Drug Use before and during Pregnancy in Japan: The Japan Environment and Children’s Study
Abstract
:1. Introduction
2. Methods
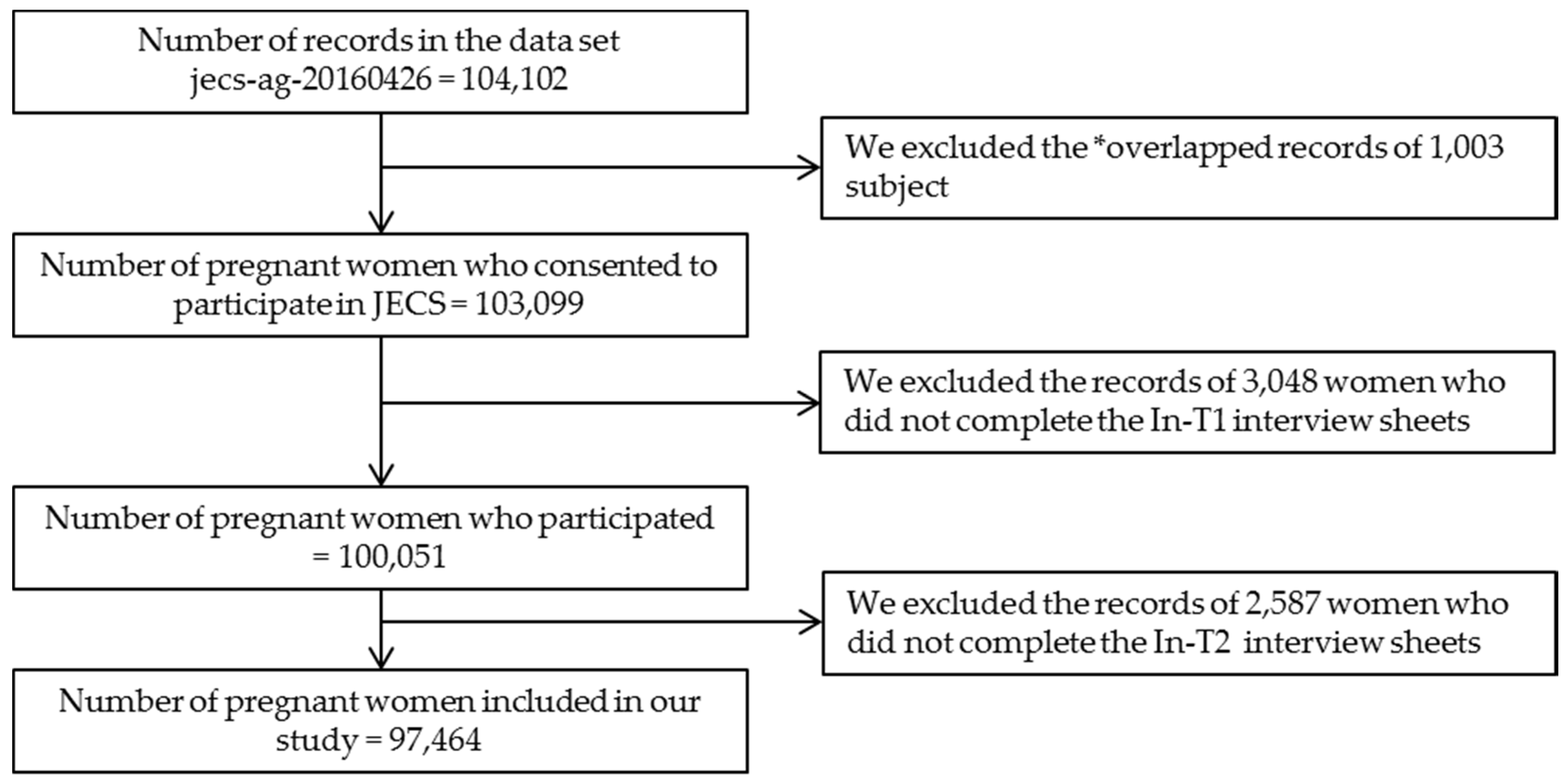
2.1. Study Settings and Subjects
2.2. Data Collection
2.3. Data Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
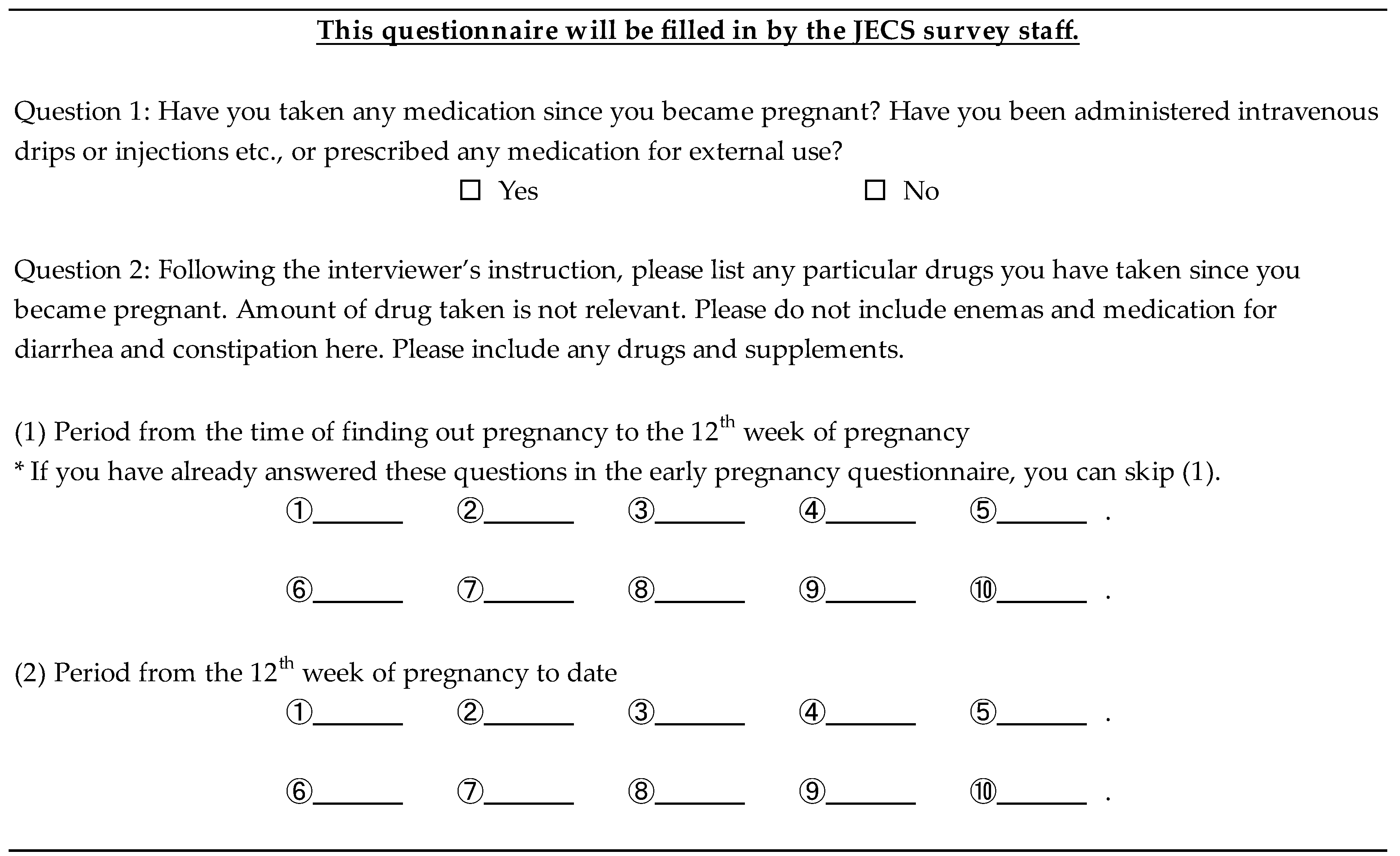
Appendix B
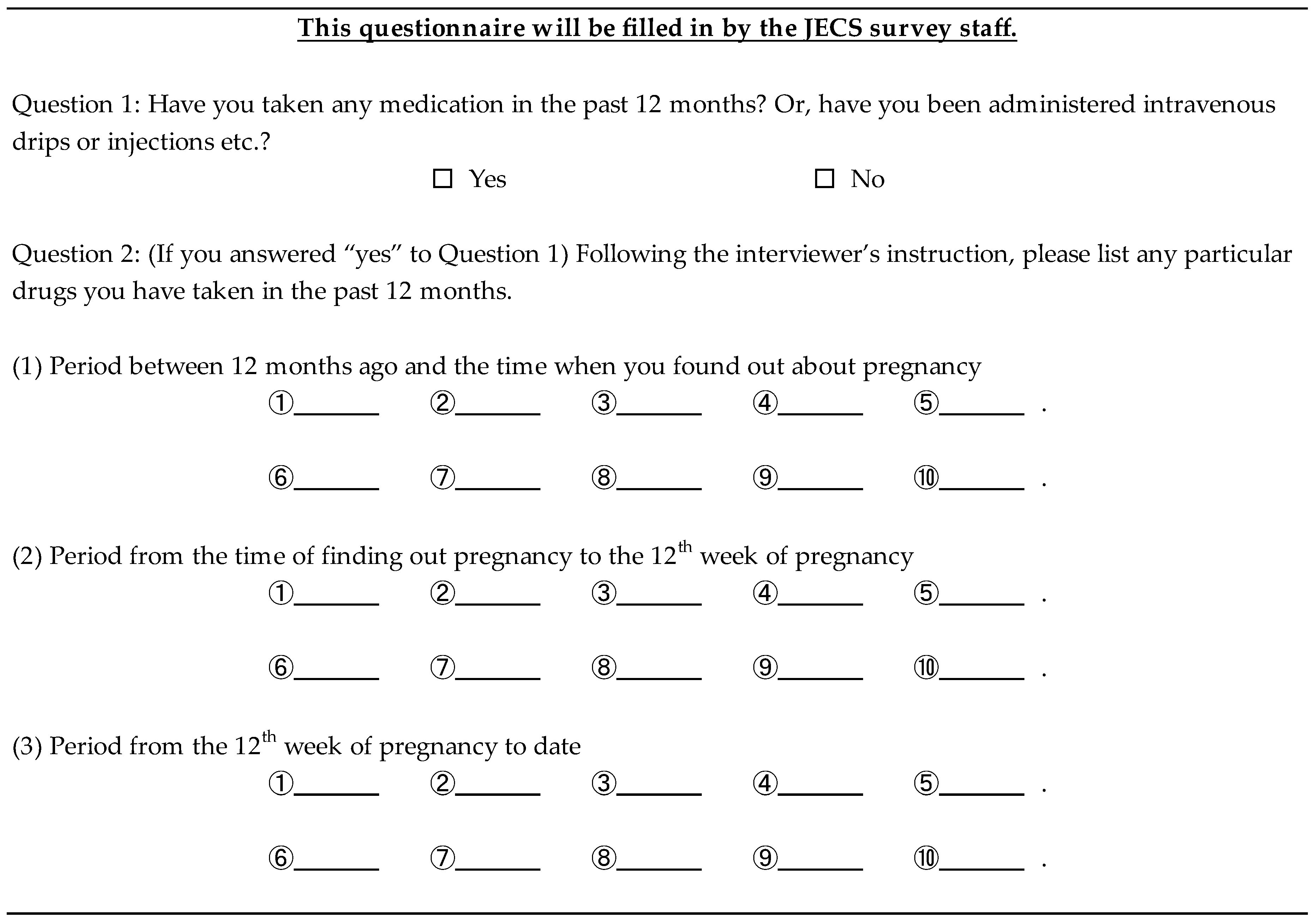
Appendix C

References
- Källén, B.A.J. Methodological issues in the epidemiological study of the teratogenicity of drugs. Congenit. Anom. 2005, 45, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kulaga, S.; Zagarzadeh, A.; Berard, A. Prescriptions filled during pregnancy for drugs with the potential of fetal harm. BJOG 2009, 116, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Malm, H.; Martikainen, J.; Klaukka, T.; Neuvonen, P.J. Prescription drugs during pregnancy and lactation—A Finnish register-based study. Eur. J. Clin. Pharmacol. 2003, 59, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Olesen, C.; Sorensen, H.T.; de Jong-van den Berg, L.; Olsen, J.; Steffensen, F.H.; Grp, E. Prescribing during pregnancy and lactation with reference to the Swedish classification system—A population-based study among Danish women. Acta Obstet. Gynecol. Scand. 1999, 78, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Olesen, C.; Steffensen, F.; Nielsen, G.; de Jong-van den Berg, L.; Olsen, J.; Sorensen, H. Drug use in first pregnancy and lactation: A population-based survey among Danish women. Eur. J. Clin. Pharmacol. 1999, 55, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Olesen, C.; Thrane, N.; Henriksen, T.; Ehrenstein, V.; Olsen, J. Associations between socio-economic factors and the use of prescription medication during pregnancy: A population-based study among 19,874 Danish women. Eur. J. Clin. Pharmacol. 2006, 62, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Beyens, M.; Guy, C.; Ratrema, M.; Ollagnier, M. Prescription of drugs to pregnantwomen in France: The HIMAGE study. Therapie 2003, 58, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.; Hurault, C.; Sarramon, M.F.; Guitard, C.; Berrebi, A.; Grau, M.; Albouy-Cossard, C.; Bourrel, R.; Elefant, E.; Montastruc, J.L.; et al. Prescription of drugs during pregnancy: A study using EFEMERIS, the new French database. Eur. J. Clin. Pharmacol. 2009, 65, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Egen-Lappe, V.; Hasford, J. Drug prescription in pregnancy: Analysis of a large statutory sickness fund population. Eur. J. Clin. Pharmacol. 2004, 60, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Baglio, G.; Spinelli, A.; Grandolfo, M.E. Drug use in pregnancy among Italian women. Eur. J. Clin. Pharmacol. 2000, 56, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gagne, J.J.; Maio, V.; Berghella, V.; Louis, D.Z.; Gonnella, J.S. Prescription drug use during pregnancy: A population-based study in Regione Emilia-Romagna, Italy. Eur. J. Clin. Pharmacol. 2008, 64, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.K.; Jentink, J.; Vroom, F.; Van Den Berg, P.B.; De Walle, H.E.; De Jong-Van Den Berg, L.T. Drug prescription pattern before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in The Netherlands. BJOG 2006, 113, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Schirm, E.; Meijer, W.; Tobi, H.; de Jong-van den Berg, L. Drug use by pregnant women and comparable non-pregnant women in The Netherlands with reference to the Australian classification system. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 114, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Engeland, A.; Bramness, J.G.; Daltveit, A.K.; Rønning, M.; Skurtveit, S.; Furu, K. Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106,000 pregnancies in Norway 2004–2006. Br. J. Clin. Pharmacol. 2008, 65, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.E.; Gurwitz, J.H.; Davis, R.L.; Chan, K.A.; Finkelstein, J.A.; Fortman, K.; McPhillips, H.; Raebel, M.A.; Roblin, D.; Smith, D.H.; et al. Prescription drug use in pregnancy. Am. J. Obstet. Gynecol. 2004, 191, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.; Damase-Michel, C.; Lapeyre-Mestre, M.; Montastruc, J.L. Prescription of drugs during pregnancy in France. Lancet 2000, 356, 1735–1736. [Google Scholar] [CrossRef]
- Reimann, I.; Karpinsky, C.; Hoffmann, A. Epidemiological data on drug use during pregnancy in Thuringia, East Germany, 1993. Int. J. Clin. Pharmacol. Ther. 1996, 34, 80–83. [Google Scholar] [PubMed]
- Riley, E.H.; Fuentes-Afflick, E.; Jackson, R.A.; Escobar, G.J.; Brawarsky, P.; Schreiber, M.; Haas, J.S. Correlates of prescription drug use during pregnancy. J. Womens Health 2005, 14, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Garriguet, D. Medication use among pregnant women. Health Rep. 2006, 17, 9–18. [Google Scholar] [PubMed]
- Rubin, J.; Ferencz, C.; Loffredo, C. Use of prescription and non-prescription drugs in pregnancy. J. Clin. Epidemiol. 1993, 46, 581–589. [Google Scholar] [CrossRef]
- Headley, J.; Northstone, K.; Simmons, H.; Golding, J. Medication use during pregnancy: Data from the Avon Longitudinal Study of Parents and Children. Eur. J. Clin. Pharmacol. 2004, 60, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qi, X.; Hao, J.; Huang, Z.; Zhang, Z.; Xing, X.; Cheng, D.; Xia, L.; Xu, Y.; Zhu, P.; et al. Pattern of drug use during the first trimester among Chinese women: Data from a population-based cohort study. Eur. J. Clin. Pharmacol. 2010, 66, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Daw, J.R.; hanley, G.E.; Greyson, D.L.; Morgan, S.G. Prescription drug use during pregnancy in developed countries: A systematic review. Pharmacoepidemiol. Drug Saf. 2011, 20, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.A.; Gilboa, S.M.; Werler, M.M.; Kelley, K.E.; Louik, C.; Hernández-Díaz, S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am. J. Obstet. Gynecol. 2011, 205, 51.e1. [Google Scholar] [CrossRef] [PubMed]
- Källén, B.; Borg, N.; Reis, M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals 2013, 10, 1221–1286. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Obara, T.; Nishigori, H.; Nishigori, T.; Metoki, H.; Ishikuro, M.; Tatsuta, N.; Mizuno, S.; Sakurai, K.; Nishijima, I.; Murai, Y.; et al. Prevalence and determinants of inadequate use of folic acid supplementation in Japanese pregnant women: The Japan Environment and Children’s Study (JECS). J. Matern. Fetal Neonatal Med. 2017, 30, 588–593. [Google Scholar] [CrossRef] [PubMed]
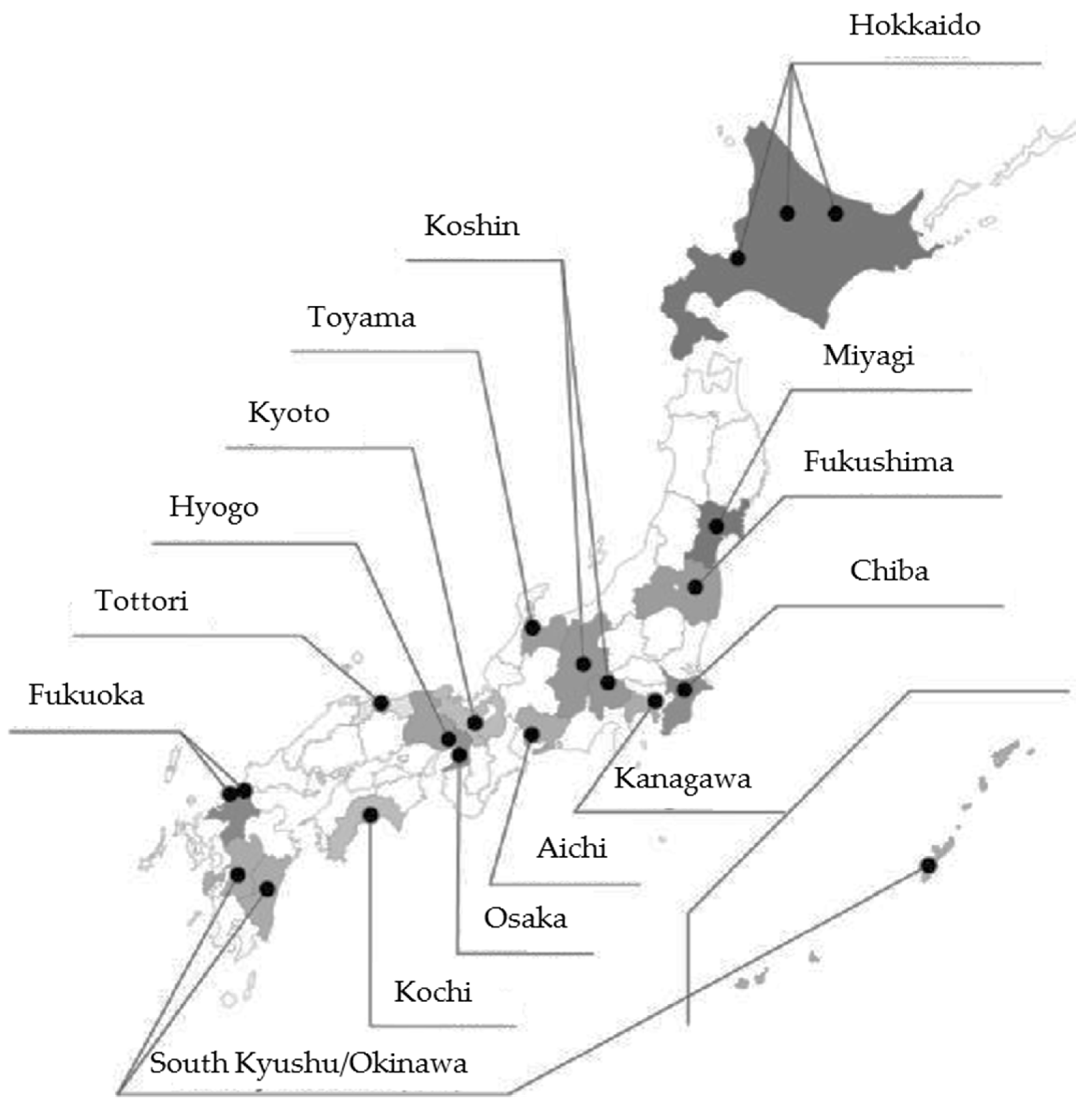
| Unit Center | n | % |
|---|---|---|
| Hokkaido | 7709 | 7.9 |
| Miyagi | 8775 | 9.0 |
| Fukushima | 12,820 | 13.2 |
| Chiba | 5461 | 5.6 |
| Kanagawa | 6288 | 6.5 |
| Koshin | 7020 | 7.2 |
| Toyama | 5326 | 5.5 |
| Aichi | 5451 | 5.6 |
| Kyoto | 3082 | 3.2 |
| Osaka | 7735 | 7.9 |
| Hyogo | 4951 | 5.1 |
| Tottori | 3007 | 3.1 |
| Kochi | 6817 | 7.0 |
| Fukuoka | 7403 | 7.6 |
| South Kyusyu and Okinawa | 5619 | 5.8 |
| n | % | ||
|---|---|---|---|
| Gestation (weeks) of In-T1 | Median(IQR) | 15.7 (12.3–22.1) | |
| No response | 530 | 0.5 | |
| Gestation (weeks) of In-T2 | Median(IQR) | 27.6 (25.3–31.7) | |
| No response | 526 | 0.5 | |
| Gestation (weeks) of M-T1 | Mean +/− SD | 16.8 +/− 7.4 | |
| Median(IQR) | 15.4 (12.4–19.4) | ||
| No response | 859 | 0.9 | |
| Gestation (weeks) of M-T2 | Mean +/− SD | 28.3 +/− 6.1 | |
| Median(IQR) | 27.7 (25.3–30.4) | ||
| No response | 913 | 0.9 | |
| Age | Mean +/− SD | 30.8 +/− 5.0 | |
| <30 years | 39,460 | 40.5 | |
| 30–34 years | 33,918 | 34.8 | |
| ≥35 years | 23,683 | 24.3 | |
| No response | 403 | 0.4 | |
| Marital status | Married | 92,478 | 94.9 |
| Single/divorced/widowed | 4183 | 4.3 | |
| No response | 803 | 0.8 | |
| Education | Junior high/high school | 35,109 | 36.0 |
| Technical/junior college | 40,517 | 41.6 | |
| University/graduate school | 20,816 | 21.4 | |
| No response | 1022 | 1.1 | |
| Family income | <4,000,000 yen | 36,280 | 37.2 |
| ≥4,000,000 and <6,000,000 yen | 29,788 | 30.6 | |
| ≥6,000,000 yen | 24,025 | 24.7 | |
| No response | 7371 | 7.6 | |
| Body mass index | <18.5 kg/m2 | 15,595 | 16.0 |
| ≥18.5 and <25 kg/m2 | 70,483 | 72.3 | |
| ≥25 kg/m2 | 10,301 | 10.6 | |
| No response | 1085 | 1.1 | |
| Smoking | Nonsmokers | 56,115 | 57.6 |
| Ever smokers | 35,632 | 36.6 | |
| Current smokers | 4615 | 4.7 | |
| No response | 1102 | 1.1 | |
| Alcohol consumption | Nondrinkers | 33,461 | 34.3 |
| Ever drinkers | 53,612 | 55.0 | |
| Current drinkers | 9570 | 9.8 | |
| No response | 821 | 0.8 | |
| Parity | Primipara | 38,397 | 39.4 |
| Multipara | 56,786 | 58.3 | |
| No response | 2281 | 2.3 | |
| Fertility treatment | Yes | 6463 | 6.6 |
| No | 90,595 | 93.0 | |
| No response | 406 | 0.4 | |
| History of spontaneous abortion | Yes | 18,410 | 18.9 |
| No | 77,476 | 79.5 | |
| No response | 1578 | 1.6 | |
| History of a mental health disorder | No | 89,531 | 91.9 |
| Yes | 7551 | 7.8 | |
| No response | 382 | 0.4 | |
| Hypertension | No | 95,766 | 98.3 |
| Yes | 1189 | 1.2 | |
| No response | 509 | 0.5 | |
| Diabetes | No | 95,909 | 98.4 |
| Yes | 1046 | 1.1 | |
| No response | 509 | 0.5 | |
| Mental health disorder | No | 96,183 | 98.7 |
| Yes | 772 | 0.8 | |
| No response | 509 | 0.5 | |
| Other pregnancy complication | No | 84,305 | 86.5 |
| Yes | 12,650 | 13.0 | |
| No response | 509 | 0.5 | |
| Hypertensive disorders of pregnancy | No | 93,896 | 96.3 |
| Mild | 2114 | 2.2 | |
| Severe | 945 | 1.0 | |
| No response | 509 | 0.5 | |
| Gestational diabetes | No | 94,302 | 96.8 |
| Yes | 2653 | 2.7 | |
| No response | 509 | 0.5 | |
| Other obstetric labor complication | No | 54,648 | 56.1 |
| Yes | 42,307 | 43.4 | |
| No response | 509 | 0.5 | |
| Before Pregnancy Diagnosis | Before Week 12 of Pregnancy | After Week 12 of Pregnancy | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Antibacterial, Antiviral, Antifungal, Carcinostatic drugs | Antimicrobial | 13,668 | 14.02 | 3223 | 3.31 | 8415 | 8.63 |
| Antiviral | 3749 | 3.85 | 1194 | 1.23 | 1521 | 1.56 | |
| Antifungal | 936 | 0.96 | 1112 | 1.14 | 1904 | 1.95 | |
| Carcinostatic | 628 | 0.64 | 503 | 0.52 | 65 | 0.07 | |
| Corticosteroids | Corticosteroids: oral administration, inhalation, infusion | 3008 | 3.09 | 1120 | 1.15 | 1660 | 1.70 |
| Corticosteroids: external use, enema | 5415 | 5.56 | 2756 | 2.83 | 4283 | 4.39 | |
| Antipyreic, Analgesic drugs | Antipyretic, Analgesic, Medicine for common cold: prescription | 29,064 | 29.82 | 7498 | 7.82 | 12,982 | 13.32 |
| Antipyretic, Analgesic, Medicine for common cold: over the counter | 33,799 | 34.68 | 3035 | 3.16 | 1162 | 1.19 | |
| Poultice which the analgesic is included in | 4352 | 4.47 | 926 | 0.97 | 1711 | 1.76 | |
| Antirheumatic drugs | Immunosuppressant, Immunoregulation | 650 | 0.67 | 470 | 0.48 | 144 | 0.15 |
| Infliximab, Etanercept | 42 | 0.04 | 20 | 0.02 | 21 | 0.02 | |
| Antirheumatic drug unidentified in detail | 14 | 0.01 | 4 | 0.00 | 4 | 0.00 | |
| Antiallergy drugs | Antiallergic drug (oral administration, inhalation, nasal drip, tape, Antihistaminic) | 12,213 | 12.53 | 2688 | 2.76 | 5389 | 5.53 |
| Respiratory drugs | β stimulative (oral administration, inhalation) | 528 | 0.54 | 286 | 0.29 | 541 | 0.56 |
| Nontypable inhalant | 144 | 0.15 | 51 | 0.05 | 77 | 0.08 | |
| Antitussive, Expectorant | 4711 | 4.83 | 1362 | 1.40 | 4131 | 4.24 | |
| Theophylline | 346 | 0.36 | 88 | 0.09 | 189 | 0.19 | |
| Other respiratory drug | 207 | 0.21 | 89 | 0.09 | 181 | 0.19 | |
| Antidiabetic drugs, Antihyperlipidemic drugs | Insulin preparation | 100 | 0.10 | 163 | 0.17 | 289 | 0.30 |
| Hypoglycemic tablet | 207 | 0.21 | 96 | 0.10 | 35 | 0.04 | |
| Antihyperlipidemic | 79 | 0.08 | 27 | 0.03 | 26 | 0.03 | |
| Antigout | 9 | 0.01 | 2 | 0.00 | 2 | 0.00 | |
| Hormone-related drugs | Thyroid hormone preparation/levothyroxine sodium | 642 | 0.66 | 640 | 0.66 | 710 | 0.73 |
| Antithyroid/Thiamazole | 360 | 0.37 | 309 | 0.32 | 311 | 0.32 | |
| Other hormone drugs | 2245 | 2.30 | 966 | 0.99 | 340 | 0.35 | |
| Blood-related drugs | Iron preparartion | 1243 | 1.28 | 1670 | 1.71 | 11,736 | 12.04 |
| Other blood-related | 1030 | 1.06 | 2181 | 2.24 | 2040 | 2.09 | |
| Cardiovasucular drugs | Antihypertensive (including diuretic) | 269 | 0.28 | 145 | 0.15 | 291 | 0.30 |
| Pressor | 32 | 0.03 | 8 | 0.01 | 18 | 0.02 | |
| Antiarrhythmic, Antianginal | 51 | 0.05 | 49 | 0.05 | 68 | 0.07 | |
| Heart failure therapeutic | 3 | 0.00 | 2 | 0.00 | 3 | 0.00 | |
| Other caridiovasucular drugs | 181 | 0.19 | 142 | 0.15 | 167 | 0.17 | |
| Gastrointestinal drugs | Antiulcer (Proton pump inhibitor, H2 blocker) | 1397 | 1.43 | 345 | 0.35 | 686 | 0.70 |
| General gastrointestinal agents | 8107 | 8.32 | 2777 | 2.85 | 5036 | 5.17 | |
| Other gastrointestinal agents | 2325 | 2.39 | 1416 | 1.45 | 2176 | 2.23 | |
| Psychoactive drugs | Selective serotonin reuptake inhibitors (SSRI) | 518 | 0.53 | 173 | 0.18 | 149 | 0.15 |
| Antidepressant drug except the SSRI | 265 | 0.27 | 91 | 0.09 | 79 | 0.08 | |
| Antianxiety | 992 | 1.02 | 326 | 0.33 | 372 | 0.38 | |
| Sleeping pill | 946 | 0.97 | 228 | 0.23 | 238 | 0.24 | |
| Antipsychotic | 233 | 0.24 | 104 | 0.11 | 119 | 0.12 | |
| Valproic acid | 180 | 0.18 | 66 | 0.07 | 68 | 0.07 | |
| Antiepileptic except the above | 142 | 0.15 | 117 | 0.12 | 133 | 0.14 | |
| Lithium carbonate | 30 | 0.03 | 3 | 0.00 | 4 | 0.00 | |
| Other psychoactive drugs | 116 | 0.12 | 32 | 0.03 | 33 | 0.03 | |
| Perinatal drugs | Utero relaxants | 522 | 0.54 | 4982 | 5.11 | 14,822 | 15.21 |
| Utero-tonic | 795 | 0.82 | 188 | 0.19 | 279 | 0.29 | |
| Ovulation inducing | 3846 | 3.95 | 277 | 0.28 | 22 | 0.02 | |
| Other perinatal related drugs | 2420 | 2.48 | 2672 | 2.74 | 1964 | 2.02 | |
| Other drugs | Anesthetic, pain block injection | 932 | 0.96 | 112 | 0.11 | 447 | 0.46 |
| Chinese herbal medicines | 8389 | 8.61 | 5865 | 6.02 | 9175 | 9.41 | |
| External application (non-identified contents) | 4905 | 5.03 | 1972 | 2.02 | 3966 | 4.07 | |
| Injection, Drip infusion (non-identified contents) | 1460 | 1.50 | 1043 | 1.07 | 942 | 0.97 | |
| Bone & Calcium metabolism | 93 | 0.10 | 95 | 0.10 | 126 | 0.13 | |
| Antimigraine headache | 308 | 0.32 | 60 | 0.06 | 87 | 0.09 | |
| Muscle relaxant | 269 | 0.28 | 23 | 0.02 | 21 | 0.02 | |
| Antiemetic drug | 985 | 1.01 | 1802 | 1.85 | 1316 | 1.35 | |
| AntiParkinson | 112 | 0.11 | 38 | 0.04 | 12 | 0.01 | |
| Hemorrhoids | 297 | 0.30 | 377 | 0.39 | 1076 | 1.10 | |
| Supplements, vitamins/minerals | Folic acid | 6810 | 6.99 | 28,153 | 28.89 | 25,494 | 26.16 |
| Vitamin A | 122 | 0.13 | 70 | 0.07 | 72 | 0.07 | |
| Vitamin B | 2648 | 2.72 | 2282 | 2.34 | 2894 | 2.97 | |
| Vitamin C | 2794 | 2.87 | 1536 | 1.58 | 1793 | 1.84 | |
| Vitamin D | 131 | 0.13 | 111 | 0.11 | 148 | 0.15 | |
| Vitamin E | 636 | 0.65 | 277 | 0.28 | 267 | 0.27 | |
| Mineralas | 1760 | 1.81 | 4797 | 4.92 | 6751 | 6.93 | |
| Multi vitamins supplement | 3766 | 3.86 | 2956 | 3.03 | 3059 | 3.14 | |
| Total supplement | 2620 | 2.69 | 2680 | 2.75 | 3380 | 3.47 | |
| Illegal drugs | Marijuana | 2 | 0.00 | 1 | 0.00 | 3 | 0.00 |
| Psychostimulant | 1 | 0.00 | 0 | 0.00 | 2 | 0.00 | |
| Ecstasy | 0 | 0.00 | 0 | 0.00 | 1 | 0.00 | |
| Thinner | 1 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Toluene | 2 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Other illegal drugs | 2 | 0.00 | 1 | 0.00 | 0 | 0.00 | |
| Vaccines | 1431 | 1.47 | 318 | 0.33 | 897 | 0.92 | |
| Drugs not included in the list mentioned above | 5064 | 5.20 | 3011 | 3.09 | 5272 | 5.41 | |
| Forgot the drug name | 1342 | 1.38 | 486 | 0.50 | 607 | 0.62 | |
| Number of Items | Before Pregnancy Diagnosis | Before Week 12 of Pregnancy | After Week 12 of Pregnancy |
|---|---|---|---|
| % | % | % | |
| 0 | 21.59 | 42.93 | 31.16 |
| 1 | 25.54 | 27.61 | 25.51 |
| 2 | 20.06 | 15.46 | 18.49 |
| 3 | 19.34 | 9.75 | 14.30 |
| 4 | 5.14 | 2.60 | 5.16 |
| 5 | 3.32 | 0.97 | 2.57 |
| 6 | 2.56 | 0.43 | 1.39 |
| 7 | 1.51 | 0.17 | 0.69 |
| 8 | 0.69 | 0.06 | 0.40 |
| 9 | 0.24 | 0.02 | 0.19 |
| 10 | 0.02 | 0.01 | 0.08 |
| 11 | - | 0.003 | 0.03 |
| 12 | - | - | 0.01 |
| 13 | - | - | 0.005 |
| 14 | - | - | 0.002 |
| >1 | 78.41 | 57.07 | 68.84 |
| Number of Items | Before Pregnancy Diagnosis | Before Week 12 of Pregnancy | After Week 12 of Pregnancy |
|---|---|---|---|
| % | % | % | |
| 0 | 24.67 | 63.98 | 48.28 |
| 1 | 28.46 | 20.54 | 25.22 |
| 2 | 20.71 | 9.03 | 13.65 |
| 3 | 15.39 | 4.10 | 7.52 |
| 4 | 4.50 | 1.39 | 2.71 |
| 5 | 2.75 | 0.58 | 1.32 |
| 6 | 1.80 | 0.26 | 0.68 |
| 7 | 1.08 | 0.08 | 0.35 |
| 8 | 0.50 | 0.03 | 0.17 |
| 9 | 0.14 | 0.01 | 0.07 |
| 10 | 0.01 | 0.005 | 0.03 |
| 11 | - | - | 0.01 |
| 12 | - | - | 0.003 |
| >1 | 75.33 | 36.02 | 51.72 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishigori, H.; Obara, T.; Nishigori, T.; Metoki, H.; Ishikuro, M.; Mizuno, S.; Sakurai, K.; Tatsuta, N.; Nishijima, I.; Fujiwara, I.; et al. Drug Use before and during Pregnancy in Japan: The Japan Environment and Children’s Study. Pharmacy 2017, 5, 21. https://doi.org/10.3390/pharmacy5020021
Nishigori H, Obara T, Nishigori T, Metoki H, Ishikuro M, Mizuno S, Sakurai K, Tatsuta N, Nishijima I, Fujiwara I, et al. Drug Use before and during Pregnancy in Japan: The Japan Environment and Children’s Study. Pharmacy. 2017; 5(2):21. https://doi.org/10.3390/pharmacy5020021
Chicago/Turabian StyleNishigori, Hidekazu, Taku Obara, Toshie Nishigori, Hirohito Metoki, Mami Ishikuro, Satoshi Mizuno, Kasumi Sakurai, Nozomi Tatsuta, Ichiko Nishijima, Ikuma Fujiwara, and et al. 2017. "Drug Use before and during Pregnancy in Japan: The Japan Environment and Children’s Study" Pharmacy 5, no. 2: 21. https://doi.org/10.3390/pharmacy5020021
APA StyleNishigori, H., Obara, T., Nishigori, T., Metoki, H., Ishikuro, M., Mizuno, S., Sakurai, K., Tatsuta, N., Nishijima, I., Fujiwara, I., Arima, T., Nakai, K., Mano, N., Kuriyama, S., Yaegashi, N., & Japan Environment & Children’s Study Group. (2017). Drug Use before and during Pregnancy in Japan: The Japan Environment and Children’s Study. Pharmacy, 5(2), 21. https://doi.org/10.3390/pharmacy5020021







