The Light-Addressable Potentiometric Sensor and Its Application in Biomedicine towards Chemical and Biological Sensing
Abstract
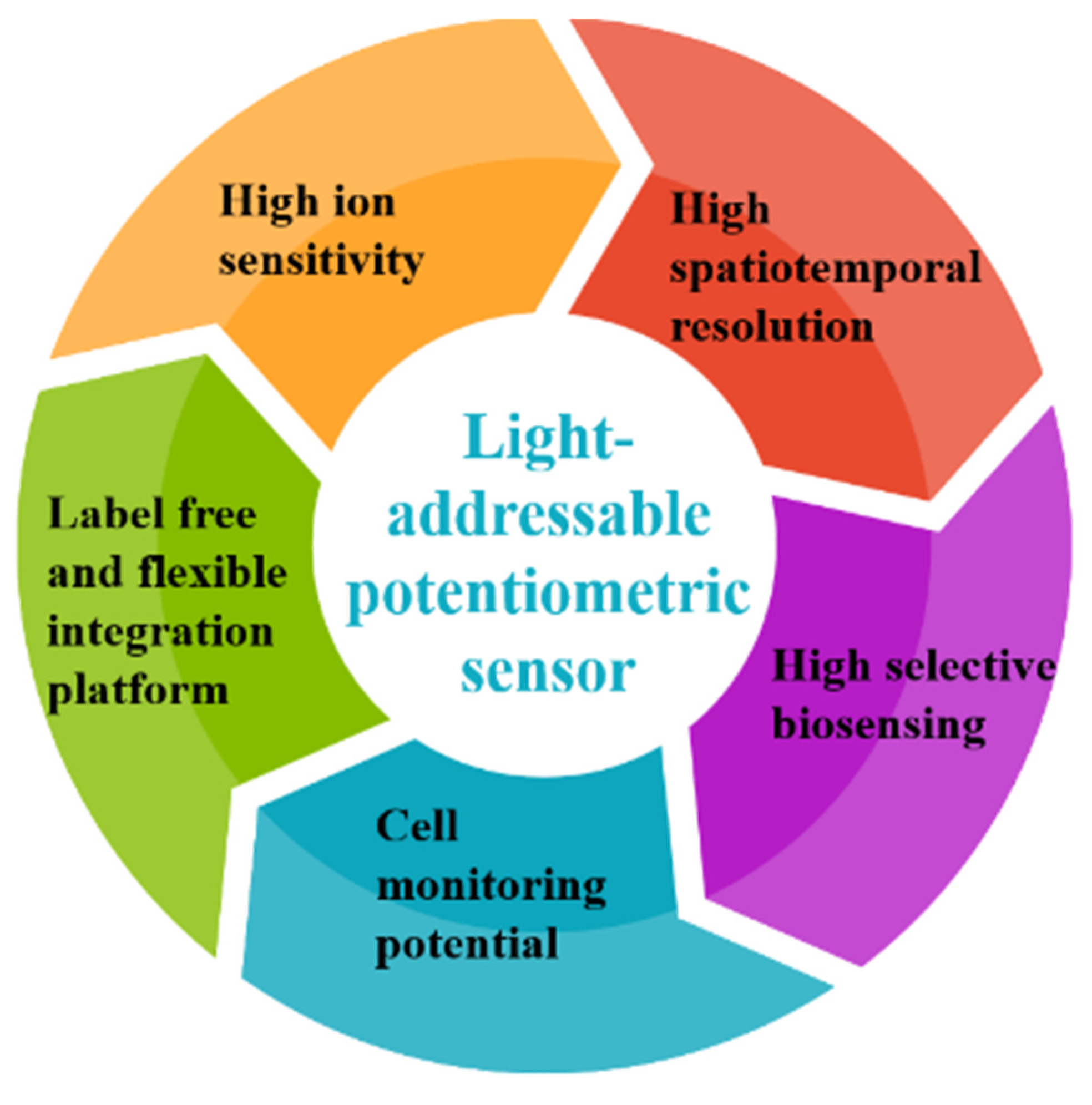
1. Introduction
2. Measurement System of a LAPS
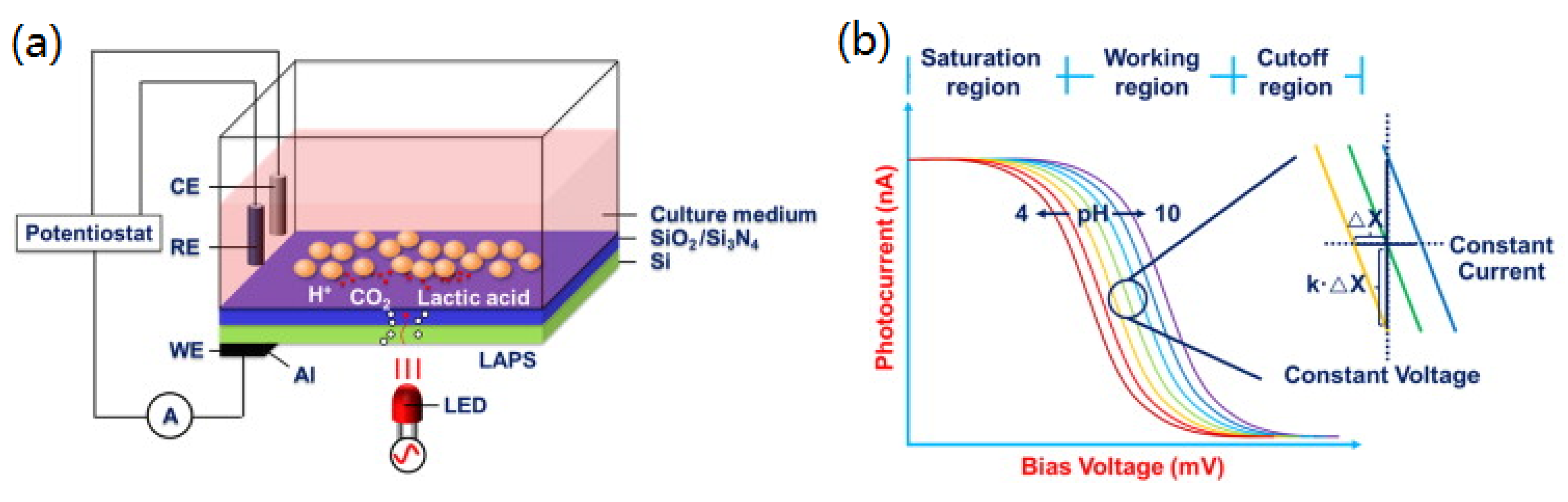
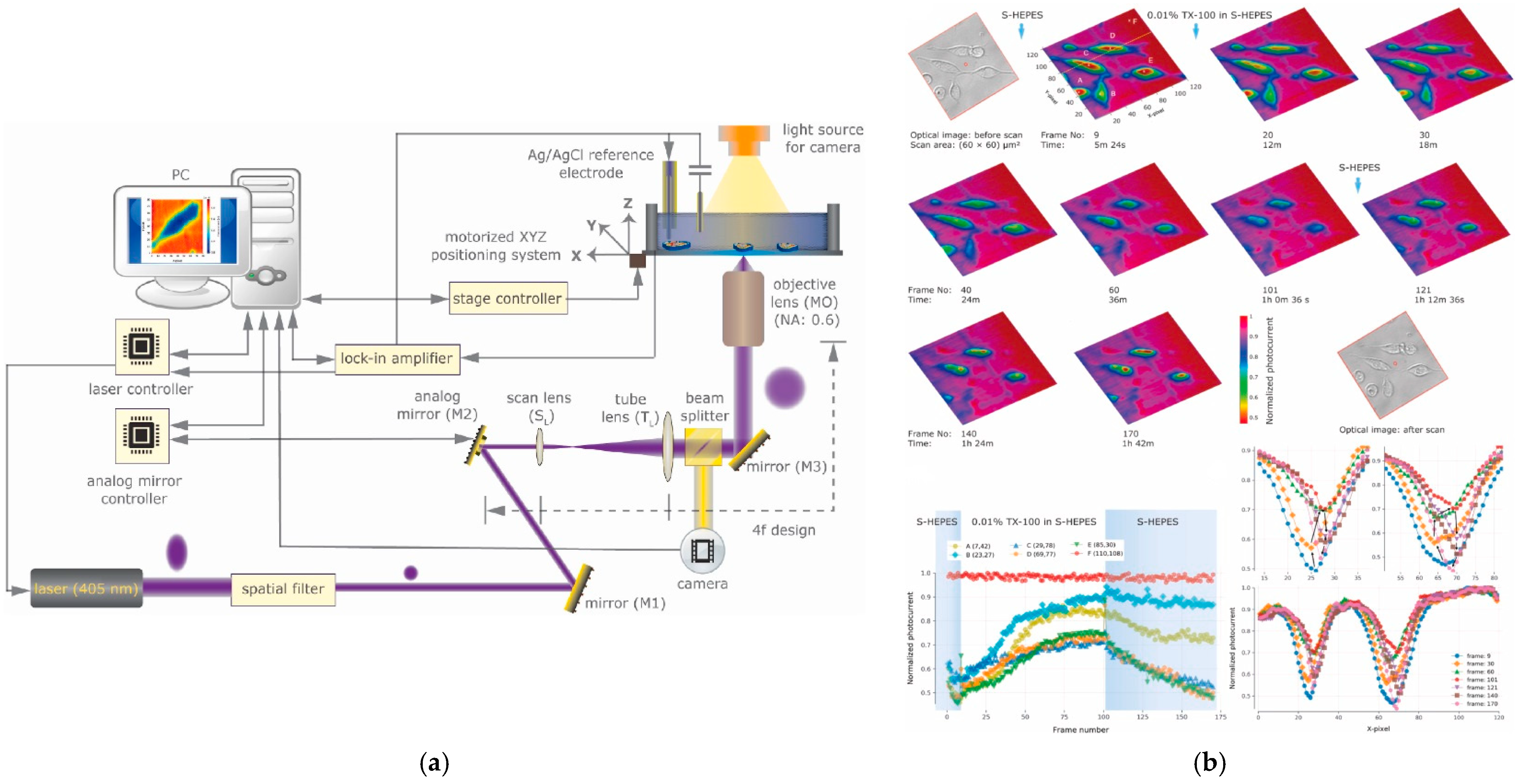
2.1. Principle and Setup
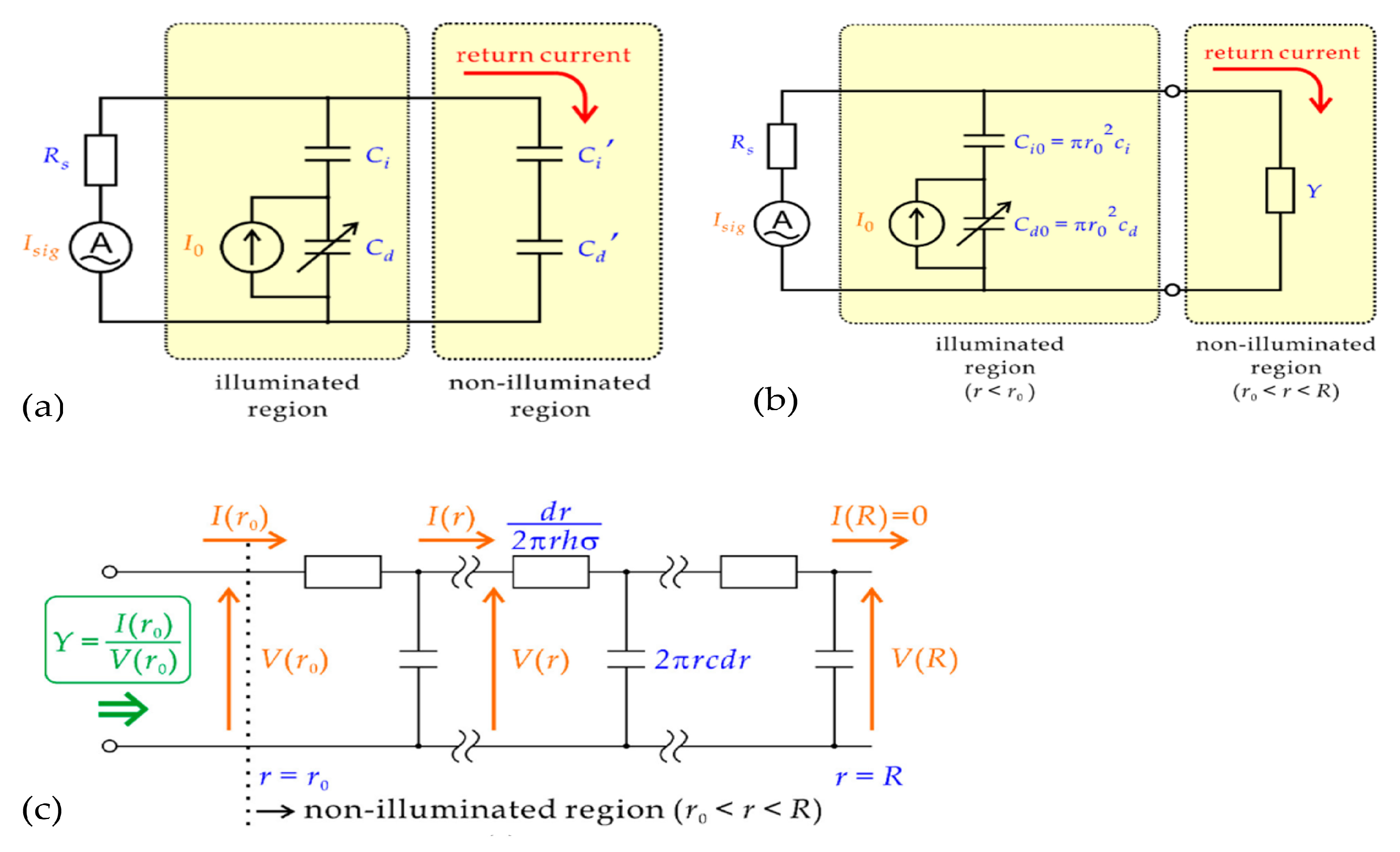
2.2. Modeling of a LAPS
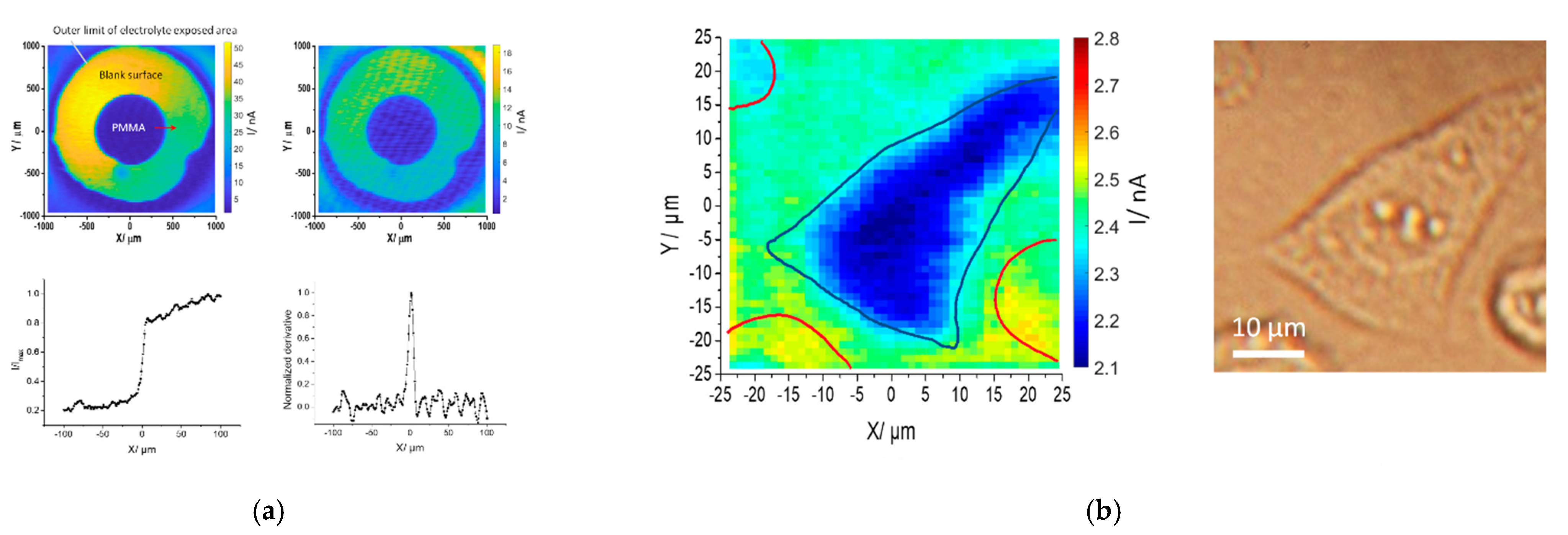
2.3. Device Simulation of a LAPS
3. LAPS for Chemical Sensing
3.1. Chemical Sensing and Application
3.2. Advanced Materials for Chemical Sensing and Imaging
4. LAPS for Biosensing
4.1. Biosensing and Imaging
4.2. Cell Monitoring
5. Optical System Improvements for LAPS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, G.; Hafeman, J.; Wallace, P.; Harden, M.M. Light-Addressable Potentiometric Sensor for Bio-chemical Systems. Science 1988, 240, 1182–1185. [Google Scholar]
- Hu, N.; Wu, C.; Ha, D.; Wang, T.; Liu, Q.; Wang, P. A novel microphysiometer based on high sensitivity LAPS and microfluidic system for cellular metabolism study and rapid drug screening. Biosens. Bioelectron. 2012, 40, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yoshinobu, T.; Miyamoto, K.I.; Werner, C.F.; Poghossian, A.; Wagner, T.; Schoning, M.J. Light-Addressable Potentiometric Sensors for Quantitative Spatial Imaging of Chemical Species. Annu. Rev. Anal. Chem. 2017, 10, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Yoshinobu, T.; Sato, D.; Guo, Y.; Werner, C.F.; Miyamoto, K.-I. Modeling of the Return Current in a Light-Addressable Potentiometric Sensor. Sensors 2019, 19, 4566. [Google Scholar] [CrossRef]
- Werner, C.F.; Wagner, T.; Yoshinobu, T.; Keusgen, M.; Schoening, M.J. Frequency behaviour of light-addressable potentiometric sensors. Phys. Status Solidi 2013, 210, 884–891. [Google Scholar] [CrossRef]
- Guo, Y.; Miyamoto, K.-I.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Device simulation of the light-addressable potentiometric sensor for the investigation of the spatial resolution. Sens. Actuators B Chem. 2014, 204, 659–665. [Google Scholar] [CrossRef]
- Guo, Y.; Seki, K.; Miyamoto, K.-I.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Device Simulation of the Light-addressable Potentiometric Sensor with a Novel Photoexcitation Method for a Higher Spatial Resolution. Procedia Eng. 2014, 87, 456–459. [Google Scholar] [CrossRef][Green Version]
- Guo, Y.; Miyamoto, K.-I.; Wagner, T.; Schoening, M.J.; Yoshinobu, T. Theoretical study and simulation of light-addressable potentiometric sensors. Phys. Status Solidi 2014, 211, 1467–1472. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Iwasaki, H.; Ui, Y.; Furuichi, K.; Ermolenko, Y.; Mourzina, Y.; Wagner, T.; Näther, N.; Schöning, M. The light-addressable potentiometric sensor for multi-ion sensing and imaging. Methods 2005, 37, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Hu, N.; Wu, C.; Kirsanov, D.; Legin, A.; Khaydukova, M.; Wang, P. Novel structured light-addressable potentiometric sensor array based on PVC membrane for determination of heavy metals. Sens. Actuators B Chem. 2012, 174, 59–64. [Google Scholar] [CrossRef]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Design of a miniaturized multisensor chip with nanoband electrode array and light addressable potentiometric sensor for ion sensing. Anal. Methods 2015, 7, 9190–9197. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Tahir, H.E.; Zou, X.; Wang, P. Rapid and wide-range determination of Cd(II), Pb(II), Cu(II) and Hg(II) in fish tissues using light addressable potentiometric sensor. Food Chem. 2017, 221, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Zou, X. Rapid determination of cadmium in rice using an all-solid RGO-enhanced light addressable potentiometric sensor. Food Chem. 2018, 261, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lue, C.E.; Lai, C.S.; Wang, J.C.; Wu, C.M.; Yang, C.M. Differential Light Addressable Potentiometric Sensor with Poly(vinyl chloride) and HfO2Membranes for pH Sensors. Jpn. J. Appl. Phys. 2010, 49, 04DL10. [Google Scholar] [CrossRef]
- DAS, A.; Das, A.; Chang, L.B.; Lai, C.S.; Lin, R.M.; Chu, F.C.; Lin, Y.H.; Chow, L.; Jeng, M.J. GaN Thin Film Based Light Addressable Potentiometric Sensor for pH Sensing Application. Appl. Phys. Express 2013, 6, 036601. [Google Scholar] [CrossRef]
- Chin, C.H.; Lu, T.F.; Wang, J.C.; Yang, J.H.; Lue, C.E.; Yang, C.M.; Li, S.S.; Lai, C.S. Effects of CF4Plasma Treatment on pH and pNa Sensing Properties of Light-Addressable Potentiometric Sensor with a 2-nm-Thick Sensitive HfO2Layer Grown by Atomic Layer Deposition. Jpn. J. Appl. Phys. 2011, 50, 04DL06. [Google Scholar] [CrossRef]
- Lue, C.E.; Lai, C.S.; Chen, H.Y.; Yang, C.M. Light Addressable Potentiometric Sensor with Fluorine-Terminated Hafnium Oxide Layer for Sodium Detection. Jpn. J. Appl. Phys. 2010, 49, 04DL05. [Google Scholar] [CrossRef]
- Nose, K.; Miyamoto, K.-I.; Yoshinobu, T. Estimation of Potential Distribution during Crevice Corrosion through Analysis of I–V Curves Obtained by LAPS. Sensors 2020, 20, 2873. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Das, A.; Lai, C.S. A Simple and Convenient Set-Up of Light Addressable Potentiometric Sensors (LAPS) for Chemical Imaging Using a Commercially Available Projector as a Light Source. Int. J. Electrochem. Sci. 2013, 8, 7062–7074. [Google Scholar]
- Miyamoto, K.-I.; Wagner, T.; Yoshinobu, T.; Kanoh, S.; Schoening, M.J. Phase-mode LAPS and its application to chemical imaging. Sens. Actuators B Chem. 2011, 154, 28–32. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Kanzaki, R.; Takahashi, H. Microscale pH gradient generation by electrolysis on a light-addressable planar electrode. Sens. Actuators B Chem. 2010, 149, 205–211. [Google Scholar] [CrossRef]
- Miyamoto, K.-I.; Kuwabara, Y.; Kanoh, S.; Yoshinobu, T.; Wagner, T.; Schöning, M.J. Chemical image scanner based on FDM-LAPS. Sens. Actuators B Chem. 2009, 137, 533–538. [Google Scholar] [CrossRef]
- Miyamoto, K.; Wagner, T.; Mimura, S.; Kanoh, S.I.; Yoshinobu, T.; Schöning, M.J. Constant-phase-mode operation of the light-addressable potentiometric sensor. Sens. Actuators B Chem. 2011, 154, 119–123. [Google Scholar] [CrossRef]
- Miyamoto, K.; Seki, K.; Guo, Y.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Enhancement of the Spatial Resolution of the Chemical Imaging Sensor by a Hybrid Fiber-Optic Illumination. Procedia Eng. 2014, 87, 612–615. [Google Scholar] [CrossRef][Green Version]
- Takenaga, S.; Schneider, B.; Erbay, E.; Biselli, M.; Schnitzler, T.; Schöning, M.J.; Wagner, T. Fabrication of bio-compatible lab-on-chip devices for biomedical applications by means of a 3D-printing process. Phys. Status Solidi 2015, 212, 1347–1352. [Google Scholar] [CrossRef]
- Yang, J.H.; Lu, T.F.; Wang, J.C.; Lue, C.E.; Lai, C.S. Functionalization of nanoscaled 2 nm-thick ALD-HfO2 layer by rapid thermal annealing and CF4 plasma for LAPS NH4+ detection. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2118–2121. [Google Scholar]
- Yang, J.-H.; Lu, T.-F.; Wang, J.C.; Yang, C.-M.; Pijanowska, D.; Chin, C.H.; Lue, C.E.; Lai, C.S. LAPS with nanoscaled and highly polarized HfO2 by CF4 plasma for NH4+ detection. Sens. Actuators B Chem. 2013, 180, 71–76. [Google Scholar] [CrossRef]
- Wang, J.-C.; Ye, Y.-R.; Lin, Y.-H.; Johnson, D. Light-Addressable Potentiometric Sensor with Nitro-gen-Incorporated Ceramic Sm2O3 Membrane for Chloride Ions Detection. J. Am. Ceram. Soc. 2015, 98, 443–447. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, N.; Li, S.; Jia, H.; Xue, Y.; Cui, L.; Huang, Y.; Li, G. Light-addressable potentiometric sensor with gold nanoparticles enhancing enzymatic silver deposition for 1,5-anhydroglucitol determination. Biochem. Eng. J. 2017, 123, 29–37. [Google Scholar] [CrossRef]
- Miyamoto, K.I.; Ichimura, H.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Chemical imaging of the concentration profile of ion diffusion in a microfluidic channel. Sens. Actuators B Chem. 2013, 189, 240–245. [Google Scholar] [CrossRef]
- Chen, T.C.; Zeng, W.Y.; Liao, Y.H.; Das, A.; Yang, C.M.; Lai, C.-S. High photocurrent and operation frequency for light-addressable potentiometric sensor by thinner Si substrate. In Proceedings of the 2014 IEEE International Nanoelectronics Conference (INEC), Sapporo, Japan, 28–31 July 2014; pp. 1–3. [Google Scholar]
- Wagner, T.; Werner, C.; Miyamoto, K.; Schöning, M.; Yoshinobu, T. A high-density multi-point LAPS set-up using a VCSEL array and FPGA control. Procedia Chem. 2009, 1, 1483–1486. [Google Scholar] [CrossRef][Green Version]
- Wan, H.; Ha, D.; Zhang, W.; Zhao, H.; Wang, X.; Sun, Q.; Wang, P. Design of a novel hybrid sensor with microe-lectrode array and LAPS for heavy metal determination using multivariate nonlinear calibration. Sens. Actuators B Chem. 2014, 192, 755–761. [Google Scholar] [CrossRef]
- Cai, W.; Zhao, H.X.; Ha, D.; Guo, H.S.; Zhang, W.; Wang, P. Design of wireless sensor node based on a novel hybrid chemical sensor for heavy metal monitoring. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing China, 5–9 June 2011; pp. 2114–2117. [Google Scholar]
- Yoshinobu, T.; Schöning, M.J. Light-addressable potentiometric sensors for cell monitoring and biosensing. Curr. Opin. Electrochem. 2021, 28, 100727. [Google Scholar] [CrossRef]
- Zeng, W.-Y.; Chen, C.-C.; Yang, C.-M.; Lai, C.-S. High photocurrent and high frequency response of light-addressable potentiometrie sensor with thin Si substrate and surface roughness. In 2015 IEEE SENSORS; IEEE: Pusan, Korea, 2015; pp. 1–3. [Google Scholar]
- Yu, H.; Wang, J.; Liu, Q.; Zhang, W.; Cai, H.; Wang, P. High spatial resolution impedance measurement of EIS sensors for light addressable cell adhesion monitoring. Biosens. Bioelectron. 2011, 26, 2822–2827. [Google Scholar] [CrossRef]
- Yang, C.-M.; Zeng, W.-Y.; Chen, Y.-P.; Chen, T.-C. Surface Modification for High Photocurrent and pH Sensitivity in a Silicon-Based Light-Addressable Potentiometric Sensor. IEEE Sens. J. 2018, 18, 2253–2259. [Google Scholar] [CrossRef]
- Das, A.; Lin, Y.-H.; Lai, C.-S. Miniaturized amorphous-silicon based chemical imaging sensor system using a mini-projector as a simplified light-addressable scanning source. Sens. Actuators B Chem. 2014, 190, 664–672. [Google Scholar] [CrossRef]
- Yang, C.M.; Liao, Y.H.; Chen, C.H.; Chen, C.C.; Lai, C.S. P-I-N Amorphous Silicon Light-Addressable Potentiometric Sensors for High-photovoltage Chemical Image. Procedia Eng. 2015, 120, 1015–1018. [Google Scholar] [CrossRef]
- Zhou, B.; Das, A.; Kappers, M.J.; Oliver, R.A.; Humphreys, C.J.; Krause, S. InGaN as a Substrate for AC Photo-electrochemical Imaging. Sensors 2019, 19, 4386. [Google Scholar] [CrossRef]
- Tu, Y.; Ahmad, N.; Briscoe, J.; Zhang, D.W.; Krause, S. Light-Addressable Potentiometric Sensors Using ZnO Nanorods as the Sensor Substrate for Bioanalytical Applications. Anal. Chem. 2018, 90, 8708–8715. [Google Scholar] [CrossRef]
- Yang, C.M.; Liao, Y.H.; Chen, C.H.; Chen, T.C.; Lai, C.S.; Pijanowska, D. P-I-N amorphous silicon for thin-film light-addressable potentiometric sensors. Sens. Actuators B Chem. 2016, 236, 1005–1010. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, C.M.; Chang, L.B.; Lai, C.S. Thickness effect of IGZO layer in light-addressable potentiometric sensor. In Proceedings of the 2016 23rd International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 6–8 July 2016; pp. 203–205. [Google Scholar]
- Siqueira, J.J.R.; Maki, R.M.; Paulovich, F.V.; Werner, C.F.; Poghossian, A.; de Oliveira, M.C.F.; Zucolotto, V.; Oliveira, J.O.N.; Schöning, M.J. Use of Information Visualization Methods Eliminating Cross Talk in Multiple Sensing Units Investigated for a Light-Addressable Potentiometric Sensor. Anal. Chem. 2009, 82, 61–65. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Ipatov, A. Recent trends in potentiometric sensor arrays—A review. Anal. Chim. Acta 2010, 678, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Watkinson, M.; Gautrot, J.; Krause, S. High-sensitivity light-addressable potentiometric sensors using silicon on sapphire functionalized with self-assembled organic monolayers. Sens. Actuators B Chem. 2015, 209, 230–236. [Google Scholar] [CrossRef]
- Yang, C.M.; Chiang, T.W.; Yeh, Y.T.; Das, A.; Lin, Y.T.; Chen, T.-C. Sensing and pH-imaging properties of niobium oxide prepared by rapid thermal annealing for electrolyte–insulator–semiconductor structure and light-addressable potentiometric sensor. Sens. Actuators B Chem. 2015, 207, 858–864. [Google Scholar] [CrossRef]
- Wei, C.K.; Peng, H.-Y.; Tsai, Y.-C.; Chen, T.-C.; Yang, C.M. Fluorographene sensing membrane in a light-addressable potentiometric sensor. Ceram. Int. 2019, 45, 9074–9081. [Google Scholar] [CrossRef]
- Yang, C.M.; Chen, C.-H.; Chang, L.B.; Lai, C.-S. IGZO Thin-Film Light-Addressable Potentiometric Sensor. IEEE Electron Device Lett. 2016, 37, 1481–1484. [Google Scholar] [CrossRef]
- Yang, C.-M.; Yang, Y.-C.; Chen, C.-H. Thin-film light-addressable potentiometric sensor with SnOx as a photo-sensitive semiconductor. Vacuum 2019, 168, 108809. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Takahashi, H. Surface passivation of the thin-film LAPS with perhydropolysilaz-ane-derived silica treated by O2 plasma. IEEJ Trans. Electr. Electron. Eng. 2011, 6, 392–393. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Jimbo, Y.; Kanzaki, R.; Takahashi, H. A light addressable electrode with a TiO2 nanocrystalline film for localized electrical stimulation of cultured neurons. Sens. Actuators B Chem. 2014, 192, 393–398. [Google Scholar] [CrossRef]
- Litvinenko, S.; Kozinetz, A.; Skryshevsky, V. Concept of photovoltaic transducer on a base of modified p–n junction solar cell. Sens. Actuators A Phys. 2015, 224, 30–35. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wu, F.; Krause, S. LAPS and SPIM Imaging Using ITO-Coated Glass as the Substrate Material. Anal. Chem. 2017, 89, 8129–8133. [Google Scholar] [CrossRef]
- Yue, Z.; Khalid, W.; Zanella, M.; Abbasi, A.Z.; Pfreundt, A.; Gil, P.R.; Schubert, K.; Lisdat, F.; Parak, W.J. Evaluation of quantum dots applied as switchable layer in a light-controlled electrochemical sensor. Anal. Bioanal. Chem. 2009, 396, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Y.; Krause, S.; Munoz, A.G.; Kunze, J.; Schmuki, P. Repair of thin thermally grown silicon dioxide by anodic oxidation. Electrochim. Acta 2008, 53, 3395–3402. [Google Scholar] [CrossRef]
- Zarei, L.; Tavallaie, R.; Choudhury, M.H.; Parker, S.G.; Bakthavathsalam, P.; Ciampi, S.; Gonçales, V.R.; Gooding, J.J. DNA-Hybridization Detection on Si(100) Surfaces Using Light-Activated Electrochemistry: A Comparative Study between Bovine Serum Albumin and Hexaethylene Glycol as Antifouling Layers. Langmuir 2018, 34, 14817–14824. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, F.; Watkinson, M.; Zhu, J.; Krause, S. "Click" Patterning of Self-Assembled Monolayers on Hydro-gen-Terminated Silicon Surfaces and Their Characterization Using Light-Addressable Potentiometric Sensors. Langmuir 2015, 31, 9646–9654. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Campos, I.; Wu, F.; Zhu, J.; Sukhorukov, G.B.; Palma, M.; Watkinson, M.; Krause, S. The effect of gold nanoparticles on the impedance of microcapsules visualized by scanning photo-induced impedance microscopy. Electrochim. Acta 2016, 208, 39–46. [Google Scholar] [CrossRef]
- Yang, C.-M.; Zeng, W.-Y.; Chen, C.-H.; Chen, Y.-P.; Chen, T.-C. Spatial resolution and 2D chemical image of light-addressable potentiometric sensor improved by inductively coupled-plasma reactive-ion etching. Sens. Actuators B Chem. 2018, 258, 1295–1301. [Google Scholar] [CrossRef]
- Özsoylu, D.; Kizildag, S.; Schöning, M.J.; Wagner, T. Effect of Plasma Treatment on the Sensor Properties of a Light-Addressable Potentiometric Sensor (LAPS). Phys. Status Solidi 2019, 216, 1900259. [Google Scholar] [CrossRef]
- Schöning, M.J.; Kloock, J.P. About 20 Years of Silicon-Based Thin-Film Sensors with Chalcogenide Glass Mate-rials for Heavy Metal Analysis: Technological Aspects of Fabrication and Miniaturization. Electroanalysis 2007, 19, 2029–2038. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wu, F.; Wang, J.; Watkinson, M.; Krause, S. Image detection of yeast Saccharomyces cerevisiae by light-addressable potentiometric sensors (LAPS). Electrochem. Commun. 2016, 72, 41–45. [Google Scholar] [CrossRef]
- Liu, J.; Jia, Y. Label-free protein chip and its detection system realization. In Proceedings of the 2011 IEEE International Conference of Electron Devices and Solid-State Circuits, Seoul, Korea, 7–10 August 2011; pp. 1–2. [Google Scholar]
- Jia, Y.-F.; Gao, C.-Y.; He, J.; Feng, D.-F.; Xing, K.L.; Wu, M.; Liu, Y.; Cai, W.-S.; Feng, X.-Z. Unlabeled multi tumor marker detection system based on bioinitiated light addressable potentiometric sensor. Analyst 2012, 137, 3806–3813. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Guan, M.; Huang, G.; Qiu, H.; Chen, Z.; Li, G.; Huang, Y. Highly sensitive covalently functionalized light-addressable potentiometric sensor for determination of biomarker. Mater. Sci. Eng. C 2016, 63, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yin, X.B.; Zhang, J.; Zhou, S.; Song, M.; Xing, K.L. Graphene oxide modified light addressable potentiometric sensor and its application for ssDNA monitoring. Analyst 2012, 137, 5866–5873. [Google Scholar] [CrossRef]
- Bronder, T.; Wu, C.; Poghossian, A.; Werner, C.; Keusgen, M.; Schöning, M. Label-free Detection of DNA Hybridization with Light-addressable Potentiometric Sensors: Comparison of Various DNA- immobilization Strategies. Procedia Eng. 2014, 87, 755–758. [Google Scholar] [CrossRef]
- Shao, C.; Zhou, S.; Yin, X.-B.; Gu, Y.; Jia, Y. Influences of Probe’s Morphology for Metal Ion Detection Based on Light-Addressable Potentiometric Sensors. Sensors 2016, 16, 701. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Men, H.; Zou, S.; Li, Y.; Wang, Y.; Ye, X.; Wang, P. A novel electronic tongue combined MLAPS with stripping voltammetry for environmental detection. Sens. Actuators B Chem. 2005, 110, 350–357. [Google Scholar] [CrossRef]
- Werner, C.F.; Groebel, S.; Krumbe, C.; Wagner, T.; Selmer, T.; Yoshinobu, T.; Baumann, M.E.M.; Keusgen, M.; Schoening, M.J. Nutrient concentration-sensitive microorganism-based biosensor. Phys. Status Solidi 2012, 209, 900–904. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, H.; Xu, Y.; Li, Y.; Li, R.; Wang, P. Olfactory cell-based biosensor: A first step towards a neurochip of bioelectronic nose. Biosens. Bioelectron. 2006, 22, 318–322. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, W.; Yu, H.; Hu, N.; Du, L.; Wang, P.; Yang, M. Olfactory mucosa tissue-based biosensor: A bioelectronic nose with receptor cells in intact olfactory epithelium. Sens. Actuators B Chem. 2010, 146, 527–533. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, W.; Hu, N.; Cai, H.; Yu, H.; Wang, P. Olfactory receptor cells respond to odors in a tissue and semi-conductor hybrid neuron chip. Biosens Bioelectron 2010, 26, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, F.; Jia, T.; Wang, Z. Meso-tetra(4-carboxyphenyl)porphine-Enhanced DNA Methylation Sensing Interface on a Light-Addressable Potentiometric Sensor. ACS Omega 2019, 4, 12567–12574. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, S.; Zhang, R.; Gu, Y.; Li, Y.; Jia, Y. Porous Graphene Oxide Enhanced Aptamer Specific Circulating-Tumor-Cell Sensing Interface on Light Addressable Potentiometric Sensor: Clinical Application and Simulation. ACS Appl Mater Interfaces 2019, 11, 8704–8709. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, F. Studies of Functional Nucleic Acids Modified Light Addressable Potentiometric Sensors: X-ray Photoelectron Spectroscopy, Biochemical Assay, and Simulation. Anal. Chem. 2018, 90, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Cai, H.; Li, Y.; Li, R.; Wang, P.; Yang, G.G. Investigation of light addressable potentiometric sensor array sensitive to heavy metal ion based on micro-lens array. J. Zhejiang Univ. Eng. Ing Sci. 2008, 42, 517. [Google Scholar]
- Jia, Y.; Gao, C.; Feng, D.; Wu, M.; Liu, Y.; Chen, X.; Xing, K.; Feng, X. Bio-initiated light addressable potentiometric sensor for unlabeled biodetection and its MEDICI simulation. Analyst 2011, 136, 4533–4538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyamoto, K.I.; Yoshida, M.; Sakai, T.; Matsuzaka, A.; Wagner, T.; Kanoh, S.I.; Yoshinobu, T.; Schöning, M.J. Differential Setup of Light-Addressable Potentiometric Sensor with an Enzyme Reactor in a Flow Channel. Jpn. J. Appl. Phys. 2011, 50, 04DL08. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zou, X.; Zhang, H.; Xu, X. Micrometer-scale light-addressable potentiometric sensor on an optical fiber for biological glucose determination. Anal. Chim. Acta 2020, 1123, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Liu, Q.; Xu, Y.; Cai, H.; Wang, P. A novel experimental research based on taste cell chips for taste transduction mechanism. Sens. Actuators B Chem. 2008, 131, 24–28. [Google Scholar] [CrossRef]
- Liu, H.L.; Chen, Y.M.; Yang, M.C.; Lai, C.S. Real-time 2D pH images by fast scanning light-addressable Potentiometrie sensor system controlled by LabVIEW program. IEEE Sens. 2015, 1–3. [Google Scholar] [CrossRef]
- Werner, C.F.; Takenaga, S.; Taki, H.; Sawada, K.; Schöning, M.J. Comparison of label-free ACh-imaging sensors based on CCD and LAPS. Sens. Actuators B Chem. 2013, 177, 745–752. [Google Scholar] [CrossRef]
- Hu, N.; Ha, D.; Wu, C.; Zhou, J.; Kirsanov, D.; Legin, A.; Wang, P. A LAPS array with low cross-talk for non-invasive measurement of cellular metabolism. Sens. Actuators A Phys. 2012, 187, 50–56. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Cai, H.; Du, L.P.; Liu, Q.J.; Wang, P. A photovoltage-based integrated sensor for nephrotoxi-city evaluation under drug stimulation. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2122–2125. [Google Scholar]
- Hu, N.; Zhou, J.; Su, K.; Zhang, D.; Xiao, L.; Wang, T.; Wang, P. An integrated label-free cell-based biosensor for simultaneously monitoring of cellular physiology multiparameter in vitro. Biomed. Microdevices 2013, 15, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cai, H.; Zhang, W.; Xiao, L.; Liu, Q.; Wang, P. A novel design of multifunctional integrated cell-based biosensors for simultaneously detecting cell acidification and extracellular potential. Biosens. Bioelectron. 2009, 24, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, T.; Cao, J.; Su, K.; Zhou, J.; Wu, J.; Wang, P. Comparison between ECIS and LAPS for establishing a cardiomyocyte-based biosensor. Sens. Actuators B Chem. 2013, 185, 238–244. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, J.; Hu, N.; Ha, D.; Miao, X.; Wang, P. Cellular impedance sensing combined with LAPS as a new means for real-time monitoring cell growth and metabolism. Sens. Actuators A Phys. 2013, 199, 136–142. [Google Scholar] [CrossRef]
- Dantism, S.; Röhlen, D.; Dahmen, M.; Wagner, T.; Wagner, P.; Schöning, M.J. LAPS-based monitoring of metabolic responses of bacterial cultures in a paper fermentation broth. Sens. Actuators B Chem. 2020, 320, 128232. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Miyamoto, K.; Wagner, T.; Schöning, M.J. Recent developments of chemical imaging sensor systems based on the principle of the light-addressable potentiometric sensor. Sens. Actuators B Chem. 2015, 207, 926–932. [Google Scholar] [CrossRef]
- Dantism, S.; Takenaga, S.; Wagner, P.; Wagner, T.; Schöning, M.J. Determination of the extracellular acidification of Escherichia coliK12 with a multi-chamber-based LAPS system. Phys. Status Solidi 2016, 213, 1479–1485. [Google Scholar] [CrossRef]
- Dantism, S.; Takenaga, S.; Wagner, T.; Wagner, P.; Schöning, M.J. Differential imaging of the metabolism of bacteria and eukaryotic cells based on light-addressable potentiometric sensors. Electrochim. Acta 2017, 246, 234–241. [Google Scholar] [CrossRef]
- Su, K.; Zhou, J.; Zou, L.; Wang, T.; Zhuang, L.; Hu, N.; Wang, P. Integrated multifunctional cell-based biosensor system for monitoring extracellular acidification and cellular growth. Sens. Actuators A Phys. 2014, 220, 144–152. [Google Scholar] [CrossRef]
- Dantism, S.; Takenaga, S.; Wagner, P.; Wagner, T.; Schöning, M. Light-addressable Potentiometric Sensor (LAPS) Combined with Multi-chamber Structures to Investigate the Metabolic Activity of Cells. Procedia Eng. 2015, 120, 384–387. [Google Scholar] [CrossRef]
- Takenaga, S.; Herrera, C.; Werner, C.F.; Biselli, M.; Thönnessen, V.; Schnitzler, T.; Öhlschläger, P.; Almajhdi, F.N.; Wagner, T.; Schöning, M.J. Toward multi-analyte bioarray sensors: LAPS-based on-chip determination of a Michaelis-Menten-like kinetics for cell culturing. Phys. Status Solidi 2014, 211, 1410–1415. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, N.; Zhang, F.; Wang, H.; Ye, W.; Wang, P. Neurosecretory cell-based biosensor: Monitoring secretion of adrenal chromaffin cells by local extracellular acidification using light-addressable potentiometric sensor. Biosens. Bioelectron. 2012, 35, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Dantism, S.; Rohlen, D.; Selmer, T.; Wagner, T.; Wagner, P.; Schoning, M.J. uantitative differential monitoring of the metabolic activity of Corynebacterium glutamicum cultures utilizing a light-addressable potentiometric sensor system. Biosens. Bioelectron. 2019, 139, 111332. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Naicker, S.; Kaur, K.; Thundat, T. Metabolic Study of Cancer Cells Using a pH Sensitive Hydrogel Nanofiber Light Addressable Potentiometric Sensor. ACS Sens 2017, 2, 151–156. [Google Scholar] [CrossRef]
- Liang, T.; Gu, C.; Gan, Y.; Wu, Q.; He, C.; Tu, J.; Pan, Y.; Qiu, Y.; Kong, L.; Wan, H.; et al. Microfluidic chip system integrated with light addressable potentiometric sensor (LAPS) for real-time extracellular acidification detection. Sens. Actuators B Chem. 2019, 301, 127004. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, H.; Tan, Z.; Cai, H.; Ye, W.; Zhang, M.; Wang, P. In vitro assessing the risk of drug-induced cardio-toxicity by embryonic stem cell-based biosensor. Sens. Actuators B Chem. 2011, 155, 214–219. [Google Scholar] [CrossRef]
- Kimmel, D.W.; Meschievitz, M.E.; Hiatt, L.A.; Cliffel, D.E. Multianalyte Microphysiometry of Macrophage Responses to Phorbol Myristate Acetate, Lipopolysaccharide, and Lipoarabinomannan. Electroanalysis 2013, 25, 1706–1712. [Google Scholar] [CrossRef]
- Guo, Y.; Werner, C.F.; Handa, S.; Wang, M.; Ohshiro, T.; Mushiake, H.; Yoshinobu, T. Miniature multiplexed label-free pH probe in vivo. Biosens. Bioelectron. 2021, 174, 112870. [Google Scholar] [CrossRef]
- Guo, Y.; Werner, C.F.; Canales, A.; Yu, L.; Jia, X.; Anikeeva, P.; Yoshinobu, T. Polymer-fiber-coupled field-effect sensors for label-free deep brain recordings. PLoS ONE 2020, 15, e0228076. [Google Scholar] [CrossRef]
- Jia, Y.; Qin, M.; Zhang, H.; Niu, W.; Li, X.; Wang, L.; Li, X.; Bai, Y.; Cao, Y.; Feng, X. Label-free biosensor: A novel phage-modified Light Addressable Potentiometric Sensor system for cancer cell monitoring. Biosens. Bioelectron. 2007, 22, 3261–3266. [Google Scholar] [CrossRef] [PubMed]
- Shaibani, P.M.; Etayash, H.R.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable Nanofiber-Light Addressable Potentiometric Sensor for Rapid Escherichia coli Detection in Orange Juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Shaibani, P.M.; Jiang, K.; Haghighat, G.; Hassanpourfard, M.; Etayash, H.; Naicker, S.; Thundat, T. The detection of Escherichia coli (E. coli) with the pH sensitive hydrogel nanofiber-light addressable potentiometric sensor (NF-LAPS). Sens. Actuators B Chem. 2016, 226, 176–183. [Google Scholar] [CrossRef]
- Wagner, T.; Shigiahara, N.; Miyamoto, K.; Suzurikawa, J.; Finger, F.; Schöning, M.J.; Yoshinobu, T. Light-addressable Potentiometric Sensors and Light–addressable Electrodes as a Combined Sensor-and-manipulator Microsystem with High Flexibility. Procedia Eng. 2012, 47, 890–893. [Google Scholar] [CrossRef]
- Werner, C.F.; Krumbe, C.; Schumacher, K.; Groebel, S.; Spelthahn, H.; Stellberg, M.; Wagner, T.; Yoshinobu, T.; Selmer, T.; Keusgen, M.; et al. Determination of the extracellular acidification of Escherichia coli by a light-addressable potentiometric sensor. Phys. Status Solidi 2011, 208, 1340–1344. [Google Scholar] [CrossRef]
- Werner, C.F.; Wagner, T.; Miyamoto, K.-I.; Yoshinobu, T.; Schöning, M.J. High speed and high resolution chemical imaging based on a new type of OLED-LAPS set-up. Sens. Actuators B Chem. 2012, 175, 118–122. [Google Scholar] [CrossRef]
- Itabashi, A.; Kosaka, N.; Miyamoto, K.I.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. High-speed chemical imaging system based on front-side-illuminated LAPS. Sens. Actuators B Chem. 2013, 182, 315–321. [Google Scholar] [CrossRef]
- Zhou, B.; Das, A.; Zhong, M.; Guo, Q.; Zhang, D.W.; Hing, K.A.; Sobrido, A.J.; Titirici, M.M.; Krause, S. Photoelectrochemical imaging system with high spatiotemporal resolution for visualizing dynamic cellular responses. Biosens. Bioelectron. 2021, 180, 113121. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Seki, K.; Suto, T.; Werner, C.F.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Improved spatial resolution of the chemical imaging sensor with a hybrid illumination that suppresses lateral diffusion of photocarriers. Sens. Actuators B Chem. 2018, 273, 1328–1333. [Google Scholar] [CrossRef]
- Werner, C.F.; Miyamoto, K.-I.; Wagner, T.; Schoening, M.J.; Yoshinobu, T. Lateral resolution enhancement of pulse-driven light-addressable potentiometric sensor. Sens. Actuators B Chem. 2017, 248, 961–965. [Google Scholar] [CrossRef]
- Guo, Y.; Seki, K.; Miyamoto, K.I.; Wagner, T.; Schoening, M.J.; Yoshinobu, T. Novel photoexcitation method for light-addressable potentiometric sensor with higher spatial resolution. Appl. Phys. Express 2014, 7, 7. [Google Scholar] [CrossRef]
- Miyamoto, K.I.; Kaneko, K.; Matsuo, A.; Wagner, T.; Kanoh, S.; Schoening, M.J.; Yoshinobu, T. Miniaturized chemical imaging sensor system using an OLED display panel. Sens. Actuators B Chem. 2012, 170, 82–87. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Ha, D.; Cai, W.; Wang, P. Light-addressable potentiometric sensor based on precise light intensity modulation for eliminating measurement error caused by light source. Sens. Actuators A Phys. 2012, 185, 139–144. [Google Scholar] [CrossRef]
- Wagner, T.; Werner, C.F.B.; Miyamoto, K.I.; Schöning, M.J.; Yoshinobu, T. A high-density multi-point LAPS set-up using a VCSEL array and FPGA control. Sens. Actuators B Chem. 2011, 154, 124–128. [Google Scholar] [CrossRef]
- Das, A.; Chen, T.-C.; Yang, C.-M.; Lai, C.-S. A high-speed, flexible-scanning chemical imaging system using a light-addressable potentiometric sensor integrated with an analog micromirror. Sens. Actuators B Chem. 2014, 198, 225–232. [Google Scholar] [CrossRef]
- Lin, Y.H.; Das, A.; Ho, K.S.; Lai, C.S. A Novel Light-Addressable Potentiometric Sensors Set-Up with LCD Projector as Scanning Light Source. In Proceedings of the 2011 6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems 2011, Kaohsiung, Taiwan, 20–23 February 2011; pp. 972–975. [Google Scholar]
- Zhang, W.; Zhao, Y.; Ha, D.; Cai, W.; Wang, P. Design of precision light intensity modulation for light-addressable potentiometric sensor. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 1404–1407. [Google Scholar]
- Wagner, T.; Werner, C.F.; Miyamoto, K.-I.; Schöning, M.J.; Yoshinobu, T. Development and characterisation of a compact light-addressable potentiometric sensor (LAPS) based on the digital light processing (DLP) technology for flexible chemical imaging. Sens. Actuators B Chem. 2012, 170, 34–39. [Google Scholar] [CrossRef]
- Werner, C.F.; Schusser, S.; Spelthahn, H.; Wagner, T.; Yoshinobu, T.; Schöning, M.J. Field-programmable gate array based controller for multi spot light-addressable potentiometric sensors with integrated signal correction mode. Electrochim. Acta 2011, 56, 9656–9660. [Google Scholar] [CrossRef]
- Wagner, K.; Miyamoto, C.F.; Werner, M.J.; Schöning; Yoshinobu, T. Flexible electrochemical imaging with “zoom-in” functionality by using a new type of light-addressable potentiometric sensor. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2133–2135. [Google Scholar]
- Wagner, T.; Werner, C.F.; Miyamoto, K.I.; Ackermann, H.J.; Yoshinobu, T.; Schöning, M.J.; Schoening, M.J. FPGA-based LAPS device for the flexible design of sensing sites on functional interfaces. Phys. Status Solidi 2010, 207, 844–849. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Jiang, S.; Kunze, J.; Schmuki, P.; Krause, S. High resolution LAPS and SPIM. Electrochem. Commun. 2010, 12, 758–760. [Google Scholar] [CrossRef]
- Chen, D.; Liu, S.-b.; Yin, S.-m.; Liang, J. Research on the enhancement of signal-to-noise ratio of light-addressable potentiometric sensor by optical focusing. Optoelectron. Lett. 2016, 12, 27–30. [Google Scholar] [CrossRef]
- Dantism, S.; Röhlen, D.; Wagner, T.; Wagner, P.; Schöning, M.J. Optimization of Cell-Based Multi-Chamber LAPS Measurements Utilizing FPGA-Controlled Laser-Diode Modules. Phys. Status Solidi 2018, 215, 215. [Google Scholar] [CrossRef]


| Category | Target | Technology | Sensitivity | Detection Limit or Range | Noise | Measurement Time | Ref. |
|---|---|---|---|---|---|---|---|
| Chemical Sensing | Ions and molecules | LAPS with pulsed laser deposition (PLD) | 58 mV/pK, 59 mV/pLi, 57 mV/pCs, 27 mV/pCa and 26 mV/pMg | 10−6–10−1 mol/L | - | - | [9] |
| pH | LAPS with a HfO2 layer | 30.1 mV/pH | pH 2–12 | - | - | [14] | |
| LAPS with Gallium nitride (GaN) film | 52.29 mV/pH | pH 2–12 | - | - | [15] | ||
| Heavy metals | LAPS with PVC membrane | 28.7–29.3 mV/dec | 10−5–10−1 mol/L | 0.5 mV | 2–4 s | [10] | |
| Four taste molecules | LAPS with surface imprinted TiO2 membranes | 40 ppm/V | 2–300 ppm | - | - | [25] | |
| Ions (pH and heavy metal) | LAPS with a miniaturized multi-sensor chip based on nano-band electrode array (NEA) | 0.510 mA/ppb(lead), 0.678 mA/ppb(copper) and f 56.49 mV/pH | 20–100 ppb | 2 mV | 120 s | [11] | |
| LAPS with Microelectrode array (MEA) on the same wafer | 36.3 nA/ppb(zinc), 11.2 nA/ppb(lead) and 4.6 nA/ppb(copper) and 52.1 mV/pH | - | 4 mV | - | [32] | ||
| - | 10−7–10−2 mol/L (Zn2+ and Pb2+) | - | 15 s | [33] | |||
| pH and pNa | LAPS with a HfO2 layer | 33.9 mV/pNa | - | - | - | [16] | |
| 31.8 mV/pNa | - | - | - | [17] | |||
| NH4+ | LAPS with functionalized ALD-HfO2 film | 37.28 mV/pNH4 | - | - | - | [26] | |
| LAPS with ALD-HfO2 films with post RTA and CF4 plasma treatment | 37 mV/pNH4 | - | - | - | [27] | ||
| K+ and Cl− | LAPS with ceramic samarium oxide (Sm2O3)-sensing membrane | 39.21 mV/pK and 36.17 mV/pCl | 10−3.5–100 M | - | - | [28] | |
| 1,5-anhydroglucitol (1,5-AG) | LAPS with gold nanoparticles enhancing enzymatic silver deposition | 2.1 mV per gland 1, 5-AG concentration (μg/mL) | 40–225 μg/mL | SNR = 3 | - | [29] | |
| Cd(II), Pb(II), Cu(II), Hg(II) in fish tissues | LAPS | - | 0.1–1000 mg/L | - | 10 s | [12] | |
| cadmium (Cd) in rice | LAPS with reduced graphene oxide (RGO) | 27.9 mV per decade | 0.002 mg/L | 0.23 mV | 10 s | [13] | |
| pH of Crevice Corrosion | LAPS | - | 12 μm | - | 145 s | [18] | |
| Chemical Imaging | Multi-ion | LAPS with stripe patterns of membranes on the surface | 58 mV/pK, 59 mV/pLi, 57 mV/pCs, 27 mV/pCa and 26 mV/pMg | - | - | - | [9] |
| pH | LAPS with a commercially available projector | - | pH 5–10 | - | 15 Hz (22 × 22 µm2) | [19] | |
| Phase-mode LAPS | 55.7 mV/pH, | pH 4–10 | - | - | [20] | ||
| LAPS with digital micromirror device (DMD) | - | - | - | 150 ms (500 × 500-pixel image) | [21] | ||
| FDM-LAPS(frequency division multiplex) | - | pH 4–10 | - | 6.4 s (16 pixels × 128 lines) | [22] | ||
| constant-phase-mode operation of the LAPS with EIS | 52.7 mV/pH | pH 4–10 | - | - | [23] | ||
| Ion diffusion | LAPS | 52.4 mV/pH | pH 4–10 | - | 24 ms per pixel (128 × 128 pixel image) | [30] | |
| Ion and pH | LAPS with Hybrid Fiber-Optic Illumination | - | 68 μm spatial resolution | - | - | [24] | |
| Imaging the impedance of an organic monolayer | LAPS and SPIM | −65 mV potential shift | - | 5 mV | 15 min (500 × 500 μm2; 5 μm step size) | [34] |
| Target | Main Improvement | Technology | Sensitivity | Detection Limit or Range | Noise | Measurement Time | Ref. |
|---|---|---|---|---|---|---|---|
| pH | High photocurrent and pH sensitivity | LAPS with thin Si substrate and surface roughness | 55.23 mV/pH | pH 2–12 | - | - | [35] |
| High photovoltage and pH sensitivity | LAPS with thin Si substrate and niobium oxide (NbOx) | 60.3 mV/pH | pH 2–10 | - | - | [31] | |
| thin-Si LAPS | 45.4 mV/pH | pH 2–12 | - | - | [37] | ||
| High-sensitivity imaging and sensing | LAPS using silicon on sapphire (SOS) functionalized with self-assembled organic monolayers | - | 49 nA | - | 0.01 V/s | [46] | |
| Improvement on photocurrent and spatial resolution | LAPS with P-I-N amorphous silicon (a-Si) on ITO/glass | 40 mV/pH | pH 2–10 | - | - | [39] | |
| Increases of calculated pH sensitivity and linearity | LAPS with a niobium oxide (NbOx) layer | 60 mV/pH | pH 3–10 | 0.3 mV | −3 mV/h | [47] | |
| pH sensing membrane | LAPS with fluorographene sensing membrane | 56.8 mV/pH | pH 2–12 | - | 2.6 mV/h | [48] | |
| LAPS with In–Ga–Zn oxide (IGZO) layer | 61.8 mV/pH | pH 2–12 | - | - | [49] | ||
| Low cost and robust | LAPS and SPIM using ITO-Coated glass as the Substrate Material | 70 mV/pH | 2.3 μm resolution | - | - | [54] | |
| Increase AC photocurrent with enhanced image resolution | LAPS with ZnO Nanorods as the Sensor Substrate | 53 mV/pH | 45.7 nA photocurrent | 0.1 nA | 6 h stability | [41] | |
| Higher photovoltage | LAPS with niobium oxide (NbOx)/P-I-N amorphous silicon (a-Si) on ITO/glass structure | 40 mV/pH | pH 2–10 | - | - | [42] | |
| Higher photocurrent and high speed imaging | LAPS with Inductively coupled plasma reactive-ion etching (ICP-RIE) (Si substrate) | 55.8 mV/pH | pH 2–12 | - | - | [60] | |
| Higher photovoltage | LAPS with In-Ga-Zn oxide (IGZO) semiconductor layer | 66 mV/pH | pH 2–12 | - | - | [43] | |
| pH sensing evaluation | LAPS with SnOx as a photosensitive semiconductor | 1.29 μA and 57.6 mV/pH | pH 4–10 | - | - | [50] | |
| Low cost and high performance | LAPS with well-ordered polystyrene (PS) colloidal monolayer | 53.47 mV/pH | pH 4–10 | - | - | [63] | |
| High spatial resolution, high speed imaging and high signal-to-noise ratio | LAPS with anisotropic etching process based on tetramethylammonium hydroxide (TMAH) | - | 20 μm spatial resolution | - | 10 Hz–120 kHz | [44] | |
| High photocurrent and immunity to room light inference | LAPS with Indium Gallium Zinc Oxide (IGZO) on Indium Tin Oxide (ITO) glass (IGZO LAPS) | 58.9 mV/pH | pH 2–12, 50 μm pattern recognition s | - | - | [45] | |
| Superhydrophilic | LAPS with Plasma Treatment | 59.6 mV/pH | pH 6–10 | 4 mV hysteresis | 117 h | [61] | |
| Neurons (from rat embryos) | Compatibility and easy to fabricate | LAPS using TiO2 as a photo-conductor | 10 µC/cm2/V | - | - | 5 min | [52] |
| photocurrent | No need to apply DC bias voltage and for gas detection | LAPS with silicon p–n junction from solar cell | - | - | - | 103 cm/s | [53] |
| Immobilizing enzymes | LAPS with quantum dots as switchable layer | - | 913 nA | - | - | [55] | |
| DNA | Resist nonspecific adsorption to the DNA-modified interface on Si(100) devices | LAPS and light-addressable amperometric sensors based on organic-monolayer-protected Si(100) | - | 1.0 × 10−11–1.0 × 10−6 M DNA | 4.5%STD | - | [57] |
| No specific target | AC photoelectrochemical imaging | LAPS with InGaN/GaN thin films | - | 10 nm narrow gap | - | - | [40] |
| Flexibility and resolution of the image | LAPS with mini-projector and miniaturized amorphous-silicon | - | 72 μm × 72 μm spot size | SNR = 10.72 | - | [38] | |
| Impedance of functionalized microcapsules | LAPS with microcapsules modified with gold nanoparticles | - | 20 μm × 25 μm spot size | - | - | [59] | |
| Repair thermally grown oxide layers | LAPS and SPIM | - | 1.31 nA/cm2 | - | - | [56] | |
| Characterization | LAPS with chemically patterned non-oxide SOS substrate | −50 mV voltage shift | - | - | - | [58] | |
| Keep the electrode stable longer | LAPS with Perhydropolysilazane-Derived Silica Treated by O2 Plasma (passivation) | - | - | - | 100 h stability | [51] |
| Category | Target | Technology | Sensitivity | Detection Limit or Range | Noise | Measurement Time | Ref. |
|---|---|---|---|---|---|---|---|
| Enzyme-based biosensor | Urease | LAPS with an enzyme reactor in a fluidic channel | - | 0.3 × 10−3–10−1 mol/L | - | 12.5 μL/min | [82] |
| Glucose oxidase | LAPS with on-board light driver and transimpedance amplifier | 101.1 mV/dec | 0.01–100 mM | - | 10 s | [83] | |
| Affinity-based biosensor | Short chain single strand DNA (ssDNA) | GO-LAPS (Graphene oxide (GO) based LAPS) | - | 1 pM–10 nM | - | 50 mV/s | [69] |
| LAPS | - | 100 mV | - | - | [70] | ||
| LAPS with different ssDNA chains | 0.514 mV/lg[ppb] | 0.01–100 ppb | 3.9%STD | - | [71] | ||
| DNA sequences related to HBV, HCV, HIV | LAPECS | 0.29 μA/logC(nM) | 0.7 pM | 0.7 μA | - | [72] | |
| Functional Nucleic Acids | FNA-LAPS | 2.86 (Pb2+) and 1.53 (Ag+) mV/lg(ppb) | 0.01 ppb | - | 30 min | [80] | |
| Antigens and antibodies of AFP CEA CA19-9 Ferritin | LAPS with chip initiated by L-Dopa | 0.39 ± 0.07 pA per ng/mL, 0.22 ± 0.04 pA per ng/mL, 0.18 ± 0.07 pA per U/mL and 0.12 ± 0.02 pA per 10 ng/mL for AFP, CEA, CA19-9 and Ferritin | 20 ng/mL(AFP) | - | - | [67] | |
| Human immunoglobulin G (hIgG) | LAPS with goat anti-human immunoglobulin G antibody | ΔV(V) = 0.00714 ChIgG(μg/mL)–0.0147 | 0.15 μg/mL | SNR = 3 | - | [68] | |
| Mouse IgG detecting rabbit anti-mouse IgG | LAPS | 65 7 μV/p[IgG] | 0.01 μg/mL | - | - | [66] | |
| 5-methylcytosine (5mC) | LAPS with DNA Methylation Sensing Interface | - | 10 pM–100 nM | - | - | [78] | |
| Alpha-fetoprotein (AFP) | LAPS with gold nanoparticles (Au NPs) | 2.5892 mV/μg/mL | 92.0 ng/mL | 2.76%RSD | - | [81] | |
| Circulating-tumor-cell (CTC) | LAPS with porous-graphene-oxide (PGO) enhanced aptamer specific CTC sensing interface | ΔVout/Vout,0 = −7.63, −9.85, −6.67%/lg[spiked cells] for A549, HeLa and MDAMB231 | 5–5000 spiked cells | - | 30 min | [79] | |
| Cell-based biosensor | Heavy metal | MLAPS with stripping voltammetry | - | 280 μg/L Fe(III), 26 μg/L Cr(VI) | - | 0.02 V/s | [73] |
| Tastants mixture | LAPS | - | 10–30 μV | - | - | [84] | |
| Nutrient concentration | LAPS | (89.0 ± 1.5) mV per decade glucose | 0.5 mM glucose | - | - | [74] | |
| Odorants or neurotransmitters | LAPS | - | 1 μM acetic acid | - | - | [75] | |
| - | 25 μV | - | - | [76] | |||
| - | 45 μV | - | 60 s | [77] | |||
| HCl | LAPS in both time domain and frequency domain | - | pH 2–7.5 | SNR = 3 | - | [85] | |
| Bio-initiator-based biosensor | L-DOPA for protein binding and immunoassay | LAPS with the surface bio-initiated by L-DOPA | 5.68 nA/p[Ag] | 0.001–4 μg/mL IgG | - | - | [82] |
| Polyion-based enzymatic membrane for acetylcholine (ACh) | LAPS and CCD | 56.5 mV/pH for LAPS and 20.5 mV/pH for CCD-type sensor | 1 μM–1 M ACh | - | 200 ms/frame | [86] |
| Category | Target | Technology | Sensitivity | Detection Limit or Range | Noise (mV) | Measurement Time | Ref. |
|---|---|---|---|---|---|---|---|
| Cell metabolism | Rat renal cells | LAPS with heavy doping and thick oxide layer | 56.99 mV/pH | 64% ECAR (extracellular acidification rate) | 5 nA | - | [87] |
| LAPS with an electrolyte-insulator-semiconductor (EIS) | 41.6 mV/pH | - | - | - | [88] | ||
| LAPS with electrical cell-substrate impedance sensor (ECIS) | 57.7 mV/pH | 24% ECAR | - | 2.5 h | [89] | ||
| LAPS with EIS (impedance measurement) | 7.87 ± 1.90% (RMS ± standard deviation) | - | - | 30 min | [37] | ||
| Cardiac myocytes | LAPS | 53.9 mV/pH | - | 0.25 mV | 90 ms (extracellular potential) | [90] | |
| LAPS and ECIS | 1 nA | - | - | 1.0 × 10−4 s (time resolution) | [91] | ||
| Four-channel LAPS | - | 20–40 µV | - | 30 to 250 times per min (beating frequencies) | [104] | ||
| Human breast cancer cells MCF-7 | LAPS with constant voltage detection mode | 1104 nA/pH | 80% ECAR | 1 nA | - | [2] | |
| Mouse embryonic fibroblast 3T6 cells | LAPS and cellular impedance sensor(LAPCIS) | - | 0.16 CI (in cell index), 9.3% ECAR | - | 24–48 h | [92] | |
| Escherichia coli (E. coli) | LAPS with polyacrylamide gel | 5.0 × 104 H+ s−1 per cell | - | 0.06 mV | 36–48 min | [112] | |
| Chinese hamster ovary (CHO) cells | LAPS | 52.8 mV/pH | 0.24 mV/min | - | 10 min | [99] | |
| LAPS with polymer (PP-ABS) multi-chamber structure | - | 2.9 mV/min | - | - | [98] | ||
| LAPS with multi-chamber structures | 54 mV/pH | 2.78 mV/min | - | - | [96] | ||
| LAPS with microfluidic unit | 56 mV/pH | 0.79 mM/pH (buffer capacity) | - | 5 h | [25] | ||
| Adrenal chromaffin cells | LAPS | - | 15% ECAR | 20 dB (SNR) | 10 min | [100] | |
| C3 cells | LAPS | - | 17–299 mM (glucose concentration) | - | 30 min | [99] | |
| Escherichia coli, Corynebacterium glutamicum, and Lactobacillus brevis | Differential LAPS | 54 mV/pH | 1.7–400 mM (glucose concentration) | 0.02 mV | 30 min | [113] | |
| 0.5 mV/min | 1.67 mM (glucose concentration) | 0.02 mV | 40 min | [94] | |||
| HeLa cell lines | LAPS and ECIS | 52.88 mV/pH | 20% ECAR | - | 24 h | [97] | |
| Cancer cells (MDA MB231 and MDA-MB-435MDR) | LAPS with pH sensitive hydrogel nanofibers (NF-LAPS) | 74 mV/pH | - | 5 nA | 90 min | [102] | |
| Hepatoma HepG2 cells | LAPS with Microfluidic chip system | 335.5 nA/pH | 17.88 mpH/min | - | 17 min | [103] | |
| Corynebacterium glutamicum (C. glutamicum ATCC13032) | LAPS with multi-chamber structures | 54 mV/pH | 0.22 mV/min | 0.03 mV | 2 nm/s | [101] | |
| Escherichia coli K12 (E. coli K12) | LAPS with with multi-chamber structure | 54 mV/pH | 0.55 mV/min | - | 60 min | [95] | |
| - | 1.97 mV/min | 0.05 mV | 20 min | [96] | |||
| Taste receptor cells (from Sprague–Dawley rat) | LAPS in both time domain and frequency domain | - | - | 10 dB (SNR) | 49.65 ± 1.92 ms (extracellular potential) | [85] | |
| Rat taste bud cells | LAPS | - | 10–30 µV | - | - | [114] | |
| LAPS and platinum electrodes | - | 0.8% (signal change) | - | 30 min | [105] | ||
| Detection of cells | Mammary adenocarcinoma cell (MDAMB231) | Phage-LAPS | - | 10–60 µV | - | - | [108] |
| Escherichia coli Detection in Orange Juice | Portable nanofiber LAPS (NF-LAPS) | 0.149 per CFU/mL | 102–106 CFU/mL | - | 60 min | [109] | |
| E. coli | NF-LAPS | 74 mV/pH | 20 CFU/ml | 0.003 mV | 60 min | [110] | |
| CTC | LAPS with anti-EpCAM on carboxylated graphene oxide (GO-COOH) | - | 5–1281 CTCs/ml | - | - | [111] | |
| pH probe in vivo | Hippocampal formation of rats | LAPS with a multimodal fiber fabricated by the convergence thermal drawing | 57.5 mV/pH | 250 μm (spatial resolution) | 2.2 mV | 30 Hz (temporal resolution), 14 pixels simultaneously | [106] |
| Deep brain recordings | Mouse hippocampus | LAPS | 50 mV/pH | 5 ± 1.5 Ω/cm | 0.8 dB/cm | 200 Hz | [107] |
| Target | Main Improvement | Technology | Sensitivity | Detection Limit or Range | Noise | Measurement Time | Ref. |
|---|---|---|---|---|---|---|---|
| pH | Resolution of the image | LAPS with an OLED display | 53.7 mV/pH | pH 4–10 | - | 1536 s (1.6 mm × 1.6 mm, spot size of 200 µm) | [101] |
| High-speed chemical imaging | LAPS by frequency division multiplex (FDM) | 0.26/pH (normalized photocurrent) | pH 4–10 | - | 70 frames per second | [104] | |
| Suppress lateral diffusion of photocarriers | LAPS with ring-shaped constant illumination | - | 100 µm spatial resolution | - | - | [116] | |
| Lateral resolution enhancement | pulse-driven LAPS | 53.5 mV/pH | pH 5–10 | 0.2 mV | 1.5 ms per spot (spot size of 100 µm) | [117] | |
| Higher spatial resolution | LAPS with a novel photoexcitation method | - | 82.32 µm spatial resolution | - | - | [118] | |
| High measurement resolution and miniaturisation, reduces measurement time | LAPS with an OLED display (new developed driving method) | 58 mV/pH | pH 5–9 | - | 25 ms per spot (spot size of 200 µm) | [119] | |
| Eliminate measurement error caused by fluctuation, distortion and noise of light source output | LAPS with a SLED | 54.8 mV/pH | pH 5–9 | 7 mV | 50 s | [120] | |
| Increasing the measurement spot density | LAPS using VCSEL array and FPGA (field-programmable gate array) control) | 59.9 mV/pH | pH 4–9 | - | 14 ms per spot | [121] | |
| High-speed measurement and flexibility of addressing | LAPS using an analog micromirror (back side) | 50.4 mV/pH | pH 2–12 | - | 40 s (14.5 × 10.5 mm2, 16 fps) | [122] | |
| Easy, flexible, and miniaturized light source | LAPS with LCD projector | 53 mV/pH | pH 1.9–9.14 | 0.27 mV | 20 Hz (spot size of 180 μm) | [123] | |
| Better linearity and accuracy | LAPS with a superluminescence LED (SLED) | 52.5 mV/pH | pH 3–11 | 7 mV | 150 Hz | [124] | |
| Reduces the size of the set-up and increases the stability and speed of the measurement | LAPS based on the digital light processing (DLP) | - | pH 7 | - | 0.39 ms per spot (step width of 43 μm, spot size of 130 μm) | [125] | |
| Simultaneous stimulation of several areas | Multi spot LAPS with FPGA based controller and integrated signal correction mode | 57 mV/pH | pH 7–9 | 3 mV | 200 ms | [126] | |
| Large chemical images | LAPS with digital mirror device (DMD) | - | - | - | 11 ms per spot (spot size of 130 μm) | [127] | |
| Increase the number of measurement spots, shorten time and improve accuracy | LAPS with FPGA | 54.6 mV/pH | pH 4–9 | 1.4 mV | - | [128] | |
| High resolution and sensitivity | LAPS and SPIM using SOS substrates with a thin anodically grown oxide illuminating the back of the semiconductor substrate | - | 0.8 µm resolution | 0.1 µm | - | [129] | |
| Improving signal-to-noise ratio (SNR) | LAPS with optical focusing | - | pH 4–9 | - | - | [130] | |
| Escherichia coli (E. coli) K12 | Reduce scattering effects | Multi-Chamber LAPS with FPGA-Controlled Laser-Diode Modules | 54 mV/pH | 0.3 × 109–4.8 × 109 cells | 0.15 mV | 5.60 mV/min (4.8 × 109 cells) | [131] |
| B50 rat neuroblastoma cells | High speed, lateral resolution and photocurrent stability | LAPS with analog micromirror and α-Fe2O3 (hematite) thin films | 23 mV/pH | - | - | 8 fps | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, P.; Liu, S.; Chen, Y.; Liang, D.; Wang, M.; Du, L.; Wu, C. The Light-Addressable Potentiometric Sensor and Its Application in Biomedicine towards Chemical and Biological Sensing. Chemosensors 2022, 10, 156. https://doi.org/10.3390/chemosensors10050156
Liu Y, Zhu P, Liu S, Chen Y, Liang D, Wang M, Du L, Wu C. The Light-Addressable Potentiometric Sensor and Its Application in Biomedicine towards Chemical and Biological Sensing. Chemosensors. 2022; 10(5):156. https://doi.org/10.3390/chemosensors10050156
Chicago/Turabian StyleLiu, Yage, Ping Zhu, Shuge Liu, Yating Chen, Dongxin Liang, Miaomiao Wang, Liping Du, and Chunsheng Wu. 2022. "The Light-Addressable Potentiometric Sensor and Its Application in Biomedicine towards Chemical and Biological Sensing" Chemosensors 10, no. 5: 156. https://doi.org/10.3390/chemosensors10050156
APA StyleLiu, Y., Zhu, P., Liu, S., Chen, Y., Liang, D., Wang, M., Du, L., & Wu, C. (2022). The Light-Addressable Potentiometric Sensor and Its Application in Biomedicine towards Chemical and Biological Sensing. Chemosensors, 10(5), 156. https://doi.org/10.3390/chemosensors10050156








