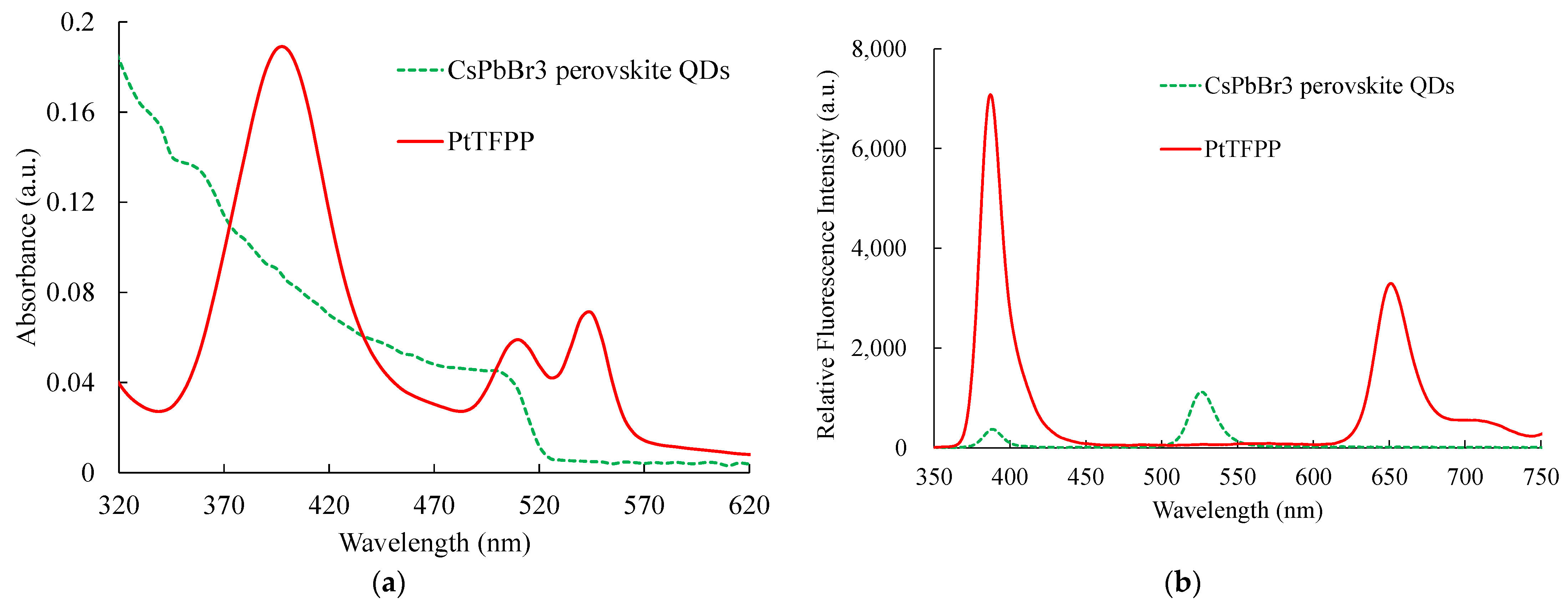
Figure 6a,b show the absorption and emission spectra results, where the sensing materials based on CsPbBr3 PQDs and PtTFPP were used to detect NO and O2, respectively. All sensing materials are excited using a 308 nm UV LED. The absorption spectra of all sensing materials are captured individually from the optical dual sensor, i.e., the combined emission spectra of CsPbBr3 PQDs and PtTFPP. The sensing materials of CsPbBr3 PQDs and PtTFPP have peak absorption spectra at around 480 nm and 400 nm, respectively. The same material for emission spectra for this optical dual sensor was observed, with a corresponding central peak wavelength at 518 nm and 650 nm, respectively. It can also be noticed that the emission signals from CsPbBr3 PQDs and PtTFPP do not overlap. Therefore, the emission spectrum of the sensing material used in this study will be further observed in the presence of optical NO and O2 dual sensors.
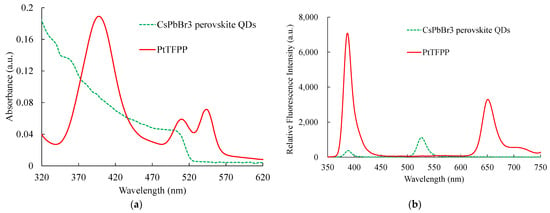
Figure 6.
(a) Absorption and (b) emission spectra of CsPbBr3 perovskite QDs and PtTFPP.
3.2. NO Sensing Properties of Optical Dual Sensor
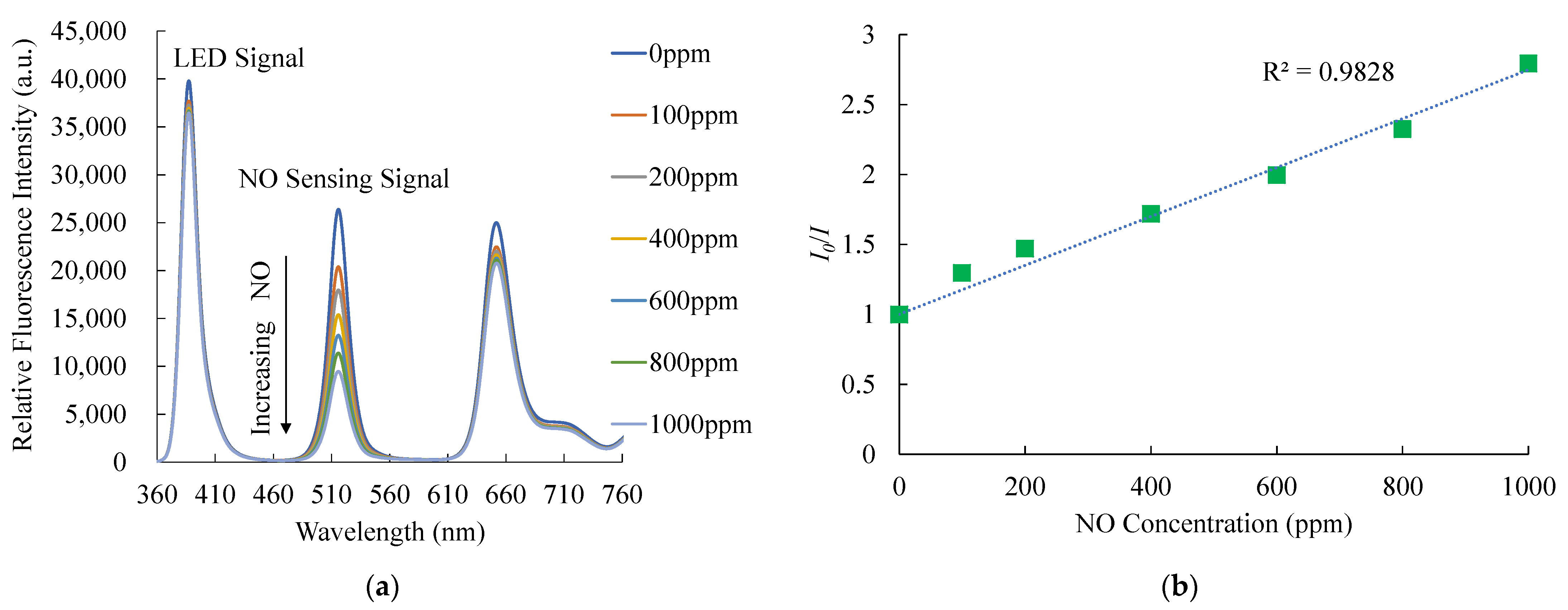
Figure 7a shows the relative emission spectra of the optical dual sensor under different NO concentrations. The CsPbBr3 perovskite QDs have a strong fluorescence emission spectrum of around 518 nm. The figures confirm that the presence of gas nitric oxide molecules results in a fluorescence quenching of the CsPbBr3 perovskite QDs. As intended, the emission spectrum of CsPbBr3 perovskite QDs is significantly decreased by the quenching process, with increased NO concentrations changing from 0 to 1000 ppm. Also, Figure 7b presents the Stern–Volmer plot of the optical NO sensor based on CsPbBr3 perovskite QDs. The relationship between relative fluorescence intensities and the NO and O2 gas concentrations can be explained by the following Stern–Volmer equation [46]:
where I0 and I are the fluorescence intensities of sensing materials in the absence and presence of analytical gas, respectively. KSV is the Stern–Volmer constant, and [Q] is the concentration of analytical gas. The typical Stern–Volmer plot depicts the relationship between the nitric oxide and the ratio of relative fluorescence intensity in sensitivity (I0/I) and NO concentrations in the optical dual sensor. The Stern–Volmer equation can explain the effect of quenching fluorescence intensity by Equation (1). The maximum sensitivity to nitric oxide gas molecules achieved by this optical dual sensor is 2.7 in a 1000 ppm nitric oxide environment, and the Stern–Volmer plot is linear. The sensitivity of the optical NO sensor is defined as the ratio I0ppmNO/I1000ppmNO, where I0ppm and I1000ppmNO represent the measured fluorescence intensities in 100% N2 and 1000 ppm NO gases, respectively. On the other hand, the sensitivity of bare CsPbBr3 perovskite QDs is lower than optical dual sensors. The optical dual sensor for NO sensing with the PtTFPP-containing electrospun fiber improves the sensitivity of CsPbBr3 perovskite QDs for optical NO sensing performance.
I0/I = 1 + KSV[Q]
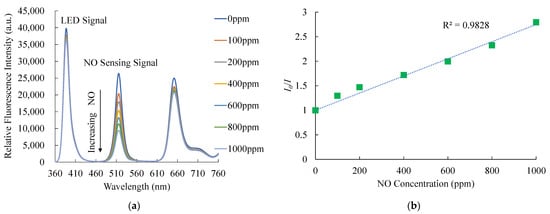
Figure 7.
(a) Response of optical dual sensor at different NO concentrations and (b) Stern–Volmer plot.
3.3. O2 Sensing Properties of Optical Dual Sensor
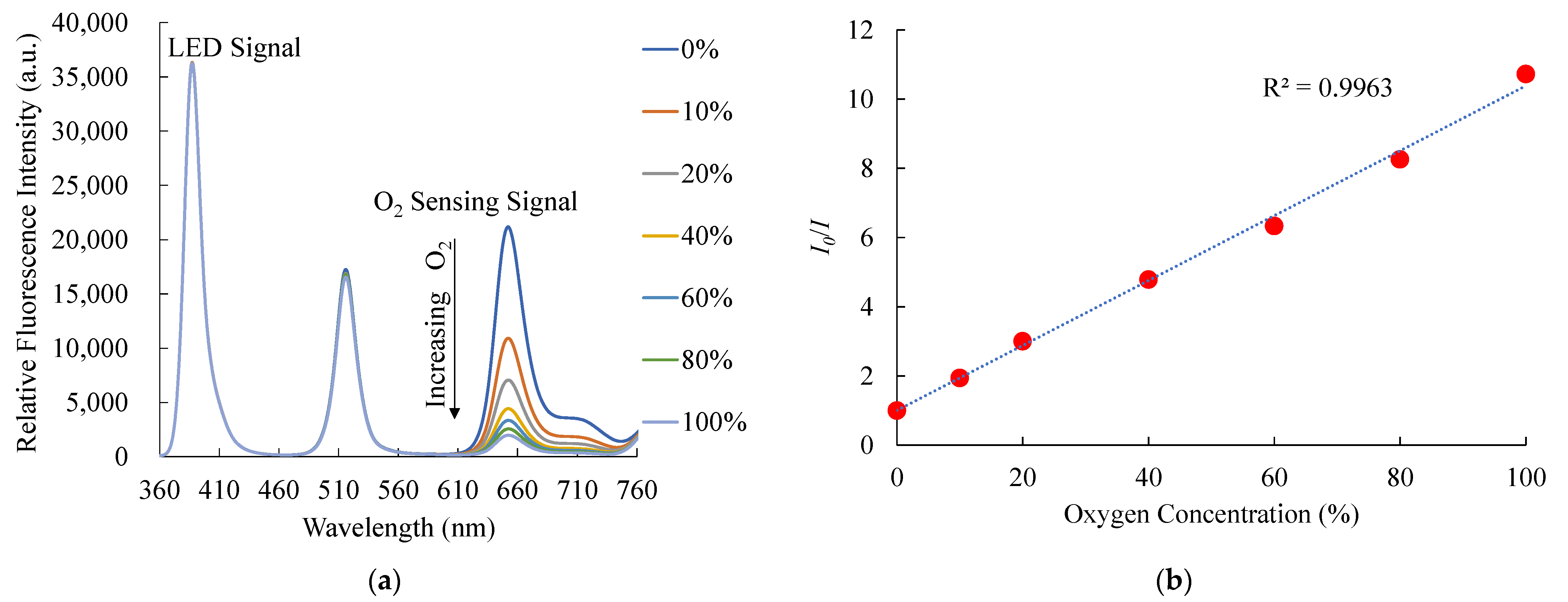
The steady-state room temperature fluorescence spectra of PtTFPP-containing electrospun fibers as an oxygen sensor under different oxygen concentrations ranging from 0% to 100% of oxygen are presented in Figure 8a. As a result, the optical oxygen sensor showed strong fluorescence spectra at 650 nm. On the other hand, oxygen does not affect the fluorescence signals of CsPbBr3 perovskite QDs, indicating the selectivity of the optical dual sensor toward the oxygen sensor. The relative fluorescence intensity of the PtTFPP-containing electrospun fibers oxygen sensor decreases when oxygen concentration increases from 0% to 100%. The results also show that the sensitivity of the optical oxygen sensor is 10.7, and the Stern–Volmer plot is linearly shown in Figure 8b. The sensitivity of the optical O2 sensor is defined as the ratio I0%O2/I100%O2, where I0%O2 and I100%O2 represent the measured fluorescence intensities in 100% N2 and 100% O2 gases, respectively. The relationship between I0/I and under different O2 concentrations was like that in the optical nitric oxide sensor and can also be described by Equation (1). We also checked the sensitivity of bare PtTFPP-containing electrospun fibers for O2 sensing and compared them with the optical dual sensor. The result was similar to this work based on the optical dual sensor for O2 sensing with no significant difference.
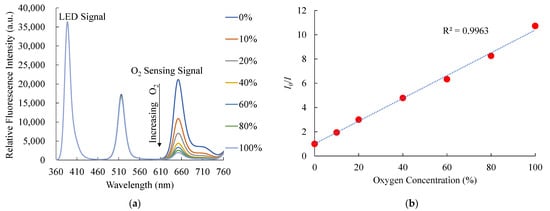
Figure 8.
(a) Response of optical dual sensor at different O2 concentrations and (b) Stern–Volmer plot.
3.4. Response Time of the Optical Dual Sensor
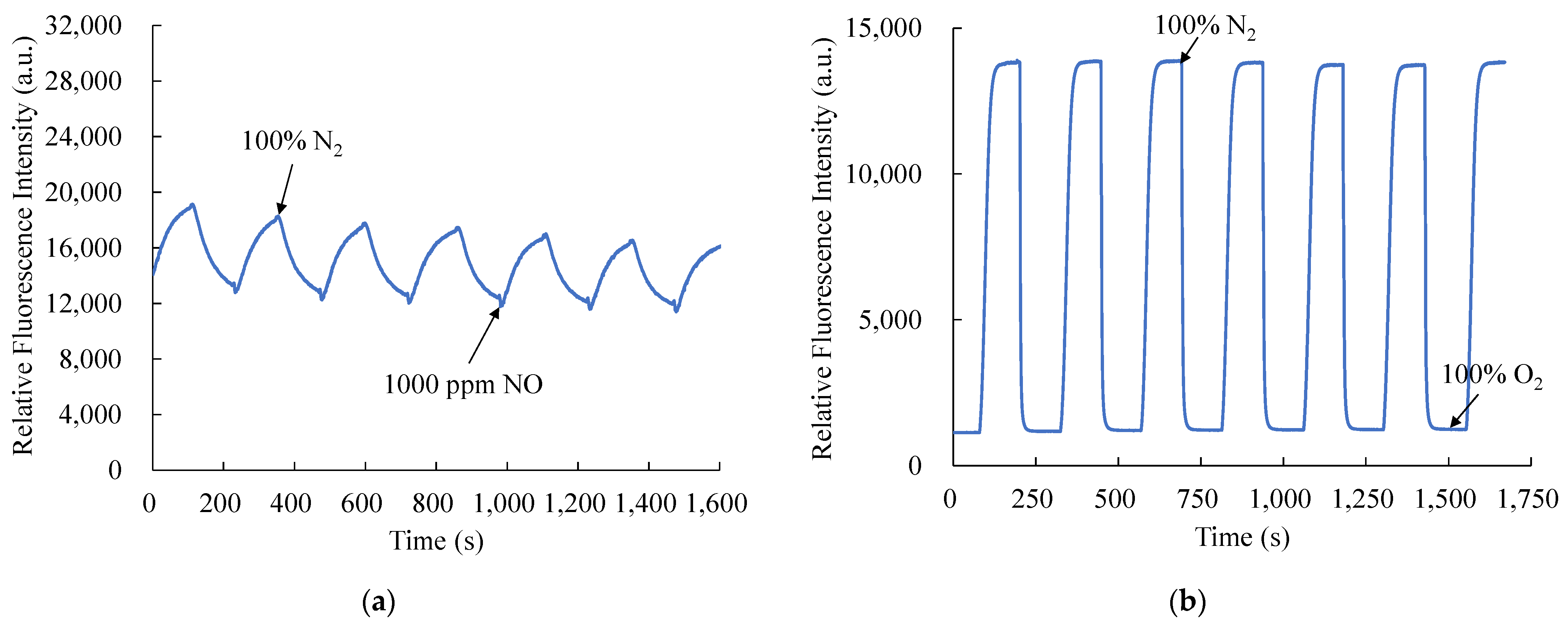
The NO and O2 response times of optical dual sensor based on CsPbBr3 perovskite QDs and PtTFPP-containing electrospun fibers are shown in Figure 9a,b, respectively. Figure 9a shows the response and recovery times for the optical NO sensor are 71 s and 109 s when switching from 100% N2 to 1000 ppm NO and 1000 ppm NO to 100% N2, respectively. Consequently, Figure 9b shows that the response time of the optical O2 sensor is 60 s when switching from 100% N2 to 100% O2, and the recovery time is about 65 s when switching from 100% O2 to 100% N2. The response and recovery time processes were repeated for six cycles, and NO and O2 gases were opened for 2 min until the fluorescence intensity was extinguished to a saturation value. The CsPbBr3 perovskite QDs and PtTFPP-containing electrospun fibers provide a stable and reproducible signal when in the environment alternately fully. So, the new optical dual sensor that has been proposed can respond quickly to real-world applications.
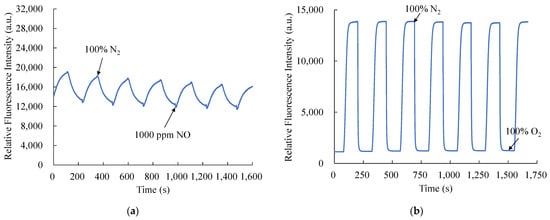
Figure 9.
Response and recovery times of the optical dual sensor for (a) NO and (b) O2 sensing.
3.5. Dynamic Response of the Optical Dual Sensor
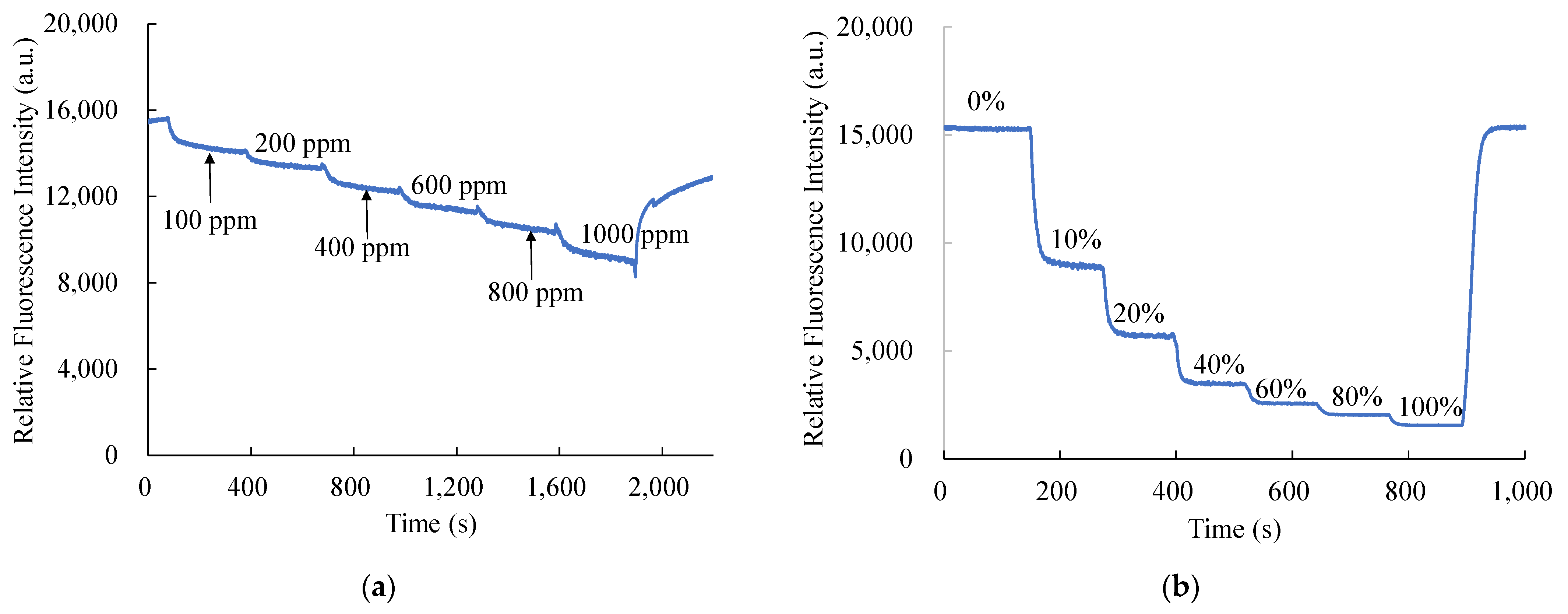
The dynamic response times of CsPbBr3 perovskite QDs and PtTFPP-containing electrospun fibers were captured in the same pattern as the response time retrieval from the optical dual sensor and shown in Figure 10. Figure 10a reveals the optical dual sensor for NO detecting switches from 100% N2 to 1000 ppm NO with a normal dynamic response. The concentration of NO gas increased stepwise at every 300 s interval of the recovery of fluorescence intensity under 100% N2 atmosphere. Similarly, in Figure 10b, dynamic response was observed by changing the oxygen concentrations from 100% N2 to 100% O2. The concentration of O2 gas increased stepwise at every 130 s interval of the recovery of fluorescence intensity under 100% N2 atmosphere.
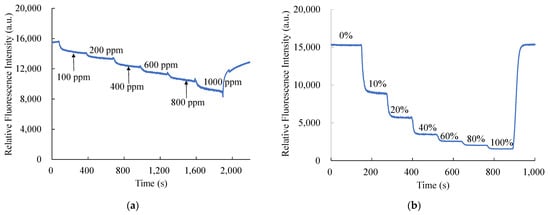
Figure 10.
Dynamic response of optical dual sensor for (a) NO and (b) O2 sensing.
3.6. Photostability of Optical Dual Sensor
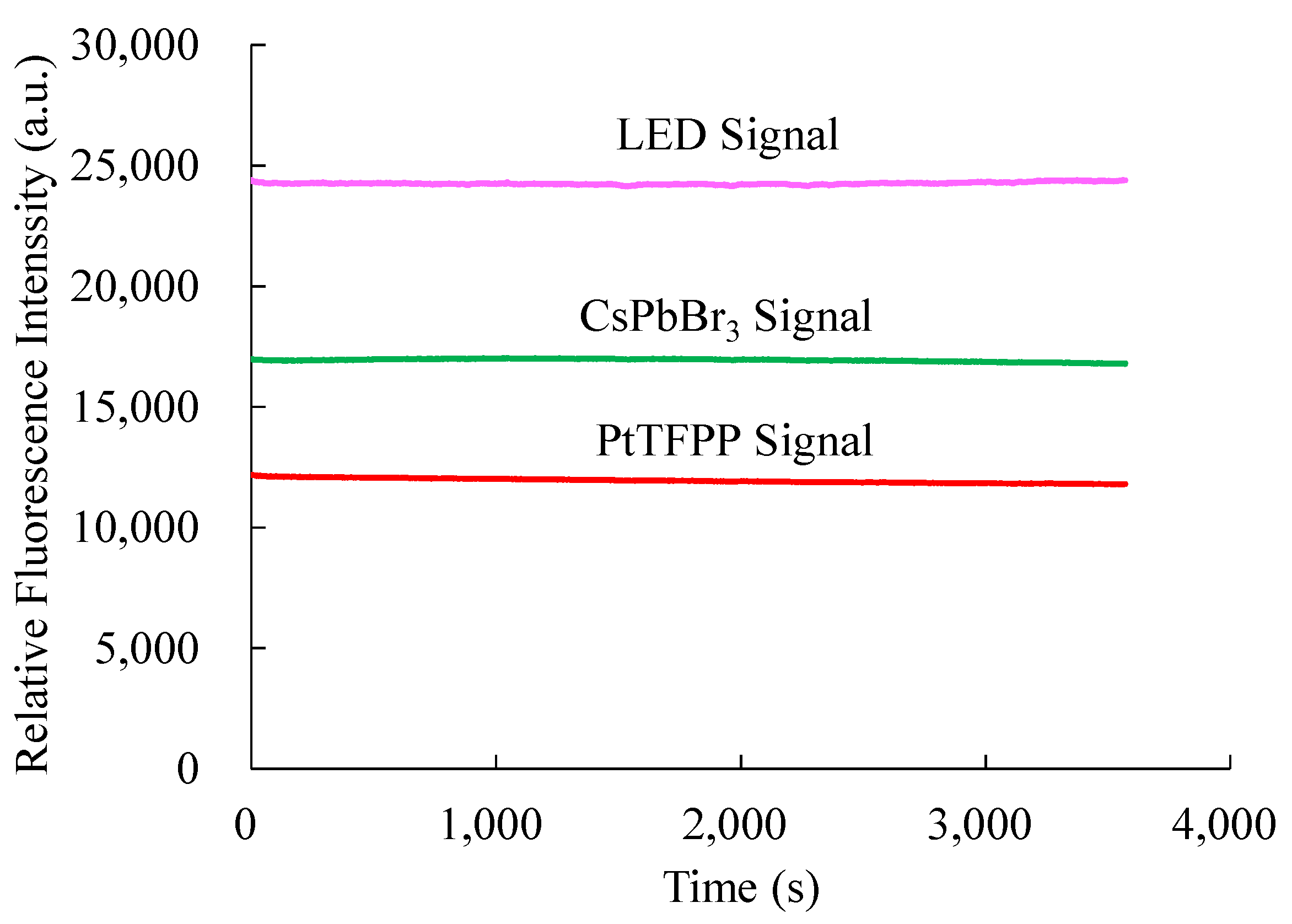
Since some materials degrade photochemically when exposed to continuous light, photostability was also investigated; this may cause the material’s fluorescence to fluctuate over time. Therefore, it is necessary to check the photostability of the optical dual sensor against light illumination. In determining photostability, the optical dual sensor is continuously illuminated by a 380 nm UV LED for an hour in the ambient environment. Changes in fluorescence intensity were observed and recorded using a strip chart for one hour, shown in Figure 11. Photostability on fluorescence intensity of the optical NO sensing signal based on the CsPbBr3 perovskite QDs and O2 sensing signal based on PtTFPP-containing electrospun fibers are around 16,900 and 12,100, respectively. The fluorescence intensities of CsPbBr3 PQDs and PtTFPP-containing electrospun fibers remained steady, according to the findings of the photostability test, which are displayed in the image above, with one hour of pulse irradiation at ambient temperature. In this experiment, we have investigated that the photostability of the optical dual sensor does not change with integral time.
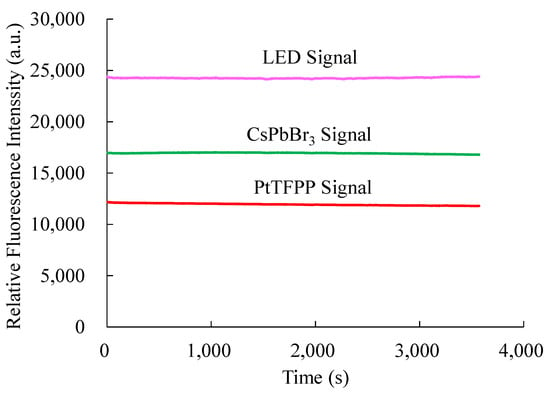
Figure 11.
Photostability of the optical dual sensor.
3.7. Selectivity of Optical Dual Sensor
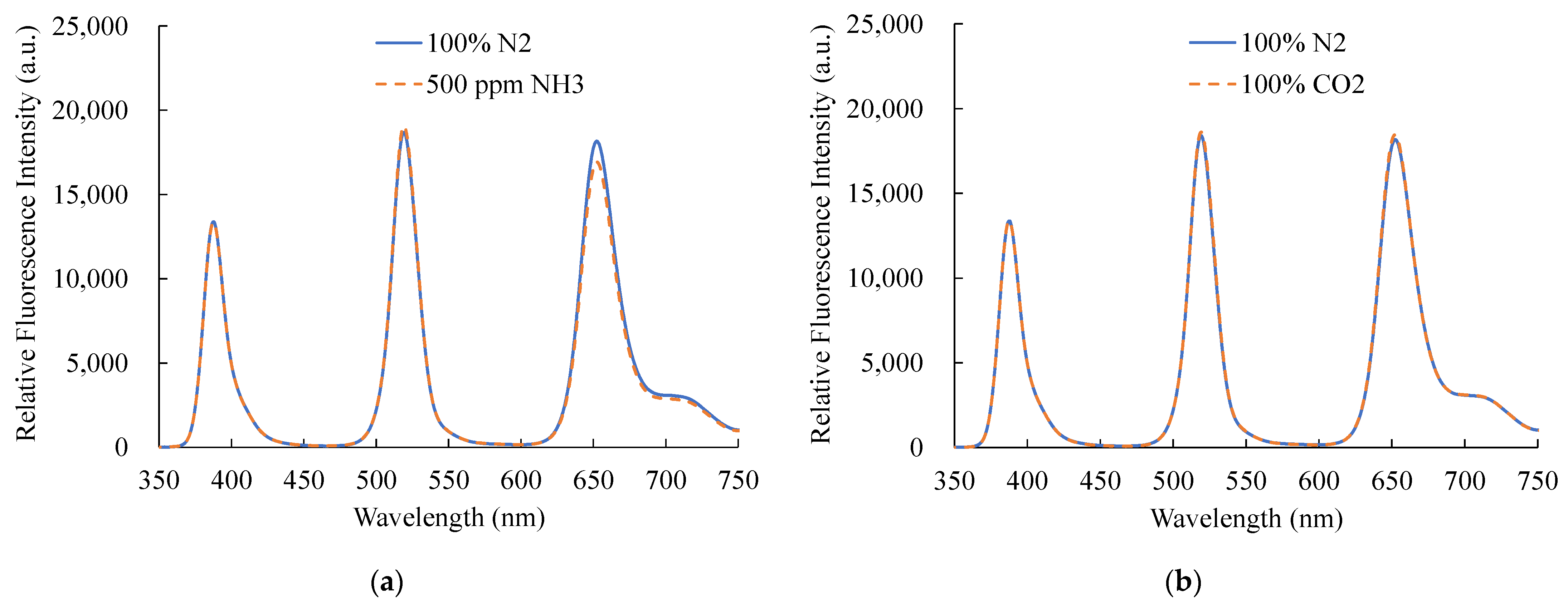
Figure 12 presents certain other gases under particular experimental circumstances, and additional gases can sometimes prevent the optical gas sensor from functioning properly. As a result, the optical dual sensor selectivity should also be considered. In this work, ammonia (NH3) and carbon dioxide (CO2) gases were presented, and the optical dual sensor response of the NO and O2 sensors was observed. The suggested optical dual sensor was alternately exposed to CO2 and NH3 gases for 20 min to monitor the change in fluorescence intensities. The results show photostability under 100% CO2 and 500 ppm NH3, respectively. The fluorescence intensity exhibits no difference and confirms the proposed optical dual sensor’s insensitivity to NH3 and CO2 gases.
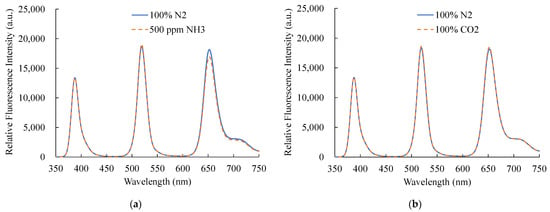
Figure 12.
Selectivity of the optical dual sensor to (a) CO2 and (b) NH3.
3.8. Cross Sensitivity of the Optical Dual Sensor
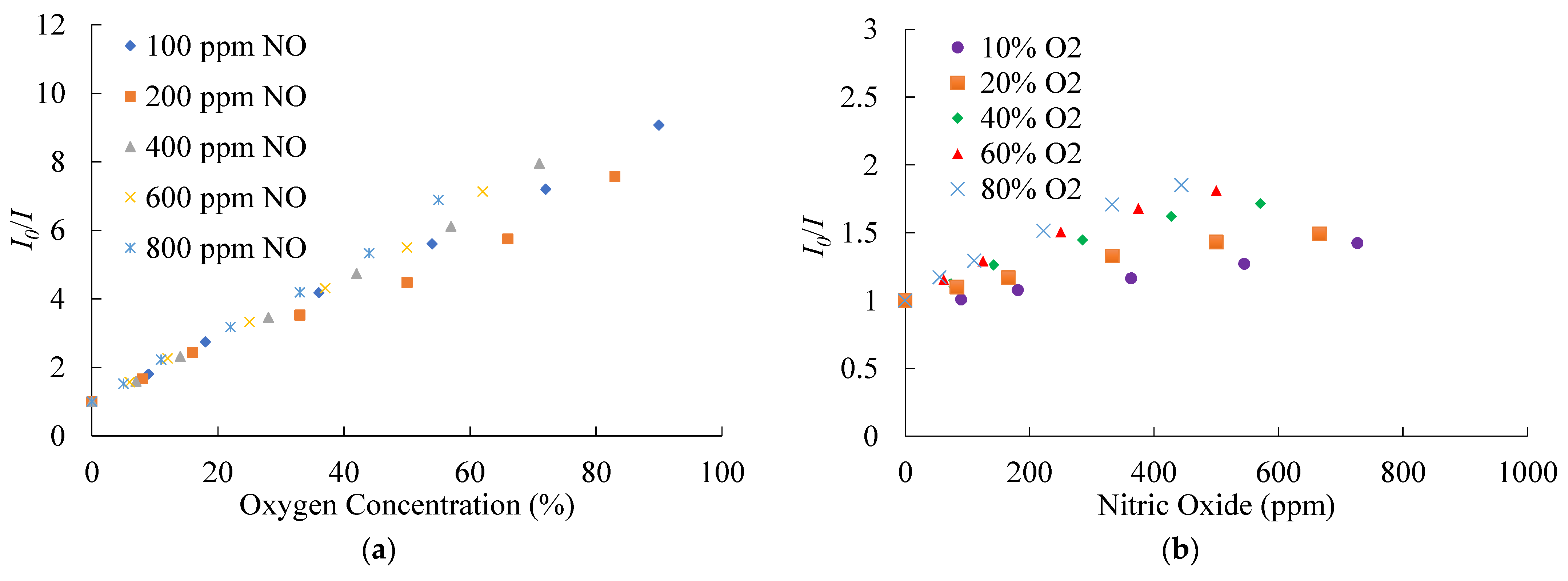
The optical dual sensor based on CsPbBr3 perovskite QDs and PtTFPP-containing electrospun fibers, especially for the mixing of NO and O2 gases, can be found in various fields such as in the industry, health, and environment applications. Therefore, it is necessary to check the cross-sensitive effect of NO and O2 on the optical dual sensor. Figure 13a,b show the cross effect of optical dual sensors for NO/O2 mixed gases. Figure 13a shows the results of a fixed NO concentration at 100, 200, 400, 600, and 800 ppm with different O2 concentrations and sensing signal ratios in the presence of O2 gas concentration. The result shows that the optical O2 sensor has several responses under different fixed NO concentrations. At each fixed NO concentration, the sensitivity of the optical oxygen sensor under different changes in O2 concentrations was recorded and calibrated. Figure 13b shows the results of fixed O2 concentrations at 10%, 20%, 40%, 60%, and 80% with different NO concentrations. Similarly, the sensing signal ratios have slight responses in the presence of gas NO. The optical dual sensor was calibrated to determine the response of the optical NO sensor at different fixed O2 concentrations. Therefore, at each fixed O2 concentration, the sensitivity of CsPbBr3 PQDs under different changes of NO concentrations was recorded and calibrated. Finally, regarding the cross-sensitivity of the optical dual sensor, it can be observed that with increasing NO and O2 gas concentrations, the NO and O2 sensitivity sensor decreases and becomes minimum under different concentrations of gas NO and O2 at 800 ppm and 80%, respectively.
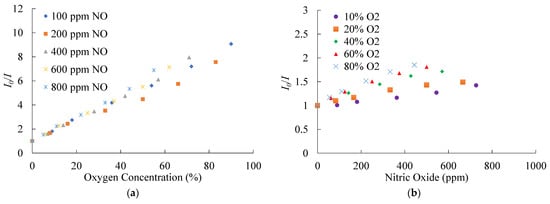
Figure 13.
Cross-sensitivity calibration of the optical dual sensor for (a) O2 and (b) NO.
3.9. Relative Humidity Effect of Optical Dual Sensor
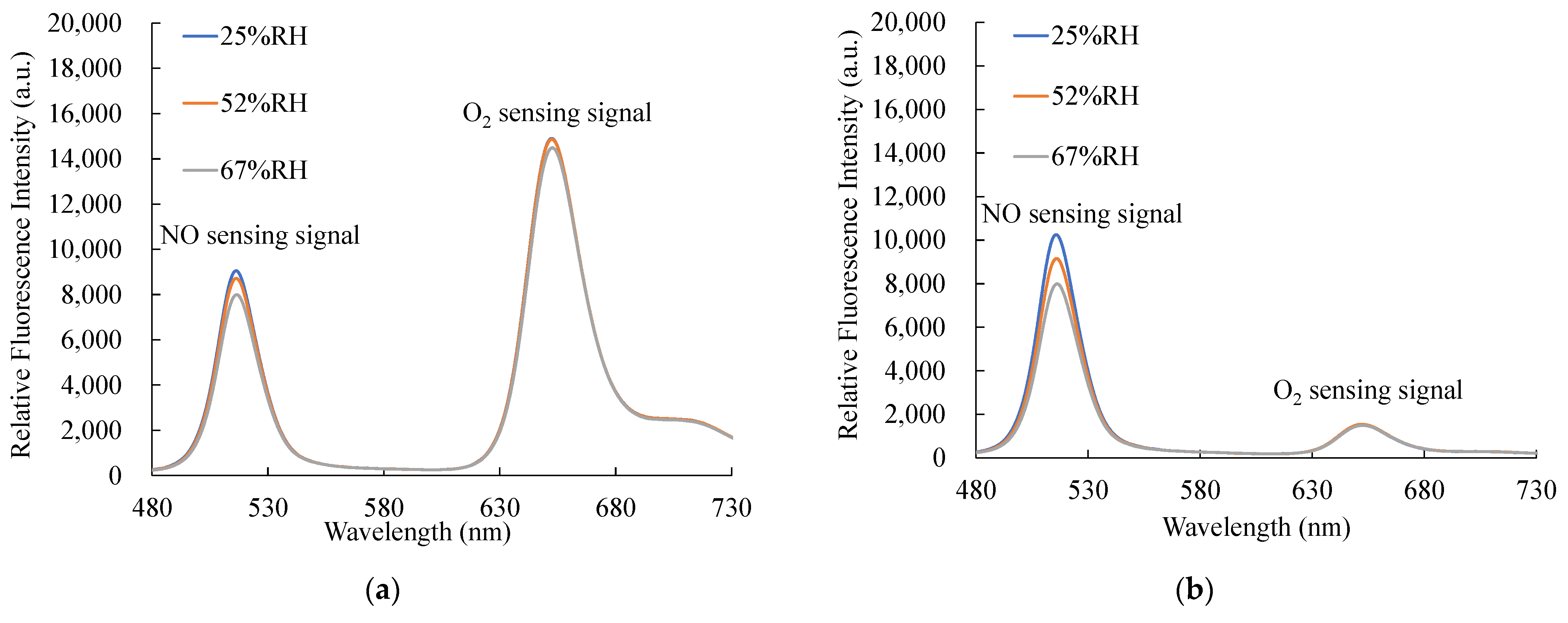
Figure 14a,b show the relative humidity (RH) effect of optical dual sensors under 1000 ppm NO and 100% O2, respectively. The fluorescence intensities were measured and recorded at three different relative humidity levels from 25% to 67%. The relative humidity experiment results show NO sensing signals decreased slightly as the RH increased, and O2 sensing signals showed no significant change under different RH levels.
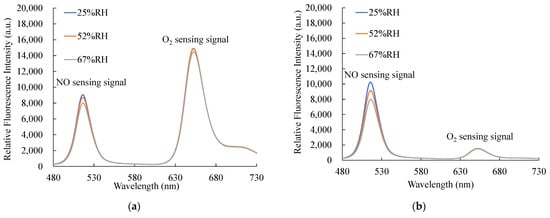
Figure 14.
Relative humidity effect of the optical dual sensor for (a) 1000 ppm NO and (b) 100% O2.
4. Conclusions
This research presents a new optical dual sensor based on fluorescence sensing material PtTFPP-containing electrospun fibers and CsPbBr3 PQDs for the simultaneous detection of O2 and NO gases. The optical dual sensor detects NO and O2 gases simultaneously with the approach of CsPbBr3 perovskite QDs coated on the surface of PtTFPP-containing electrospun fibers. The sensing materials are excited by a 380 nm UV LED to obtain the emission spectra. The experimental results show that the sensitivities of optical dual sensors for NO and O2 sensing are 10.7 and 2.7, respectively. The Stern–Volmer plots for NO and O2 sensing are linear relationships with NO and O2 concentrations in the tested range of 0 to 1000 ppm and 0 to 100%, respectively. Also, the response and recovery time for nitric oxide sensing is 71 s and 109 s, respectively. Response and recovery are 60 s and 65 s for oxygen sensing, respectively. In conclusion, the new optical dual gas sensor developed in this research enables the simultaneous sensing of NO and O2 gas concentrations in various applications.
Author Contributions
Conceptualization and problem formulation, C.-S.C.; experiment and writing—original draft preparation, R.M.; funding acquisition, C.-S.C.; writing—review and editing, R.M. and C.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided to this study by the Ministry of Science and Technology of Taiwan under Grant No. MOST 109-2221-E-131-005-MY2 and NSCT 111-2221-E-131-016.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allso, T.; Neal, R. A review: Application and implementation of optic fiber sensors for gas detection. Sensors 2021, 21, 6755. [Google Scholar]
- Mirzaei, A.; Kordrostami, Z.; Shahbaz, M.; Kim, J.Y.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors using quantum dots: A review. Sensors 2022, 22, 4369. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.O.; Bierer, B.; Scholz, L.; Wollenstein, J.; Palzer, S. A wireless gas sensor network to monitor indoor environmental quality in schools. Sensors 2018, 18, 4345. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Xia, S.Y.; Tan, Y.X.; Huang, Z.Y. Zr-doped h-BN monolayer: A high-sensitivity atmospheric pollutant monitoring sensor. Sensors 2022, 22, 4103. [Google Scholar] [CrossRef]
- Marr, I.; Reiss, S.; Hagen, G.; Moos, R. Planar zeolite film-based potentiometric gas sensors manufactured by a combined thick-film and electroplating technique. Sensors 2011, 11, 7736–7748. [Google Scholar] [CrossRef]
- Goldenstein, C.S.; Spearrin, R.M.; Jeffries, J.B.; Hanson, R.K. Infrared laser-absorption sensing for combustion gases. Prog. Energy Combust. Sci. 2017, 60, 132–176. [Google Scholar] [CrossRef]
- Senf, B.; Yeo, W.H.; Kim, J.H. Recent advances in portable biosensors for biomarker detection in body fluids. Biosensors 2020, 10, 127. [Google Scholar] [CrossRef]
- Kim, K.S.; Baek, W.H.; Kim, J.M.; Yoon, T.S.; Lee, H.H.; Kang, C.J.; Kim, Y.S. A nanopore structured high-performance toluene gas sensor made by the nanoimprinting method. Sensors 2010, 10, 765–774. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef]
- Iverson, N.M.; Hofferber, E.M.; Stapleton, J.A. Nitric oxide sensors for biological applications. Chemosensors 2018, 6, 8. [Google Scholar] [CrossRef]
- Klyamer, D.; Shutilov, R.; Basova, T. Recent advances in phthalocyanine and porphyrin-based materials as active layers for nitric oxide chemical sensors. Sensors 2022, 22, 895. [Google Scholar] [CrossRef] [PubMed]
- Goshi, E.; Zhou, G.X.; He, Q.J. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019, 9, 192–207. [Google Scholar]
- Calabrese, C.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Bedioui, F.; Villeneuve, N. Electrochemical nitric oxide sensors for biological samples-principle, selected examples, and applications. Electroanalysis 2003, 15, 5–18. [Google Scholar] [CrossRef]
- Ding, L.Y.; Ruan, Y.L.; Li, T.; Huang, J.; Warren-Simth, S.C.; Ebendorff-Heidepriem, H.; Monro, T.M. Nitric oxide optical fiber sensor based on exposed core fibers and CdTe/CdS quantum dots. Sens. Actuators B Chem. 2018, 273, 9–17. [Google Scholar] [CrossRef]
- Ghidelli, C.; Perez-Gago, M.B. Recent advances in modified atmosphere packaging and edible coatings to maintain the quality of fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef]
- Tsukada, K.; Sakai, S.; Hase, K.; Minamitani, H. Development of the catheter-type optical oxygen sensor and applications to bio instrumentation. Biosens. Bioelectron. 2003, 18, 1439–1445. [Google Scholar] [CrossRef]
- Amao, Y. Probes, and polymers for optical sensing of oxygen. Microchim. Acta 2003, 143, 1–12. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L.; Sung, T.W. Review on recent developments of fluorescent oxygen and carbon dioxide optical fiber sensors. Photonic Sens. 2011, 1, 234–250. [Google Scholar] [CrossRef]
- Lee, S.K.; Okura, I. Photostable optical oxygen sensing material: Platinum tetrakis (pentafluorophenyl) porphyrin immobilized in polystyrene. Anal. Commun. 1997, 34, 185–188. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. Highly sensitive and linear calibration optical fiber oxygen sensor based on Pt (II) complex embedded in a sol-gel matrix. Sens. Actuators B Chem. 2011, 155, 53–57. [Google Scholar] [CrossRef]
- Amao, Y.; Miyashita, T.; Okura, I. Platinum tetrakis (pentafluorophenyl) porphyrin immobilized in polytrifluoroethylmethacrylate film as a photostable optical oxygen detection material. J. Fluor. Chem. 2001, 107, 101–106. [Google Scholar] [CrossRef]
- Yeh, T.S.; Chu, C.S.; Lo, Y.L. Highly sensitive optical fiber oxygen sensor using Pt (II) complex embedded in sol-gel matrices. Sens. Actuators B Chem. 2006, 119, 701–707. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. High-performance fiber-optic oxygen sensors based on fluorinated xerogels doped with Pt (II) complexes. Sens. Actuators B Chem. 2007, 124, 376–382. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L.; Sung, T.W. Enhanced oxygen sensing properties of Pt (II) complex and dye entrapped core-shell silica nanoparticles embedded in a sol-gel matrix. Talanta 2010, 82, 1044–1051. [Google Scholar] [CrossRef]
- Chu, F.H.; Yang, J.J.; Cai, H.W.; Qu, R.H.; Fang, Z.J. Characterization of a dissolved oxygen sensor made of plastic optical fiber coated with ruthenium-incorporated sol-gel. Appl. Opt. 2009, 48, 338–342. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Leiner, M.J.P.; Posch, H.E. A new sensing material for optical oxygen measurement, with the indicator embedded in an aqueous phase. Mikrochim. Acta. 1986, 90, 359–366. [Google Scholar] [CrossRef]
- Huynh, L.; Wang, Z.U.; Yang, J.; Stoeva, V.; Lough, A.; Manners, I.; Winnik, M.A. Evaluation of phosphorescent rhenium and iridium complexes in poly thionyl phosphazene films for oxygen sensor applications. Chem. Mater. 2005, 17, 4765–4773. [Google Scholar] [CrossRef]
- Xu, W.Y.; Kneas, K.A.; Demas, J.N.; DeGraff, B.A. Oxygen sensors based on luminescence quenching of metal complexes: Osmium complexes suitable for laser diode excitation. Anal. Chem. 1996, 68, 2605–2609. [Google Scholar] [CrossRef]
- Borisov, S.M.; Lehner, P.; Klimant, I. Novel optical trace oxygen sensors based on platinum(II) and palladium(II) complexes with 5,10,15,20-Meso-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin covalently immobilized on silica-gel particles. Anal. Chim. Acta 2011, 690, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Amao, Y.; Asai, K.; Miyashita, T.; Okura, I. Novel optical oxygen sensing material: Platinum porphyrin-fluoropolymer film. Polym. Adv. Technol. 2000, 11, 705–709. [Google Scholar] [CrossRef]
- Dolanský, J.; Henke, P.; Kubát, P.; Fraix, A.; Sortino, S.; Mosinger, J. Polystyrene Nanofiber Materials for Visible-Light-Driven Dual Antibacterial Action via Simultaneous Photogeneration of NO and O2(1Δg). ACS Appl. Mater. Interfaces 2015, 7, 22980–22989. [Google Scholar] [CrossRef]
- DiRosa, M.D.; Chang, A.Y.; Davidson, D.F.; Hanson, R.K. CW Laser Strategies for Multi-Parameter Measurements of High-Speed Flows Containing Either NO or O2. In Proceedings of the Twenty-Ninth Aerospace Sciences Meeting of the American Institute of Aeronautics and Astronautics, Reno, NV, USA, 7–10 January 1991. [Google Scholar]
- Schmidt-Zhang, P.; Sandow, K.P.; Adolf, F.; Gopel, W.; Guth, U. A novel thick film sensor for simultaneous O2 and NO monitoring in exhaust gases. Sens. Actuator B. Chem. 2000, 70, 25–29. [Google Scholar] [CrossRef]
- Ding, Q.F.; Hayashi, T.; Packiasamy, A.R.J.; Miyazaki, A.; Fukatsu, A.; Shiraishi, H.; Nomura, T.; Iguchi, A. The effect of high glucose on NO and O2− through endothelial GTPCH1 and NADPH oxidase. Life Sci. 2004, 75, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Dacres, H.; Narayanaswamy, R. A new optical sensing reaction for nitric oxide. Sens. Actuators B Chem. 2003, 90, 222–229. [Google Scholar] [CrossRef]
- Dacres, H.; Narayanaswamy, R. Sensitivity optical NO sensor based on bis [(2,9-dimethyl-1,10-phenanthroline)] copper(II) complex. Sens. Actuators B Chem. 2005, 107, 14–23. [Google Scholar] [CrossRef]
- Dacres, H.; Narayanaswamy, R. Evaluation of Copper(ii) Eriochrome Cyanine R (ECR) Complex Immobilized in Anion Exchange Membrane as a Potential Nitric Oxide Optical Sensor. Aust. J. Chem. 2008, 61, 189–196. [Google Scholar] [CrossRef]
- Fabregat, V.; Izquierdo, M.A.; Burguete, M.I.; Galindo, F.; Luis, S.V. Quantum dot-polymethacrylate composites for the analysis of NOx by fluorescence spectroscopy. Inorganica Chim. Acta 2012, 381, 212–217. [Google Scholar] [CrossRef]
- Miki, H.; Matsubara, F.; Nakashima, S.; Ochi, S.; Nakagawa, K.; Matsuguchi, M.; Sadaoka, Y. A fractional exhaled nitric oxide sensor based on optical absorption of cobalt tetraphenylporphyrin derivatives. Sens. Actuators B Chem. 2016, 231, 458–468. [Google Scholar] [CrossRef]
- Wang, S.H.; Shen, C.Y.; Lien, Z.J.; Wang, J.H. Nitric oxide sensing properties of a surface acoustic wave sensor with copper-ion-doped polyaniline/tungsten oxide nanocomposite film. Sens. Actuators B Chem. 2017, 243, 1075–1082. [Google Scholar] [CrossRef]
- Xuan, W.; Shan, H.; Zhu, L.; Guan, T.; Zhao, Y.; Qiang, Y.; Song, J.; Zhang, J.; Sui, M.; Gu, X.; et al. In-situ synthesis of stable ZnO-coated CsPbBr3 nanocrystals for room temperature heptanal sensors. Mater. Today Chem. 2022, 26, 101155. [Google Scholar] [CrossRef]
- Kumar, D.; Mesin, R.; Chu, C.S. Optical fluorescent sensor based on perovskite QDs for nitric oxide gas detection. Appl. Opt. 2023, 62, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yeh, S.Y.; Huang, W.L.; Xu, Y.X.; Huang, Y.S.; Yeh, T.H.; Tien, C.H.; Chen, L.C.; Tseng, Z.L. Using thermally cross-linkable hole transporting layer to improve interface characteristics for perovskite CsPbBr3 quantum-dot light emitting diodes. Polymers 2020, 12, 2243. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic/Plenum Press: New York, NY, USA, 1999; Chapters 8 and 9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).