Abstract
This study introduces an innovative approach involving the injection of hydrogen into a low-swirl, non-premixed flame, which operates with gaseous fuels derived from an air-blast atomizer designed for aero-engine applications. The aim is to characterize how hydrogen enrichment influences flame structures while maintaining a constant thermal output of 4.6 kW. Using high-speed chemiluminescence imaging, three fueling conditions were compared: the first involved pure methane/air, while the second and third conditions introduced varying levels of hydrogen to an air–methane mixture. The results reveal significant effects of hydrogen enrichment on flame characteristics, including a slightly shorter length and a wider angle attributed to heightened expansion within the Combustion Recirculation Zone. Moreover, the emission of UV light underwent considerable changes, resulting in a shifted luminosity zone and reduced variance. To delve deeper into the underlying mechanisms, the researchers employed Proper Orthogonal Decomposition (POD) and Spectral Proper Orthogonal Decomposition (SPOD) analyses, showing coherent structures and energetic modes within the flames. Hydrogen enrichment led to the development of smaller structures near the nozzle exit, accompanied by longitudinal oscillations and vortex shedding phenomena. These findings contribute to an advanced understanding of hydrogen’s impact on flame characteristics, thereby propelling efforts toward improved flame stability. Additionally, these insights hold significance in the exploration of hydrogen as an alternative energy source with potential environmental benefits.
1. Introduction
Combustion plays a fundamental role in air transportation, given the high energy density of liquid fuels. However, the current inefficiency of existing aero-engines and the production of harmful emissions, contributing to climate change, exhibit significant challenges.
The EU’s Fit-for-55, emphasizing the pressing demand for a 55% reduction in carbon dioxide (CO2) emissions by 2030 compared to 1990 levels, underscores the critical imperative to achieve a climate-neutral Europe by 2050. This initiative seeks to propel the continent towards environmental sustainability and resilience in the face of evolving climate challenges [1].
As per the International Energy Agency (IEA), the essential pillars for decarbonizing the global energy system encompass energy efficiency, behavioral change, electrification, renewables, hydrogen and hydrogen-based fuels, and carbon capture, utilization, and storage (CCUS) [2].
To attain this objective, a substantial augmentation in the utilization of renewable energy sources aiming to reduce dependency on fossil fuels is imperative [3A].
In accordance with stringent emission standards and in pursuit of enhanced fuel efficiency, multiple international organizations are investigating the viability of lean combustor concepts.
In recent years, gas turbine (GT) research has prominently shifted towards developing novel combustion systems that can concurrently ensure low pollutant emissions and safe operability across diverse operating conditions. The pursuit of reduced emissions has led to the exploration of lean burn concepts, introducing new challenges related to flame stability, flashback occurrences, and Lean Blow-Off (LBO) limits.
Lean fuel burning serves as an effective approach to decrease NOx emissions by reducing flame temperature. Nonetheless, these lower-temperature flames are susceptible to critical instabilities, posing challenges such as re-ignition and flame blowout issues [3].
In many existing applications, these challenges are addressed through the implementation of a partially premixed system, albeit with limitations on the potential benefits in terms of nitrogen oxide (NOx) emissions.
Burning hydrogen produces primarily water (H2O), making it a genuinely emission-free fuel in terms of CO2. The ultimate objective is to transition to 100% green hydrogen for combustion, replacing natural gas entirely. In the interim, a more immediate goal involves blending hydrogen with both natural gas and liquid fuels for use in gas turbines and other industrial combustion applications, thereby achieving a partial reduction in CO2 emissions.
Moreover, the versatility of hydrogen extends to the transportation and aviation sectors, where it can be blended with liquid fuels such as aviation kerosene (jet A). This strategic blending allows for a smoother transition and integration of hydrogen into existing infrastructure and combustion technologies, promoting a gradual reduction in greenhouse gas emissions across various industries. The term “fuel-flexibility” remains critical in these applications, ensuring stable, safe, and reliable operation as hydrogen blends are introduced, providing adaptability to the evolving landscape of renewable energy sources.
To address issues with methane combustion, the gas turbine industry is also actively investigating lean premixed combustion with hydrogen-enriched fuel blends as a promising strategy to mitigate both greenhouse gas emissions and NOx emissions [4]. In the present scenario, numerous gas turbines have the capability to combust blends of hydrogen in varied proportions [5,6].
Adding a more reactive and cleaner fuel, such as hydrogen, could offer a practical solution [7]. The blending of methane with hydrogen has demonstrated the ability to improve performance and reduce emissions without necessitating modifications to existing combustors [8].
When utilizing hydrogen, either in conjunction with natural gas or liquid fuels, as a complete substitute, it is essential to understand the distinctions between these two fuels.
Hydrogen, with a density one-ninth that of natural gas and being the smallest known molecule, poses challenges in terms of transportation and sealing. Additionally, hydrogen’s heating value is only one-third that of natural gas on a volumetric basis, meaning three times the amount of hydrogen fuel is required to generate an equivalent power output compared to natural gas.
In hydrogen combustion, despite the need for a higher volume flow of fuel for equivalent energy production, approximately 20% less air by volume is necessary to generate a flame comparable to natural gas. This reduction in the air volume requirement leads to a decreased mass flow through the combustor, consequently reducing convective heat transfer.
Furthermore, hydrogen exhibits a considerably wider flammability range than conventional liquid or gaseous fuels, raising heightened concerns regarding environmental, health, and safety aspects during both hydrogen transportation and combustion.
Numerous studies [7,8,9] have concentrated on assessing the influence of hydrogen on the flame propagation of hydrogen-enriched mixtures.
Halter et al. [9] conducted a study on the impact of hydrogen blends and inlet pressure on the laminar flame speed. The findings suggested that the laminar flame speed increased with higher hydrogen contents and decreased with elevated inlet pressures. Mandilas et al. [10] investigated the influence of hydrogen on iso-octane-air and methane mixtures under both laminar and turbulent conditions. Their findings revealed that the introduction of hydrogen resulted in earlier flame instabilities, yet it improved laminar flame speed at lean limits in turbulent combustion.
The introduction of hydrogen into methane marginally enhanced reactivity under lean conditions but introduced complexities, safety concerns, and thermoacoustic instabilities [7,8]. However, flame speed demonstrated a slight improvement under lean conditions compared to rich conditions [6].
Fruzza et al. [11] investigate flashback in different H2/CH4 mixtures at an equivalence ratio (φ) of 0.8, proposing a numerical model for predicting critical flashback velocity in laminar premixed flames. Two flashback regimes are identified based on H2 content. Results suggest that relying solely on laminar flame speed is inadequate for designing safe operation in practical premixed burners.
Besides the significant benefits of hydrogen addition, achieving effective mixing of hydrogen and air before combustion presents a significant challenge. To address this, micro-mixing technologies have been developed to avert the formation of high-temperature stoichiometric reaction layers at the hydrogen injector outlet, which have the potential to result in elevated NOx emissions and thermal stress [12,13,14].
This study introduces an innovative approach involving the injection of hydrogen into a low-swirl, non-premixed flame, which operates with gaseous fuels derived from an air-blast atomizer designed for aero-engine applications [15,16]. Previous experimental investigations by Sedlmaier [15] and Fokaides [16] extensively explored this burner using both gaseous methane and liquid jet A fuels, offering a comprehensive description of the flame topology. Langone et al. [17] complemented the experimental work with numerical simulations using Large Eddy Simulation (LES) and three different combustion models.
The distinctive feature of this burner configuration lies in its capacity for partial premixing with methane before combustion, effectively preventing the formation of diffusion–reaction layers. Importantly, this achievement does not necessitate extensive redesign of the combustor [18]. The underlying principle of this concept hinges on the intricate interaction between the swirling jet and the adjacent confinement walls. This interaction establishes a robust outer recirculation zone, facilitating the efficient upstream transport of combustion products from the primary reaction region to the flame’s base.
To the best of the authors’ knowledge, no previous studies have explored the application of this nozzle concept fueled with hydrogen, especially in a blend.
The simulations [17] underlined that the flame attains stability when the velocity decreases to a sufficiently low level, as evident from both the diminishing methane concentration and the rising mean temperature in the axial direction of the flame tube. The flame’s base is firmly anchored along the outer shear layer of the swirling jet, discernible by the zero axial velocity isoline tangential to the heat-release zones. Additionally, the instantaneous fields of temperature and equivalence ratio reveal turbulent instabilities at the jet’s base, causing the entrainment of hot vitiated products into the fresh mixture jet and facilitating continuous re-ignition of the reactants.
The present study investigates how H2 enrichment impacts flame characteristics and dynamics for this burner, employing modal decomposition techniques.
In recent times, the combustion process has undergone thorough examination through high-speed acquisition of OH* and CH* chemiluminescence images and flow field images. The high-speed chemiluminescence technique serves as a valuable tool for generating extensive time-resolved datasets, serving as a representative sample for in-depth analysis to extract characteristic frequencies and identify corresponding combustion modes.
The analysis of chemiluminescence acquisitions has been carried out employing advanced data science concepts, such as Fast Fourier Transform (FFT), Proper Orthogonal Decomposition (POD), Dynamic Mode Decomposition (DMD), and Spectral Proper Orthogonal Decomposition (SPOD). These techniques enable the extraction of frequency characteristics, fluctuation patterns, identification of unstable heat release structures, and reconstruction of the flame. Additionally, they facilitate the analysis of multi-physics correlations within the combustion system.
The derived frequency is pivotal in comprehending the feedback loop associated with combustion instability. SPOD and DMD facilitate the elucidation of oscillation regions and the growth patterns of instability. This analytical capability allows for a detailed examination of the mechanisms underlying combustion instability.
Hence, for insight identification, segregation, and temporal resolution of dynamic components, the study employs Spectral Proper Orthogonal Decomposition (SPOD). This method represents an advanced iteration of the conventional POD method, incorporating spectral analysis into the process [19,20]. SPOD is essentially the frequency domain counterpart of POD. It furnishes spatially orthogonal modes characterized by oscillations at individual, distinct frequencies. This method retains the energy-ranking capability inherent in POD and offers a comprehensive understanding of the spatial distribution of energy content at specific frequencies.
The SPOD method was employed to meticulously study and compare flames with and without hydrogen (H2) blends. This advanced analytical approach allowed for an in-depth analysis of the main fluctuation structure and frequency domain characteristics of the flames, shedding light on the factors contributing to their formation. The comparative study facilitated a comprehensive understanding of the impact of hydrogen blends on flame behavior, providing valuable insights into the combustion dynamics under different fuel conditions. The integration of hydrogen into methane combustion represents a complex and multifaceted research domain that demands thorough exploration. Consequently, there is a discernible gap in understanding the combustion characteristics, stability, and efficiency of such blended fuels. Closing this research gap is imperative for advancing the knowledge and optimizing combustion systems for improved performance and reduced environmental impact.
The application of these decomposition methods enables the extraction of the most energetic spatial modes. This approach sheds light on strategies to enhance specific modes crucial for the mixing process while concurrently offering insights on mitigating or eliminating modes that may pose challenges. For instance, if a mode’s frequency aligns closely with the resonance frequency of the chamber or the downstream turbine blades, identifying and modifying such frequencies becomes crucial. Furthermore, discerning the frequencies of different modes opens avenues for geometric alterations that can redistribute spectral energy to a more favorable frequency band, potentially concealing it within the ambient noise generated by other sources like the compressor or jet mixing process.
2. Experimental Characterization
An experimental facility operating under atmospheric conditions was employed for this study. The test stand includes a nozzle holder for mounting and positioning the investigated nozzle, along with an optically accessible combustion chamber for implementing the measurement techniques. A box with four rectangular surfaces measuring 105 mm in width and 360 mm in height was used. It featured a single burner with an atmospheric nozzle and was used to study the attached swirl flame, as depicted in Figure 1. To induce swirled flames, a nozzle based on the air-blast concept, with an effective area of Aeff = 319 mm2, was employed. This design aimed to replicate the realistic geometrical scale of aero-engines, like the nozzle investigated by Kasabov et al. [21].
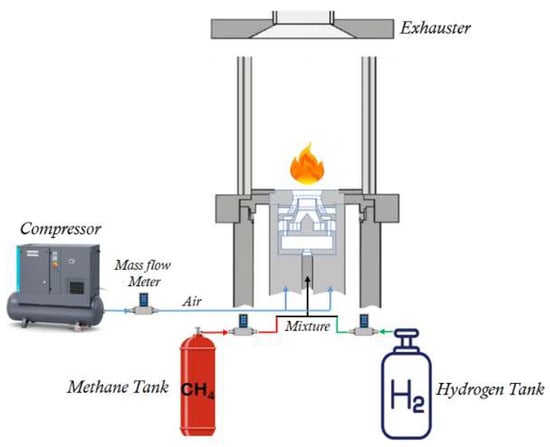
Figure 1.
An illustrative diagram depicting the experimental arrangement.
The nozzle comprises five parts, notably two swirlers designed to induce rotary movement in the air before entering the burner. The system comprises a modular configuration involving two radial swirl generators, an atomizer lip that segregates the two air streams within the nozzle, and an air diffuser. Methane is supplied to the atomizer through a central pipe along the nozzle’s axis. The primary airflow is introduced at the lower half of the nozzle holder and is subsequently enveloped by secondary air supplied through a connecting section at the upper half of the nozzle assembly.
The choice of gaseous fuel, specifically methane, under atmospheric conditions was made to facilitate a detailed investigation of the flame, even though the injector was originally designed to be fueled with jet A for aeronautical applications. This deliberate selection aimed to focus on key aspects of the combustion process, disregarding secondary parameters that might have arisen, such as the two-phase flow within the flow pattern.
Hydrogen and methane from bottles (purity 99.9%) were used as fuels. The flow rates of the fuels were accurately calculated to sustain a consistent thermal power output of 4.6 kW. Firstly, only pure methane was used at a rate of 8.33 L/min. Secondly, a blend of hydrogen and methane was employed, with a hydrogen flow rate of 3.33 L/min (approximately constituting 30% of the total mixture flow rate) and a methane flow rate of 7.33 L/min. The third case involved a hydrogen flow rate of 5 L/min (approximately 40% of the total mixture flow rate) and a methane flow rate of 6.83 L/min. The initial ignition equivalence ratio (ϕ) was established at 0.8, and it experienced a slight reduction to 0.77 in the case of the mixed fuel. Throughout these experiments, a steady inlet airflow rate of 100 L/min was maintained.
In this research, we employed high-speed imaging of reaction zone chemiluminescence to study the spatio-temporal distribution of the flame front and primary combustion zone. Emissions were captured using a Phantom Miro M320S High speed camera by Vision Research Inc. (Charlottetown, Canada), equipped with a Lambert image intensifier. Notably, no spectral filtering was employed during the imaging process. Grayscale images were recorded at a frame rate of 1000 Hz, with each pixel quantized using 8 bits, covering a range from 0 to 255. To conduct modal spatio-temporal characterization of the flame based on high-speed acquisitions, we utilized both Proper Orthogonal Decomposition (POD) and Spectral Proper Orthogonal Decomposition (SPOD) methods [8,9].
3. Results and Discussions
3.1. High-Speed Imaging of Chemiluminescence in Reaction Zones
The chemiluminescence of a combustion process originates from two distinct sources. Firstly, there is the contribution from the flame front, primarily constituted by discrete emissions from radicals such as OH*, CH*, and C2*. Secondly, there is the continuous emission resulting from the hot gases during the post-combustion period, commonly referred to as afterglow emissions.
High-speed chemiluminescence images, recorded at a rate of 1000 frames per second, were acquired for all conditions, involving both methane-only and hydrogen-enriched methane conditions. No spectral filtering was employed during image acquisition. The objective of this imaging was to assess visual distinctions in reaction zones arising from the introduction of hydrogen into the methane fuel.
Broadband chemiluminescence techniques were utilized to qualitatively examine the impact of hydrogen addition on flame structure. Image capture settings were kept uniform for both conditions with and without hydrogen while maintaining a constant thermal power output. This consistency facilitated a direct comparison of the pertinent distinctions in flame appearance. In Figure 2 the image in the first row was obtained with pure methane/air, and in the second and third rows, progressively increasing amounts of hydrogen were added.
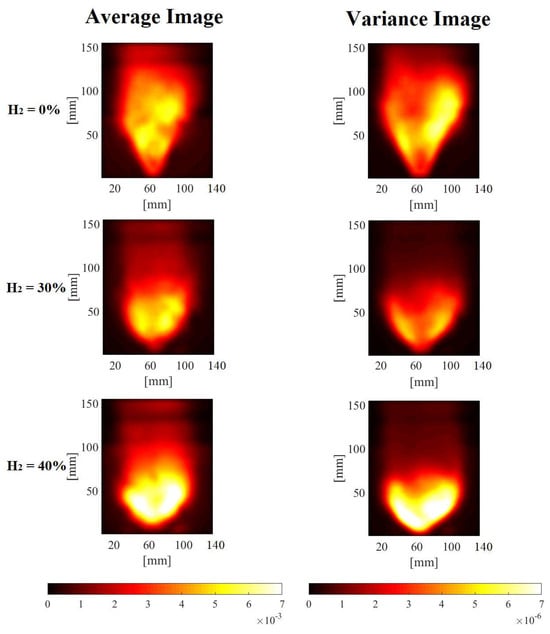
Figure 2.
Time-averaged and variance of the broadband chemiluminescence signal (line-of-sight) without and with H2 presence.
Figure 2 provides an analysis of average and variance flame structures, offering a comprehensive perspective on the impact of hydrogen addition. With a gradual increase in hydrogen concentration within the mixture, it becomes apparent that the average flame length is reduced compared to the pure methane case, and the flame angle gradually expands.
While the position of the flame root remains relatively consistent among the three cases, the location of the maximum heat release rate is notably closer to the burner outlet in the case of H2 addition. This phenomenon of a shorter flame length and wider angle is attributed to the increased expansion of burned gases within the combustion recirculation zone (CRZ) owing to the higher heat release rate. This is also evident in the gradually increasing flame intensity. The impact of hydrogen presence on UV emission was notably more pronounced, with the observation that the brightness zone shifted toward the nozzle exit.
The UV variance experienced a significant reduction with the introduction of hydrogen. This decrease in fluctuations contributes to enhanced flame stability. To gain deeper insights into how hydrogen improves combustion, the study employed the Proper Orthogonal Decomposition (POD) and the Spectral Proper Orthogonal Decomposition (SPOD) methods to identify coherent structures and energetic modes within the flame.
Figure 3 provides a comparative analysis of the initial four POD modes in UV chemiluminescence images using three different fueling conditions: the first without hydrogen addition and the other two with the presence of 30% and 40% hydrogen, respectively. The contour plots illustrating mode shapes are presented without a specific scale or sign, as they are scaled based on their individual time coefficients. The colors hot red and yellow are used to highlight areas with the highest intensity of heat fluctuations. Importantly, these colors denote contrasting directional changes, indicating a phase difference of π between different regions. It is crucial to note that these modes represent heat fluctuations, starting from the most energetic mode (first mode). The Proper Orthogonal Decomposition (POD) technique divides dynamic data into orthogonal modes ordered by descending energy levels, where the initial mode possesses the highest energy. The energy contribution of each mode to the reconstruction of the flow field is assessed based on its energy content. In Figure 3b, the energy contributions, namely the relative flame fluctuations of eigenvalues 1–8, are illustrated for all three cases, both with and without the addition of hydrogen. Typically, it was noticed that the initial eight modes consumed most of the relative energy. Moreover, it was observed that the energy content of the primary mode in the hydrogen-enriched methane flame was lower than that in the pure methane flame. Clearly, the introduction of hydrogen resulted in reduced heat fluctuations in modes 1–8. This decline in energy content in the mixed fuel flame can be attributed to the stabilizing influence of hydrogen, leading to diminished heat release fluctuations that manifest as energy content in the higher modes.
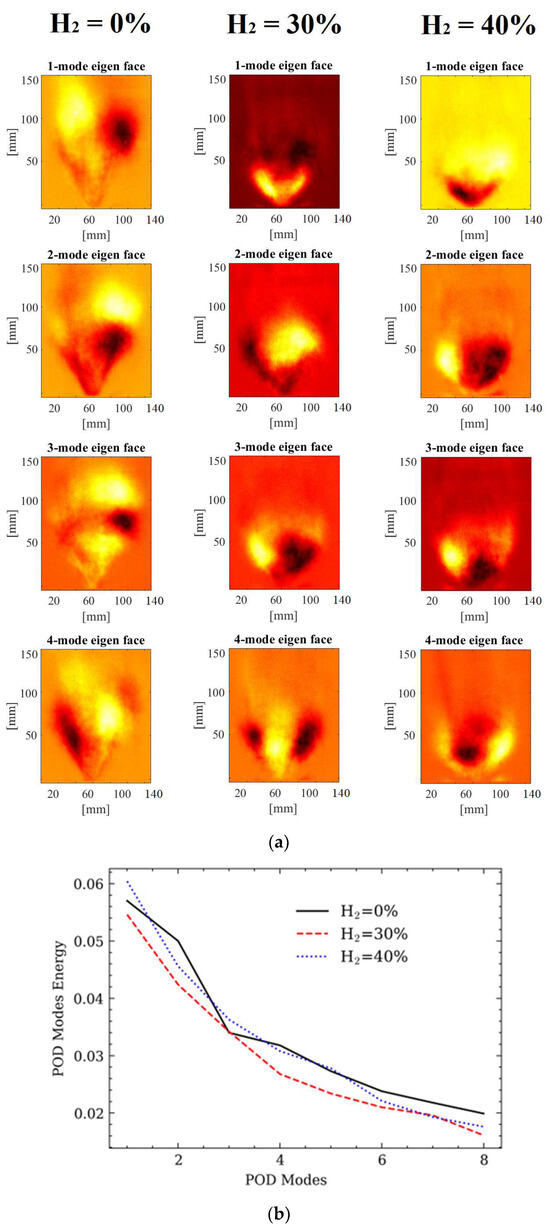
Figure 3.
Proper Orthogonal Decomposition of chemiluminescence images: (a) POD modes images; (b) POD Energy content.
At higher modes, distinct, coherent structures become apparent, and these can be associated with specific flow characteristics. As the mode number increases incrementally, the corresponding wavenumber of this motion also experiences an increase. In the pure methane flame, noticeable larger-scale coherent structures originate at the burner exit and subsequently evolve downstream. Specifically, the first two modes highlight rotational fluctuations, while the third and fourth modes manifest vortex shedding along the shear layers near the burner exit. Moreover, the fourth mode illustrates an alternate propagation of ring vortices in the swirling combustion mode.
In the final two cases, the incorporation of hydrogen gives rise to smaller-scale structures, notably near the nozzle exit, marked by longitudinal oscillations in the second mode. Simultaneously, modes 3 and 4 distinctly display vortex shedding.
3.2. Spectral Proper Orthogonal Decomposition
Figure 4a provides the SPOD analysis of chemiluminescence images at selected frequency values and energy contents. Analyzing the methane flame in Figure 4 through SPOD reveals interesting insights. Notably, it underlines a substantial concentration of energy in the low-frequency range, with a prominent peak around 15 Hz. Furthermore, a distinct peak is noticed at approximately 180 Hz in the high-frequency range.
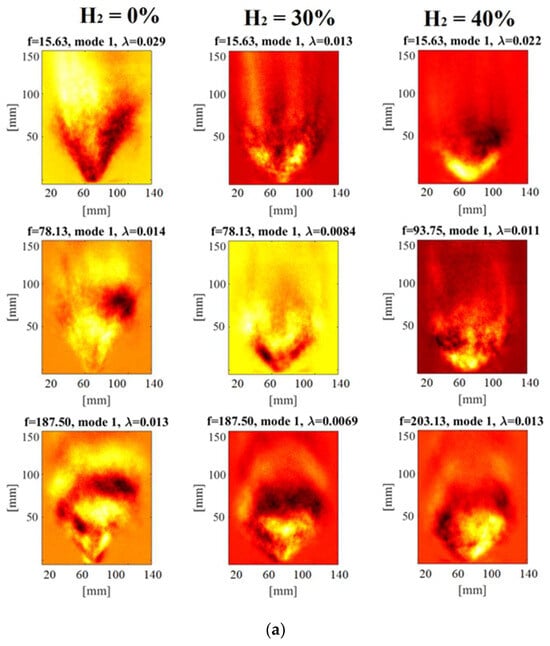
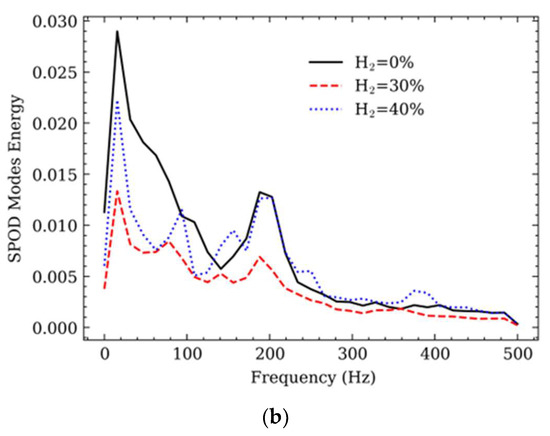
Figure 4.
SPOD of chemiluminescence images: (a) Mode 1 at selected frequency values; (b) SPOD energy content.
Enriching the methane fuel with H2 in the second and third cases results in a noticeable decrease in energy intensity within the low-frequency range. Interestingly, with H2 enrichment, new peaks emerge at around 78 Hz for a 30% H2 enrichment and 90 Hz for a 40% H2 enrichment. It is crucial to note that despite this H2 enrichment, the original peaks at 15 Hz and 180–200 Hz remain unchanged. The initial SPOD mode at around 15 Hz settings rotating structures corresponding to a dominant mode that was not as distinctly identified in the POD analysis. These rotating structures indicate the existence of transversal flame oscillation, suggesting a swirling motion in the reaction zone. Particularly, these rotating structures are more evident in the case of pure methane flame.
On the contrary, the first mode at the high-frequency range of approximately 180–200 Hz shows evident longitudinal oscillations in all test cases. In the case of H2 addition, at a frequency of 93.75 Hz, the first mode displays an approximately axisymmetric fluctuation. In this mode, the strong response area exchanges between upward and downward directions, indicating a robust axial movement of the flame within the spatial domain. These characteristics indicate an axial oscillation mode where the flame shifts forcefully along the axial direction.
3.3. Frequency Analysis of POD Modes
In order to gain a better understanding of flame fluctuations, an evaluation of the power spectral density (PSD) has been conducted from the time coefficients of POD modes of broadband chemiluminescence images for the case without hydrogen presence and with two percentages of hydrogen addition. This study has been performed to identify the main characteristics of frequency contents for the different flame structures, as shown in Figure 5.
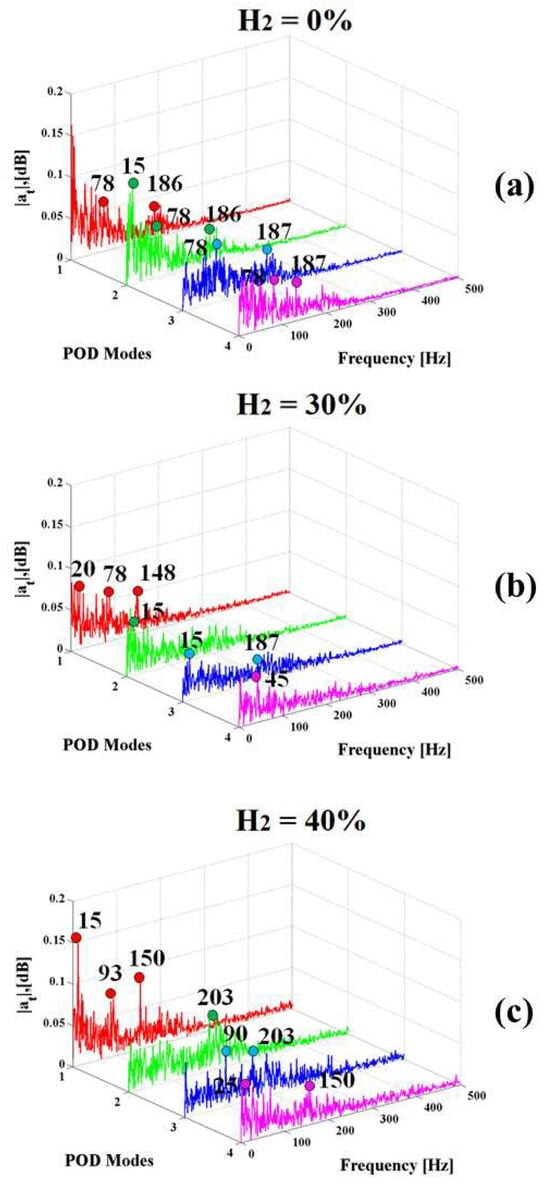
Figure 5.
The Power spectral density of the time coefficients of POD modes of broadband chemiluminescence images for the case H2 = 0% (a), H2 = 30% (b), and H2 = 40% (c).
The presence of hydrogen has a notable influence on the frequency of fluctuations observed in the POD modes, which can be qualitatively linked to heat transfer. Clearly, distinct frequencies emerge as dominant within the combustor when comparing fuels with and without hydrogen addition.
Regarding the POD eigenmodes, the peaks are within the frequency range of 0–200 Hz for all three cases. Specifically, for methane flames without hydrogen (a) and for H2 = 30% (b), high peaks were observed in the range of 15–187 Hz for the first four modes, which are characterized by rotating and transversal oscillations for the range 0–100 Hz and there was evident content around 200 Hz, indicating longitudinal oscillations. In the case of H2 = 40% (c), longitudinal oscillations were present from the second mode onwards, leading to an increase in content around 200 Hz. It has been observed that the introduction of hydrogen has a mitigating effect on the amplitude of the power spectral density of Mode 1, indicating a tangible reduction in heat fluctuations. The presence of hydrogen in the flame induces a consistent decrease in amplitude fluctuations across all modes, signifying the stabilizing influence of hydrogen. The effective mitigation of spectral density and heat fluctuations within a flame can be achieved through the introduction of hydrogen. This is attributed to various factors, encompassing the improvement of ignition, advancement in flame propagation, acceleration of chemical reactions, regulation of the flame front, and utilization of dilution effects. Notably, the reaction H + O2 = OH + O has been identified as a prominent consumer of atomic hydrogen and a pivotal chain-branching reaction that generates OH radicals. Consequently, an increase in H2 levels holds the potential to augment the concentration of OH radicals at the flame front, thereby fostering heightened reaction rates and stability. Additionally, the localized addition of H2 led to an augmentation in the equivalence ratio at the base of the flame.
4. Conclusions
This study conducted a comparative analysis of flame structures using chemiluminescence images under three distinct fueling conditions: pure methane/air and methane with varying levels of hydrogen enrichment. The introduction of hydrogen exerted notable effects on the flames, resulting in a slightly shorter flame length and a wider flame angle, primarily attributed to increased expansion within the Combustion Recirculation Zone. These alterations correlated with significant changes in UV emissions, including a shift in the luminosity zone and reduced variability. To gain deeper insights into these changes, we applied Proper Orthogonal Decomposition (POD) and Spectral Proper Orthogonal Decomposition (SPOD) analyses. These analytical methods facilitated the identification of coherent structures and energetic modes within the flames.
The enrichment of hydrogen was identified to induce specific effects on flame structures. These effects encompassed the presence of smaller structures near the nozzle exit, the emergence of longitudinal oscillations, and the occurrence of vortex shedding. In essence, hydrogen played a crucial role in shaping the characteristics of the flames, thereby contributing to enhanced stability. Furthermore, the introduction of hydrogen exerted a substantial influence on the frequency of flame fluctuations.
The observed alterations in flame characteristics, such as a shorter flame length and wider flame angle, directly influence combustion efficiency. The enhanced stability induced by hydrogen enrichment suggests potential benefits for gas turbine operation, where maintaining stable combustion is crucial for efficiency and emissions control. Understanding the frequency and nature of flame fluctuations provides insights into how combustion systems can be tuned for optimal performance, particularly in applications where combustion stability is paramount.
While this study sheds light on the effects of hydrogen enrichment on flame structures, there remains a wealth of unexplored nuances within hydrogen-methane combustion. Future research efforts could delve into specific aspects, such as investigating a broader range of hydrogen-methane ratios to discern optimal conditions for stability and efficiency and exploring variations in combustion conditions, including pressure and temperature, to understand their impact on flame characteristics.
These targeted investigations could provide a more comprehensive understanding of hydrogen-enriched combustion, guiding the development of cleaner and more efficient combustion systems.
Finally, it should be underlined that the utilization of hydrogen in combustion applications presents intricate challenges that demand careful consideration. Employing a fuel composed entirely of hydrogen requires an additional volumetric flow of approximately 208%, nearly three times more than that necessary for methane to produce equivalent power. Consequently, depending on the blend percentage, there may be a need to expand the size of piping and valves to accommodate the higher volumetric flow demanded by hydrogen. The unique physical characteristics of hydrogen introduce complexities in terms of production, storage, and transportation, distinguishing it from natural gas.
Safety concerns emerge due to hydrogen’s broader flammability range, lower vapor density, and faster flame speed. These factors mandate meticulous considerations in system design. Safety mechanisms, such as gas detection and fire protection systems, must be customized to address hydrogen’s unique volatility and distinctive detection characteristics. Additionally, measures for explosion-proofing should be implemented to contain more substantial explosions, and modifications to ventilation systems become essential.
Moreover, hydrogen presents distinctive challenges related to supply and infrastructure. Pipelines and valves within the fuel system must be compatible with hydrogen, requiring materials specifically designed with safety factors in mind. The design must also incorporate hydrogen-tight seals capable of accommodating the small hydrogen gas molecules. Addressing these intricacies is pivotal for the successful and secure integration of hydrogen into combustion applications.
Author Contributions
Conceptualization, M.G.D.G. and G.M. (Guido Marseglia); Methodology, G.M. (Guido Marseglia) and M.G.D.G.; Software, S.B.; Validation; Formal analysis, S.B. and Z.A.S.; Investigation, P.D.G., P.D.G. and R.A.O.M.; Data curation. S.B. and P.D.G.; Writing—original draft preparation, S.B. and G.M. (Ghazanfar Mehdi); Writing—review and editing, M.G.D.G.; Supervision, M.G.D.G.; Project administration, M.G.D.G.; Fundings: A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021 of the Italian Ministry of University and Research, financed by the European Union—NextGenerationEU [Award Number: National Sustainable Mobility Center CN00000023, named MOST, Concession Decree No. 1033 of 17 June 2022, adopted by the Italian Ministry of University and Research, Spoke 14 “Hydrogen and New Fuels”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- EU Commission Press Release European Green Deal: The Commission Proposes Transformation of EU Economy and Society to Meet Climate Ambitions. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_3541 (accessed on 15 December 2023).
- International Energy Agency. Global Hydrogen Review; Technical Report; IEA: Paris, France, 2021.
- Mehdi, G.; Bonuso, S.; De Giorgi, M.G. Plasma Assisted Re-Ignition of Aeroengines under High Altitude Conditions. Aerospace 2022, 9, 66. [Google Scholar] [CrossRef]
- Chiesa, P.; Lozza, G.; Mazzocchi, L. Using hydrogen as gas turbine fuel. J. Eng. Gas Turbines Power 2005, 127, 73–80. [Google Scholar] [CrossRef]
- Lam, K.-K.; Geipel, P.; Larfeldt, J. Hydrogen enriched combustion testing of Siemens industrial SGT-400 at atmospheric conditions. J. Eng. Gas Turbines Power 2015, 137, 21502. [Google Scholar] [CrossRef]
- Bothien, M.R.; Ciani, A.; Wood, J.P.; Fruechtel, G. Toward decarbonized power generation with gas turbines by using sequential combustion for burning hydrogen. J. Eng. Gas Turbines Power 2019, 141, 121013. [Google Scholar] [CrossRef]
- Jackson, G.S.; Sai, R.; Plaia, J.M.; Boggs, C.M.; Kiger, K.T. Influence of H2 on the response of lean premixed CH4 flames to high strained flows. Combust. Flame 2003, 132, 503–511. [Google Scholar] [CrossRef]
- Hawkes, E.R.; Chen, J.H. Direct Numerical Simulation of Hydrogen-Enriched Lean Premixed Methane—Air Flames. Combust. Flame 2004, 138, 242–258. [Google Scholar] [CrossRef]
- Halter, F.; Chauveau, C.; Djebaili-Chaumeix, N.; Gokalp, I. Characterization of the effects of pressure and hydrogen concentration on laminar burning velocities of methane–hydrogen–air mixtures. Proc. Combust. Inst. 2005, 30, 201–208. [Google Scholar] [CrossRef]
- Mandilas, C.; Ormsby, M.P.; Sheppard, C.G.W.; Woolley, R. Effects of hydrogen addition on laminar and turbulent premixed methane and iso-octane–air flames. Proc. Combust. Inst. 2007, 31, 1443–1450. [Google Scholar] [CrossRef]
- Fruzza, F.; Lamioni, R.; Tognotti, L.; Galletti, C. Flashback of H2-enriched premixed flames in perforated burners: Numerical prediction of critical velocity. Int. J. Hydrogen Energy 2023, 48, 31790–31801. [Google Scholar] [CrossRef]
- Dahl, G.; Suttrop, F. Engine control and low-NOx combustion for hydrogen fuelled aircraft gas turbines. Int. J. Hydrogen Energy 1998, 23, 695–704. [Google Scholar] [CrossRef]
- Cozzi, F.; Coghe, A. Behavior of hydrogen-enriched nonpremixed swirled natural gas flames. Int. J. Hydrogen Energy 2006, 31, 669–677. [Google Scholar] [CrossRef]
- Oh, J.; Hwang, J.; Yoon, Y. EI NOx scaling in a non-premixed turbulent hydrogen jet with swirled coaxial air. Int. J. Hydrogen Energy 2010, 35, 8715–8722. [Google Scholar] [CrossRef]
- Sedlmaier, J.; Habisreuther, P.; Zarzalis, N.; Jansohn, P. Influence of liquid and gaseous fuel on lifted flames at elevated pressure stabilized by outer recirculation. In Proceedings of the ASME Turbo Expo 2014: Turbine Technical Conference and Exposition, Düsseldorf, Germany, 16–20 June 2014; Volume 45684. [Google Scholar] [CrossRef]
- Fokaides, P.A.; Kasabov, P.; Zarzalis, N. Experimental investigation of the stability mechanism and emissions of a lifted swirl nonpremixed flame. J. Eng. Gas Turbines Power 2008, 130, 011508-1–011508-9. [Google Scholar] [CrossRef]
- Langone, L.; Amerighi, M.; Andreini, A. Large Eddy Simulations of a Low-Swirl Gaseous Partially Premixed Lifted Flame in Presence of Wall Heat Losses. Energies 2022, 15, 788. [Google Scholar] [CrossRef]
- Funke, H.H.-W.; Beckman, N.; Keinz, J.; Horikawa, A. 30 years of dry-low-NOx Micromix combustor research for hydrogenrich fuels: An overview of past and present activities. J. Eng. Gas Turbines Power 2021, 143, 71002. [Google Scholar] [CrossRef]
- Lumley, J.L. The structure of inhomogeneous turbulence. Atmos. Turbul. Wave Propag. 1967, 5, 166–178. [Google Scholar]
- Sirovich, L. Turbulence and the dynamics of coherent structures part iii: Dynamics and scaling. Q. Appl. Math. 1987, 45, 583–590. [Google Scholar] [CrossRef]
- Kasabov, P.; Zarzalis, N.; Habisreuther, P. Experimental study on lifted flames operated with liquid kerosene at elevated pressure and stabilized by outer recirculation. Flow Turbul. Combust. 2013, 90, 605–619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).