Abstract
The introduction of solid-phase imprinting has had a significant impact in the molecular imprinting field, mainly due to its advantage of orienting the template immobilisation, affinity separation of nanoMIPs and faster production time. To date, more than 600 documents on Google Scholar involve solid-phase synthesis, mostly relying on silanes mediating template immobilisation on the solid phase. Organosilanes are the most explored functionalisation compounds due to their straightforward use and ability to promote the binding of organic molecules to inorganic substrates. However, they also suffer from well-known issues, such as lack of control in the layer’s deposition and poor stability in water. Since the first introduction of solid-phase imprinting, few efforts have been made to overcome these limitations. The work presented in this research focuses on optimising the silane stability on glass beads (GBs) and iron oxide nanoparticles (IO-NPs), to subsequently function as solid phases for imprinting. The performance of three different aminosilanes were investigated; N-(6-aminohexyl) aminomethyltriethoxy silane (AHAMTES), 3-Aminopropyltriethoxysilane (APTES), and N-(2-aminoethyl)-3-aminopropyltriethoxysilane (AEAPTES), as well as studying the effect of dipodal silane bis(triethoxysilyl)ethane (BTSE). A stable solid phase was consequently achieved with 3% v/v AEAPTES and 2.4% BTSE, providing an upgraded protocol from Canfarotta et al. for the silanisation of the solid phase for molecular imprinting purposes.
1. Introduction
Since Wolff and Mosbach’s original work on molecularly imprinted polymers (MIPs), many different strategies for their synthesis have been explored. For a long time, bulk polymerisation remained the gold standard in MIP synthesis, mainly due to the initial use of MIPs as adsorbents, in chromatography columns, and in sensor coating.
In following years, MIPs began to be applied in the diagnostic field in the form of MIP nanoparticles (nanoMIPs), having achieved a size comparable to that of antibodies, and the idea of nanoMIPs becoming a synthetic counterpart of antibodies became more prominent. Novel methods of synthesis were explored [1,2,3,4], providing the ability to synthesise high affinity nanoparticles to be applied in different diagnostic devices [5,6]. However, they still suffered from some drawbacks such as labour-intensive production, large affinity distribution, and difficulties in the template removal [7].
A turning point was presented by the introduction of a novel solid-phase synthesis approach first described in 2013 by Piletsky’s group (Poma et al. [7]). This consisted of the covalent immobilisation of the template of interest onto glass beads (around 70–100 microns), which were subsequently used as substrate during the imprinting process. An affinity separation was then performed, which allowed the removal of unreacted monomers and low-affinity nanoMIPs, thus selectively collecting high affinity nanoMIPs via a high temperature elution step.
This approach overcame some of the drawbacks from previous methodologies as it facilitates template-oriented immobilisation, with consequent homogeneity in the MIPs binding site generation. Through the aforementioned affinity separation, the product collection is limited to high-affinity nanoMIPs, effectively removing the need for time-consuming purification steps.
Due to its numerous advantages, this strategy had a significant impact in the molecular imprinting field, as evidenced from the number of works published mentioning solid-phase imprinting (to date, more than 600 documents on Google Scholar). As a follow-up from the original protocol, a more universal protocol for the synthesis of nanoMIPs was published in 2016 by Piletsky’s group (F. Canfarotta et al. [8]). This work detailed a generic approach for the synthesis of nanoMIPs in organic or aqueous solvents, via the immobilisation of small molecules, peptides, and proteins.
Shortly after, K. Haupt’s group also demonstrated the use of solid-phase synthesis for the oriented immobilisation of proteins via the binding of trypsin to its inhibitor p-aminobenzamidine (PAB), anchored to the solid phase [9]. Later on, the same group published a more generic method for the oriented immobilisation of proteins by exploiting the known high affinity of proteins’ His-tag to metal chelates. The latter were immobilised on the solid phase and oriented immobilization was achieved by introducing the HIS-tagged target protein [10]. Both methods were successful in orienting the imprinting; however, these approaches are only applicable to proteins. In recent years, a generic method for immobilisation was developed, where click chemistry is utilised to immobilise a modified azide-functionalized template molecule to glass beads functionalized with terminal alkyne groups [11].
An alternative to the use of glass beads as solid-phase support for the synthesis of nanoMIPs was proposed by R. Mahajan et al. [12], where iron oxide nanoparticles were utilised as a solid support, leading to a higher nanoMIP yield. This work also reports the use of template-functionalised iron oxide nanoparticles in a competitive magnetic molecularly imprinted nanoparticles assay (MINA), an assay format recently demonstrated to be a valid alternative to ELISAs in the detection of trypsin and pepsin [12].
All the approaches so far introduced include the use of organosilanes as an intermediate step between template immobilisation and solid phase. Organosilanes have been studied and used for several years and, at present, still represent one of the most-explored functionalisation strategies due to their straightforward use and the advantage of promoting the binding of organic molecules to inorganic particles, surfaces, or substrates [13]. However, they also suffer from well-known issues, such as a lack of control in the layer’s deposition [14] and poor stability in water due to hydrolysis occurring between the silanol groups and the hydroxyl groups on surfaces [15].
Hydrolysis of the silanes represents a severe issue for the solid-phase synthesis of nanoMIPs because, if the template-silane construct is hydrolysed in the low-temperature elution step, the final nanoMIPs yield will be lower due to partial elution of nanoMIPs formed around the template and washed away with the waste material (unreacted monomers and low-affinity nanoMIPs). If the silane, instead, hydrolyses in the high-temperature elution step, the final sample will be contaminated with template-silane constructs, requiring an extra separation step to avoid the template-silane competing with the testing analyte in the final application, therefore effectively decreasing the performance of the sensor platform developed. Since the first introduction of solid-phase imprinting, little efforts were made to overcome this limitation.
Very recently, S. Piletsky et al. [16] reported the successful use of iodo silanes for the immobilisation of peptides. 3-iodopropyl)trimethoxysilane (IPTMS) showed an increased stability compared to its amino counterpart aminomethyltriethoxysilane (AHAMTES). However, this approach is limited to the immobilisation of peptides or templates bearing thiol groups.
The work presented in this research focuses on optimising the silane stability on glass beads (GBs) and iron oxide nanoparticles (IO-NPs) as solid phases. The performance of three different aminosilanes among some of the most used silanes, namely AHAMTES, 3-Aminopropyltriethoxysilane (APTES), and N-(2-aminoethyl)-3-aminopropyltriethoxysilane (AEAPTES), was investigated, as well as studying the effect of dipodal silane bis(triethoxysilyl)ethane (BTSE) on the stability of the silane layer.
2. Materials and Methods
Deionised water, toluene, ethanol, methanol, sulphuric acid, and sodium hydroxide were purchased from ThermoFisher. Ninhydrin and bis(triethoxysilyl)ethane (BTSE) were purchased from Alpha Aesar (Haverhill, MA, USA). Aminomethyltriethoxysilane (AHAMTES) was from Fluorechem. 3-Aminopropyltriethoxysilane (AEPTES), N-(2-aminoethyl)-3-aminopropyltriethoxysilane (AEAPTES), dodecylamine phosphate saline buffer (PBS) and Fe3O4 < 50 nm were from Merck KGaA (Darmstadt, Germany).
2.1. Glass Beads Activation
Glass beads (GBs) (70–110 μm, from Microbeads ag, Gebenstorf, Switzerland) (300 g) were first washed with water (3 × GB volume) and ethanol (1 × GB volume) under vacuum, and then boiled in NaOH (4 M, 300 mL) for 15 min prior to washing with water (3 × 2 L). The beads were subsequently placed in a solution of sulphuric acid (50%, 120 mL) for 1 h before again being washed with water (2 × 2 L) and incubated in buffer for 5 min (PBS (0.01 M or phosphate buffer 0.1 M, 200 mL). Further water washes (10 × 2 L) were performed to ensure the final pH was between 6 and 8. The solid phase was dried by washing with ACN (2 × GB volume) and placing under vacuum for 15 min, before leaving it in an oven (2 h, 150 °C).
2.2. Glass Beads Silanisation
The three silanes (Table 1) were individually added to a toluene solution (0.4 mL × g of GB, 80 °C) in addition to BTSE 0.12% v/v (successively varied to 2.4% v/v). Meanwhile, a Duran bottle was flushed with nitrogen for 5 min and the glass beads were added while still warm, and the toluene mixture added. An airtight cap was then applied, and the mixture swirled to get all the GB in suspension. This was then left to react overnight (80 °C).

Table 1.
Summary of silanes used to investigate stability study.
The GBs were subsequently washed with MeOH (3 × GB volume), and ACN (5 × GB volume) to remove any residual silane, before drying under vacuum and further oven drying (2 h, 150 °C). Once dried, a sample of GB was collected for analysis, the remaining incubated in DI water (40 °C, overnight). Afterwards, beads were washed with DI water (5 × GB volume), (3 × GB volume) to remove any residual silane, dried (2 h, 150 °C) and stored in closed bottle (RT). A sample was collected for analysis.
2.3. Glass Beads Washes Simulation and Amino Groups Quantification
The GBs were washed with DI water (10 × GB volume, RT) and with EtOH (10 × GB volume, 65 °C). A sample (3 × 1 g) was collected for analysis. A 1:1 dilution series of dodecylamine (3 × 1 mL, 0.1–5 mM (0.93 mg mL−1)) in EtOH was prepared for calibration. A sample of the silanised solid phase (3 × 1 g) to be tested was also prepared. Ninhydrin (2 mL, 1.07 mg mL−1, 6 mM) was added to each sample and incubated under exclusion of light (180 min, 60 °C). Once complete, the samples were allowed to cool and UV spectra obtained (300–700 nm, λmax = 580 ± 4 nm), using ninhydrin (6 mM) as reference.
2.4. Iron Oxide Nanoparticles Silanisation
The protocol was adapted from Bioconjugate techniques [13] and optimised by F. Grillo [17]. A 50 mL solution 5% water in EtOH v/v was prepared and pH adjusted to 4.5–5.5 with acetic acid. The silane coupling agents, APTES and AEAPTMS, were dissolved in aqueous solution and left to react (5 min, RT). After flashing with N2, IO-NPs were added and left to react under continuous sonication (4 h). NPs were washed with 5% EtOH in DI water (5 × 250 mL). After drying IO-NPs with N2, they were dried in an oven (60 min, 110 °C). IO-NPs were left overnight in a vacuum chamber.
2.5. Iron Oxide Nanoparticles Aminogroups Quantification
Samples of 5 mg of silanised IO-NPs were suspended in 2 mL of MilliQ water and sonicated for 10 min, 30 min, and 60 min. In each sample, the supernatant was removed, IO-NPs washed with DI water (3 × 2 mL), and resuspended in EtOH (1 mL) for Kaiser test. Ninhydrin (1 mL, 2.14 mg mL−1, 12 mM) was added to each sample and incubated for 180 min at 60 °C under exclusion of light. A 1:1 dilution series of dodecylamine (3 × 1 mL, 0.1–5 mM (0.93 mg mL−1)) in EtOH was prepared for calibration. Once complete, the samples were allowed to cool and UV spectra obtained (300–700 nm, λmax = 580 ± 4 nm), using ninhydrin (6 mM) as reference. Each test was run in triplicate.
3. Results
3.1. Silanes Stability on Glass Beads
3.1.1. Amino Silanes Investigation
As described in the introduction, the solid-phase synthesis approach consists of low and high affinity elution steps performed at RT and 65 °C. In this stability study, to monitor silane hydrolysis in the same conditions as the solid phase would be subjected to during nanoMIPs synthesis in an aqueous environment, GB samples were analysed at three stages. Initially, samples of GBs were taken after silanisation (step 1). Smith et al. [18] had previously observed that most of the hydrolytic degradation occurred at 40 °C within 24 h. Hence, after silanisation, GBs were incubated overnight in DI water at 40 °C and a sample was collected for analysis (step 2). Washes in DI water at RT and ethanol (EtOH) at 65 °C were then performed on silanised GBs to simulate nanoMIP elutions (step 3) (Figure 1). The silanisation protocol was adapted from Canfarotta et al. [8] and Hermanson [19], and the quantification of silanes on glass beads was attained via adaptation of the Kaiser test [20], with a dodecylamine calibration curve (0.1–5 mM) prepared and used as reference [17].
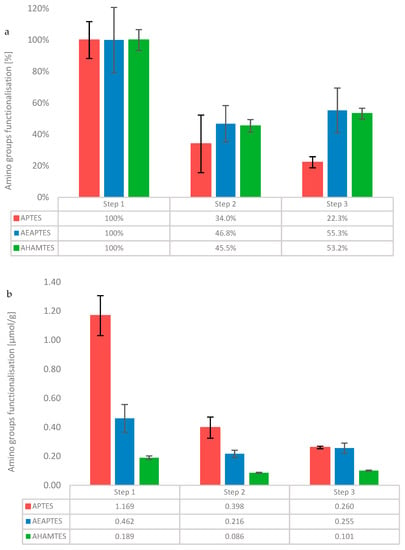
Figure 1.
Kaiser test for quantification of amino groups in APTES (red), AEAPTES (blue), and AHAMTES (green). Quantification after silane deposition (step 1); 40 °C water soaking (step 2); and nanoMIPs elution simulation (step 3). Data is reported in percentage (a) and absolute values [µmol/g of GBs] (b).
The data reported in Figure 1 show a drop in concentration of amino groups in all silanes tested when exposed to 40 °C incubation in water to 34.0 ± 18.3%, 46.8 ± 11.6%, and 45.5 ± 4.0%, for APTES, AEAPTES and AHAMTES, respectively. However, AEPTES and AHAMTES, after an initial drop in step 2, maintain constant levels, with a percentage of amino groups of 55.3 ± 14.2% and 53.2 ± 3.4%, respectively. APTES, however, reports a further drop of 11.7% (22.3 ± 3.5% on GB) (Figure 1a) after step 2, indicating the poorest stability among the silanes tested. Interestingly, when the data are plotted in absolute values (µmol/g of GB, (Figure 1b)), APTES showed the highest amount of silanisation (1.169 µmol/g).
3.1.2. Dipodal Silane in Stability Study
A known factor influencing the stability of silane layers is the accessibility of water at the base of the layer facilitating hydrolysis [18]. Surfaces modified with dipodal silanes have previously demonstrated remarkably improved resistance to hydrolysis compared to surfaces prepared from conventional silanes [21]. It was therefore hypothesised that the addition of a hydrophobic non-functional silane could increase the hydrophobicity of the solid-phase silane layer and stabilise it. GBs were therefore functionalised with AHAMTES in 0.12 BTSE, and this was compared to AHMTES in the absence of BTSE (Figure 2).
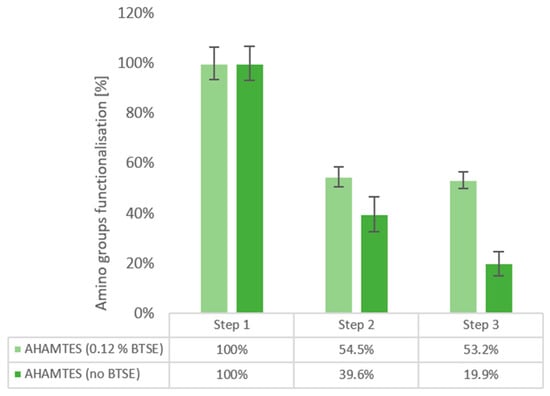
Figure 2.
Kaiser test for quantification of amino groups in AHAMTES (0.12% BTSE) (light green), AHAMTES (no BTSE) (dark green). After silane deposition (step 1), after 40 °C water soaking (step 2), and nanoMIPs elution simulation (step 3).
In the absence of BTSE, a linear drop in silane is detected (100 ± 6.7%, 33.4 ± 6.9%, 16.7 ± 4.7%). On the contrary, in the presence of BTSE, after an initial drop to 54.4 ± 3.4% (step 2), no significant silane loss occurs in the nanoMIPs simulated elution phase (step 3, 52.3 ± 3.4%).
Following the same logic, BTSE percentage was increased by 20 times, to a final v/v% of 2.4% and tested using AEPTES (Figure 3).
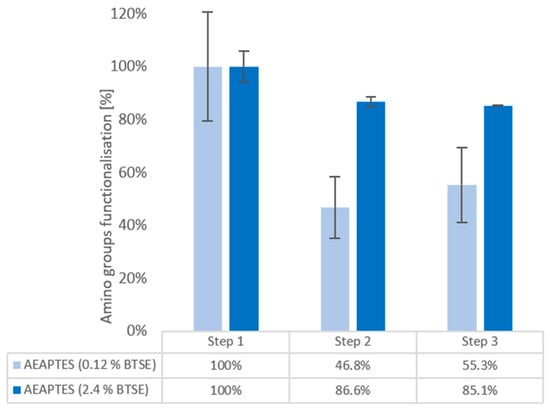
Figure 3.
Kaiser test for quantification of amino groups in AEAPTES (0.12% BTSE) (light blue), AEAPTES (2.4% BTSE) (dark blue). After silane deposition (step 1), after 40 °C water soaking (step 2), and nanoMIPs elution simulation (step 3).
Also, in this case it was found that BTSE stabilises the silane, decreasing the initial silane loss to only 13.4% after step 2 (86.6 ± 1.7%), maintained constant to 85.1 ± 0.14% after nanoMIPs elution simulation. In addition, increasing the percentage of BTSE decreased the loss from the first incubation in water (step 2) by almost 50%, from 46.8 ± 11.6 with 0.12% BTSE to 86.6 ± 1.7% with 2.4% BTSE.
3.2. Silane Stability on Iron Oxide Nanoparticles (IO-NPs)
3.2.1. Silane Investigation
A stability study was conducted on commercially available Fe3O4 nanoparticles with diameter < 50 nm. IO-NPs were purchased and silanised in-house using a protocol previously optimised in previous work [17].
AEAPTES and APTES were used in the presence and absence of BTSE. To study the stability of the silanes on IO-NPs, an induced stress was introduced by sonicating silanised IO-NPs (sIO-NPs) in DI for different time intervals (10, 30, 60 min). After removing the supernatant, the sIO-NPs were resuspended and the amount of remaining amino groups quantified by Kaiser test. The results are given in Figure 4.
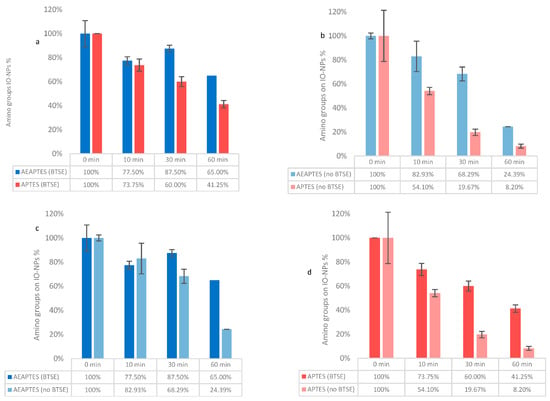
Figure 4.
Kaiser test for quantification of amino groups. Comparison between IO-NPs AEAPTES and AEAPTES in the presence of 0.12% BTSE (a) and the absence of BTSE (b). Comparison between IO-NPs with BTSE 0.12% on AEAPTES (c) and APTES (d).
The results collected for sIO-NPs coincide with the investigation on GBs, where the use of AEAPTES produces a more stable layer of silane, with 65.00% amino groups retained after 60 min of sonication, compared to 41.25% in case of APTES (Figure 4a). Additionally, the presence of BTSE stabilises both silanes, going from 24.39% in the absence of BTSE to 65.00% in its presence for AEAPTES (Figure 4c) and from 8.20% 41.25% to for APTES (Figure 4d).
3.2.2. Stability over Time
Finally, if IO-NPs were going to be used in an assay with template immobilised, stability over time is also pivotal for storage purposes. Stability over time was studied by immersing sIO-NPs in DI water for a week and compared to freshly dispersed sIO-NPs (Figure 5).
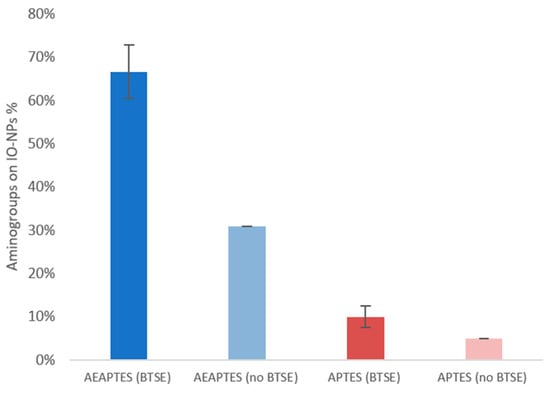
Figure 5.
Kaiser test for quantification of amino groups; percentage of amino groups on sIO-NPs after one week in DI water.
Studies of the stability over time also agree with the previous data where the AEAPTES layer produced more stability than APTES, with 66.67% to 10.00% amino groups retained, respectively, for AEAPTES compared to APTES in the presence of BTSE. The presence of BTSE stabilises the layer in both silanes tested. When BTSE was absent, only a percentage of 30.29% silane was retained compared to 66.67% in the presence.
In the case of APTES, the retention went from 4.92% silane retained in the absence of BTSE to 10% in its presence. As already observed for glass beads, APTES has resulted in the silane forming the most stable layer and the presence of BTSE contributed to an increased stability.
4. Discussion
The data collected in this study are in agreement with what observed by Zhu et al. [14], reporting a decrease in silane layer thickness on silicon wafer after exposure to 40 °C water, and yet remaining stable to hydrolysis afterwards. APTES emerged to be the least stable despite the highest number aminogroups originally quantified (Figure 1b). This is presumably due to APTES forming multilayers [14], and can be justified by the nature of the aminopropyl silanes, which tend to be prone to form irregular structures with intra- and intermolecular siloxane bonds as shown in Figure 6. In addition, the formation of irregular structures and multilayers exposes the silane to hydrolysis.
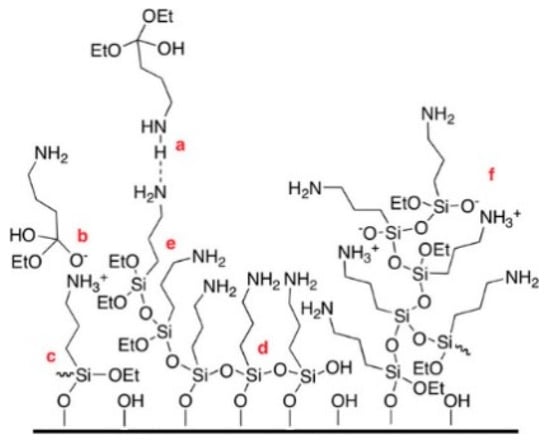
Figure 6.
An APTES-derived layer with structural irregularities: individual silane molecules can be incorporated into the layer via (a) hydrogen bonding, (b) electrostatic attraction, (c) covalent bonding with the substrate, and (d) horizontal and (e) vertical polymerization with neighbouring silanes; (f) oligomeric/polymeric silanes can also react/interact with functionalities present at the interface. Image reprinted with permission from [14]. Copyright © 2023, American Chemical Society.
Although a high number of amino groups on the surface would be desirable to maximise template immobilisation, the stability of the silane layer is crucial, since template molecules (and silane) may otherwise be released from the GB, thus contaminating the nanoMIP dispersion.
AHAMTES showed a similar behaviour to AEAPTES in stability terms; however, the moles immobilised were 2.5 times lower than AEAPTES, possibly due to the low density monolayer produced by AHAMTES [14]. As previously highlighted, maximising the surface covering is an important parameter which can lead to a higher chance of immobilisation of the template in the following steps. AEAPTES was found to be the best performant silane overall, which is also in agreement with what reported by Smith et al. [18] and Zhu et al. [14].
In all cases explored, BTSE resulted improving the stability of the solid phase. BTSE is capable of condensing on the surface similarly to functional silanes; however, it possesses two silicon atoms that can covalently bind to a surface, and this contributes to the stabilisation of the silane layer by creating a hydrophobic environment more hostile to water [21]. In addition, the alternation of BTSE to AEAPTES (Figure 7) might prevent AEPTES from creating intramolecular binding, a known issue with aminoethyl silanes [14].
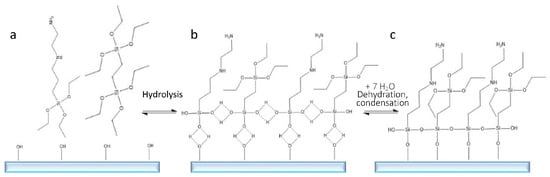
Figure 7.
Schematic representation of silanisation; AEPTES + BTSE (a); hydrolysis of the –R group (b); dehydration and condensation of the silanes on the glass surface (c).
The use of 3% v/v of AEAPTES and 2.4% v/v was found to be the conditions able to preserve GB silanisation with a drop in silane percentage of only 13% after 40 °C overnight incubation.
In order to prevent silane contamination during the MIPs synthesis the authors therefore recommend introducing a water incubation step after every GB silanisation.
5. Conclusions
An updated protocol for the silanisation of glass beads from the original solid-phase synthesis protocol described by Canfarotta et al. is here outlined, and has shown improved silane stability on both glass beads and iron oxide nanoparticles. Three different aminosilanes were tested, APTES, AEAPTES and AHAMTES, with AEAPTES resulting the most stable overall in both solid phases tested. The introduction of BTSE improved the stability across all silanes examined and, in all conditions tested, with best results achieved using 3% v/v AEAPTES and 2.4% BTSE. A 40 °C overnight incubation in DI water was introduced to remove the lose silanes that are likely not to be cross-linked to the solid phase. The authors recommend the addition of this incubation step to avoid template leaking during the solid-phase synthesis of nanoMIPs.
Author Contributions
Conceptualization, F.G., F.C. and T.S.B.; methodology, F.C.; formal analysis, F.G.; investigation, F.G., M.A., W.L.S., K.L., R.S., T.H. and J.C.; resources, data curation, F.G. and T.S.B.; writing—original draft preparation, F.G.; writing—review and editing, F.G., T.S.B., F.C. and E.P.; supervision, S.P.; project administration, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Engineering and Physical Sciences Research Council, grant number EP/R51326X/1.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Umpleby Ii, R.J.; Bode, M.; Shimizu, K.D. Measurement of the continuous distribution of binding sites in molecularly imprinted polymers. Analyst 2000, 125, 1261–1265. [Google Scholar] [CrossRef]
- Pérez, N.; Whitcombe, M.J.; Vulfson, E.N. Molecularly imprinted nanoparticles prepared by core-shell emulsion polymerization. J. Appl. Polym. Sci. 2000, 77, 1851–1859. [Google Scholar] [CrossRef]
- Vaihinger, D.; Landfester, K.; Kräuter, I.; Brunner, H.; Tovar, G.E.M. Molecularly imprinted polymer nanospheres as synthetic affinity receptors obtained by miniemulsion polymerisation. Macromol. Chem. Phys. 2002, 203, 1965–1973. [Google Scholar] [CrossRef]
- Ye, L.; Weiss, R.; Mosbach, K. Synthesis and Characterization of Molecularly Imprinted Microspheres. Macromolecules 2000, 33, 8239–8245. [Google Scholar] [CrossRef]
- Hoshino, Y.; Koide, H.; Urakami, T.; Kanazawa, H.; Kodama, T.; Oku, N.; Shea, K.J. Recognition, Neutralization, and Clearance of Target Peptides in the Bloodstream of Living Mice by Molecularly Imprinted Polymer Nanoparticles: A Plastic Antibody. J. Am. Chem. Soc. 2010, 132, 6644–6645. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, K.; Koide, H.; Hoshino, Y.; Shea, K.J. Preparation of abiotic polymer nanoparticles for sequestration and neutralization of a target peptide toxin. Nat. Protoc. 2015, 10, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template—“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443. [Google Scholar] [CrossRef]
- Ambrosini, S.; Beyazit, S.; Haupt, K.; Tse Sum Bui, B. Solid-phase synthesis of molecularly imprinted nanoparticles for protein recognition. Chem. Commun. 2013, 49, 6746–6748. [Google Scholar] [CrossRef]
- Xu, J.; Ambrosini, S.; Tamahkar, E.; Rossi, C.; Haupt, K.; Tse Sum Bui, B. Toward a Universal Method for Preparing Molecularly Imprinted Polymer Nanoparticles with Antibody-like Affinity for Proteins. Biomacromolecules 2016, 17, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Medina Rangel, P.X.; Laclef, S.; Xu, J.; Panagiotopoulou, M.; Kovensky, J.; Tse Sum Bui, B.; Haupt, K. Solid-phase synthesis of molecularly imprinted polymer nanolabels: Affinity tools for cellular bioimaging of glycans. Sci. Rep. 2019, 9, 3923. [Google Scholar] [CrossRef]
- Mahajan, R.; Rouhi, M.; Shinde, S.; Bedwell, T.; Incel, A.; Mavliutova, L.; Piletsky, S.; Nicholls, I.A.; Sellergren, B. Highly Efficient Synthesis and Assay of Protein-Imprinted Nanogels by Using Magnetic Templates. Angew. Chem. Int. Ed. Engl. 2019, 58, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhu, M.; Lerum, M.Z.; Chen, W. How To Prepare Reproducible, Homogeneous, and Hydrolytically Stable Aminosilane-Derived Layers on Silica. Langmuir 2012, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J. Hydrolysis and Condensation of Silicates: Effects on Structure. J. Non-Cryst. Solids 1988, 100, 31–51. [Google Scholar] [CrossRef]
- Piletsky, S.S.; Garcia Cruz, A.; Piletska, E.; Piletsky, S.A.; Aboagye, E.O.; Spivey, A.C. Iodo Silanes as Superior Substrates for the Solid Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles. Polymers 2022, 14, 1595. [Google Scholar] [CrossRef]
- Grillo, F. Development of a novel assay for drugs of abuse based on Molecularly Imprinted Polymers as Synthetic Antibodies. Master’s Thesis, University of Leicester Library, Leicester, UK, 2018. [Google Scholar]
- Asenath Smith, E.; Chen, W. How to Prevent the Loss of Surface Functionality Derived from Aminosilanes. Langmuir ACS J. Surf. Colloids 2008, 24, 12405–12409. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugated Techniques, 2nd ed.; Elsevier: Alpharetta, GA, USA, 2008. [Google Scholar]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Arkles, B.; Pan, Y.; Larson, G.L.; Singh, M. Enhanced hydrolytic stability of siliceous surfaces modified with pendant dipodal silanes. Chemistry 2014, 20, 9442–9450. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).