Abstract
Objective: Chronic stress affects the immune system via the hypothalamic–pituitary–adrenal (HPA) axis and autonomic system. Chronic inflammation is a risk factor for cardiovascular diseases, cancer onset and progression, susceptibility to infection, and cognitive impairment. Mind–body interventions (MBIs) could affect the immune and neuroendocrine systems, and we aimed to assess the correlations among these systems through a meta-analysis. Methods: RCTs were identified by searching three databases: PubMed, Embase, and Scopus. Of the 1697 studies identified, 89 were included in this study. Risk of bias was examined using the Cochrane risk-of-bias assessment tool. Data were pooled using a random-effects model, and SMDs were calculated. I2 statistics and Egger’s test were used to assess the significance of the asymmetry. Influence diagnostics were used to assess whether pooled effects were disproportionately dependent on any single study. The trim-and-fill method was applied to all identified asymmetric instances. Meta-regression was used to examine the moderating effect of MBI efficacy on biomarkers. Results: MBIs generally decreased the levels of inflammatory factors, such as the CRP, IL-6, TNF-α, IL-1, IL-8, IL-17, ESR, and cortisol, and increased IL-10, IFN-γ, IL-1ra, BDNF, and secretory IgA. In a subgroup analysis of the CNS and cancer, qigong and yoga showed increased BDNF and IL-6, respectively. Notably, IL-10 was increased in inflammatory diseases, and IFN-γ was increased in viral infections. Conclusions: This study revealed MBIs decrease inflammatory cytokine and increase anti-inflammatory, antiviral, and immune-activating factors. These results suggest the MBIs including gentle physical exercise may be beneficial for neuropsychiatric disorders or tumors. Prospero registration number: CRD42024507646.
1. Introduction
Mind–body interventions (MBIs) including mindfulness-based therapies are behavioral health interventions and mind–body practices with a focus on cultivating mind–body awareness and attitudinal qualities of kindness, compassion, joy, and equanimity [1]. MBIs include breathing exercises, meditation, and physical movements. Mindfulness-based therapies are approaches integrating mindfulness into daily life using static or dynamic methods to achieve mental and physical adjustment. Static practices include mindfulness-based stress reduction, mindfulness-based cognitive therapy, acceptance and commitment therapy, and meditation [2,3,4,5]. Dynamic practices include breathing and gentle physical exercises, such as tai chi chuan, tai chi chih, and yoga [6,7,8,9].
The immune, nervous, and endocrine systems are interconnected [10]. Chronic stress affects the immune system via the hypothalamic–pituitary–adrenal (HPA) axis and autonomic system (increased sympathetic activity and decreased parasympathetic tone). Subsequent production of proinflammatory cytokines further stimulates the HPA axis, leading to increased cortisol secretion to combat the stress [5]. Acute stress can strengthen immunity and promote protection during infection; however, chronic stress causes cortisol increase and dysregulates or inhibits immune function [11]. Chronic stress leads to chronic inflammation and disease, increased susceptibility to infections, and increased risk of cancer onset and progression [12]. Patients with chronic conditions, such as heart failure and cancer, exhibit an increased risk of mild cognitive impairment (MCI) and subsequent progression to dementia [13,14,15]. In addition to cognitive symptoms, patients with MCI often exhibit neuropsychiatric symptoms, such as depression, anxiety, apathy, and irritability [16]. A meta-analysis revealed that older adults with high circulating IL-6 levels are 1.5-times more likely to experience cognitive decline than young individuals [17]. During stress or injury, many systems are activated and transmit messages to each other [18]. Therefore, any imbalance or lack of coordination among these systems leads to immune dysregulation and disease development.
To date, most meta-analyses have focused on the immune system, with only a few studies investigating the nervous and endocrine systems [2,19]. One previous meta-analysis presented that MBIs including mindfulness-based therapy, tai chi, and yoga may reduce markers of inflammation and influence virus-specific immune responses to vaccination [20]. Another meta-analysis mentioned that mind–body interventions influence both psychological and physiological regulation, as well as the body’s reactivity to stressors, thereby decreasing the risk and progression of stress-related diseases [19]. Therefore, in this study, we aimed to identify the specific correlations among these systems and MBIs to determine the mechanisms by which different MBIs affect different health conditions.
2. Methods
2.1. Study Selection and Data Extraction
Based on the eligibility criteria outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [21], studies reporting the effects of MBIs on at least one of the following biomarkers were selected for this meta-analysis: immune function received C-reactive protein (CRP), interleukin (IL)-6, IL-1, IL-1 receptor antagonist (IL-1ra), tumor necrosis factor (TNF)-α, IL-8, IL-10, IL-17, interferon (IFN)-γ, nuclear factor (NF)-κB, salivary immunoglobulin A (sIgA), and neuroendocrine function included brain-derived neurotrophic factor (BDNF) and cortisol. Inclusion criteria were as follows: (1) articles written in English, (2) randomized controlled trial (RCT) design, (3) including a measure of at least one of the target biomarkers, (4) published in a peer-reviewed journal, and (5) including MBIs. Exclusion criteria were as follows: (1) articles with “unpublished” status, (2) non-peer reviewed articles, (3) non-RCT design, and (4) not including a measure of at least one target biomarker. The full search protocol (registration number: CRD42024507646) can be accessed on the International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO).
Three databases (PubMed, Embase, and Scopus) were searched for articles published between January, 2000 and January, 2024. Predetermined search terms related to MBI (meditation OR qigong OR yoga OR mindfulness OR mindful OR tai chi), markers (inflammatory OR cytokine OR immunity OR immune OR BDNF OR brain-derived neurotrophic factor), and design type (randomized controlled trial OR RCT) were used (Supplementary: prospero search protocol).
Two authors (SL and TC) independently conducted the electronic search. Disagreements were resolved by a third author (KY). All participant information and design data were extracted separately by the two authors to reduce the likelihood of bias. When multiple articles were published in a single study, the most relevant publication was used and supplemented with data from other relevant publications, as required. The authors of these studies were contacted when the pertinent information was unavailable in the published version.
The following data were extracted from each study by the PICO (Population, Intervention, Comparator, Outcome), which was used to define the eligibility criteria for selecting suitable studies [22]. (1) P (Population): Participants in any clinical or experimental context. No restrictions based on age, sex, or health condition were applied; (2) I (Intervention): Studies involving mind–body interventions (such as mindfulness-based stress reduction, mindfulness-based cognitive therapy, acceptance and commitment therapy, tai chi, and yoga). Refs. [2,3,4,5,6,7,8,9] and any session and duration; (3) C (Comparator): Studies involving control type (such as active control, waitlist, and treatment as usual). However, studies that compared the effects of different MBIs were excluded; (4) O (Outcome): The measurement of biomarker levels and statistics (mean, standard deviation [SD], and effect size). Most biomarkers were obtained from serum samples, except for IgA, which was obtained from saliva samples, and cortisol, which was obtained from serum, hair, and saliva samples. As hair cortisol accumulates continuously during hair growth, it has no set specific sampling time. For the serum and saliva samples, we used either the average value provided in the original study or the value from the morning collection.
2.2. Risk of Bias Within Individual Studies
We examined the risk of bias in the included studies according to the following six key domains using the Cochrane risk-of-bias assessment tool (Rob 2.0) [23]: (a) randomization, (b) deviation from intervention, (c) missing data, (d) measurement of outcome, (e) selection of reported results, and (f) overall risk of bias. Each potential source of bias was graded as high, low, or some concerns.
2.3. Summary Measures
Standardized mean difference (SMD) was calculated using primary effect size statistics. Effect sizes were calculated exclusively from the means and SDs. Pooled effect sizes were calculated separately for each biomarker at baseline and post-intervention and for the differences between the intervention and control effects.
2.4. Synthesis of Results
All meta-analyses and plots were estimated using the metafor package in R 4.3.0 [24]. SMD, corresponding p values, and 95% confidence intervals (95% CIs) were calculated. All meta-analyses were specified as random-effects models using a restricted maximum likelihood estimator. A random-effects model accounted for within- and between-study variations. Heterogeneity across the studies was assessed using I2 statistics. An I2 statistic > 50% indicated substantial heterogeneity [25]. Subgroup analyses were conducted to detect substantial heterogeneity. Based on MBI intervention type, medical condition, and control intervention type, we categorized the studies into three groups. The MBI interventions included static, qigong, and yoga. For medical conditions, the categories were central nervous system (CNS), medical disease (MD), and cancer. The control interventions included active control (AC), waitlist (WL), and treatment as usual (TAU). The static group received acceptance and compassion therapy, cognitive behavioral therapy, mindfulness-based and awareness therapy, and meditation. The qigong group included tai chi chuan, tai chi chih, and qigong. The CNS group included healthy participants experiencing stress and loneliness, patients with cognitive impairment, Parkinson’s disease, anxiety, depression, schizophrenia, or fibromyalgia. The AC group included treatment approaches other than MBI and did not simply continue with the original treatment alone, such as group counseling, health education program, psychosocial therapy, and relaxation exercise, etc. The WL group initially maintained their original lifestyle or treatment approach until the research intervention was completed, after which they joined the study treatment program. As for the TAU group, they continued maintaining their original lifestyle and treatment approach throughout the entire study.
2.5. Risk of Bias Across Studies
When three or more studies were identified, publication bias was assessed through visual inspection of funnel plots, where asymmetry in the distribution of effect size to standard error was suggestive of publication bias [24]. Influence diagnostics were used to assess whether pooled effects were disproportionately dependent on a single study [26]. Egger’s test was used to assess the significance of asymmetry [27]. The trim-and-fill method was applied to any identified instances of asymmetry, and the effect sizes were recalculated [28]. Meta-regression was used to examine whether the intervention method and medical conditions moderated the MBI efficacy.
3. Results
3.1. Study Selection and Characteristics
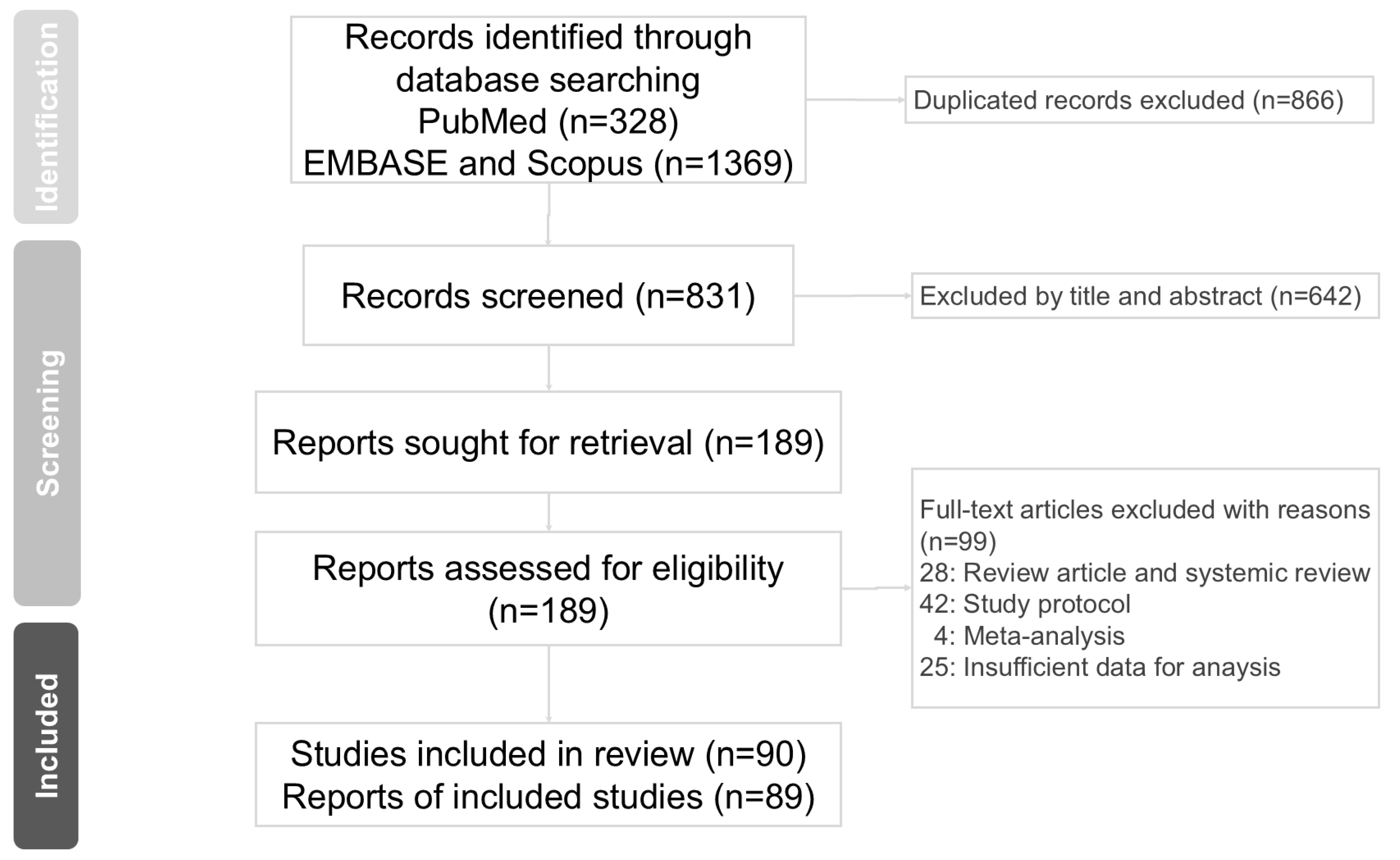
In this study, we identified 1697 articles from online databases. After excluding 831 duplicated articles, 189 articles were assessed for eligibility. Finally, 89 articles [3,4,5,6,7,8,9,12,13,14,15,16,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] were assessed for suitability according to the inclusion criteria for this meta-analysis. The PRISMA flow diagram for studies retrieved through electronic searches and the selection process for study inclusion are shown in Figure 1. Characteristics of the 89 qualifying studies are presented in Table 1. The quality of the included studies was assessed using the Cochrane risk-of-bias assessment tool (Rob 2.0) [23] (Table 2). The number of studies for each biomarker varied, with 47 for CRP, 57 for IL-6, 26 for TNF-α, 10 for IL-1, 4 for IL-1ra, 13 for IL-8, 11 for IL-10, 4 for IL-17, 7 for IFN-γ, 3 for ESR, 2 for NF-κB, 11 for BDNF, 20 for cortisol, and 3 for sIgA (Table 3; Supplementary Figures S1 and S2, Supplementary Table S1).
Figure 1.
PRISMA flow diagram for studies retrieved through the electronic search and the selection processes.

Table 1.
Characteristics of the included studies.

Table 2.
Risk of bias for each study. Domains, D1: Bias arising from the randomization process; D2: Bias due to deviations from intended interventions; D3: Bias due to missing outcome data; D4: Bias in measurement of the outcome; D5: Bias in selection of the reported result; D6: Overall risk of bias.

Table 3.
Meta-analysis of MBI post-intervention effect on biomarkers.
3.2. Statistical Pooling of Outcomes and Meta-Analysis
A meta-analysis was conducted for each biomarker post-intervention, and the difference between the MBI and control groups was assessed. Post-intervention, MBIs slightly affected the levels of CRP (SMD = −0.12; 95% CI, −0.23 to −0.01) and IL-1ra (SMD = 0.02; 95% CI, −0.15 to 0.19), moderately affected the levels of IL-6 (SMD = −0.24; 95% CI, −0.57 to 0.08), IFN-γ (SMD = 0.32; 95% CI, −0.10 to 0.74), TNF-α (SMD = −0.37; 95% CI, −0.73 to −0.01), BDNF (SMD = 0.30; 95% CI, −0.25 to 0.85), IL-1 (SMD = −0.40; 95% CI, −0.72 to −0.08), IL-8 (SMD = −0.24; 95% CI, −0.56 to 0.08), IL-17 (SMD = −0.30; 95% CI, −0.88 to 0.28), IL-10 (SMD = 0.38; 95% CI, −0.21 to 0.97), and cortisol (SMD = −0.33; 95% CI, −0.55 to −0.12), and significantly affected the levels of sIgA (SMD = 0.59; 95% CI, −0.21 to 1.40) (Table 3; Supplementary Figure S1).
Analysis of the differences between the post-intervention effect of MBI and post-intervention effect of control groups revealed that MBIs slightly affected the levels of CRP (SMD = −0.13; 95% CI, −0.21 to −0.05), IL-6 (SMD = −0.11; 95% CI, −0.30 to 0.08), IL-8 (SMD = −0.13; 95% CI, −0.29 to 0.03), IL-1ra (SMD = −0.02; 95% CI, −0.23 to 0.19), and cortisol (SMD = −0.16; 95% CI, −0.34 to 0.03), moderately affected the levels of TNF-α (SMD = −0.24; 95% CI, −0.42 to −0.06), BDNF (SMD = 0.29; 95% CI, −0.19 to 0.78), IL-1 (SMD = −0.24; 95% CI, −0.37 to −0.11), IL-10 (SMD = 0.27; 95% CI, −0.06 to 0.61), IFN-γ (SMD = 0.46; 95% CI, −0.06 to 0.99), and sIgA (SMD = 0.39; 95% CI, −0.23 to 1.01), and significantly affected the levels of IL-17 (SMD = −0.75; 95% CI, −1.74 to 0.23) (Table 3; Supplementary Figure S3).
3.3. Subgroup Analysis
The I2 statistic showed potential heterogeneity for publication bias in IL-6, TNF-α, IL-1, IL-8, IL-10, IFN-γ, cortisol, and BDNF levels post-intervention (Table 3). Therefore, subgroup analysis was conducted.
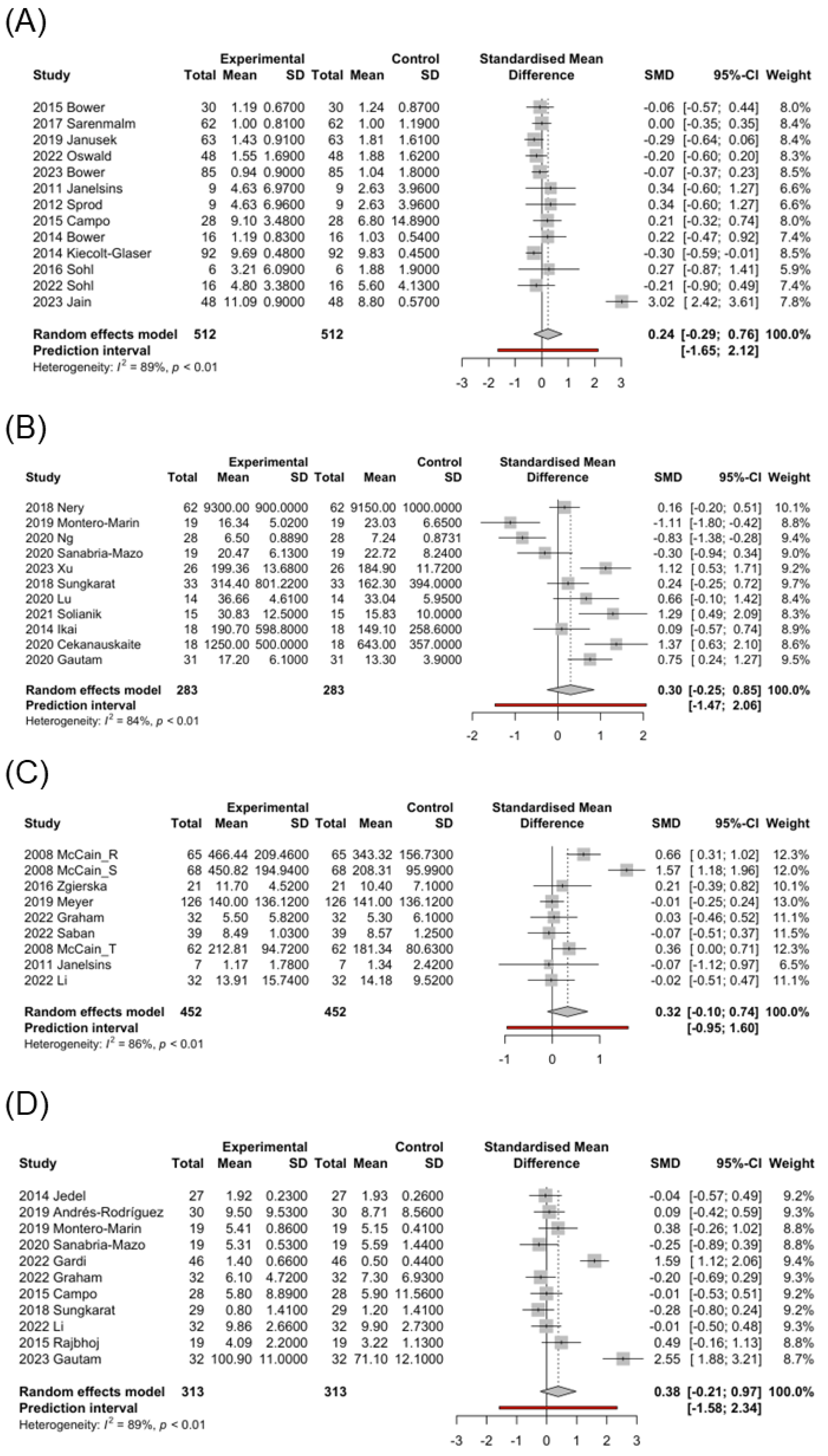
In the MBI post-intervention studies, most groups showed a mild-to-moderate decrease in IL-6 levels, with only the cancer group showing a moderate increase in IL-6 levels (SMD = 0.24; 95% CI, −0.29 to 0.76; Figure 2A). Therefore, the cancer group was further analyzed by intervention type. Qigong (SMD = 0.29; 95% CI, 0.10 to 0.47) and yoga (SMD = 0.61; 95% CI, −1.14 to 2.36) exerted moderate-to-high increasing effects on IL-6 levels, whereas static exerted a mild decreasing effect on IL-6 levels (SMD = −0.12; 95% CI, −0.27 to 0.02). However, the control group exhibited a mild-to-moderate decrease in IL-6 levels (Table 3). We further analyzed the control post-intervention studies in the cancer group: the AC (SMD = 0.07; 95% CI, −0.30 to 0.44) exerted a mild increasing effect, and the TAU (SMD = −0.64; 95% CI, −3.08 to 1.80) exerted an obvious decreasing effect on IL-6 (Table 3).
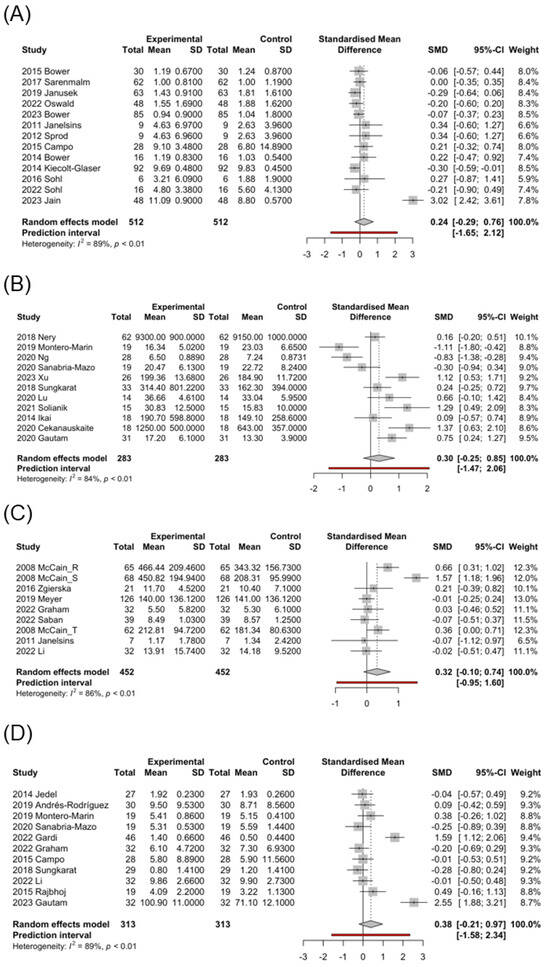
Figure 2.
Forest plot of the standardized mean difference of mind–body interventions effect in biomarkers. (A) IL-6-cancer [6,8,9,38,42,44,47,49,56,91,94,96,100], (B) BDNF [3,15,41,51,53,61,63,66,68,78,105], (C) IFN-γ [5,8,30,57,83,86,92], (D) IL-10 [3,6,40,45,53,54,68,82,83,86,99]. Control: pre-MBI effect. Experimental: post-MBI effect.
In the MBI post-intervention on IL-6 levels in the CNS group, all groups showed a mild-to-moderate decrease. However, in the control post-intervention studies in the CNS group, the AC (SMD = 0.03; 95% CI, −0.10 to 0.16) and WL (SMD = 0.05; 95% CI, −0.11 to 0.20) exerted a mild increasing effect, and the TAU (SMD = −0.13; 95% CI, −0.48 to 0.22) exerted a moderate decreasing effect on IL-6.
In the MBI post-intervention studies in TNF-α levels, the intervention groups for static (SMD = −0.09; 95% CI, −0.34 to 0.17) and qigong (SMD = −0.01; 95% CI, −0.24 to 0.22) exhibited a mild decrease, but the group for yoga exhibited a significant decrease (SMD = −0.92; 95% CI, −1.88 to 0.03). However, when compared separately with the control group, all exhibited a mild-to-moderate decrease. In the medical condition subgroups, a significant decrease in TNF-α levels was observed in the cancer (SMD = −0.66; 95% CI, −2.42 to 1.11) and MD (SMD = −0.66; 95% CI, −1.61 to 0.30) groups, but only a slight decrease was observed in the CNS group (SMD = −0.10; 95% CI, −0.34 to 0.13) (Table 3). In the control post-intervention studies in TNF-α levels, the intervention groups for AC (SMD = −0.06; 95% CI, −0.25 to 0.13) and WL (SMD = −0.03; 95% CI, −0.67 to 0.62) exhibited a mild decrease, but the group for TAU exhibited a mild increase (SMD = 0.05; 95% CI, −0.17 to 0.27).
In the MBI post-intervention studies, a significant increase in BDNF levels was observed in the qigong (SMD = 0.70; 95% CI, −0.62 to 2.02) and yoga (SMD = 0.73; 95% CI, −0.83 to 2.29) groups, whereas a mild decrease in BDNF levels was observed in the static group (SMD = −0.18; 95% CI, −1.26 to 0.91) (Table 3). Interestingly, a mild-to-moderate increase in BDNF levels was observed in the control group. However, when comparing the post-intervention effect of MBI with the control group, the qigong (SMD = 0.68; 95% CI, −1.03 to 2.38) and yoga (SMD = 0.65; 95% CI, −0.93 to 2.24) groups exhibited a high difference, whereas the static group exhibited a slight decrease (SMD = −0.14; 95% CI, −0.92 to 0.65) (Table 3). In the control post-intervention studies, a significant increase in BDNF levels was observed in the AC (SMD = 0.59; 95% CI, −0.31 to 1.48) group, whereas a mild decrease was observed in the TAU group (SMD = −0.07; 95% CI, −0.44 to 0.30).
3.4. Risk of Bias Across Studies
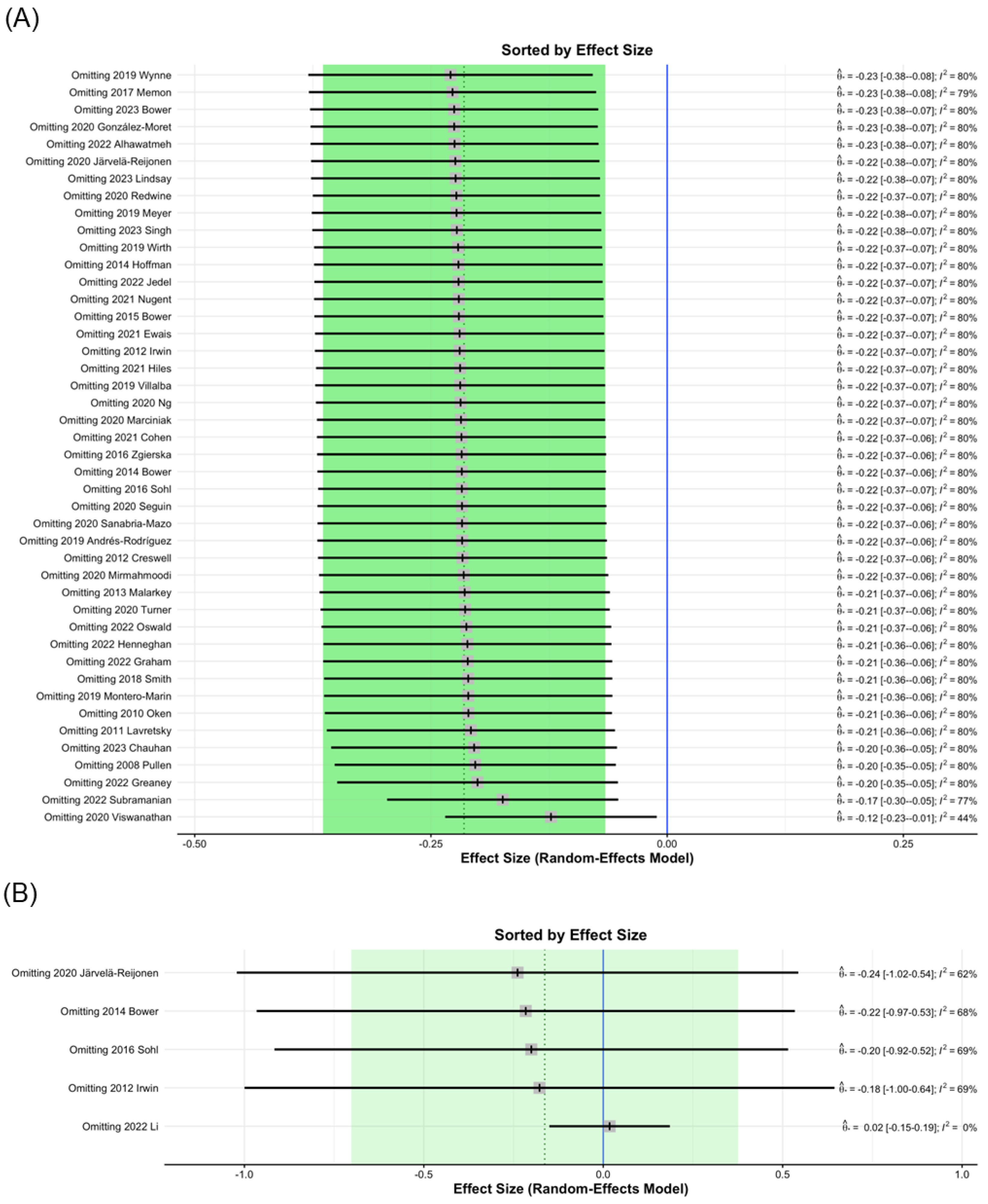
Influence diagnostics identified 2020 Viswanathan [71] as a study that, when excluded from analysis, contributed to significant changes in the MBI post-intervention study I2 values of CRP from 80% to 44% (Figure 3A); therefore, the data of 2020 Viswanathan was removed. This finding may be attributed to the relatively large number of participants. In the analysis of IL-1ra levels, the 2022 Li study [86], which was excluded from analysis, contributed to significant changes in I2 values from 60% to 0% (Figure 3B). This result may be due to the testing being conducted six months after MBI treatment.
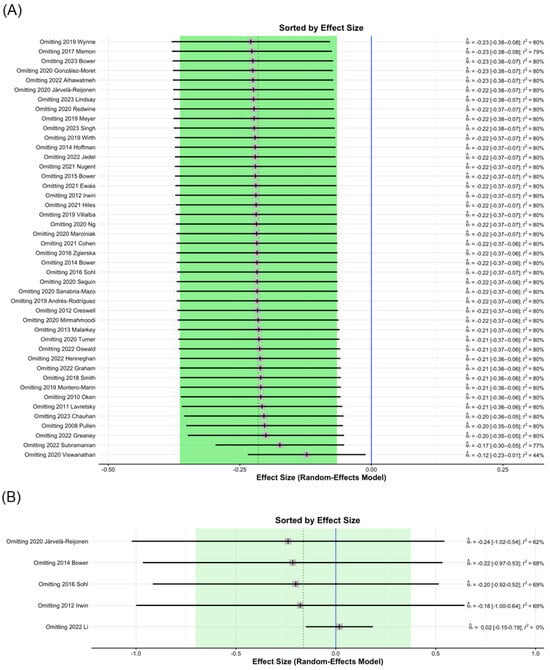
Figure 3.
Sorting plot of influence diagnostics of biomarkers. (A) CRP [3,4,5,7,9,12,13,15,16,35,36,37,40,44,47,48,52,54,57,58,59,60,64,65,67,68,69,70,71,72,73,74,75,79,83,84,85,87,91,95,96,97,101,104], (B) IL-1ra [9,37,47,65,86].
Results from the I2 statistic show the potential for publication bias for CRP, IL-6, TNF-α, IL-10, IFN-γ, BDNF, and cortisol levels at post-intervention (Table 3; Supplementary Figure S4). The trim-and-fill method was used to estimate the effect sizes of potentially suppressed studies [28]. This did not alter the parameter estimates for IL-6, IL-10, BDNF, and cortisol levels, as no supplementary studies were included (Table 4; Supplementary Figure S5). However, the trim-and-fill method results increased the I2 statistic of the CRP (post-intervention) from 44% to 65%. Heterogeneity at post-intervention was moderate-to-high for CRP, IL-6, IL-6-CNS, IL-6-cancer, TNF-α, IL-10, IFN-γ, BDNF, and cortisol (Table 4). However, in the subgroup analysis, the number of studies may be smaller, which could lead to small-study effects-related heterogeneity.

Table 4.
Risk of bias across studies and subgroup meta-regression of MBI effect on biomarkers at post-intervention.
3.5. Meta-Regression
Next, meta-regression was performed to account for between-study sources of heterogeneity using study-specific characteristics. In trim-and-fill analysis, the potentially missing studies for subgroups of CRP, IL-6-CNS, TNF-α, and IFN-γ were noted. However, the subgroup meta-regression based on intervention types did not reveal statistically significant effects, indicating that the observed heterogeneity may primarily stem from publication bias rather than differences in intervention methods (Table 4). Moderators of the intervention type showed a significant association with IL-6 levels in the cancer subgroup (Q = 37.52, p < 0.001; Table 4). This may also reflect the impact of the intervention method on cytokine IL-6 in the cancer subgroup.
4. Discussion
Bidirectional communication is observed between the neuroendocrine and immune systems, which is essential to maintain physiological homeostasis and good health. The hormonal and neuropeptide mediators that connect the endocrine, central nervous, and immune systems form distinct interaction pathways, such as the hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous system [18]. Chronic stress may cause immune and endocrine system dysregulation, then chronic inflammation and pathological outcomes are further exacerbated. MBIs can regulate psychological and physiological reactivity to stressors, decrease sympathetic tone, and increase parasympathetic activity, thereby reducing the likelihood and progression of stress-related disease [19]. MBIs affect the physiological markers integral to immune function, such as those involved in inflammation (like CRP and IL-6) and improve the response to infection [106]. Here, MBIs generally decreased the levels of inflammatory factors, such as CRP, IL-6, TNF-α, IL-1, IL-8, IL-17, ESR, and cortisol, and increased the levels of anti-inflammatory, antiviral, and immune-activating factors, such as IL-10, IFN-γ, IL-1ra, BDNF, and sIgA.
Biomarkers of stress, inflammation, and neuroplasticity are implicated in MCI [15]. Some biomarkers, such as CRP, IL-6, and IL-1β, related to aging or diseases, such as heart failure and cancer, are associated with MCI [13,107]. BDNF is a neurotrophin essential for neurogenesis and maintenance of neuronal plasticity, and its expression is influenced by epigenetic changes and upstream regulators, such as pro-inflammatory cytokines [107,108]. In a study by Devasahayam et al. [109], no differences were reported in BDNF levels at rest between patients with multiple sclerosis (MS) and controls; however, IL-6 levels were significantly higher in patients with MS. In the MS group, increased BDNF/IL-6 ratio was associated with fast walking speed exercise. Here, under the influence of MBIs, the qigong (SMD = 0.70; 95% CI, −0.62 to 2.02) and yoga (SMD = 0.73; 95% CI, −0.83 to 2.29) groups showed significantly increased BDNF levels, whereas the static group exhibited no significant difference or a slight decrease in BDNF levels (SMD = −0.18; 95% CI, −1.26 to 0.91) (Table 3). Interestingly, the control group showed a mild overall increase in BDNF levels, and the subgroup of AC (SMD = 0.59; 95% CI, −0.31 to 1.48) exhibited a high increase, possibly because patients in the AC group received supportive treatment including relaxation exercise, group counseling, and cognitive training. This implies that a combination of physical and gentle exercise, along with active psychological support, positively affects the BDNF levels (Figure 2B).
Here, in the subgroup analysis of IL-6, the CNS subgroup exhibited consistently decreased IL-6 levels (SMD = −0.19; 95% CI, −0.34 to −0.03; Supplementary Figure S1C), whereas the control group showed increasing IL-6 levels (SMD = 0.03; 95% CI, −0.08 to 0.15) (Table 3). Additionally, when we further analyzed this CNS subgroup according to different types of MBIs individually, we found that IL-6 levels were always decreased regardless of the type of MBI. However, in the control post-intervention studies in the CNS group on IL-6, the AC (SMD = 0.03; 95% CI, −0.10 to 0.16) and WL (SMD = 0.05; 95% CI, −0.11 to 0.20) exerted a mild increasing effect, and the TAU (SMD = −0.13; 95% CI, −0.48 to 0.22) exerted a moderate decreasing effect on IL-6. This may imply that MBI can reduce pro-inflammatory cytokine IL-6 in patients with neurological and psychosomatic symptoms, whereas other exercise methods without MBI may not necessarily have the same effect.
Additionally, IL-6 levels were increased in the cancer subgroup (SMD = 0.24; 95% CI, −0.29 to 0.76; Figure 2A). To further understand the influence of MBIs on this subgroup, we conducted individual analyses of different types of MBIs in the cancer subgroup. Both the qigong (SMD = 0.29; 95% CI, 0.10 to 0.47) and yoga (SMD = 0.61; 95% CI, −1.14 to 2.36) groups exhibited increased IL-6 levels, and only the static group showed a slight decrease in IL-6 levels (SMD = −0.12; 95% CI, −0.27 to 0.02). In contrast, the control group exhibited decreased IL-6 levels (Table 3). In the subgroup analysis, the AC (SMD = 0.07; 95% CI, −0.30 to 0.44) exerted a mild increasing effect, and the TAU (SMD = −0.64; 95% CI, −3.08 to 1.80) exerted an obvious decreasing effect on IL-6. This shows that exercise can indeed increase IL-6 levels in cancer patients, but MBI combined with gentle exercise has an even more significant effect in this participant group.
IL-6 is a cytokine abundantly expressed in the tumor microenvironment of various tumor types [110]. Direct stimulation of tumor cells via IL-6 leads to increased cell proliferation and invasiveness. IL-6 is produced by multiple cell types in the tumor microenvironment, including tumor-infiltrating immune cells, stromal cells, and tumor cells themselves. Paracrine or autocrine IL-6 signaling prompts stromal and immune cells to secrete signaling molecules, such as the vascular endothelial growth factor (VEGF) for angiogenesis or pro-inflammatory cytokine IL-1β [111]. As no increase in IL-6 levels was observed in the static group in this study, the increase in IL-6 levels may not be due to the impact of the tumor.
Some studies have reported the anti-inflammatory effects of exercise [10,112]. At rest, approximately 30% of circulating IL-6 levels are attributed to the adipose tissue, with only approximately 10% attributed to adipocytes, and the remainder are mostly attributed to adipose tissue-resident macrophages. Other sources of circulating IL-6 include blood leukocytes (predominantly monocytes), brain, and liver [10]. The plasma level of IL-6 increases exponentially with exercise duration and returns to resting levels within 1 h of exercise [8,113,114]. In our analysis, the qigong and yoga groups showed a significant increase in IL-6 levels. In contrast, the control group showed a decrease in IL-6 levels. In the study by Campo et al. [6] on 28 cancer survivors, IL-6 levels were significantly increased from the baseline 6.8 (SD 14.89) pg/mL to 1-week post-intervention 9.1 (SD 3.48) pg/mL after 12 weeks of tai chi training. This indicates that treatment combining MBIs with gentle physical exercise continuously increases the IL-6 levels in patients with cancer. Regular exercise reduces the resting levels of intratumoral IL-6 correlating with a reduced tumor size in breast cancer-bearing mice [115]. Exercise-induced acute release of IL-6 promotes antitumor adaptive immunity by inducing the migration of cytotoxic T cells to tumor-draining lymph nodes and tumor vasculature and stimulating lymphocyte trafficking [116,117].
IFN-γ is the only type II interferon produced by natural killer and T cells that is an important antiviral cytokine and activator of macrophages [118]. It promotes T cell activation, Th1 differentiation, and production of pro-inflammatory cytokines (particularly TNF) [119]. Here, patients receiving MBIs showed a moderate increase in IFN-γ levels (SMD = 0.32; 95% CI, −0.10 to 0.74; Figure 2C), whereas the control group exhibited decreased IFN-γ levels (SMD = −0.17; 95% CI, −0.54 to 0.20) (Table 3). In the study by McCain et al. [30] on 62 patients undergoing treatment for HIV infection, IFN-γ levels were significantly increased from 181.34 (SE 10.24) pg/mL to 212.81 (SE 12.03) pg/mL after 10 weeks of tai chi training but significantly decreased from 599.51 (SE 38.66) pg/mL to 366.94 (SE 23.31) pg/mL in the control group, showing a marked difference. This suggests that IFN-γ levels are increased by MBI therapy, initiating an immune response to combat viral infections.
IL-10 is a cytokine with potent anti-inflammatory properties that plays key roles in immune and inflammatory responses [120]. In this analysis, MBIs moderately increased the IL-10 levels (SMD = 0.38; 95% CI, −0.21 to 0.97; Figure 2D). Subgroup analysis showed the significant impact on patients with inflammatory diseases, such as rheumatoid arthritis [99] and ulcerative colitis [40], but no noticeable effects on patients with cancer [6] (Table 3). This suggests that MBIs increase the anti-inflammatory IL-10 levels in inflammatory conditions. However, this effect is not as pronounced in non-inflammatory conditions.
Physiological responses to stress can be evaluated using cortisol and sIgA levels [39]. Here, stress-prone populations, such as young students or nurses, exhibited decreased cortisol levels (SMD = −0.33; 95% CI, −0.55 to −0.12; Supplementary Figure S1J) and increased sIgA levels (SMD = 0.59; 95% CI, −0.21 to 1.40; Supplementary Figure S1K) after their participation in the research project. This trend can be observed regardless of whether they received MBI therapy. However, patients who underwent MBI therapy exhibited moderate-to-high changes in these biomarker levels.
This study has some limitations. First, although we observed that MBIs increase the circulatory IL-6 levels in cancer, whether they exert inhibitory effects on tumors or prevent their recurrence warrants further cohort studies. Additionally, whether and for how long changes in these biomarkers are sustained following interventions remain unclear. In the analysis results, some small effect sizes can be observed. However, the long-term cumulative effect of MBI on these biomarkers may still have a significant impact. Third, whether IL-6 serves as a primary assessment tool for outcome measures in cancer patients undergoing MBI therapy remains unclear. Future research should focus on longitudinal studies to determine whether these changes in biomarkers lead to meaningful clinical outcomes.
5. Conclusions
We found that MBIs generally decrease the inflammatory cytokine levels and increase the anti-inflammatory and antiviral cytokine levels. In the subgroup analysis of CNS, qigong and yoga increased the levels of BDNF but decreased IL-6; however, in the subgroup analysis of cancer, the levels of IL-6 were increased. These effects respectively imply neuroprotection and tumor suppression. Additionally, MBI may elevate the IFN-γ and sIgA levels during the condition of virus infection and under stress. In inflammatory diseases, such as rheumatoid arthritis and ulcerative colitis, MBIs significantly increase the levels of anti-inflammatory cytokine IL-10.
Disease and stress cause an imbalance in the immune and neuroendocrine systems. MBIs may restore the balance among these systems, and help the body from external stress, infections, and internal tumors. The MBIs including gentle physical exercise may be beneficial for neuropsychiatric disorders and tumors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare13080952/s1, Figure S1. Forest plot of the standardized mean difference of mind-body interventions effect on biomarkers. (A) CRP, (B) IL-6, (C) IL-6-CNS, (D) TNF-α, (E) IL-1, (F) IL-8, (G) IL-17, (H) IL-1ra, (I) ESR, (J) Cortisol, (K) sIgA. Control: pre-MBI effect. Experimental: post-MBI effect. Figure S2. Forest plot of the standardized mean difference of control interventions effect on biomarkers. (A) CRP, (B) IL-6, (C) BDNF, (D) TNF-α, (E) IL-1, (F) IL-8, (G) IL-17, (H) IL-1ra, (I) ESR, (J) Cortisol, (K) sIgA. Control: pre-intervention effect. Experimental: post-intervention effect. Figure S3. Forest plot of the standardized mean difference of postintervention effect between mind-body interventions and controls on biomarkers. (A) CRP, (B) IL-6, (C) BDNF, (D) TNF-α, (E) IL-1, (F) IL-8, (G) IL-17, (H) IL-1ra, (I) ESR, (J) Cortisol, (K) sIgA. Control: Control group. Experimental: MBI group. Figure S4. Funnel plot for publication bias of the standardized mean difference of mind-body interventions effect on biomarkers. (A) CRP, (B) IL-6, (C) TNF-α, (D) IL-1, (E) IL-8, (F) Cortisol. Figure S5. Funnel plot of the Trim-and-fill analysis of mind-body interventions effect on biomarkers. (A) CRP, (B) IL-6, (C) IL-6-CNS, (D) IL-6-cancer, (E) TNF-α, (F) IL-10, (G) IFN-γ, (H) BDNF, (I) Cortisol. Table S1. Statistical information between groups for each biomarker.
Author Contributions
S.-C.L.: Conceptualization, Data curation, Investigation, Risk-of-bias assessment, Formal analysis, Methodology, Validation, Visualization, Writing of the original draft. P.-H.T.: Formal analysis, Methodology, Validation. K.-H.Y.: Conceptualization, Methodology feedback, Risk-of-bias assessment. T.-M.C.: Data curation, Investigation, Risk-of-bias assessment, Writing of the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Hsueh-Fen Juan of the Department of Life Science and Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, for kind support and critical reading of this manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
BDNF, brain-derived neurotrophic factor; CNS, central nervous system; CRP, C-reactive protein; HPA, hypothalamic–pituitary–adrenal; MBI, mindfulness-based intervention; MCI, mild cognitive impairment; RCT, randomized controlled trial; SMD, standardized mean difference; TNF-α, tumor necrosis factor-alpha.
References
- Dahlgaard, J.; Jørgensen, M.M.; van der Velden, A.M.; Sumbundu, A.; Gregersen, N.; Olsen, R.K.; Mehlsen, M.Y. Chapter 22—Mindfulness, Health, and Longevity. In The Science of Hormesis in Health and Longevity; Academic Press: Cambridge, MA, USA, 2019; pp. 243–255. ISBN 9780128142530. [Google Scholar] [CrossRef]
- Dunn, T.J.; Dimolareva, M. The effect of mindfulness-based interventions on immunity-related biomarkers: A comprehensive meta-analysis of randomised controlled trials. Clin. Psychol. Rev. 2022, 92, 102124. [Google Scholar] [CrossRef] [PubMed]
- Montero-Marin, J.; Andres-Rodriguez, L.; Tops, M.; Luciano, J.V.; Navarro-Gil, M.; Feliu-Soler, A.; López-Del-Hoyo, Y.; Garcia-Campayo, J. Effects of attachment-based compassion therapy (ABCT) on brain-derived neurotrophic factor and low-grade inflammation among fibromyalgia patients: A randomized controlled trial. Sci. Rep. 2019, 9, 15639. [Google Scholar] [CrossRef]
- Oken, B.S.; Fonareva, I.; Haas, M.; Wahbeh, H.; Lane, J.B.; Zajdel, D.; Amen, A. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J. Altern. Complement. Med. 2010, 16, 1031–1038. [Google Scholar] [CrossRef]
- Zgierska, A.E.; Burzinski, C.A.; Cox, J.; Kloke, J.; Stegner, A.; Cook, D.B.; Singles, J.; Mirgain, S.; Coe, C.L.; Bačkonja, M. Mindfulness Meditation and Cognitive Behavioral Therapy Intervention Reduces Pain Severity and Sensitivity in Opioid-Treated Chronic Low Back Pain: Pilot Findings from a Randomized Controlled Trial. Pain Med. 2016, 17, 1865–1881. [Google Scholar] [CrossRef]
- Campo, R.A.; Light, K.C.; O’Connor, K.; Nakamura, Y.; Lipschitz, D.; LaStayo, P.C.; Pappas, L.M.; Boucher, K.M.; Irwin, M.R.; Hill, H.R.; et al. Blood pressure, salivary cortisol, and inflammatory cytokine outcomes in senior female cancer survivors enrolled in a tai chi chih randomized controlled trial. J. Cancer Surviv. 2015, 9, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Pullen, P.R.; Nagamia, S.H.; Mehta, P.K.; Thompson, W.R.; Benardot, D.; Hammoud, R.; Parrott, J.M.; Sola, S.; Khan, B.V. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J. Card. Fail. 2008, 14, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Davis, P.G.; Wideman, L.; Katula, J.A.; Sprod, L.K.; Peppone, L.J.; Palesh, O.G.; Heckler, C.E.; Williams, J.P.; Morrow, G.R.; et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin. Breast Cancer 2011, 11, 161–170. [Google Scholar] [CrossRef]
- Bower, J.E.; Greendale, G.; Crosswell, A.D.; Garet, D.; Sternlieb, B.; Ganz, P.A.; Irwin, M.R.; Olmstead, R.; Arevalo, J.; Cole, S.W. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology 2014, 43, 20–29. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Alotiby, A. Immunology of Stress: A Review Article. J. Clin. Med. 2024, 13, 6394. [Google Scholar] [CrossRef]
- Malarkey, W.B.; Jarjoura, D.; Klatt, M. Workplace based mindfulness practice and inflammation: A randomized trial. Brain Behav. Immun. 2013, 27, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Redwine, L.S.; Pung, M.A.; Wilson, K.; Bangen, K.J.; Delano-Wood, L.; Hurwitz, B. An exploratory randomized sub-study of light-to-moderate intensity exercise on cognitive function, depression symptoms and inflammation in older adults with heart failure. J. Psychosom. Res. 2020, 128, 109883. [Google Scholar] [CrossRef]
- Oh, B.; Butow, P.N.; Mullan, B.A.; Clarke, S.J.; Beale, P.J.; Pavlakis, N.; Lee, M.S.; Rosenthal, D.S.; Larkey, L.; Vardy, J. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: A randomized controlled trial. Support. Care Cancer 2012, 20, 1235–1242. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Fam, J.; Feng, L.; Cheah, I.K.; Tan, C.T.; Nur, F.; Wee, S.T.; Goh, L.G.; Chow, W.L.; Ho, R.C.-M.; et al. Mindfulness improves inflammatory biomarker levels in older adults with mild cognitive impairment: A randomized controlled trial. Transl. Psychiatry 2020, 10, 21. [Google Scholar] [CrossRef]
- Marciniak, R.; Sumec, R.; Vyhnalek, M.; Bendickova, K.; Laznickova, P.; Forte, G.; Jeleník, A.; Římalová, V.; Frič, J.; Hort, J.; et al. The Effect of Mindfulness-Based Stress Reduction (MBSR) on Depression, Cognition, and Immunity in Mild Cognitive Impairment: A Pilot Feasibility Study. Clin. Interv. Aging 2020, 15, 1365–1381. [Google Scholar] [CrossRef] [PubMed]
- Bradburn, S.; Sarginson, J.; Murgatroyd, C.A. Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Front. Aging Neurosci. 2017, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D. Neuroendocrine interactions in the immune system. Cell. Immunol. 2008, 252, 1–6. [Google Scholar] [CrossRef]
- Oyler, D.L.; Hulett, J.M.; Pratscher, S.D.; Price-Blackshear, M.A.; Murphy, E.A.; Bettencourt, B.A. The Influence of Meditative Interventions on Immune Functioning: A Meta-Analysis. Mindfulness 2023, 14, 1815–1851. [Google Scholar] [CrossRef]
- Morgan, N.; Irwin, M.R.; Chung, M.; Wang, C. The effects of mind-body therapies on the immune system: Meta-analysis. PLoS ONE 2014, 9, e100903. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Borenstein, M. Fixed-effect versus random-effects models. In Introduction to Meta-Analysis; Borenstein, M., Higgins, H.L., Rothstein, J.P.T., Eds.; John Wiley & Sons Ltd.: Cornwall, UK, 2009; pp. 77–85. [Google Scholar]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey, S.G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Chen, H.-H.; Yeh, M.-L.; Lee, F.-Y. The Effects of Baduanjin Qigong in the Prevention of Bone Loss for Middle-Aged Women. Am. J. Chin. Med. 2006, 34, 741–747. [Google Scholar] [CrossRef]
- McCain, N.L.; Gray, D.P.; Elswick, R.K.; Robins, J.W.; Tuck, I.; Walter, J.M.; Rausch, S.M.; Ketchum, J.M. A randomized clinical trial of alternative stress management interventions in persons with HIV infection. J. Consult. Clin. Psychol. 2008, 76, 431–441. [Google Scholar] [CrossRef]
- Banasik, J.; Williams, H.; Haberman, M.; Blank, S.E.; Bendel, R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J. Am. Acad. Nurse Pract. 2011, 23, 135–142. [Google Scholar] [CrossRef]
- Fan, Y.; Tang, Y.-Y.; Ma, Y.; Posner, M.I. Mucosal Immunity Modulated by Integrative Meditation in a Dose-Dependent Fashion. J. Altern. Complement. Med. 2010, 16, 151–155. [Google Scholar] [CrossRef]
- Oh, B.; Butow, P.; Mullan, B.; Clarke, S.; Beale, P.; Pavlakis, N.; Kothe, E.; Lam, L.; Rosenthal, D. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: A randomized controlled trial. Ann. Oncol. 2010, 21, 608–614. [Google Scholar] [CrossRef]
- Pullen, P.R.; Thompson, W.R.; Benardot, D.; Brandon, L.J.; Mehta, P.K.; Rifai, L.; Vadnais, D.S.; Parrott, J.M.; Khan, B.V. Benefits of yoga for African American heart failure patients. Med. Sci. Sports Exerc. 2010, 42, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Lavretsky, H.; Alstein, L.L.; Olmstead, R.E.; Ercoli, L.M.; Riparetti-Brown, M.; Cyr, N.S.; Irwin, M.R. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: A randomized controlled trial. Am. J. Geriatr. Psychiatry 2011, 19, 839–850. [Google Scholar] [CrossRef]
- Creswell, J.D.; Irwin, M.R.; Burklund, L.J.; Lieberman, M.D.; Arevalo, J.M.; Ma, J.; Breen, E.C.; Cole, S.W. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav. Immun. 2012, 26, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R. Mitigating cellular inflammation in older adults: A randomized controlled trial of Tai Chi Chih. Am. J. Geriatr. Psychiatry 2012, 20, 764–772. [Google Scholar] [CrossRef]
- Sprod, L.K.; Janelsins, M.C.; Palesh, O.G.; Carroll, J.K.; Heckler, C.E.; Peppone, L.J.; Mohile, S.G.; Morrow, G.R.; Mustian, K.M. Health-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuan. J. Cancer Surviv. 2012, 6, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Koh, D.; Teo, Y.C.; Hj Tamin, R.; Lim, A.; Fredericks, S. Biochemical and psychometric evaluation of Self-Healing Qigong as a stress reduction tool among first year nursing and midwifery students. Complement. Ther. Clin. Pract. 2013, 19, 179–183. [Google Scholar] [CrossRef]
- Jedel, S.; Hoffman, A.; Merriman, P.; Swanson, B.; Voigt, R.; Rajan, K.B.; Shaikh, M.; Li, H.; Keshavarzian, A. A randomized controlled trial of mindfulness-based stress reduction to prevent flare-up in patients with inactive ulcerative colitis. Digestion 2014, 89, 142–155. [Google Scholar] [CrossRef]
- Ikai, S.; Suzuki, T.; Uchida, H.; Saruta, J.; Tsukinoki, K.; Fujii, Y.; Mimura, M. Effects of weekly one-hour Hatha yoga therapy on resilience and stress levels in patients with schizophrenia-spectrum disorders: An eight-week randomized controlled trial. J. Altern. Complement. Med. 2014, 20, 823–830. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Bennett, J.M.; Andridge, R.; Peng, J.; Shapiro, C.L.; Malarkey, W.B.; Emery, C.F.; Layman, R.; Mrozek, E.E.; Glaser, R. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: A randomized controlled trial. J. Clin. Oncol. 2014, 32, 1040–1049. [Google Scholar] [CrossRef]
- Black, D.S.; O’Reilly, G.A.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 494–501. [Google Scholar] [CrossRef]
- Bower, J.E.; Crosswell, A.D.; Stanton, A.L.; Crespi, C.M.; Winston, D.; Arevalo, J.; Ma, J.; Cole, S.W.; Ganz, P.A. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer 2015, 121, 1231–1240. [Google Scholar] [CrossRef]
- Rajbhoj, P.H.; Shete, S.U.; Verma, A.; Bhogal, R.S. Effect of yoga module on pro-inflammatory and anti-inflammatory cytokines in industrial workers of lonavla: A randomized controlled trial. J. Clin. Diagn. Res. 2015, 9, CC01–CC05. [Google Scholar]
- Creswell, J.D.; Taren, A.A.; Lindsay, E.K.; Greco, C.M.; Gianaros, P.J.; Fairgrieve, A.; Marsland, A.L.; Brown, K.W.; Way, B.M.; Rosen, R.K.; et al. Alterations in Resting-State Functional Connectivity Link Mindfulness Meditation With Reduced Interleukin-6: A Randomized Controlled Trial. Biol. Psychiatry 2016, 80, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sohl, S.J.; Danhauer, S.C.; Birdee, G.S.; Nicklas, B.J.; Yacoub, G.; Aklilu, M.; Avis, N.E. A brief yoga intervention implemented during chemotherapy: A randomized controlled pilot study. Complement. Ther. Med. 2016, 25, 139–142. [Google Scholar] [CrossRef]
- Memon, A.A.; Sundquist, K.; Ahmad, A.; Wang, X.; Hedelius, A.; Sundquist, J. Role of IL-8, CRP and epidermal growth factor in depression and anxiety patients treated with mindfulness-based therapy or cognitive behavioral therapy in primary health care. Psychiatry Res. 2017, 254, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kenne Sarenmalm, E.; Martensson, L.B.; Andersson, B.A.; Karlsson, P.; Bergh, I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017, 6, 1108–1122. [Google Scholar] [CrossRef]
- Hoge, E.A.; Bui, E.; Palitz, S.A.; Schwarz, N.R.; Owens, M.E.; Johnston, J.M.; Pollack, M.H.; Simon, N.M. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Res. 2018, 262, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Nery, S.F.; Paiva, S.P.C.; Vieira, E.L.; Barbosa, A.B.; Sant’Anna, E.M.; Casalechi, M.; Cruz, C.D.; Teixeira, A.L.; Reis, F.M. Mindfulness-based program for stress reduction in infertile women: Randomized controlled trial. Stress Health 2019, 35, 49–58. [Google Scholar] [CrossRef]
- Smith, B.W.; Shelley, B.M.; Sloan, A.L.; Colleran, K.; Erickson, K. A Preliminary Randomized Controlled Trial of a Mindful Eating Intervention for Post-menopausal Obese Women. Mindfulness 2017, 9, 836–849. [Google Scholar] [CrossRef]
- Sungkarat, S.; Boripuntakul, S.; Kumfu, S.; Lord, S.R.; Chattipakorn, N. Tai Chi Improves Cognition and Plasma BDNF in Older Adults With Mild Cognitive Impairment: A Randomized Controlled Trial. Neurorehabil. Neural Repair. 2018, 32, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Rodríguez, L.; Borras, X.; Feliu-Soler, A.; Perez-Aranda, A.; Rozadilla-Sacanell, A.; Montero-Marin, J.; Maes, M.; Luciano, J.V. Immune-inflammatory pathways and clinical changes in fibromyalgia patients treated with Mindfulness-Based Stress Reduction (MBSR): A randomized, controlled clinical trial. Brain Behav. Immun. 2019, 80, 109–119. [Google Scholar] [CrossRef]
- Cheung, D.S.T.; Deng, W.; Tsao, S.W.; Ho, R.T.H.; Chan, C.L.W.; Fong, D.Y.T.; Chau, P.H.; Hong, A.W.L.; Fung, H.Y.K.Y.; Ma, J.L.C.; et al. Effect of a Qigong Intervention on Telomerase Activity and Mental Health in Chinese Women Survivors of Intimate Partner Violence: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e186967. [Google Scholar] [CrossRef]
- Witek Janusek, L.; Tell, D.; Mathews, H.L. Mindfulness based stress reduction provides psychological benefit and restores immune function of women newly diagnosed with breast cancer: A randomized trial with active control. Brain Behav. Immun. 2019, 80, 358–373. [Google Scholar]
- Meyer, J.D.; Hayney, M.S.; Coe, C.L.; Ninos, C.L.; Barrett, B.P. Differential Reduction of IP-10 and C-Reactive Protein via Aerobic Exercise or Mindfulness-Based Stress-Reduction Training in a Large Randomized Controlled Trial. J. Sport. Exerc. Psychol. 2019, 41, 96–106. [Google Scholar] [PubMed]
- Villalba, D.K.; Lindsay, E.K.; Marsland, A.L.; Greco, C.M.; Young, S.; Brown, K.W.; Smyth, J.M.; Walsh, C.P.; Gray, K.; Chin, B.; et al. Mindfulness training and systemic low-grade inflammation in stressed community adults: Evidence from two randomized controlled trials. PLoS ONE 2019, 14, e0219120. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Franco, R.; Wagner Robb, S.; Daniels, K.; Susko, K.; Hudson, M.F.; O’rourke, M.A. Randomized Controlled Trial of a 4-Week Mindfulness Intervention among Cancer Survivors Compared to a Breathing Control. Cancer Investig. 2019, 37, 227–232. [Google Scholar] [CrossRef]
- Wynne, B.; McHugh, L.; Gao, W.; Keegan, D.; Byrne, K.; Rowan, C.; Hartery, K.; Kirschbaum, C.; Doherty, G.; Cullen, G.; et al. Acceptance and Commitment Therapy Reduces Psychological Stress in Patients With Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 935–945.e1. [Google Scholar]
- Cekanauskaite, A.; Skurvydas, A.; Zlibinaite, L.; Mickeviciene, D.; Kilikeviciene, S.; Solianik, R. A 10-week yoga practice has no effect on cognition, but improves balance and motor learning by attenuating brain-derived neurotrophic factor levels in older adults. Exp. Gerontol. 2020, 138, 110998. [Google Scholar] [CrossRef]
- Ganesan, S.; Gaur, G.S.; Negi, V.S.; Sharma, V.K.; Pal, G.K. Effect of Yoga Therapy on Disease Activity, Inflammatory Markers, and Heart Rate Variability in Patients with Rheumatoid Arthritis. J. Altern. Complement. Med. 2020, 26, 501–507. [Google Scholar]
- Gautam, S.; Kumar, M.; Kumar, U.; Dada, R. Effect of an 8-Week Yoga-Based Lifestyle Intervention on Psycho-Neuro-Immune Axis, Disease Activity, and Perceived Quality of Life in Rheumatoid Arthritis Patients: A Randomized Controlled Trial. Front. Psychol. 2020, 11, 2259. [Google Scholar]
- Gonzalez-Moret, R.; Cebolla, A.; Cortes, X.; Banos, R.M.; Navarrete, J.; de la Rubia, J.E.; Lisón, J.F.; Soria, J.M. The effect of a mindfulness-based therapy on different biomarkers among patients with inflammatory bowel disease: A randomised controlled trial. Sci. Rep. 2020, 10, 6071. [Google Scholar] [CrossRef]
- Jarvela-Reijonen, E.; Puttonen, S.; Karhunen, L.; Sairanen, E.; Laitinen, J.; Kolehmainen, M.; Pihlajamäki, J.; Kujala, U.M.; Korpela, R.; Ermes, M.; et al. The Effects of Acceptance and Commitment Therapy (ACT) Intervention on Inflammation and Stress Biomarkers: A Randomized Controlled Trial. Int. J. Behav. Med. 2020, 27, 539–555. [Google Scholar]
- Lu, E.Y.; Lee, P.; Cai, S.; So, W.W.Y.; Ng, B.F.L.; Jensen, M.P.; Cheung, W.M.; Tsang, H.W. Qigong for the treatment of depressive symptoms: Preliminary evidence of neurobiological mechanisms. Int. J. Geriatr. Psychiatry 2020, 35, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Mirmahmoodi, M.; Mangalian, P.; Ahmadi, A.; Dehghan, M. The Effect of Mindfulness-Based Stress Reduction Group Counseling on Psychological and Inflammatory Responses of the Women With Breast Cancer. Integr. Cancer Ther. 2020, 19, 1534735420946819. [Google Scholar] [CrossRef]
- Sanabria-Mazo, J.P.; Montero-Marin, J.; Feliu-Soler, A.; Gasion, V.; Navarro-Gil, M.; Morillo-Sarto, H.; Colomer-Carbonell, A.; Borràs, X.; Tops, M.; Luciano, J.V.; et al. Mindfulness-Based Program Plus Amygdala and Insula Retraining (MAIR) for the Treatment of Women with Fibromyalgia: A Pilot Randomized Controlled Trial. J. Clin. Med. 2020, 9, 3246. [Google Scholar] [CrossRef]
- Seguin-Fowler, R.; Graham, M.; Ward, J.; Eldridge, G.; Sriram, U.; Fine, D. Feasibility of a yoga intervention to decrease pain in older women: A randomized controlled pilot study. BMC Geriatr. 2020, 20, 400. [Google Scholar]
- Turner, L.; Galante, J.; Vainre, M.; Stochl, J.; Dufour, G.; Jones, P.B. Immune dysregulation among students exposed to exam stress and its mitigation by mindfulness training: Findings from an exploratory randomised trial. Sci. Rep. 2020, 10, 5812. [Google Scholar] [CrossRef]
- Viswanathan, V.; Sivakumar, S.; Sai Prathiba, A.; Devarajan, A.; George, L.; Kumpatla, S. Effect of yoga intervention on biochemical, oxidative stress markers, inflammatory markers and sleep quality among subjects with type 2 diabetes in South India: Results from the SATYAM project. Diabetes Res. Clin. Pract. 2021, 172, 108644. [Google Scholar]
- Cohen, Z.P.; Cosgrove, K.T.; Akeman, E.; Coffey, S.; Teague, K.; Hays-Grudo, J.; Paulus, M.P.; Aupperle, R.L.; Kirlic, N. The effect of a mindfulness-based stress intervention on neurobiological and symptom measures in adolescents with early life stress: A randomized feasibility study. BMC Complement. Med. Ther. 2021, 21, 123. [Google Scholar] [CrossRef]
- Ewais, T.; Begun, J.; Kenny, M.; Hay, K.; Houldin, E.; Chuang, K.H.; Tefay, M.; Kisely, S. Mindfulness based cognitive therapy for youth with inflammatory bowel disease and depression—Findings from a pilot randomised controlled trial. J. Psychosom. Res. 2021, 149, 110594. [Google Scholar] [CrossRef]
- Hiles, S.A.; Urroz, P.D.; Gibson, P.G.; Bogdanovs, A.; McDonald, V.M. A feasibility randomised controlled trial of Novel Activity Management in severe ASthma-Tailored Exercise (NAMASTE): Yoga and mindfulness. BMC Pulm. Med. 2021, 21, 71. [Google Scholar] [CrossRef]
- Nugent, N.R.; Brick, L.; Armey, M.F.; Tyrka, A.R.; Ridout, K.K.; Uebelacker, L.A. Benefits of Yoga on IL-6: Findings from a Randomized Controlled Trial of Yoga for Depression. Behav. Med. 2021, 47, 21–30. [Google Scholar] [CrossRef]
- Rodrigues de Oliveira, D.; Wilson, D.; Palace-Berl, F.; de Mello Ponteciano, B.; Fungaro Rissatti, L.; Sardela de Miranda, F.; Pollizi, V.P.; Fuscella, J.C.; Terzi, A.M.; Lepique, A.P.; et al. Mindfulness meditation training effects on quality of life, immune function and glutathione metabolism in service healthy female teachers: A randomized pilot clinical trial. Brain Behav. Immun. Health 2021, 18, 100372. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wong, N.M.L.; Shao, R.; Man, I.S.C.; Wong, C.H.Y.; Yuen, L.P.; Chan, C.C.; Lee, T.M. Qigong exercise enhances cognitive functions in the elderly via an interleukin-6-hippocampus pathway: A randomized active-controlled trial. Brain Behav. Immun. 2021, 95, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Solianik, R.; Mickeviciene, D.; Zlibinaite, L.; Cekanauskaite, A. Tai chi improves psychoemotional state, cognition, and motor learning in older adults during the COVID-19 pandemic. Exp. Gerontol. 2021, 150, 111363. [Google Scholar] [CrossRef] [PubMed]
- Alhawatmeh, H.N.; Rababa, M.; Alfaqih, M.; Albataineh, R.; Hweidi, I.; Abu Awwad, A. The Benefits of Mindfulness Meditation on Trait Mindfulness, Perceived Stress, Cortisol, and C-Reactive Protein in Nursing Students: A Randomized Controlled Trial. Adv. Med. Educ. Pract. 2022, 13, 47–58. [Google Scholar] [CrossRef]
- Chanta, A.; Klaewsongkram, J.; Mickleborough, T.D.; Tongtako, W. Effect of Hatha yoga training on rhinitis symptoms and cytokines in allergic rhinitis patients. Asian Pac. J. Allergy Immunol. 2022, 40, 126–133. [Google Scholar]
- Diez, G.G.; Anitua, E.; Castellanos, N.; Vazquez, C.; Galindo-Villardon, P.; Alkhraisat, M.H. The effect of mindfulness on the inflammatory, psychological and biomechanical domains of adult patients with low back pain: A randomized controlled clinical trial. PLoS ONE 2022, 17, e0276734. [Google Scholar] [CrossRef]
- Gardi, C.; Fazia, T.; Stringa, B.; Giommi, F. A short Mindfulness retreat can improve biological markers of stress and inflammation. Psychoneuroendocrinology 2022, 135, 105579. [Google Scholar] [CrossRef]
- Graham, B.; Jin, Y.; Bazeley, P.; Husni, E.; Calabrese, L.H. Online, low-volume meditation does not alter immune-related biomarkers. Brain Behav. Immun. Health 2022, 26, 100531. [Google Scholar] [CrossRef]
- Greaney, S.K.; Amin, N.; Prudner, B.C.; Compernolle, M.; Sandell, L.J.; Tebb, S.C.; Weilbaecher, K.N.; Abeln, P.; Luo, J.; Tao, Y.; et al. Yoga Therapy During Chemotherapy for Early-Stage and Locally Advanced Breast Cancer. Integr. Cancer Ther. 2022, 21, 15347354221137285. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Fico, B.G.; Wright, M.L.; Kesler, S.R.; Harrison, M.L. Effects of meditation compared to music listening on biomarkers in breast cancer survivors with cognitive complaints: Secondary outcomes of a pilot randomized control trial. Explore 2022, 18, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, P.; Cui, S.S.; Tan, Y.Y.; He, Y.C.; Shen, X.; Jiang, Q.-Y.; Huang, P.; He, G.-Y.; Li, B.-Y.; et al. Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl. Neurodegener. 2022, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Jedel, S.; Beck, T.; Swanson, G.; Hood, M.M.; Voigt, R.M.; Gorenz, A.; Jakate, S.; Raeisi, S.; Hobfoll, S.; Keshavarzian, A. Mindfulness Intervention Decreases Frequency and Severity of Flares in Inactive Ulcerative Colitis Patients: Results of a Phase II, Randomized, Placebo-Controlled Trial. Inflamm. Bowel Dis. 2022, 28, 1872–1892. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, E.K.; Creswell, J.D.; Stern, H.J.; Greco, C.M.; Walko, T.D.; Dutcher, J.M.; Wright, A.G.; Brown, K.W.; Marsland, A.L. Mindfulness-based stress reduction increases stimulated IL-6 production among lonely older adults: A randomized controlled trial. Brain Behav. Immun. 2022, 104, 6–15. [Google Scholar] [CrossRef]
- Martinez-Borras, R.; Navarrete, J.; Bellosta-Batalla, M.; Martinez-Brotons, C.; Martinez-Rubio, D. Changes in Salivary Immunoglobulin A, Stress, and Burnout in a Workplace Mindfulness Intervention: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 6226. [Google Scholar] [CrossRef]
- Ng, S.M.; Yin, M.X.C.; Chan, J.S.M.; Chan, C.H.Y.; Fong, T.C.T.; Li, A.; So, K.F.; Yuen, L.P.; Chen, J.P.; Chung, K.F.; et al. Impact of mind-body intervention on proinflammatory cytokines interleukin 6 and 1beta: A three-arm randomized controlled trial for persons with sleep disturbance and depression. Brain Behav. Immun. 2022, 99, 166–176. [Google Scholar] [CrossRef]
- Oswald, L.B.; Fox, R.S.; Murphy, K.M.; Salsman, J.M.; Sanford, S.D.; McDade, T.W.; Victorson, D.E. Preliminary Effects of Mindfulness Training on Inflammatory Markers and Blood Pressure in Young Adult Survivors of Cancer: Secondary Analysis of a Pilot Randomized Controlled Trial. Int. J. Behav. Med. 2022, 29, 676–684. [Google Scholar] [CrossRef]
- Saban, K.L.; Collins, E.G.; Mathews, H.L.; Bryant, F.B.; Tell, D.; Gonzalez, B.; Bhoopalam, S.; Chroniak, C.P.; Janusek, L.W. Impact of a Mindfulness-Based Stress Reduction Program on Psychological Well-Being, Cortisol, and Inflammation in Women Veterans. J. Gen. Intern. Med. 2022, 37 (Suppl. S3), 751–761. [Google Scholar] [CrossRef]
- Sharma, P.; Yadav, R.K.; Khadgawat, R.; Dada, R. Transcriptional modulation of inflammation, and aging in Indian obese adults following a 12-week yoga-based lifestyle intervention: A randomized controlled trial. Front. Med. 2022, 9, 898293. [Google Scholar] [CrossRef] [PubMed]
- Sohl, S.J.; Tooze, J.A.; Johnson, E.N.; Ridner, S.H.; Rothman, R.L.; Lima, C.R.; Ansley, K.C.; Wheeler, A.; Nicklas, B.; Avis, N.E.; et al. A Randomized Controlled Pilot Study of Yoga Skills Training Versus an Attention Control Delivered During Chemotherapy Administration. J. Pain Symptom Manag. 2022, 63, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.K.; Sripad, V.D.; Dharmalingam, A.; Guhan, V.N.; Kalidoss, V.K.; Gautam, N.; Shankaralingappa, A.; Rajendran, R.; Mohiuddin, S.G. Effect of 4-Week Heartfulness Meditation on Stress Scores, Sleep Quality, and Oxidative and Inflammatory Biochemical Parameters in COVID-19 Patients after Completion of Standard Treatment—A Randomized Controlled Trial. Int. J. Yoga 2022, 15, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Partridge, A.H.; Wolff, A.C.; Cole, S.W.; Irwin, M.R.; Thorner, E.D.; Joffe, H.; Petersen, L.; Crespi, C.M.; Ganz, P.A. Improving biobehavioral health in younger breast cancer survivors: Pathways to Wellness trial secondary outcomes. J. Natl. Cancer Inst. 2023, 115, 83–92. [Google Scholar] [CrossRef]
- Chauhan, S.; Patra, S.; Singh, S.P.; Lakhani, J.D. Combined effect of yoga and naturopathy in uncomplicated varicose vein disease—A prospective randomized controlled trial. J. Ayurveda Integr. Med. 2023, 14, 100718. [Google Scholar] [CrossRef]
- Dua, R.; Malik, S.; Kumari, R.; Naithani, M.; Panda, P.K.; Saroha, A.; Omar, B.; Pathania, M.; Saxena, S. The Role of Yoga in Hospitalized COVID-19 Patients: An Exploratory Randomized Controlled Trial. Cureus 2023, 15, e39320. [Google Scholar]
- Gautam, S.; Kumar, R.; Kumar, U.; Kumar, S.; Luthra, K.; Dada, R. Yoga maintains Th17/Treg cell homeostasis and reduces the rate of T cell aging in rheumatoid arthritis: A randomized controlled trial. Sci. Rep. 2023, 13, 14924. [Google Scholar] [CrossRef]
- Jain, M.; Mishra, A.; Yadav, V.; Shyam, H.; Kumar, S.; Mishra, S.K.; Ramakant, P. Long-term yogic intervention decreases serum interleukins IL-10 and IL-1beta and improves cancer-related fatigue and functional scale during radiotherapy/chemotherapy in breast cancer patients: A randomized control study. Support. Care Cancer 2022, 31, 6. [Google Scholar] [CrossRef]
- Lindsay, E.K.; Marsland, A.L.; Cole, S.W.; Dutcher, J.M.; Greco, C.M.; Wright, A.G.C.; Brown, K.W.; Creswell, J.D. Mindfulness-Based Stress Reduction reduces pro-inflammatory gene regulation but not systemic inflammation among older adults: A randomized controlled trial. Psychosom. Med. 2023, 86, 463–472. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.M.; Huang, Y.L.; Yu, P.M.; Zhang, X.W.; Zhao, C.; Mao, B.; Min, J.; Jiang, H.-L. Tai Chi as a complementary exercise for pulmonary rehabilitation in chronic obstructive pulmonary disease: A randomised controlled trial. Complement. Ther. Med. 2023, 78, 102977. [Google Scholar] [CrossRef]
- Mullapudi, T.; Debnath, M.; Govindaraj, R.; Raj, P.; Banerjee, M.; Varambally, S. Effects of a six-month yoga intervention on the immune-inflammatory pathway in antipsychotic-stabilized schizophrenia patients: A randomized controlled trial. Asian J. Psychiatr. 2023, 86, 103636. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Metri, K.; Tekur, P.; Mohanty, S.; Singh, A.; Raghuram, N. Tele-yoga in the management of ankylosing spondylitis amidst COVID pandemic: A prospective randomized controlled trial. Complement. Ther. Clin. Pract. 2023, 50, 101672. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Yuan, J.; Wu, Y.; Wang, X.; Meng, J.Y.; Liu, W.; Wei, Y.; Kang, C.-Y.; Yang, J.-Z. A randomized controlled trial of mindfulness-based cognitive therapy (MBCT) for major depressive disorder in undergraduate students in China: The efficacy, serum proinflammatory cytokines and brain-derived neurotrophic factor. Asian J. Psychiatr. 2023, 88, 103718. [Google Scholar] [CrossRef]
- Black, D.S.; Slavich, G.M. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef]
- Yap, N.Y.; Toh, Y.L.; Tan, C.J.; Acharya, M.M.; Chan, A. Relationship between cytokines and brain-derived neurotrophic factor (BDNF) in trajectories of cancer-related cognitive impairment. Cytokine 2021, 144, 155556. [Google Scholar] [CrossRef]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Devasahayam, A.J.; Kelly, L.P.; Williams, J.B.; Moore, C.S.; Ploughman, M. Fitness Shifts the Balance of BDNF and IL-6 from Inflammation to Repair among People with Progressive Multiple Sclerosis. Biomolecules 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Raskova, M.; Lacina, L.; Kejik, Z.; Venhauerova, A.; Skalickova, M.; Kolar, M.; Jakubek, M.; Rosel, D.; Smetana, K., Jr.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Mathur, N.; Pedersen, B.K. Exercise as a mean to control low-grade systemic inflammation. Mediat. Inflamm. 2008, 2008, 109502. [Google Scholar] [CrossRef]
- Keller, C.; Steensberg, A.; Hansen, A.K.; Fischer, C.P.; Plomgaard, P.; Pedersen, B.K. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J. Appl. Physiol. 2005, 99, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Fischer, C.P. Beneficial health effects of exercise--the role of IL-6 as a myokine. Trends Pharmacol. Sci. 2007, 28, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Shalamzari, S.A.; Agha-Alinejad, H.; Alizadeh, S.; Shahbazi, S.; Khatib, Z.K.; Kazemi, A.; Saei, M.A.; Minayi, N. The effect of exercise training on the level of tissue IL-6 and vascular endothelial growth factor in breast cancer bearing mice. Iran. J. Basic Med. Sci. 2014, 17, 231–236. [Google Scholar] [PubMed]
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398. [Google Scholar] [CrossRef]
- Orange, S.T.; Leslie, J.; Ross, M.; Mann, D.A.; Wackerhage, H. The exercise IL-6 enigma in cancer. Trends Endocrinol. Metab. 2023, 34, 749–763. [Google Scholar] [CrossRef]
- Mohning, M.P.; Downey, G.P.; Cosgrove, G.P.; Redente, E.F. Chapter 3—Mechanisms of Fibrosis. In Idiopathic Pulmonary Fibrosis; Swigris, J.J., Brown, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 9–31. [Google Scholar]
- Markey, K.A.; Hill, G.R. Chapter 13—Cytokines in Hematopoietic Stem Cell Transplantation. In Cytokine Effector Functions in Tissues; Foti, M., Locati, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 219–236. [Google Scholar]
- Shankar Subramanian Iyer, G.C. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).