Abstract
The light-addressable potential sensor (LAPS) was invented in 1988 and has developed into a multi-functional platform for chemical and biological sensing in recent decades. Its surface can be flexibly divided into multiple regions or pixels through light addressability, and each of them can be sensed independently. By changing sensing materials and optical systems, the LAPS can measure different ions or molecules, and has been applied to the sensing of various chemical and biological molecules and cells. In this review, we firstly describe the basic principle of LAPS and the general configuration of a LAPS measurement system. Then, we outline the most recent applications of LAPS in chemical sensing, biosensing and cell monitoring. Finally, we enumerate and analyze the development trends of LAPS from the aspects of material and optical improvement, hoping to provide a research and application perspective for chemical sensing, biosensing and imaging technology.
1. Introduction
The light-addressable potential sensor (LAPS) is an electrochemical sensing platform based on field-effect capacitor photocurrent measurement. It detects ions and molecules in an electrolyte in a spatially resolved manner. Since its invention in 1988, LAPS has been widely developed and applied for chemical and biomedical research [1]. LAPS is suitable for detecting the potential changes caused by the changes in ion concentrations. It can also be used for the measurement of local impedance (SPIM). It can be based on an electrolyte insulator semiconductor (EIS) or electrolyte semiconductor (ES) substrate. Compared with ion-selective field-effect transistors with interdigital electrodes for measuring conductivity, redox potential or impedance parameters, LAPS combines chemical sensors and optical addressability, and has great potential for electrochemical imaging and biological imaging.
LAPS combines the generation and measurement of photocurrent with the ion environment on the substrate surface. First, we discuss how this combination realizes the measurement and imaging of ion concentration, photocurrent and photovoltage. Further, we use modeling and simulation methods to help explain this process and try to theoretically explain the effects of light source, substrate thickness and doping concentration on the lateral resolution of LAPS. Then, we select and list the applications of LAPS in chemical sensing, biosensing and imaging, along with the improvements in materials and optical systems, in detail, in order to achieve better sensing and imaging process in the future. When facing different targets of interest, we always need different sensing and imaging strategies according to the actual needs. Figure 1 shows the main advantages of a light-addressable potentiometric sensor compared to other sensors for chemical sensing and biosensing. We illustrate these advantages in detail and discuss the disadvantages of LAPS as well, followed by the challenges faced by today’s research and possible solutions. We strongly believe that this review can clarify the current research directions and application methods for LAPS, and help readers use LAPS to develop practical high-resolution biological imaging methods and biosensors with high sensitivity, high selectivity and stability.
Figure 1.
Main advantages of the light-addressable potentiometric sensor.
2. Measurement System of a LAPS
2.1. Principle and Setup
A complete LAPS system includes three basic units: a light source (such as LED or laser beam), a LAPS chip and an electronic circuit for reading photocurrent. For example, Figure 2a shows a typical setup of LAPS based on EIS structure, using culture medium as the electrolyte, SiO2/Si2N4 as the insulator, Si as the semiconductor, an LED light source and a constant potential circuit for measurement.
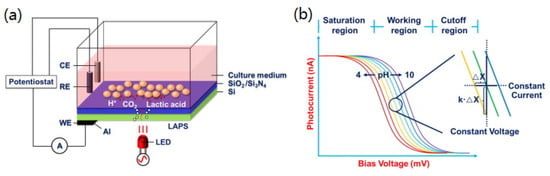
Figure 2.
(a) A typical setup of a light-addressable potentiometric sensor for acid detection; (b) typical photocurrent–voltage curves for a LAPS sensor under different pHs, under constant current detection mode and constant voltage detection mode measuring parameters [2].
Most of the carriers are swept away from the surface when a bias voltage is applied to the metal electrode, resulting in a space charge region. When the modulated light is irradiated on the back of the LAPS through the optical probe, according to band gap theory, that is the internal photoelectric effect. During illumination, electron–hole pairs will be generated on the back surface of the silicon substrate. After the photon-induced carriers are generated, they will begin to diffuse immediately, and some of them will recombine in the path. Only the molecules reaching the space charge region can be bent and separated by the energy band, generating external photocurrent and affecting the spatial resolution. In the absence of light, the excess carriers in the space charge region are released and gradually recombined. Therefore, under modulated illumination, alternating photocurrent can be detected in an external circuit. This is the principle of the LAPS sensing process, and Figure 2b shows typical photocurrent–voltage curves of this process using a constant potential circuit applied to pH sensing.
LAPS devices are optically addressable, which means the signal from each local area (point) on the grid surface can be read by illuminating the area with a modulated light source. This characteristic makes LAPS not only useful for chemical sensing, but also suitable for chemical imaging and multi-sensor applications [1]. When the surface of a semiconductor is scanned by a focused laser beam (scanning setting) or a light source array (e.g., light emitting diode (LED)), the two-dimensional distribution of the change in surface potential caused by the local concentrations of some chemical or biological substances can be detected by measuring the photocurrent at each point.
2.2. Modeling of a LAPS
We know little about the characteristics of the LAPS, even if it has been used in the sensing and imaging of various ions and molecules. For example, it is generally believed that one of the biggest characteristics of a LAPS is its spatial resolution, but so far, the optical characteristics of LAPS devices have not been analyzed in detail. In addition, in an experiment, a LAPS device often cannot obtain a satisfactory spatial resolution and signal-to-noise ratio at the same time, so it is necessary to find a compromise between the two. To reasonably explain these problems, it is essential to establish a LAPS equivalent circuit model to analyze its photocurrent characteristics.
A. Poghossian et al. discussed the impedance effect and crosstalk of a non-illumination area on the measurement of the photocurrent and optical addressability of LAPS devices by designing an extended equivalent circuit model [3]. Photocurrent depends not only on the local interface potential in the illumination area, but also on the possible change in interface potential in the dark area; that is, the change in bypass impedance in the equivalent circuit produces crosstalk effect. In order to minimize the influence of non-illumination area, the experiment proves that when selecting the measuring circuit in the design of LAPS, the input resistance must be much lower than the impedance of the non-illumination area, so that most of the photocurrent flows into the measuring circuit to achieve the best measurement effect.
Tatsuo et al. proposed a circuit model for reflux in LAPS, which is shown in Figure 3 [4]. They discussed the dependence of signal current on various parameters, such as contact area diameter, modulation frequency and solution-specific conductivity, and then calculated the series resistance of the circuit. It was found that due to the differences in reflux in calibration and measurement, the local change in analyte concentration in imaging may be underestimated. In addition, these equivalent circuit models were also used to study the impedance measurements and frequency characteristics of LAPS under different bias voltages. It was found that the input of data acquisition card can be maximized through a three-electrode configuration and a gain scaling network. A better operational amplifier with better noise performance and higher bandwidth can be used to improve the signal-to-noise ratio and bandwidth [5].
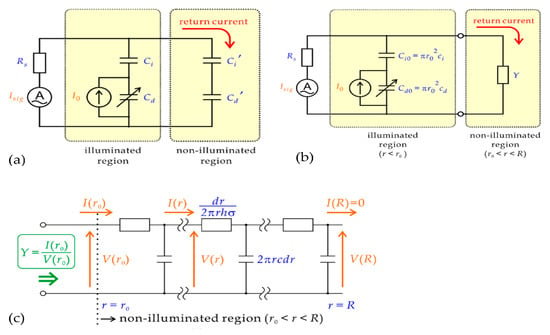
Figure 3.
(a) A simple circuit model of the return current; (b) the path of the return current is represented by admittance Υ; (c) a circuit model of the non-illuminated region [4].
2.3. Device Simulation of a LAPS
Differently from establishing the equivalent circuit model, a simplified division simulation is proposed to simulate the carrier distribution and photocurrent response, which provides new insights into the amplitude mode and phase mode operation of LAPS. Similarly, the division simulation also focuses on the spatial resolution of LAPS, which can check various equipment parameters to effectively design and optimize LAPS structure and settings in order to improve performance. By calculating the temporal and spatial variation in electron and hole distribution in semiconductor layer with modulated illumination, the photocurrent response and spatial resolution are obtained. At the same time, the specific relationship between LAPS spatial resolution and substrate thickness, doping concentration and light intensity can be found. Firstly, higher spatial resolution can be obtained by the utilization of a thinner silicon substrate, which can be explained by considering the geometric effect in minority carrier diffusion. Secondly, the spatial resolution is dependent on the minority carrier diffusion length, and the doping concentration has an effect on the minority carrier diffusion length, which is why the doping concentration usually affects the spatial resolution of LAPS in experiments. Third, when the silicon substrate is thick, higher spatial resolution can be obtained using a light source with a longer wavelength and lower illumination intensity. Finally, the simulation results show that the incident angle of constant illumination will also affect the spatial resolution of LAPS, as Figure 4 shows. When combined illumination with large incident angle is used, the spatial resolution can be improved, because the optical carriers are limited near the depletion layer without enhancing the recombination near the back. At this stage, the simulation mainly considers the insulator semiconductor part of LAPS. In the future, it will be necessary to add the electrolyte insulator interface to the division simulation to consider its electrochemical effect [6,7,8].
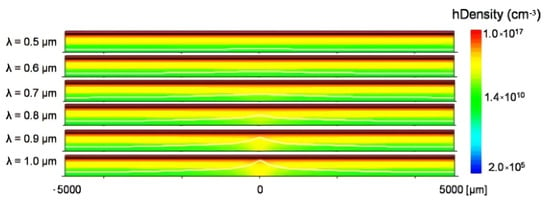
Figure 4.
Distribution of minority carriers near the space charge region. The white lines show the border of the space charge region [6].
3. LAPS for Chemical Sensing
3.1. Chemical Sensing and Application
LAPS chemical sensing has been reported for various metal ions by researchers, such as Yoshinobu et al. In the early stage, LAPS combined with a microfluidic system was applied to chemical sensing and imaging at the same time, which was used for the detection of heavy metal ions Pb2+, Cu2+, Cd2+ and Hg2+; and other metal ions, Li+, K+, CS+, Mg2+ and Ca2+ [9]. LAPS chemical sensing targets are usually heavy metals [10,11,12,13], pH [11,14,15,16,17,18], pH imaging [19,20,21,22,23,24], other metals and nonmetals [9,25,26,27,28,29,30,31]. In addition, microfluidics combined with LAPS have been used to monitor ion diffusion for a long time [30]. A LAPS-based method has also been proposed to measure corrosion, combined with biosensor corrosion of materials [18].
Food safety has always been one of the most important application fields of chemical sensors. When detecting video pollutants, SPR and optical biosensors are usually used to detect pesticides, pathogens, heavy metal ions and toxic substances. With the progress in microfluidic and optical technology, the demand for optical biosensors in food safety is increasing. LAPS has been applied to the detection of food safety in recent years, such as heavy metals in fish [12] and Cd content in rice [13]. Figure 5 shows great potential for food safety monitoring using a LAPS. More detailed information of LAPS’s application to chemical sensing is shown in Table 1.
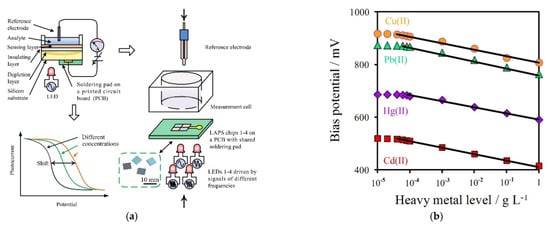
Figure 5.
(a,b) A rapid method for the tracing of Cd (II), Pb (II), Cu (II) and Hg (II) in fish tissues [12].

Table 1.
Categories, targets, technology, detection limits or ranges, noise and measurement times of articles on LAPS chemical sensing and imaging published in recent years.
3.2. Advanced Materials for Chemical Sensing and Imaging
LAPS chip materials will affect the spatial resolution [35,36,37,38,39,40,41,42,43,44,45], sensitivity [31,35,37,46,47,48,49,50], stability [51], manufacturing simplicity and costs [38,52,53,54], specific molecular binding ability [55,56,57,58,59], imaging speed [60], super hydrophilic analysis [61] and multi-component analysis ability [62] of LAPS. Therefore, people continue to improve the performances of LAPS devices with new substrate materials and doping methods. For example, Zhou et al. found that as a photocurrent imaging substrate without any modifications, In0.175Ga0.825N/GaN obtains greater photocurrent under a semiconductor laser, and clearer PMMA dots and photocurrent images of mesenchymal stem cells under a focused laser beam, as shown in Figure 6, giving it greater advantages compared with ITO and ZnO substrates [40].
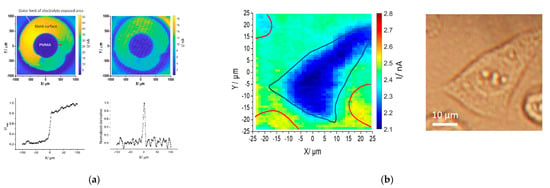
Figure 6.
(a) AC photocurrent images of a PMMA dot on InGaN measured at 0.6V; (b) AC photocurrent image of a mesenchymal stem cell on InGaN surface [40].
The LAPS has shown high sensitivity, resolution and imaging speed with various ions and pH levels, since ion and pH measurement became the major application from the very invention of the LAPS. As Table 2 shows, a well designed chemical sensor with LAPS can not only achieve high sensitivity and a low signal-to-noise ratio, but also become a candidate for the development of a biosensor. As we mentioned above, LAPS has great potential for food safety detection, in which the boundary between chemical sensing and biosensing is getting blurred. Therefore, detection of heavy metals and bacteria is suggested to be combined together using LAPS as a platform in the future, but we will face more challenges on specificity, stability and standardization problems.

Table 2.
Targets, main improvements, technology, detection limits or ranges, noise and measurement times of articles on the improvements in LAPS chip materials published in recent years.
4. LAPS for Biosensing
4.1. Biosensing and Imaging
A biosensor using LAPS uses enzymes [19,38,64], antigens or antibodies [65,66,67], DNA [68,69,70,71], cells [72,73,74,75,76], sensitive materials [77,78,79,80] or biological initiators [81] to detect specific biochemical molecules of interest, which greatly expands the detection range of LAPS and generally provides good specificity, as shown in Table 3. In addition, the rapid detection of LAPS can overcome the disadvantage of poor binding stability between the LAPS chip and biomolecules or cells to a certain extent.

Table 3.
Categories, targets, technology, sensitivities, detection limits or ranges, noise and measurement times of articles on LAPS biosensing and imaging published in recent years.
4.2. Cell Monitoring
A LAPS has the ability to characterize the chemical processes of cells cultured on the LAPS’s surface, which is a unique advantage of LAPS among electrochemical sensors. As a result, people can use LAPS for monitoring and imaging of cell metabolism. The target cells monitored in recent studies were rat renal cells [87,88,89], cardiac myocytes [90,91], human breast cancer cells [2], mouse embryonic fibroblasts [92], Escherichia coli [65,93,94,95,96], HeLa cell lines [97], Chinese hamster oval (CHO) cells [25,96,98,99], adrenal chromaffin cells [100], C3 cells [99], Corynebacterium glutamicum [93,94], Lactobacillus brevis [93,94,101], MDA MB231 and MDA-MB-435MDR [102] and hepatoma HepG2 cells [103]. Stimulated cells have also been reportedly monitored by LAPS [84,85,104,105]. On this basis, LAPS cell monitoring was further applied to in vivo pH probes [106], deep brain recordings [107] and detection of cells [108,109,110,111].
LAPS can realize more effective monitoring of cell activities, especially high-speed monitoring of cell metabolites, cell responses to different targets and the extracellular environment in a label free sensing process, which can hardly be achieved by other optical imaging or chemical sensing methods. However, the biosensing processes, especially cell monitoring, are limited mainly because of the low biocompatibility of LAPS chips and the difficulty of raising photocurrent and spatial resolution with materials and optical methods. The imaging times are too long to get more detailed information of cell activities as well, according to Table 4. Therefore, for the development of biosensors using LAPS, we should continually focus on achieving higher photocurrent, spatial resolution and scanning speed, and better stability and biocompatibility. More aspects of cell interaction with LAPS chips and electrolyte and optical systems should be considered in the development of biosensors using LAPS.

Table 4.
Categories, targets, technology, sensitivities, detection limits or ranges, noise and measurement times of articles on LAPS cell monitoring published in recent years.
5. Optical System Improvements for LAPS
Higher lateral resolution, sensitivity, stability, measurement and imaging speed are the main improvements achieved by improving the optical system of a LAPS. These improvements include light sources [64], optical devices, optical control systems, etc. For example, Zhou et al. proposed a new high-spatio-temporal resolution photoelectrochemical imaging system (PEIS) which uses an analog micromirror to obtain a diffraction-limited laser spot to scan the sensor’s surface (see Figure 7a). The multifunctional system is based on an electrolyte insulator semiconductor (EIS). This structure achieves very fast LAPS measurements and high-speed AC/DC photoelectrochemical imaging based on its electrolyte semiconductor (ES) structure, along with high lateral resolution. The use of PEIS makes the details of cell viability clearer than with a typical fluorescence microscope. In addition, EIS can image multiple cells simultaneously and continuously, and monitor the concentrations of ions and metabolites at the same time, as shown in Figure 7b, which provides electrochemical information that other electrochemical imaging devices do not [115]. Miyamoto et al. used mixed illumination composed of a modulated beam and annular constant illumination to suppress the transverse diffusion of optical carriers through enhanced recombination [116]. The spatial resolution of the chemical imaging sensor has been improved and can distinguish chemical images of 100 μm. More detailed information of overall progress by improving optical system for LAPS is shown in Table 5.
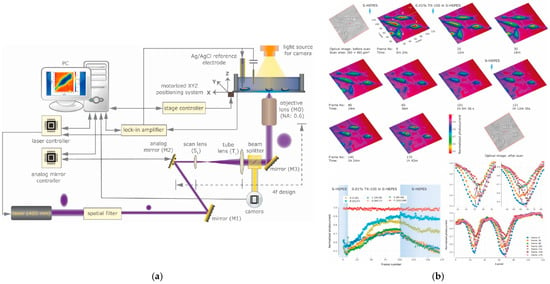
Figure 7.
(a) Schematic of the photoelectrochemical imaging setup with an analog micro-mirror-based fast beam steering was achieved with 4f design lens relay system. (b) Photocurrent changes under cells during exposure to a TX-100 concentration smaller than the critical micelle concentration. Including time-lapse photocurrent images of B50 cells exposed to 0.01% TX-100 in S-HEPES buffer, time-dependent photocurrent traces for individual cells and photocurrent X-axis line scan analysis [115].

Table 5.
Targets, main improvements, technology, detection limits or ranges, noise and measurement times of articles on the improvement of LAPS optical systems published in recent years.
6. Conclusions
LAPS has great application prospects in chemical and biomedical sensing and imaging, but many new problems have emerged. There are many problems and challenges to be solved for emerging chemical materials: the affinity and stability of biomaterials combined with LAPS chips, and physical simulation and optical system construction. Both chemical sensors and biosensors based on LAPS are facing the challenge of difficulties improving sensitivity, selectivity and detection limits in time and space, including photocurrent, special resolution and speed of imaging. The difference is that chemical sensors care more about sensitivity, whereas specificity, stability and biocompatibility are highly valued in the development of biosensors. In the future, the abiotic parts of a LAPS could be considered as a whole system in the device simulation process, combining the electrolyte insulator interface with the insulator semiconductor part of a LAPS and optical system during sensor developing and data processing. It is predictable that the developments in electrodes and optical systems will provide higher photocurrents and imaging speeds for LAPS. Integrating the field-effect charge sensing process in LAPS with microfluidic technologies could further improve the sensitivity and accuracy. Moreover, more strategies for interface functionalization of biomacromolecule and cell culturing should be used to take more advantage of the light addressability in biosensing process. At the same time, miniaturization and performance improvements are being carried out, which may allow more applications in biomedicines.
Author Contributions
Y.L.: Conceptualization, Writing—Original draft preparation; P.Z.: Writing —Review and Editing, Visualization; S.L.: Writing—Review and Editing, Visualization; Y.C.: Writing—Review and Editing; D.L.: Writing—Review and Editing; M.W.: Conceptualization, Writing—Review and Editing; L.D.: Conceptualization, Writing—Review and Editing, Supervision, Funding acquisition; C.W.: Writing—Review and Editing, Supervision, Funding Acquisition, Project Administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported in part by grants from the National Natural Science Foundation of China (grant numbers 32071370, 51861145307, and 31700859), and the Key Research and Development Program of Shaanxi Province—International Science and Technology Cooperation General Project (grant number 2022KW-23).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could appeared to have influenced the work reported in this paper. The authors declare no conflict of interest.
References
- Dean, G.; Hafeman, J.; Wallace, P.; Harden, M.M. Light-Addressable Potentiometric Sensor for Bio-chemical Systems. Science 1988, 240, 1182–1185. [Google Scholar]
- Hu, N.; Wu, C.; Ha, D.; Wang, T.; Liu, Q.; Wang, P. A novel microphysiometer based on high sensitivity LAPS and microfluidic system for cellular metabolism study and rapid drug screening. Biosens. Bioelectron. 2012, 40, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yoshinobu, T.; Miyamoto, K.I.; Werner, C.F.; Poghossian, A.; Wagner, T.; Schoning, M.J. Light-Addressable Potentiometric Sensors for Quantitative Spatial Imaging of Chemical Species. Annu. Rev. Anal. Chem. 2017, 10, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Yoshinobu, T.; Sato, D.; Guo, Y.; Werner, C.F.; Miyamoto, K.-I. Modeling of the Return Current in a Light-Addressable Potentiometric Sensor. Sensors 2019, 19, 4566. [Google Scholar] [CrossRef]
- Werner, C.F.; Wagner, T.; Yoshinobu, T.; Keusgen, M.; Schoening, M.J. Frequency behaviour of light-addressable potentiometric sensors. Phys. Status Solidi 2013, 210, 884–891. [Google Scholar] [CrossRef]
- Guo, Y.; Miyamoto, K.-I.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Device simulation of the light-addressable potentiometric sensor for the investigation of the spatial resolution. Sens. Actuators B Chem. 2014, 204, 659–665. [Google Scholar] [CrossRef]
- Guo, Y.; Seki, K.; Miyamoto, K.-I.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Device Simulation of the Light-addressable Potentiometric Sensor with a Novel Photoexcitation Method for a Higher Spatial Resolution. Procedia Eng. 2014, 87, 456–459. [Google Scholar] [CrossRef][Green Version]
- Guo, Y.; Miyamoto, K.-I.; Wagner, T.; Schoening, M.J.; Yoshinobu, T. Theoretical study and simulation of light-addressable potentiometric sensors. Phys. Status Solidi 2014, 211, 1467–1472. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Iwasaki, H.; Ui, Y.; Furuichi, K.; Ermolenko, Y.; Mourzina, Y.; Wagner, T.; Näther, N.; Schöning, M. The light-addressable potentiometric sensor for multi-ion sensing and imaging. Methods 2005, 37, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Hu, N.; Wu, C.; Kirsanov, D.; Legin, A.; Khaydukova, M.; Wang, P. Novel structured light-addressable potentiometric sensor array based on PVC membrane for determination of heavy metals. Sens. Actuators B Chem. 2012, 174, 59–64. [Google Scholar] [CrossRef]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Design of a miniaturized multisensor chip with nanoband electrode array and light addressable potentiometric sensor for ion sensing. Anal. Methods 2015, 7, 9190–9197. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Tahir, H.E.; Zou, X.; Wang, P. Rapid and wide-range determination of Cd(II), Pb(II), Cu(II) and Hg(II) in fish tissues using light addressable potentiometric sensor. Food Chem. 2017, 221, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Zou, X. Rapid determination of cadmium in rice using an all-solid RGO-enhanced light addressable potentiometric sensor. Food Chem. 2018, 261, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lue, C.E.; Lai, C.S.; Wang, J.C.; Wu, C.M.; Yang, C.M. Differential Light Addressable Potentiometric Sensor with Poly(vinyl chloride) and HfO2Membranes for pH Sensors. Jpn. J. Appl. Phys. 2010, 49, 04DL10. [Google Scholar] [CrossRef]
- DAS, A.; Das, A.; Chang, L.B.; Lai, C.S.; Lin, R.M.; Chu, F.C.; Lin, Y.H.; Chow, L.; Jeng, M.J. GaN Thin Film Based Light Addressable Potentiometric Sensor for pH Sensing Application. Appl. Phys. Express 2013, 6, 036601. [Google Scholar] [CrossRef]
- Chin, C.H.; Lu, T.F.; Wang, J.C.; Yang, J.H.; Lue, C.E.; Yang, C.M.; Li, S.S.; Lai, C.S. Effects of CF4Plasma Treatment on pH and pNa Sensing Properties of Light-Addressable Potentiometric Sensor with a 2-nm-Thick Sensitive HfO2Layer Grown by Atomic Layer Deposition. Jpn. J. Appl. Phys. 2011, 50, 04DL06. [Google Scholar] [CrossRef]
- Lue, C.E.; Lai, C.S.; Chen, H.Y.; Yang, C.M. Light Addressable Potentiometric Sensor with Fluorine-Terminated Hafnium Oxide Layer for Sodium Detection. Jpn. J. Appl. Phys. 2010, 49, 04DL05. [Google Scholar] [CrossRef]
- Nose, K.; Miyamoto, K.-I.; Yoshinobu, T. Estimation of Potential Distribution during Crevice Corrosion through Analysis of I–V Curves Obtained by LAPS. Sensors 2020, 20, 2873. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Das, A.; Lai, C.S. A Simple and Convenient Set-Up of Light Addressable Potentiometric Sensors (LAPS) for Chemical Imaging Using a Commercially Available Projector as a Light Source. Int. J. Electrochem. Sci. 2013, 8, 7062–7074. [Google Scholar]
- Miyamoto, K.-I.; Wagner, T.; Yoshinobu, T.; Kanoh, S.; Schoening, M.J. Phase-mode LAPS and its application to chemical imaging. Sens. Actuators B Chem. 2011, 154, 28–32. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Kanzaki, R.; Takahashi, H. Microscale pH gradient generation by electrolysis on a light-addressable planar electrode. Sens. Actuators B Chem. 2010, 149, 205–211. [Google Scholar] [CrossRef]
- Miyamoto, K.-I.; Kuwabara, Y.; Kanoh, S.; Yoshinobu, T.; Wagner, T.; Schöning, M.J. Chemical image scanner based on FDM-LAPS. Sens. Actuators B Chem. 2009, 137, 533–538. [Google Scholar] [CrossRef]
- Miyamoto, K.; Wagner, T.; Mimura, S.; Kanoh, S.I.; Yoshinobu, T.; Schöning, M.J. Constant-phase-mode operation of the light-addressable potentiometric sensor. Sens. Actuators B Chem. 2011, 154, 119–123. [Google Scholar] [CrossRef]
- Miyamoto, K.; Seki, K.; Guo, Y.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Enhancement of the Spatial Resolution of the Chemical Imaging Sensor by a Hybrid Fiber-Optic Illumination. Procedia Eng. 2014, 87, 612–615. [Google Scholar] [CrossRef][Green Version]
- Takenaga, S.; Schneider, B.; Erbay, E.; Biselli, M.; Schnitzler, T.; Schöning, M.J.; Wagner, T. Fabrication of bio-compatible lab-on-chip devices for biomedical applications by means of a 3D-printing process. Phys. Status Solidi 2015, 212, 1347–1352. [Google Scholar] [CrossRef]
- Yang, J.H.; Lu, T.F.; Wang, J.C.; Lue, C.E.; Lai, C.S. Functionalization of nanoscaled 2 nm-thick ALD-HfO2 layer by rapid thermal annealing and CF4 plasma for LAPS NH4+ detection. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2118–2121. [Google Scholar]
- Yang, J.-H.; Lu, T.-F.; Wang, J.C.; Yang, C.-M.; Pijanowska, D.; Chin, C.H.; Lue, C.E.; Lai, C.S. LAPS with nanoscaled and highly polarized HfO2 by CF4 plasma for NH4+ detection. Sens. Actuators B Chem. 2013, 180, 71–76. [Google Scholar] [CrossRef]
- Wang, J.-C.; Ye, Y.-R.; Lin, Y.-H.; Johnson, D. Light-Addressable Potentiometric Sensor with Nitro-gen-Incorporated Ceramic Sm2O3 Membrane for Chloride Ions Detection. J. Am. Ceram. Soc. 2015, 98, 443–447. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, N.; Li, S.; Jia, H.; Xue, Y.; Cui, L.; Huang, Y.; Li, G. Light-addressable potentiometric sensor with gold nanoparticles enhancing enzymatic silver deposition for 1,5-anhydroglucitol determination. Biochem. Eng. J. 2017, 123, 29–37. [Google Scholar] [CrossRef]
- Miyamoto, K.I.; Ichimura, H.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Chemical imaging of the concentration profile of ion diffusion in a microfluidic channel. Sens. Actuators B Chem. 2013, 189, 240–245. [Google Scholar] [CrossRef]
- Chen, T.C.; Zeng, W.Y.; Liao, Y.H.; Das, A.; Yang, C.M.; Lai, C.-S. High photocurrent and operation frequency for light-addressable potentiometric sensor by thinner Si substrate. In Proceedings of the 2014 IEEE International Nanoelectronics Conference (INEC), Sapporo, Japan, 28–31 July 2014; pp. 1–3. [Google Scholar]
- Wagner, T.; Werner, C.; Miyamoto, K.; Schöning, M.; Yoshinobu, T. A high-density multi-point LAPS set-up using a VCSEL array and FPGA control. Procedia Chem. 2009, 1, 1483–1486. [Google Scholar] [CrossRef][Green Version]
- Wan, H.; Ha, D.; Zhang, W.; Zhao, H.; Wang, X.; Sun, Q.; Wang, P. Design of a novel hybrid sensor with microe-lectrode array and LAPS for heavy metal determination using multivariate nonlinear calibration. Sens. Actuators B Chem. 2014, 192, 755–761. [Google Scholar] [CrossRef]
- Cai, W.; Zhao, H.X.; Ha, D.; Guo, H.S.; Zhang, W.; Wang, P. Design of wireless sensor node based on a novel hybrid chemical sensor for heavy metal monitoring. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing China, 5–9 June 2011; pp. 2114–2117. [Google Scholar]
- Yoshinobu, T.; Schöning, M.J. Light-addressable potentiometric sensors for cell monitoring and biosensing. Curr. Opin. Electrochem. 2021, 28, 100727. [Google Scholar] [CrossRef]
- Zeng, W.-Y.; Chen, C.-C.; Yang, C.-M.; Lai, C.-S. High photocurrent and high frequency response of light-addressable potentiometrie sensor with thin Si substrate and surface roughness. In 2015 IEEE SENSORS; IEEE: Pusan, Korea, 2015; pp. 1–3. [Google Scholar]
- Yu, H.; Wang, J.; Liu, Q.; Zhang, W.; Cai, H.; Wang, P. High spatial resolution impedance measurement of EIS sensors for light addressable cell adhesion monitoring. Biosens. Bioelectron. 2011, 26, 2822–2827. [Google Scholar] [CrossRef]
- Yang, C.-M.; Zeng, W.-Y.; Chen, Y.-P.; Chen, T.-C. Surface Modification for High Photocurrent and pH Sensitivity in a Silicon-Based Light-Addressable Potentiometric Sensor. IEEE Sens. J. 2018, 18, 2253–2259. [Google Scholar] [CrossRef]
- Das, A.; Lin, Y.-H.; Lai, C.-S. Miniaturized amorphous-silicon based chemical imaging sensor system using a mini-projector as a simplified light-addressable scanning source. Sens. Actuators B Chem. 2014, 190, 664–672. [Google Scholar] [CrossRef]
- Yang, C.M.; Liao, Y.H.; Chen, C.H.; Chen, C.C.; Lai, C.S. P-I-N Amorphous Silicon Light-Addressable Potentiometric Sensors for High-photovoltage Chemical Image. Procedia Eng. 2015, 120, 1015–1018. [Google Scholar] [CrossRef]
- Zhou, B.; Das, A.; Kappers, M.J.; Oliver, R.A.; Humphreys, C.J.; Krause, S. InGaN as a Substrate for AC Photo-electrochemical Imaging. Sensors 2019, 19, 4386. [Google Scholar] [CrossRef]
- Tu, Y.; Ahmad, N.; Briscoe, J.; Zhang, D.W.; Krause, S. Light-Addressable Potentiometric Sensors Using ZnO Nanorods as the Sensor Substrate for Bioanalytical Applications. Anal. Chem. 2018, 90, 8708–8715. [Google Scholar] [CrossRef]
- Yang, C.M.; Liao, Y.H.; Chen, C.H.; Chen, T.C.; Lai, C.S.; Pijanowska, D. P-I-N amorphous silicon for thin-film light-addressable potentiometric sensors. Sens. Actuators B Chem. 2016, 236, 1005–1010. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, C.M.; Chang, L.B.; Lai, C.S. Thickness effect of IGZO layer in light-addressable potentiometric sensor. In Proceedings of the 2016 23rd International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 6–8 July 2016; pp. 203–205. [Google Scholar]
- Siqueira, J.J.R.; Maki, R.M.; Paulovich, F.V.; Werner, C.F.; Poghossian, A.; de Oliveira, M.C.F.; Zucolotto, V.; Oliveira, J.O.N.; Schöning, M.J. Use of Information Visualization Methods Eliminating Cross Talk in Multiple Sensing Units Investigated for a Light-Addressable Potentiometric Sensor. Anal. Chem. 2009, 82, 61–65. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Ipatov, A. Recent trends in potentiometric sensor arrays—A review. Anal. Chim. Acta 2010, 678, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Watkinson, M.; Gautrot, J.; Krause, S. High-sensitivity light-addressable potentiometric sensors using silicon on sapphire functionalized with self-assembled organic monolayers. Sens. Actuators B Chem. 2015, 209, 230–236. [Google Scholar] [CrossRef]
- Yang, C.M.; Chiang, T.W.; Yeh, Y.T.; Das, A.; Lin, Y.T.; Chen, T.-C. Sensing and pH-imaging properties of niobium oxide prepared by rapid thermal annealing for electrolyte–insulator–semiconductor structure and light-addressable potentiometric sensor. Sens. Actuators B Chem. 2015, 207, 858–864. [Google Scholar] [CrossRef]
- Wei, C.K.; Peng, H.-Y.; Tsai, Y.-C.; Chen, T.-C.; Yang, C.M. Fluorographene sensing membrane in a light-addressable potentiometric sensor. Ceram. Int. 2019, 45, 9074–9081. [Google Scholar] [CrossRef]
- Yang, C.M.; Chen, C.-H.; Chang, L.B.; Lai, C.-S. IGZO Thin-Film Light-Addressable Potentiometric Sensor. IEEE Electron Device Lett. 2016, 37, 1481–1484. [Google Scholar] [CrossRef]
- Yang, C.-M.; Yang, Y.-C.; Chen, C.-H. Thin-film light-addressable potentiometric sensor with SnOx as a photo-sensitive semiconductor. Vacuum 2019, 168, 108809. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Takahashi, H. Surface passivation of the thin-film LAPS with perhydropolysilaz-ane-derived silica treated by O2 plasma. IEEJ Trans. Electr. Electron. Eng. 2011, 6, 392–393. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Jimbo, Y.; Kanzaki, R.; Takahashi, H. A light addressable electrode with a TiO2 nanocrystalline film for localized electrical stimulation of cultured neurons. Sens. Actuators B Chem. 2014, 192, 393–398. [Google Scholar] [CrossRef]
- Litvinenko, S.; Kozinetz, A.; Skryshevsky, V. Concept of photovoltaic transducer on a base of modified p–n junction solar cell. Sens. Actuators A Phys. 2015, 224, 30–35. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wu, F.; Krause, S. LAPS and SPIM Imaging Using ITO-Coated Glass as the Substrate Material. Anal. Chem. 2017, 89, 8129–8133. [Google Scholar] [CrossRef]
- Yue, Z.; Khalid, W.; Zanella, M.; Abbasi, A.Z.; Pfreundt, A.; Gil, P.R.; Schubert, K.; Lisdat, F.; Parak, W.J. Evaluation of quantum dots applied as switchable layer in a light-controlled electrochemical sensor. Anal. Bioanal. Chem. 2009, 396, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Y.; Krause, S.; Munoz, A.G.; Kunze, J.; Schmuki, P. Repair of thin thermally grown silicon dioxide by anodic oxidation. Electrochim. Acta 2008, 53, 3395–3402. [Google Scholar] [CrossRef]
- Zarei, L.; Tavallaie, R.; Choudhury, M.H.; Parker, S.G.; Bakthavathsalam, P.; Ciampi, S.; Gonçales, V.R.; Gooding, J.J. DNA-Hybridization Detection on Si(100) Surfaces Using Light-Activated Electrochemistry: A Comparative Study between Bovine Serum Albumin and Hexaethylene Glycol as Antifouling Layers. Langmuir 2018, 34, 14817–14824. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, F.; Watkinson, M.; Zhu, J.; Krause, S. "Click" Patterning of Self-Assembled Monolayers on Hydro-gen-Terminated Silicon Surfaces and Their Characterization Using Light-Addressable Potentiometric Sensors. Langmuir 2015, 31, 9646–9654. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Campos, I.; Wu, F.; Zhu, J.; Sukhorukov, G.B.; Palma, M.; Watkinson, M.; Krause, S. The effect of gold nanoparticles on the impedance of microcapsules visualized by scanning photo-induced impedance microscopy. Electrochim. Acta 2016, 208, 39–46. [Google Scholar] [CrossRef]
- Yang, C.-M.; Zeng, W.-Y.; Chen, C.-H.; Chen, Y.-P.; Chen, T.-C. Spatial resolution and 2D chemical image of light-addressable potentiometric sensor improved by inductively coupled-plasma reactive-ion etching. Sens. Actuators B Chem. 2018, 258, 1295–1301. [Google Scholar] [CrossRef]
- Özsoylu, D.; Kizildag, S.; Schöning, M.J.; Wagner, T. Effect of Plasma Treatment on the Sensor Properties of a Light-Addressable Potentiometric Sensor (LAPS). Phys. Status Solidi 2019, 216, 1900259. [Google Scholar] [CrossRef]
- Schöning, M.J.; Kloock, J.P. About 20 Years of Silicon-Based Thin-Film Sensors with Chalcogenide Glass Mate-rials for Heavy Metal Analysis: Technological Aspects of Fabrication and Miniaturization. Electroanalysis 2007, 19, 2029–2038. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wu, F.; Wang, J.; Watkinson, M.; Krause, S. Image detection of yeast Saccharomyces cerevisiae by light-addressable potentiometric sensors (LAPS). Electrochem. Commun. 2016, 72, 41–45. [Google Scholar] [CrossRef]
- Liu, J.; Jia, Y. Label-free protein chip and its detection system realization. In Proceedings of the 2011 IEEE International Conference of Electron Devices and Solid-State Circuits, Seoul, Korea, 7–10 August 2011; pp. 1–2. [Google Scholar]
- Jia, Y.-F.; Gao, C.-Y.; He, J.; Feng, D.-F.; Xing, K.L.; Wu, M.; Liu, Y.; Cai, W.-S.; Feng, X.-Z. Unlabeled multi tumor marker detection system based on bioinitiated light addressable potentiometric sensor. Analyst 2012, 137, 3806–3813. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Guan, M.; Huang, G.; Qiu, H.; Chen, Z.; Li, G.; Huang, Y. Highly sensitive covalently functionalized light-addressable potentiometric sensor for determination of biomarker. Mater. Sci. Eng. C 2016, 63, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yin, X.B.; Zhang, J.; Zhou, S.; Song, M.; Xing, K.L. Graphene oxide modified light addressable potentiometric sensor and its application for ssDNA monitoring. Analyst 2012, 137, 5866–5873. [Google Scholar] [CrossRef]
- Bronder, T.; Wu, C.; Poghossian, A.; Werner, C.; Keusgen, M.; Schöning, M. Label-free Detection of DNA Hybridization with Light-addressable Potentiometric Sensors: Comparison of Various DNA- immobilization Strategies. Procedia Eng. 2014, 87, 755–758. [Google Scholar] [CrossRef]
- Shao, C.; Zhou, S.; Yin, X.-B.; Gu, Y.; Jia, Y. Influences of Probe’s Morphology for Metal Ion Detection Based on Light-Addressable Potentiometric Sensors. Sensors 2016, 16, 701. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Men, H.; Zou, S.; Li, Y.; Wang, Y.; Ye, X.; Wang, P. A novel electronic tongue combined MLAPS with stripping voltammetry for environmental detection. Sens. Actuators B Chem. 2005, 110, 350–357. [Google Scholar] [CrossRef]
- Werner, C.F.; Groebel, S.; Krumbe, C.; Wagner, T.; Selmer, T.; Yoshinobu, T.; Baumann, M.E.M.; Keusgen, M.; Schoening, M.J. Nutrient concentration-sensitive microorganism-based biosensor. Phys. Status Solidi 2012, 209, 900–904. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, H.; Xu, Y.; Li, Y.; Li, R.; Wang, P. Olfactory cell-based biosensor: A first step towards a neurochip of bioelectronic nose. Biosens. Bioelectron. 2006, 22, 318–322. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, W.; Yu, H.; Hu, N.; Du, L.; Wang, P.; Yang, M. Olfactory mucosa tissue-based biosensor: A bioelectronic nose with receptor cells in intact olfactory epithelium. Sens. Actuators B Chem. 2010, 146, 527–533. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, W.; Hu, N.; Cai, H.; Yu, H.; Wang, P. Olfactory receptor cells respond to odors in a tissue and semi-conductor hybrid neuron chip. Biosens Bioelectron 2010, 26, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, F.; Jia, T.; Wang, Z. Meso-tetra(4-carboxyphenyl)porphine-Enhanced DNA Methylation Sensing Interface on a Light-Addressable Potentiometric Sensor. ACS Omega 2019, 4, 12567–12574. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, S.; Zhang, R.; Gu, Y.; Li, Y.; Jia, Y. Porous Graphene Oxide Enhanced Aptamer Specific Circulating-Tumor-Cell Sensing Interface on Light Addressable Potentiometric Sensor: Clinical Application and Simulation. ACS Appl Mater Interfaces 2019, 11, 8704–8709. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, F. Studies of Functional Nucleic Acids Modified Light Addressable Potentiometric Sensors: X-ray Photoelectron Spectroscopy, Biochemical Assay, and Simulation. Anal. Chem. 2018, 90, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Cai, H.; Li, Y.; Li, R.; Wang, P.; Yang, G.G. Investigation of light addressable potentiometric sensor array sensitive to heavy metal ion based on micro-lens array. J. Zhejiang Univ. Eng. Ing Sci. 2008, 42, 517. [Google Scholar]
- Jia, Y.; Gao, C.; Feng, D.; Wu, M.; Liu, Y.; Chen, X.; Xing, K.; Feng, X. Bio-initiated light addressable potentiometric sensor for unlabeled biodetection and its MEDICI simulation. Analyst 2011, 136, 4533–4538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyamoto, K.I.; Yoshida, M.; Sakai, T.; Matsuzaka, A.; Wagner, T.; Kanoh, S.I.; Yoshinobu, T.; Schöning, M.J. Differential Setup of Light-Addressable Potentiometric Sensor with an Enzyme Reactor in a Flow Channel. Jpn. J. Appl. Phys. 2011, 50, 04DL08. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zou, X.; Zhang, H.; Xu, X. Micrometer-scale light-addressable potentiometric sensor on an optical fiber for biological glucose determination. Anal. Chim. Acta 2020, 1123, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Liu, Q.; Xu, Y.; Cai, H.; Wang, P. A novel experimental research based on taste cell chips for taste transduction mechanism. Sens. Actuators B Chem. 2008, 131, 24–28. [Google Scholar] [CrossRef]
- Liu, H.L.; Chen, Y.M.; Yang, M.C.; Lai, C.S. Real-time 2D pH images by fast scanning light-addressable Potentiometrie sensor system controlled by LabVIEW program. IEEE Sens. 2015, 1–3. [Google Scholar] [CrossRef]
- Werner, C.F.; Takenaga, S.; Taki, H.; Sawada, K.; Schöning, M.J. Comparison of label-free ACh-imaging sensors based on CCD and LAPS. Sens. Actuators B Chem. 2013, 177, 745–752. [Google Scholar] [CrossRef]
- Hu, N.; Ha, D.; Wu, C.; Zhou, J.; Kirsanov, D.; Legin, A.; Wang, P. A LAPS array with low cross-talk for non-invasive measurement of cellular metabolism. Sens. Actuators A Phys. 2012, 187, 50–56. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Cai, H.; Du, L.P.; Liu, Q.J.; Wang, P. A photovoltage-based integrated sensor for nephrotoxi-city evaluation under drug stimulation. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2122–2125. [Google Scholar]
- Hu, N.; Zhou, J.; Su, K.; Zhang, D.; Xiao, L.; Wang, T.; Wang, P. An integrated label-free cell-based biosensor for simultaneously monitoring of cellular physiology multiparameter in vitro. Biomed. Microdevices 2013, 15, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cai, H.; Zhang, W.; Xiao, L.; Liu, Q.; Wang, P. A novel design of multifunctional integrated cell-based biosensors for simultaneously detecting cell acidification and extracellular potential. Biosens. Bioelectron. 2009, 24, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, T.; Cao, J.; Su, K.; Zhou, J.; Wu, J.; Wang, P. Comparison between ECIS and LAPS for establishing a cardiomyocyte-based biosensor. Sens. Actuators B Chem. 2013, 185, 238–244. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, J.; Hu, N.; Ha, D.; Miao, X.; Wang, P. Cellular impedance sensing combined with LAPS as a new means for real-time monitoring cell growth and metabolism. Sens. Actuators A Phys. 2013, 199, 136–142. [Google Scholar] [CrossRef]
- Dantism, S.; Röhlen, D.; Dahmen, M.; Wagner, T.; Wagner, P.; Schöning, M.J. LAPS-based monitoring of metabolic responses of bacterial cultures in a paper fermentation broth. Sens. Actuators B Chem. 2020, 320, 128232. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Miyamoto, K.; Wagner, T.; Schöning, M.J. Recent developments of chemical imaging sensor systems based on the principle of the light-addressable potentiometric sensor. Sens. Actuators B Chem. 2015, 207, 926–932. [Google Scholar] [CrossRef]
- Dantism, S.; Takenaga, S.; Wagner, P.; Wagner, T.; Schöning, M.J. Determination of the extracellular acidification of Escherichia coliK12 with a multi-chamber-based LAPS system. Phys. Status Solidi 2016, 213, 1479–1485. [Google Scholar] [CrossRef]
- Dantism, S.; Takenaga, S.; Wagner, T.; Wagner, P.; Schöning, M.J. Differential imaging of the metabolism of bacteria and eukaryotic cells based on light-addressable potentiometric sensors. Electrochim. Acta 2017, 246, 234–241. [Google Scholar] [CrossRef]
- Su, K.; Zhou, J.; Zou, L.; Wang, T.; Zhuang, L.; Hu, N.; Wang, P. Integrated multifunctional cell-based biosensor system for monitoring extracellular acidification and cellular growth. Sens. Actuators A Phys. 2014, 220, 144–152. [Google Scholar] [CrossRef]
- Dantism, S.; Takenaga, S.; Wagner, P.; Wagner, T.; Schöning, M. Light-addressable Potentiometric Sensor (LAPS) Combined with Multi-chamber Structures to Investigate the Metabolic Activity of Cells. Procedia Eng. 2015, 120, 384–387. [Google Scholar] [CrossRef]
- Takenaga, S.; Herrera, C.; Werner, C.F.; Biselli, M.; Thönnessen, V.; Schnitzler, T.; Öhlschläger, P.; Almajhdi, F.N.; Wagner, T.; Schöning, M.J. Toward multi-analyte bioarray sensors: LAPS-based on-chip determination of a Michaelis-Menten-like kinetics for cell culturing. Phys. Status Solidi 2014, 211, 1410–1415. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, N.; Zhang, F.; Wang, H.; Ye, W.; Wang, P. Neurosecretory cell-based biosensor: Monitoring secretion of adrenal chromaffin cells by local extracellular acidification using light-addressable potentiometric sensor. Biosens. Bioelectron. 2012, 35, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Dantism, S.; Rohlen, D.; Selmer, T.; Wagner, T.; Wagner, P.; Schoning, M.J. uantitative differential monitoring of the metabolic activity of Corynebacterium glutamicum cultures utilizing a light-addressable potentiometric sensor system. Biosens. Bioelectron. 2019, 139, 111332. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Naicker, S.; Kaur, K.; Thundat, T. Metabolic Study of Cancer Cells Using a pH Sensitive Hydrogel Nanofiber Light Addressable Potentiometric Sensor. ACS Sens 2017, 2, 151–156. [Google Scholar] [CrossRef]
- Liang, T.; Gu, C.; Gan, Y.; Wu, Q.; He, C.; Tu, J.; Pan, Y.; Qiu, Y.; Kong, L.; Wan, H.; et al. Microfluidic chip system integrated with light addressable potentiometric sensor (LAPS) for real-time extracellular acidification detection. Sens. Actuators B Chem. 2019, 301, 127004. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, H.; Tan, Z.; Cai, H.; Ye, W.; Zhang, M.; Wang, P. In vitro assessing the risk of drug-induced cardio-toxicity by embryonic stem cell-based biosensor. Sens. Actuators B Chem. 2011, 155, 214–219. [Google Scholar] [CrossRef]
- Kimmel, D.W.; Meschievitz, M.E.; Hiatt, L.A.; Cliffel, D.E. Multianalyte Microphysiometry of Macrophage Responses to Phorbol Myristate Acetate, Lipopolysaccharide, and Lipoarabinomannan. Electroanalysis 2013, 25, 1706–1712. [Google Scholar] [CrossRef]
- Guo, Y.; Werner, C.F.; Handa, S.; Wang, M.; Ohshiro, T.; Mushiake, H.; Yoshinobu, T. Miniature multiplexed label-free pH probe in vivo. Biosens. Bioelectron. 2021, 174, 112870. [Google Scholar] [CrossRef]
- Guo, Y.; Werner, C.F.; Canales, A.; Yu, L.; Jia, X.; Anikeeva, P.; Yoshinobu, T. Polymer-fiber-coupled field-effect sensors for label-free deep brain recordings. PLoS ONE 2020, 15, e0228076. [Google Scholar] [CrossRef]
- Jia, Y.; Qin, M.; Zhang, H.; Niu, W.; Li, X.; Wang, L.; Li, X.; Bai, Y.; Cao, Y.; Feng, X. Label-free biosensor: A novel phage-modified Light Addressable Potentiometric Sensor system for cancer cell monitoring. Biosens. Bioelectron. 2007, 22, 3261–3266. [Google Scholar] [CrossRef] [PubMed]
- Shaibani, P.M.; Etayash, H.R.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable Nanofiber-Light Addressable Potentiometric Sensor for Rapid Escherichia coli Detection in Orange Juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Shaibani, P.M.; Jiang, K.; Haghighat, G.; Hassanpourfard, M.; Etayash, H.; Naicker, S.; Thundat, T. The detection of Escherichia coli (E. coli) with the pH sensitive hydrogel nanofiber-light addressable potentiometric sensor (NF-LAPS). Sens. Actuators B Chem. 2016, 226, 176–183. [Google Scholar] [CrossRef]
- Wagner, T.; Shigiahara, N.; Miyamoto, K.; Suzurikawa, J.; Finger, F.; Schöning, M.J.; Yoshinobu, T. Light-addressable Potentiometric Sensors and Light–addressable Electrodes as a Combined Sensor-and-manipulator Microsystem with High Flexibility. Procedia Eng. 2012, 47, 890–893. [Google Scholar] [CrossRef]
- Werner, C.F.; Krumbe, C.; Schumacher, K.; Groebel, S.; Spelthahn, H.; Stellberg, M.; Wagner, T.; Yoshinobu, T.; Selmer, T.; Keusgen, M.; et al. Determination of the extracellular acidification of Escherichia coli by a light-addressable potentiometric sensor. Phys. Status Solidi 2011, 208, 1340–1344. [Google Scholar] [CrossRef]
- Werner, C.F.; Wagner, T.; Miyamoto, K.-I.; Yoshinobu, T.; Schöning, M.J. High speed and high resolution chemical imaging based on a new type of OLED-LAPS set-up. Sens. Actuators B Chem. 2012, 175, 118–122. [Google Scholar] [CrossRef]
- Itabashi, A.; Kosaka, N.; Miyamoto, K.I.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. High-speed chemical imaging system based on front-side-illuminated LAPS. Sens. Actuators B Chem. 2013, 182, 315–321. [Google Scholar] [CrossRef]
- Zhou, B.; Das, A.; Zhong, M.; Guo, Q.; Zhang, D.W.; Hing, K.A.; Sobrido, A.J.; Titirici, M.M.; Krause, S. Photoelectrochemical imaging system with high spatiotemporal resolution for visualizing dynamic cellular responses. Biosens. Bioelectron. 2021, 180, 113121. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Seki, K.; Suto, T.; Werner, C.F.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Improved spatial resolution of the chemical imaging sensor with a hybrid illumination that suppresses lateral diffusion of photocarriers. Sens. Actuators B Chem. 2018, 273, 1328–1333. [Google Scholar] [CrossRef]
- Werner, C.F.; Miyamoto, K.-I.; Wagner, T.; Schoening, M.J.; Yoshinobu, T. Lateral resolution enhancement of pulse-driven light-addressable potentiometric sensor. Sens. Actuators B Chem. 2017, 248, 961–965. [Google Scholar] [CrossRef]
- Guo, Y.; Seki, K.; Miyamoto, K.I.; Wagner, T.; Schoening, M.J.; Yoshinobu, T. Novel photoexcitation method for light-addressable potentiometric sensor with higher spatial resolution. Appl. Phys. Express 2014, 7, 7. [Google Scholar] [CrossRef]
- Miyamoto, K.I.; Kaneko, K.; Matsuo, A.; Wagner, T.; Kanoh, S.; Schoening, M.J.; Yoshinobu, T. Miniaturized chemical imaging sensor system using an OLED display panel. Sens. Actuators B Chem. 2012, 170, 82–87. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Ha, D.; Cai, W.; Wang, P. Light-addressable potentiometric sensor based on precise light intensity modulation for eliminating measurement error caused by light source. Sens. Actuators A Phys. 2012, 185, 139–144. [Google Scholar] [CrossRef]
- Wagner, T.; Werner, C.F.B.; Miyamoto, K.I.; Schöning, M.J.; Yoshinobu, T. A high-density multi-point LAPS set-up using a VCSEL array and FPGA control. Sens. Actuators B Chem. 2011, 154, 124–128. [Google Scholar] [CrossRef]
- Das, A.; Chen, T.-C.; Yang, C.-M.; Lai, C.-S. A high-speed, flexible-scanning chemical imaging system using a light-addressable potentiometric sensor integrated with an analog micromirror. Sens. Actuators B Chem. 2014, 198, 225–232. [Google Scholar] [CrossRef]
- Lin, Y.H.; Das, A.; Ho, K.S.; Lai, C.S. A Novel Light-Addressable Potentiometric Sensors Set-Up with LCD Projector as Scanning Light Source. In Proceedings of the 2011 6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems 2011, Kaohsiung, Taiwan, 20–23 February 2011; pp. 972–975. [Google Scholar]
- Zhang, W.; Zhao, Y.; Ha, D.; Cai, W.; Wang, P. Design of precision light intensity modulation for light-addressable potentiometric sensor. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 1404–1407. [Google Scholar]
- Wagner, T.; Werner, C.F.; Miyamoto, K.-I.; Schöning, M.J.; Yoshinobu, T. Development and characterisation of a compact light-addressable potentiometric sensor (LAPS) based on the digital light processing (DLP) technology for flexible chemical imaging. Sens. Actuators B Chem. 2012, 170, 34–39. [Google Scholar] [CrossRef]
- Werner, C.F.; Schusser, S.; Spelthahn, H.; Wagner, T.; Yoshinobu, T.; Schöning, M.J. Field-programmable gate array based controller for multi spot light-addressable potentiometric sensors with integrated signal correction mode. Electrochim. Acta 2011, 56, 9656–9660. [Google Scholar] [CrossRef]
- Wagner, K.; Miyamoto, C.F.; Werner, M.J.; Schöning; Yoshinobu, T. Flexible electrochemical imaging with “zoom-in” functionality by using a new type of light-addressable potentiometric sensor. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2133–2135. [Google Scholar]
- Wagner, T.; Werner, C.F.; Miyamoto, K.I.; Ackermann, H.J.; Yoshinobu, T.; Schöning, M.J.; Schoening, M.J. FPGA-based LAPS device for the flexible design of sensing sites on functional interfaces. Phys. Status Solidi 2010, 207, 844–849. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Jiang, S.; Kunze, J.; Schmuki, P.; Krause, S. High resolution LAPS and SPIM. Electrochem. Commun. 2010, 12, 758–760. [Google Scholar] [CrossRef]
- Chen, D.; Liu, S.-b.; Yin, S.-m.; Liang, J. Research on the enhancement of signal-to-noise ratio of light-addressable potentiometric sensor by optical focusing. Optoelectron. Lett. 2016, 12, 27–30. [Google Scholar] [CrossRef]
- Dantism, S.; Röhlen, D.; Wagner, T.; Wagner, P.; Schöning, M.J. Optimization of Cell-Based Multi-Chamber LAPS Measurements Utilizing FPGA-Controlled Laser-Diode Modules. Phys. Status Solidi 2018, 215, 215. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).