Abstract
Cannibalism is a behavior that different species of fish exhibit in the early stages of their life, and it has been widely reported. In Tabasco, Mexico, the ancestral species Atractosteus tropicus is farmed, which is a freshwater fish with a high nutritional and economic value. This species exhibits high cannibalistic behavior both in its larval and juvenile stages, which considerably decreases its production. Therefore, strategies have been developed to mitigate the effects of this behavior. One of them is the placement of shelters (rocks and artificial vegetation), which allow the vulnerable population to protect themselves from cannibals. The goal of this work is to study the effect of shelters on the cannibalistic behavior of the A. tropicus population through a mathematical model. The population is divided into two classes, the vulnerable population (prey) and the cannibal population (predator). Moreover, a system of ordinary differential equations is established, which is analyzed, and sufficient conditions for the coexistence of the two species are shown. Numerical simulations show coexistence by varying levels of refuge. The results obtained in this work can be applied to other populations that exhibit cannibalistic behavior.
MSC:
34D23; 37G15; 37N25
1. Introduction
Cannibal behavior has been identified in different organisms, such as arachnids, amphibians, mollusks, marine mammals, gastropods, crustaceans (see [1,2,3,4,5,6,7]), and it is defined as the act of completely or partially ingesting an individual of its own species [8,9]. Types of cannibalism have been observed in around 390 fish species [10]. In this aspect, three types of cannibalism in fish have been categorized: (a) Type 1, the cannibal is smaller in relation to the prey. (b) Type 2, the cannibal is larger and can completely ingest the prey. (c) Type 3, several cannibals dismember the prey and consume it (see [11,12,13,14]). The tropical gar (A. tropicus) is a species cataloged as ancestral that lives in Central American countries and in southeastern Mexico. Due to its local trade, it is considered a species of economic and cultural importance [15,16]. Despite the development of its activity and advances in research on its biology, nutrition, and physiology, one of the main limitations of its cultivation is the cannibalism present in the larval and juvenile stages, both in the natural environment and in captivity [17,18,19]. The effect of cannibalism in larval stages can cause a maximum survival of 33%, with this being caused by consumption among the larvae or by lacerations produced by cannibalistic behavior [20]. Some factors that have been reported in A. tropicus larvae related to cannibalism are culture density, variability in size and/or color between larvae, insufficient feeding and/or low nutritional value, and the presence of abnormally larvae [19,21]. In this sense, different biotic and abiotic factors have been identified as triggers of cannibalistic behavior in fish, including the absence of refuges [22]. A strategy to reduce stress in fish and control aggressive and cannibalistic events is to implement enriched environments through the use of various types of refuges, which aims at replicating the conditions of natural environments. The use of refuges has been shown to mimic natural conditions and decrease energy expenditure, injury, or illness [19,23]. In black rockfish (Sebastes schlegelii) fry, the use of a high density of refuges (8 per tank) had a direct effect on the reduction of cannibalism [24]. On the other hand, the use of refuges (PVC 15 × 8 × 3 cm) and low light decreased cannibalism in juvenile Barramundi (Lates calcarifer), where the frequency of aggressive behaviors such as chasing and biting were reduced with the presence of refuges [25].
In an effort to understand the different phenomena that occur in the interaction of populations, such as predation and cannibalism, mathematical models have been established (see [26,27]). In Hassell’s book, it is recorded that adding a large shelter to a model, which exhibits divergent oscillations in the absence of shelters, replaces the oscillatory behavior with a stable equilibrium [28]. In order to establish a mathematical model that describes cannibalistic behavior in A. tropicus, a Gause-type model is proposed:
where x and y denote the density of prey and predators, respectively, which present a rich diversity of dynamics. Laboratory experiments indicate that shelters have a stabilizing effect on predator–prey relationships [19]. In [27], Kar proposed the followed prey–predator model incorporating a prey refuge:
where the term mx is incorporated, which measures the defense of the prey through a refuge level given by the parameter m. The conditions for the existence of equilibrium points, limit cycles, and the dependence on the parameter m are studied in [27].
In this work, System (2) is adapted to simulate cannibalistic interactions in tropical gar (A. tropicus). The aim of this paper is to evaluate the use of refuges and their effect on cannibal behavior in tropical gar (A. tropicus). This project proposes a way to analyze the different interactions that can occur in tropical gar (A. tropicus) classified into two classes, prey and cannibal, which are denoted with x and y, respectively. The model considers logistic growth for the prey and Holling type II functional response incorporating a refuge parameter m for the prey x. Furthermore, the model considers a predation rate c on prey without defense mechanisms due to the observed malformations [19]. In the population of predators, it is considered that they have the ability to capture individuals that are capable of taking refuge. This is measured by the conversion parameter γ, and the percentage of the use of vulnerable prey is obtained by δ. A small mortality rate d is considered for the predator class. Under the previous hypotheses, the proposed model is
where x, and y denote the population of prey and cannibals, respectively, in any time t, and α, K, β, a, c, γ, and d are positive constants, with . The parameter α represents the intrinsic growth rate of the prey class, K is the carrying capacity of the medium, c is the predation rate of undefended prey, β is the predation rate of defended prey, γ is the conversion factor that indicates the use for the cannibal class for each captured prey, d represents the mortality rate of the cannibal class, denotes the functional response of the predator and is better known as Holling type II functional response with the incorporation of the refuge parameter m for the class x, and is the maximum number of prey that a predator can eat per time unit.
In this paper, we analyze the global dynamics and the bifurcation in System (3). The conditions are given for System (3) to present global stability for the coexistence equilibrium point. We prove that there exists a set in the parameter space where System (3) presents a Hopf bifurcation. This bifurcation is obtained in the (m, γ)-parameter space. System (3) generalizes system (2) since it incorporates the predation of the vulnerable class through the term cxy, which does not appear in (2). Moreover, it provides mathematical support to the experimental work carried out in [19].
2. Equilibrium Points and Their Stability
In order to have expressions that allow us to analyze the equilibrium points of the system, we solve in (3) for the parameters c and d, and we obtain
where
Since we want c > 0 and d > 0, we need to assume
The trivial equilibrium points of differential System (3) are and The local behavior of the equilibrium points and are summarized in Table 1 with
for more details, see Appendix A.1.

Table 1.
Equilibrium points and local stability.
In order to find the coexistence equilibrium points, we solve the first equation of (3)
From the second equation, we have
Since y is different from zero,
As
by Descartes’ rule of signs, there is a only one positive root which is the first coordinate of the equilibrium point.
From (6) and (8), it follows that if x < K, there is a only one coexistence equilibrium point
where and
The following theorem shows the global stability of the equilibrium point . Its proof can be found in Appendix A.2.
Theorem 1.
If and , then the equilibrium point is globally asymptotically stable.
3. Hopf Bifurcation at Equilibrium Point p∗
In order to establish the necessary conditions to have a Hopf bifurcation, we set the following conditions on the parameters of the System: (3),
and
Lemma 1.
Proof details are shown in Appendix A.3.
Proposition 1.
If the hypotheses of Lemma 1 are satisfied, then there exists a curve in the parameter space , such that the eigenvalues of are , and the first Lyapunov coefficient is negative.
The details of the proof are in Appendix A.4.
4. Numerical Simulation
In the following examples, we take the parameter values corresponding to A. tropicus and we show the effect of refuge on the survival of the prey population [19].
4.1. Example 1
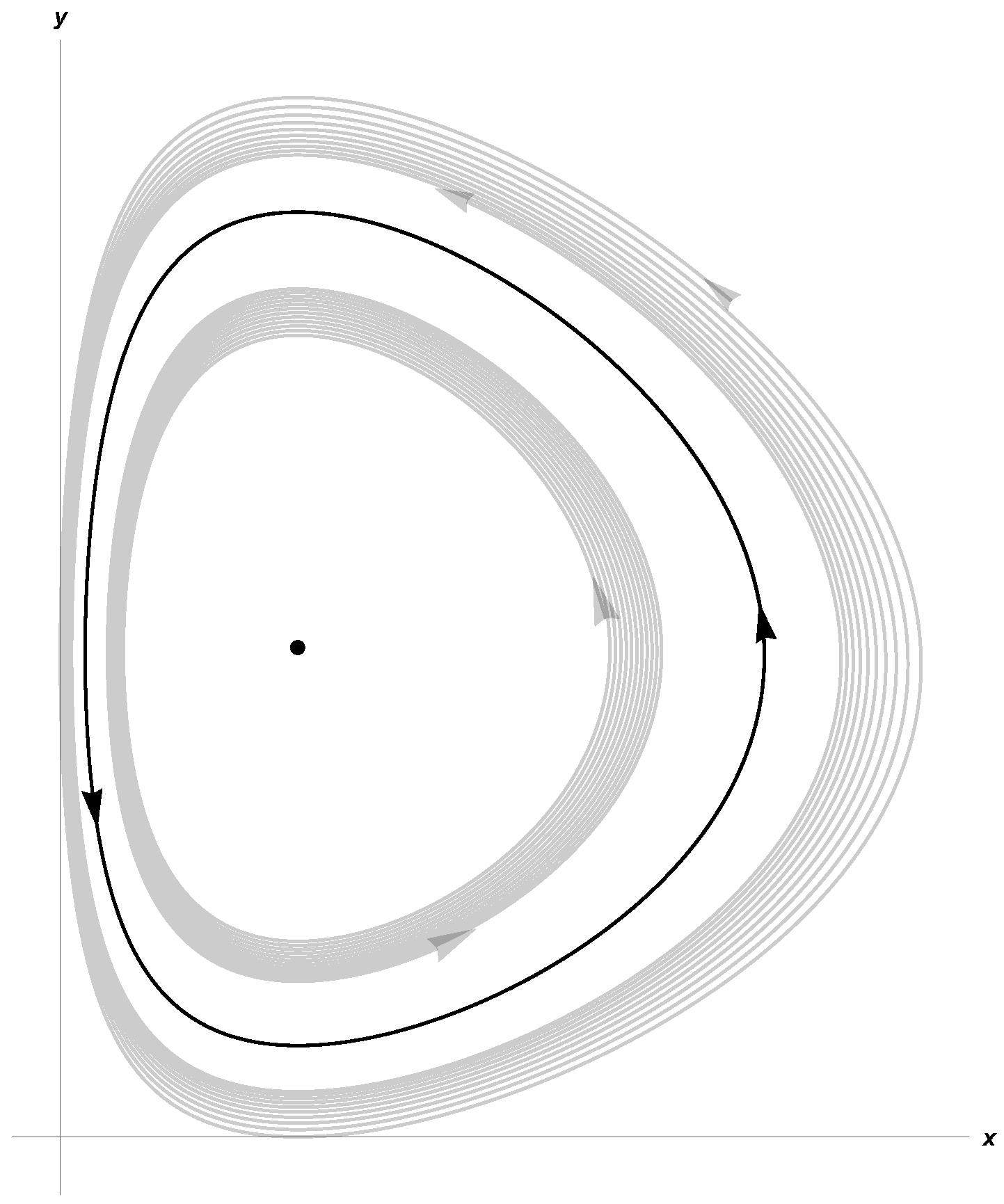
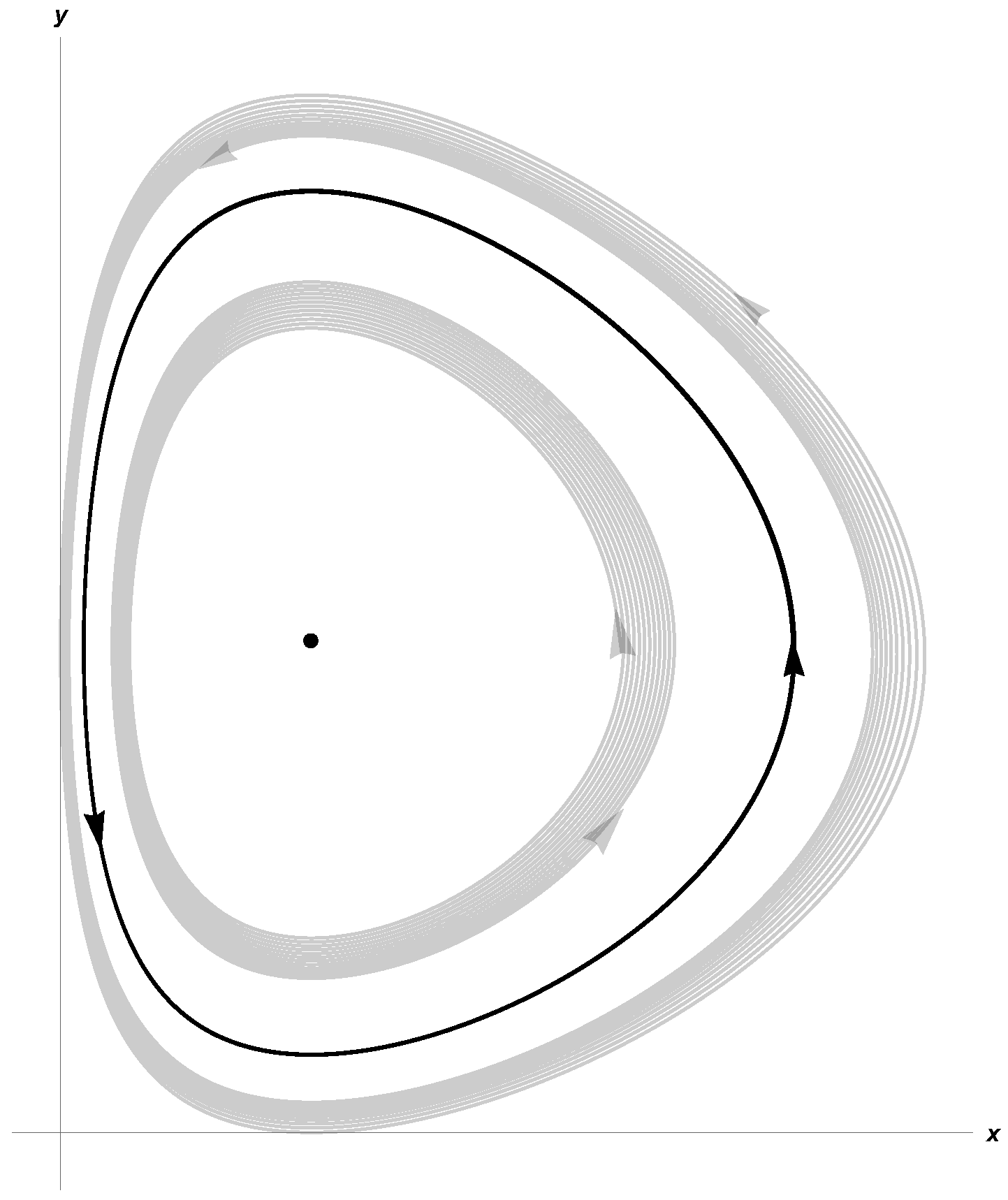
Taking β = 0.27, d = 0.15, γ = 0.8, a = 0.27, c = 0.61, δ = 0.25, System (3) satisfies (10) and (11). In Figure 1 and Figure 2, we show stable limit cycles corresponding to m = 0.15 and m = 0.2, respectively. The first Lyapunov coefficient for m = 0.15 is . For m = 0.2,

Figure 1.
Limit cycle for K = 18.89, α = 0.64, and m = 0.15.
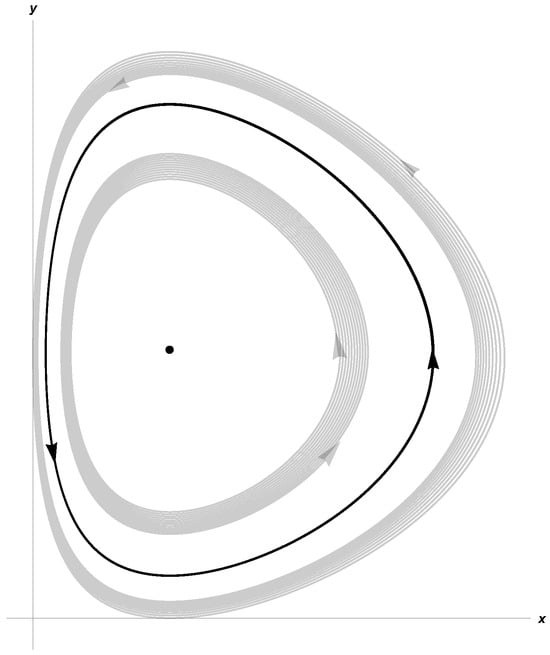
Figure 2.
Limit cycle for K = 21.83, α = 0.64, and m = 0.2.
4.2. Example 2
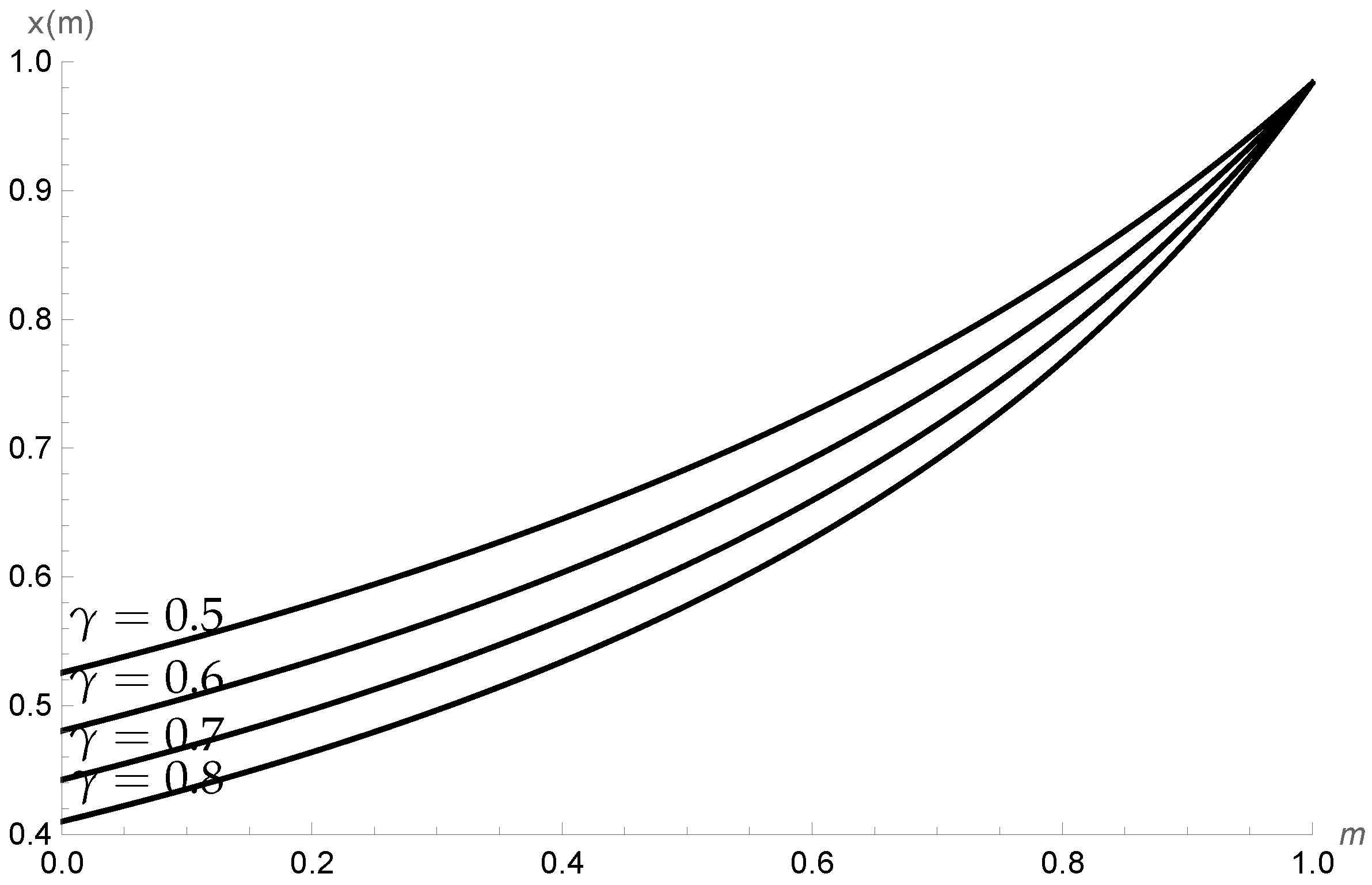
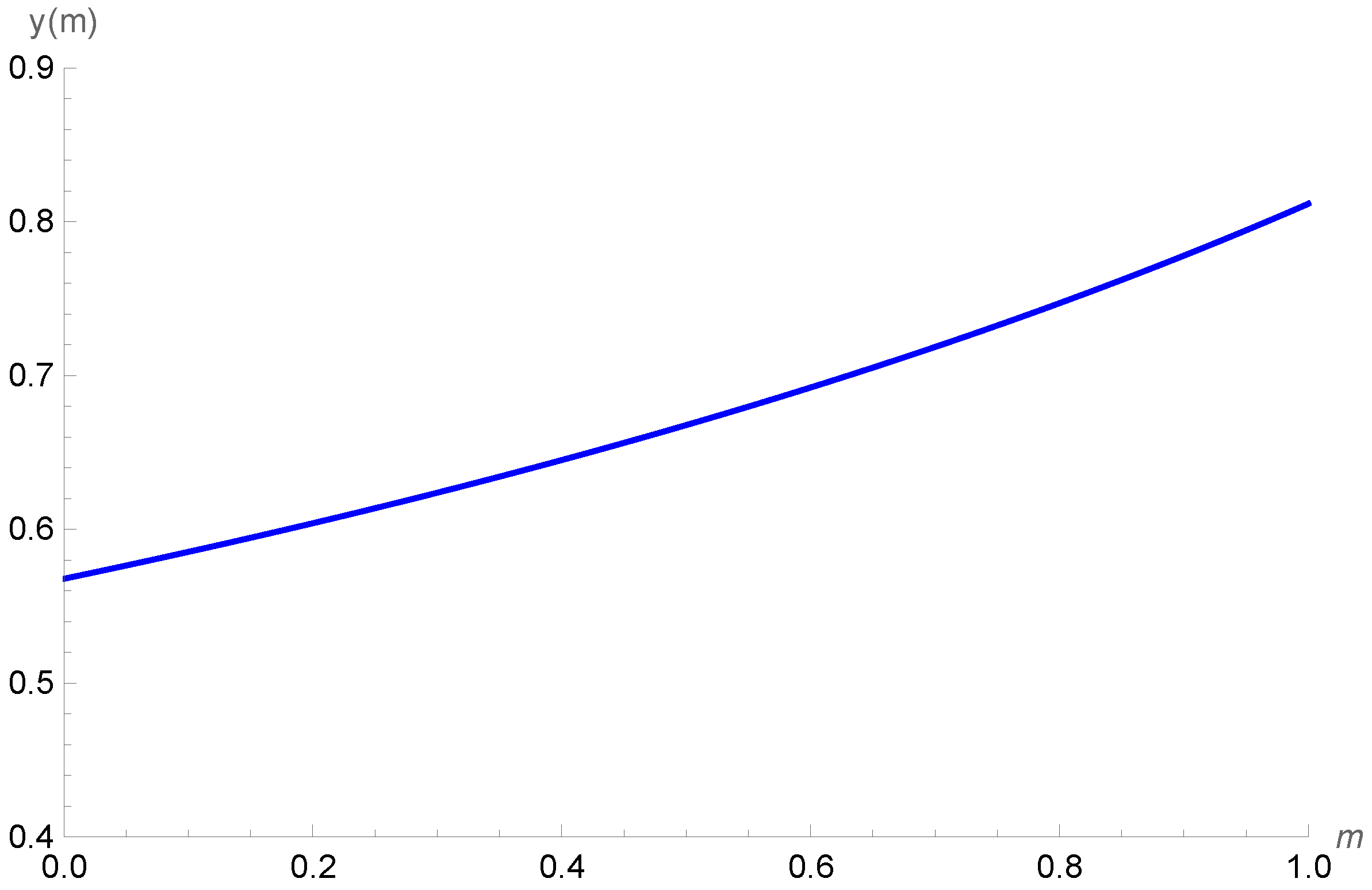
We take β = 0.27, d = 0.15, c = 0.61, δ = 0.25, α = 0.5, K = 100, and a = 0.03 p∗ as being locally stable. Figure 3 shows the increase in density in prey class x when the refuge level is increased. It is possible to observe that by increasing the profit rate γ, the initial densities of prey class x decrease but its trend as a function of m is increasing, as expected. On the other hand, in Figure 4, we show that the variation in the density of the cannibal population is negligible when faced with variations in the profit rate of the cannibal, since the different curves are superimposed.

Figure 3.
Behavior of the density of the vulnerable population x as a function of the refuge level m for the profit rates γ = 0.5, γ = 0.6, γ = 0.7, and γ = 0.8.
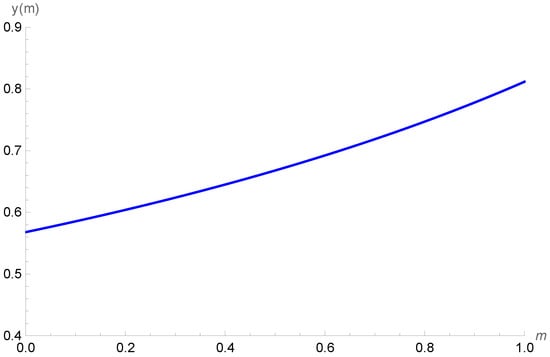
Figure 4.
Behavior of the cannibal class y as a function of the refuge level m for the profit rates γ = 0.5, γ = 0.6, γ = 0.7, and γ = 0.8.
4.3. Example 3
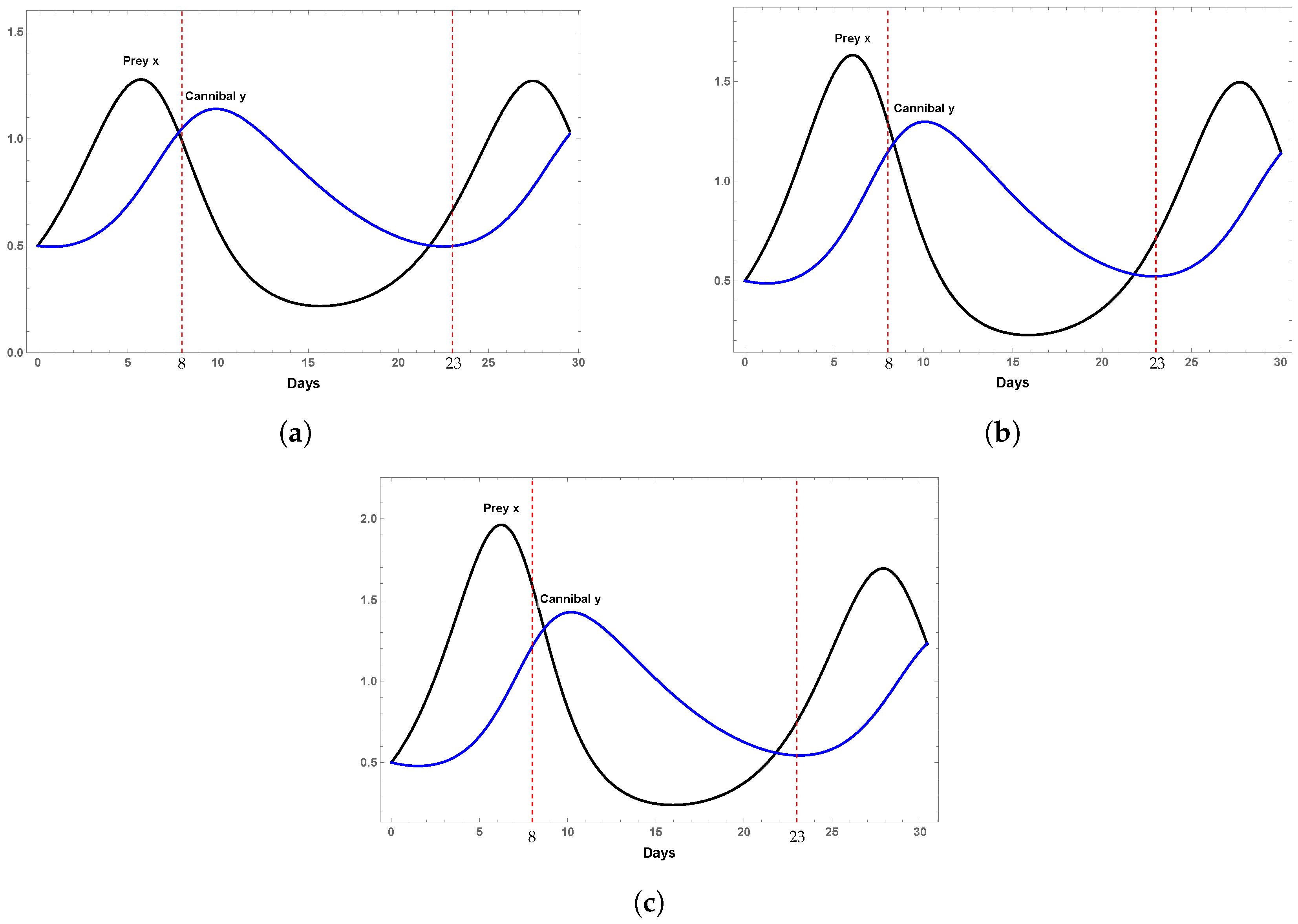
We set the parameters β = 0.27, d = 0.15, c = 0.61, δ = 0.25, α = 0.64, K = 21.83, a = 0.27, and γ = 0.5. Taking the initial condition and varying the refuge level m from 0.2 to 0.7, we obtain the time series corresponding to the vulnerable population and the cannibal population shown in Figure 5. It can be observed how the increase in the refuge rate from 0.2 to 0.7 contributes to raising the density of the vulnerable population and, consequently, to the preservation of the species. It is worth mentioning that we have not included simulations for refuge rates higher than 70% since they contribute very little to increasing population density levels and, on the other hand, cause environmental saturation.

Figure 5.
Time series with , for K = 21.83, α = 0.64, β = 0.27, d = 0.15, c = 0.61, δ = 0.25, a = 0.27, and γ = 0.5. (a) Behavior of the vulnerable population x and the cannibal class y for m = 0.2. (b) Behavior of the vulnerable population x and the cannibal class y for m = 0.5. (c) Behavior of the vulnerable population x and the cannibal class y for m = 0.7.
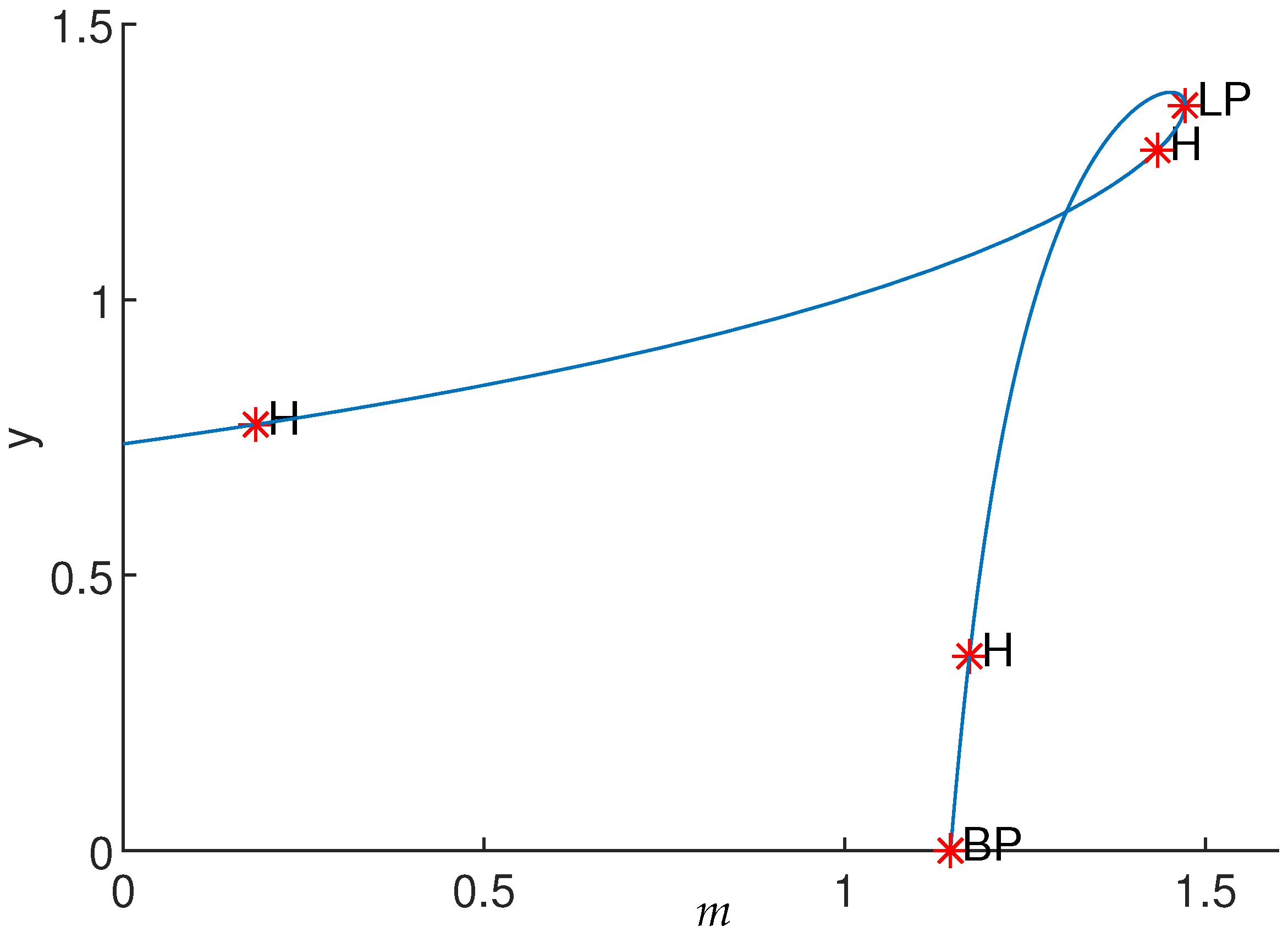
Numerical simulations show that apart from the Hopf bifurcation, the system presents other bifurcations. By varying parameter m using MatCont software, one saddle-node bifurcation (LP) and branch point (BP) are also observed (see Figure 6). Furthermore, bifurcations of codimension 2 were found in the negatives; thus, they are not of interest.
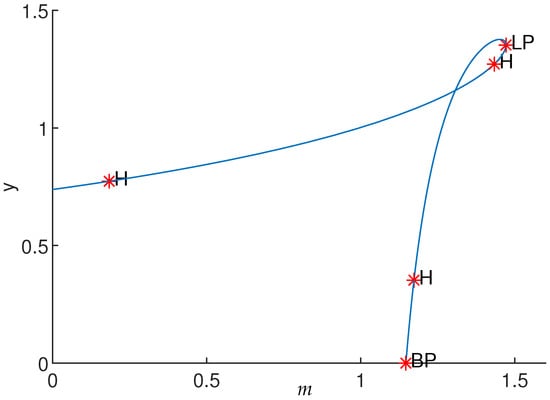
Figure 6.
Equilibrium curve of System (3) for γ = 0.5, K = 21.83, c = 0.61, β = 0.27, δ = 0.25, a = 0.27 and d = 0.15. Equilibrium point and bifurcation parameter m = 0.18. The first Lyapunov coefficient .
5. Conclusions
In this work, we studied cannibalism in A. tropicus because it is an important species in tropical ecosystems and has a commercial value. The results obtained are important and consistent with the work carried out in [19]. There are parameter conditions for system (3), which has a Hopf bifurcation. For different levels of shelter (m = 0.15, m = 0.2), it is possible to show the Hopf bifurcation of the supercritical type. The presence of the Hopf bifurcation in system (3) guarantees the existence of a stable limit cycle and the coexistence of the two classes in the species A. tropicus. In order to obtain some insight into the role of the shelter level, some numerical simulations were performed to explore different scenarios. Figure 3 and Figure 5 show how the density of class x is benefited by increasing the refuge level for different values of the profit rate γ. In [19], the authors describe the results obtained from experimental tests that investigated the behavior of cannibalistic attacks in tropical gar (A. tropicus) larvae. Experimentally, they showed that shelters decreased attacks in pairs and groups compared to using rocks as shelter. Numerical simulations show that it is possible to guarantee the coexistence of both classes, prey and cannibals, during the cannibalism period from the 8th day to the 23rd day, for different shelter levels m = 0.2, 0.5, 0.7 (see Figure 5). However, the inappropriate use of refuges can modify the social structure of a population, causing changes in behavior mainly caused by the competition for resources [29], which can translate into an increase in cannibalistic or aggressive behavior. The inappropriate use of shelters can stress organisms, causing even more aggression: for example, in sharptooth catfish (Cirrius gariepinus), the use of plastic shelters increased aggression, most likely due to the increase in territoriality caused by these shelters [30]. Shelters, if any, can also modify the entry of light into the aquarium. These changes in luminosity trigger cannibalistic behaviors; therefore, the relationship between shelters and luminosity can be another trigger [24]. Furthermore, stress, low availability of swimming area, and reduced space for feeding can be triggering factors that increase such aggressive behaviors.
The model within this paper (3) clearly explains the effect of refuges on cannibalistic behavior in A. tropicus larvae through experimental data. Hence, the data obtained through observation with mathematical modeling can be a fundamental tool to understand the predator–prey relationships in natural or artificial environments. System (3) allows us to describe the dynamics of A. tropicus in captivity. However, this system also applies to other species that meet the hypotheses raised in this paper. Furthermore, we are working on other improvements to the models that consider characteristics such as the type of feeding and the effect of colors in the bottom of fish tanks for A. tropicus, according to experimental results [19].
Author Contributions
Conceptualization, C.A.S.-Q., R.M.-G. and C.A.Á.-G.; methodology, L.M.V. and G.B.; software, L.M.V. and G.B.; validation, L.M.V., G.B. and C.A.S.-Q.; formal analysis, L.M.V. and G.B.; investigation, C.A.S.-Q., C.A.Á.-G., L.M.V. and G.B.; writing—original draft preparation, C.A.S.-Q., L.M.V., G.B. and A.L.-M.; writing—review and editing, L.M.V. and G.B.; supervision, C.A.S.-Q., R.M.-G., C.A.Á.-G., G.B. and A.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The first author thanks Consejo Nacional de Ciencia y Tecnología (CONACYT) for their thesis fellowship for their doctoral graduate studies (CVU 858306).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| PVC | Polyvinyl chloride |
Appendix A
Appendix A.1. Local Dynamics
The linear approximation of the system around the point is given by the Jacobian matrix in , as follows:
Its eigenvalues are and . Since , if , then we have an unstable node in (0, 0) and a saddle when . Finally, if , the equilibrium point is not hyperbolic and is a saddle node (see Sotomayor’s theorem [31]).
For , the Jacobian matrix is
where
Its eigenvalues are and . Since , if N < 0, then we have a stable node in (K, 0) and a saddle when N > 0. If N = 0, the equilibrium point is a saddle node (see Sotomayor’s theorem [31]).
Appendix A.2. Proof of Theorem 1
Proof.
In order to demonstrate global stability, the following Lyapunov function is proposed:
where μ is a positive parameter that needs to be defined.
Since
it follows .
As the Hessian matrix of the v computation in is
which is positive definite, the function v has a local minimum at . The derivative of v along the paths of System (3) is
Taking , we have
From the first equation of System (3), we have
On the other hand,
By hypothesis, it follows that
Then, .
Therefore, , and is globally asymptotically stable. □
Appendix A.3. Purely Imaginary Eigenvalues
Proof.
The Jacobian matrix computation at is
where
and
The characteristic polynomial of is
By taking and , we have
Thus, the eigenvalues of are
□
Appendix A.4. Hopf Bifurcation
Proof.
With Lemma 1, the eigenvalues of are . In order to calculate the first Lyapunov coefficient , we will use Kuznetsov’s formula (see [31]).
By moving to the origin, we obtain the equivalent system, as follows:
where
The bilinear and trilinear forms B and C at the equilibrium are
where
and
The eigenvectors of A are
where
which satisfy
By normalizing these eigenvectors in such a way that we obtain
see
The eigenvectors for the transpose matrix AT are
where
They satisfy
There exists such that
In fact,
Taking then
Using Kuznetsov’s formula, the first Lyapunov coefficient is
where
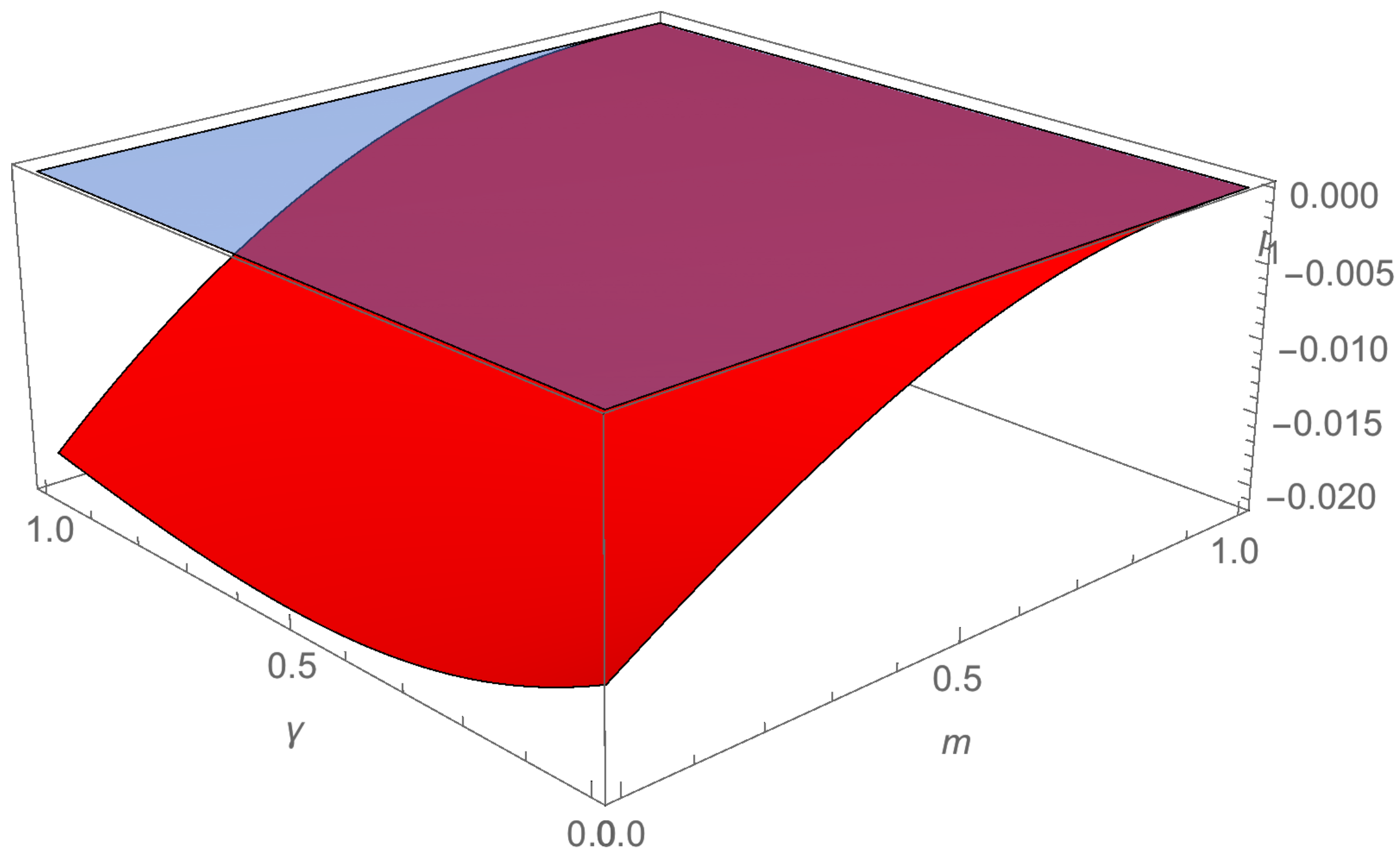
Since H = 0.09, R = 0.33, c = 0.61, γ = 0.8, a = 0.27, β = 0.27, δ = 0.25, d = 0.15, m = 0.2, and ω = 0.3, is a continuous function of m and γ which takes negative values (see Figure A1). □
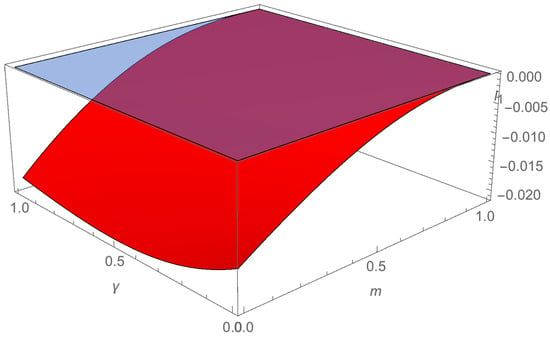
Figure A1.
The first Lyapunov coefficient .
Figure A1.
The first Lyapunov coefficient .
References
- Ironside, J.E.; Dalgleish, S.T.; Kelly, S.J.; Payne, W. Sex or food? Effects of starvation, size and diet on sexual cannibalism in the amphipod crustacean. Aquat. Ecol. 2019, 53, 1–7. [Google Scholar] [CrossRef]
- Johnson, J.C.; Halpin, R.; Stevens, R., II. Extreme developmental synchrony reduces sibling cannibalism in the black widow spider, Latrodectus hesperus. Anim. Behav. 2016, 120, 61–66. [Google Scholar] [CrossRef]
- Nishimura, K. An interaction-driven cannibalistic reaction norm. Ecol. Evol. 2018, 8, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Polis, G.A. The evolution and dynamics of intraspecific predation. Ann. Rev. Ecol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Rosas-Luis, R.; Jiménez-Badillo, M.L.; Montoliu-Elena, L.; Morillo-Velarde, P.S. Food and feeding habits of Octopus insularis in the Veracruz Reef System National Park and confirmation of its presence in the southwest Gulf of Mexico. Mar. Ecol. 2019, 40, e12535. [Google Scholar] [CrossRef]
- Neer, A.V.; Gross, S.; Kesselring, T.; Wohlsein, P.; Leitzen, E.; Siebert, U. Behavioural and pathological insights into a case of active cannibalism by a grey seal. (Halichoerus grypus) on Helgoland, Germany. J. Sea Res. 2019, 148, 12–16. [Google Scholar] [CrossRef]
- Yu, Z.L.; Wang, H.; Song, H.; Bai, Y.C.; Sun, J.C.; Qian, Y.S.; Hu, N.; Yang, M.J.; Zhang, T. Cannibalism by the juveniles of the gastropod Rapana venosa (Muricidae) reared under laboratory conditions. J. Molluscan Stud. 2018, 84, 303–309. [Google Scholar] [CrossRef]
- Pfennig, D.W. Kinship and cannibalism. Bioscience 1997, 47, 667–675. [Google Scholar] [CrossRef]
- Smith, C.; Reay, P. Cannibalism in teleost fishes. Rev. Fish Biol. Fish. 1991, 1, 41–64. [Google Scholar] [CrossRef]
- Pereira, L.S.; Agostinho, A.A.; Winemiller, K.O. Revisiting cannibalism in fishes. Rev. Fish Biol. Fish. 2017, 27, 499–513. [Google Scholar] [CrossRef]
- Baras, E.; Ndao, M.; Maxi, M.Y.Y.; Jeandrain, D.; Thomé, J.P.; Vanadewalle, P.; Melard, C. Sibling cannibalism in dorada under experimental conditions. I. Ontogeny, dynamics, bioenergetics of cannibalism and prey size selectivity. J. Fish Biol. Fish. 2000, 57, 1001–1020. [Google Scholar] [CrossRef]
- Baras, E.; Jobling, M. Dynamics of intracohort cannibalism in cultured fish. Aquac. Res. 2002, 33, 461–479. [Google Scholar] [CrossRef]
- Baras, E. Cannibalism in fish larvae: What have we learned. In Larval Fish Aquaculture; Nova Science Publishers: New York, NY, USA, 2013; pp. 167–199. [Google Scholar]
- Cuff, W.R. Behavioral aspects of cannibalism in larval walleye, Stizostedion vitreum. Can. J. Zool. 1980, 58, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J. Estado del arte de la biología y cultivo de pejelagarto (Atractosteus tropicus). Agroproductividad 2015, 8, 44–51. [Google Scholar]
- Nelson, J.S. Fishes of the World; John Wiley Sons: Edmonton, AB, Canada, 2006. [Google Scholar]
- Aguilera, C.; Mendoza, R.; Iracheta, I.; Marquez, G. Digestive enzymatic activity on Tropical gar (Atractosteus tropicus) larvae fed different diets. Fish. Physiol. Biochem. 2012, 38, 679–691. [Google Scholar] [CrossRef]
- Márquez-Couturier, G. Biología y tecnología para el cultivo del pejelagarto Atractosteus tropicus en el sureste de México. Inst. Nac. Pesca 2000, 8, 265–267. [Google Scholar]
- Sepúlveda-Quiroz, C.A.; Alvarez-Villagomez, C.S.; Mendoza-Porras, O.; Peña-Marín, E.S.; Maytorena-Verdugo, C.S.; Pérez-Jiménez, G.M.; Jesus-Contreras, R.; Álvarez-González, C.A.; Martínez-García, R. Attack behavior leading cannibalism in tropical gar (Atractosteus tropicus) larvae under different tank colors and shelter type. Aquaculture 2023, 563, 738991. [Google Scholar] [CrossRef]
- Palma-Cancino, D.J.; Martinez-Garcia, R.; Alvarez-Gonzalez, C.A.; Camarillo-Coop, S.; Peña-Marin, E.S. Esquemas de alimentación para larvicultura de pejelagarto (Atractosteus tropicus Gill): Crecimiento, supervivencia y canibalismo. Ecosist. Recur. Agropec. 2019, 6, 273. [Google Scholar] [CrossRef]
- Márquez-Couturier, G.; Vázquez-Navarrete, C.; Contreras-Sánchez, W.M.; Álvarez-González, C.A. Acuicultura Tropical Sustentable: Una Estrategia Para la Producción y Conservación del Pejelagarto (Atractosteus tropicus) en Tabasco, México; Universidad Juárez Autónoma de Tabasco: Tabasco, México, 2013; pp. 1–223. [Google Scholar]
- Duck, K.; Pajdak, J.; Terech-Majewska, E.; Szarek, J. Intracohort cannibalism and methods for its mitigation in cultured freshwater fish. Rev. Fish Biol. Fish. 2017, 27, 193–208. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 2014, 17, 1–30. [Google Scholar] [CrossRef]
- Xi, D.; Zhang, X.; Lü, H.; Zhang, Z. Cannibalism in juvenile black rockfish, Sebastes schlegelii (Hilgendorf, 1880) Reared under controlled conditions. Aquaculture 2017, 479, 682–689. [Google Scholar] [CrossRef]
- Qin, J.G.; Mittiga, L.; Ottolenghi, F. Cannibalism Reduction in Juvenile Barramundi Lates calcarifer by Providing Refuges and Low Light. J. World Aquac. Soc. 2004, 35, 113–118. [Google Scholar] [CrossRef]
- Beay, L.K.; Saija, M. A Stage-Structure Rosenzweig-MacArthur Model with Effect of Prey Refuge. Jambura J. Biomath. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Kar, T.K. Stability analysis of a prey–predator model incorporating a prey refuge. Commun. Nonlinear Sci. Numer. Simul. 2005, 10, 681–691. [Google Scholar] [CrossRef]
- Nelson, J.S. The Dynamics of Arthropod Predator-Prey Systems; Princeton University Press: Princenton, NJ, USA, 1978. [Google Scholar]
- Nilsson, K.A.; Persson, L. Refuge availability and within-species differences in cannibalism determine population variability and dynamics. Ecosphere 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Hecht, T.; Appelbaum, S. Observations on intraspecific aggression and coeval sibling cannibalism by larval and juvenile Claias gariepinus (Clariidae: Pisces) under controlled conditions. J. Zool. 1998, 214, 21–44. [Google Scholar] [CrossRef]
- Kuznetsov, Y.A. Elements of Applied Bifurcation Theory; Springer: New York, NY, USA, 2004. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).