Effect of Motion-Controlled Video Games-Based Virtual Reality Exercise on Patients with Post-COVID-19 Condition: A Randomized Controlled Trial
Abstract
1. Introduction
2. Method
2.1. Study Design, Setting, and Ethical Considerations
2.2. Participants
2.3. Evaluation
2.4. Outcome Measures
2.4.1. Primary Outcome
2.4.2. Secondary Outcomes
2.5. Randomization
2.6. Intervention
2.6.1. CTG
2.6.2. VRG
2.7. Adverse Events Monitoring
2.8. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19): Post COVID-19 Condition. 2023. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 27 August 2024).
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Peluso, M.J.; Deeks, S.G. Mechanisms of long COVID and the path toward therapeutics. Cell 2024, 187, 5500–5529. [Google Scholar] [CrossRef] [PubMed]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef]
- Saunders, E.G.; Pouliopoulou, D.V.; Miller, E.; Billias, N.; MacDermid, J.C.; Brunton, L.; Pereira, T.V.; Quinn, K.L.; Bobos, P. Rehabilitation interventions and outcomes for post-COVID condition: A scoping review. BMJ Public Health 2025, 3, e001827. [Google Scholar] [CrossRef]
- Groenveld, T.; Achttien, R.; Smits, M.; de Vries, M.; van Heerde, R.; Staal, B.; van Goor, H.; COVID Rehab Group. Feasibility of Virtual Reality Exercises at Home for Post-COVID-19 Condition: Cohort Study. JMIR Rehabil. Assist. Technol. 2022, 9, e36836. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors 2021, 21, 7389. [Google Scholar] [CrossRef]
- Soleimani, M.; Ghazisaeedi, M.; Heydari, S. The efficacy of virtual reality for upper limb rehabilitation in stroke patients: A systematic review and meta-analysis. BMC Med. Inform. Decis. Mak. 2024, 24, 135. [Google Scholar] [CrossRef]
- Bashir, Z.; Misquith, C.; Shahab, A.; Has, P.; Bukhari, S. The impact of Virtual Reality on Anxiety and Functional Capacity in Cardiac Rehabilitation: A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2023, 48, 101628. [Google Scholar] [CrossRef]
- Blasco-Peris, C.; Fuertes-Kenneally, L.; Vetrovsky, T.; Sarabia, J.M.; Climent-Paya, V.; Manresa-Rocamora, A. Effects of Exergaming in Patients with Cardiovascular Disease Compared to Conventional Cardiac Rehabilitation: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 3492. [Google Scholar] [CrossRef]
- Darabseh, M.Z.; Aburub, A.; Davies, S. The Effects of Virtual Reality Physiotherapy Interventions on Cardiopulmonary Function and Breathing Control in Cystic Fibrosis: A Systematic Review. Games Health J. 2023, 12, 13–24. [Google Scholar] [CrossRef]
- Gava, V.; Fialho, H.R.F.; Calixtre, L.B.; Barbosa, G.M.; Kamonseki, D.H. Effects of Gaming on Pain-Related Fear, Pain Catastrophizing, Anxiety, and Depression in Patients with Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Games Health J. 2022, 11, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Y.; Wu, Z.; Yao, X.; Fan, Y. Effectiveness of Active Exergames for Improving Cognitive Function in Patients with Neurological Disabilities: A Systematic Review and Meta-Analysis. Games Health J. 2023, 12, 198–210. [Google Scholar] [CrossRef]
- Polat, M.; Kahveci, A.; Muci, B.; Günendi, Z.; Kaymak Karataş, G. The Effect of Virtual Reality Exercises on Pain, Functionality, Cardiopulmonary Capacity, and Quality of Life in Fibromyalgia Syndrome: A Randomized Controlled Study. Games Health J. 2021, 10, 165–173. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Vavougios, G.D.; Kalogiannis, P.; Tachoulas, K.; Touloudi, E.; Astara, K.; Mysiris, D.S.; Tsirimona, G.; Papayianni, E.; Boutlas, S.; et al. Breathlessness and exercise with virtual reality system in long-post-coronavirus disease 2019 patients. Front. Public Health 2023, 11, 1115393. [Google Scholar] [CrossRef]
- Rutkowski, S.; Bogacz, K.; Czech, O.; Rutkowska, A.; Szczegielniak, J. Effectiveness of an Inpatient Virtual Reality-Based Pulmonary Rehabilitation Program among COVID-19 Patients on Symptoms of Anxiety, Depression and Quality of Life: Preliminary Results from a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16980. [Google Scholar] [CrossRef]
- Rutkowski, S.; Bogacz, K.; Rutkowska, A.; Szczegielniak, J.; Casaburi, R. Inpatient post-COVID-19 rehabilitation program featuring virtual reality-Preliminary results of randomized controlled trial. Front. Public Health 2023, 11, 1121554. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Rutkowska, A.; Kirejczyk, Ł.; Radosz, B.; Bogacz, K.; Szczegielniak, J. Implementation of a Rehabilitation Program in a Virtual Reality for Post-COVID-19 Patients-Preliminary Results. Slovak J. Sport Sci. 2022, 8, 77–89. [Google Scholar] [CrossRef]
- Dalko, K.; Elsuson, H.A.; Kalter, I.; Zilezinski, M.; Hofstetter, S.; Stoevesandt, D.; Paulicke, D.; Jahn, P. Virtual Reality Applications for the Implementation of Domestic Respiratory Rehabilitation Programs for Patients with Long COVID and Post-COVID Condition: Scoping Review. JMIR Serious Games 2024, 12, e52309. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O. Hastane anksiyete ve depresyon olcegi Turkce formunun gecerlilik ve guvenilirligi. Turk. Psikiyatr. Derg. 1997, 8, 187–280. [Google Scholar]
- Gencay-Can, A.; Can, S.S. Validation of the Turkish version of the fatigue severity scale in patients with fibromyalgia. Rheumatol. Int. 2012, 32, 27–31. [Google Scholar] [CrossRef]
- Soylu, C.; Kütük, B. Reliability and Validity of the Turkish Version of SF-12 Health Survey. Turk. J. Psychiatry 2022, 33, 108–117. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bushman, B.A. Developing the P (for Progression) in a FITT-VP Exercise Prescription. ACSM’s Health Fit. J. 2018, 22, 6–9. [Google Scholar] [CrossRef]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Pollini, E.; Lazzarini, S.G.; Cordani, C.; Del Furia, M.J.; Kiekens, C.; Negrini, S.; Arienti, C. Effectiveness of Rehabilitation Interventions on Adults with COVID-19 and Post-COVID-19 Condition. A Systematic Review with Meta-analysis. Arch. Phys. Med. Rehabil. 2024, 105, 138–149. [Google Scholar] [CrossRef]
- Cai, M.; Xie, Y.; Topol, E.J.; Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 2024, 30, 1564–1573. [Google Scholar] [CrossRef]
- The Lancet. Long COVID: 3 years in. Lancet 2023, 401, 795. [Google Scholar] [CrossRef]
- Gloeckl, R.; Leitl, D.; Schneeberger, T.; Jarosch, I.; Koczulla, A.R. Rehabilitative interventions in patients with persistent post COVID-19 symptoms—A review of recent advances and future perspectives. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, X.K.; Sit, C.H.; Liang, X.; Li, M.H.; Ma, A.C.H.; Wong, S.H.S. Effect of Physical Exercise-Based Rehabilitation on Long COVID: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2024, 56, 143–154. [Google Scholar] [CrossRef]
- Fricke-Comellas, H.; Heredia-Rizo, A.M.; Casuso-Holgado, M.J.; Salas-González, J.; Fernández-Seguín, L.M. Exploring the Effects of Qigong, Tai Chi, and Yoga on Fatigue, Mental Health, and Sleep Quality in Chronic Fatigue and Post-COVID Syndromes: A Systematic Review with Meta-Analysis. Healthcare 2024, 12, 2020. [Google Scholar] [CrossRef]
- Sick, J.; Steinbacher, V.; Kotnik, D.; König, F.; Recking, T.; Bengsch, D.; König, D. Exercise rehabilitation in post COVID-19 patients: A randomized controlled trial of different training modalities. Eur. J. Phys. Rehabil. Med. 2025, 61, 130–140. [Google Scholar] [CrossRef]
- Jorge, M.S.G.; Nepomuceno, P.; Schneider, R.H.; Wibelinger, L.M. Eight weeks of Pilates Method improves physical fitness and sleep quality of individuals with post-COVID-19 syndrome: A randomized clinical trial blinded. J. Bodyw. Mov. Ther. 2025, 41, 238–245. [Google Scholar] [CrossRef]
- Tryfonos, A.; Pourhamidi, K.; Jörnåker, G.; Engvall, M.; Eriksson, L.; Elhallos, S.; Asplund, N.; Mandić, M.; Sundblad, P.; Sepic, A.; et al. Functional Limitations and Exercise Intolerance in Patients with Post-COVID Condition: A Randomized Crossover Clinical Trial. JAMA Netw. Open 2024, 7, e244386. [Google Scholar] [CrossRef]
- Barz, A.; Berger, J.; Speicher, M.; Morsch, A.; Wanjek, M.; Rissland, J.; Jäger, J. Effects of a symptom-titrated exercise program on fatigue and quality of life in people with post-COVID condition—A randomized controlled trial. Sci. Rep. 2024, 14, 30511. [Google Scholar] [CrossRef]
- Ahmadi Hekmatikar, A.H.; Ferreira Junior, J.B.; Shahrbanian, S.; Suzuki, K. Functional and Psychological Changes after Exercise Training in Post-COVID-19 Patients Discharged from the Hospital: A PRISMA-Compliant Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2290. [Google Scholar] [CrossRef] [PubMed]
- Magni, O.; Arnaoutis, G.; Panagiotakos, D. The impact of exercise on chronic systemic inflammation: A systematic review and meta–meta-analysis. Sport Sci. Health 2025, 21, 1405–1417. [Google Scholar] [CrossRef]
- Sánchez-García, J.C.; Reinoso-Cobo, A.; Piqueras-Sola, B.; Cortés-Martín, J.; Menor-Rodríguez, M.J.; Alabau-Dasi, R.; Rodríguez-Blanque, R. Long COVID and Physical Therapy: A Systematic Review. Diseases 2023, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Hadjiat, Y.; Marchand, S. Virtual Reality and the Mediation of Acute and Chronic Pain in Adult and Pediatric Populations: Research Developments. Front. Pain. Res. 2022, 3, 840921. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Georgieva, I.; Gong, Z.; Nanjappan, V.; Georgiev, G.V. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sci. 2021, 11, 221. [Google Scholar] [CrossRef]
- Nagamine, T. Challenges in using virtual reality technology for pain relief. World J. Clin. Cases 2025, 13, 103372. [Google Scholar] [CrossRef]
- Nagamine, T. Application of virtual reality technology improves the functionality of brain networks in individuals experiencing pain. World J. Clin. Cases 2025, 13, 97856. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Mohamed Awad Allah, S.A.; Abd Elhaseeb, G.A.; Elsharawy, D.E.; Ahmed, H.S.; Mohamed Abdelwahab, M.A. Effects of conventional versus virtual reality-simulated treadmill exercise on fatigue, cognitive function, and participant satisfaction in post-COVID-19 subjects: A randomized trial. J. Exerc. Sci. Fit. 2024, 22, 316–321. [Google Scholar] [CrossRef]
- Smits, M.; Staal, J.B.; van Goor, H. Could Virtual Reality play a role in the rehabilitation after COVID-19 infection? BMJ Open Sport. Exerc. Med. 2020, 6, e000943. [Google Scholar] [CrossRef]
- Janaudis-Ferreira, T.; Beauchamp, M.K.; Rizk, A.; Tansey, C.M.; Sedeno, M.; Barreto, L.; Bourbeau, J.; Ross, B.A.; Benedetti, A.; Li, P.Z.; et al. Virtual rehabilitation for individuals with Long COVID: A randomized controlled trial. medRxiv 2024. [Google Scholar] [CrossRef]
- Lee, S.A.; Heo, S.; Kim, S.; Park, C.; Jung, Y.; Ji, G.; Lee, H.-A.; Kim, K.; Kim, S.; Kim, B.-N.; et al. The Effectiveness of Virtual Reality Intervention for COVID-19-Related Psychological Distress: A Systematic Review. Psychiatry Investig. 2023, 20, 357–368. [Google Scholar] [CrossRef]
- Cano, N.; Gómez-Hernández, J.; Ariza, M.; Mora, T.; Roche, D.; Porras-García, B.; Garolera, M. A multimodal group-based immersive virtual reality intervention for improving cognition and mental health in patients with post-COVID-19 condition. A quasi-experimental design study. Front. Psychol. 2024, 15, 1441018. [Google Scholar] [CrossRef]
- Hamdani, S.U.; Zill, E.H.; Zafar, S.W.; Suleman, N.; Um-Ul-Baneen; Waqas, A.; Rahman, A. Effectiveness of relaxation techniques ‘as an active ingredient of psychological interventions’ to reduce distress, anxiety and depression in adolescents: A systematic review and meta-analysis. Int. J. Ment. Health Syst. 2022, 16, 31. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Long, X.; Tu, H.; Chen, J. Effectiveness of Virtual Reality-Complemented Pulmonary Rehabilitation on Lung Function, Exercise Capacity, Dyspnea, and Health Status in Chronic Obstructive Pulmonary Disease: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e64742. [Google Scholar] [CrossRef]
- Gidoni, M.; Russo, R.; Alessandro, P.; Pirani, R.; Gatti, C.; D’Ambrosio, L.; Esposito, M. Virtual and augmented reality training on digital mirror D-wall. Case study: Long COVID-19 rehabilitation protocol. Arch. Phys. Med. Rehabil. 2023, 104, e63. [Google Scholar] [CrossRef]
- Zampolini, M.; Selb, M.; Boldrini, P.; Branco, C.A.; Golyk, V.; Hu, X.; Kiekens, C.; Negrini, S.; Nulle, A.; Oral, A.; et al. The Individual Rehabilitation Project as the core of person-centered rehabilitation: The Physical and Rehabilitation Medicine Section and Board of the European Union of Medical Specialists Framework for Rehabilitation in Europe. Eur. J. Phys. Rehabil. Med. 2022, 58, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Manocchio, N.; Ljoka, C.; Ferdinandi, V.; Cicchi, L.; Foti, C. Commentary on “The learning rehabilitation system: Strengthening an intersectoral strategy to improve functioning of an ageing population” by Bickenbach et al. Health Policy 2025, 155, 105303. [Google Scholar] [CrossRef] [PubMed]



| VRG (n = 37) | CTG (n = 37) | p Value | |
|---|---|---|---|
| Age, years | 43.3 (8.4) | 45.3 (8.6) | 0.30 |
| Gender, female | 30 | 28 | 0.77 |
| BMI, kg/m2 | 26.4 (3.4) | 27.2 (3.5) | 0.31 |
| Comorbidity, yes | 10 | 8 | 0.81 |
| Duration since COVID-19 diagnosis, months | 9.1 (2.2) | 8.7 (3.2) | 0.67 |
| Duration since PCC diagnosis, months | 6.8 (1.3) | 7.3 (1.7) | 0.42 |
| Quantitative variables are expressed as mean (standard deviation), categorical variables as n (%). | |||
| 0. Week | 8. Week | Change VRG | Change CTG | |||
|---|---|---|---|---|---|---|
| VRG | CTG | VRG | CTG | |||
| Primary Outcome | ||||||
| VAS, 0–10 cm | 6.4 (1.3) | 6.1 (1.2) | 4.2 (1.1) | 5.3 (1.2) | −2.22 ± 0.95 | −0.85 ± 0.57 |
| Secondary Outcomes | ||||||
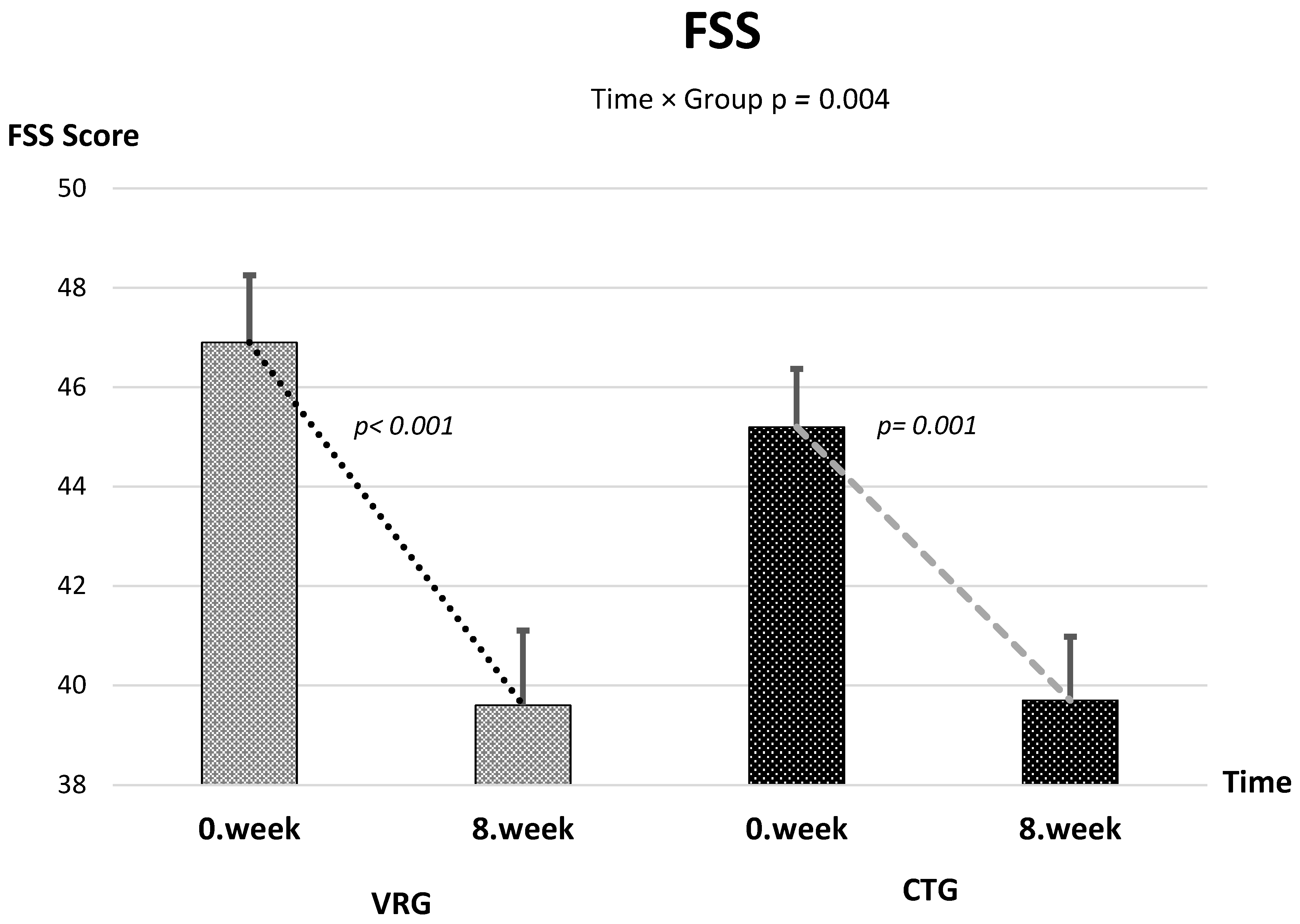
| FSS | 46.9 (8.2) | 45.2 (7.1) | 39.6 (9.1) | 39.7 (7.8) | −8.16 ± 6.70 | −3.97 ± 4.61 |
| HADS-A | 8.6 (2.2) | 7.9 (3.2) | 7.6 (2.1) | 7.1 (2.8) | −1.03 ± 0.99 | −1.19 ± 1.60 |
| HADS-D | 9.1 (3.0) | 8.7 (3.1) | 7.1 (2.1) | 7.8 (2.5) | −2.00 ± 2.30 | −0.84 ± 1.21 |
| 6MWT, meters | 461.6 (88.8) | 450.6 (44.5) | 499.3 (74.1) | 478.8 (42.1) | 37.7 ± 40.3 | 27.8 ± 17.1 |
| SF-12 M | 42.4 (11.6) | 44.8 (11.9) | 48.7 (10.3) | 53.2 (7.8) | 6.23 ± 9.91 | 8.33 ± 10.84 |
| SF-12 F | 34.0 (9.4) | 30.8 (9.4) | 40.9 (7.0) | 37.9 (9.9) | 6.23 ± 9.91 | 7.04 ± 8.91 |
| Group | Time | Time × Group Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Mean Diff [95% CI] | F (df1, df2) | p | Mean Diff [95% CI] | F (df1, df2) | p | Mean Diff [95% CI] | F (df1, df2) | p | Partial η2 |
| Primary Outcome | ||||||||||
| VAS, 0–10 cm | −0.26 [−0.84–0.32] | 0.74 (1, 59.4) | 0.387 | −2.22 [−2.50–−1.93] | 232.3 (1, 59.4) | <0.001 | −1.37 [−1.73–−1.00] | 56.31 (1, 59.4) | <0.001 | 0.487 |
| Secondary Outcomes | ||||||||||
| FSS | +1.73 [−1.38–4.84] | 1.21 (1, 61.5) | 0.275 | −6.07 [−7.11–−5.03] | 93.8 (1, 61.5) | <0.001 | −4.19 [−6.99–−1.39] | 8.96 (1, 61.5) | 0.004 | 0.127 |
| HADS-A | +0.73 [−0.67–2.13] | 1.10 (1, 60.0) | 0.298 | −1.11 [−1.62–−0.60] | 17.3 (1, 60.0) | <0.001 | +0.16 [−0.45–0.78] | 0.28 (1, 60.0) | 0.601 | 0.005 |
| HADS-D | +0.32 [−1.16–1.80] | 0.17 (1, 54.6) | 0.680 | −1.42 [−1.92–−0.92] | 34.9 (1, 54.6) | <0.001 | −1.16 [−2.02–−0.31] | 7.40 (1, 54.6) | 0.009 | 0.119 |
| 6MWT, meters | +11.0 [−12.1–34.1] | 0.92 (1, 48.8) | 0.340 | +32.7 [19.8–45.6] | 45.6 (1, 48.8) | <0.001 | +9.9 [−4.6–24.4] | 1.90 (1, 48.8) | 0.175 | 0.037 |
| SF-12 Physical | +3.16 [−1.01–7.33] | 2.28 (1, 69.5) | 0.136 | +6.95 [5.25–8.65] | 74.8 (1, 69.5) | <0.001 | −0.18 [−3.97–3.61] | 0.009 (1, 69.5) | 0.926 | 0.0001 |
| SF-12 Mental | −2.39 [−7.33–2.55] | 0.92 (1, 71.4) | 0.340 | +7.53 [5.12–9.94] | 41.2 (1, 71.4) | <0.001 | −2.10 [−6.91–2.72] | 0.76 (1, 71.4) | 0.388 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polat, M.; Oba, P.; Karadağ, A. Effect of Motion-Controlled Video Games-Based Virtual Reality Exercise on Patients with Post-COVID-19 Condition: A Randomized Controlled Trial. Healthcare 2025, 13, 2914. https://doi.org/10.3390/healthcare13222914
Polat M, Oba P, Karadağ A. Effect of Motion-Controlled Video Games-Based Virtual Reality Exercise on Patients with Post-COVID-19 Condition: A Randomized Controlled Trial. Healthcare. 2025; 13(22):2914. https://doi.org/10.3390/healthcare13222914
Chicago/Turabian StylePolat, Musa, Pınar Oba, and Ahmet Karadağ. 2025. "Effect of Motion-Controlled Video Games-Based Virtual Reality Exercise on Patients with Post-COVID-19 Condition: A Randomized Controlled Trial" Healthcare 13, no. 22: 2914. https://doi.org/10.3390/healthcare13222914
APA StylePolat, M., Oba, P., & Karadağ, A. (2025). Effect of Motion-Controlled Video Games-Based Virtual Reality Exercise on Patients with Post-COVID-19 Condition: A Randomized Controlled Trial. Healthcare, 13(22), 2914. https://doi.org/10.3390/healthcare13222914








