Abstract
Bacterial keratitis is a serious ocular infection that can cause severe visual loss if treatment is not initiated at an early stage. It is most commonly caused by Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumoniae, or Serratia species. Depending on the invading organism, bacterial keratitis can progress rapidly, leading to corneal destruction and potential blindness. Common risk factors for bacterial keratitis include contact lens wear, ocular trauma, ocular surface disease, ocular surgery, lid deformity, chronic use of topical steroids, contaminated ocular medications or solutions, and systemic immunosuppression. The pathogenesis of bacterial keratitis, which depends on the bacterium-host interaction and the virulence of the invading bacterium, is complicated and not completely understood. This review highlights some of the proteomic technologies that have been used to identify virulence factors and the host response to infections of bacterial keratitis in order to understand the disease process and develop improved methods of diagnosis and treatment. Although work in this field is not abundant, proteomic technologies have provided valuable information toward our current knowledge of bacterial keratitis. More studies using global proteomic approaches are warranted because it is an important tool to identify novel targets for intervention and prevention of corneal damage caused by these virulent microorganisms.
1. Introduction
Infectious keratitis is a serious, sight-threatening ocular condition. Early diagnosis, identification of the etiologic organism, and prompt antimicrobial therapy are required for successful treatment. Infectious keratitis is characterized by corneal inflammation and can be caused by bacteria, fungi, viruses, or parasites [1] with bacteria causing the most threatening condition [2]. The incidence of corneal infections continues to rise proportionately with the increased use of contact lenses across the globe. Despite advances in clinical diagnosis, laboratory investigations, and the availability of potent antibiotics, visual morbidity remains high in underdeveloped countries. The prevalence of infectious keratitis ranges in different regions of the world from 6.3 to 710 cases per 100,000 individuals per year, with increased incidence among contact lens wearers [3].
Bacterial keratitis is caused by a variety of species including Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), Streptococcus pneumoniae (S. pneumoniae) and Serratia species with P. aeruginosa being the most commonly isolated Gram-negative organism (40%–70%) [4], followed by Serratia marcescens (S. marcescens) among contact lens wearers [5]. Possible sources of these bacteria could be environmental, the patient’s normal flora, ocular devices, contact lens care solutions/cases, or topical drug/ irrigation solutions. Typical findings associated with bacterial keratitis include pain, presence of anterior chamber reaction or hypopyon, poor vision, and corneal ulcer [6]. Bacterial keratitis rarely occurs in a normal healthy eye, due to the human cornea’s natural resistance to infection. However, contact lens wear, corneal surgery, trauma, ocular surface disease, and systemic disease, such as diabetes mellitus or immunosuppression, are predisposing factors associated with increased risk of infection [7]. Identification of virulence factors and the host response to the invading bacterium are critical to understanding the disease process in order to develop effective treatment modalities.
Virulence of the invading organism depends on its ability to penetrate and colonize the cornea, resist host defense mechanisms, and produce corneal damage [8]. Colonization of the host cells is mediated by adhesins expressed on the bacterial surface that bind to receptors on the host cell surface. Adhesins may also act as toxins [9]. Many bacteria display several adhesins on fimbriae and non-fimbriae structures. These adhesive proteins recognize carbohydrates on host cells and bind to these cells via protein-protein interactions. Tissue damage is usually mediated by exogenous proteins secreted by the bacterium or secondary effector molecules that assist in the infective process. Upregulation or downregulation of host defense mechanisms may also be involved. Some bacteria such as S. aureus, S. pneumoniae, and P. aeruginosa adhere to ulcerated corneal epithelium at relatively higher rates than other bacteria, making them the most commonly isolated organisms [10].
Although many virulence factors have been identified thus far using traditional approaches such as cloning, polymerase chain reaction (PCR), gene knockout, and antisense technology, proteomic methods such as enzyme-linked immunosorbent assay (ELISA), antibody arrays and Western blotting used in combination with these approaches have contributed enormously to our current knowledge of the pathogenesis of keratitis. This short review paper will discuss the use of the few proteomic approaches used to date for the study of bacterial keratitis, including identification of virulence factors and bacteria-host interactions for the most frequently isolated organisms. Furthermore, we will address the value of expanding these studies and the need for more global proteomic approaches to the study of bacterial keratitis.
2. Staphylococcus Aureus
S. aureus is one of the most significant pathogens in bacterial keratitis [11,12]. Its incidence varies worldwide, but its increased resistance to certain antibiotics makes it an important global healthcare issue [13,14]. Staphylococcal keratitis is characterized by destruction of the cornea via bacterial exoprotein deposition and the host inflammatory response to infection [15]. Although antibiotic therapies may succeed in reducing or eliminating the bacterial load, damage from scarring, loss of visual acuity and blindness may still result. Additionally, the emergence of multidrug-resistant S. aureus strains further complicates therapeutic strategies [16].
The mechanisms involved in the initiation of S. aureus keratitis are not yet understood. S. aureus has been shown to adhere to corneal epithelial cells via fibronectin and collagen [17,18,19]. Virulence factors produced by S. aureus in keratitis include α-toxin as the major factor, with β and γ-toxins to a lesser extent [15,20,21]. O’Callagan et al., used proteomic approaches including polyacrylamide gel electrophoresis (PAGE), Western blotting, silver staining and enzyme assays to purify the α and β-toxins and assess their ocular toxicity in New Zealand white rabbits [15]. These studies confirmed the contribution of α-toxin to ocular damage and identified the role of β-toxin in keratitis. The administration of purified α-toxin was found to directly destroy the epithelium, and mutants deficient in α-toxin caused less corneal edema than their isogenic parent strains. For the β-toxin, its administration to the eye demonstrated that it can mediate edema in the sclera and conjunctiva. This suggested that the two bacterial proteins identified, α-toxin and β-toxin, could be targeted for a new type of chemotherapy designed to limit the ocular damage caused by these toxins and reduce the major tissue damage and scarring reactions associated with Staphylococcus keratitis.
In order to understand the contribution of the host response to S. aureus infection, a keratitis mouse model was developed in both C57BL/6 and BALB/c mice using both virulent and non-virulent strains of S. aureus. Using ELISA, the authors detected significant upregulation of IL-4, IL-10, IL-6, and macrophage inflammatory peptide (MIP)-2 in the mice infected with the virulent strain [22]. The author suggested that IL-4, IL-10 and IL-6 cytokines may be protective during Gram-positive corneal infection and therefore, may be useful as adjunct therapies during treatment.
In an effort to test the efficacy of antimicrobials on a co-culture of the bacterium S. aureus and the fungus Fusarium solani, a recent study used 2D gel electrophoresis and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) to study the proteomic profile of the co-culture with and without antimicrobials. In the presence of antimicrobials, S. aureus and F. solani were found to interact and the co-culture showed differential protein expression when grown without antimicrobial agents [23]. This may suggest that the bacterial-fungal interaction affects protein expression and pathogenesis.
3. Pseudomonas Aeruginosa
Pseudomonas aeruginosa (P. aeruginosa) is a ubiquitous Gram-negative bacterium associated with bacterial keratitis and one of the most destructive among opportunistic pathogens. P. aeruginosa keratitis progresses rapidly and is characterized by infiltration of inflammatory cells (Figure 1) and tissue destruction leading to corneal perforation [24]. Recent reports confirm that P. aeruginosa is the most commonly isolated organism from contact lens wearers, the group with highest risk for keratitis infection [4,25,26]. In 2002, it was reported that 25,000–30,000 contact lens wearers developed microbial keratitis annually in the United States [27], and that 6%–39% of the cases are caused by P. aeruginosa [28,29]. Currently, there are at least 34 million contact lens users in the United States and 140 million worldwide [30].
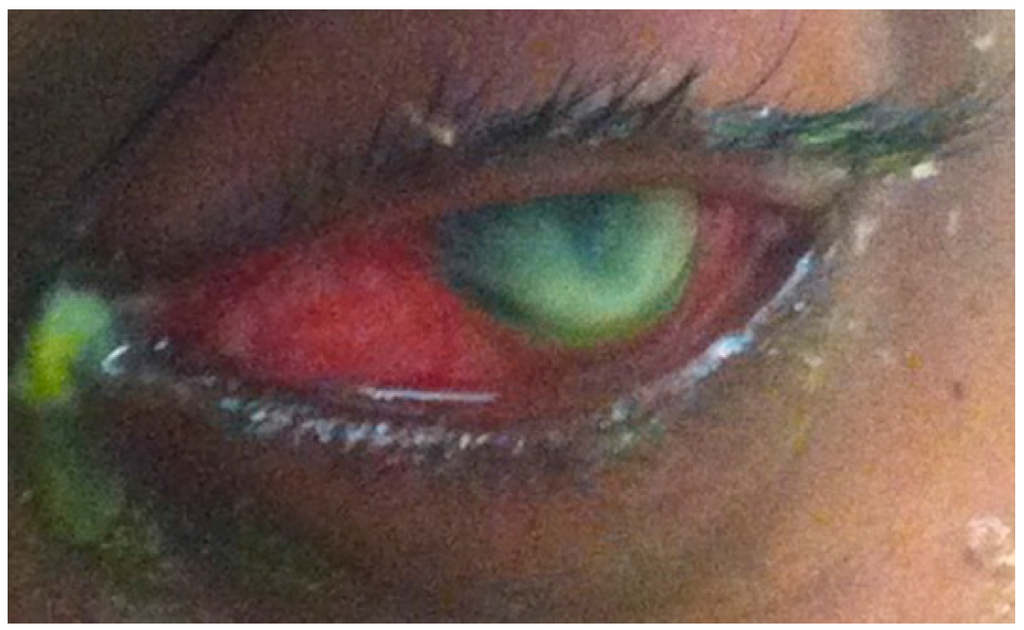
Figure 1.
A photograph showing a bacterial keratitis infection caused by P. aeruginosa.
The pathogenesis of P. aeruginosa is mediated through a plethora of virulence factors. These include cell-associated structures, such as pili [31] and flagella [32], and extracellular products, such as alkaline protease [33], elastase B (LasB) [34], exoenzyme S [9], exotoxin A [35], endotoxin [36], slime polysaccharide, phospholipase C, leukocidin, protease IV [37], and P. aeruginosa small protease (PASP) [38]. Whereas Gram-positive bacteria, including S. aureus, adhere to host tissues via fibronectin and collagen [17], P. aeruginosa attach to cell surfaces that lack fibronectin [39]. P. aeruginosa adhere to injured cornea [40], exposed corneal stroma [41], or immature non-wounded cornea [42]. The corneal epithelial receptors for Pseudomonas spp. have been identified as glycoproteins [43]. SDS-PAGE and Western blot analysis were performed on purified PASP and LasB to determine their role in keratitis [38].
An important factor that contributes to the destruction of the cornea during bacterial keratitis is excessive activation of the host defense system. P. aeruginosa can activate several pathways of the immune system during infection, and activation often involves toll-like receptors (TLRs) on the corneal epithelium. These TLRs recognize lipopolysaccharides or flagella from P. aeruginosa and activate the epithelial cells to produce inflammatory mediators such as cytokines and chemokines. These cytokines or chemokines recruit white blood cells, predominantly polymorphonuclear leukocytes, to the site of infection in order to eliminate the P. aeruginosa. Karthikeyan et al. examined corneal ulcers from patients with P. aeruginosa and found elevated expression of the pathogen recognition receptors TLR2, TLR4 and TLR9, pro-inflammatory cytokines IL-1α, IL-1β, and IFN-γ, and the inflammasome components NLRP3, NLRC4 and ASC compared with donor corneas [44]. Because neutrophils were the predominant cell types in these corneal ulcers, the author suggested that they may be the source of the majority of these factors. The authors used Western blotting to characterize the exotoxins (ExoS, ExoT and ExoU) secreted by P. aeruginosa clinical isolates [44]. Using a P. aeruginosa keratitis mouse model, several ELISA studies have shown increased IL-1β, IL-6, IFN-γ, TNF-α, and IL-12 p40 compared to uninfected control eyes [45,46]. The kinetics and identities of inflammatory cytokines produced were found to be bacterial strain- and time-dependent [47,48,49]. The role of IL-12 in ocular P. aeruginosa infection has also been explored using ELISA and immunocytochemistry in combination with standard molecular techniques [50].
Protein arrays have also been used to study P. aeruginosa keratitis. Sack et al. used this approach to delineate the spectrum of angiogenic bioactive protein modulators that might be secreted and upregulated by the corneal epithelium in response to killed P. aeruginosa products and revealed that the immortalized cell lines constitutively secrete several proteins and upregulate secretion of IL-6, IL-8, and GRO in response to killed bacteria [51]. These studies revealed the role of innate and adaptive immune defense system in keratitis.
Recently, Sewell et al. [52] performed a global proteomic approach, using liquid chromatography followed by tandem mass spectrometry, to compare a clinical isolate of P. aeruginosa from an active corneal ulcer with a non-pathogenic laboratory strain of P. aeruginosa (ATCC10145) and found a total of 133 proteins that were significantly different between the two strains. The upregulated proteins were associated with virulence and pathogenicity [52] and included flagellin B, lipotoxin F, organic solvent tolerant protein, polyhydroxyalkanoate synthesis protein and dehydrocarnitine CoA transferase subunit B. In addition, two putative nonribosomal peptide synthetases (NRPS) were detected in the corneal strain but not ATCC10145. The NRPSs are responsible for the production of the secondary metabolite l-2-amino-4-methoxy-trans-3-butenoic acid (AMB), a potent toxin produced by P. aeruginosa [52]. This suggests that P. aeruginosa might be using AMB as a virulent factor in keratitis. Further studies are warranted to confirm this hypothesis.
4. Streptococcus Pneumoniae
S. pneumoniae (pneumococcus) is also a common cause of infectious keratitis after P. aeruginosa and/or S. aureus [11,29,53,54,55,56,57,58]; however, some epidemiologic studies identify it as the top cause of bacterial keratitis [59,60,61,62,63,64,65]. Unlike P. aeruginosa, pneumococcal keratitis is not typically associated with contact lens use but rather with predisposing conditions such as ocular trauma or surgery [53,58,59,60,63,65,66,67,68,69].
The outer capsule of S. pneumoniae, composed of polysaccharide necessary to establish virulence and survive the host immune response, is the most studied virulence factor for this bacterium [70,71]. With infections, such as pneumoniae, otitis media, meningitis and septicemia, the noncapsular forms of bacteria are avirulent. Whereas with keratitis, noncapsular strains have been shown to cause as severe keratitis as their capsular counterparts in intrastromal infection models [72,73].
Another virulence factor, pneumolysin (PLY), is a pore-forming toxin that was first identified by Johnson and Allen [74] as being responsible for ocular tissue damage during pneumococcal keratitis [74,75,76]. This toxin causes both direct cellular damage by forming pores in host cell membranes and immune-derived damage by activating the complement system and inducing inflammation. It has also been found that PLY reduces the opsonic activity of S. pneumoniae, which could allow for more bacterial replication and more toxin release [77,78]. Proteomic approaches have been used to determine the structure and function of PLY [79,80,81], as well as to investigate whether passive immunization with pneumolysin antiserum could reduce corneal damage associated with pneumococcal keratitis [82]. These studies used ELISA, Western blotting and purification of recombinant PLY and found that passive immunization with antiserum to PLY can significantly minimize the initial corneal damage commonly observed with pneumococcal keratitis and promote full recovery from keratitis. The finding suggests a novel treatment for pneumococcal keratitis by using antibodies to PLY, or peptides constructed of antibody epitopes, in addition to the customary antibiotic therapy [82]. No other protein contributing to virulence in pneumococcal keratitis has been identified.
As for the host response in corneal ulcers from patients with culture positive S. pneumoniae, similar to P. aeruginosa, Karthikeyan et al. also found elevated expression of TLR2, TLR4, TLR9, IL-1α, IL-1β, IFN-γ, NLRP3, NLRC4 and ASC compared with control non-infected corneas. The authors used Western blotting to confirm the expression of PLY in S. pneumoniae clinical isolates [44].
5. Serratia Species
Serratia species are opportunistic Gram-negative bacteria that belong to the large family of Enterobacteriaceae, with Serratia marcescens being the primary pathogenic species [83]. Risk for Serratia keratitis is associated with abnormal corneal surface, topical medication use, and contact lens wear [5,84]. It can also cause refractory keratitis resulting in corneal perforation and blindness.
S. marcescens produces four different proteases, as well as two nucleases [85,86], all of which were isolated and characterized using proteomic approaches such as protein precipitation, isoelectric focusing and gel electrophoresis [85]. One protease of 56 kilodaltons (56K protease), which is the major pathogenic factor in Serratial keratitis [87], was purified from the culture supernatant of a strain of S. marcescens isolated from a severe corneal ulcer of a human eye. Purification of this protease was achieved using different proteomic methods that included ammonium sulfate precipitation, DEAE-cellulose ion-exchange chromatography, Sephadex gel filtration chromatography, polyacrylamide gel electrophoresis and immunodiffusion [87]. The 56 kDa protease activates Hageman factor, initiating the Hageman factor-kallikrein-kinin cascade, which leads to enhanced vascular permeability [88,89]. This study also used chromatography techniques for purification of 56K protease and Hageman factor. Subsequently, the production and purification of an anti-56K protease antibody that was used for immunization purposes [89]. The protein structure of the 56 kDa protein was recently determined by Bhaskar et al. [90]. These studies were performed to determine the role of the 56 kDa protein in the pathogenesis of serratial infections in the eye as well as confirming the inflammatory process in the infection. To study the host response to serratial infections of the cornea, Zhou et al. also used ELISA to measure cytokine production in infected corneas as well as in the supernatants of stimulated bone marrow-derived macrophages confirming an inflammatory process in serratial keratitis [91].
6. Other Studies
6.1. Host Response Studies
Multiple laboratories have studied the host response mechanisms to corneal infection by different bacteria, some of which are mentioned in the above sections. Using keratitis animal models and ELISA, these studies investigated the inflammatory processes and the immune response of the host to the infection [92,93,94,95,96,97,98,99,100,101,102]. Although, the majority of these studies were focused on pseudomonas keratitis, findings from other studies of different bacteria were similar. All studies confirmed the role of toll like receptors (TLR 2, 6, 4 and 9), interleukins (IL-8, IL-18, IL-6, and IL-1β, IL-10, IL-17) and metalloproteinases (MMP-9) in addition to NFκB, TNF-α, JNK, and p38 among other proteins in bacterial keratitis. In addition, because of their role in clearance of debris and pathogens from the surface of the eye and protection against infections, secreted mucins and their O-glycans in the tear film have been studied, and their protein structure has been elucidated [103,104].
6.2. Contact Lens Studies
Because contact lens wear is a major risk factor for bacterial keratitis, studies to investigate the mechanisms of host responses to infections resulting from contact lens wear [105,106] were conducted. These studies investigated contact lens protein deposits. Zhao et al. used liquid chromatography combined with tandem mass spectrometry (LC-MS-MS) and found that the worn contact lenses contained a wide array of proteins deposited from tear film and other sources and that these protein deposit profiles were varied and specific for each lens material tested [106]. Green-Church et al. used a similar approach, nano-liquid chromatography tandem mass spectrometry (nano-LC-MS/MS), to investigate the proteomes of two daily wear silicone hydrogel contact lenses when used with two multipurpose care solutions [105]. The authors reported that the contact lens protein deposition profiles showed a high degree of similarity between the two silicone hydrogels, consisting mainly of six proteins including lipocalin, lysozyme, lacritin, lactoferrin, proline rich 4, and Ig-Alpha. However, some unique proteins were also detected for each polymer type, not only providing valuable information about the tear film proteome itself but also yielding insight about the interaction between these polymers and tear film proteins [105]. These studies were conducted in an effort to determine the optimal contact lens material because it has been estimated that 80% of clinical issues and 30% of aftercare visits relating to extended wear of conventional contact lenses were due to deposition of tear-derived substances on the lens surface [107]. However, a recent comprehensive review suggests that deposition of proteins, such as lysozyme and lactoferrin, on contact lens materials may actually be beneficial during contact lens wear, as these proteins can reduce the viability of bacteria on the contact lens, thus slowing or preventing the pathogenesis of contact lens-related microbial keratitis and inflammation [108].
7. Concluding Remarks and Future Perspectives
In addition to the identification of virulence factors, the studies described above have helped to increase our understanding of the innate immune system’s ability to recognize invading bacteria at the corneal surface through Toll-like receptors (TLRs) expressed on the surface of epithelial cells, macrophages, and dendritic cells in the stroma. These TLRs recognize conserved bacterial surface proteins, such as lipopolysaccharide (LPS), leading to rapid production of proinflammatory and chemotactic cytokines and recruitment of neutrophils to the corneal stroma. This process seems to be common to all bacteria described. These studies led to the development of current therapies and the design of better contact lens materials. However, more studies are needed to further characterize the mediators of innate and adaptive immunity, as well as to identify other virulence factors that could potentially be used as targets for novel therapies. In addition, studies to understand mechanisms of bacterial resistance are also needed. All these can be studied using proteomic approaches. Proteomic analysis has been used in medical microbiology for the identification of novel pathogenic mechanisms, investigation of the epidemiology and taxonomy of microbial pathogens, the analysis of drug resistance [109], and in the design and development of antimicrobial vaccines [110]. In each of these areas, proteomics has provided new insights that complement genomic-based investigations, as a genomics approach alone is typically insufficient. As mentioned in this review, most studies of bacterial keratitis to date have used standard molecular techniques, such as cloning, PCR, and gene knockout while very few have incorporated a proteomic approach. Although limited, the few proteomic studies described in this short review have helped tremendously to gain insight into the pathophysiology of keratitis; however, compared to studies performed on fungal or viral keratitis, these studies were limited. There is an urgent need for more proteomic studies such as those performed by Sewell et al., comparing a corneal strain of P. aeruginosa to a non-corneal/non-pathogenic laboratory strain [52] by Hare et al. in the study of cystic fibrosis [111], and those performed to study the tear proteome in fungal keratitis [112,113]. Global proteomic methods such as Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS) have been widely used for molecular characterization of diseases [114,115,116,117]. Global comparisons of clinical and non-clinical strains would undoubtedly reveal novel virulence factors and aid in the diagnosis, prognosis and treatment of bacterial keratitis. Finally, studies focusing on the functional properties of proteins implicated in the pathogenesis of bacterial keratitis may also contribute to a better understanding of the disease and development of new drugs for treatment.
Acknowledgments
The authors would like to thank Heidi Rassavong for her critical reading of the manuscript.
Author Contributions
Drafting of the manuscript: RB, JD, TR, JB; Critical revision of the manuscript: RB.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keay, L.; Edwards, K.; Naduvilath, T.; Taylor, H.R.; Snibson, G.R.; Forde, K.; Stapleton, F. Microbial keratitis predisposing factors and morbidity. Ophthalmology 2006, 113, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Al-Mujaini, A.; Al-Kharusi, N.; Thakral, A.; Wali, U.K. Bacterial keratitis: Perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ. Med. J. 2009, 9, 184–195. [Google Scholar] [PubMed]
- Parmar, P.; Salman, A.; Kalavathy, C.M.; Kaliamurthy, J.; Thomas, P.A.; Jesudasan, C.A. Microbial keratitis at extremes of age. Cornea 2006, 25, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D. Pseudomonas aeruginosa infection and inflammation during contact lens wear: A review. Optom. Vis. Sci. 2007, 84, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Mah-Sadorra, J.H.; Najjar, D.M.; Rapuano, C.J.; Laibson, P.R.; Cohen, E.J. Serratia corneal ulcers: A retrospective clinical study. Cornea 2005, 24, 793–800. [Google Scholar] [CrossRef] [PubMed]
- American academy of ophthalmology cornea/external disease panel—preferred practice pattern guidelines, bacterial keratitis, Limited Revision. American Academy of Ophthalmology: San Francisco, CA, USA, 2011; Available online: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx (accessed on 8 October 2015).
- Wilhelmus, K.R. Review of clinical experience with microbial keratitis associated with contact lenses. CLAO J. 1987, 13, 211–214. [Google Scholar] [PubMed]
- Jones, D.B. Pathogenesis of bacterial and fungal keratitis. Trans. Ophthalmol. Soc. U.K. 1978, 98, 367–371. [Google Scholar] [PubMed]
- Baker, N.R.; Minor, V.; Deal, C.; Shahrabadi, M.S.; Simpson, D.A.; Woods, D.E. Pseudomonas aeruginosa exoenzyme s is an adhesion. Infect. Immun. 1991, 59, 2859–2863. [Google Scholar] [PubMed]
- Reichert, R.; Stern, G. Quantitative adherence of bacteria to human corneal epithelial cells. Arch. Ophthalmol. 1984, 102, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, F.; Bruttin, O.; Zografos, L.; Guex-Crosier, Y. Bacterial keratitis: A prospective clinical and microbiological study. Br. J. Ophthalmol. 2001, 85, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Otri, A.M.; Fares, U.; Al-Aqaba, M.A.; Miri, A.; Faraj, L.A.; Said, D.G.; Maharajan, S.; Dua, H.S. Profile of sight-threatening infectious keratitis: A prospective study. Acta Ophthalmol. 2012, 91, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, G.; Alfonso, E.C.; Miller, D. Shifting trends in bacterial keratitis in south florida and emerging resistance to fluoroquinolones. Ophthalmology 2000, 107, 1497–1502. [Google Scholar] [CrossRef]
- Lichtinger, A.; Yeung, S.N.; Kim, P.; Amiran, M.D.; Iovieno, A.; Elbaz, U.; Ku, J.Y.; Wolff, R.; Rootman, D.S.; Slomovic, A.R. Shifting trends in bacterial keratitis in toronto: An 11-year review. Ophthalmology 2012, 119, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, R.J.; Callegan, M.C.; Moreau, J.M.; Green, L.C.; Foster, T.J.; Hartford, O.M.; Engel, L.S.; Hill, J.M. Specific roles of α-toxin and β-toxin during Staphylococcus aureus corneal infection. Infect. Immun. 1997, 65, 1571–1578. [Google Scholar] [PubMed]
- Moreira, B.M.; Daum, R.S. Antimicrobial resistance in staphylococci. Pediatr. Clin. N. Am. 1995, 42, 619–648. [Google Scholar]
- Patti, J.M.; Allen, B.L.; McGavin, M.J.; Hook, M. Mscramm-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 1994, 48, 585–617. [Google Scholar] [CrossRef] [PubMed]
- Jett, B.D.; Gilmore, M.S. Internalization of Staphylococcus aureus by human corneal epithelial cells: Role of bacterial fibronectin-binding protein and host cell factors. Infect. Immun. 2002, 70, 4697–4700. [Google Scholar] [CrossRef] [PubMed]
- Rhem, M.N.; Lech, E.M.; Patti, J.M.; McDevitt, D.; Hook, M.; Jones, D.B.; Wilhelmus, K.R. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect. Immun. 2000, 68, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Dajcs, J.J.; Thibodeaux, B.A.; Girgis, D.O.; O’Callaghan, R.J. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 2002, 21, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Callegan, M.C.; Engel, L.S.; Hill, J.M.; O’Callaghan, R.J. Corneal virulence of Staphylococcus aureus: Roles of α-toxin and protein A in pathogenesis. Infect. Immun. 1994, 62, 2478–2482. [Google Scholar] [PubMed]
- Hume, E.B.; Cole, N.; Khan, S.; Garthwaite, L.L.; Aliwarga, Y.; Schubert, T.L.; Willcox, M.D. A Staphylococcus aureus mouse keratitis topical infection model: Cytokine balance in different strains of mice. Immunol. Cell Biol. 2005, 83, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Antonio, B.; Reyes-Grajeda, J.P.; Mariana, O.; Verónica, R.A.; Carolina, G.; Luz, L.N.; Herlinda, M.; Victor, M.B. Proteomic analysis of the interaction fusarium solani-Staphylococcus aureus isolated from human keratitis in presence of antimicrobial agents. Investig. Ophthalmol. Vis. Sci. 2014, 55, 392. [Google Scholar]
- Rattanatam, T.; Heng, W.J.; Rapuano, C.J.; Laibson, P.R.; Cohen, E.J. Trends in contact lens-related corneal ulcers. Cornea 2001, 20, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Schornack, M.M.; Faia, L.J.; Griepentrog, G.J. Pseudomonas keratitis associated with daily wear of silicone hydrogel contact lenses. Eye Contact Lens 2008, 34, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Apel, A.; Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 2008, 27, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Khatri, S.; Lass, J.H.; Heinzel, F.P.; Petroll, W.M.; Gomez, J.; Diaconu, E.; Kalsow, C.M.; Pearlman, E. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2278–2284. [Google Scholar]
- Forster, R.K. Conrad berens lecture. The management of infectious keratitis as we approach the 21st century. CLAO J. 1998, 24, 175–180. [Google Scholar] [PubMed]
- Varaprasathan, G.; Miller, K.; Lietman, T.; Whitcher, J.P.; Cevallos, V.; Okumoto, M.; Margolis, T.P.; Yinghui, M.; Cunningham, E.T., Jr. Trends in the etiology of infectious corneal ulcers at the f. I. Proctor foundation. Cornea 2004, 23, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Keay, L.; Edwards, K.; Naduvilath, T.; Brian, G.; Jacobs, R. Studies of contact lens-related microbial keratitis in australia and new zealand: New learnings. Eye Contact Lens 2007, 33, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Doig, P.; Todd, T.; Sastry, P.A.; Lee, K.K.; Hodges, R.S.; Paranchych, W.; Irvin, R.T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect. Immun. 1988, 56, 1641–1646. [Google Scholar] [PubMed]
- Holder, I.A.; Naglich, J.G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: Immunization using divalent flagella preparations. J. Trauma 1986, 26, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Hobden, J.A. Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol. 2002, 21, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Twining, S.S.; Kirschner, S.E.; Mahnke, L.A.; Frank, D.W. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin a on corneal proteinases and proteins. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2699–2712. [Google Scholar]
- Iglewski, B.H.; Liu, P.V.; Kabat, D. Mechanism of action of Pseudomonas aeruginosa exotoxin aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect. Immun. 1977, 15, 138–144. [Google Scholar] [PubMed]
- Cryz, S.J., Jr.; Pitt, T.L.; Furer, E.; Germanier, R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect. Immun. 1984, 44, 508–513. [Google Scholar] [PubMed]
- Engel, L.S.; Hill, J.M.; Caballero, A.R.; Green, L.C.; O’Callaghan, R.J. Protease iv, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J. Biol. Chem. 1998, 273, 16792–16797. [Google Scholar] [CrossRef] [PubMed]
- Marquart, M.E.; Caballero, A.R.; Chomnawang, M.; Thibodeaux, B.A.; Twining, S.S.; O’Callaghan, R.J. Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.E.; Straus, D.C.; Johanson, W.G., Jr.; Bass, J.A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J. Infect. Dis. 1981, 143, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; Moon, M.M.; Strejc, M.; Berk, R.S. Evidence for n-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1978–1985. [Google Scholar]
- Stern, G.A.; Weitzenkorn, D.; Valenti, J. Adherence of Pseudomonas aeruginosa to the mouse cornea. Epithelial v stromal adherence. Arch. Ophthalmol. 1982, 100, 1956–1958. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; Moon, M.; Berk, R.S. In vivo identification of sialic acid as the ocular receptor for Pseudomonas aeruginosa. Infect. Immun. 1986, 51, 687–689. [Google Scholar] [PubMed]
- Rudner, X.L.; Zheng, Z.; Berk, R.S.; Irvin, R.T.; Hazlett, L.D. Corneal epithelial glycoproteins exhibit Pseudomonas aeruginosa pilus binding activity. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2185–2193. [Google Scholar]
- Karthikeyan, R.S.; Priya, J.L.; Leal, S.M., Jr.; Toska, J.; Rietsch, A.; Prajna, V.; Pearlman, E.; Lalitha, P. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS ONE 2013, 8, e64867. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; Rudner, X.L.; McClellan, S.A.; Barrett, R.P.; Lighvani, S. Role of IL-12 and IFN-γ in Pseudomonas aeruginosa corneal infection. Investig. Ophthalmol. Vis. Sci. 2002, 43, 419–424. [Google Scholar]
- Kernacki, K.A.; Berk, R.S. Characterization of the inflammatory response induced by corneal infection with Pseudomonas aeruginosa. J. Ocul. Pharmacol. 1994, 10, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.; Krockenberger, M.; Stapleton, F.; Khan, S.; Hume, E.; Husband, A.J.; Willcox, M. Experimental Pseudomonas aeruginosa keratitis in interleukin-10 gene knockout mice. Infect. Immun. 2003, 71, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.; Bao, S.; Willcox, M.; Husband, A.J. Expression of interleukin-6 in the cornea in response to infection with different strains of Pseudomonas aeruginosa. Infect. Immun. 1999, 67, 2497–2502. [Google Scholar] [PubMed]
- Kernacki, K.A.; Goebel, D.J.; Poosch, M.S.; Hazlett, L.D. Early cytokine and chemokine gene expression during Pseudomonas aeruginosa corneal infection in mice. Infect. Immun. 1998, 66, 376–379. [Google Scholar] [PubMed]
- O’Brien, T.P. Management of bacterial keratitis: Beyond exorcism towards consideration of organism and host factors. Eye 2003, 17, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Sack, R.; Sathe, S.; Beaton, A.R.; McNamara, N.; Fleiszig, S.; Ni, M. Protein array characterization of bioactive proteins secreted by immortalized human corneal epithelium in response to pseudomonas constituents. Curr. Eye Res. 2009, 34, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sewell, A.; Dunmire, J.; Wehmann, M.; Rowe, T.; Bouhenni, R. Proteomic analysis of keratitis-associated Pseudomonas aeruginosa. Mol. Vis. 2014, 20, 1182–1191. [Google Scholar] [PubMed]
- Boonpasart, S.; Kasetsuwan, N.; Puangsricharern, V.; Pariyakanok, L.; Jittpoonkusol, T. Infectious keratitis at king chulalongkorn memorial hospital: A 12-year retrospective study of 391 cases. J. Med. Assoc. Thail. 2002, 85 (Suppl. 1), S217–S230. [Google Scholar]
- Lalitha, P.; Srinivasan, M.; Manikandan, P.; Bharathi, M.J.; Rajaraman, R.; Ravindran, M.; Cevallos, V.; Oldenburg, C.E.; Ray, K.J.; Toutain-Kidd, C.M.; et al. Relationship of in vitro susceptibility to moxifloxacin and in vivo clinical outcome in bacterial keratitis. Clin. Infect. Dis. 2012, 54, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Norina, T.J.; Raihan, S.; Bakiah, S.; Ezanee, M.; Liza-Sharmini, A.T.; Wan Hazzabah, W.H. Microbial keratitis: Aetiological diagnosis and clinical features in patients admitted to hospital universiti sains malaysia. Singap. Med. J. 2008, 49, 67–71. [Google Scholar]
- Ramesh, S.; Ramakrishnan, R.; Bharathi, M.J.; Amuthan, M.; Viswanathan, S. Prevalence of bacterial pathogens causing ocular infections in south india. Indian J. Pathol. Microbiol. 2010, 53, 281–286. [Google Scholar] [PubMed]
- Shalchi, Z.; Gurbaxani, A.; Baker, M.; Nash, J. Antibiotic resistance in microbial keratitis: Ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology 2011, 118, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, M.D.; Al-Swailem, S.A.; Sutphin, J.E.; Zimmerman, M.B. Bacterial keratitis after penetrating keratoplasty: Incidence, microbiological profile, graft survival, and visual outcome. Ophthalmology 2007, 114, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Al Otaibi, A.G.; Allam, K.; Damri, A.J.; Shamri, A.A.; Kalantan, H.; Mousa, A. Childhood microbial keratitis. Oman J. Ophthalmol. 2012, 5, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Bashir, G.; Shah, A.; Thokar, M.A.; Rashid, S.; Shakeel, S. Bacterial and fungal profile of corneal ulcers--a prospective study. Indian J. Pathol. Microbiol. 2005, 48, 273–277. [Google Scholar] [PubMed]
- Bharathi, M.J.; Ramakrishnan, R.; Vasu, S.; Palaniappan, R. Aetiological diagnosis of microbial keratitis in south India—A study of 1618 cases. Indian J. Med. Microbiol. 2002, 20, 19–24. [Google Scholar] [PubMed]
- Bharathi, M.J.; Ramakrishnan, R.; Vasu, S.; Meenakshi, R.; Shivkumar, C.; Palaniappan, R. Epidemiology of bacterial keratitis in a referral centre in south india. Indian J. Med. Microbiol. 2003, 21, 239–245. [Google Scholar] [PubMed]
- Bharathi, M.J.; Ramakrishnan, R.; Meenakshi, R.; Padmavathy, S.; Shivakumar, C.; Srinivasan, M. Microbial keratitis in south india: Influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007, 14, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Feilmeier, M.R.; Sivaraman, K.R.; Oliva, M.; Tabin, G.C.; Gurung, R. Etiologic diagnosis of corneal ulceration at a tertiary eye center in kathmandu, nepal. Cornea 2010, 29, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, S.; Katiyar, R.; Saini, S. Epidemiological features and laboratory results of bacterial and fungal keratitis: A five-year study at a rural tertiary-care hospital in western maharashtra, india. Singap. Med. J. 2012, 53, 264–267. [Google Scholar]
- Mulet, M.E.; Perez-Santonja, J.J.; Ferrer, C.; Alio, J.L. Microbial keratitis after intrastromal corneal ring segment implantation. J. Refract. Surg. 2010, 26, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Cosar, C.B.; Cohen, E.J.; Rapuano, C.J.; Laibson, P.R. Clear corneal wound infection after phacoemulsification. Arch. Ophthalmol. 2001, 119, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, T.; Levy, J.; Raiskup, F.; Klemperer, I.; Frucht-Pery, J. Two cases of pneumococcal keratitis following myopic lasik. J. Refract. Surg. 2005, 21, 498–501. [Google Scholar] [PubMed]
- Nubile, M.; Carpineto, P.; Lanzini, M.; Ciancaglini, M.; Zuppardi, E.; Mastropasqua, L. Multilayer amniotic membrane transplantation for bacterial keratitis with corneal perforation after hyperopic photorefractive keratectomy: Case report and literature review. J. Cataract Refract. Surg. 2007, 33, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Griffith, F. The significance of pneumococcal types. J. Hyg. 1928, 27, 113–159. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; Macleod, C.M.; McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 1944, 79, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Norcross, E.W.; Tullos, N.A.; Taylor, S.D.; Sanders, M.E.; Marquart, M.E. Assessment of Streptococcus pneumoniae capsule in conjunctivitis and keratitis in vivo neuraminidase activity increases in nonencapsulated pneumococci following conjunctival infection. Curr. Eye Res. 2010, 35, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.M.; O’Callaghan, R.J.; Girgis, D.O.; McCormick, C.C.; Caballero, A.R.; Marquart, M.E. Ocular virulence of capsule-deficient Streptococcus pneumoniae in a rabbit keratitis model. Investig. Ophthalmol. Vis. Sci. 2005, 46, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Hobden, J.A.; Hagenah, M.; O’Callaghan, R.J.; Hill, J.M.; Chen, S. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr. Eye Res. 1990, 9, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Allen, J.H. Ocular toxin of the pneumococcus. Am. J. Ophthalmol. 1971, 72, 175–180. [Google Scholar] [CrossRef]
- Johnson, M.K.; Allen, J.H. The role of cytolysin in pneumococcal ocular infection. Am. J. Ophthalmol. 1975, 80, 518–521. [Google Scholar] [CrossRef]
- Paton, J.C.; Rowan-Kelly, B.; Ferrante, A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 1984, 43, 1085–1087. [Google Scholar] [PubMed]
- Alcantara, R.B.; Preheim, L.C.; Gentry-Nielsen, M.J. Pneumolysin-induced complement depletion during experimental pneumococcal bacteremia. Infect. Immun. 2001, 69, 3569–3575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelly, S.J.; Jedrzejas, M.J. Structure and molecular mechanism of a functional form of pneumolysin: A cholesterol-dependent cytolysin from Streptococcus pneumoniae. J. Struct. Biol. 2000, 132, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.; Katsnelson, M.; Malak, H.A.; Greene, N.G.; Howell, S.J.; Hise, A.G.; Camilli, A.; Kadioglu, A.; Dubyak, G.R.; Pearlman, E. Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and Caspase-1 activation and is dependent on K+ efflux. J. Immunol. 2015, 194, 1763–1775. [Google Scholar] [PubMed]
- Taylor, S.D.; Sanders, M.E.; Tullos, N.A.; Stray, S.J.; Norcross, E.W.; McDaniel, L.S.; Marquart, M.E. The cholesterol-dependent cytolysin pneumolysin from Streptococcus pneumoniae binds to lipid raft microdomains in human corneal epithelial cells. PLoS ONE 2013, 8, e61300. [Google Scholar] [CrossRef] [PubMed]
- Green, S.N.; Sanders, M.; Moore, Q.C., III; Norcross, E.W.; Monds, K.S.; Caballero, A.R.; McDaniel, L.S.; Robinson, S.A.; Onwubiko, C.; O’Callaghan, R.J.; et al. Protection from Streptococcus pneumoniae keratitis by passive immunization with pneumolysin antiserum. Investig. Ophthalmol. Vis. Sci. 2008, 49, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, A.; Falkiner, F.R. Serratia marcescens. J. Med. Microbiol. 1997, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Parment, P.A. The role of Serratia marcescens in soft contact lens associated ocular infections. A review. Acta Ophthalmol. Scand. 1997, 75, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Maeda, H.; Takata, K.; Kamata, R.; Okamura, R. Purification and characterization of four proteases from a clinical isolate of Serratia marcescens kums 3958. J. Bacteriol. 1984, 157, 225–232. [Google Scholar] [PubMed]
- Yonemura, K.; Matsumoto, K.; Maeda, H. Isolation and characterization of nucleases from a clinical isolate of Serratia marcescens kums 3958. J. Biochem. 1983, 93, 1287–1295. [Google Scholar] [PubMed]
- Kamata, R.; Matsumoto, K.; Okamura, R.; Yamamoto, T.; Maeda, H. The serratial 56K protease as a major pathogenic factor in serratial keratitis. Clinical and experimental study. Ophthalmology 1985, 92, 1452–1459. [Google Scholar] [CrossRef]
- Kamata, R.; Yamamoto, T.; Matsumoto, K.; Maeda, H. A serratial protease causes vascular permeability reaction by activation of the hageman factor-dependent pathway in guinea pigs. Infect. Immun. 1985, 48, 747–753. [Google Scholar] [PubMed]
- Matsumoto, K.; Yamamoto, T.; Kamata, R.; Maeda, H. Pathogenesis of serratial infection: Activation of the hageman factor-prekallikrein cascade by serratial protease. J. Biochem. 1984, 96, 739–749. [Google Scholar] [PubMed]
- Bhaskar, A.; Upgade, A.; Kavitha, P. Characterization of keratitis disease causing 56k cysteine protease encoding gene from Serratia marcescens. J. Proteom. Bioinform. 2012, 5–10. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, R.; Sun, Y.; Platt, S.; Szczotka-Flynn, L.; Pearlman, E. Innate immune regulation of Serratia marcescens-induced corneal inflammation and infection. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7382–7388. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.J.; Berk, J.M.; Hazlett, L.D.; Berk, R.S. Serum antibody response to Pseudomonas aeruginosa antigens during corneal infection. Infect. Immun. 1991, 59, 1984–1990. [Google Scholar] [PubMed]
- Hazlett, L.D. Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 2004, 23, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; McClellan, S.A.; Barrett, R.P.; Liu, J.; Zhang, Y.; Lighvani, S. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Investig. Ophthalmol. Vis. Sci. 2007, 48, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Heinzel, F.P.; Diaconu, E.; Sun, Y.; Hise, A.G.; Golenbock, D.; Lass, J.H.; Pearlman, E. Activation of Toll-like Receptor (TLR)2, TLR4, and TLR9 in the Mammalian Cornea Induces MyD88-Dependent Corneal Inflammation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 589–595. [Google Scholar] [CrossRef] [PubMed]
- McClellan, S.A.; Huang, X.; Barrett, R.P.; van Rooijen, N.; Hazlett, L.D. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J. Immunol. 2003, 170, 5219–5227. [Google Scholar] [CrossRef] [PubMed]
- McClellan, S.A.; Huang, X.; Barrett, R.P.; Lighvani, S.; Zhang, Y.; Richiert, D.; Hazlett, L.D. Matrix metalloproteinase-9 amplifies the immune response to Pseudomonas aeruginosa corneal infection. Investig. Ophthalmol. Vis. Sci. 2006, 47, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.L.; Willcox, M.D.; Lloyd, A.; Wakefield, D.; Thakur, A. Regulatory role of IL-1β in the expression of IL-6 and IL-8 in human corneal epithelial cells during Pseudomonas aeruginosa colonization. Clin. Exp. Ophthalmol. 2001, 29, 171–174. [Google Scholar] [CrossRef]
- Li, C.; McClellan, S.A.; Barrett, R.; Hazlett, L.D. Interleukin 17 regulates Mer tyrosine kinase-positive cells in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6886–6900. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, J.; Yu, F.S. Innate immune response of corneal epithelial cells to Staphylococcus aureus infection: Role of peptidoglycan in stimulating proinflammatory cytokine secretion. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.N.; Redfern, R.; Frikeche, J.; Pinglay, S.; Foster, J.W.; Lema, C.; Cope, L.; Chakravarti, S. Functions of peptidoglycan recognition proteins (Pglyrps) at the ocular surface: Bacterial keratitis in gene-targeted mice deficient in Pglyrp-2, -3 and -4. PLoS ONE 2015, 10, e0137129. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Scott, S.G.; Nakata, C.; Hamad, A.R.; Chakravarti, S. Extracellular matrix protein lumican promotes clearance and resolution of Pseudomonas aeruginosa keratitis in a mouse model. PLoS ONE 2013, 8, e54765. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Vergnes, J.P.; Brown, S.I. O-glycosidic linkage in glycoprotein isolates from human ocular mucus. Exp. Eye Res. 1983, 37, 533–541. [Google Scholar] [CrossRef]
- Chao, C.C.; Butala, S.M.; Herp, A. Studies on the isolation and composition of human ocular mucin. Exp. Eye Res. 1988, 47, 185–196. [Google Scholar] [CrossRef]
- Green-Church, K.B.; Nichols, J.J. Mass spectrometry-based proteomic analyses of contact lens deposition. Mol. Vis. 2008, 14, 291–297. [Google Scholar] [PubMed]
- Zhao, Z.; Wei, X.; Aliwarga, Y.; Carnt, N.A.; Garrett, Q.; Willcox, M.D. Proteomic analysis of protein deposits on worn daily wear silicone hydrogel contact lenses. Mol. Vis. 2008, 14, 2016–2024. [Google Scholar] [PubMed]
- Stenson, S. Superior limbic keratoconjunctivitis associated with soft contact lens wear. Arch. Ophthalmol. 1983, 101, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Omali, N.B.; Subbaraman, L.N.; Coles-Brennan, C.; Fadli, Z.; Jones, L.W. Biological and clinical implications of lysozyme deposition on soft contact lenses. Optom. Vis. Sci. 2015, 92, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Cash, P. Proteomics in medical microbiology. Electrophoresis 2000, 21, 1187–1201. [Google Scholar] [CrossRef]
- Grandi, G. Antibacterial vaccine design using genomics and proteomics. Trends Biotechnol. 2001, 19, 181–188. [Google Scholar] [CrossRef]
- Hare, N.J.; Solis, N.; Harmer, C.; Marzook, N.B.; Rose, B.; Harbour, C.; Crossett, B.; Manos, J.; Cordwell, S.J. Proteomic profiling of Pseudomonas aeruginosa AES-1R, PAO1 and PA14 reveals potential virulence determinants associated with a transmissible cystic fibrosis-associated strain. BMC Microbiol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, S.; Chitra, T.; Bini, R.; Prajna, N.V.; Lalitha, P.; Dharmalingam, K. Comparative analysis of the tear protein profile in mycotic keratitis patients. Mol. Vis. 2008, 14, 500–507. [Google Scholar] [PubMed]
- Ananthi, S.; Venkatesh Prajna, N.; Lalitha, P.; Valarnila, M.; Dharmalingam, K. Pathogen induced changes in the protein profile of human tears from fusarium keratitis patients. PLoS ONE 2013, 8, e53018. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Lu, Q.; Xue, P.; Zhang, H.; Dong, Z.; Yang, F.; Wang, N. Proteomic analysis of aqueous humor from patients with myopia. Mol. Vis. 2008, 14, 370–377. [Google Scholar] [PubMed]
- Niwa, T. Biomarker discovery for kidney diseases by mass spectrometry. J. Chromatogr. B 2008, 870, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, P.; Sjogren, M. Proteome studies of CSF in ad patients. Mech. Ageing Dev. 2006, 127, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.R.; Verma, M.; Zhao, Y.; Srivastava, S. Proteomics for cancer biomarker discovery. Clin. Chem. 2002, 48, 1160–1169. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).