Abstract
When tunable diode laser absorption spectroscopy is used to measure the concentration of gas, the second harmonic signal of demodulation is changed due to the influence of temperature change, and the error in concentration measurement is great. In order to solve the problem of large errors in atmospheric quality monitoring equipment due to the change in gas temperature, this paper, based on the tunable semiconductor laser absorption spectroscopy (TDLAS) theory, measured methane gas with 1000 ppm standard gas as the target and selected the central absorption wavelength of 1650 nm. The influence of temperature change on gas injection and the laser absorption spectrometer is studied. A temperature compensation algorithm based on an empirical formula is designed. Firstly, by analyzing the variable temperature test data of the detection module, it is proposed to divide the influence factors of temperature into two parts and study the influence of injection gas temperature and detector temperature, respectively. Secondly, the temperature compensation is carried out by polynomial fitting the concentration inversion results. Finally, according to the compensation effect, a scheme was proposed to compensate the measured gas by applying a constant temperature treatment to the detector at 313 K. After compensation, the average error of the system measurement is reduced from 8.4% to 1.08% when the gas temperature changes from 233 K to 343 K, which effectively reduces the deviation of the measured value caused by the abrupt temperature change. It further improves the accuracy and reliability of measuring gas concentration when gas inspection equipment is working outdoors and has strong practicability.
Keywords:
tunable diode laser absorption spectroscopy (TDLAS); methane concentration; second harmonic; temperature compensation MSC:
78A55
1. Introduction
Since the Industrial Revolution, global warming has been remarkable, seriously threatening human survival and social development. Global warming will cause sea levels to rise, increase the frequency of natural disasters such as floods, and affect the balance of marine ecosystems. Exacerbating natural disasters such as droughts, high temperatures, and storms, which threaten agriculture, water resources, and energy and destabilize the global economy. It even leads to species extinction and ecosystem degradation, causing sustained and serious damage to the ecological environment and bringing many risks to human society. Methane () has 22 times more effect on the greenhouse effect than carbon dioxide [1] and is the second global warming greenhouse gas in the earth’s atmosphere after carbon dioxide [2]. In addition, as important precursors of ozone formation, they can be synthesized by photochemical reactions under solar radiation [3,4], thus indirectly causing air quality problems. Accurate measurement of methane content in the air of different ground environments is very necessary to analyze the source of atmospheric methane and can provide a very important basis for the study of atmospheric chemistry [5].
At present, the principle of greenhouse gas navigation monitoring vehicles is mostly based on CRDS technology; the accuracy can reach the ppb (one billion) level, but the cost is very expensive and the technical threshold is high, hindering the popularity of greenhouse gas navigation vehicles. Although Tunable Diode Laser Absorption Spectroscopy (TDLAS) has lower precision than CRDS, it can reach ppm (PPM), fully meeting the need for gas monitoring. However, due to the great influence of temperature on loading and measurement, laboratory performance cannot be maintained. Moreover, according to market research, there is no greenhouse gas navigation vehicle based on TDLAS technology available at present. Therefore, it is very important for the popularization of greenhouse gas transport vehicles to improve the anti-temperature interference ability of TDLAS technology through a temperature compensation algorithm.
In order to study the effects of temperature changes on TDLAS gas measurements, many scholars utilize spectral parameters from the High-Resolution Transmission Molecular Absorption Database (HITRAN) [6]. For example, the intensity of the spectral line, the absorption coefficient, and the absorption linear parameters have been studied. In order to solve the effect of temperature on actual measurement, there have been some corresponding solutions. Cheng Siyang and Gao Minguang [7] et al. used the nonlinear least squares algorithm based on a numerical calculation to invert and correct the gas concentration at high temperatures, and the accuracy of the measurement results was greatly improved compared with that before the correction. Zhang Zhirong [8] from the Anhui Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, proposed a second harmonic and first harmonic elimination method to eliminate the influence of light intensity on detection results. The influence of temperature fluctuation on the harmonic signal was corrected according to the empirical formula obtained by polynomial fitting and the theoretical correction formula obtained by HITRAN database line fitting after eliminating light intensity. A DFB laser with a central absorption wavelength of 760.77 nm was used to detect 21% oxygen in the temperature range of 300–900 K. The results show that both correction methods can effectively reduce the influence of temperature change. He Ying [9] from the University of Science and Technology of China tested the mixture at 500 K, 600 K, and 700 K, respectively, and corrected it by using the relative relationship between absorption line strength and temperature. The experiment verified that the relative error between the ammonia concentration and the actual value after inversion correction was less than 4%. Shu Xiawen and Zhang Yujun [10] of the Anhui Institute of Optics and Mechanical Engineering, Chinese Academy of Sciences, used a DFB laser with a central wavelength of 1.7 μm to measure 0.0146% gas at 295–500 K. An empirical formula was obtained according to the fitting of concentration data and temperature at different temperatures, and then the measurement results of gas concentration at different temperatures were corrected. The average error of the gas test under low concentrations is 9%, and the rationality of the empirical formula is verified. In addition, Zhu Yu and Wei Zhen [11] et al. from Anhui Environmental Monitoring Station used the HITRAN database to quantitatively analyze the Fourier transform infrared spectrum of methane at 300–600 K temperature and obtained more accurate synthetic spectral data by using the sectional correction of the relationship between line intensity and temperature to solve the problem of large measurement errors caused by temperature. Zhu [12] et al. chose a distributed feedback diode laser of 1579 nm as the laser source for measurement and measured 10–20% of the gas at a temperature of 298–338 K. Two methods based on direct absorption (DA) and wavelength modulation (WMS) were proposed for measurement, and temperature compensation was carried out by taking advantage of the influence of temperature on spectral line intensity. The experimental results show that the relative standard deviation of DA and WMS decreases from 0.84% and 0.35% to 0.42% and 0.31%, respectively, after temperature compensation, which verifies the rationality of temperature compensation. Qi [13] et al. calculated the absorption spectrum data by using the HITRAN spectral database and gave the temperature correction curve within the range of atmospheric ambient temperature. Liu et al. [14] used nonlinear and linear regularization methods to invert the non-uniform temperature and concentration distribution in the band of 1300–1350 nm, respectively, and used a regularization algorithm to reduce the noise caused by the non-uniform temperature field. Although some temperature compensation methods have been used in industrial monitoring, there is a lack of research on the actual monitoring of methane concentration measurements in the environment based on TDLAS technology.
From the discussion and analysis of the above literature, it can be seen that temperature compensation is very important for the accuracy and stability of spectral measurement results. This paper solves the problem of temperature compensation in TDLAS technology measurement from the perspective of engineering. Aiming at the problem that the concentration value measured by the detection module is obviously interfered with by temperature and the regularity is not strong, the influence factors of temperature are divided into two parts, and the temperature compensation algorithm based on the empirical formula is designed to correct the detector and the sampling gas, respectively. According to the compensation effect, the temperature compensation scheme is designed so that the detector adopts constant temperature control while the sampling gas temperature compensation reduces the interference of temperature with the detection equipment and improves the application value of the detector. The main research contents of this paper and their relationships are shown in Figure 1.
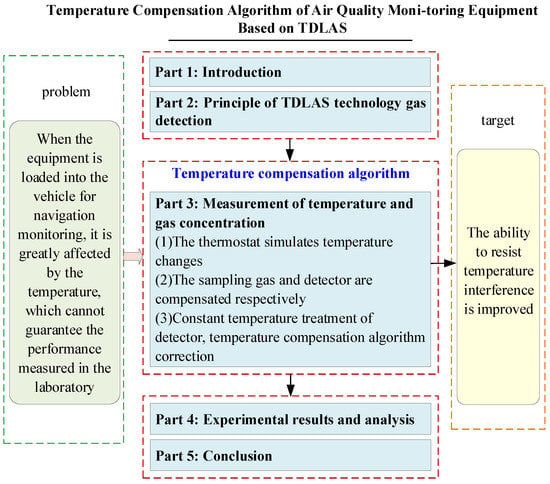
Figure 1.
The main research elements and interrelationships in this thesis.
2. Materials and Methods
The interior of a gas molecule is in a state of constant motion, such as electron motion, rotation, vibration, translation, etc. Among them, there are three main forms of motion, which are the movement of electrons, the rotation of the whole molecule, and the vibration between the nuclei. From the perspective of quantum mechanics, it is known that the energy of molecules and atoms is quantized; in other words, the energy of various motions inside gas molecules is certain and discontinuous. Therefore, the above three modes of motion correspond to three energy levels: electron, rotational, and vibrational. Because the electron energy level, rotational energy level, and vibrational energy level inside the molecule are specific, when the light source irradiates the gas molecule, the gas molecule will selectively absorb the specific wavelength of light, forming the gas absorption spectrum, and transition from the low energy level to the high energy level; this phenomenon is called the specific absorption of gas molecules. After the energy in the incident light is absorbed by the gas, the intensity of the light decreases. Therefore, the concentration of the measured gas can be inversely deduced according to the attenuation degree of the beam.
The absorption spectrum theory of gas molecules reveals the relationship between the energy absorbed by gas molecules and their internal energy level structure. The Lambert-Beer law describes the attenuation relationship between transmitted light and incident light when monochromatic light with a specific wavelength passes through the gas to be measured, as shown in Figure 2. When an incident laser passes through the measured gas, if the wavelength and frequency of the laser are consistent with a certain absorption frequency of the measured gas, the energy of the laser will be absorbed by the measured gas [15], and the incident laser will be attenuated into the outgoing laser. The attenuation of laser energy can be obtained by Lambert–Beer’s law.
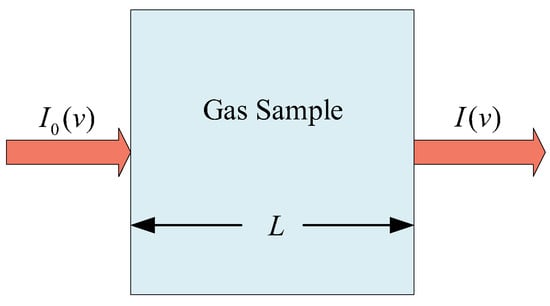
Figure 2.
Schematic diagram of the Lambert–Beer’s law.
The laser with light intensity passes through the gas absorption medium with length L to absorb the light from the gas [16], as shown in Equation (1):
among them, is the transmitted light intensity (attenuated laser intensity), namely the light intensity of the detected light [17], and the unit is milliwatt (mW); is the incident light intensity (unattenuated laser intensity), namely the light intensity before the laser passes through the gas, and the unit is milliwatt (mW). represents molar absorption coefficient, unit: square centimeter per mole (cm2 mol−1); represents gas pressure, unit: ; represents light length [18], unit: cm; and C represents the gas concentration to be measured. In the near-infrared band, the absorption coefficient of gas is very small. When the gas absorption optical path is short or the gas concentration is low, it generally satisfies , and Equation (1) can be rewritten as:
among them, is the spectral intensity at a certain temperature, unit: ; is expressed as a linear absorption function of the gas spectrum. Combining Equations (1) and (2) can obtain:
The system environment is under atmospheric pressure experimental conditions, which are mainly collision broadening. The linear function of collision broadening is usually regarded as a Lorentz function in mathematical form, and its function expression is as follows:
among them, is the full line width of the line function, and its calculation formula is:
among them, and P are the pressure broadening coefficient and pressure of the gas to be measured, n is the temperature coefficient, and and represent the pressure broadening coefficient and pressure of the colliding gas, respectively.
In order to improve the accuracy and sensitivity of the detection, this paper adopts the wavelength modulation spectroscopy (WMS) technique [19,20]. In this paper, a high-frequency sinusoidal current signal is superimposed on a low-frequency sawtooth current signal to drive a semiconductor laser, so that the laser wavelength is scanned near the centre of the gas absorption spectrum, and the light source frequency and output light intensity will also be modulated accordingly, at which time the laser light source power is:
among them, is the laser center frequency, is the modulation amplitude, f is the frequency, and t is the time. If the initial light intensity of the semiconductor laser is , the output light intensity after modulation is I, which is the laser light intensity signal input into the gas chamber to be measured, then by the Equation (6) relationship:
among them, the output optical frequency of the semiconductor laser can be modulated by changing the temperature or injecting current [21]. The temperature modulation range is large and the precision is low; the current modulation range is small and the precision is high. Because the absorption line of gas is very narrow, the absorption coefficient in the band range varies greatly, and a small change in frequency will lead to a huge difference in the absorption coefficient. Therefore, this paper adopts the current modulation method.
Ideally, the output optical power of a semiconductor laser has nothing to do with frequency v, and it can be assumed that modulation has no effect on , that is, . When the injection current is modulated by a cosine signal with frequency ω, the output optical frequency of the semiconductor laser can be expressed as:
among them, is the central frequency of the modulation, and is the frequency modulation amplitude.
After gas absorption, the relationship between the output optical power and the input optical power conforms to the Lambert–Beer’s law.
among them, is the optical power output by the semiconductor laser; is the optical power received by the photodetector; is the light collection efficiency, which is the difference between the optical power received by the photodetector and the output of the semiconductor laser when there is no gas absorption; α(v) is the light absorption coefficient of the unit concentration and unit length medium at the frequency v; C is the volume concentration of the gas to be measured; and L is the length of the gas absorption optical path.
In the near infrared band, the absorption coefficient of the gas is very small. When the gas absorption optical path is short or the gas concentration is low, it generally satisfies α(v)CL<<1. Equation (10) can be rewritten as:
combine Equation (9) and expanding α(v) into a Fourier series, it can be expressed as:
among them, is the nth Fourier coefficient of the absorption coefficient. When the modulation amplitude is sufficiently small, there is:
as the amplitude of the second harmonic signal is the peak value of its waveform and is linearly related to the concentration value of the gas to be measured. Under the same conditions, the second harmonic achieves a higher resolution compared to the other harmonics, and since the harmonic amplitude decreases with the number of harmonics, the higher amplitude of the second harmonic is beneficial for improving gas detection. Therefore, when choosing a wavelength modulation, the second harmonic is usually combined to calculate the concentration information of the gas. According to the Lambert–Beer’s law, this gives:
in order to eliminate the influence of light intensity, this paper uses the double frequency of the modulation signal for demodulation, and the second harmonic signal is proportional to the measured gas concentration, namely:
3. Experimental Apparatus and Process
3.1. Construction of Methane Concentration Measurement System
The gas concentration detection system is shown in Figure 3. The system adopts pumping to extract the gas to be measured. First, the microprocessor outputs the tuning signal, drives the laser to emit laser, and enters the gas pool after collimation by the collimator to scan the absorption peak of the gas to be measured. Photoelectric detection completes the detection of the outgoing laser from the gas chamber so that the optical signal containing the concentration information of the gas to be measured is converted into an electrical signal. After pre-amplification, the required frequency [22] is selectively extracted by the phase-locked amplifier, and the data is processed on the microprocessor to calculate the concentration value of the gas to be measured.
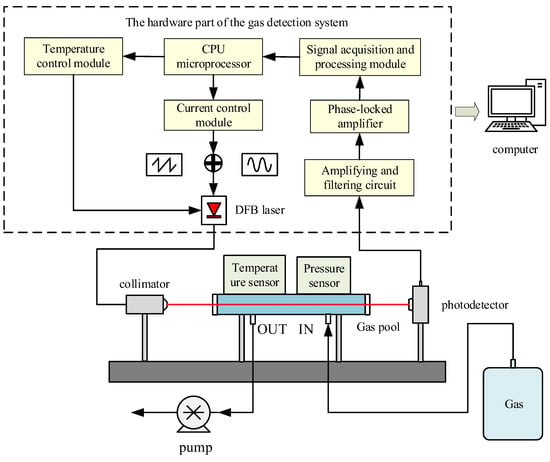
Figure 3.
Methane concentration detection systems.
The overall block diagram of the system scheme based on TDLAS technology is shown in Figure 4.
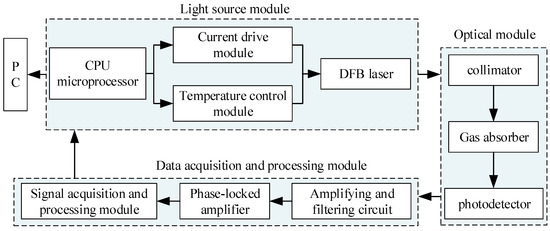
Figure 4.
Methane concentration detection systems.
In order to study the influence of temperature change on concentration detection results, an incubator was used to simulate ambient temperature change. The experiment adopts the small, vertical, constant temperature and humidity test chamber produced by Aerospace Zhida. The equipment can operate accurately in high and low temperature, and humid and hot environments. PID (proportional-integral-derivative control) automatically calculates and outputs the output of the heater according to the set temperature points to realize dynamic balance and finally achieve the purpose of simulating the change in environmental temperature.
According to the system block diagram, the test platform is built, and the experimental environment is shown in Figure 5a. The 1000 ppm air bag is connected to the laser absorption spectrum detector and put into the thermostat together. The air pump is turned on, and the power supply is switched on. The laser emits modulated light through the collimator into the gas pool module. After amplification, the signal enters the lock-in amplifier to extract the second harmonic information and send it to the MCU microprocessor for data analysis. After concentration inversion, the detected value can be converted into the real concentration. The concentration data of the measured gas at different temperatures can be displayed by the computer.
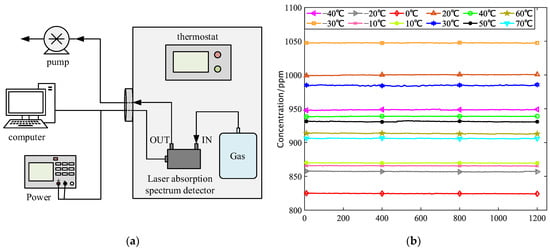
Figure 5.
(a) Thermostatic control system; (b) 1000 ppm methane gas 10 min concentration collection data.
After the construction is completed, the variable temperature test of 1000 ppm standard gas is carried out. When the vehicle gas detector works, the ambient temperature is generally between 233 and 343 K. Therefore, in the whole experiment process, the experimental temperature range is selected as 233 K to 343 K, and every 10 K is a test condition. By adjusting the thermostat to 233 K, 243 K, 253 K, 263 K, 273 K, 283 K, 293 K, 303 K, 313 K, 323 K, 333 K, and 343 K, twelve temperature points is obtained. Change the temperature of the thermostat from low to high. After preheating each temperature point for 10 min, open the air pump to start the test. After the system is stable, test data for each temperature point is taken continuously for 10 min, as shown in Figure 5b. The measured concentration information for each test condition was processed on average, and the recorded results were drawn into a table, as shown in Table 1.

Table 1.
Concentration detection data and analysis at different temperatures.
The phase is shown in Table 1. When the temperature rises from 233 K to 343 K, the concentration value at 293 K is closest to the true value with the smallest relative error. Plot the gas concentration data in Table 1 as Figure 6.
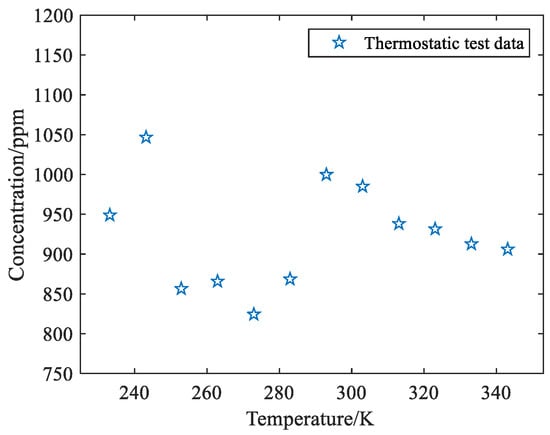
Figure 6.
Scatter plot of overall system temperature change test results.
As can be seen from the scatter plot in Figure 7 drawn by the variable temperature test results, with the increase in temperature in the thermostatic chamber, there is a great difference between the real and measured values of methane gas concentration at 1000 ppm in the air bag. The concentration measured by the system at 273 K is only 824 ppm, while the concentration measured by the system at 243 K is as high as 1047 ppm. Additionally, the correlation coefficient is R = −0.0359, and the correlation between concentration data and temperature change is low. This is because temperature has a complex effect on the system, the gas to be measured and the detector are affected by temperature. In summary, this paper puts forward the method of “splitting in two” to study the influence of temperature on the measured gas and detector, respectively.
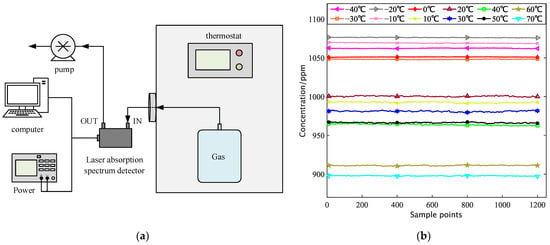
Figure 7.
(a) Schematic diagram of the feed gas temperature change test; (b) 1000 ppm Methane Variable Temperature Test 10 min concentration collection data.
3.2. The Influence of Temperature Change of Gas to Be Measured on Measurement
The 1000 ppm methane gas is sealed in the thermostat to control the temperature of the test chamber. The methane air bag is led out of the temperature cycle test chamber through the hose, and the heat loss is reduced by wrapping the thermal insulation cotton, which is connected to the system at room temperature, as shown in Figure 7a.
By adjusting the internal temperature of the incubator to twelve temperature points of 233 K, 243 K, 253 K, 263 K, 273 K, 283 K, 293 K, 303 K, 313 K, 323 K, 333 K, and 343 K, each temperature point preheating for 10 min, open the air pump to start the test. After the system is stable, the gas concentration values corresponding to different temperatures are collected from the host, and the test data for each temperature point is continuously taken for 10 min, as shown in Figure 7b. The measured concentration information for each test condition was processed on average, and the recorded results were drawn into a table, as shown in Table 2.

Table 2.
Table of results of the average treated feed gas temperature change test.
According to Table 2, when the temperature rises from 233 K to 343 K, the maximum methane gas measured at 1000 ppm is 1069 ppm at 263 K, with a relative error of 6.9%. At the lowest level of 343 K, the methane concentration of 1000 ppm was 897 ppm after inversion, with a relative error of 10.3%. The concentration value at 293 K is closest to the true value, and the relative error is minimal. The test data in Table 2 are graphically analyzed, and Figure 8a is obtained.
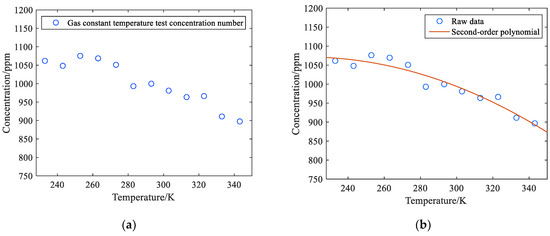
Figure 8.
(a) Scatter plot of feed gas temperature test results; (b) Fitting curve of the results of the 1000 ppm methane temperature change experiment.
As the ambient temperature rises, the methane concentration measured by the monitoring system shows a decreasing trend, and the correlation coefficient R = −0.943 shows an obvious linear relationship. This is not caused by the decrease in concentration but precisely reflects the decrease in gas absorption coefficient when the temperature rises, which is consistent with the theoretical analysis. According to the test results in Table 2, quadratic polynomial fitting was performed for the relationship between methane gas concentration and temperature, and the equation obtained is shown in Equation (19).
The methane concentration measured at each temperature is substituted into the quadratic multinomial fitting equation, and A = −0.0112, B = 4.8319, and C = 548.0504 are obtained. The curve for fitting normalized concentration with temperature change is shown in Figure 8b. In the figure, the quadratic multinomial fitting equation has a good fitting relationship with the change in methane concentration value in the temperature range of 233–343 K. Assume that the initial measured temperature is , the temperature at this concentration moment is substituted into Equation (19) as a constant, and then all concentrations will be calibrated to obtain the corrected concentration through this formula.
The concentration before correction is and is the corrected methane concentration, and the empirical formula for methane temperature correction is shown in Equation (20).
The methane concentration data measured at different temperatures were put into Equation (20) for correction. Compared with the experimental results of methane gas with a 1000 ppm concentration in Figure 8a, it can be seen that the influence of external temperature on test results can be effectively reduced after adding temperature correction. The concentration results after compensation are plotted in Figure 9.
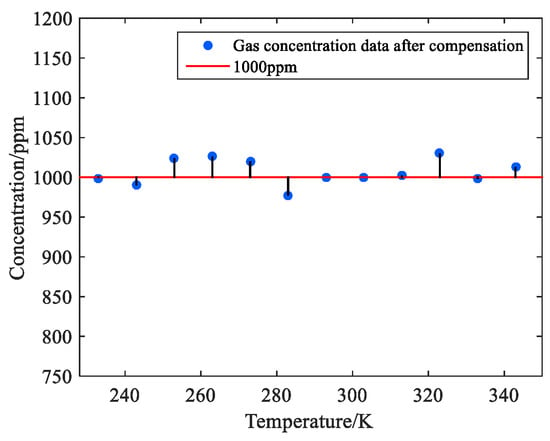
Figure 9.
Scatter plot after compensation for the 1000 ppm methane variable temperature test.
In Figure 9, the measured methane concentration value is compensated, and the value is stable around 970–1030 ppm. The relative errors before and after compensation of the system are plotted in Table 3.

Table 3.
System error.
As shown in Table 3, the temperature rise test of 233 K to 343 K for 1000 ppm of methane gas is carried out. If not corrected, the measured value of the system will gradually decrease with the increase in temperature. As the absorption spectral line of gas changes significantly during the temperature rise process, the amplitude of an effective spectral signal in the measured signal will also decrease [10]. If the methane gas of 1000 ppm to be measured is not corrected, the inversion result exceeds 1070 ppm at 253 K. When the temperature is 343 K, the inversion result will be less than 890 ppm, and the measurement error is too large. After temperature correction, the average measurement error of the system is reduced from 5% to 1.25%, and the stability of the system is improved.
3.3. The Effect of Ambient Temperature Change on Measurement
Next, the influence of temperature on the laser absorption spectrometer is studied. Seal the gas detector in the thermal cycle chamber and control the temperature of the temperature cycle test chamber. The methane gas detector in the thermostatic chamber leads the temperature cycle test chamber to the 1000 ppm methane gas bag at room temperature through the soft gas pipe, as shown in Figure 10a.
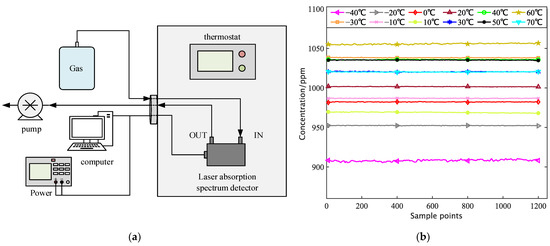
Figure 10.
(a) Laser Absorption Spectroscopy Detector Variable Temperature Test Schematic; (b) Laser Absorption Spectroscopy Detector Variable Temperature Test 10 min concentration acquisition data.
By adjusting the internal temperature of the incubator to twelve temperature points of 233 K, 243 K, 253 K, 263 K, 273 K, 283 K, 293 K, 303 K, 313 K, 323 K, 333 K, and 343 K, each temperature point preheating for 10 min, open the air pump to start the test. After the system is stable, the gas concentration values corresponding to different temperatures are collected from the host, and the test data for each temperature point is continuously taken for 10 min, as shown in Figure 10b. The measured concentration information for each test condition was processed on average, and the recorded results were plotted in Table 4.

Table 4.
Table of results of the average treated feed gas temperature change test.
As shown in Table 4, when the temperature rose from 233 K to 343 K, the maximum methane gas measured at 1000 ppm was 1036 ppm at 313 K, with a relative error of 3.6%. At the lowest temperature of 233 K, a methane concentration of 1000 ppm was only 908 ppm after inversion, and the relative error reached 9.2%. The concentration value at 293 K is closest to the true value, and the relative error is minimal. Plot the gas concentration data in Table 4 as Figure 11a.
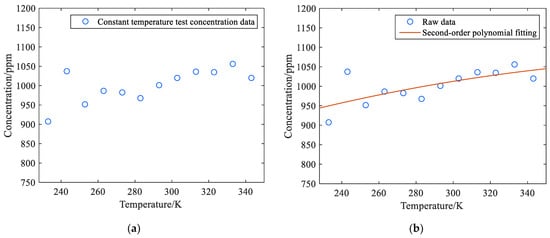
Figure 11.
(a) Scatter plot of tester variable temperature test results; (b) Fitted curve of the results of the tester’s variable temperature experiment.
As shown in Figure 11a, as the temperature of the laser absorption spectrum detector increases, the methane concentration obtained by the system test shows an overall upward trend, and the correlation coefficient R = 0.1893 is significantly lower than the correlation coefficient in the gas temperature experiment to be measured. The correlation degree is not high because the temperature change has a great impact on the laser, photoelectric sensor, and other components inside the detector. According to the test results in Table 4, quadratic polynomial fitting was performed for the relationship between methane gas concentration and temperature, and the equation obtained is shown in Equation (21).
The methane concentration measured at each temperature is substituted into the quadratic multinomial fitting equation, and D = −0.0025, E = 2.2639, and F = 557.1625 are obtained. The curve for fitting normalized concentration with temperature change is shown in Figure 11b. In the figure, the quadratic multinomial fitting equation has a good fitting relationship with the change in methane concentration value in the temperature range of 233–343 K. Assume that the initial measured temperature is , the temperature at this concentration moment is substituted into Equation (19) as a constant, and then all concentrations will be calibrated to obtain the corrected concentration through this formula.
The concentration before correction is and is the corrected methane concentration, then the empirical formula of methane temperature correction is shown in Equation (22).
The methane concentration data measured at different temperatures were substituted into Equation (22) for correction, and the experimental results with temperature correction were drawn as Figure 12. By comparing the experimental results of methane gas with a 1000 ppm concentration in Figure 11a, it can be seen that the influence of external temperature on test results can be effectively reduced after adding temperature correction.
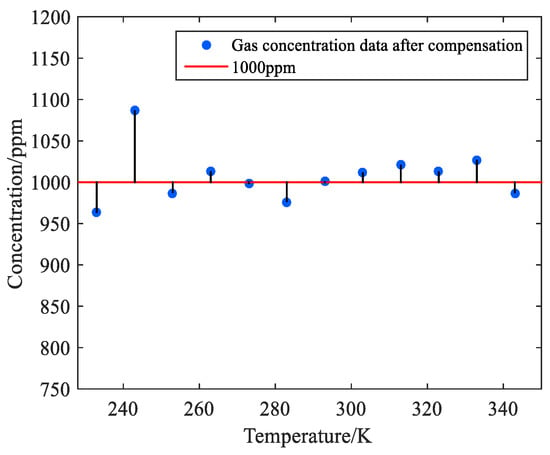
Figure 12.
Scatter plot after compensation for the 1000 ppm methane variable temperature test.
In Figure 12, after compensation, the measured methane concentration is stable around 963–1080 ppm. The relative errors before and after compensation of the system are plotted in Table 5.

Table 5.
System error.
The experimental data show that the average error of the temperature compensation system decreases from 3.41% to 2.2%, and the relative error of the laser absorption spectrum detector at 243 K is still as high as 8.7% after the temperature compensation. Although the stability of the system has improved, the average error still reaches 2.2%. Compared with the temperature compensation result of the gas to be measured, 1.25%, the compensation effect of the algorithm is not so good, which is far from the expectation. Therefore, this paper decided to add a hardware thermostat to the laser infrared absorption spectrum detector to maintain a constant temperature, so as to reduce the impact of environmental temperature changes on the methane concentration monitoring system.
3.4. Correction of Monitoring System under Constant Temperature State of Detector
The temperature of the constant-temperature device equipped with a laser infrared absorption spectrum detector is set to 40 °C. On the one hand, the influence of temperature change on the detector can be avoided. On the other hand, the constant temperature of 40 °C can avoid the condensation of water vapor in the air. The 1000 ppm air bag was placed in the thermostat and connected to the laser infrared absorption spectrum detector under constant temperature, as shown in Figure 13a.
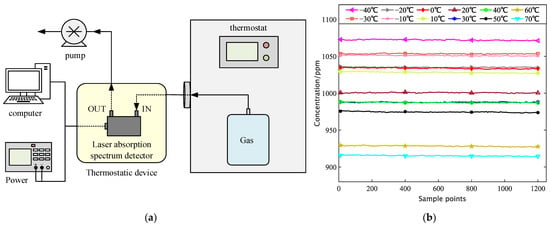
Figure 13.
(a) Schematic diagram of the inlet gas variable temperature detector for constant temperature testing; (b) Inlet gas variable temperature detector for a 10 min concentration test.
Adjust the internal temperature of the incubator to twelve temperature points: 233 K, 243 K, 253 K, 263 K, 273 K, 283 K, 293 K, 303 K, 313 K, 323 K, 333 K, and 343 K. After preheating for 10 min at each temperature point, open the air pump to start the test. After the system is stable, the gas concentration values corresponding to different temperatures are collected from the host, and the test data for each temperature point is continuously taken for 10 min, as shown in Figure 13b. The measured concentration information for each test condition was processed on average, and the recorded results were plotted in Table 6.

Table 6.
Table of results of the average treated feed gas temperature change test.
According to Table 6, when the temperature rose from 233 K to 343 K, the maximum methane gas measured at 1000 ppm was 1071 ppm at 233 K, with a relative error of 7.1%. At the lowest level of 343 K, the methane concentration of 1000 ppm was 814 ppm after inversion, with a relative error of 8.6 percent. The concentration value at 293 K is closest to the true value, and the relative error is minimal. Table 4, Table 5 and Table 6 test data is plotted in Figure 14a.
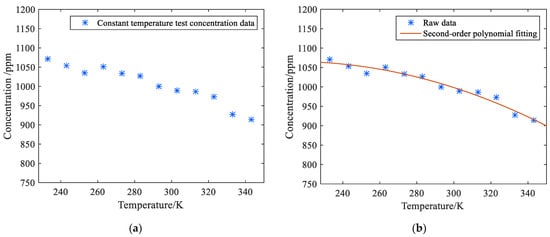
Figure 14.
(a) Gas Variable Temperature Detector Scatter plot of thermostatic test results; (b) Gas Concentration Fitted Curve Graph.
As the ambient temperature rises, the methane concentration measured by the monitoring system decreases gradually, and the correlation coefficient R = −0.943 shows an obvious linear relationship. This is not caused by the decrease in concentration but precisely reflects the change when the temperature rises, which is consistent with the theoretical analysis. According to the test results in Table 6, quadratic polynomial fitting was performed for the relationship between methane gas concentration and temperature, and the equation obtained is shown in Equation (23).
The methane concentration measured at each temperature is substituted into the quadratic multinomial fitting equation, and G = −0.0088, H = 3.7162, and I = 671.0139 are obtained. The curve for fitting normalized concentration with temperature change is shown in Figure 14b. In the figure, the quadratic multinomial fitting equation has a good fitting relationship with the change in methane concentration value in the temperature range of 233–343 K. Assume that the initial measured temperature is , the temperature at this concentration moment is substituted into Equation (19) as a constant, and then all concentrations will be calibrated to obtain the corrected concentration through this formula.
The concentration before correction is and is the corrected methane concentration, and the empirical formula for methane temperature correction is shown in Equation (24).
4. Experimental Results and Analysis
The methane concentration data measured at different temperatures were substituted into Equation (24) for correction, and the concentration results after compensation were drawn as Figure 15.
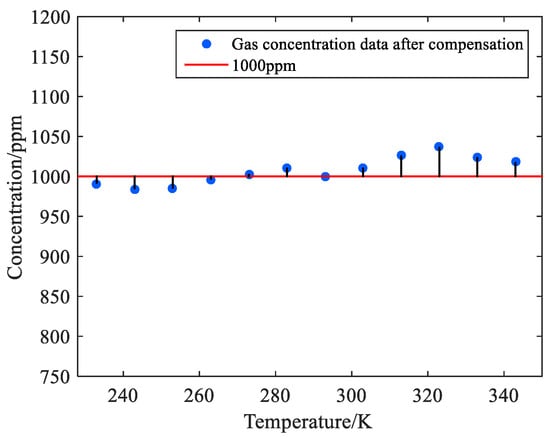
Figure 15.
Scatter plot after correction by the temperature compensation algorithm.
In Figure 15, after compensation, the measured methane concentration is stable around 998–1025 ppm, fluctuating no more than 3%. The relative errors before and after compensation of the system are plotted in Table 7.

Table 7.
System error.
As shown in Table 7, when 1000 ppm methane gas is heated with the detector, if it is not corrected, the real value of methane gas concentration is significantly different from the measured value as the temperature rises. At 273 K, the concentration measured by the system is only 824 ppm, with a relative error of 17.57%. At 243 K, the concentration measured by the system is 1047 ppm, and the relative error is 4.75%. After the infrared detector was treated at 40 °C and the temperature was corrected, the measured value of methane was stable at about 1000 ppm. Compared with before compensation, the average error of system measurement decreased from 8.42% to 1.08%, and the compensation effect was obvious. The methane concentration before and after system compensation is drawn in Figure 16.
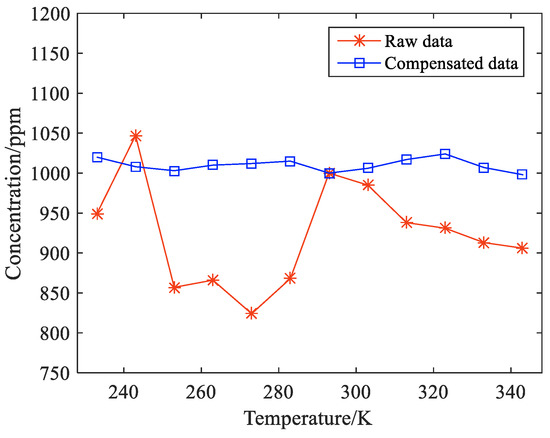
Figure 16.
Comparison of the results before and after compensation.
As shown in Figure 16, compared with the original data obtained from the temperature rise test of methane gas and detector at 233 K to 343 K, the compensation effect of the algorithm is obvious. The measurement value of methane is obviously disturbed by temperature before correction, and the anti-interference ability of the system is poor. After the correction, the measured value of methane is stable at about 1000 ppm, and the fluctuation is less than 3%, which improves the stability of the system. It is proven that the experimental scheme can eliminate the influence of temperature fluctuation and realize the detection of trace methane gas concentrations in a wide temperature range.
5. Discussion
In this paper, aiming at the application environment of the vehicle detector, the temperature test platform is built, and the temperature is simulated by the thermostat to study the influence of temperature on the monitoring system. Secondly, through the analysis of experimental test data, it is proposed to divide the influence factors of temperature into two parts, respectively, for the sampling gas and the detector temperature compensation. According to the compensation effect, a compensation scheme was proposed, that is, the detector was treated at 40 °C to study the influence of gas temperature on the measured gas. Finally, a temperature compensation algorithm based on an empirical formula is designed to correct the system. The experimental results show that the average error of the system measurement is reduced from 8.4% to 1.08%, and the compensation effect is obvious, which effectively reduces the impact of the abrupt change of ambient temperature on the measurement results.
The temperature compensation algorithm designed in this paper has universality. Due to the short time, only methane gas is actually tested, and the detection of other greenhouse gases can be realized by replacing lasers and photodetectors with different wavelengths. In the future, carbon dioxide and nitrous oxide will be tested and verified.
Author Contributions
Conceptualization, Y.W.; methodology, Y.W.; validation, Y.W.; investigation, Y.W.; resources, X.W.; data curation, Y.W. and X.W.; writing—original draft preparation, Y.W.; writing—review and editing, Y.W.; visualization, Y.W.; supervision, X.W.; project administration, Y.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that has been used is confidential.
Acknowledgments
On the occasion of the completion of this thesis, I would like to sincerely thank my supervisor, Wang, X.L. From the opening of the thesis to the finalization of the thesis, in the past nearly a year, he has carefully guided me and put forward a lot of valuable suggestions, which made me more confident and gave me great encouragement in the writing process of the thesis, so that my thesis work can be successfully completed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- D’Amato, F.; Mazzinghi, P. Methane analyzer based on TDL’s for measurements in the lower stratosphere. Appl. Phys. B 2002, 75, 195–202. [Google Scholar] [CrossRef]
- Iwaszenko, S.; Kalisz, P.; Sota, M. Detection of Natural Gas Leakages Using a Laser-Based Methane Sensor and UAV. Remote Sens. 2021, 13, 510. [Google Scholar] [CrossRef]
- Liu, C.R.; Lian, X.B.; Huang, P.J. Research Review on the spatio-temporal Distribution of Ozone Pollution and Its Causes in China. J. Arid. Meteorol. 2020, 38, 355–361. [Google Scholar]
- Staniaszek, Z.; Griffiths, P.; Folberth, G. The role of future anthropogenic methane emissions in air quality and climate. Npj Clim. Atmos. Sci. 2022, 5, 21. [Google Scholar] [CrossRef]
- Kan, R.F.; Liu, W.Q.; Zhang, Y.J. Tunable diode laser absorption spectrometer monitors the ambient Methane with high sensitivity. Chin. J. Lasers 2005, 32, 1217–1220. [Google Scholar]
- Phillips, W.J.; Plemmons, D.H.; Plemmons Galyen, N.A. HITRAN/HITEMP Spectral Databases and Uncertainty Propagation by Means of Monte Carlo Simulation with Application to Tunable Diode Laser Absorption Diagnostics; Defense Technical Information Center: Fort Belvoir, VA, USA, 2011.
- Cheng, S.Y.; Gao, M.G.; Xu, L. Temperature correction method in spectral analysis of high temperature gas concentration. Infrared Laser Eng. 2013, 42, 413–417. [Google Scholar]
- Zhang, Z.R.; Wu, B.; Xia, H.; Pang, T.; Wang, G.X.; Sun, P.S.; Wang, Y. Study on the temperature modified method for monitoring gas concentrations with tunable diode laser absorption spectroscopy. Acta Phys. Sin. 2013, 62, 234204. [Google Scholar] [CrossRef]
- He, Y. Study on On-Line Detection Technology and Application of Main Anthropogenic Ammonia Emissions Based on Laser Absorption Spectroscopy. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2017. [Google Scholar]
- Shu, X.W.; Zhang, Y.J.; Kan, R.F. An Investigation of Temperature Compensation of HCL Gas Online Monitoring Based on TDLAS Method. Spectrosc. Spectr. Anal. 2010, 30, 1352–1356. [Google Scholar]
- Zhu, Y.; Wei, Z.; Zhang, J.S. Temperature Correction Method of Absorption Spectrum with FTIR. J. Atmos. Environ. Opt. 2016, 11, 191–196. [Google Scholar]
- Zhu, X.; Yao, S.; Ren, W. TDLAS Monitoring of Carbon Dioxide with Temperature Compensation in Power Plant Exhausts. Appl. Sci. 2019, 9, 442. [Google Scholar] [CrossRef]
- Qi, R.B.; He, S.K.; Li, X.T. Simulation of TDLAS direct absorption based on HITRAN database. Spectrosc. Spectr. Analy 2015, 35, 172–177. [Google Scholar]
- Liu, C.; Xu, L.; Gao, Z. Measurement of nonuniform temperature and concentration distributions by combining line-of-sight tunable diode laser absorption spectroscopy with regularization methods. Appl. Opt. 2013, 52, 4827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.K.; Zheng, X.Y.; Chen, S.Z. Research on laser water vapor concentration detection technology in the air-sea flux observation. IOP Conference Series. Mater. Sci. Eng. 2020, 711, 012079. [Google Scholar]
- Yang, C.; Wang, Z.; Zhao, L. Development of an Online Detection Setup for Dissolved Gas in Transformer Insulating Oil. Appl. Sci. 2021, 11, 12149. [Google Scholar] [CrossRef]
- Perlitz, J.; Bro, H.; Will, S. Measurement of Water Mole Fraction from Acoustically Levitated Pure Water and Protein Water Solution Droplets via Tunable Diode Laser Absorption Spectroscopy (TDLAS) at 1.37 µm. Appl. Sci. 2021, 11, 5036. [Google Scholar] [CrossRef]
- Kan, R.F.; Liu, W.; Zhang, Y. Application of α–β–γ filtering to real-time atmosphere methane concentration measurement. Chin. Phys. 2006, 15, 1379–1383. [Google Scholar]
- Telle, J.M.; Tang, C.L. Very rapid tuning of cw dye laser. Appl. Phys. Lett. 1975, 26, 572–574. [Google Scholar] [CrossRef]
- Pavone, F.S.; Lnguscio, M. Frequency- and wavelength-modulation spectroscopies: Comparison of experimental methods using an AlGaAs diode laser. Appl. Phys. 1993, 56, 118–122. [Google Scholar] [CrossRef]
- Yan, G. Research on Embedded Remote Monitoring Technology of Urban Atmospheric Trace Methane. Ph.D. Thesis, Jilin University, Changchun, China, 2022. [Google Scholar]
- Liger, V.; Mironenko, V.; Kuritsyn, Y. Temperature Measurements by Wavelength Modulation Diode Laser Absorption Spectroscopy with Logarithmic Conversion and 1f Signal Detection. Sensors 2023, 23, 622. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).