Abstract
Of all the substances that can be present in water intended for human consumption, arsenic (As) is one of the most toxic. Many treatment technologies can be used for removing As from water, for instance, adsorption onto iron media, where commercially available adsorbents are removed and replaced with new media when they are exhausted. Since this is an expensive operation, in this work, a novel and portable plant for regenerating iron media has been developed and tested in four real case studies in Central Italy. The obtained results highlight the good efficiency of the system, which was able, from 2019 to 2023, to regenerate the iron media and to restore its capability to adsorb the As from water almost entirely. Indeed, when the legal threshold value of 10 μg/L is exceeded, the regeneration process is performed and, after that, the As concentration in the water effluent is at the minimum level in all the investigated case studies.
1. Introduction
According to Directive 2020/2184 of the European Parliament and the Council on the quality of water intended for human consumption, “Water intended for human consumption” is treated or untreated water intended for drinking, for preparing food, drink, or other domestic uses, and water used for the manufacture, treatment, and storage of substances intended for human consumption [1]. Water intended for human consumption, particularly drinking water, must be healthy and clean, and must not contain microorganisms, parasites, or other substances in quantities or concentrations representing a potential danger to human health.
Arsenic (As) is a chemical element with the atomic number 33. It is a semimetal that occurs in three different allotropic forms: yellow, black, and gray. It is found in rocks, water, and animal and vegetable organisms and its content in the rocks of the Earth’s crust has been estimated at 1.5 g per ton of rock. As is a common contaminant in water. The most dangerous problem in this context is that in many areas of the world, drinking water is contaminated with As. Due to its high toxicity, especially with regard to arsenite, drinking water regulations allow only very low concentrations of arsenic. Of all the substances that can be present in water, As is one of the most toxic, potentially resulting in skin cancer or other cancers [2]. Indeed, arsenic is easily found in nature, from the atmosphere to soils and rocks, but also in natural waters and organisms [3], being the twentieth most abundant element in all-natural metalloids [4]. Since millions of people are nowadays being exposed to excessive As through the consumption of contaminated drinking water [5], it is mandatory to remove this substance from the water intended for human consumption, respecting the maximum level for As in drinking water fixed at 10 μg/L by many Agencies such as the World Health Organization (WHO) or the US Environmental Protection Agency [6].
In Italy, the data on the presence of As in water comprise a rapidly evolving set of information; following new abstractions, this element can be found in significant concentrations even in aquifers whose hydrogeological conditions would exclude its presence. However, it is evident that this element constitutes a strong criticality in the water use system, especially for water intended for human consumption.
Arsenic is a water contaminant widespread in many areas of the Earth, not only in Italy, of course. Some of the territories where arsenic is found in water in high concentrations are western India, Alaska, Mexico, Chile, and Argentina.
In general terms, it can be stated that in Italy, the supply of qualitatively suitable water is pursued with rigorous and consolidated practices of management of the water system, is controlled through a tested surveillance system, and is regulated on a legislative level, with regard to frequency, typology, and methods of control. Without prejudice to certain circumstances, generally limited in terms of time and territory, for which non-compliance may occur due to the presence of non-standard chemical or microbiological parameters following which limitations on the use of water may also be ordered with adequate information actions on the populations concerned, the water distributed is fit for human consumption and can be consumed in safe conditions from a health point of view, so there is no need for its treatment downstream of the “point of delivery”.
In any case, the As presence in water poses the problem of its removal before the water’s distribution to the population, so many treatment technologies can be used for removing As from water, including the following [7]:
- (1)
- Oxidation (oxidation and filtration; photochemical oxidation; photocatalytic oxidation; biological oxidation; in situ oxidation);
- (2)
- Membrane technologies (microfiltration; ultrafiltration; nanofiltration; reverse osmosis);
- (3)
- Coagulation/flocculation;
- (4)
- Ion exchange;
- (5)
- Adsorption onto solid media (AM) (activated alumina; iron-based sorbents; zero-valent iron; indigenous filters; miscellaneous sorbents; metal–organic frameworks).
A review of such technologies has been made by a number of authors [8,9,10,11,12]. In many cases, these technologies have been proven as expensive or complex, with the exception of AM, which has been accepted as a suitable removal technology, particularly for developing regions, because of its simple operation, potential for regeneration, and little toxic sludge generation [13].
In AM technology, As and other anions are adsorbed onto a packed bed of media. The employed removal mechanism is usually an exchange of anions for surface hydroxides of the media [14]. When the As concentration of the effluent from an adsorption system reaches the regulatory limit of 10 μg/L, the media are usually removed and replaced with new media.
In the literature, a lot of research can be found dealing with the adsorbent materials employed in AM technology, i.e., with metal oxide/hydroxides, including iron, aluminum, zirconium, and titanium, constituting the majority of the commercially available adsorbents [8,11,15]. In particular, many iron media products have been introduced in the drinking water treatment market in recent years since iron-based adsorbents generally have been found to have higher arsenic adsorptive capacity and efficiency [16,17]. As aforementioned, when the adsorptive iron media no longer has the ability to reduce the As in water effluent to values lower than the maximum contaminant level of 10 μg/L, it is removed and replaced with the media, and the exhausted media can be disposed of in a sanitary landfill [18], but this is an expensive operation. The price of the adsorptive iron media on the market remains quite high and there does not seem to be any sign at the moment of a sensible decrease. In addition, the presence of competitive and/or inhibiting species in the treated waters, perhaps underestimated during the design phase, has led to a reduction in the estimated durations of activity of these products before their exhaustion, thus aggravating the cost of water purification. Indeed, the substitution of the adsorptive iron media accounts on average for 80% of all the operation and maintenance costs of the system, including media replacement, chemicals, electricity, and labor [16].
In order to diminish the operation and maintenance costs, one option to exhaust iron media substitution is its regeneration and reuse. The regeneration process is based on the mechanism of adsorption of As on ferric hydroxides, a chemical exchange that by its nature is reversible. The reactivation process brings the adsorbent material back to its initial state by extracting and bringing back into solution the species that are bound to it during the “work” phase of the masses, leading to the achievement of the desired result: the restoration of the absorption capacity of the As [17].
In the literature, few studies have been conducted on the regeneration and reuse of adsorptive iron media [19,20], while some authors have even suggested that it is not possible to regenerate such media because the process will cause particle degradation [21], although it is true that regeneration offers a potential option to reduce the cost of the system. Starting from these premises, the aim of the present manuscript is the introduction of a novel technological plant that can be used for removing As from water intended for human consumption, employing adsorptive iron media and pursuing its regeneration and reuse. The novelty of this work consists particularly of the presentation of a technological system characterized by its portability. Indeed, it is worth noting that the majority of existing systems for regenerating iron media are non-portable.
The present manuscript is organized as follows. In Section 2, the iron media formation and its use in removing As from water are briefly presented. In Section 3, the proposed portable technological plant is introduced. In Section 4, the selected case studies that have been analyzed are presented. Section 5 describes the performed analysis, while the results are described and discussed in Section 6. Finally, the conclusions are summarized in Section 7.
2. Iron Media Formation and Its Use for Removing As from Water
The process of formation of iron oxide/hydroxide, like the type used in the proposed technological plant, mainly consists of the following three macro-phases: a chemical reaction, a dehydration phase, and a grinding/granulating phase.
In the first macro-phase, i.e., the chemical reaction, iron oxide/hydroxide can be obtained by combining a positive trivalent iron ion with a hydroxide ion. For instance, the following formula shows the chemical reaction between ferric chloride (FeCl3) and sodium hydroxide (NaOH):
Based on the reaction of the two selected reagents (sometimes, instead of ferric chloride, ferric sulfate can be used), the necessary time passes for the ferric hydroxide to flocculate, and then it is pressed in a filter press.
The sludge resulting from the first phase contains a significant percentage of water; therefore, a dehydration process is required, i.e., the second macro-phase. Such a process occurs in two ways. (1) Sludge freezing allows further separation from the sludge related to the portion of water that is not mechanically separated; the product obtained has a percentage of residual water of about 50% and is translucent and granular in appearance. (2) Sludge drying by a thermal process such as a rotary drum or belt dryers can reduce the water content to less than 20% in the media.
The third macro-phase, i.e., the grinding/granulating phase, consists of the grinding of the dehydrated sludge (e.g., ferric hydroxide), in order to obtain the required granulometry.
The obtained iron media can be used for removing As from water based on the mechanism of adsorption of arsenic on ferric hydroxides, a chemical exchange that by its nature is reversible. Various studies have found that a classic three-step regeneration process of (1) backwashing the iron media, (2) caustic regeneration, and (3) acid neutralization conditioning has been shown to be effective in removing As and other contaminants absorbed by the iron media [22,23]. Hence, the study of the reactivation process and the subsequent development of the design of the novel technological plant presented here were based on experiences conducted by the international scientific community, in particular by the US Environmental Protection Agency, both as laboratory tests and small pilot plants on real-scale interventions.
3. The Proposed Portable Plant and the Regeneration Process
Based on the aforementioned, the novel technological plant presented here has been designed to be portable and work on site and is constituted by the following elements.
- Two centrifugal pumps for the reagents’ (acid and base) recirculation, characterized by a flow rate of 16 m3/h and a pressure head of 20 m;
- A static mixer, made of plastic material, with a maximum flow rate of 30 m3/h;
- A piston pump for ferric hydroxide dosing, in 30% solution, in plastic material, with a flow rate of 1500 L/h;
- A piston pump for 50% sulfuric acid solution dosing, in plastic material, with a flow rate range of 100–300 L/h;
- Polyvinyl chloride (PVC), with a flow rate range of 0–30 m3/h, diameter (D) 50 mm; various valves (1′’ PVC ball and check valves);
- Pipes in plastic material;
- Electromagnetic flowmeter, with a range of 0–30 m3/h, D 40 mm.
Additionally, the following on-site accessories are required for the completion of the regeneration:
- A polyethylene tank, the volume of which must be defined based on that of the specific filter, for depositing the eluates;
- Chemical reagent tanks.
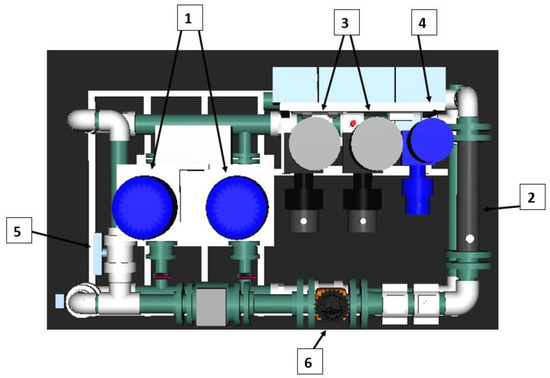
Figure 1.
Technological plant aerial view (dimensions 1.7 m × 1 m). (1) Recirculation pumps. (2) Static mixer. (3) Sodium hydroxide dosing pumps. (4) Sulfuric acid dosing pump. (5) Manual regulation valve. (6) Electromagnetic flowmeter.
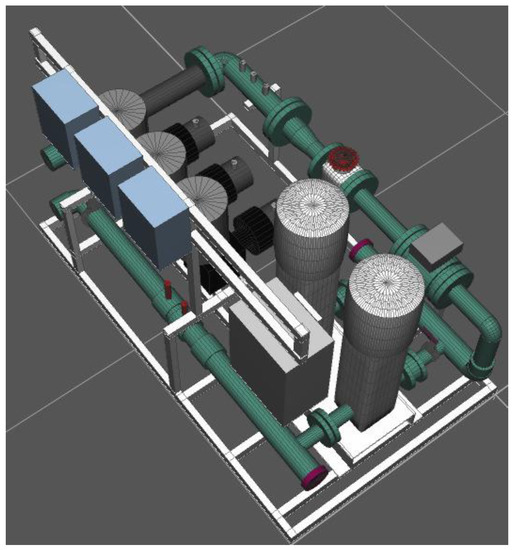
Figure 2.
Technological plant isometric view.

The filter filled with iron media is used as a reactor for the reactivation process while the remaining part of the equipment necessary for the process (hereafter called the skid) is brought directly on site, as well as a tank of adequate capacity where the process eluates will flow. Hoses and hydraulic fittings complete the system equipment. Once the inspection has been carried out and the data for the process are collected, a specific procedure is created in order to plan all the operations that will be carried out during the intervention itself.
From a descriptive point of view, the regeneration process presents a preliminary phase in which the filter containing the oxides to be reactivated is isolated, partially or totally, based on the characteristics of the plant, from the rest of the system. At the same time, the skid and the eluate collection tank are positioned. Once the various equipment has been hydraulically connected via flexible pipes, the system is ready to be started.
The first phase of the regeneration process consists of an alkaline washing of the iron media masses with an aqueous solution of sodium hydroxide that is recirculated through the filter. The aim of alkaline solution recirculation is to increase the contact time with the media and bring a favorable environment to the ion exchange process.
At the end of the alkaline phase of the process, the eluates produced are progressively segregated in the collection tank and replaced with water. This operation continues until a conspicuous lowering of the saline concentration of the water leaving the filter is obtained.
During this phase of “displacement” of the alkaline solution, the eluates are neutralized before reaching the collection tank. At this point, almost all of the species absorbed in the mass were transferred to the alkaline solution and segregated in the storage tank. It is therefore possible to proceed to the second phase of the process in order to restore the alkalinity of the system. The water still present in the filter is recirculated, proceeding at the same time with the controlled injection of sulfuric acid, which acts as a neutralizer. The neutralization operation will continue until a neutral pH is obtained. The process ends with the restoration of the original hydraulic connections and with the backwashing of the filter in order to ensure uniform hydraulic conductivity through the filtering masses.
From an operational point of view, the operational phases regarding the regeneration process are as follows:
- Before starting the regeneration process, a backwashing of the filter media is performed;
- The loading manhole (located at the top of the filter) is opened, and water is allowed to flow about 10–15 cm above the filter media bed;
- The outlet of the pilot system is connected to the previously opened loading manhole, and the return of the system is connected to the lower part of the filter;
- The pumps of the pilot system are activated, and water is circulated in a closed loop;
- The dosing pumps for soda are activated, and soda is circulated inside the filter;
- After the soda has been completely introduced, it continues to circulate for about an hour;
- Subsequently, the eluate is discharged into an existing tank;
- The upper loading manhole is closed, and a backwash with clean water is performed. It is then reopened when the water level is 10–15 cm above the filter media bed. The dosing pump for acid is activated, and the mass is neutralized;
- The last step is to close the upper loading manhole and wash the filter before putting it back into operation.
It should be noted that the procedure is well-suited for regenerating a conventional pressure filter with nozzles (perforated plate with nozzles) for use in series or parallel. The filter must have vents and drains for emptying, as well as a loading/unloading manhole for the filter media.
On the other hand, during the installation of a new filter, the stages of filling the filter media are as follows:
- Placement of a layer of quartz sand support (particle size 2.00–3.15 mm) to cover the nozzles;
- G-OX (filter media bed height between 0.8 and 1.6 m);
- Partial filling with water to protect the nozzles during the sand-filling process;
- Introduction of quartz sand (DIN EN 12904 grade) as a support layer according to the supplier’s instructions; leveling and rinsing the layer;
- Introduction of ferric hydroxide through the upper loading manhole;
- Free space, approximately 50% of the G-OX bed, for expansion during backwashing.
4. The Selected Case Studies
The novel technological plant is a potential solution to address the issue of As contamination in water sources and was tested in four different case studies to examine its performance in real-world applications. The four case studies were located in the Viterbo province, in Central Italy, situated 80 km north of Rome.
Italy is generally characterized by high As concentrations in water. High As concentrations have been historically observed (since 1999) in the Central Italian Alps [24], where water is collected from both cold springs and thermal springs. Also, Central Italy has been known to have high levels of As in its water sources. Zuzolo et al. [25], in their comprehensive study aimed at evaluating the occurrence, distribution, and potential health impacts of As on a national scale in Italy, found that significant As concentrations in tap water and soil (up to 27.20 μg/L and 62.20 mg/kg, respectively) are mainly governed by geological features, and that in the central parts of Italy, where alkaline volcanic materials and consequently high levels of As occur, there can be health issues for residents. Baiocchi et al. [26] and Cinti et al. [27] analyzed many water samples from springs and wells in the Sabatini and Vicano-Cimino Volcanic Districts (Central Italy), determining high As concentrations and highlighting risks to the population due to the fact that water mostly sourced from shallow and cold aquifers hosted within volcanic rocks represents the main public drinking water supply. The aforementioned studies highlight that high carcinogenic and non-carcinogenic risk is associated with water ingestion for those living in Northern Italy and Central and Southern Italy (including the capital Rome), also pointed out by [28]. This is true, although it is well known in the literature that As can be found in different oxidation states (As(III) and As(V), i.e., arsenites and arsenates) which have different effects on different organisms, from moderate (skin diseases) to severe (cancer) [29].
The European Union (EU) allows a maximum concentration of 10 μg/L for As in drinking water. However, in the Viterbo area, the concentration levels far exceed the limit, with the highest value recorded at 75 μg/L.
Being a volcanic area, the Viterbo province has a natural composition that includes arsenic. As a result, the water in the region is considered undrinkable by law and must be purified before it can be consumed. The installation of As removal plants is common in the region, and in 2014, 50 million euros were invested in dedicated purifiers to help mitigate the situation. Despite this, many municipalities have reported difficulties in managing the purifiers due to their high operational costs.
The novel technological plant presents a promising solution to improve the situation in the Viterbo province while keeping operational costs low. The plant has been designed to remove As from water sources efficiently, and its effectiveness has been demonstrated in the four case studies mentioned earlier. By implementing the new plant, the region can improve the quality of its water sources and ensure that the concentration of As in drinking water remains within the limits set by the EU.
The four case studies were selected with the primary goal of thoroughly testing the system under different circumstances in order to ensure its effectiveness and durability. Such a decision was driven by the need to ensure that the system is robust enough to handle various environmental factors and external influences that may affect its functionality. Testing the same portable plant multiple times provided valuable insight into how the system performs in the real world and allowed us to identify any potential issues or weaknesses that needed to be addressed. Through the testing process, the system’s design and functionality have been optimized, ensuring that it met the required performance standards. The testing of the plant also served as a validation of the system’s reliability, which is critical for ensuring the success of any large-scale technological project.
The novel portable technological plant has been employed in different dearsenification plants in the Viterbo province of Central Italy, named WT01, WT02, WT03, and WT04 (Figure 4).
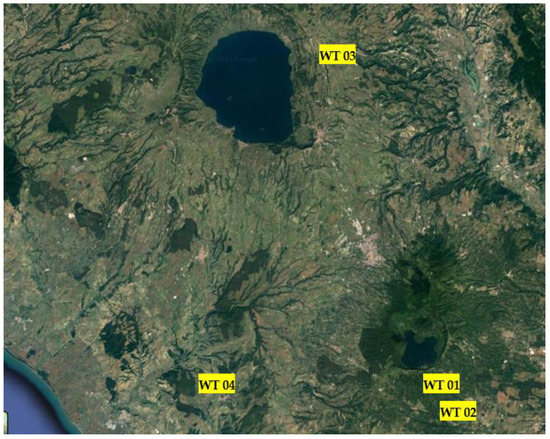
Figure 4.
Map of the 4 technological plants in the province of Viterbo, Central Italy.
The WT01 dearsenification plant has two filters in series, each one containing 1800 L of ferric hydroxides. The nominal plant flow rate is 12 L/s and the input As concentration is 34 μg/L.
The WT02 dearsenification plant has two filters in series, each one containing 1000 L of ferric hydroxides. The input water is particularly critical in terms of concentration not only for As (58 μg/L) but also vanadium (12 μg/L) and silica (64 μg/L). The nominal plant flow rate is 4.5 L/s.
The WT03 dearsenification plant has two filters in series, each one containing 3200 L of ferric hydroxides. The nominal plant flow rate is 17 L/s.
Finally, the WT04 dearsenification plant has one filter (named F1) of 4700 L of ferric hydroxides and two filters (named F2-F3) each with 5700 L of ferric hydroxides, connected in parallel. The nominal plant flow rate is 26 L/s.
5. The Performed Analysis
For the four investigated case studies, the As concentrations were periodically monitored in the water effluent, before and after the iron media regeneration, employing national and international guidelines [30,31,32]. Each analysis was performed two times and then the results were averaged. Other than As, pH and hydraulic conductivity were measured (continuously), but for the sake of brevity, only As and pH results are presented in detail in the following section. It is noteworthy that pH could have an effect on the regeneration process and its efficiency, and this could be a subject of future research, as specified in the conclusion section.
6. Results and Discussion
Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9 show the As time series monitored in the water effluent in the four investigated case studies.
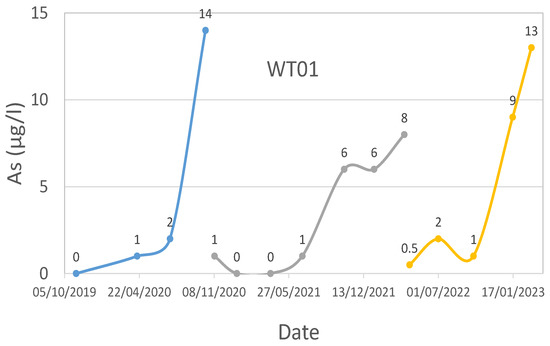
Figure 5.
WT01 technological plant water effluent’s As time series.
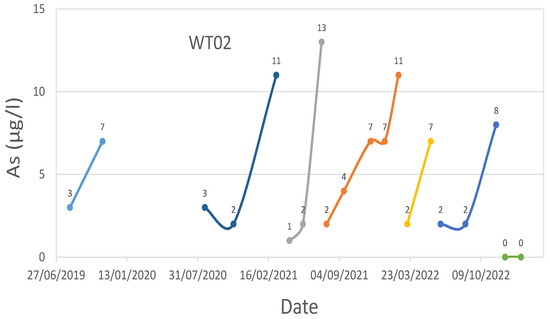
Figure 6.
WT02 technological plant water effluent’s As time series.
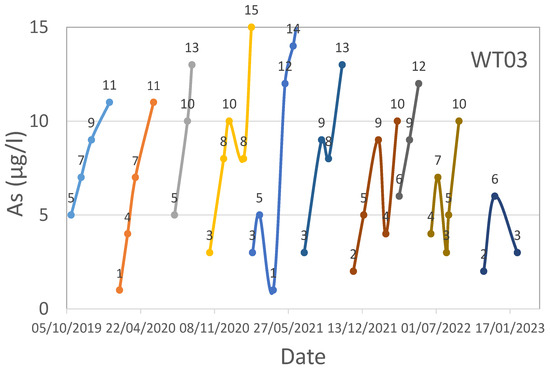
Figure 7.
WT03 technological plant water effluent’s As time series.
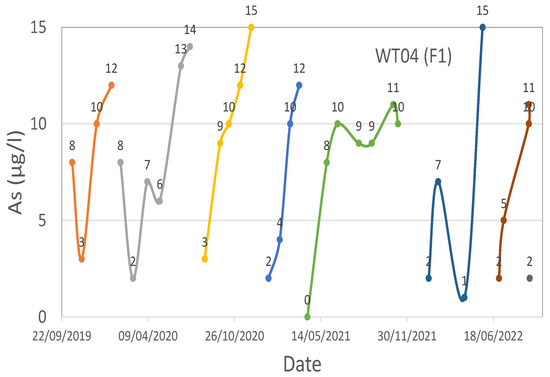
Figure 8.
WT04 (F1) technological plant water effluent’s As time series.
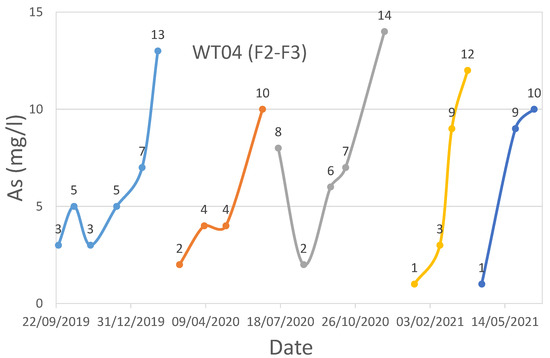
Figure 9.
WT04 (F2-F3) technological plant water effluent As time series.
Iron media regenerations were performed for WT01 in October 2019, September 2020, March 2022, and March 2023.
Iron media regenerations were performed for WT02 in March 2019, January 2020, March 2021, July 2021, March 2022, June 2022, and November 2022.
For WT03, iron media regenerations were performed in September 2019, February 2020, July 2020, October 2020, February 2021, July 2021, November 2021, March 2022, June 2022, and October 2022.
Iron media regenerations were performed for WT04 (F1) in October 2019, February 2020, July 2020, December 2020, March 2021, December 2021, June 2022, and September 2022.
For WT04 (F2-F3), iron media regenerations were performed in August 2019, March 2020, July 2020, December 2020, March 2021, July 2021, and December 2021.
Regarding WT01, the iron media kept the As level below the legal threshold of 10 μg/L until the passage of about 67,000 bed volumes (BV), equal to about 240,000 m3 of treated water. Then, it was decided to regenerate only one filter. The regeneration was performed not at the exact moment when the threshold was reached but sometime later, when the system had reached 74,000 BV (approximately 267,000 m3).
The new As legal threshold value of 10 μg/L achievement occurred at around 104,000 BV. It is interesting to note how the duration of the regeneration (104,000 − 74,000 = 30,000 BV) is aligned with the expected value, i.e., just under half of the previous duration, equal to 67,000/2 = 33,500 BV. In view of this second exceeding of the legal limit, it was decided to proceed with a new regeneration of the iron media.
In particular, the decision was again to reactivate only one filter, the one at the top of the series. Given the excellent response that brought the plant over 28,000 BV, the second filter was reactivated the following year. The response in terms of duration was also higher than expected, with approximately 31,000 BV.
The excellent results meant that the regeneration of the iron media can be carried out on both filters also not at the same moment, but one after the other. At present, the iron media continue to absorb arsenic and produce water with pollutant levels well below the legal limits.
Figure 5 shows the As concentration from 2019 to today, and it can be seen that periodically, the value of As increases (close to or above the limit for water intended for human consumption of 10 μg/L) and then decreases.
This happens in correspondence with the regenerations carried out at this plant in October 2019, September 2020, March 2022, and March 2023. It is noteworthy that after the iron media regeneration, the As concentration in the effluent is close to 0 μg/L, testifying to the effectiveness of the process.
Regarding WT02, it was decided to proceed with the regeneration of the iron media, as their duration did not allow for the treatment of more than 50,000 m3 before the performance decreased.
Since the first reactivation, carried out in March 2019, the iron media have been regenerated other five times (January 2020, March 2021, July 2021, November 2022, and July 2022) and, presumably, this operation will also be carried out in the future. Figure 6 shows the As concentration from 2019 to today: also for this case study, it can be seen how periodically, the value of As increases (close to or above the limit for water intended for human consumption of 10 μg/L) and then decreases. This happens in correspondence with the regenerations carried out at this plant. Unlike the previous case study, the As concentration in the effluent after the iron media regeneration is not close to 0 μg/L, having values close to 1 or 2 μg/L. This circumstance could be due to the input water that is particularly critical in terms of concentration not only for As but also for other elements. In any case, the water effluent As concentration is much lower than the legal threshold equal to 10 μg/L.
Regarding WT03 and WT04, as can be seen from Figure 7, Figure 8 and Figure 9, the trend is rather variable, but there are evident drops in the value of As near the regenerations, which took place in September 2019, February 2020, July 2020, October 2020, February 2021, July 2021, November 2021, March 2022, and October 2022 for WT03, while for WT04, the regenerations were performed at different moments based on the filter to reactivate (F1 or F2-F3).
Regarding the pH analysis, a general trend was observed in all four investigated case studies. The monitored pH was high (values greater than 12) during the desorption of As and other adsorbed elements, low (values not greater than 3–4) during the reacidification process, and neutral (values approximately equal to 7) during the last phases of the regeneration process.
In any case, the investigated case studies testify to the effectiveness of the proposed system related to the regeneration of the iron media, with the following fundamental advantages:
- Environmental sustainability: Iron media are no longer considered as waste to be disposed of in landfills (according to the European Waste Catalogue, 19 September 2001, “Solid waste produced by primary filtration and screening processes”) but indeed can be reused effectively according to the principles of a circular economy.
- Cost saving: Considering as an example a standard filter with a diameter of 2 m and a filter bed height of 1.1 m, the volume to be considered for the unique purchase of the filling material is approximately 3–4 m3, which corresponds to an expense of EUR 15,000–20,000 for the replacement of the masses. Moreover, other costs that should be taken into account are related to the landfill disposal of the exhausted iron media, which can be approximately quantified as EUR 2500, and the disposal of eluates, which can be approximately quantified as EUR 3500 (in the same example of the aforementioned standard filter). Of course, we also have to consider the cost of the reagents used in the regeneration process. Their exact quantity depends on the initial characteristics of the filter media, but in the same example of the aforementioned standard filter, we can roughly estimate an approximate cost of EUR 1000–2000.
- Using the regenerated material significantly reduces the production of the “new” media, with considerable savings in raw materials and convenience in terms of energy and economics.
- Low environmental impact, due to the reuse of materials and savings on disposal costs.
- Less dependence on producers (that at the moment are no more than 2–3 in Europe).
Moreover, the advantages deriving from regeneration with a mobile plant, directly on site, like in the here-investigated case studies, are as follows:
- Brief shutdown of the plant.
- Washing of the iron media on site, avoiding the emptying/filling of the iron media.
- Low environmental impact.
It is noteworthy that oxidation is known as a suitable method for the removal of As, but it is also true that oxidation alone can only transform As(III) to As(V), which is less toxic, and that the sorption processes of arsenite and arsenates on iron oxides are quite different and complex, as shown by [33]. In our opinion, this circumstance does not diminish the effectiveness and relevance of the presented technological plant and the related technical and economic implications.
7. Conclusions
Adsorptive media technology is a commonly employed approach for removing arsenic (As) from water, especially for drinking purposes. However, this method usually requires iron-based media, which are disposed of after one use. Since the replacement of iron media accounts for approximately 80% of the total operational cost, it would be more cost-effective to regenerate and reuse the media. In light of this issue, a novel and portable technological plant has been designed and tested in four real case studies to regenerate iron media, proving its feasibility. The following conclusions can be drawn:
- (1)
- The obtained results highlight the good efficiency of the system, which is able to regenerate the iron media and restore its capability to adsorb As from water almost entirely. When the legal threshold value of 10 μg/L is exceeded, the regeneration process is performed, and after that, the As concentration in the water effluent is at the minimum level in all the investigated case studies.
- (2)
- Multiple regenerations occurring from 2019 to 2023 seem to validate the system’s reliability, which is critical for ensuring the success of any large-scale technological project.
- (3)
- The main advantages of the proposed portable technological plant that emerged from the results are the renewal of the filter bed with the restoration of its original adsorption capacity; no solid waste to dispose of, thereby eliminating disposal costs; low environmental impact; a reduction in the production of “new” media, saving raw materials and improving energy efficiency; minimized system downtime; and limited equipment movement.
Further research is needed to test various types of iron media to investigate the possibility of extending the time between regenerations. The system’s good efficiency indicates that it has the potential to be used on a larger scale, which would help minimize the amount of iron media being thrown away, making the technology more sustainable and cost-effective. As aforementioned, the pH of the water to be treated could have an effect on the regeneration process and its efficiency, so this could also be a subject of future research.
The innovative approach described here has the potential to significantly reduce the operational cost of As removal from water. This is particularly important in developing countries where the cost of As removal is often prohibitive, leading to the consumption of As-contaminated water, which can have serious health implications. By using this technology, we can provide clean and safe drinking water to the affected population, improving their overall health and well-being.
In conclusion, the development of this novel and portable system for regenerating iron media is a promising step forward in As removal technology. Its potential for large-scale implementation could make it an attractive solution for developing countries where As contamination is a significant problem. Future research will continue to explore and optimize this technology, making it more effective, sustainable, and accessible.
Author Contributions
Conceptualization, I.C., L.F. and M.P.; methodology, I.C., L.F. and M.P.; software, I.C., L.F. and M.P.; validation, I.C., L.F. and M.P.; formal analysis, I.C., L.F. and M.P.; investigation, I.C., L.F. and M.P.; resources, I.C., L.F. and M.P.; data curation, I.C., L.F. and M.P.; writing—original draft preparation, I.C., L.F., M.P., C.A. and A.P.; writing—review and editing, I.C., L.F., M.P., C.A. and A.P.; project administration, I.C., L.F. and M.P.; funding acquisition, I.C., L.F. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
We thank the company Gajarda Srl for its technical and operational support (materials for experiments and highly specialized technicians).
Conflicts of Interest
The authors declare no conflict of interest.
References
- EU. Directive 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast). Off. J. EU. 2020, 435, 1–62. [Google Scholar]
- Liu, Y.; Chen, Z.; Yin, X.; Chen, Y.; Liu, Y.; Yang, W. Selective and efficient removal of As(V) and As(III) from water by resin-based hydrated iron oxide. J. Mol. Struct. 2023, 1273, 134361. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. A review on sources, identification and treatment strategies for the removal of toxic arsenic from water system. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef]
- He, Z.L.; Tian, S.L.; Ning, P. Adsorption of arsenate and arsenite from aqueous solutions by cerium-loaded cation exchange resin. J. Rare Earths 2012, 30, 563–572. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations; Arsenic and Clarification to Compliance and New Source Contaminant Monitoring. Final Rule Fed. Reg. 2001, 66, 6076. [Google Scholar]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef]
- Choong, T.S.Y.; Chuah, T.G.; Robiah, Y.; Gregory, F.L.; Koay, G.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Cundy, A.B.; Hopkinson, L.; Whitby, L.D. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Singh, R.D. Technological options for the removal of arsenic with special reference to South East Asia. J. Environ. Manag. 2012, 107, 1–18. [Google Scholar] [CrossRef]
- Mondal, P.; Bhowmick, S.; Chatterjee, D.; Figoli, A.; Wan der Bruggen, B. Remediation of inorganic arsenic in groundwater for safe water supply: A critical assessment of technological solutions. Chemosphere 2013, 92, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.A.; Sorg, T.J.; Ghurye, G.L. Ion exchange and adsorption of inorganics contaminants. In Water Quality and Treatment; A Handbook on Drinking Water; AWWA: Denver, CO, USA, 2011. [Google Scholar]
- Lakshmanan, D.; Clifford, D.; Samanta, G. Arsenic removal by coagulation with aluminum, iron, titanium and zirconium. J. Am. Water Work. Assoc. 2008, 100, 76–88. [Google Scholar] [CrossRef]
- Wang, L.; Chen, A.S.C. Costs of Arsenic Removal Technologies for Small Water Systems: U.S. EPA Arsenic Removal Technology Demonstration Program; EPA/600/R-04/210; USEPA, Office of Research and Development: Cincinnati, OH, USA, 2011. [Google Scholar]
- Chen, A.S.C.; Sorg, T.J.; Wang, L. Regeneration of iron-based adsorptive media used for removing arsenic from groundwater. Water Res. 2015, 77, 85–97. [Google Scholar] [CrossRef]
- Cornwell, D.A.; Roth, D.K. Water treatment plant residuals management. In Water Quality and Treatment; A Handbook on Drinking Water; AWWA: Denver, CO, USA, 2011. [Google Scholar]
- Mamindy-Pajany, Y.; Hure, C.; Marmier, N.; Romoe, M. Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-based iron: Effects of pH, concentration and reversibility. Desalination 2011, 281, 93–99. [Google Scholar] [CrossRef]
- Roy, P.; Mondal, N.K.; Das, K. Modeling of the adsorptive removal of arsenic: A statistical approach. J. Environ. Chem. Eng. 2013, 189, 1–13. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents e a critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Sorg, T.J.; Chen, A.S.C.; Wang, L.; Kolisz, R. Regenerating an arsenic removal iron-based adsorptive media system, part 1: The regeneration process. J. Am. Water Work. Assoc. 2017, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sorg, T.J.; Chen, A.S.C.; Wang, L.; Kolisz, R. Regenerating an arsenic removal iron-based adsorptive media system, part 2: Performance and cost. J. Am. Water Work. Assoc. 2017, 109, E122–E128. [Google Scholar] [CrossRef]
- Peña Reyes, F.A.; Crosta, G.B.; Frattini, P.; Basiricò, S.; Della Pergola, R. Hydrogeochemical overview and natural arsenic occurrence in groundwater from alpine springs (upper Valtellina, Northern Italy). J. Hydrol. 2015, 529, 1530–1549. [Google Scholar] [CrossRef]
- Zuzolo, D.; Cicchella, D.; Demetriades, A.; Birke, M.; Albanese, S.; Dinelli, E.; Lima, A.; Valera, P.; De Vivo, B. Arsenic: Geochemical distribution and age-related health risk in Italy. Environ. Res. 2020, 182, 109076. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, A.; Lotti, F.; Piscopo, V.; Chiocchini, U.; Madonna, S.; Manna, F. Hydraulic interactions between aquifers in Viterbo area (Central Italy). In Urban Groundwater Meeting the Challenge; Howard, K.W.F., Ed.; Taylor & Francis Group: London, UK, 2007; Volume 8, pp. 223–238. [Google Scholar]
- Cinti, D.; Vaselli, O.; Poncia, P.P.; Brusca, L.; Grassa, F.; Procesi, M.; Tassi, F. Anomalous concentrations of arsenic, fluoride and radon in volcanic sedimentary aquifers from central Italy: Quality indexes for management of the water resource. Environ. Pollut. 2019, 253, 525–537. [Google Scholar] [CrossRef]
- Flora, S.J.S. Handbook of Arsenic Toxicology; Academic Press: Cambridge, MA, USA, 2023; p. 954. [Google Scholar]
- Ozturk, M.; Metin, M.; Altay, V.; Ahmad Bhat, R.; Ejaz, M.; Gul, A.; Turkyilmaz Unal, B.; Hasanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and Human Health: Genotoxicity, Epigenomic Effects, and Cancer Signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- APAT/IRSA-CNR. Metodi Analitici per le Acque; Alluminio: Roma, Italy, 2003. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Dixit, S.; Hering, J.G. Comparison of Arsenic(V) and Arsenic(III) Sorption onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).