Abstract
Naphthol is a widely used chemical and medical detection biomarker, but it is harmful to human health and the environment. Therefore, a highly sensitive detection method for naphthol is urgently required. Herein, an electrochemical microsensor for the simultaneous detection of naphthol isomers was fabricated by the in situ growth of a three-dimensional graphene network (3DGN) on screen-printed electrodes. The microsensor exhibited good electrochemical sensing responses to typical isomers of naphthol (1-NAP and 2-NAP). Using the differential pulse voltammetry (DPV) method, the microsensor successfully realized the electrochemical detection of 1-NAP, 2-NAP, and naphthol isomer mixtures. Whether detecting naphthol isomers individually or simultaneously, the microsensor exhibited a good linear relationship for 1-NAP and 2-NAP in a wide range of concentrations. For the simultaneous detection of naphthol isomers, the limit of detection (LOD) of the microsensor to 1-NAP reached 10 nM, and the LOD for 2-NAP was about 20 nM. The microsensor also showed good selectivity, reproducibility, and stability. The simultaneous quantitative detection of 1-NAP and 2-NAP was also successfully achieved in synthetic urine samples.
1. Introduction
Naphthol is essential for chemical manufacturing or synthesis as they are widely used as precursors of dyes, insecticides, and pesticides [1,2]. However, they have been found to be harmful to human health and the environment. The naphthol isomers 1-naphthol (1-NAP) and 2-naphthol (2-NAP) can cause serious damage to the human reproductive system and circulatory system, and long-term exposure can even cause chronic severe kidney and skin diseases [3,4]. Moreover, one of the World Health Organization (WHO)-classified carcinogens, naphthalene, can enter the human body through inhalation or dermal exposure and can cause serious damage to human health. As metabolites of naphthalene, naphthol isomers present in urine can also be used as public and occupational naphthalene exposure markers for diagnosis [5,6]. Therefore, the development of a method for the simultaneous detection of two structurally and chemically similar isomers of naphthol in human urine is urgently required.
However, due to the similarities in molecular structure and properties of naphthol isomers and their coexistence in urine with complex components, the simultaneous detection of naphthol isomers in urine is a challenging task. Therefore, it is necessary to establish a rapid, sensitive, simple, and selective analytical method for the simultaneous quantitative detection of naphthol isomers. At present, the detection methods and technologies of naphthol isomers are mainly based on chromatography [7,8,9], the quartz crystal microbalance (QCM) [10], spectrofluorimetry [11], enzyme-linked immunosorbent assays [12], and electroanalytical techniques [13,14,15]. Due to the similarities of naphthol isomers and interference from the complex biochemical environment of urine, the above methods all require a lot of time and effort for sample pretreatment or complex data analysis, making these methods difficult to put into practical application. With the rapid development of microsensors and nanomaterials, microelectrochemical sensors have become a powerful and potential detection method due to their fast response, high sensitivity, low cost, and simple operation [16,17]. Naphthol can be oxidized on electrodes to produce an electrical signal, so the use of electrochemical sensors for naphthol detection has attracted much interest in recent years. However, there are two main difficulties in using electrochemical methods for the detection of naphthol. Firstly, naphthol as a monomer can be electropolymerized on the electrode interface to form non-conductive polynaphthol, which affects the reusability of the electrode. More importantly, for the two naphthol isomers, some electrochemical tests are not able to distinguish their oxidation peaks, making it difficult to achieve simultaneous detection.
Some nanomaterials were used to modify the electrode for the direct electrochemical oxidation of naphthol isomers. Graphene has the merits of a large surface area, good stability, and high conductivity and is a widely used electrode modification material. For example, graphene-based nanomaterials have been used for the detection of single naphthol isomers [18,19] and the simultaneous detection of both isomers [20,21] due to their π-electron system and excellent conductivity. However, graphene tends to aggregate due to the contact resistance and van der Waals force between layers, which reduces the surface area and conductivity [22]. In recent years, three-dimensional graphene networks (3DGNs) have attracted more and more attention due to their special nano-porous morphology and other properties. A 3DGN not only has all the advantages of graphene but also has good mechanical strength and a large pore volume. This special merit can effectively avoid graphene layer aggregation or the restacking of graphene layers, which can promote electrolyte diffusion and analyte entry into the active site at the sensing interface [23,24].
In this work, as illustrated in Figure 1, modified sensing electrodes were fabricated by in situ growth of 3DGN in the working electrode region of a microsensor using the laser method. Based on the catalytic properties of sensitive graphene nanomaterials on naphthol molecules and the synergistic effect of π-π conjugation, a new method for the detection of 1-NAP and 2-NAP in complex biological sample environments using an electrochemical sensor chip was established. In order to ensure that true simultaneous test conditions are achieved and to guarantee the reliability of the test method, calibration curves were obtained by fixing the concentration of one isomer and varying the concentration of the other monomer. Our results demonstrate that the chip-based electrochemical microsensor can clearly separate the oxidation peaks of the naphthol isomers and has high sensitivity, good reproducibility, and strong selectivity for the detection of naphthol isomers in urine samples. The disposable sensor chip can avoid interface fouling from the naphthol monomer deposition.
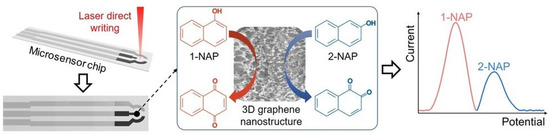
Figure 1.
Schematic showing the simultaneous detection of naphthol isomers with a 3D-graphene-nanostructure-fabricated microsensor.
2. Materials and Methods
2.1. Materials
The 1-NAP standard was purchased from Macklin; 2-NAP standard, urea, and uric acid were purchased from Sangon Biotech (Shanghai, China); the sodium hydroxide was purchased from Aladdin (Shanghai, China); and the hydrochloric acid, calcium chloride, potassium carbonate, and sodium sulfate were purchased from Shanghai Ling Feng Chemical Reagent Corp. (Shanghai, China). The urine sample (synthetic urine) was purchased from Phygene Biotechnology Corp. (Fuzhou, China). All chemicals were of analytical reagent grade and used without further purification. Deionized water was used for all solution preparations.
2.2. In Situ Growth of 3DGN on Electrodes of Microsensors
In this work, a three-electrode sensor chip was fabricated in two steps. As shown in Figure 2, firstly, a carbon counter electrode, a silver–silver chloride reference electrode, and silver wires were screen-printed onto a polyimide (PI) substrate with a thickness of 175 μm. The laser writing method was then used to synthesize the 3DGN to be the working electrode, using PI as the carbon source. The laser writing process was carried out using a Trotec Speedy 100R laser engraver with a scanning speed of 20 cm/s and a power of 7.5 W.
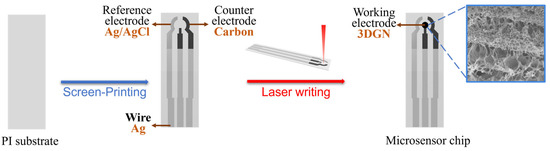
Figure 2.
The fabrication process of the microsensor chip.
2.3. 3DGN Nanostructure Characterization
Raman spectroscopy was performed using a Renishaw inVia Reflex Raman system with a laser wavelength of 514 nm. Scanning electron microscopy (SEM) characterization was performed with a Hitachi S4800 scanning electron microscope (Tokyo, Japan). Transmission electron microscopy (TEM) investigations were performed with a FEI Tecnai G20 transmission electron microscope (Hillsboro, OR, USA) at 200 kV.
2.4. 3DGN Surface Area Determination
The specific surface area was measured using the method based on UV-Vis absorption with methylene blue (MB) dye as the probes [25]. The 3DGN was in situ synthesized on a PI substrate with laser direct-writing technology in a square pattern (1 mm × 1 mm); then, the 3DGN was carefully collected with a scalpel. Next, 0.5 mg/mL of the synthesized 3DGN was mixed with 1 mg/mL of MB in DI water. After gentle stirring for 24 h, the 3DGN was removed by centrifugation (12,000 rpm, 20 min). The MB concentrations in water before and after adsorption were determined by UV–vis measurements at 445 nm (Jasco V-670 spectrophotometer, Tokyo, Japan). Then, the adsorbed MB amount could be calculated from the UV–vis absorbance difference before and after the stirring (mg/g of material).
2.5. 3DGN Electrochemistry Characterization
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) detection were carried out at a CHI 660E electrochemical workstation (Shanghai, China). The scan range for CV was from 0 to 0.8 V, and the scan rate was 20 mV/s. In contrast to CV, DPV shows better sensitivity and resolution for the simultaneous detection of naphthol isomers, so the experimental condition optimization and sensor performance were investigated with DPV. The DPV curves were obtained with the following parameters: an amplitude of 0.05 V, a pause width of 0.06 s, a sampling width of 0.02 s, and a pulse period of 0.5 s. The baseline correction for DPV was carried out by measuring a control sample. All the electrochemical characterizations and measurements were carried out with a relative humidity of 75%.
2.6. Naphthol Isomer Electrochemical Detection
The standard stock solution of the naphthol isomers (10 μM) was prepared with phosphate-buffered saline (PBS) for the electrochemical experiments. The solutions of the isomers with different pH values were prepared from 10 mM sodium phosphate salt (pH 7). The pH values ranging from 5 to 8 were adjusted with 1 M NaOH and 1 M HCl solutions monitored by a digital acidimeter model Phs-25 (Shanghai Leici, Shanghai, China). A series of isomer solutions with different concentrations ranging from 1 nM to 10 μM were diluted from the stock solution. For each characterization or test, 10 µL of each sample was applied to the working electrode for CV or DPV. The sensor was used only once to avoid surface deposition of the naphthol isomers, and triplicate tests were performed for each condition.
2.7. Real Sample Analysis
The synthetic urine (pH 4.7) used for the actual sample test was adjusted to pH 6.0 with 0.1 M NaOH and 0.1 M HCl. Then, the stock solutions of the naphthol isomer mixtures were spiked with synthetic urine, and 10 μL of each sample was dropped onto the detection area of a sensor chip directly, without any pretreatments. A DPV test was carried out to measure the oxidation peak current of each sample, and triplicate tests were performed for each concentration.
3. Results
3.1. Characterization of the 3DGN-Functionalized Microsensor
The surface area of the in situ grown 3DGN material was determined via the methylene blue adsorption method. According to previous reports [26,27], the measured surface covered per mg of adsorbed MB is 2.63 m2. Using this method, the surface area of 3DGN was measured to be 464.63 m2/g, which is in good agreements with previous studies [28]. Compared with graphene, the porous nanostructure provides a larger surface area, which means the 3DGN can adsorb more naphthol isomers and can facilitate electrochemical catalysis.
As shown in Figure 3a, the in situ prepared 3DGN nanostructure was characterized by Raman spectra. The D band at 1350 cm−1 and G band at 1580 cm−1 were attributed to the sp2 and sp3 hybridized carbon atoms on the graphene structure, respectively [29]. The 2D band at 2670 cm−1 provides evidence of the 2D structure of graphene [30,31]. The SEM and TEM characterization images in Figure 3b,c further reveal the 3D porous network morphology of the in situ synthesized working electrode. As shown in Figure 3d, a lattice fringe spacing of 0.34 nm can be obtained from the high-resolution TEM (HRTEM), which indicates the inter-planar distance of the (002) crystal plane of graphite, suggesting the successful growth of multi-layered graphene. Moreover, the few-layer (from 3 to 10 layers) structure in Figure 3d also shows that the in situ prepared 3DGN are graphene-based nanocomposites, but not graphite films. The porous nanostructure with good mechanical strength can effectively avoid graphene restacking or aggregation, and the huge surface area helps to accumulate more naphthol isomers, providing a promoting catalytic nanomaterial for electrochemical detection.
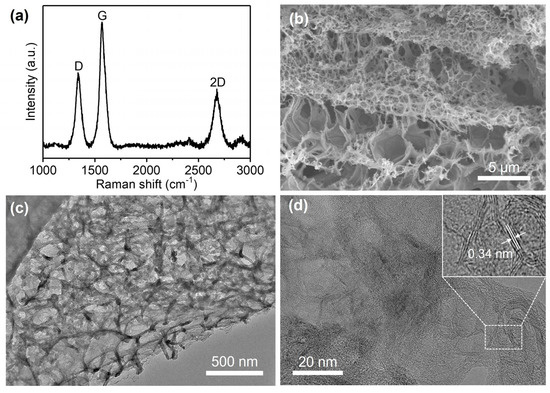
Figure 3.
Characterization of the in situ synthesized 3DGN as working electrode: (a) Raman spectra demonstrate the structure of graphene; (b) SEM and (c) TEM images show the porous nanostructure of 3DGN; (d) HRTEM image reveals the few-layer nanostructure and lattice fringe spacing of 3DGN.
3.2. Electrochemical Characterization of Naphthol Isomers on the Microsensors
As shown in Figure 4a, the electrochemical behaviors of 1-NAP and 2-NAP were investigated by CV with a scan rate of 20 mV/s, and both naphthol isomers can be oxidized on the 3DGN-modified sensor. The same test was carried out for the naphthol isomer mixture, which generated two independent oxidation peaks at 0.23 V and 0.43 V. The obtained CV curve shapes are roughly consistent with those of the individual 1-NAP and 2-NAP peaks. The CV results indicated that no additional electrochemical reaction occurred with the mixture. The potential difference between the oxidation peaks was approximately 200 mV, which is sufficient to quantify both naphthol isomers simultaneously. Moreover, there is no reduction peak for individual naphthol isomers or the mixture, indicating that electrochemical oxidation is an irreversible process.
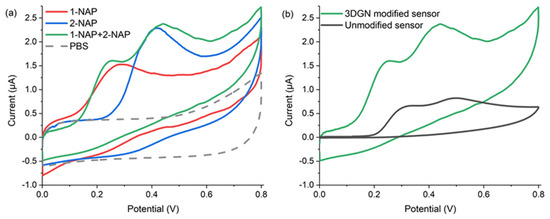
Figure 4.
CV measurements of (a) 50 µM 1-NAP, 50 µM 2-NAP, the mixture of 50 µM 1-NAP and 50 µM 2-NAP, and PBS with the 3DGN-modified sensor; (b) 50 µM naphthol isomer mixture with the 3DGN-modified sensor and unmodified one.
Figure 4b shows the electrochemical oxidation behavior of the naphthol isomers on 3DGN-modified and carbon-ink-printed electrodes. Compared with the unmodified electrode, the peaks obtained from the 3DGN-functionalized microsensor were more clearly separated, and the peak currents were also significantly increased. Therefore, the 3DGN-functionalized microsensors have good electrocatalytic activity and electrochemical oxidation performance for 1-NAP and 2-NAP. The good electrochemical sensing performance can be attributed to the in situ grown 3DGN providing more catalytic sites and larger surface area and facilitating and accelerating electron transfer at the electrode interface. On the other hand, the large π-conjugated structure possessed by 3DGN materials can enrich naphthol isomers through π-π conjugation aggregation [18].
3.3. Study on the Electrochemical Response of Naphthol Isomers as a Function of pH
The pH value of the test solution affects the electron transfer and redox reactions in the electrocatalytic oxidation process. The effect of pH on the oxidation potential and current of the naphthol isomers was studied by DPV with 50 μM 1-NAP or 2-NAP, respectively. As shown in Figure 5, in the pH range from 5.0 to 9.0, the oxidation potential of both isomers shifted to lower values with increasing pH. This phenomenon indicates that protons accompany the electron transfer process, which means that protons were directly involved in the oxidation of both naphthol isomers in the chosen pH range [13,32]. The maximum oxidation currents were obtained at pH 6.0 for both isomers; thus, considering the sensitivity of the detection of naphthol isomers, a pH of 6.0 was optimal for the following experiments.
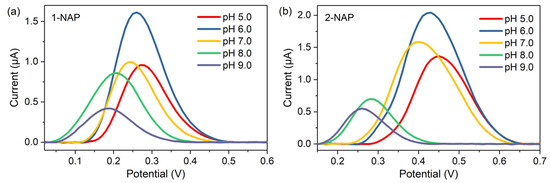
Figure 5.
DPV responses of the microsensor to (a) 1-NAP and (b) 2-NAP solutions with various pH values. The 1-NAP and 2-NAP molecules were at an identical concentration of 50 µM.
3.4. Selectivity to the Naphthol Isomers
In this work, the selectivity of the sensor was also investigated. Referring to the real target sample, the main possible interfering substances in the urine were tested on the sensor with a concentration of 10 mM at pH 6.0, and the obtained responses were compared with that of the 20 μM target naphthol isomer. To directly demonstrate the detection of naphthol isomers with the interfering substances in urine, the DPV curves obtained from the interfering substances are shown in Figure 6. The results from the selectivity investigation indicated that the sensor has no obvious electrochemical response to inorganic ions such as K+, Na+, Ca2+, Cl−, CO32−, and SO42− but has a weak electrochemical response to urea and uric acid (UA), the main organic substances in urine. However, considering that the concentration difference between the interfering substances and the target naphthol isomer is 500-fold, this influence is negligible. The results in Figure 6 demonstrated that the sensor has a good selectivity for detecting both naphthol isomers. In addition, the influence of the isomer can be obtained in Figure 6.
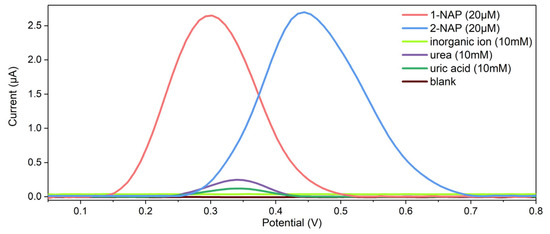
Figure 6.
The selectivity investigation on the 3DGN-modified sensor with the coexisting substances in real samples for 1-NAP and 2-NAP.
3.5. Detection of 1-NAP and 2-NAP with the Microsensor
The solutions of different concentrations of 1-NAP and 2-NAP were measured using the microsensor at pH 6.0. The DPV measurements of various concentrations of 1-NAP are shown in Figure 7a. In the dynamic range from 1 nM to 10 µM, the maximal currents of 1-NAP obtained at 0.26 V increased linearly with the concentrations, which can be expressed in the following equations:
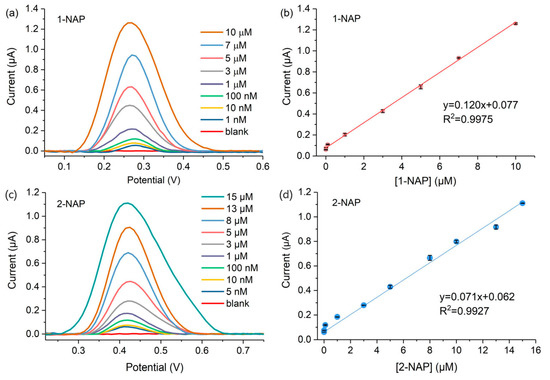
Figure 7.
(a) DPV responses of the microsensor to 1-NAP molecules with concentrations in the range of 1 nM to 10 µM; (b) linear relationship between the DPV responses and 1-NAP concentrations; (c) DPV responses of the microsensor to the 2-NAP molecules; (d) linear relationship between the DPV responses and 1-NAP concentrations.
Obviously, the observed limit of detection (LOD) of the microsensor for 1-NAP is lower than 1 nM. As for 2-NAP, the microsensors also output a linear response (maximum current obtained at 0.43 V) with the increased 2-NAP concentrations, and the LOD is about 5 nM. The linear relationship between the sensing response and the 2-NAP concentrations can be fitted as follows:
3.6. Simultaneous Determination of Naphthol Isomers with the Microsensor
The sensors were used for the simultaneous quantitative determination of naphthol isomers under optimized experimental conditions. In the mixed solution in which two naphthol isomers exist, a calibration curve was obtained by changing the concentration of the target naphthol molecule while fixing the concentration of its isomer at 5 µM. As shown in Figure 8a, the oxidation current obtained from one naphthol isomer does not interfere with that of the other isomer, and the oxidation peaks of 1-NAP and 2-NAP were separated, indicating that the sensor can detect naphthol isomers simultaneously. Figure 8b plotted the electrochemical response increases with the 1-NAP concentrations in the dynamic range from 10 nM to 10 µM. Figure 8a,b also indicated that the experimentally measured LOD of the sensor for 1-NAP was 10 nM. The linear relationship between the concentration of 1-NAP and the peak current value can be expressed as follows:
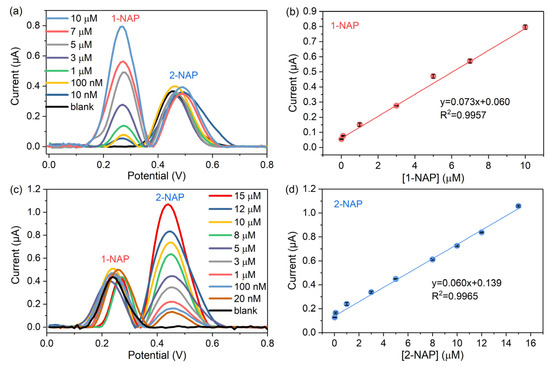
Figure 8.
DPV responses on the sensor (a) in the 1-NAP concentration range from 10 nM to 10 µM in the presence of 5 µM 2-NAP; (b) in the 2-NAP concentration range from 20 nM to 15 µM in the presence of 5 µM 1-NAP; (c) calibration curve of 1-NAP in the isomer mixture; (d) calibration curve of 2-NAP in the isomer mixture.
Similarly, Figure 8c,d show the simultaneous test results of the sensor for 2-NAP molecules co-existing with 5 μM 1-NAP. The sensor also exhibited a linear response to 2-NAP molecules in the concentration range from 20 nM to 15 µM, and its LOD was 20 nM. The linear relationship between the 2-NAP concentration and the peak current value was fitted as follows:
In the mixed solution, the detection performance of the sensor for naphthol molecules is influenced by the isomers, resulting in a slight decrease in the detection response. As summarized in Table 1, the 3DGN/SPE sensor has good sensitivity compared with other nanomaterials. The eletroreduced graphene oxide/glassy carbon electrodes (GCE) had a lower LOD, but considering the long electrochemical deposition and reducing process on GCE, the fabrication method for the 3DGN/SPE is more efficient.

Table 1.
Naphthol detection results in this work were compared with the results in the literature.
3.7. Reproducibility and Stability for the Detection of Naphthol Isomers
The reproducibility and stability of the proposed microsensor were also investigated in this work. Five randomly selected 3DGN-modified electrodes were used to evaluate the reproducibility by testing the 5 µM 1-NAP and 2-NAP mixtures. As shown in Figure 9a, the relative standard deviations (RSD) between the measured 1-NAP and 2-NAP peak current values were 1.33% and 4.18%, respectively, indicating that the electrode has good reproducibility.
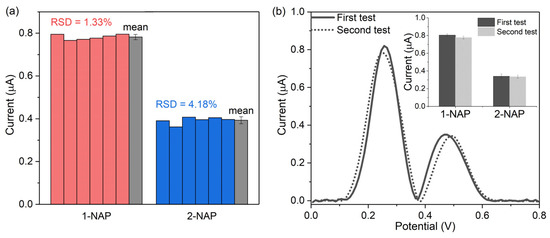
Figure 9.
(a) Reproducibility test from five parallel measurements from randomly chosen 3DGNs/SPE electrodes in 10 µM 1-NAP and 5 µM 2-NAP mixtures; (b) long-term stability of the sensor after 60 days in a mixture containing 10 µM 1-NAP and 5 µM 2-NAP.
Stability was assessed by measuring the two naphthol isomers simultaneously with the microsensor fabricated in the same batch for a duration of 60 days. Three freshly fabricated, randomly selected microsensors were used to measure the naphthol isomers, and the rest of the microsensors fabricated in the same batch were kept in a dry condition and used to conduct the same test after 60 days. As shown in Figure 9b, the curved shape obtained on day 60 is close to the initial one. The inset shows that the 1-NAP current obtained after 60 days was 96.6% of that from the first-time detection, and the 2-NAP current measured after two month was 98.5% of that on day 1, indicating that the sensor has good stability.
3.8. The Application of the Microsensor in the Detection of Naphthol Isomers in a Urine Sample
The microsensor was further exploited for the simultaneous quantitative detection of naphthol isomers in urine to evaluate its practical applicability. The pH value of the urine sample was adjusted to 6.0 before the addition of the naphthol isomers. With reference to the naphthol content in clinical samples, 1-NAP, 2-NAP, and the isomer mixture in desired amounts were added to the urine samples, respectively. The results are listed in Table 2, and the recovery for the analysis of samples spiked with 8 µM of 1-NAP or 2-NAP ranged from 101 to 105%. When the naphthol isomers were detected simultaneously, the recovery for the samples spiked with 4 µM 1-NAP, and 2-NAP ranged from 100 to 107%. The results demonstrate that the microsensor has great potential for the individual and simultaneous detection of naphthol isomers in real complex samples.

Table 2.
The detection of naphthol isomers in synthetic urine samples.
4. Conclusions
In this work, a three-dimensional graphene network (3DGN) was in situ grown on screen-printed electrodes to form electrochemical microsensors for the simultaneous detection of naphthol isomers. The synergistic effect of nanoporous graphene and the conjugation between graphene and naphthol molecules significantly enhanced the electrochemical activity towards 1-NAP and 2-NAP, thereby improving the sensitivity of naphthol detection. Under optimal detection conditions, the electrochemical detection of 1-NAP, 2-NAP, and their mixtures was successfully realized using the DPV method. For the detection of naphthol isomers, either individually or simultaneously, 1-NAP and 2-NAP showed a good linear relationship in wide detection ranges. The sensor has high sensitivity to naphthol, the LOD to 1-NAP reaches 10 nM, and the LOD to 2-NAP is about 20 nM. The sensor also has good selectivity, reproducibility, and stability for naphthol isomer detection. Finally, single and simultaneous quantitative detections of 1-NAP and 2-NAP were successfully achieved in synthetic urine samples. Compared with sensors that can only detect one of the isomers individually, the detection ability of the microsensor to simultaneously test naphthol isomers contributes to a simpler and more accurate understanding of the true content of naphthol isomers in urine. The electrochemical microsensor in this work provides a novel, rapid, simple, and low-cost method for detecting naphthol isomers, which is expected to be able to diagnose naphthol-exposed individuals in clinical practice in the future.
Author Contributions
Conceptualization, X.W. and M.L.; methodology, X.W., M.L. and Y.Z.; validation, J.S., W.S. and M.L.; resources, P.X., X.L. and Y.Z.; data curation, X.W., J.S. and W.S.; writing—original draft preparation, J.S., W.S. and X.W.; writing—review and editing, P.X., Y.Z., X.L. and X.W.; visualization, X.W. and J.S.; supervision, X.L.; funding acquisition, P.X. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2021YFB3200800), the National Natural Science Foundation of China (82171011, 61974155, 61874130, 61831021, 62171275, and 62104241), the Shanghai “Road and Belt” International Young Scientist Exchange Program (19510744600), the Shanghai Pujiang Program (20PJ1415600), and the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202002 and ZYYCXTD-D-202003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Niwa, S.-I.; Eswaramoorthy, M.; Nair, J.; Raj, A.; Itoh, N.; Shoji, H.; Namba, T.; Mizukami, F. A One-Step Conversion of Benzene to Phenol with a Palladium Membrane. Science 2002, 295, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry: Environmental impacts and remediation. Environ. Sci. Pollut. Res. 2019, 27, 3803–3818. [Google Scholar] [CrossRef]
- Croera, C.; Ferrario, D.; Gribaldo, L. In Vitro toxicity of naphthalene, 1-naphthol, 2-naphthol and 1,4-naphthoquinone on human CFU-GM from female and male cord blood donors. Toxicol. Vitr. 2008, 22, 1555–1561. [Google Scholar] [CrossRef]
- Sun, H.; Shen, O.-X.; Xu, X.-L.; Song, L.; Wang, X.-R. Carbaryl, 1-naphthol and 2-naphthol inhibit the beta-1 thyroid hormone receptor-mediated transcription in vitro. Toxicology 2008, 249, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Preuss, R.; Angerer, J.; Drexler, H. Naphthalene—An environmental and occupational toxicant. Int. Arch. Occup. Environ. Health 2003, 76, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Hardt, J.; Schulz, C.; Angerer, J. New reference value and the background exposure for the PAH metabolites 1-hydroxypyrene and 1- and 2-naphthol in urine of the general population in Germany: Basis for validation of human biomonitoring data in environmental medicine. Int. J. Hyg. Environ. Health 2008, 211, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Kishikawa, N.; Matayoshi, K.; Adutwum, L.A.; Wada, M.; Nakashima, K.; Kuroda, N. Sensitive determination of 1- and 2-naphthol in human plasma by HPLC-fluorescence detection with 4-(4,5-diphenyl-1H-imidazol-2-yl)benzoyl chloride as a labeling reagent. J. Sep. Sci. 2009, 32 13, 2218–2222. [Google Scholar] [CrossRef]
- Preuss, R.; Angerer, J. Simultaneous determination of 1- and 2-naphthol in human urine using on-line clean-up column-switching liquid chromatography–fluorescence detection. J. Chromatogr. B 2004, 801, 307–316. [Google Scholar] [CrossRef]
- Lim, H.-H.; Shin, H.-S. Simultaneous determination of 2-naphthol and 1-hydroxypyrene in fish and shellfish contaminated with crude oil by gas chromatography–mass spectrometry. Food Chem. 2013, 138, 791–796. [Google Scholar] [CrossRef]
- Yuan, Y.-K.; Xiao, X.-L.; Wang, Y.-S.; Xue, J.-H.; Li, G.-R.; Kang, R.-H.; Zhang, J.-Q.; Shi, L.-F. Quartz crystal microbalance with β-cyclodextrin/TiO2 composite films coupled with chemometrics for the simultaneous determination of urinary 1- and 2-naphthol. Sens. Actuators B Chem. 2010, 145, 348–354. [Google Scholar] [CrossRef]
- Jia, G.; Li, L.; Qiu, J.; Wang, X.; Zhu, W.; Sun, Y.; Zhou, Z. Determination of carbaryl and its metabolite 1-naphthol in water samples by fluorescence spectrophotometer after anionic surfactant micelle-mediated extraction with sodium dodecylsulfate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.M.; Marco, M.P.; Hammock, B.D. Development of a selective enzyme-linked immunosorbent assay for 1-naphthol - The major metabolite of carbaryl (1-naphthyl N-methylcarbamate). J. Agric. Food Chem. 1994, 42, 934–943. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, P.; Zhan, T.; Yu, X.; Wen, Y.; Liu, X.; Gao, H.; Wang, P.; She, X. In situ growth of ZIF-67 on ultrathin CoAl layered double hydroxide nanosheets for electrochemical sensing toward naphthol isomers. J. Colloid Interface Sci. 2020, 576, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mei, Q.; Ding, Y.; Guo, K.; Yang, X.; Zhao, J. Ordered Mesoporous NiCo2O4 Nanospheres as a Novel Electrocatalyst Platform for 1-Naphthol and 2-Naphthol Individual Sensing Application. ACS Appl. Mater. Interfaces 2017, 9, 29771–29781. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, L.; Tang, Y.; Qin, Q.; Wu, C. Nitrogen and phosphorus co-doped porous carbon framework with superior electrochemical activity for naphthol isomers sensing. Anal. Chim. Acta 2020, 1138, 158–167. [Google Scholar] [CrossRef]
- Smutok, O.; Katz, E. Biosensors: Electrochemical Devices—General Concepts and Performance. Biosensors 2022, 13, 44. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 74–83. [Google Scholar] [CrossRef]
- Pang, Y.H.; Huang, Y.Y.; Li, W.Y.; Yang, N.C.; Shen, X.F. Electrochemical Detection of Three Monohydroxylated Polycyclic Aromatic Hydrocarbons Using Electroreduced Graphene Oxide Modified Screen-printed Electrode. Electroanalysis 2020, 32, 1459–1467. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Feng, H.; Zhang, Y.; Jiang, J.; Feng, Y.; Chen, M.; Qian, D. A facile one-step in situ synthesis of copper nanostructures/graphene oxide as an efficient electrocatalyst for 2-naphthol sensing application. Electrochim. Acta 2015, 153, 352–360. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, Y.; Li, W.; Ding, H.; Shen, X. Synergetic accumulation and simultaneous determination of naphthol isomers on electrochemically reduced graphene oxide modified electrode. J. Electroanal. Chem. 2016, 769, 89–96. [Google Scholar] [CrossRef]
- Zhu, G.; Gai, P.; Wu, L.; Zhang, J.; Zhang, X.; Chen, J. β-Cyclodextrin-Platinum Nanoparticles/Graphene Nanohybrids: Enhanced Sensitivity for Electrochemical Detection of Naphthol Isomers. Chem. Asian J. 2012, 7, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Z.; Huang, X.; Wang, Y.; Huang, Y.; Duan, X. Functionalized Graphene Hydrogel-Based High-Performance Supercapacitors. Adv. Mater. 2013, 25, 5779–5784. [Google Scholar] [CrossRef] [PubMed]
- Schaper, A.K.; Wang, M.S.; Xu, Z.; Bando, Y.; Golberg, D. Comparative Studies on the Electrical and Mechanical Behavior of Catalytically Grown Multiwalled Carbon Nanotubes and Scrolled Graphene. Nano Lett. 2011, 11, 3295–3300. [Google Scholar] [CrossRef]
- Yan, M.; Wang, F.; Han, C.; Ma, X.; Xu, X.; An, Q.; Xu, L.; Niu, C.; Zhao, Y.; Tian, X.; et al. Nanowire Templated Semihollow Bicontinuous Graphene Scrolls: Designed Construction, Mechanism, and Enhanced Energy Storage Performance. J. Am. Chem. Soc. 2013, 135, 18176–18182. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Kuzenkova, A.S.; Iakunkov, A.; Romanchuk, A.Y.; Trigub, A.L.; Egorov, A.V.; Bauters, S.; Amidani, L.; Retegan, M.; Kvashnina, K.O.; et al. Enhanced Sorption of Radionuclides by Defect-Rich Graphene Oxide. ACS Appl. Mater. Interfaces 2020, 12, 45122–45135. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaría, R.; Menéndez, R.; Corma, A.; García, H. Surface Area Measurement of Graphene Oxide in Aqueous Solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene Materials: From Understanding to Design and Synthesis Control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, M.; Yang, L.; Tan, Y.; Deng, W.; Ma, M.; Su, X.; Xie, Q. Three-dimensional graphene networks as a new substrate for immobilization of laccase and dopamine and its application in glucose/O2 biofuel cell. ACS Appl. Mater. Interfaces 2014, 6, 12808–12814. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Jiang, J.; Gao, C.W.; Nan, H.Y.; Ni, Z.H.; Wang, D.; Zhong, B.; Wen, G.W. Raman spectroscopy study of twisted tetralayer graphene. J. Raman Spectrosc. 2016, 47, 668–673. [Google Scholar] [CrossRef]
- Ranjan, P.; Tulika; Laha, R.; Balakrishnan, J. Au concentration-dependent quenching of Raman 2D peak in graphene. J. Raman Spectrosc. 2017, 48, 586–591. [Google Scholar] [CrossRef]
- Zhan, T.; Song, Y.; Tan, Z.; Hou, W. Electrochemical bisphenol A sensor based on exfoliated Ni2Al-layered double hydroxide nanosheets modified electrode. Sens. Actuators B Chem. 2017, 238, 962–971. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Silva, T.A.; Lourencao, B.C.; Fatibello-Filho, O.; Rocha-Filho, R.C. Use of a boron-doped diamond electrode to assess the electrochemical response of the naphthol isomers and to attain their truly simultaneous electroanalytical determination. Electrochim. Acta 2017, 243, 374–381. [Google Scholar] [CrossRef]
- Li, L.; Liu, E.; Wang, X.; Chen, J.; Zhang, X. Simultaneous determination of naphthol isomers at poly(3-methylthiophene)-nano-Au modified electrode with the enhancement of surfactant. Mater. Sci. Eng. C 2015, 53, 36–42. [Google Scholar] [CrossRef]
- Peng, L.; Dong, S.; An, Y.; Qu, M. Controllable generation of ZnO/ZnCo2O4 arising from bimetal–organic frameworks for electrochemical detection of naphthol isomers. Analyst 2021, 146, 3352–3360. [Google Scholar] [CrossRef]
- Li, K.; Kang, J.; Zhan, T.; Cao, W.; Liu, X.; Gao, H.; Si, C.; She, X. Electrochemical sensing platform for naphthol isomers based on in situ growth of ZIF-8 on reduced graphene oxide by a reaction-diffusion technique. J. Colloid Interface Sci. 2021, 581, 576–585. [Google Scholar] [CrossRef]
- Zhu, G.; Gai, P.; Yang, Y.; Zhang, X.; Chen, J. Electrochemical sensor for naphthols based on gold nanoparticles/hollow nitrogen-doped carbon microsphere hybrids functionalized with SH-β-cyclodextrin. Anal. Chim. Acta 2012, 723, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).