Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness
Abstract
1. Introduction
2. Materials and Methods
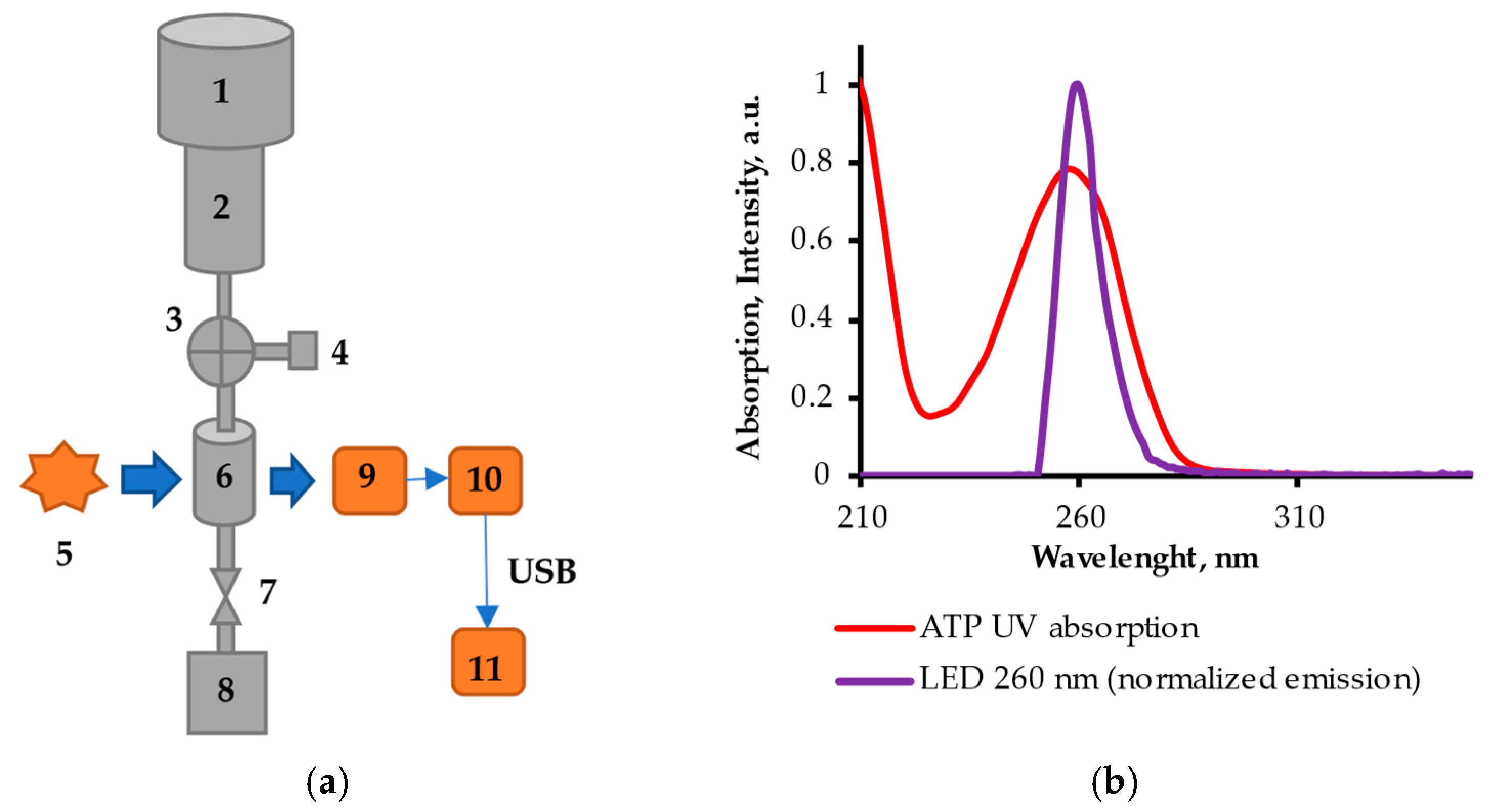
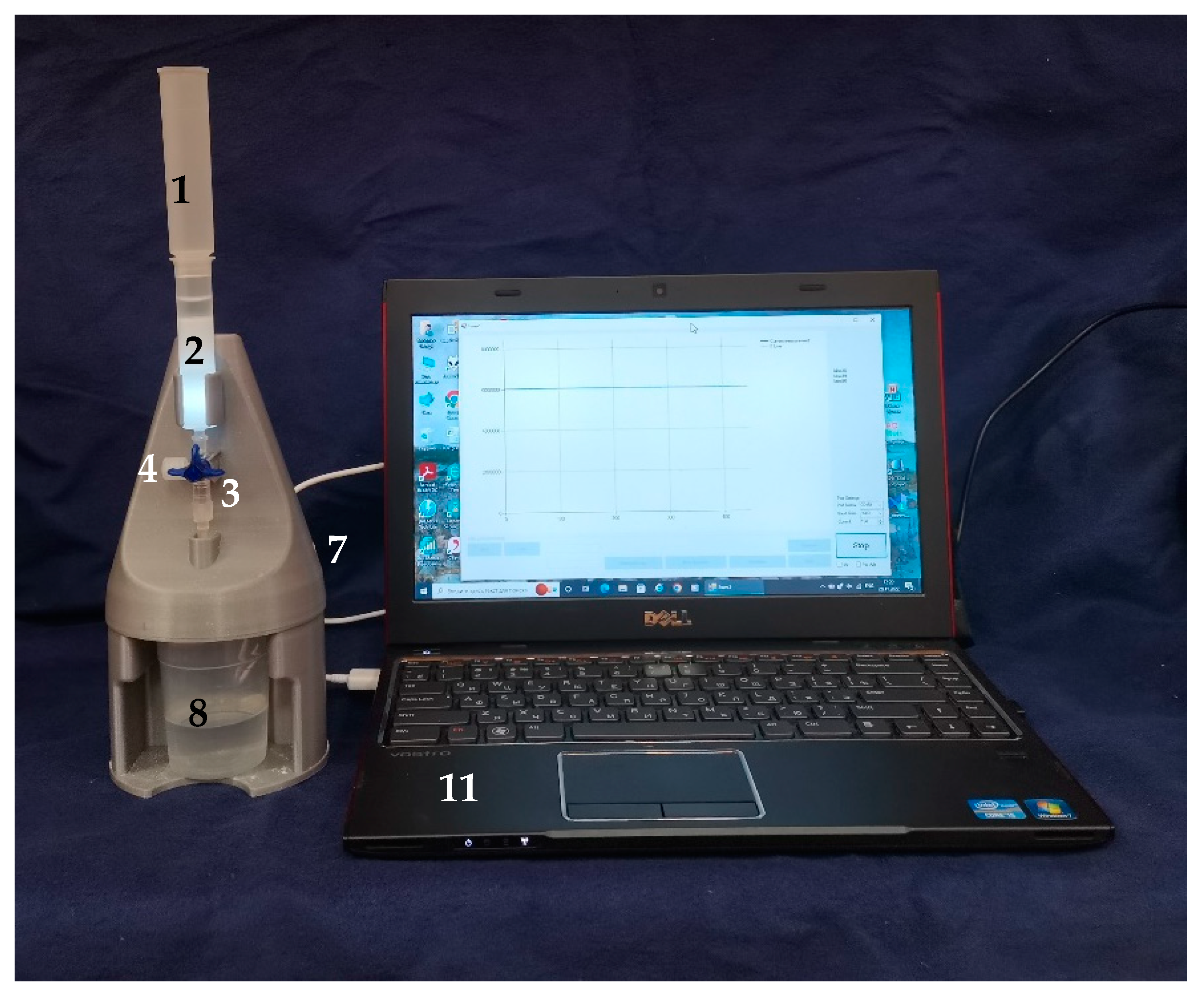
2.1. FPLMC Optical Chemical Sensor
2.2. Chemicals
2.3. Sample Preparation
2.4. pH Determination
2.5. FPMLC Measurement Protocol and New Freshness Index H*
2.6. Validation Methods
2.6.1. LC-DAD MS
2.6.2. NMR Spectroscopy
2.6.3. TVB-N and VFA Determination
3. Results
3.1. FPLMC Sensor Calibration
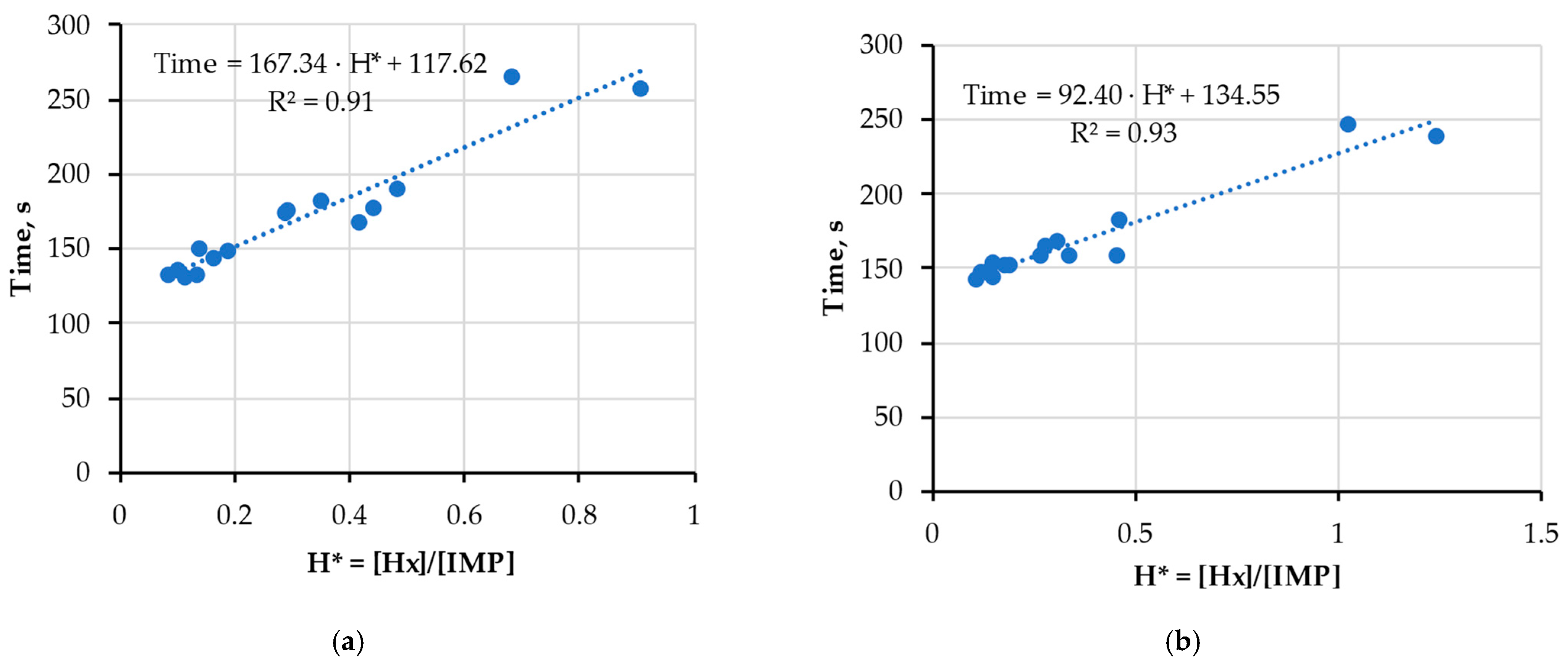
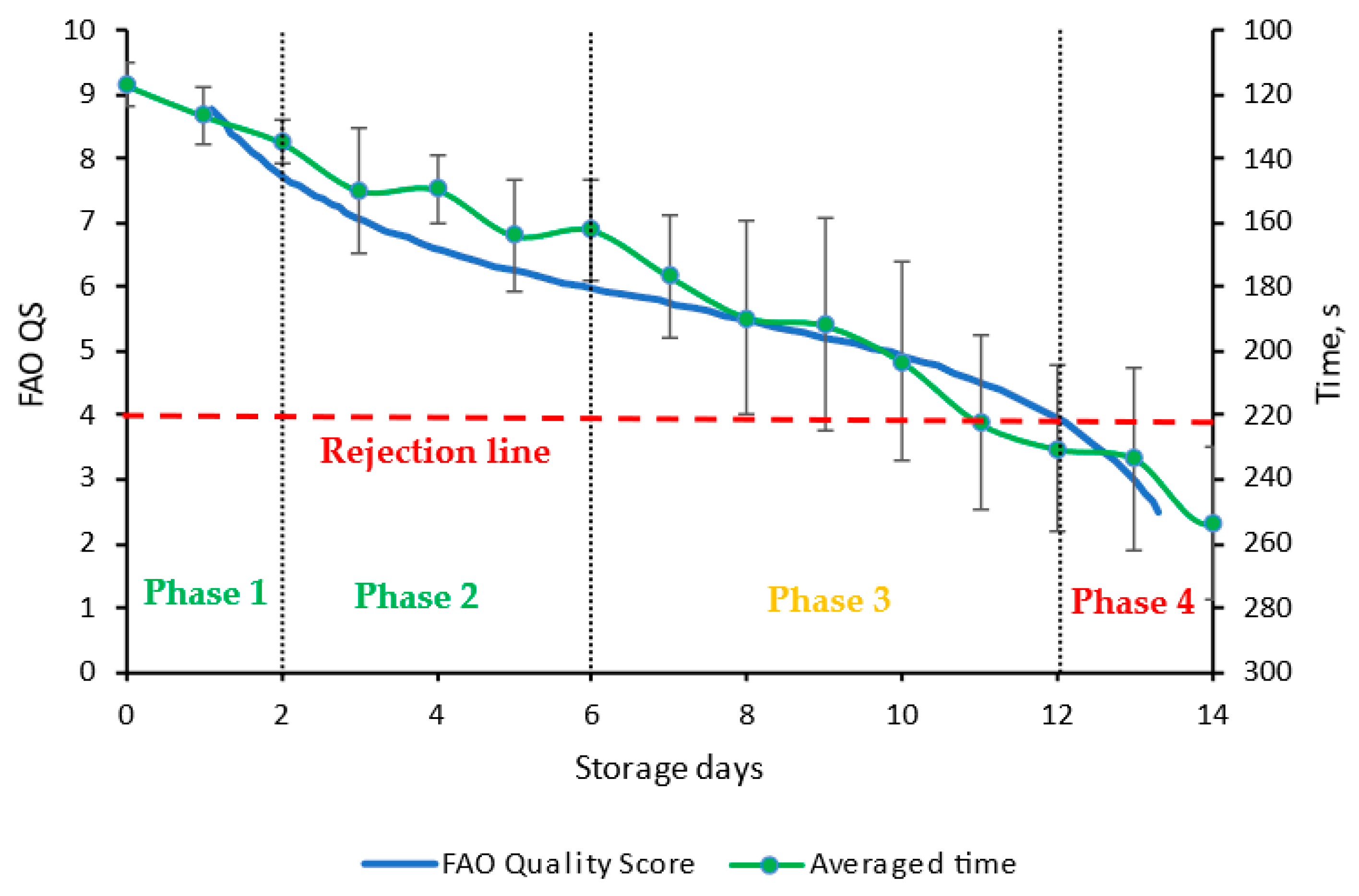
3.2. Verification of Compatibility of New Index H* and FPMLC Data
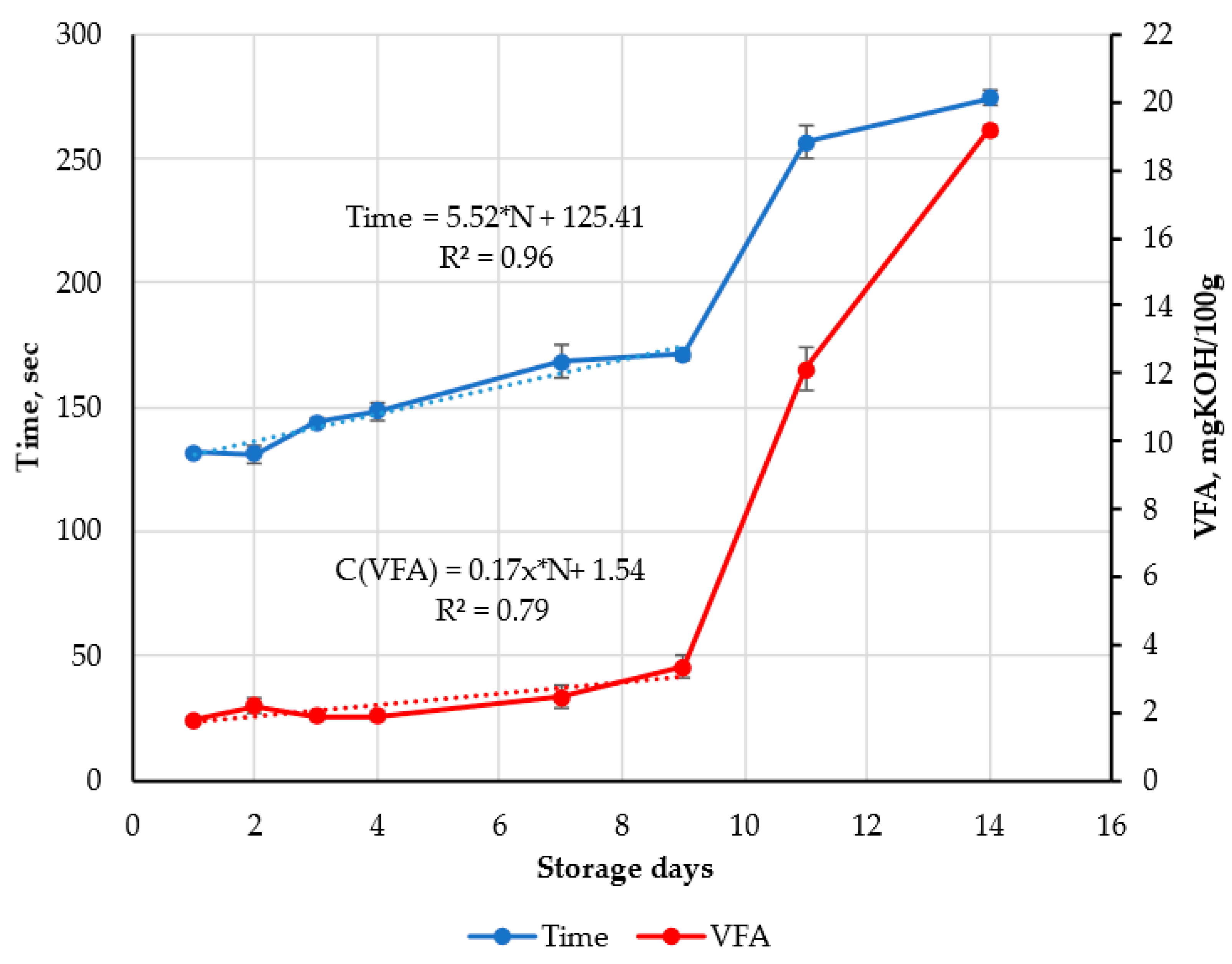
3.3. Results of Validation of FPMLC Data by VFA and TVB-N Methods
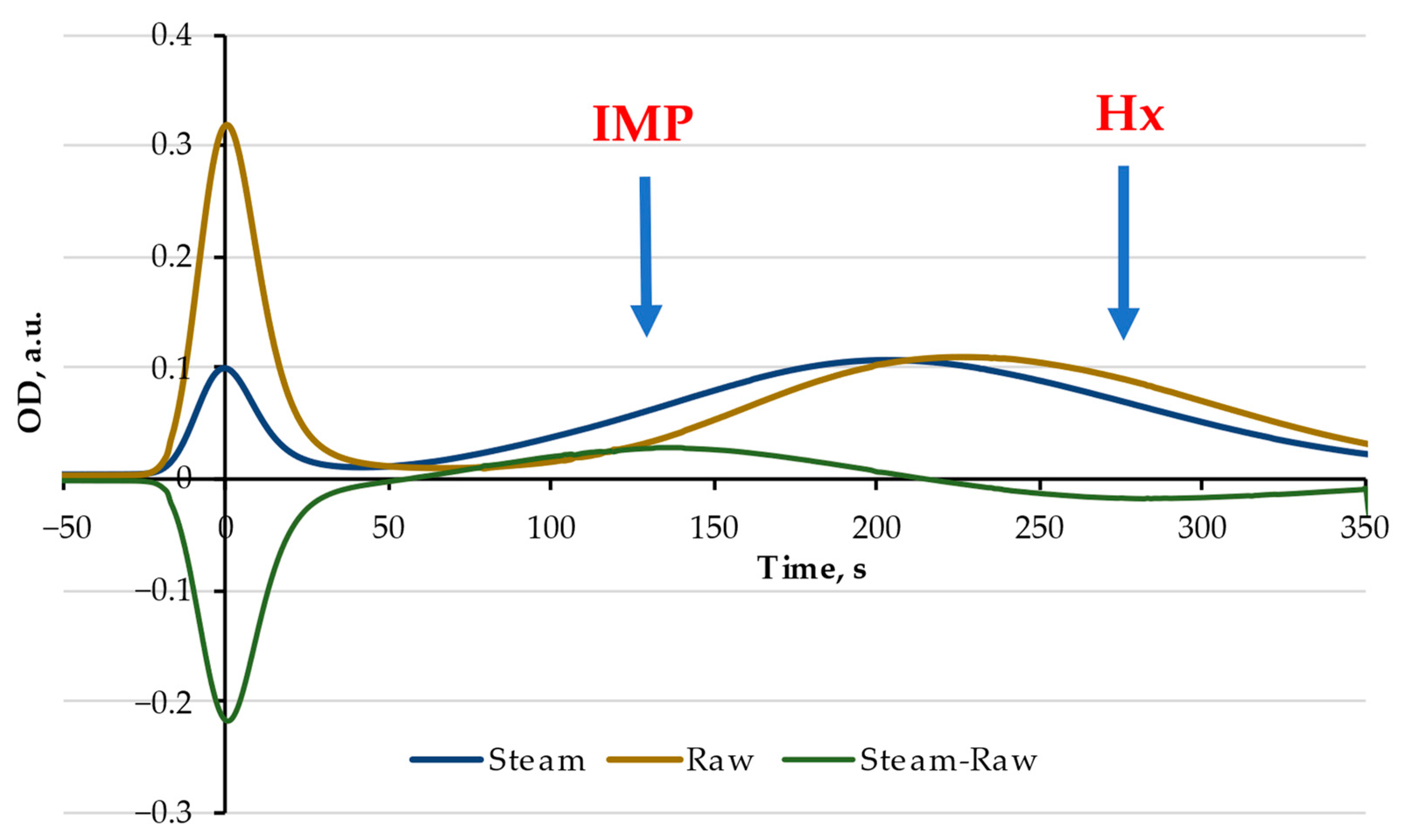
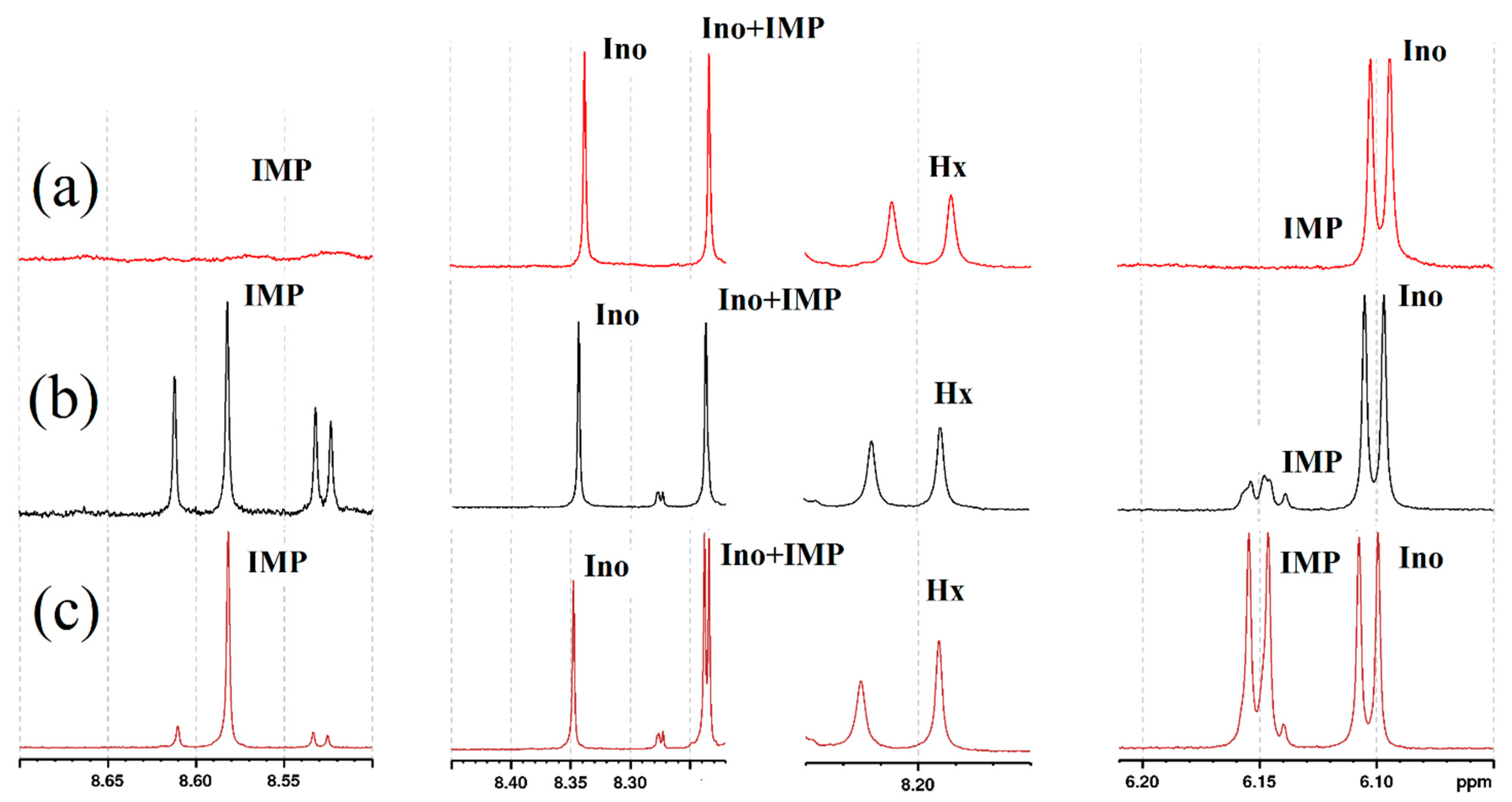
3.4. Effects of Heat Treatment and Putative Nucleotide Salvage
4. Discussion
5. Concluding Remarks
- Quickest determination of fish freshness available until now,
- Can be used in labs as well as in retail chains,
- Environment friendly,
- Low cost and reliable,
- Less qualified staff can also work,
- Non-destructive and less time consuming, and
- Quickly determine shelf life of almost all varieties of fish.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richey, J. FPLC: A comprehensive separation technique for biopolymers. Am. Lab. 1982, 14, 104–129. [Google Scholar]
- Tangvarasittichai, S.; Tangvarasittichai, O.; Jermnim, N. Comparison of fast protein liquid chromatography (FPLC) with HPLC, electrophoresis & microcolumn chromatography techniques for the diagnosis of beta-thalassaemia. Indian J. Med. Res. 2009, 129, 242–248. [Google Scholar] [PubMed]
- Verbeke, K.; Verbruggen, A. Usefulness of fast protein liquid chromatography as an alternative to high performance liquid chromatography of 99mTc-labelled human serum albumin preparations. J. Pharm. Biomed. Anal. 1996, 14, 1209–1213. [Google Scholar] [CrossRef]
- Madadlou, A.; O’Sullivan, S.; Sheehan, D. Fast Protein Liquid Chromatography. Methods Mol. Biol. 2017, 1485, 365–373. [Google Scholar] [CrossRef]
- Deori, S.; Hurri, E.; Karkehabadi, S.; Morrell, J.M. Fast protein liquid chromatography profiles of seminal plasma proteins in young bulls: A biomarker of sperm maturity? Livest. Sci. 2021, 250, 104600. [Google Scholar] [CrossRef]
- Sünter, A.; Frorip, A.; Korsakov, V.; Kurruk, R.; Kuznetsov, A.; Ots-Rosenberg, M. Optical method for screening and a new proteinuria focus group. J. Biomed. Photon. Eng. 2015, 1, 236–247. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Frorip, A.; Sünter, A.; Korsakov, V.; Konoplev, G.; Stepanova, O.; Roschina, N.; Ovsyannikov, N.; Lialin, D.; Gerasimchuk, R.; et al. Optical Chemical Sensor Based on Fast-Protein Liquid Chromatography for Regular Peritoneal Protein Loss Assessment in End-Stage Renal Disease Patients on Continuous Ambulatory Peritoneal Dialysis. Chemosensors 2022, 10, 232. [Google Scholar] [CrossRef]
- Noonin, C.; Kapincharanon, C.; Sueksakit, K.; Kanlaya, R.; Thongboonkerd, V. Application of tandem fast protein liquid chromatography to purify intact native monomeric/aggregated Tamm-Horsfall protein from human urine and systematic comparisons with diatomaceous earth adsorption and salt precipitation: Yield, purity and time-consumption. Anal. Methods 2021, 13, 3359–3367. [Google Scholar] [CrossRef]
- Tsay, F.-R.; Haidar Ahmad, I.A.; Henderson, D.; Schiavone, N.; Liu, Z.; Makarov, A.A.; Mangion, I.; Regalado, E.L. Generic anion-exchange chromatography method for analytical and preparative separation of nucleotides in the development and manufacture of drug substances. J. Chromatogr. A 2019, 1587, 129–135. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Howgate, P. A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage. Int. J. Food Sci. Technol. 2006, 41, 341–353. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Umamification of food facilitates the green transition. Soil Ecol. Lett. 2022, 5, 9. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Sang, S.; Jia, L.; Ou, C. Emerging Approach for Fish Freshness Evaluation: Principle, Application and Challenges. Foods 2022, 11, 1897. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Arai, K.-I.; Matsuyoshi, M. A New Method for Estimating the Freshness of Fish. Nippon Suisan Gakkaishi 1959, 24, 749–750. [Google Scholar] [CrossRef]
- García, M.R.; Ferez-Rubio, J.A.; Vilas, C. Assessment and Prediction of Fish Freshness Using Mathematical Modelling: A Review. Foods 2022, 11, 2312. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Hori, Y.; Otani, T.; Kunita, Y.; Sawa, S.; Sakai, S.; Tanaka, Y.; Takagahara, I.; Nakatani, Y. Applicability of the K0 Value as an Index of Freshness for Porcine and Chicken Muscles. Agric. Biol. Chem. 1988, 52, 107–112. [Google Scholar] [CrossRef]
- Batlle, N.; Aristoy, M.-C.; Toldrá, F. ATP Metabolites During Aging of Exudative and Nonexudative Pork Meats. J. Food Sci. 2001, 66, 68–71. [Google Scholar] [CrossRef]
- Nakatani, Y.; Fujita, T.; Sawa, S.; Otani, T.; Hori, Y.; Takagahara, I. Changes in ATP-Related Compounds of Beef and Rabbit Muscles and a New Index of Freshness of Muscle. Agric. Biol. Chem. 1986, 50, 1751–1856. [Google Scholar] [CrossRef]
- Nanda, P.K.; Bhattacharya, D.; Das, J.K.; Bandyopadhyay, S.; Ekhlas, D.; Lorenzo, J.M.; Dandapat, P.; Alessandroni, L.; Das, A.K.; Gagaoua, M. Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products. Chemosensors 2022, 10, 322. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat-A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Franceschelli, L.; Berardinelli, A.; Dabbou, S.; Ragni, L.; Tartagni, M. Sensing Technology for Fish Freshness and Safety: A Review. Sensors 2021, 21, 1373. [Google Scholar] [CrossRef] [PubMed]
- Sveinsdottir, K.; Martinsdottir, E.; Hyldig, G.; Jorgensen, B.; Kristbergsson, K. Application of Quality Index Method (QIM) Scheme in Shelf-life Study of Farmed Atlantic Salmon (Salmo salar). J. Food Sci. 2002, 67, 1570–1579. [Google Scholar] [CrossRef]
- Bakhchova, L.; Jantaree, P.; Gupta, A.; Isermann, B.; Steinmann, U.; Naumann, M. On-a-Chip-Based Sensitive Detection of Drug-Induced Apoptosis in Polarized Gastric Epithelial Cells. ACS Biomater. Sci. Eng. 2021, 7, 5474–5483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bei, J.; Guo, X.; Ding, Y.; Chen, T.; Lu, B.; Wang, Y.; Du, Y.; Yao, Y. Ultrasensitive photoelectrochemical immunosensor for carcinoembryonic antigen detection based on pillar5arene-functionalized Au nanoparticles and hollow PANI hybrid BiOBr heterojunction. Biosens. Bioelectron. 2022, 208, 114220. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Bei, J.; Zhao, Q.; Li, X.; He, J.; Cai, Y.; Chen, T.; Du, Y.; Yao, Y. An enhanced photo-electrochemical sensor constructed from pillar 5arene functionalized Au NPs for ultrasensitive detection of caffeic acid. Talanta 2022, 243, 123322. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, Y.; Mubango, E.; Shi, C.; Regenstein, J.M.; Yang, Q.; Hong, H.; Luo, Y. Rapid freshness and survival monitoring biosensors of fish: Progress, challenge, and future perspective. Trends Food Sci. Technol. 2022, 129, 61–73. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Sun, D.-W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Nimbkar, S.; Auddy, M.; Manoj, I.; Shanmugasundaram, S. Novel Techniques for Quality Evaluation of Fish: A Review. Food Rev. Int. 2021, 1996, 1–24. [Google Scholar] [CrossRef]
- Mustafa, F.; Othman, A.; Andreescu, S. Cerium oxide-based hypoxanthine biosensor for Fish spoilage monitoring. Sens. Actuators B Chem. 2021, 332, 129435. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Yue, F.; Shen, Z.; Xu, D.; Fang, H.; Chen, W.; Wang, Z.; Li, P.; Guo, Y.; et al. Dual enzyme electrochemiluminescence sensor based on in situ synthesis of ZIF-67@AgNPs for the detection of IMP in fresh meat. LWT 2022, 165, 113658. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarker, D.; Misra, T.N. Development of an amperometric enzyme electrode biosensor for fish freshness detection. Sens. Actuators B Chem. 1998, 53, 58–62. [Google Scholar] [CrossRef]
- Gil, L.; Barat, J.M.; Garcia-Breijo, E.; Ibañez, J.; Martínez-Máñez, R.; Soto, J.; Llobet, E.; Brezmes, J.; Aristoy, M.-C.; Toldrá, F. Fish freshness analysis using metallic potentiometric electrodes. Sens. Actuators B Chem. 2008, 131, 362–370. [Google Scholar] [CrossRef]
- Hu, S.; Liu, C.-C. Amperometric sensor for fish freshness based on immobilized multi-enzyme modified electrode. Electroanalysis 1997, 9, 1229–1233. [Google Scholar] [CrossRef]
- Itoh, D.; Sassa, F.; Nishi, T.; Kani, Y.; Murata, M.; Suzuki, H. Droplet-based microfluidic sensing system for rapid fish freshness determination. Sens. Actuators B Chem. 2012, 171–172, 619–626. [Google Scholar] [CrossRef]
- Postmortem ATP Catabolism in Fish Muscle. ATP Breakdown in Post Mortem Muscle. Available online: https://www.novocib.com/ATP_breakdown_salmon.html (accessed on 26 November 2022).
- Konoplev, G.; Agafonova, D.; Bakhchova, L.; Mukhin, N.; Kurachkina, M.; Schmidt, M.-P.; Verlov, N.; Sidorov, A.; Oseev, A.; Stepanova, O.; et al. Label-Free Physical Techniques and Methodologies for Proteins Detection in Microfluidic Biosensor Structures. Biomedicines 2022, 10, 207. [Google Scholar] [CrossRef]
- Wojnowski, M.; Issakov, V.; Knoblinger, G.; Pressel, K.; Sommer, G.; Weigel, R. High-Q embedded inductors in fan-out eWLB for 6 GHz CMOS VCO. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011; pp. 1363–1370. [Google Scholar] [CrossRef]
- Issakov, V.; Wojnowski, M.; Thiede, A.; Weigel, R. Considerations on the de-embedding of differential devices using two-port techniques. Int. J. Microw. Wirel. Technol. 2010, 2, 349–357. [Google Scholar] [CrossRef]
- Zur Nedden, S.; Eason, R.; Doney, A.S.; Frenguelli, B.G. An ion-pair reversed-phase HPLC method for determination of fresh tissue adenine nucleotides avoiding freeze-thaw degradation of ATP. Anal. Biochem. 2009, 388, 108–114. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Frorip, A.; Korsakov, R.; Korsakov, V.; Püssa, T.; Sünter, A.; Kasvand, N. New Freshness Index, Method and Device to Determine Freshness Status in Fish and Meat According to FAO Recommendations. Available online: https://www.researchgate.net/publication/321213587_New_freshness_index_method_and_device_to_determine_freshness_status_in_fish_and_meat_according_to_FAO_recommendations#fullTextFileContent (accessed on 11 January 2020).
- Püssa, T.; Frorip, A.; Kuznetsov, A.; Sünter, A.; Anton, D.; Raudsepp, P. New Freshness Index, Method and Device to Determine Freshness Status of Meat and Fish. Available online: https://www.researchgate.net/publication/326059014_NEW_FRESHNESS_INDEX_METHOD_AND_DEVICE_TO_DETERMINE_FRESHNESS_STATUS_OF_MEAT_AND_FISH (accessed on 11 January 2021).
- Walls, D.; Loughran, S.T. Protein Chromatography: Methods and Protocols, 2011th ed.; Humana Press: Totowa, NJ, USA, 2011; p. 681. ISBN 9781607619123. [Google Scholar]
- Determann, H. Gel Chromatography, Gel Filtration, Gel Permeation, Molecular Sieves: A Laboratory Hand Book, 2nd ed.; Springer: New York, NY, USA, 1969; p. 208. ISBN 978-3642950841. [Google Scholar]
- Kasvand, N.; Frorip, A.; Kuznetsov, A.; Püssa, T.; Rusalepp, L.; Sünter, A. A new approach for evaluation of meat freshness. In Proceedings of the 5th International Conference on Optical Characterization of Materials (OCM 2021), Karlsruhe, Germany, 17–18 March 2021; Beyerer, J., Längle, T., Eds.; KIT Scientific Publishing: Karlsruhe, Germany, 2021; pp. 21–32. [Google Scholar] [CrossRef]
- Ciampa, A.; Picone, G.; Laghi, L.; Nikzad, H.; Capozzi, F. Changes in the amino acid composition of Bogue (Boops boops) fish during storage at different temperatures by 1H-NMR spectroscopy. Nutrients 2012, 4, 542–553. [Google Scholar] [CrossRef]
- Abramova, L.S.; Kozin, A.V.; Shashkov, A.S. NMR—Spectroscopy technique for salmon fish species quality assessment. Food Syst. 2019, 2, 4–9. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Alvares, T.d.S.; Sampaio, G.S.L.; Cabral, C.C.; Araujo, J.V.A.; Franco, R.M.; Mano, S.B.; Conte Junior, C.A. Influence of vacuum and modified atmosphere packaging in combination with UV-C radiation on the shelf life of rainbow trout (Oncorhynchus mykiss) fillets. Food Control 2016, 60, 596–605. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, Y.; Liu, X.; Luo, Y.; Gao, L. Quality assessment of rainbow trout (Oncorhynchus mykiss) fillets during super chilling and chilled storage. J. Food Sci. Technol. 2015, 52, 5204–5211. [Google Scholar] [CrossRef]
- Ježek, F.; Buchtová, H. The effect of vacuum packaging on physicochemical changes in rainbow trout (Oncorhynchus mykiss) during cold storage. Acta Vet. Brno 2014, 83, S51–S58. [Google Scholar] [CrossRef]
- Quality and Quality Changes in Fresh Fish. Postmortem Changes in Fish. Available online: https://www.fao.org/3/v7180e/V7180e06.htm#5.1%20Sensory%20changes (accessed on 26 November 2022).
- Guidelines for the Interpretation of Results of Microbiological Testing of Ready-to-Eat Foods Placed on the Market (Revision 4). Available online: https://www.fsai.ie/food_businesses/micro_criteria/guideline_micro_criteria.html (accessed on 15 November 2022).
- Arya, S.S.; Parihar, D.B.; Vijayaraghavan, P.K. Changes in free nucleotides, nucleosides and bases during preparation of pre-cooked dehdyrated minced meats. Nahrung 1979, 23, 495–499. [Google Scholar] [CrossRef]
- Rotola-Pukkila, M.K.; Pihlajaviita, S.T.; Kaimainen, M.T.; Hopia, A.I. Concentration of Umami Compounds in Pork Meat and Cooking Juice with Different Cooking Times and Temperatures. J. Food Sci. 2015, 80, C2711–C2716. [Google Scholar] [CrossRef] [PubMed]
- Madruga, M.S.; Elmore, J.S.; Oruna-Concha, M.J.; Balagiannis, D.; Mottram, D.S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010, 123, 513–520. [Google Scholar] [CrossRef]
- Sasaki, K.; Motoyama, M.; Mitsumoto, M. Changes in the amounts of water-soluble umami-related substances in porcine longissimus and biceps femoris muscles during moist heat cooking. Meat Sci. 2007, 77, 167–172. [Google Scholar] [CrossRef]
- Cambero, M.I.; Pereira-Lima, C.I.; Ordoez, J.A.; Garca de Fernando, G.D. Beef broth flavour: Relation of components with the flavour developed at different cooking temperatures. J. Sci. Food Agric. 2000, 80, 1519–1528. [Google Scholar] [CrossRef]
- Belton, P.S.; Hills, B.P.; Webb, G.A. Advances in Magnetic Resonance in Food Science; Elsevier: Amsterdam, The Netherlands, 1999; ISBN 9781855737730. [Google Scholar]
- Lou, X.; Zhai, D.; Yang, H. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 2021, 123, 107697. [Google Scholar] [CrossRef]
- Shumilina, E.; Ciampa, A.; Capozzi, F.; Rustad, T.; Dikiy, A. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4 °C. Food Chem. 2015, 184, 12–22. [Google Scholar] [CrossRef]
- Handumrongkul, C.; Silva, J.L. Aerobic Counts, Color and Adenine Nucleotide Changes in CO2 Packed Refrigerated Striped Bass Strips. J. Food Sci. 1994, 59, 67–69. [Google Scholar] [CrossRef]
- Jones, N.R.; Murray, J.; Livingston, E.I.; Murray, C.K. Rapid estimations of hypoxanthine concentrations as indices of the freshness of chill-stored fish. J. Sci. Food Agric. 1964, 15, 763–774. [Google Scholar] [CrossRef]
- Spinelli, J.; Eklund, M.; Miyauchi, D. Measurement of Hypoxanthine in Fish as a Method of Assessing Freshness. J. Food Sci. 1964, 29, 710–714. [Google Scholar] [CrossRef]
- Kyrana, V.R.; Lougovois, V.P.; Valsamis, D.S. Assessment of shelf-life of maricultured gilthead sea bream (Sparus aurata) stored in ice. Int. J. Food Sci. Technol. 1997, 32, 339–347. [Google Scholar] [CrossRef]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Carver, J.D. Dietary nucleotides: Effects on the immune and gastrointestinal systems. Acta Paediatr. 1999, 88, 83–88. [Google Scholar] [CrossRef]
- Hess, J.R.; Greenberg, N.A. The role of nucleotides in the immune and gastrointestinal systems: Potential clinical applications. Nutr. Clin. Pract. 2012, 27, 281–294. [Google Scholar] [CrossRef]

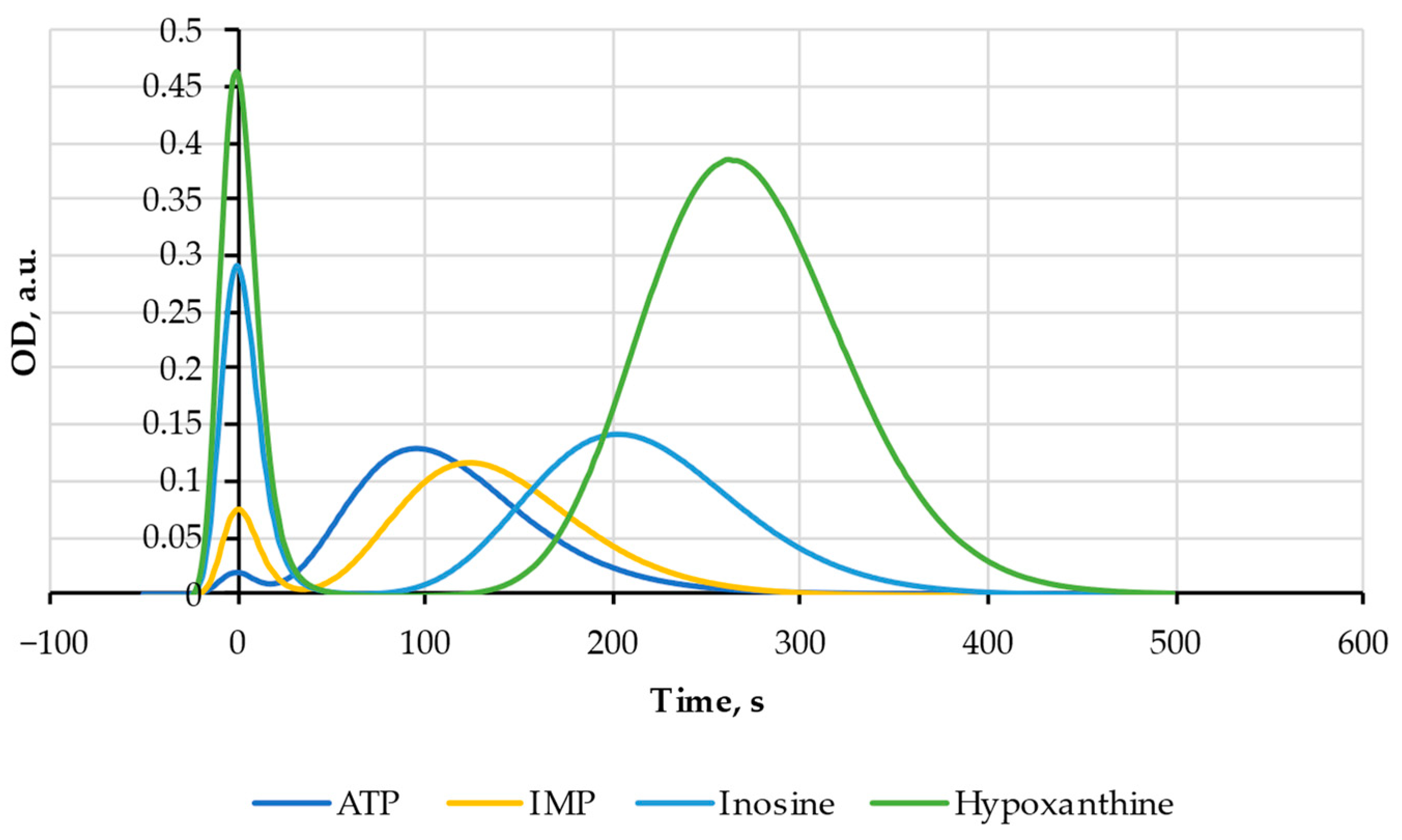

| Substance | Molecular Weight, Da | Elution Time, s |
|---|---|---|
| ATP | 507 | 95 |
| IMP | 348 | 130 |
| Ino | 268 | 200 |
| Hx | 136 | 275 |
| Fish Specie | ΔTime, s | Note |
|---|---|---|
| Alaska pollack | −21 | – |
| Pangasius | −17 | – |
| Norway haddock | −18 | Two peaks at 130 and 260 s emerged after treatment |
| Wolffish | −37 | Unstable shifts of the broad band ±10 s |
| Hake | −30 | – |
| CFU/g Unsterile Pork, Trout Initial pH 5.5–5.8 | Time, s | Index K | Index H* | Characteristic and Accordance to FAO Phases | Objects | Suits for | Some Other Characteristics |
|---|---|---|---|---|---|---|---|
| – 1 | <130 | <0.3 | H* < 0.1 | Absolutely fresh Phase 1 | All rapidly frozen fish or meat | Many purposes. Aging can be needed | Quickly (in hours) ending condition in fridge at +4 °C |
| 3 × 103 ÷ 104 | 130÷160 | 0.3 < K < 0.5 | 0.1 < H* < 0.2 | Fresh Phase 2 | High quality meat and fish | Haute cuisine | Rich in endogenous IMP; no need for artificial E630 |
| ≥104 ÷ 105 | 160÷190 | 0.5 < K < 0.75 | 0.2 < H* < 0.45 | Rather fresh Status “Use before” The first half of Phase 3 | Stored or thawed meat and fish | Ordinary consumption | Often coincides with end of bacterial lag phase |
| ≥105 ÷ 107 2 | 190 ÷210 | K ≈ 0.8 | H* ≈ 0.5 | Start or middle point of exponential bacterial growth Ending of Phase 3 | Can be all kinds of samples | Standard shelf-life end | May be in quickly changing condition |
| ≥107 ÷ 108 | 210÷240 | 0.8 < K < 0.95 | 0.8 < H*≈ 0.9 | Heavy contamination but may not be spoiled irreversibly Overlapping of Phases 3 and 4 | Often reduced-price foods or aged meat | Intense temperature treatment needed | Bacterial stationary phase: oscillating contamination and crash |
| ˃108 | ˃240 | K ≈ 1 | 1 ≈ H*˃˃1 | Phase 4 | Spoiled | Rejection | Smell, bad appearance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, A.; Frorip, A.; Sünter, A.; Kasvand, N.; Korsakov, V.; Konoplev, G.; Stepanova, O.; Rusalepp, L.; Anton, D.; Püssa, T.; et al. Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness. Chemosensors 2023, 11, 69. https://doi.org/10.3390/chemosensors11010069
Kuznetsov A, Frorip A, Sünter A, Kasvand N, Korsakov V, Konoplev G, Stepanova O, Rusalepp L, Anton D, Püssa T, et al. Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness. Chemosensors. 2023; 11(1):69. https://doi.org/10.3390/chemosensors11010069
Chicago/Turabian StyleKuznetsov, Artur, Aleksandr Frorip, Alar Sünter, Nensi Kasvand, Vadim Korsakov, Georgii Konoplev, Oksana Stepanova, Linda Rusalepp, Dea Anton, Tõnu Püssa, and et al. 2023. "Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness" Chemosensors 11, no. 1: 69. https://doi.org/10.3390/chemosensors11010069
APA StyleKuznetsov, A., Frorip, A., Sünter, A., Kasvand, N., Korsakov, V., Konoplev, G., Stepanova, O., Rusalepp, L., Anton, D., Püssa, T., Roasto, M., Abramova, L., Kozin, A., Toom, L., Hirsch, S., & Mukhin, N. (2023). Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness. Chemosensors, 11(1), 69. https://doi.org/10.3390/chemosensors11010069






