Root-growth Characteristics Contributing to Genotypic Variation in Nitrogen Efficiency of Bottle Gourd and Rootstock Potential for Watermelon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments and Experimental Design
2.2. Harvest, Shoot and Root Dry Weight Measurments
2.3. Root Morphological Measurements
2.4. Leaf Area and Photosynthetic Activity Measurements
2.5. Shoot Nitrate Reductase (NRA) Activity Measurement
2.6. Shoot Nitrogen Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Screening Experiment (Exp. 1)
3.2. Grafting Experiment (Exp. 2)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Population Prospects, The 2017 Revision, Volume I, United Nations. Available online: https://esa.un.org/unpd/wpp/publications/Files/WPP2017_Volume-I_Comprehensive-Tables.pdf (accessed on 20 January 2019).
- Byrnes, B.H.; Bumb, B.L. Population growth, food production and nutrient requirements. In Journal of Crop Production; Rengel, Z., Ed.; The Haworth Press: New York, NY, USA, 1998; pp. 1–27. [Google Scholar]
- Ercolano, M.R.; Gomez, L.D.; Andolfi, A.; Simister, R.; Troise, C.; Angelino, G.; Borrelli, C.; McQueen-Mason, S.J.; Evidente, A.; Frusciante, L.; et al. Residual biomass saccharification in processing tomato is affected by cultivar and nitrogen fertilization. Biomass Bioenergy 2015, 72, 242–250. [Google Scholar] [CrossRef]
- Amalfitano, C.; Del Vacchio, L.; Somma, S.; Cuciniello, A.; Caruso, G. Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of “Friariello” pepper grown in hydroponics. Horticult. Sci. 2017, 44, 91–98. [Google Scholar]
- Grindlay, D.J.C. Towards an explanation of crop nitrogen demand based on the optimization of leaf nitrogen per unit leaf area. J. Agric. Sci. 1997, 128, 377–396. [Google Scholar] [CrossRef]
- Brégard, A.; Bélanger, G.; Michaud, R. Nitrogen use efficiency and morphological characteristics of timothy populations selected for low and high forage nitrogen concentrations. Crop Sci. 2000, 40, 422–429. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Laegreid, M.; Bockman, O.C.; Kaarstad, O. Agriculture, Fertilisers and the Environment; CABI Publishing: Wallingford, UK, 1999; p. 294. [Google Scholar]
- Wiesler, F.; Behrens, T.; Horst, W.J. The role of nitrogen-efficient cultivars in sustainable agriculture. Sci. World J. 2001, 1, 61–69. [Google Scholar] [CrossRef]
- Hoffer, G.N. Some differences in the functioning of selfed lines of corn under varying nutritional conditions. Agron. J. 1926, 18, 322–334. [Google Scholar] [CrossRef]
- Graham, R.D. Breeding characteristics in cereals. Adv. Plant Nutr. 1984, 1, 57–102. [Google Scholar]
- Sattelmacher, B.; Horst, W.J.; Becker, H.C. Factors that contribute to genetic variation for nutrient efficiency of crop plants. Zeitschrift für Pflanzenernährung und Bodenkunde 1994, 157, 215–224. [Google Scholar] [CrossRef]
- Harley, S.M. Use of a simple colorimetric assay to determine conditions for induction of nitrate reductase in plants. Am. Biol. Teacher 1993, 55, 161–164. [Google Scholar] [CrossRef]
- Labconco, C. A guide to Kjeldahl Nitrogen Determination Methods and Apparatus; Labconco Corporation: Houston, TX, USA, 1998. [Google Scholar]
- Sattelmacher, B.; Klotz, F.; Marschner, H. Influence of the Nitrogen Level on Root-Growth and Morphology of 2 Potato Varieties Differing in Nitrogen Acquisition. Plant Soil 1990, 123, 131–137. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.I.; Sayre, K.D.; Rajaram, S.; McMahon, M. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 1997, 37, 898–904. [Google Scholar] [CrossRef]
- Schulte, auf’m Erley, G.; Wijaya, K.A.; Ulas, A.; Becker, H.; Wiesler, F.; Horst, W.J. Leaf senescence and N uptake parameters as selection traits for nitrogen efficiency of oilseed rape cultivars. Physiol. Plant. 2007, 130, 519–531. [Google Scholar] [CrossRef]
- Ulas, A.; Schulte auf’m Erley, G.; Kamh, M.; Wiesler, F.; Horst, W.J. Root-growth characteristics contributing to genotypic variation in nitrogen efficiency of oilseed rape. J. Plant Nutr. Soil Sci. 2012, 175, 489–498. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef]
- Mae, T. Physiological nitrogen efficiency in rice: Nitrogen utilization, photosynthesis and yield potential. Plant Soil 1997, 196, 201–210. [Google Scholar] [CrossRef]
- Grosse, F. Untersuchungen zur Ertragsbildung und Ertragsstruktur in einem Winterrapssortiment. Ph.D. Thesis, Christian-Albrechts-University, Kiel, Germany, 1989. [Google Scholar]
- Hirasawa, T.; Hsiao, T.C. Some characteristics of reduced leaf photosynthesis at midday in maize growing in the field. Field Crops Res. 1999, 62, 53–62. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Austin, R.B.; Bingham, J.; Blackwell, R.D.; Evans, L.T.; Ford, M.A.; Morgan, C.L.; Taylor, M. Genetic improvements in winter wheat yields since 1900 and associated physiological changes. J. Agric. Sci. 1980, 94, 675–689. [Google Scholar] [CrossRef]
- Presterl, T.; Groh, S.; Landbeck, M.; Seitz, G.; Schmidt, W.; Geiger, H.H. Nitrogen uptake and utilization efficiency of European maize hybrids developed under conditions of low and high nitrogen input. Plant Breed. 2002, 121, 480–486. [Google Scholar] [CrossRef]
- Sinebo, W.; Gretzmacher, R.; Edelbauer, A. Genotypic variation for nitrogen use efficiency in Ethiopian barley. Field Crops Res. 2004, 85, 43–60. [Google Scholar] [CrossRef]
- Gardner, J.C.; Maranville, J.W.; Paparozzi, E.T. Nitrogen use efficiency among diverse sorghum cultivars. Crop Sci. 1994, 34, 728–733. [Google Scholar] [CrossRef]
- Lewin, S.A.; Mooney, H.A.; Field, C. The dependence of plant root: Shoot ratios on internal nitrogen concentration. Ann. Botany 1989, 64, 71–75. [Google Scholar]
- Merrill, S.D.; Tanaka, D.L.; Hanson, J.D. Root length growth of eight crop species in haplustoll soils. Soil Sci. Soc. Am. J. 2002, 66, 913–923. [Google Scholar] [CrossRef]
- Clarkson, D.T. Factors affecting mineral nutrient acquisition by plants. Annu. Rev. Plant Physiol. 1985, 36, 77–115. [Google Scholar] [CrossRef]
- Jackson, W.A.; Pan, W.L.; Moll, R.H.; Kamprath, E.J. Uptake, translocation, and reduction of nitrate. Biochem. Basis Plant Breed. 1986, 2, 73–108. [Google Scholar]
- Claassen, N.; Syring, K.M.; Jungk, A. Verification of a mathematical model by simulating potassium uptake from soil. Plant Soil 1986, 95, 209–220. [Google Scholar] [CrossRef]
- Colla, G.; Suarez, C.M.C.; Cardarelli, M. Improving nitrogen use efficiency in melon by grafting. HortScience 2010, 45, 559–565. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Wang, L.; Jiao, Y.; Chen, C.; Zhao, L.; Mei, M.; Yu, Y.; Bie, Z.; Huang, Y. Pumpkin rootstock improves nitrogen use efficiency of watermelon scion by enhancing nutrient uptake, cytokinin content, and expression of nitrate reductase genes. Plant Growth Regul. 2017, 82, 233–246. [Google Scholar] [CrossRef]
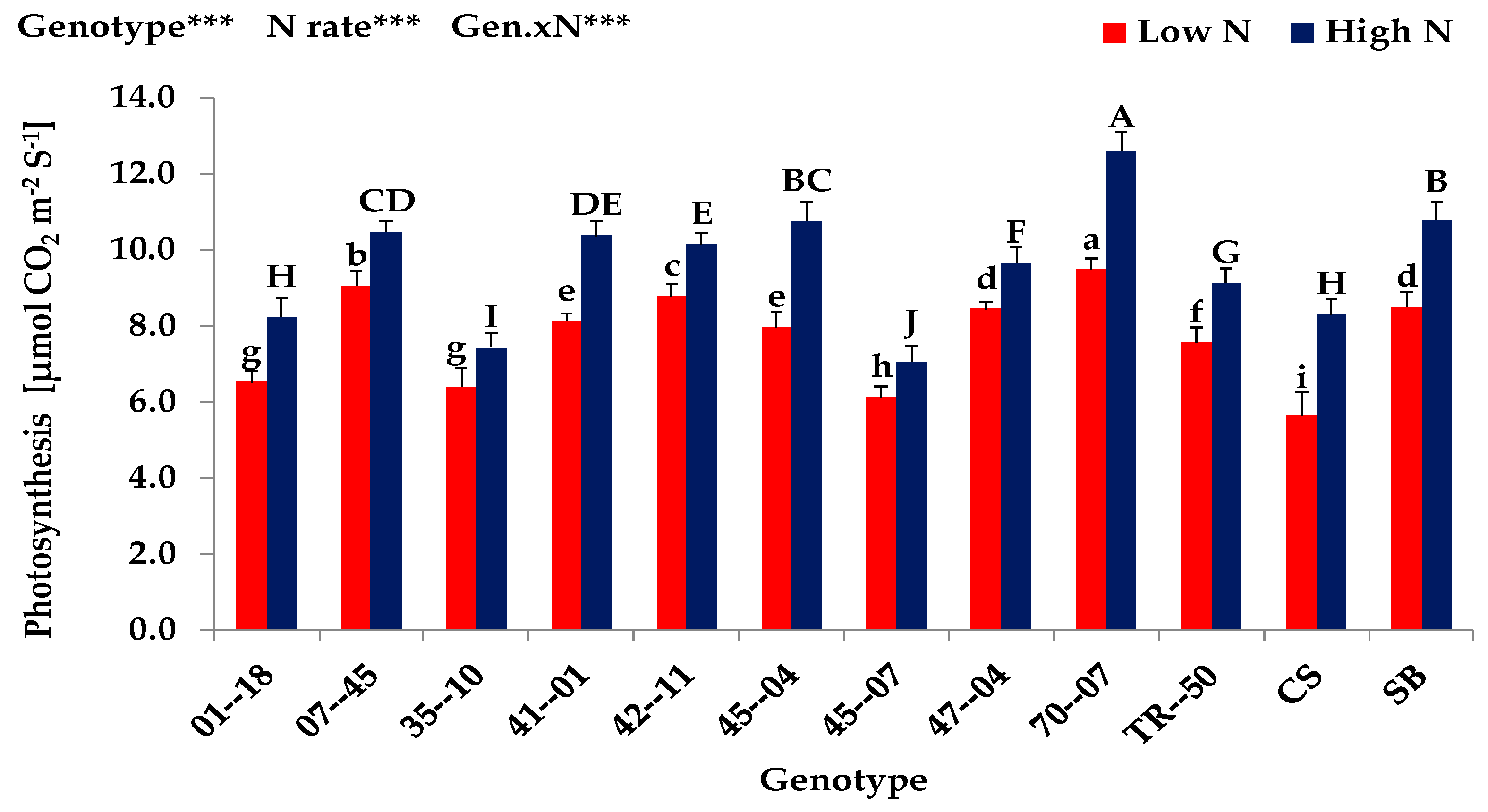
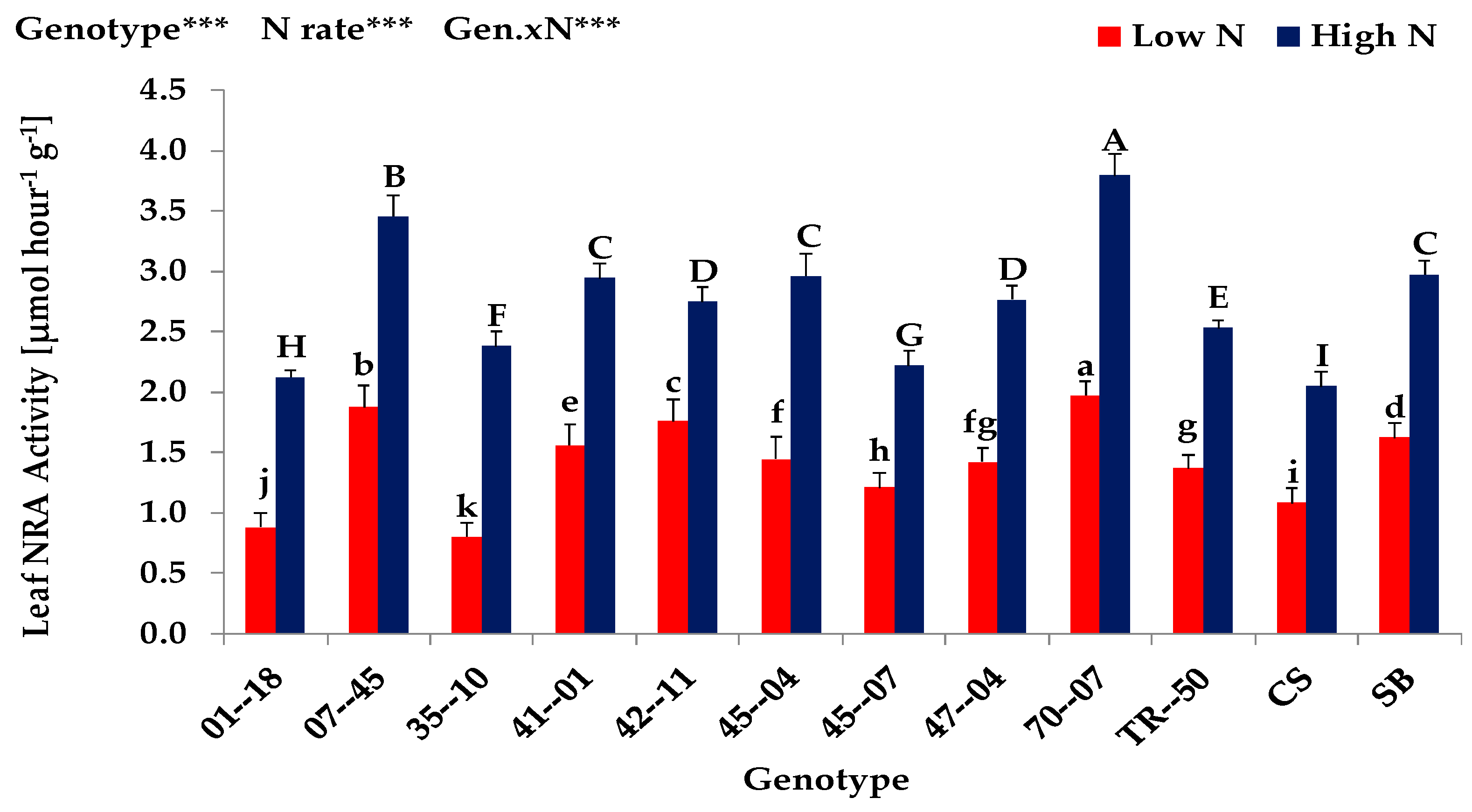

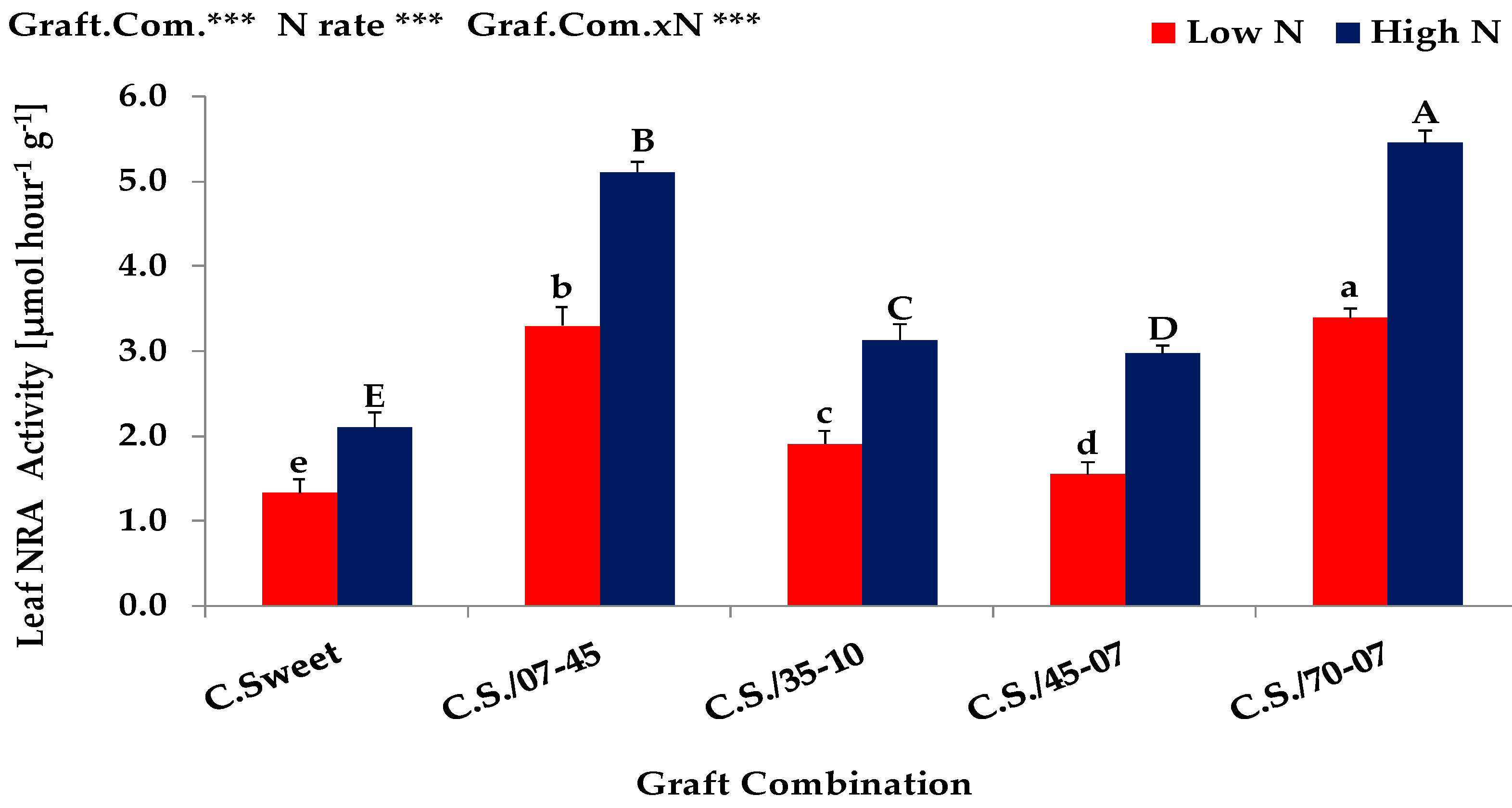
| Expt. 1 | Shoot Dry Matter (g plant−1) | Nitrogen Uptake (mg plant−1) | Nitrogen Concentration (mg g dw.−1) | Leaf Area (cm2 plant−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Low N | High N | Low N | High N | Low N | High N | Low N | High N |
| 01-18 | 7.4 de | 34.8 D | 104.6 e | 937.8 E | 14.2 hi | 26.9 EF | 0.14 ef | 0.75F |
| 07-45 | 14.6 a | 41.9 A | 202.3 b | 1032.6 D | 13.9 I | 24.6 HI | 0.20 ab | 0.97B |
| 35-10 | 7.3 de | 30.2 E | 106.6 e | 866.5 F | 14.5 gh | 28.7 BC | 0.15 de | 0.62G |
| 41-01 | 9.3 bc | 37.7 C | 155.5 c | 1144.5 B | 16.7 d | 29.3 BC | 0.18 c | 0.93BC |
| 42-11 | 10.0 b | 42.8 A | 146.4 cd | 1089.5 C | 14.6 gh | 25.5 GH | 0.19 bc | 0.91CD |
| 45-04 | 8.7 c | 39.1 B | 140.2 d | 1157.3 B | 16.2 e | 29.6 B | 0.18 c | 0.96B |
| 45-07 | 6.9 de | 26.8 F | 102.5 e | 790.2 G | 14.8 g | 29.5 B | 0.13 g | 0.56H |
| 47-04 | 9.4 bc | 38.6 BC | 134.8 d | 932.6 E | 14.4 gh | 24.2 I | 0.18 c | 0.87D |
| 70-07 | 14.7 a | 43.2 A | 229.2 a | 1226.4 A | 15.5 f | 28.4 CD | 0.21 a | 1.26A |
| TR-50 | 7.6 d | 35.1 D | 139.6 d | 919.8 E | 18.4 c | 26.2 FG | 0.16 d | 0.80E |
| C. Sweet | 5.8 f | 8.1 H | 112.4 e | 222.8 I | 19.3 b | 27.5 DE | 0.10 h | 0.12J |
| S. Baby | 6.8 e | 12.7 G | 139.2 d | 424.9 H | 20.6 a | 33.6 A | 0.13 fg | 0.18I |
| Genotype | *** | *** | *** | *** | ||||
| N rate | *** | *** | *** | *** | ||||
| Gen. × N | *** | *** | ** | *** | ||||
| Expt. 1 | Root Dry Matter (g plant−1) | Root:Shoot (g g−1) | Root Length (m plant−1) | Root Volume (cm3 plant−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Low N | High N | Low N | High N | Low N | High N | Low N | High N |
| 01-18 | 2.60 f | 2.11 F | 0.35 d | 0.06 G | 410.4 g | 336.4 I | 54.2 f | 38.9 G |
| 07-45 | 7.53 b | 5.83 A | 0.52 b | 0.14 A | 650.4 b | 523.6 B | 129.2 ab | 94.7 AB |
| 35-10 | 3.09 e | 2.64 E | 0.42 c | 0.09 E | 484.5 f | 408.7 H | 102.9 d | 73.5 E |
| 41-01 | 5.26 d | 4.90 B | 0.56 b | 0.13 B | 578.2 cd | 512.4 C | 113.5 cd | 90.2 BC |
| 42-11 | 5.52 c | 4.13 D | 0.55 b | 0.10 D | 582.6 cd | 457.6 F | 117.5 bc | 82.8 CD |
| 45-04 | 5.57 c | 4.62 C | 0.64 a | 0.12 C | 597.4 c | 486.7 E | 126.8 bc | 91.8 AB |
| 45-07 | 2.44 f | 1.86 G | 0.35 d | 0.07 F | 386.2 h | 279.7 J | 84.1 e | 54.9 F |
| 47-04 | 5.28 d | 4.12 D | 0.56 b | 0.11 C | 568.7 de | 448.1 G | 120.8 bc | 81.1 DE |
| 70-07 | 7.90 a | 6.03 A | 0.54 b | 0.14 A | 730.0 a | 550.7 A | 143.3 a | 99.6 A |
| TR-50 | 5.11 d | 4.78 BC | 0.67 a | 0.14 A | 556.0 e | 497.7 D | 113.6 cd | 87.5 CD |
| C. Sweet | 1.26 h | 0.56 I | 0.22 e | 0.07 F | 229.3 j | 114.5 L | 26.0 g | 11.7 H |
| S. Baby | 1.78 g | 0.92 H | 0.26 e | 0.07 F | 330.2 i | 205.6 K | 35.5 g | 19.8 H |
| Genotype | *** | *** | *** | *** | ||||
| N rate | *** | *** | *** | *** | ||||
| Geno. × N | *** | *** | ** | *** | ||||
| Expt. 2 | Shoot Dry Matter (g plant−1) | Nitrogen Uptake (mg plant−1) | Nitrogen Concent. (mg g dw−1) | Leaf Area (cm2 plant−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Graft.Com. | Low N | High N | Low N | High N | Low N | High N | Low N | High N |
| C. Sweet | 4.7 e | 9.8 E | 77.7 e | 331.6 E | 16.4 c | 33.8 B | 0.11 d | 0.15 E |
| C.S./07-45 | 11.2 b | 33.0 B | 186.2 b | 1153.6 B | 16.7 bc | 34.9 A | 0.17 b | 0.52 B |
| C.S./35-10 | 8.8 c | 20.3 C | 151.1 c | 613.3 C | 17.2 a | 30.2 D | 0.13 c | 0.37 C |
| C.S./45-07 | 8.5 d | 17.5 D | 143.5 d | 487.4 D | 16.9 b | 27.8 E | 0.13 c | 0.30 D |
| C.S./70-07 | 11.6 a | 38.5 A | 191.2 a | 1266.8A | 16.4 c | 32.9 C | 0.20 a | 0.64 A |
| Grafting | *** | *** | *** | *** | ||||
| N rate | *** | *** | *** | *** | ||||
| Graft. × N | *** | *** | ** | *** | ||||
| Expt. 2 | Root Dry Matter (g plant−1) | Root:Shoot (mg plant−1) | Root Length (m plant−1) | Root Volume (cm3 plant−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Graft.Com. | Low N | High N | Low N | High N | Low N | High N | Low N | High N |
| C. Sweet | 0.94 d | 0.71 E | 0.20 d | 0.07 B | 172.7 e | 144.1 E | 19.6 d | 14.7 D |
| C.S./07-45 | 3.14 b | 2.42 B | 0.28 b | 0.07 B | 263.5 b | 218.8 B | 63.0 a | 44.8 A |
| C.S./35-10 | 2.30 c | 2.16 C | 0.26 c | 0.11 A | 212.2 c | 202.3 C | 42.5 c | 37.5 B |
| C.S./45-07 | 2.25 c | 1.81 D | 0.26 c | 0.10 A | 206.9 d | 174.7D | 39.6 c | 29.7 C |
| C.S./70-07 | 3.52 a | 2.52 A | 0.30 a | 0.07 B | 286.5 a | 228.4A | 55.4 b | 45.6 A |
| Grafting | *** | *** | *** | *** | ||||
| N rate | *** | *** | *** | *** | ||||
| Graft. × N | *** | *** | ** | *** | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulas, A.; Doganci, E.; Ulas, F.; Yetisir, H. Root-growth Characteristics Contributing to Genotypic Variation in Nitrogen Efficiency of Bottle Gourd and Rootstock Potential for Watermelon. Plants 2019, 8, 77. https://doi.org/10.3390/plants8030077
Ulas A, Doganci E, Ulas F, Yetisir H. Root-growth Characteristics Contributing to Genotypic Variation in Nitrogen Efficiency of Bottle Gourd and Rootstock Potential for Watermelon. Plants. 2019; 8(3):77. https://doi.org/10.3390/plants8030077
Chicago/Turabian StyleUlas, Abdullah, Esat Doganci, Firdes Ulas, and Halit Yetisir. 2019. "Root-growth Characteristics Contributing to Genotypic Variation in Nitrogen Efficiency of Bottle Gourd and Rootstock Potential for Watermelon" Plants 8, no. 3: 77. https://doi.org/10.3390/plants8030077
APA StyleUlas, A., Doganci, E., Ulas, F., & Yetisir, H. (2019). Root-growth Characteristics Contributing to Genotypic Variation in Nitrogen Efficiency of Bottle Gourd and Rootstock Potential for Watermelon. Plants, 8(3), 77. https://doi.org/10.3390/plants8030077






