Abstract
A new class of solid-state miniaturised reference electrodes with a deep eutectic solvent as an alternate enhancement electrode system is described. A simple and accurate stable electrochemical sensor was prepared by developing a conventional reference electrode using an Ag/AgCl planar micro-reference electrode covered with a PVC polymer. A conductive deep eutectic solvent (DES), ethaline, was added in small quantities and mixed with an internal electrolyte to maintain the Cl− ion concentration in the constructed electrode. The fabricated microelectrode showed good stability, reproducibility, and long-term stability against varying concentrations of different ions. The potential response of the fabricated microelectrode was studied under varying concentrations of Cl− ions in the presence of 0.1 to 1.0% DES in a concentrated electrolyte system (20 mM Na2SO4). The stability of the fabricated microelectrode was addressed against Br− and Cl− ions using different inorganic salts, and the potential measurements were found to be insensitive toward all responsive ions. The stability response of the fabricated microelectrode against Cl− ions was optimised in the presence of 1.0% DES. The experimental data showed good agreement with the potential change of the fabricated electrode in the presence of the supporting DES electrolyte. The liquid junction-free PVC solid-state miniaturised reference electrode demonstrated a constant potentiometric measurement over a long period of time. The concentrated supporting DES electrolyte solution (20 mM) exhibited better stability values and was a more suitable fabricated microelectrode than other additive concentrations. The long-term stability of the developed microelectrode displayed a good lifetime and high stability of around 60 days.
1. Introduction
A reference electrode (RE) is an essential element in redox systems and electrochemical processes [1]. In particular, the basic concept of the reference electrode is to provide stable potential measurements performed in an electrochemical cell. Alternative approaches have been achieved in scientific studies to improve reference electrode stabilities [2]. Many advances in experimental additive electrodes have contained certain deficiencies, including a relatively expensive price and challenges in the manufacture and preparation of a truly alternative electrode with acceptable stability [3]. In recent studies, some researchers have confirmed the importance of the conventional reference electrode with a solid-state miniaturised technique, particularly when the fabrication of the electrode utilises a polymer membrane [4,5,6]. These types of electrodes often promise numerous advantages in electrochemical sensor applications, such as ion-selective electrodes [7], the impedance measuring chips [8], voltage measuring chips [9], and field-effect transistors [10].
The traditional Ag/Ag− reference electrode contains solid wire and an electrolyte solution that is enclosed in a glass tube and separated from the analyte solution by a membrane. Some successful improvements to the reference electrode have been achieved using a polymer fabrication Ag/AgCl electrode [11]. However, the fabrication method of Ag/AgCl suffers from problems such as the rapid diffusion of chloride ions from the sample and membrane (polymer matrix); a cost-effective fabrication method should consider important issues related to control electrolyte interference, potential drift, and liquid junction potential (Ej) [12].
The half-reaction that occurs in the Ag/AgCl reference electrode is given in Equation (1).
According to the Nernst equation, the potential stability of the conventional Ag/AgCl reference electrode depends on the Cl− ion activity. To maintain its potential, the concentration of Cl− ions are kept constant in the electrolyte solution. The maintenance of chloride concentrations leads in some cases to decreases in the guaranteed accuracy, and contamination limits their application in the electrochemical cell. After considering these limitations, researchers have shifted their interest from conventional reference electrodes to the fabrication of solid-state electrodes that do not need the electrolyte reservoir [13]. The fabrication and modification of solid-state miniaturised reference electrodes for their better performance are novel and productive areas of research.
To avoid electrolyte interference with the conventional reference electrode, the quasi-reference electrode has also been used in previous studies. In the quasi-reference electrode, the electrode is directly immersed in the test solutions without a membrane because it contains no internal electrolyte. This presents a challenge because it causes a disordering in the potential values, with the low stability of a high ionic strength system owing to the potential of varying Cl− concentrations in the test samples. This is one of the main reasons that the quasi-reference electrode is considered undesirable in many electrochemical systems using high ionic strength species samples. However, it can be used in low ion strength electrochemical methods such as the sensing of glucose and other low-charge macromolecules [14].
Recently, the screen-printing method was used to fabricate a solid-state reference electrode that offered very effective outcomes. It provided a cost-effective disposable solid-state microelectrode, and the method had control over the dimension and reproducibility of the product. Isao Shitanda et al. [15] constructed a solid-state Ag/AgCl reference electrode through the screen-printing method to improve the potential stability of the reference electrode against various ions. The stability of the electrode was boosted for up to 2 months. The electrode showed no significant potential changes against K+, Cl−, Na+, and ions that indicated the stability of the electrode in a high ionic strength system. In the same manner, Wei Yen et al. [16] fabricated an Ag/AgCl reference electrode using screen-printing technology as a potentiometric biosensor. The ager gel was used as the inner electrolyte and the chloroprene rubber was used as the liquid junction. The results showed that the fabricated planer solid-state reference electrode was insensitive toward many important ionic species such as Li+, Ca+2, Na+, and Cl− ions.
The insensitivity of the liquid-free solid-state electrode towards various important electroactive ions makes it a promising reference electrode candidate. However, the stability against Cl− ions is still a crucial issue under debate among researchers. Various methodologies have been proposed. One significant method is to fabricate the Ag/AgCl reference electrode over the electrolyte-doped polymeric material [17]. Recently Ag/AgCl was deposited over the PVAc/KCl composite material [18]. The constructed solid-state reference electrode showed good stability against Cl− ions. Another novel solid-state electrode was fabricated to facilitate the use of the ion-sensitive field-effect transistor. The saturated KCl electrolyte was immobilised in agarose gel [12].
Deep eutectic solvents (DESs) are a unique generation of solvents that act as a liquid electrolyte at room temperature. DESs are mainly known as surface-active compounds that are functionalised to improve the wettability of electrode surfaces. This can help to produce better charge storage performance [19]. Because of the high ionic conductivity of DESs, a liquid junction-free reference electrode was recently constructed using DES concentrations [20]. Our study attempted to improve the fabrication of miniaturised Ag/AgCl by enhancing a DES solvent micro-reference electrode. The main proposal behind this work was to develop electrode stability and reproducibility. The printed circuit board was used as base material to fabricate the solid-state electrode.
2. Materials and Methods
2.1. Materials and Chemicals
The inorganic salts of chloride and bromide anion were purchased from DEAJUNG and used without further purification. The polymer polyvinyl chloride (PVC) of high molecular weight was also purchased from Fluka and used without further purification. Ethaline as the DES solvent was prepared by mixing 1:1 mole ration of ethylene glycol (Fluka) and choline chloride (Fluka 99.9%) at 40 °C. All aqueous solutions were prepared in double-distilled water that had pH 6.7–7.0 in acetate buffer. Tetrahydrofuran (THF) purchased from Fluka was used for the membrane component.
2.2. Synthesis of Fabrication and Deposition of Membrane
Fabrication of the microelectrode membrane of Ag/AgCl was carried out according to the procedure given in the literature [12]. The membrane solution contained 0.1 to 1.0% DES, 25–30% (m/m) polyvinylchloride (PVC) membrane. The required component amounts were dissolved in 0.3 mL THF and then deposited on the surface of the Ag/AgCl microelectrode to achieve the DES-based membrane of the fabricated microelectrode. The general schematic diagram of the constructed fabricated microelectrode is shown in Figure 1.
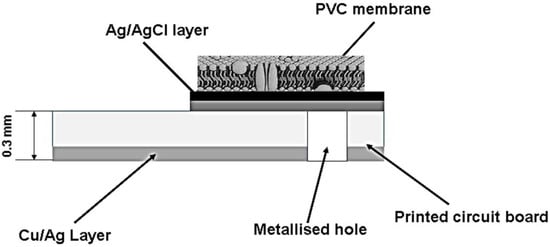
Figure 1.
Schematic diagram of the fabricated Ag/AgCl micro-reference electrode based on PVC membrane.
2.3. EMF Measurements
The automated measuring system was used in the current study to validate the fabricated electrode against different electroactive species [21]. The system consisted of a peristaltic pump (Miniplus 3, Gilson, Middleton, WI, USA), a burette (Dosiman 665, Metrohm, Herisau, Switzerland), and a data acquisition PC-based system (with 16-channel amplifier: EMF-16, Lawson Lab, San Diego, CA, USA) with the LabVIEW application. All measurements were carried out under standard conditions (room temperature (25 ± 2) °C and atmospheric pressure). The potential stability of the fabricated reference microelectrode was examined in the electrolyte solution, i.e., 0.5 mM Na2SO4 (dilute solution) and 20 mM Na2SO4 (concentrated solution). The potential stability was studied in a range of concentrations of Cl− and bromide ions (10−5 to 10−2 M). Various electrolytes of inorganic salts were separately prepared, such as sodium chloride (NaCl), sodium bromide (NaBr), potassium chloride (KCl), and potassium bromide (KBr).
The selectivity coefficients for the foreign ions were calculated by applying the KMPM function, which measures the ratio between the primary ion and the interfering ion activity, as seen in Equation (2) [5].
KMPM = ¼ Δa2A/a1B
2.4. Conditions
Conditioning in KCl is an essential factor that should be considered when activating the electrode. Time is an important factor influencing the electrochemical process, which is generally dependent on several aspects such as the thickness of the coated layer, type of membrane, the supporting electrolyte of the soaking solutions, and temperature. In this work, the main conditioning time was around 45 min in 0.2 M KCl.
3. Results and Discussion
3.1. Optimisation of EMF Measurements for the Electrode
To validate whether the electrode truly measured the potential of ions, it was important to address the reproducible potential and the stability of interfering species of anions to the sample solution. This was achieved by measuring the selectivity coefficient and the stability or unfluctuating quantities in the presence of foreign ionic species [11]. To achieve this goal, ionic liquids were considered very active ionic species that could provide an electrode additive to enhance the potential stability of the solid-state electrode against other ions owing to its high ionic conductivity [22]. This elucidated the role of ionic liquids in providing the potential for boosting ion-exchange-based micro membranes, which could easily control the efficiency of ion extraction from water into the ionic phase. In this context, ethaline was applied as a conductive ionic liquid to improve the stability and reproducibility of the fabricated PVC membrane-based Ag/AgCl microelectrode. Ethaline is composed of a choline chloride unsymmetrical large cation with ethylene glycol as an anion [19]. The cationic portion is attached by the hydrophobic chain that remains in contact with the polymer membrane, whereas the inorganic anion participates in ion exchange. Ion selectivity is the response of the fabricated electrode towards various ions that determined the stability of the electrode towards that ion [22].
The ion selectivity of the fabricated microelectrode will respond to DES quantities because the deep eutectic solvent acts as an ion exchanger. It should consider the influence of the selectivity coefficient values. To interpret this effect, the selectivity coefficient values of the constructed Ag/AgCl microelectrode in the presence of the different amounts of DES for various anions were determined. The obtained values of the ion selectivity coefficient are plotted in Figure 2. Interestingly, the results showed that the ion selectivity coefficient values of the constructed microelectrode showed very small changes in the presence of different amounts of DES. This indicated that DES was a very good ion exchanger in the polymer membrane and controlled the ion selectivity values. In this way, ethaline maintained the ionic constraint of the constructed microelectrode. Indeed, this observation was very important, and for the first time confirmed that almost no studied DESs interacted with the ion of choice and were consequently appropriate for customising as ion-exchange membranes.
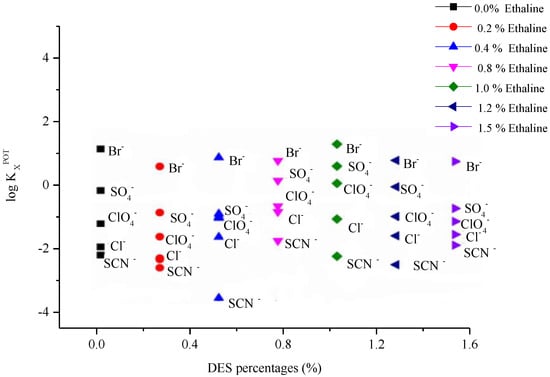
Figure 2.
Selectivity coefficients of the fabricated Ag/AgCl microelectrode in the presence of different ethaline concentrations.
The stability of the Cl− ion concentration is the main limitation of the conventional reference electrode [20]. The potential stability of the fabricated Ag/AgCl microelectrode was tested against Cl− ions for both ionic liquid-free and ionic liquid-containing systems. Figure 3 represents the potential stability profile of the fabricated reference microelectrode (Ag/AgCl) in the presence and absence of different amounts of DES (0–1.5%) in 20 mM Na2SO4 solution containing different concentrations of KCl (10−5 to 10−2 M). The results indicated that the potential stability of the Ag/AgCl reference electrode was influenced by Cl− ions in the absence of DES. When increasing the concentration of Cl− ions, a cationic response was observed (Figure 3), indicating that the obtained potential of the reference electrode was not constant. The DES additive to the membrane of the fabricated reference microelectrode assessed good results in the Cl− ion concentration owing to the constant stability potential. Optimum stability was observed in the presence of 1.0% ethaline. For this system, the change in potential was about ±0.25 mV.
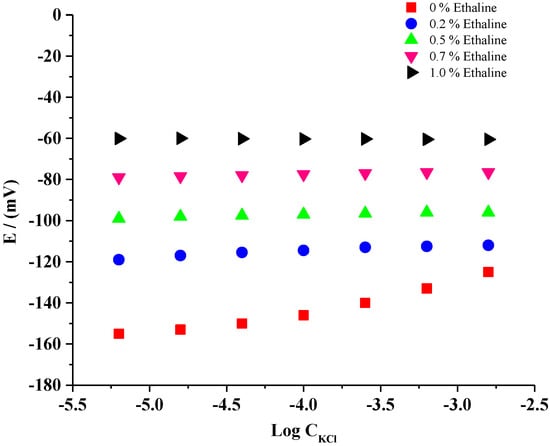
Figure 3.
Potential dependency of the fabricated microelectrode in the presence and absence of DESs under various concentrations of KCl obtained in 20 mM Na2SO4.
Results showed that the fabricated Ag/AgCl microelectrode was insensitive to different concentrations of Cl− ions owing to the presence of DESs. This finding supported our assumption that the ionic activity of ethaline acts as an ion exchanger in the polymer membrane, playing a very important role in maintaining the Cl− ion concentration in the fabricated microelectrode. The next step was to check whether either Cl− or Br− ions affected the potential stability of the constructed microelectrode with different salts. This would strengthen our hypothesis. The interference of Cl− and Br− ions of different salts in the potential stability of the DES-based fabricated reference electrode was also studied [22]. Figure 4 shows the potential dependency of the microelectrode on a range of concentrations of different anions (Cl− and Br−) obtained from different salts. The results showed a higher than −9 mV ± 0.31 potential increase in the electrode for Br− anions, while better stability was exhibited against Cl− anions. The change in the potential for the electrode in the presence of 1.0% ethaline displayed the least drift in NaCl (±0.19 mV) for the Cl− anion, whereas the greatest change in KBr (±1.77) over continuous measurement was for the Br− anion. This behaviour was not surprising owing to previous studies [16].
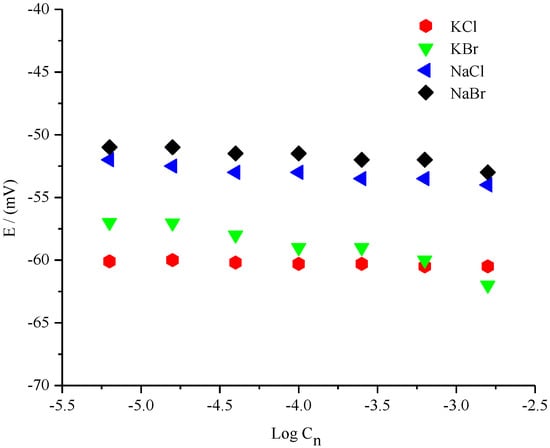
Figure 4.
Potential dependency profile of the fabricated microelectrode as a function of the log concentration of different salts in the presence of 1.0% ethaline in 20 mM Na2SO4.
The concentration of the supporting electrolyte may also influence the sensitivity and stability of the fabricated micro-reference electrode. Another factor for the supporting electrolyte was the potential stability of the fabricated microelectrode in the presence of both the diluted electrolyte (0.5 mM Na2SO4) and concentrated electrolyte solutions (20 mM Na2SO4). The data in Figure 5 show the effect of 0.5 and 1.0% DES contents. Comparatively, the microelectrode that contained 1.0% DES possessed better stability against Cl− ions for both the diluted and concentrated electrolyte systems. For the diluted Na2SO4 (0.5 mM) solution the potential reference electrode was not constant under the studied concentration of Cl− ion, which indicated that the reference electrode did not show potential stability against the studied range of Cl− ions in the presence of a diluted supporting electrolyte. The amounts of the supporting electrolytes should be considered an important aspect of the stability of the fabricated reference electrode. In our experiment, they gave a good range of potential stability, particularly at low concentrations such as 20 mM, as shown in Figure 5.
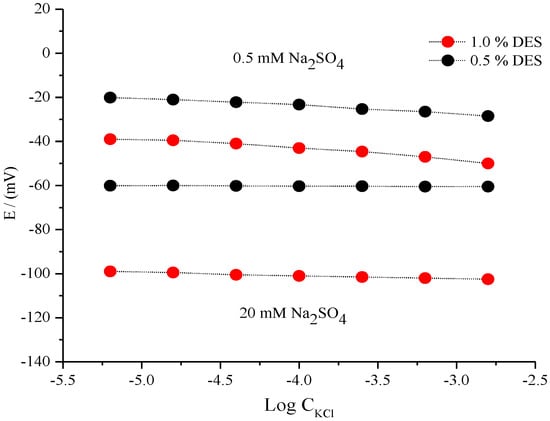
Figure 5.
Potential dependency profile of the fabricated Ag/AgCl reference electrode containing 0.5 and 1.0% IL under various concentrations of KCl in diluted Na2SO4 (0.5 mM) and concentrated Na2SO4 (20 mM).
3.2. Additive DES Effect
All the measurements were carried out in concentrated electrolyte solution, i.e., 20 mM Na2SO4. The EMF response of the microelectrode system was repeatedly measured after several days. The obtained results of the potential dependency of the electrode for varying Cl− ion concentrations are reported in Figure 5. There, the potential dependency of the microelectrode in the range of tested concentrations of Cl− ions were not significantly altered over a long period. The variation in potential on varying the Cl− concentration was almost constant during the studied time for both the 0.5 and 1.0% IL systems. However, the measured values of potential after varying times were different. The results suggested that the DES-based microelectrode showed good stability against varying Cl− ions over a period of up to 60 days. In some instances, mechanisms of ion exchange between the ion-selective membrane and DES layer could exist, engaging anion or cation movement [23,24]. PVC doped with large and immobile anions, such as a high DES amount, collected a few additional anions likely because of the satirical difficulties. Thus, this system exhibited long period stability by controlling the amount of DES in the generation of this type of reference electrode.
Regarding the time stability for the fabricated membrane, Figure 6 shows the observation stabilities for the potentiometric signal. The potential of the fabricated membrane-based Ag/AgCl electrode was examined in two cases: one in the presence of 0.5% DES and the other one in 1.0% DES under various concentrations of Cl− ions for 10 to 60 days. There were many uncertain results in the potentiometric signal due to the large resistance in the electrochemical cell, as seen in Figure 5. A reasonable observed potential consequence justified the influence of DES additives on the performance of the electrode. We assessed that the stability of the EMF response of the same microelectrode system was in good agreement with the concentrated electrolyte solution, i.e., 20 mM Na2SO4, which was previously reported [21].
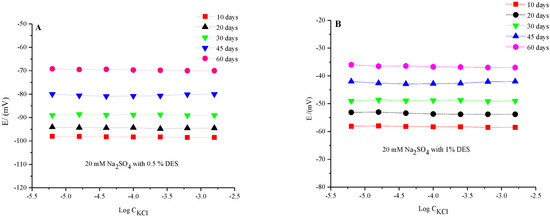
Figure 6.
Potential dependency profile of the DES-based fabricated reference microelectrode against varying concentrations of Cl− ions in a 20 mM Na2SO4 supporting electrolyte measured for 60 days.
3.3. Time Effect
As the fabricated solid-state reference electrode showed good results in our various tests, it was fabricated for the sensing application, and the next experiment was the long-term stability test. Here, the stability of the fabricated solid-state microelectrode was also studied over a long period to determine the time dependency of a DES-based reference electrode on its reproducibility and stability. To this end, the potential of the fabricated membrane-based Ag/AgCl electrode was measured in the presence of 0.5 and 1.0% DES under varying concentrations of Cl− ions for 10 to 60 days. It was clearly seen that the liquid junction-free DES membrane-based reference electrode was capable of constant potentiometric measurements for an extended period.
3.4. Comparison of ISC vs. DES-Based Microelectrode
To determine the optimum operating DES-based fabricated microelectrode, we initially assessed the validity of the fabricated membrane by measuring the ability of the junction to slow chloride ion leakage by monitoring the open circuit potential (OCP). The reason behind this step was to understand the limiting diffusion of chloride ions (Cl−) in the presence of small quantities of ethaline. In Figure 7 it can be seen that increasing the time period for different concentrations of Cl led to the exhibited potential of −4 mV ±0.177. This was most likely due to the activity of Cl ions, which is not surprising as it was previously reported by Enriquez et al. [23,24]. They improved the junction potentials of Cl− activity by using a 3D printed reference electrode and found a good relation ratio between the junction and the highest stability for gel electrolyte layers in the 3D reference electrode. They also reported that over 12 h the potential of the chloride ion increased rapidly in the calomel electrode (SCE) owing to direct contact of the chloride ions with the KCl solution.
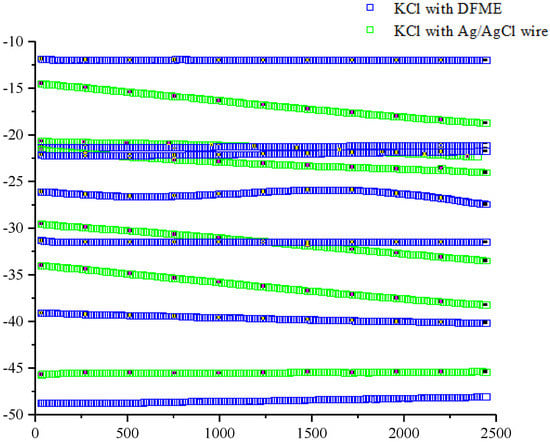
Figure 7.
Open circuit potential (OCP) for different concentrations of KCl using comparison measurements between the traditional Ag/AgCl and the DES fabricated micro-reference electrode (DFME) at room temperature.
4. Conclusions
In summary, the current work successfully fabricated a novel polymer-based solid-state reference electrode using a small quantity of deep eutectic solvent. A simple and quick method was clearly described for the preparation of a PVC polymer membrane by depositing on the surface of an Ag/AgCl microelectrode a small amount of conductive ionic solvent. The current results for additives of ethaline under various chemical examinations showed that the microelectrode had very good potential stability towards varying concentrations of Cl− and Br− ions. Different amounts of DES in the electrode were investigated in varying concentrations of Cl−. The best experimental data were achieved for the constructed electrode and the widest linear range stability of the potential of around ±0.25 in 1.0% ethaline content. The long-term stability indicated that, after more than 2 months, the reference electrode remained an active sensor. This suggested that DESs as unique liquids are probably tied to increasing the performances of electrochemical sensors when they are doped in the electrode. It can be concluded that the fabricated microelectrode demonstrated excellent electroanalytical activity, and it can be used in many specific micro electroanalytical applications.
Author Contributions
Conceptualization, S.S.M.A. and A.M.A.; methodology, S.S.M.A.; software, S.S.M.A. and A.M.A.; validation, S.S.M.A., A.M.A. and H.G.S.; formal analysis, S.S.M.A.; investigation, S.S.M.A., A.M.A. and H.G.S.; resources, S.S.M.A. and A.M.A.; data curation, S.S.M.A.; writing—original draft preparation, S.S.M.A.; writing—review and editing, S.S.M.A.; visualization, S.S.M.A.; supervision, S.S.M.A.; project administration, S.S.M.A.; funding acquisition, S.S.M.A., A.M.A. and H.G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
In chemistry laboratory in AL-Nahrain university.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Y.-M.; Chen, L.-C. Fabrication of a PVC-Based Solid-State Ag/AgCl Reference Electrode. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–3. [Google Scholar]
- Wang, S.; Wu, Y.; Gu, Y.; Li, T.; Luo, H.; Li, L.H.; Bai, Y.; Li, L.; Liu, L.; Cao, Y.; et al. Wearable sweatband sensor platform based on gold nanodendrite array as efficient solid contact of ion-selective electrode. Anal. Chem. 2017, 89, 10224–10231. [Google Scholar] [CrossRef]
- Solchenbach, S.; Pritzl, D.; Kong, E.J.Y.; Landesfeind, J.; Gasteiger, H.A. A gold micro-reference electrode for impedance and potential measurements in lithium ion batteries. J. Electrochem. Soc. 2016, 163, A2265. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated reference electrodes and their biosensing applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef]
- Abass, A.M.; Alabdullah, S.S.; Hassan, O.S.; Ahmed, A. Novel potentiometric sensors for determination of ondansetron hydrochloride in pure and dosage form. RSC Adv. 2021, 11, 34820–34827. [Google Scholar] [CrossRef] [PubMed]
- Abass, A.M. Preparation Pilocarpine Hydrochloride Selective Electrodes. Al-Nahrain J. Sci. 2017, 20, 13–19. [Google Scholar] [CrossRef]
- Alabdullah, S.S.; AL-Bassam, A.Z.; Asaad, N. Electrochemical sensors and its applications. Int. J. Res. Eng. Innov. 2021, 20, 85–262. [Google Scholar] [CrossRef]
- Hoja, J.; Lentka, G. Interface circuit for impedance sensors using two specialized single-chip microsystems. Sens. Actuators A Phys. 2010, 163, 191–197. [Google Scholar] [CrossRef]
- Tanyanyiwa, J.; Abad-Villar, E.M.; Fernández-Abedul, M.T.; Costa-García, A.; Hoffmann, W.; Guber, A.E.; Herrmann, D.; Gerlach, A.; Gottschlich, N.; Hauser, P.C. High-voltage contactless conductivity-detection for lab-on-chip devices using external electrodes on the holder. Analyst 2003, 128, 1019–1022. [Google Scholar] [CrossRef]
- Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwödiauer, R.; Bauer, S.; Lacour, S.P.; Wagner, S. Flexible ferroelectret field-effect transistor for large-area sensor skins and microphones. Appl. Phys. Lett. 2006, 89, 073501. [Google Scholar] [CrossRef]
- Lewenstam, A.; Bartoszewicz, B.; Migdalski, J.; Kochan, A. Solid contact reference electrode with a PVC-based composite electroactive element fabricated by 3D printing. Electrochem. Commun. 2019, 109, 106613. [Google Scholar] [CrossRef]
- Cicmil, D.; Anastasova, S.; Kavanagh, A.; Diamond, D.; Mattinen, U.; Bobacka, J.; Lewenstam, A.; Radu, A. Ionic Liquid-Based, Liquid-junction-free reference electrode. Electroanalysis 2011, 23, 1881–1890. [Google Scholar] [CrossRef]
- Mamińska, R.; Dybko, A.; Wróblewski, W. All-solid-state miniaturised planar reference electrodes based on ionic liquids. Sens. Actuators B Chem. 2006, 115, 552–557. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, C.; Hu, H.; Li, Z.; Xiao, G.; Yang, Q.; Sun, P.; Cheng, L.; Niu, W.; Bi, J.; et al. ISFET and Dex-AgNPs based portable sensor for reusable and real-time determinations of concanavalin A and glucose on smartphone. Biosens. Bioelectron. 2020, 151, 111962. [Google Scholar] [CrossRef] [PubMed]
- Shitanda, I.; Kiryu, H.; Itagaki, M. Improvement in the long-term stability of screen-printed planar type solid-state Ag/AgCl reference electrode by introducing poly (dimethylsiloxane) liquid junction. Electrochim. Acta 2011, 58, 528–531. [Google Scholar] [CrossRef]
- Liao, W.Y.; Chou, T.C. Fabrication of a planar-form screen-printed solid electrolyte modified Ag/AgCl reference electrode for application in a potentiometric biosensor. Anal. Chem. 2006, 78, 4219–4223. [Google Scholar] [CrossRef] [PubMed]
- Idegami, K.; Chikae, M.; Nagatani, N.; Tamiya, E.; Takamura, Y. Fabrication and characterization of planar screen-printed Ag/AgCl reference electrode for disposable sensor strip. Jpn. J. Appl. Phys. 2010, 49, 097003. [Google Scholar] [CrossRef]
- Macedo, D.S.; Vepsäläinen, M.; Acharya, D.; Wood, C.D.; Wen, D.; Thomson, L.; Peacock, S.; Rodopoulos, T.; Hogan, C.F. An unusually stable solid state Ag|AgCl reference electrode for long term continuous measurements based on a crosslinked poly (vinyl acetate)/KCl composite. Electrochim. Acta 2021, 368, 137636. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, J.; Uchiyama, K.; Yoshida, Y. Application of a novel ionic-liquid-based membrane reference electrode with inorganic insertion material paste to a calibration-free all-solid-state ion sensor chip. Sens. Actuators B Chem. 2021, 347, 130625. [Google Scholar] [CrossRef]
- Hu, J.; Ho, K.T.; Zou, X.U.; Smyrl, W.H.; Stein, A.; Bühlmann, P. All-solid-state reference electrodes based on colloid-imprinted mesoporous carbon and their application in disposable paper-based potentiometric sensing devices. Anal. Chem. 2015, 87, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Mendecki, L.; Callan, N.; Ahern, M.; Schazmann, B.; Radu, A. Influence of Ionic Liquids on the Selectivity of Ion Exchange-Based Polymer Membrane Sensing Layers. Sensors 2016, 16, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Joh, H.; Seong, M.; Lee, W.S.; Choi, J.H.; Oh, S.J. Engineering surface ligands of nanocrystals to design high performance strain sensor arrays through solution processes. J. Mater. Chem. C 2017, 5, 2442–2450. [Google Scholar] [CrossRef]
- Sibug-Torres, S.M.; Go, L.P.; Enriquez, E.P. Fabrication of a 3D-printed porous junction for Ag|AgCl|gel-KCl reference electrode. Chemosensors 2020, 8, 130. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).