Abstract
This work constructed an ultrasensitive electrochemical bisphenol AF (BPAF) sensor using ultra-stable graphdiyne-templated platinum nanoparticles (PtNPs@GDY) as a sensing platform. PtNPs@GDY nanocomposite was synthesized by a chemical reduction method, and the preparation process was simple and rapid. GDY, with its natural porous structure, was used as substrate to stabilize PtNPs. Due to the high adsorption ability of GDY, it can prevent PtNPs from aggregation and inactivation. Transmission electron microscopy (TEM), high-resolution TEM (HRTEM) and Energy-dispersive X-ray spectroscopy (EDS) were used to characterize the microstructure and morphologies of the materials. Cyclic voltammetry (CV), Electrochemical impedance spectroscopy (EIS) and differential pulse voltammetry (DPV) were employed to investigate the electrochemical properties of the material and the performance of the sensor. At an optimized condition, the sensor exhibited excellent catalytic activities towards BPAF. The linear ranges were from 0.4 to 15.4 μM and 35.4 to 775.4 μM. The limit of detection was 0.09 μM. In addition, the electrochemical sensor showed good reproducibility, stability and anti-interference.
1. Introduction
Bisphenol AF (1,1,1,3,3,3-hexafluoro-2,2-bis(4-hydroxyphenyl) propane, BPAF) is a homologue of bisphenol A (BPA) in which the methyl groups are perfluorinated [1]. It is mainly used as crosslinking agent of fluorine rubber, which can give rubber products good compression resistance, chemical corrosion resistance and thermal stability [2]. BPAF can also be used as monomer to synthesize special fluorine-containing polyimide, fluorine-containing polyamide, fluorine-containing polyester, fluorine-containing polycarbonate and other fluorine-containing polymers, which can be used as gas separation membranes, dielectric coatings, optical fiber sheaths, photocell substrates and binders [3]. Therefore, it has been widely used in microelectronics, optics, space technology and other fields [4]. BPA has been proven to be an endocrine-disrupting compound. As a homologue of BPA, BPAF may also become endocrine disruptors in humans and wild animals by binding with hormone receptors. Because the -CF3 of BPAF may be much more electronegative and reactive than the -CH of BPA. It has been reported that in vitro, the binding efficiency of BPAF with estrogen receptor-α (ER α) is about 20 times that of BPA, and the binding efficiency of BPAF with ER β is about 50 times that of BPA, which indicates that BPAF appears to shift endocrine action toward greater toxicity [2,5]. Therefore, the detection of trace BPAF is very important and necessary.
At present, there are many studies in the literature about the detection of BPA [6,7,8,9], but few for BPAF. High-performance liquid chromatography (HPLC) and ultra-high-pressure liquid chromatography-electrospray tandem mass spectrometry (LC-MS/MS) are the most widely used methods in the actual sample detection of BPAF [10,11]. Although these methods display high accuracy, good reproducibility and a reliable capacity for qualitative analysis, they require complex sample pretreatments and expensive instruments. Electrochemical methods have attracted great interest among environmental researchers because of their low cost, miniaturization, rapid reaction, high sensitivity and selectivity with simple sample pre-treatments [12,13,14]. Recently, an electrochemical method for the detection of BPAF was reported [14]. It is based on a carboxy-functionalized multi-walled carbon nanotubes (COOH-MWCNTs)-modified glassy carbon electrode (GCE).
As we all know, the core of electrochemical detection is the construction of working electrodes, and the key to improving the performance of the modified electrode lies in the design of the interface between the target and the electrode and the construction of efficient electron transport. Nowadays, various nanomaterials have been used to construct electrochemical sensors and improve the performance of the sensors. However, there are still challenges in improving the sensitivity and stability of the sensors.
Graphdiyne (GDY) is a new allotrope of 2D carbon nanostructure as a big network of diacetylenic linkages, with the configurations composed by six carbon hexagons (6C-hexagon) like graphene and eighteen carbon hexagons (18C-hexagon) [15]. GDY has good conductivity and semiconducting behavior. Because of its unique structure and properties, GDY has been widely used in various fields, such as catalysis and battery and energy storage [16,17,18]. Recently, it has been reported that GDY possesses adequate sp-hybridized carbon that may coordinate with metal atoms and dock them on the surface of GDY [19].
In this work, an ultra-stable GDY-templated platinum nanoparticle (PtNPs@GDY) was prepared. The preparation process was simple and rapid. GDY with natural porous structure was used as substrate to stabilize PtNPs. Due to the high adsorption ability of GDY, it can prevent PtNPs from aggregation and inactivation, which will lead to catalytic activity increasing. The electrochemical sensor based on PtNPs@GDY displayed good selectivity and sensitivity for the determination of BPAF.
2. Materials and Methods
2.1. Reagents and Apparatus
GDY was purchased from Pioneer Nanotechnology Co. (Nanjing, China), H2PtCl6, NaBH4, Na2HPO4, KH2PO4, H3PO4, CC, HQ, RC and BPAF were bought from Aladdin Chemical Reagents Co. Ltd. (Shanghai, China). All reagents involved are analytical grade and are used without further purification. Phosphate buffer (PB) solution with various pH were prepared by using the stock solution of 0.1 M Na2HPO4, 0.1 M NaH2PO4, and the supporting electrolyte was 0.1 M KCl. The experimental water is the deionized water.
All electrochemical experiments were carried out on a CHI 660E electrochemical workstation (Chenhua Instruments Co., Shanghai, China), which contained a three-electrode system; a working electrode (bare or modified electrode), a reference electrode (saturated calomel electrode, saturated with KCl) and an auxiliary electrode (a platinum wire). The surface morphologies of composites were characterized using a high-resolution transmission electron microscopy (HRTEM, FEI Talos F200X, USA) and energy-dispersive X-ray spectroscopy (EDS, FEI Talos F200X, Super X, USA).
2.2. Synthesis of PtNPs@GDY
Firstly, 5 mg GDY powder was dissolved in water and processed by sonification for 10 min. Then, 0.5 mL H2PtCl6 aqueous solution (m:m, 1%) was added dropwise into 1 mL GDY suspension under vigorous stirring in an ice bath for 10 min. The resulting product was collected and washed with pure water by centrifugation to remove the excess H2PtCl6. The product was PtCl62−@GDY. Finally, 0.5 mL NaBH4 aqueous solution (10 mg/mL) was rapidly added to 1 mL PtCl62−@GDY suspension. The whole process was still carried out under the condition of ice bath and continuous stirring. After centrifugation, the excess NaBH4 was removed, and the product was obtained PtNPs@GDY. The illustration of the synthesis steps was shown in Scheme 1.
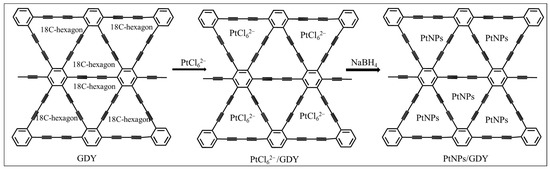
Scheme 1.
Illustration for the synthesis of PtNPs@GDY.
2.3. Fabrication of the Modified Electrodes
GCE (3 mm in diameter) was polished to a mirror surface with 0.05 mm and 0.3 mm alumina slurry. After ultrasonic treatment with water, ethanol and water for 5 min, the obtained electrode was used as a bare GCE. Then, 5 mL homogeneous suspensions of PtNPs@GDY (1 mg/mL) was dropped onto the bare GCE surface and dried in air (used as PtNPs@GDY/GCE). Other contrast-modified electrodes were prepared by the same method.
3. Results and Discussion
3.1. Characterization of PtNPs@GDY
The morphology of the prepared PtNPs@GDY was characterized by TEM. As shown in Figure 1A, there were many nanoparticles uniformly loaded on the GDY sheets, which revealed that platinum ions were successfully reduced to platinum nanoparticles by sodium borohydride. Figure 1B presents the elemental mapping images of PtNPs@GDY, which showed the homogeneous spatial distribution of Pt and C elements on the GDY. The EDS measurement further proved the existence of Pt and C atoms (Figure 1C).
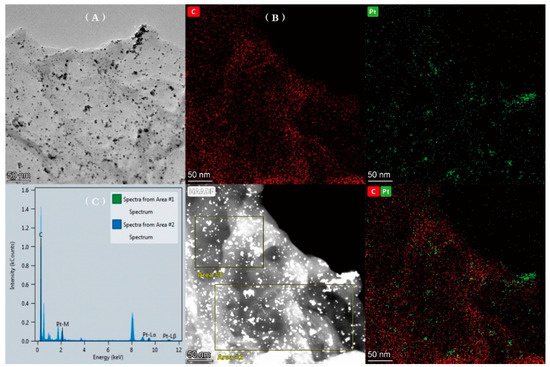
Figure 1.
TEM image (A), elemental mapping (B) and EDS (C) of PtNPs@GDY.
3.2. Electrochemical Behaviors of the Different Modified Electrodes
Electrochemical impedance spectroscopy (EIS) is a common and effective method to characterize the electrochemical performance of the modified electrode in the process of construction. According to the Nyquist diagram, the impedance diagram consists of a linear part, which represents the process of finite diffusion, and a semicircle part, which represents the resistance of electron transfer (the greater the diameter of the semicircle, the greater the resistance) [20,21,22]. The electrode-modification materials were characterized by EIS measurements carried out in the presence of K3[Fe(CN)6]/K4[Fe(CN)6] (5.0 mM) and KCl (0.1 M) (Figure 2). The amplitude of the perturbing signal is 0.005 V, and the frequency range is from 0.1 Hz to 100,000 Hz. DC potential is 0.15 V. It can be seen that the bare GCE had a small charge transfer resistance and, after modification with GDY, the semicircle significantly reduced, suggesting that GDY promoted the transfer of electrons on the electrode surface. After adsorption of PtCl62−, the diameter of the curves increased because electron transfer was retarded between the redox probe and the electrochemical layer. When the PtCl62−/GDY was reduced to PtNPs@GDY by NaHB4, the diameter of the semicircle decreases significantly, implying that the PtNPs@GDY promoted the charge transfer between the redox pair and electrode surface.
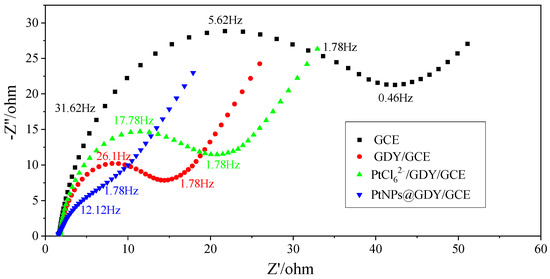
Figure 2.
The impedimetric characteristics of bare GCE, GDY/GCE, PtCl62−/GDY/GCE, and PtNPs@GDY/GCE.
Figure 3 showed CVs of the bare GCE (A), GDY/GCE (B), PtCl62−/GDY/GCE (C), PtNPs@GDY/GCE and the above three modified electrodes (D) in 0.1 M PB with and without 0.5 μM BPAF. The results showed that BPAF had a response at about 0.75 V on all electrodes including bare GCE but, at the same concentration, the response on the PtNPs@GDY/GCE was the largest and most obvious.
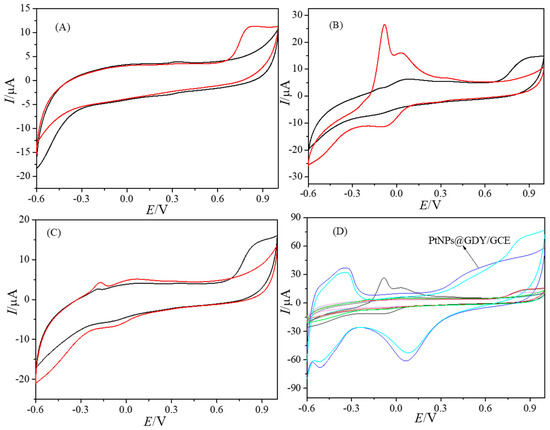
Figure 3.
CVs of the bare GCE (A), GDY/GCE (B), PtCl62−/GDY/GCE (C), PtNPs@GDY/GCE and the above three modified electrodes (D) in 0.1 M PB with the absence and presence of 0.5 mM BPAF.
3.3. Effect of pH and Scan Rate
The optimal pH value was investigated by differential pulse voltammetry (DPV) technique in the range of 3.0 to 8.0 (Figure 4A). The relationship between peak currents, peak potentials and pH values was shown in Figure 4B. The results showed that with the increasing of pH, the peak potential decreased gradually, and the peak current first decreased and then increased, reaching a peak at 6.0. Therefore, pH 6.0 PB was selected as the conductive liquid in the following experiments.
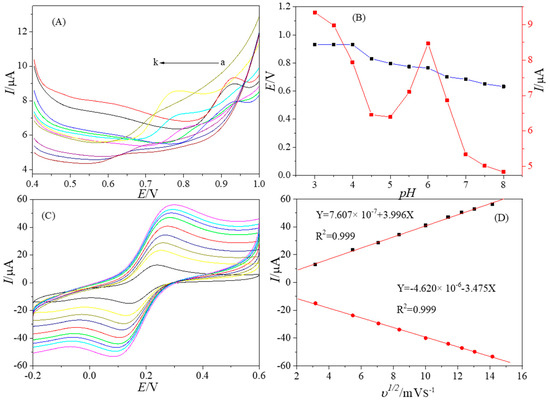
Figure 4.
DPVs of PtNPs@GDY/GCE in 0.1 M PB with 0.5 μM BPAF at different pH (from a to k: 3.0 to 8.0) (A); The relationship between pH and peak currents and peak potentials (B); CVs of PtNPs@GDY/GCE in 5 mM K3[Fe(CN6)] solution with 0.1 M KCl at different scan rates (10, 30, 50, 70, 100, 130, 150, 170, 200 mVs−1) (C); The relationship between peak currents and square root of the scan rates (D).
Figure 4C showed the CVs of PtNPs@GDY/GCE in 5 mM K3[Fe(CN6)] solution with 0.1 M KCl at different scan rates. The relationship between peak currents and the scan rates was shown in Figure 4D. It can be seen that the peak current is proportional to the square root of the sweep speed (Ip = 0.7607 × 10−6 + 3.996 υ 1/2, R2 = 0.999). The effective surface area of the modified electrode can be calculated by using the Randles–Sevcik equation [23]:
where Ip is the peak currents (A), A is the effective surface area (cm2), D (6.67 × 10−6 cm2 s−1) is the diffusion coefficient of K3[Fe(CN6)], n is the number of electron transfer (n = 1), u is the scan rate (V/s) and c is the bulk concentration of K3[Fe(CN6)](mol/cm3). The effective surface area is 1.15 cm2.
3.4. DPV Determination of BPAF
Under the optimum conditions, the response of the PtNPs@GDY/GCE to BPAF was studied by the DPV method (Figure 5A). The results showed that the peak current increased with the increase of the concentration of BPAF and showed a two-stage linear relationship (Figure 5B). The linear equations were Ip = 1.05 × 10−5 + 0.098C (R2 = 0.946) and Ip = 1.21 × 10−5 + 0.0059C (R2 = 0.995) with the linear range of 0.4–15.4 μM and 35.4–775.4 μM. According to the formula LOD = 3 S/m [24]. Where S is the standard deviation of peak current, m is the slope of linear equation. The LOD was 0.09 μM. The oxidation mechanism for BPAF can refer to the report of Wang [1] and Chan [25].
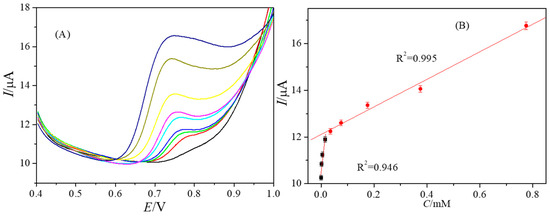
Figure 5.
DPVs of PtNPs@GDY/GCE in 0.1 M PB (pH 6.0) with different concentrations of BPAF (0.4, 2.4, 5.4, 15.4, 35.4, 75.4, 175.4, 375.4, 775.4 μM) at the scan rate of 100 mVs−1 (A); The linear relationship between the peak current and concentration (B).
3.5. Interference, Stability and Practical Application
Anti-interference ability is an important parameter to evaluate the performance of electrochemical sensors. In this work, some phenolic compounds and inorganic ions were used to detect their effects on the sensor response signal. It can be seen from Figure 6A that catechol (CC), hydroquinone (HQ) and Resorcinol (RC) were oxidized on PtNPs@GDY/GCE at about 0.1 V, 0.25 V and 0.65 V, respectively. The oxidation peak potential difference and peak current between these substances showed that they had no obvious interference with BPAF; 500-fold Na+, K+, Cl−, SO42−, NO3− and PO43− also had no interference on the determination of BPAF. These experiments revealed that the PtNPs@GDY/GCE has good selectivity for BPAF.
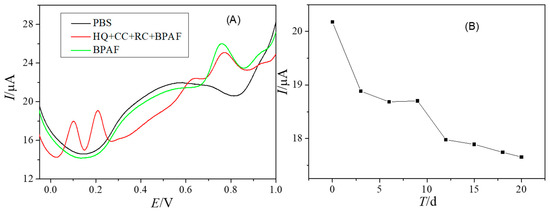
Figure 6.
DPVs of PtNPs@GDY/GCE in 0.1 M PB (pH 6.0) with 0.5 μM BPAF, 2 μM HQ, 1 μM CC and 1 μM RC (A), and the study of the stability tested with the proposed sensor (B).
After the modified electrode was prepared, it was placed in the refrigerator at 4 °C for 20 days. During this period, 0.5 μM BPAF was tested by DPV every 3 days. The results showed that the peak current remained between 87.5% and 93.6% of the original peak current (Figure 6B). This indicated that the modified electrode had good stability.
In order to test the potential of the modified electrode in practical application, we used DPV and standard addition method to test the recovery of BPAF in river water (from Liucang river, Bijie, Guizhou, China). The data obtained were listed in Table 1. It can be seen that the recoveries ranged from 85% to 118.3%, indicating that the PtNPs@GDY/GCE can be applied to determine BPAF in real samples.

Table 1.
Determination of BPAF in river water (n = 3).
4. Conclusions
In this work, a graphdiyne-templated platinum (PtNPs@GDY) nanocomposite was prepared and used to construct a sensitive electrochemical BPAF sensor. The preparation of PtNPs@GDY and the construction of the sensor are very simple and fast. GDY, with its natural porous structure and high adsorption capacity, can prevent the aggregation and deactivation of PtNPs. In addition to their respective good catalytic performance, the large specific surface area and adsorption performance of GDY and the high conductivity of PtNPs give the PtNPs@GDY/GCE not only excellent catalytic performance, but also excellent selectivity and stability. The recovery experiment also shows that the PtNPs@GDY/GCE has the potential to detect BPAF in real samples.
Author Contributions
Y.Z. is the corresponding author; Y.Z., Z.X. and G.G. conceived the experiments. P.Z., Q.L. and L.M. conducted the experiments. Z.X. and G.G. acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Natural Science Foundation of China (NSFC22064005), the Department Education Project of Guizhou Province (KY[2018]471, KY[2019]159), Guizhou Province Key Laboratory of Ecological Protection and Restoration of Typical Plateau Wetlands (QKHPTRC [2020] 2002) and Guizhou Coal Chemical Engineering Collaborative Innovation Center ([2014]08).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.; Yang, J.; Wang, Y.; Li, Y.; Wang, F.; Zhang, L. Studies on electrochemical oxidation of estrogenic disrupting compound bisphenol AF and its interaction with human serum albumin. J. Hazard. Mater. 2014, 276, 105–111. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, A.D.; Schug, K.A. A review of separation methods for the determination of estrogens and plastics-derived estrogen mimics from aqueous systems. Anal. Chim. Acta 2011, 696, 6–26. [Google Scholar] [CrossRef]
- Song, S.; Ruan, T.; Wang, T.; Liu, R.; Jiang, G. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China. Environ. Sci. Technol. 2012, 46, 13136–13143. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, J.; Zhang, Y.; Zhou, P.; Wang, J.; Liu, Y. Heterogeneous catalytic oxidation degradation of BPAF by peroxymonosulfate active with manganic manganous oxide: Mineralization, mechanism and degradation pathways. Chemosphere 2021, 263, 127950. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Suyama, K.; Shiki, J.; Torikai, K.; Nose, T.; Shimohigashi, M.; Shimohigashi, Y. Bisphenol AF: Halogen bonding effect is a major driving force for the dual ERα-agonist and ERβ-antagonist activities. Bioorg. Med. Chem. 2020, 28, 115274. [Google Scholar] [CrossRef] [PubMed]
- Deiminiat, B.; Rounaghi, G.H.; Arbab-Zavar, M.H.; Razavipanah, I. A novel electrochemical aptasensor based on f-MWCNTs/AuNPs nanocomposite for label-free detection of bisphenol A. Sens. Actuators B 2017, 242, 158–166. [Google Scholar] [CrossRef]
- Ali, M.Y.; Alam, A.U.; Howlader, M.M. Fabrication of highly sensitive bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuators B 2020, 320, 128319. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Y.; Wang, J.; Xiao, L.; Yang, X.; Cui, R.; Han, Z. Nanocomposites consisting of nanoporous platinum-silicon and graphene for electrochemical determination of bisphenol A. Microchim. Acta 2020, 187, 241. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Campiña, J.M.; Silva, A.F. A layered nanocomposite of laccase, chitosan, and Fe3O4 nanoparticles-reduced graphene oxide for the nanomolar electrochemical detection of bisphenol A. Microchim. Acta 2020, 187, 262. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, J.; Yang, Y.; Zhou, N.; Zhang, J.; Shao, B.; Wu, Y. Determination of bisphenol AF (BPAF) in tissues, serum, urine and feces of orally dosed rats by ultra-high-pressure liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B 2012, 901, 93–97. [Google Scholar] [CrossRef]
- Li, Y.; Lu, P.; Cheng, J.; Wang, Q.; He, C. Simultaneous solid-phase extraction and determination of three bisphenols in water samples and orange juice by a porous β-cyclodextrin polymer. Food Anal. Methods 2018, 11, 1476–1484. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Li, H.; Wang, W.; Lv, Q.; Xi, G.; Bai, H. Disposable paper-based electrochemical sensor based on stacked gold nanoparticles supported carbon nanotubes for the determination of bisphenol A. Electrochem. Commun. 2016, 68, 104–107. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, D.; Wang, L.; Li, Q.; Zhang, L. Simultaneous determination of endocrine disrupting compounds bisphenol F and bisphenol AF using carboxyl functionalized multi-walled carbon nanotubes modified electrode. Talanta 2014, 130, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, S.; Lv, P.; He, C.; Ma, D.; Yang, Z. First principles study on the interfacial properties of NM/graphdiyne (NM = Pd, Pt, Rh and Ir): The implications for NM growing. Appl. Surf. Sci. 2016, 360, 1–7. [Google Scholar] [CrossRef]
- Hui, L.; Xue, Y.; Yu, H.; Liu, Y.; Fang, Y.; Xing, C.; Li, Y. Highly efficient and selective generation of ammonia and hydrogen on a graphdiyne-based catalyst. J. Am. Chem. Soc. 2019, 141, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, N.; He, J.; Yang, Z.; Shen, X.; Huang, C. Preparation of 3D architecture graphdiyne nanosheets for high-performance sodium-ion batteries and capacitors. ACS Appl. Mater. Interfaces 2017, 9, 40604–40613. [Google Scholar] [CrossRef]
- Wang, N.; He, J.; Wang, K.; Zhao, Y.; Jiu, T.; Huang, C.; Li, Y. Graphdiyne-Based Materials: Preparation and Application for Electrochemical Energy Storage. Adv. Mater. 2019, 31, 1803202. [Google Scholar] [CrossRef]
- Lin, Z.Z. Graphdiyne as a promising substrate for stabilizing Pt nanoparticle catalyst. Carbon 2015, 86, 301–309. [Google Scholar] [CrossRef]
- Yakubu, S.; Xiao, J.; Gu, J.; Cheng, J.; Wang, J.; Li, X.; Zhang, Z. A competitive electrochemical immunosensor based on bimetallic nanoparticle decorated nanoflower-like MnO2 for enhanced peroxidase-like activity and sensitive detection of Tetrabromobisphenol A. Sens. Actuators B 2020, 325, 128909. [Google Scholar] [CrossRef]
- Shi, Q.; Tao, H.; Wu, Y.; Chen, J.; Wang, X. An ultrasensitive label-free electrochemical aptasensing platform for thiamethoxam detection based on ZIF-67 derived Co-N doped porous carbon. Bioelectrochemistry 2022, 149, 108317. [Google Scholar] [CrossRef]
- Wei, P.; Wang, S.; Wang, W.; Niu, Z.; Rodas-Gonzalez, A.; Li, K.; Yang, Q. CoNi bimetallic metal–organic framework and gold nanoparticles-based aptamer electrochemical sensor for enrofloxacin detection. Appl. Surf. Sci. 2022, 604, 154369. [Google Scholar] [CrossRef]
- Nehru, R.; Hsu, Y.F.; Wang, S.F.; Dong, C.D.; Govindasamy, M.; Habila, M.A.; AlMasoud, N. Graphene oxide@ Ce-doped TiO2 nanoparticles as electrocatalyst materials for voltammetric detection of hazardous methyl parathion. Microchim. Acta 2021, 188, 216. [Google Scholar] [CrossRef] [PubMed]
- Skoog, D.; Holler, J.; Nieman, T. Principles of Instrumental Analysis, 5th ed.; Harrcourt Brace College Publishers: Orlando, FL, USA, 1998; pp. 13–14. [Google Scholar]
- Chan, Y.Y.; Yue, Y.N.; Li, Y.X.; Webster, R.D. Electrochemical/chemical oxidation of bisphenol A in a four-electron/two-proton process in aprotic organic solvents. Electrochim. Acta 2013, 112, 287–294. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).