Abstract
Chlorogenic acid (CGA) is an active ingredient widely found in plants and has been shown to have potential blood-glucose-lowering effects. However, the research on the efficient extraction processes of CGA from sweet potatoes and its systematic mechanisms underlying hypoglycemic effects is still insufficient. This study optimized the extraction of CGA from various sweet potato parts and varieties using ethanol, and predicted the hypoglycemic mechanism of sweet potato leaves via network pharmacology and molecular docking. The efficacy of the leaf extracts was demonstrated through the in vitro inhibition of hepatic glucose output, accompanied by minimal cytotoxicity, and was further validated in an acute mouse model. The results demonstrated that the optimal extraction conditions were an ethanol concentration of 65.48%, a liquid–solid ratio of 39.00 mL·g−1, an ultrasonic time of 50.00 min, and a temperature of 45 °C. The final extraction yield of CGA crude extract was 3.54%, with the highest content in sweet potato leaves, suggesting a multi-target synergistic mechanism of action for sweet potato leaves. Further in vitro experiments indicated that the CGA crude extract can exert hypoglycemic effects by inhibiting hepatic gluconeogenesis. In conclusion, the study lays a foundation for the further purification and utilization of sweet potato CGA, and establishes a theoretical basis for the development of sweet potato leaf resources as hypoglycemic functional ingredients.
1. Introduction
Sweet potato (Ipomoea batatas L.) is the world’s seventh-largest crop. According to statistics from the Food and Agriculture Organization (FAO) [1], China cultivated sweet potatoes on a total area of 2,750,400 hm2 in 2022, with a total fresh weight production of 5,945,500 tons. The total cultivation area and production accounted for 30% and 56% of the world’s total, respectively. As the world’s leading producer of sweet potatoes, China conserves more than 2000 conserved sweet potato germplasm resources [2]. Research by Wang Zhen et al. [3] demonstrated that sweet potato stems and leaves contain high levels of chlorogenic acid (CGA), with the content in leaves harvested in September reaching as high as 4.49%. Despite their high nutritional and functional value and the capacity for multiple annual harvests, a substantial proportion (95–98%) of sweet potato stems and leaves is discarded as waste, with only a minor fraction (2–5%) utilized as animal feed, leading to substantial resource waste [4]. According to the literature, there are more studies on polyphenols [5,6] and substances such as anthocyanins in sweet potato stems and leaves and fewer studies on CGA. In view of the waste of resources where 95–98% of sweet potato stems and leaves are discarded, based on existing research, advanced methods such as response surface methodology (RSM) are used to systematically optimize the CGA extraction process, aiming to achieve breakthroughs in higher extraction efficiency and lower cost.
Diabetes mellitus, a severe global public health threat and the third leading cause of death among chronic non-communicable diseases, significantly impairs human health [7]. A key pathophysiological hallmark of type 2 diabetes is elevated hepatic glucose output, which raises fasting and postprandial blood glucose levels, largely attributable to insulin resistance [8]. However, clinical management has mainly focused on the insulin pathway, while the role of glucagon was ignored, a hormone promoting hepatic glucose production [9,10]. In this context, natural products from sweet potato have shown promising bioactive potential for managing metabolic disorders. According to the reported studies, phenolic acids, cellulose, anthocyanins, polysaccharides, carotenoids, vitamins, proteins, and flavonoids extracted from sweet potatoes demonstrated biological activities in tests of bioactive components in studies worldwide [11,12,13]. Beyond their established antioxidant and anti-inflammatory properties [14,15], sweet potato constituents demonstrate specific hypoglycemic activity. For instance, sweet potato polysaccharides can improve insulin resistance and induce islet regeneration [16,17]. Notably, polyphenols from sweet potato upregulate the PI3K/AKT/GSK3 signaling pathway in the liver and the PI3K/AKT/GLUT4 pathway in muscle, thereby enhancing the activity of enzymes related to glycogen synthesis and glucose metabolism [18,19]. These findings align with the paradigm of network pharmacology, which utilizes systems biology to elucidate multi-target mechanisms of natural products [20], suggesting that sweet potato extracts likely exert effects via synergistic, multi-target mechanisms rather than a single pathway.
CGA, a dietary phenolic acid, exhibits its diverse biological activities, including anti-inflammatory, antihypertensive, antitumor, antioxidant, and glucose metabolism-regulating effects [21,22,23]. While most studies on CGA’s hypoglycemic mechanism focus on the insulin signaling pathway, its role in modulating the glucagon pathway remains underexplored. CGA has been shown to inhibit glucose-6-phosphatase, the key enzyme catalyzing the final step of gluconeogenesis, and inhibits hepatic glucagon response to reduce fasting blood glucose in mice [24,25,26]. CGA extracts from Cecropia obtusifolia and Cecropia peltata can improve hyperglycemia by specifically blocking hepatic glucose output and regulating glucose homeostasis through pathways such as PI3K-AKT [27,28]. Building on this potential and the aforementioned research, this study employed an integrated approach combining network pharmacology, molecular docking, and experimental validation, to examine how CGA from sweet potato leaves exerts its hypoglycemic effect by inhibiting glucagon-regulated hepatic glucose production.
Our study aimed to optimize the extraction of CGA from sweet potato stems and leaves using response surface methodology. The extraction yield of the CGA crude extract was the evaluation index, with ethanol concentration, liquid–solid ratio, and ultrasonic time as independent variables. The objectives were to establish an efficient extraction protocol and evaluate the hypoglycemic potential of the crude extract, thereby promoting the comprehensive utilization of sweet potato resources and reducing waste, as well as to provide new ideas for the prevention and treatment of diabetes by CGA.
2. Materials and Methods
2.1. Materials and Animals
The sweet potato plant material was sourced from Tai’an, Shandong Province. The six newly grown leaves at the top of the stem and vine are young, and the six leaves at the bottom are old. The older leaves, younger leaves, and stems were dried, crushed, sieved (100 mesh), sealed in a brown paper bag, and placed in the refrigerator.
SPF-grade 18–22 g male C57BL/6J mice, C60-sterilized laboratory animal feed, C60-sterilized laboratory animal bedding were utilized in this study. All operations were carried out in strict accordance with the relevant regulations on laboratory animal ethics. All experiments and animal care were approved by the Animal Ethics Committee of Shandong Agricultural University (SDAUA-2023-025). The mice were housed in an animal facility with constant temperature (22 ± 1 °C) and humidity, following a 12 h light/dark cycle, with free access to water and food, and regular changes.
For the pyruvate tolerance test, 24 fasted mice were randomly divided into four groups (blank, pyruvate, CGA crude extract, met) with six mice in each group, and fasting blood glucose was measured. The blank and pyruvate groups were given 10 mL·kg−1 of normal saline, the other groups to be tested were given 150 mg·kg−1 of CGA crude extract or 200 mg·kg−1 of metformin (Met). After 0.5 h, the blank group was injected with normal saline, and the other three groups were intraperitoneally injected with 2 g·kg−1 pyruvate, and a change in blood glucose within 2 h was observed. The area under the curve (AUC) for blood glucose was calculated as 0.5 × (glucose0 h + glucose0.5 h)/2 + 0.5 × (glucose0.5 h + glucose1 h)/2 + (glucose1 h + glucose2 h)/2. The selection of CGA crude extract treatment dosage and safety evaluation are based on previous research conducted by our research team [26].
2.2. Extraction Process of Chlorogenic Acid from Old Leaves of Sweet Potato
Based on previous literature on the extraction of CGA from dandelion [29], an ultrasonic-assisted method was selected for CGA extraction. Precisely 0.50 g of leaf powder was weighed into a 25 mL ground-glass conical flask. A specified volume of absolute ethanol was added, and the mixture was subjected to ultrasonication in an ultrasonic bath (SB-5200DTD, Ningbo Xinzhi Biotechnology Co., Ltd., Ningbo, China) at 45 °C, 90 W, and 40 kHz for a predetermined duration. The water bath temperature was maintained by circulating water and monitored with a thermometer. The flask was secured to the rack in the water bath using a rubber band. Following centrifugation at 5000× g for 15 min, the supernatant was collected as the CGA crude extract.
2.3. Single-Factor Experimental Design
The single-factor experiment was performed by following the method of Mangiapelo et al. [30] to investigate CGA extraction yield under different extraction conditions. The extraction yield was employed as the metric to evaluate the CGA crude extract from sweet potato leaves using a single-factor experimental design, as outlined in Table 1.

Table 1.
Single-factor experimental factors and levels.
2.4. Response Surface Experimental Design
Based on the previous experiments, a three-factor and three-level experimental design was carried out according to the Box–Behnken design principle, and the specific process is shown in Table 2 to determine the optimal process conditions for the CGA crude extract in sweet potato. Each group performs ten repetitions.

Table 2.
Response surface factor levels.
2.5. Chlorogenic Acid Standard Curve Plotting
The absorbance of CGA standard solutions at various concentrations (0, 0.002, 0.004, 0.006, 0.008, 0.010, 0.012, 0.014, 0.016 g·L−1) was measured at a wavelength of 331 nm [31]. A standard curve was then established using the least squares method, yielding the linear regression equation A = 70.796C1 − 0.0068, R2 = 0.9992. A is the absorbance, and C1 is the CGA concentration, g·L−1.
2.6. Calculation of Chlorogenic Acid Extraction Rate
Using anhydrous ethanol as a blank control, the absorbance of CGA crude extracts was measured by colorimetry, and the content of CGA crude extracts was calculated based on the standard curve. The extraction yield of CGA was calculated using the following formula: yield of CGA/% = C2 × V × n/m. C2: Calculated concentration of CGA standard, mg·mL−1; V: ethanol volume, mL; n: dilution factor; m: weight of sweet potato powder, mg.
2.7. Determination of CGA Crude Extracts of Sweet Potato Leaf by HPLC
Method changes were made based on previous research [32]. Use Dionex Ultimate 3000 high-performance liquid chromatography (HPLC) system (Thermo Fisher Scientific, Waltham, MA, USA) to establish a gradient elution system. Chromatographic conditions: chromatographic column: COSMOSIL 5C18-MS-II (4.6 × 250 mm, Nacalai Tesque, Kyoto, Japan); mobile phase: 10% acetonitrile-water (0.1% phosphoric acid), filtered through a 0.45 μm microporous membrane and degassed by ultrasound before use; column temperature: 35 °C; flow rate: 1 mL/min; injection volume 10 μL. CGA standards of 20, 40, 60, 80, 100, and 1000 μg/mL were prepared, and the standard curve was plotted with the peak area (mAU*s) as the vertical coordinate and the concentration (μg·mL−1) as the horizontal coordinate, and then the CGA crude extract was analyzed by the same method in HPLC, and its concentrations were calculated.
2.8. Collection of Active Components and Targets of Sweet Potato Leaves
Chemical components of sweet potatoes were collected through the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP). Screening was conducted based on an oral bioavailability (OB) threshold ≥30% or a drug-likeness (DL) threshold ≥0.18. The TCMSP platform was also used to identify the target proteins of the active components of honeysuckle.
2.9. Screening of Disease Targets for Diabetes
Target genes associated with diabetes were retrieved from the human gene compendium (GeneCards, https://www.genecards.org/, accessed on 19 November 2025) and the Online Mendelian Inheritance in Man (OMIM, https://www.omim.org/, accessed on 19 November 2025) databases. The retrieved targets were integrated, and duplicate entries were removed. The targets related to the active ingredients were then mapped against the disease targets, and a Venn diagram was plotted to identify the potential therapeutic targets for diabetes.
2.10. Construction of Protein-Protein Interaction (PPI) Network and Screening of Core Targets
Using the data obtained in Section 2.9, apply Cytoscape 3.10.3 to construct a “drug-active ingredient-target gene-disease” relationship network to analyze the mechanism of sweet potato leaves in treating diabetes. Import the obtained intersecting targets into the STRING platform (https://cn.string-db.org/, accessed on 19 November 2025), set the species to “Homo sapiens” and the confidence level to 0.400, construct a PPI network, and save the resulting data.
2.11. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
The selected potential targets were subjected to functional enrichment analysis, including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, via the DAVID database. The results of these analyses were subsequently visualized as bubble plots.
2.12. Component–Target Molecular Docking
The compound’s 3D structure (PubChem) and AKT1, EGFR, STAT3, TNF, GAPDH structure (PDB: 2OCB, 3P0V, 6TLC, 1RJ7, 1ZNQ, RCSB) were prepared in AutoDockTools-1.5.7 by removing solvents/ligands and adding hydrogens. Protein–ligand docking conformations were rendered using PyMOL (https://pymol.org, accessed on 19 November 2025).
2.13. Isolation of Primary Mouse Hepatocytes
Primary mouse hepatocytes were isolated by an improved two-step collagenase perfusion, as previously described [33]. Briefly, fasted C57BL/6J mice were anesthetized with 1% sodium pentobarbital. Livers were then perfused and digested using collagenase type IV solutions (Liquid I and II). Isolated hepatocytes were filtered, centrifuged, and cultured in DMEM with 10% FBS on 48- or 6-well plates.
2.14. Cytotoxicity Assay
Primary hepatocytes were plated at a density of 1 × 105 cells per well in a 24-well plate; after attachment, medium was replaced with serum-free DMEM for starvation. Cells were cultured overnight, and 5 replicate wells were incubated with different concentration CGA crude extract for 24 h. MTT solution was added, culture was continued, supernatant was removed, dimethyl sulfoxide was added, and the absorbance value was measured at 490 nm by shaking at 37 °C at low speed for 10 min.
2.15. Glucose Output
Primary hepatocytes were plated at a certain density (60–70%) and cultured overnight. Prior to the assay, cells were serum-starved in KRH solution for 2–4 h. The cells were then incubated for 6 h with the relevant substrates (10 mM pyruvate, 100 nM glucagon, or 1, 10, 100, 1000, 10,000 μg/mL CGA crude extract) in KRH solution, and the absorbance was measured using a glucose assay kit (Shanghai Rongsheng Biopharmaceutical Co., Ltd., Shanghai, China) at 505 nm.
2.16. Data Statistics and Analysis
All experiments were repeated at least three times, the results were analyzed by IBM SPSS Statistics software (version 25.0; IBM Corp., Armonk, NY, USA) and expressed as mean ± standard deviation, the statistical differences between the groups were evaluated by one-way ANOVA, the differences were considered to be statistically significant at p < 0.05, and the statistical data were plotted using OriginPro 2021 and GraphPad Prism 8 software. The extraction optimization was analyzed using Design-Expert 13 software.
3. Results and Discussion
3.1. Single-Factor Test Results
The effects of ultrasonic time, liquid–solid ratio, and ethanol concentration on the extraction yield of chlorogenic acid in sweet potato were investigated. As can be seen from Figure 1a, the CGA yield peaked at 30 min. Shorter times (e.g., 10 min) were likely insufficient for complete dissolution, while prolonged ultrasonication (e.g., 50 min) may have promoted the extraction of impurities or potential degradation of CGA [34]. Consequently, 10, 30, and 50 min were chosen as the levels for the subsequent experimental design. As can be seen from Figure 1b, the extraction yield of CGA was highest under the condition of a liquid–solid ratio of 30 mL·g−1, and then it showed a decreasing trend, which may increase the surface area of the ethanol solution and the diffusion rate of CGA. Therefore, the liquid–solid ratios of 10, 30, and 50 mL·g−1 were selected for the response surface test. As can be seen from Figure 1c, when the ethanol volume fraction was 70%, the extraction of CGA reached its peak, and at low ethanol concentrations, some of the CGA was not dissolved. Under the high concentration of ethanol, it precipitated other compounds or induced undesirable interactions with CGA. Therefore, 50%, 70%, and 90% ethanol concentrations were selected for the response surface test.

Figure 1.
The effect of single factor on the extraction yield of chlorogenic acid from sweet potato. (a): ultrasonic time; (b): liquid-solid ratio; (c): ethanol concentration.
3.2. Response Surface Test Results
3.2.1. Significance Test and Variance Analysis of Regression Model
In the response surface test, we obtained a quadratic multiple regression equation as follows (Table 3):
Y = −2.8524 + 0.0060A + 0.0215B + 0.1791C + 0.0010AB + 0.0003AC − 0.0001BC − 0.0009A2 − 0.0006B2 − 0.0014C2

Table 3.
Response surface test design results.
Based on the results in Table 4, the results indicate that the p-value of the model was less than 0.01, indicating that the regression equation was highly significant. The lack of fit was 0.0863, which was not significant, indicating that there was no obvious lack-of-fit factor in the equation. This indicates that the fit of the experimental model was good, thus validating the feasibility of the experimental approach. According to the results of the F value, it could be seen that the extraction yield of CGA in older leaves of sweet potato was affected by the linear terms C > B > A, among which the linear item C had the greatest effect on the extraction yield of CGA extract crude, and the linear item A had the smallest effect. In addition, AB, C, A2, and C2 had an extremely significant effect on the extraction rate, p < 0.01; the quadratic term B2 had a significant effect on the extraction yield of CGA, p < 0.05; and the linear terms A and B and the interactions AC and BC had no significant effect (p > 0.05).

Table 4.
Analysis of the extraction yield of CGA crude extracts.
3.2.2. Responsive Surface Experimental Design
In the 3D surface response plots (Figure 2), the contour lines form an elliptical shape with a close distribution, and the color gradient intensifies from light green to dark red. These features indicate a strong interaction between the corresponding two factors. Furthermore, the steep slope of the three-dimensional surface plot suggests that these factors have a significant influence on the extraction yield of the CGA crude extract.

Figure 2.
Response surface result.
The optimal extraction conditions were as follows: ethanol concentration of 65.48%, liquid–solid ratio of 39.00 mL·g−1, ultrasonic time of 50.00 min, and setting temperature of 45 °C, under which the extraction yield of CGA crude extract was 3.54%. Based on the optimal conditions, three replicate validation experiments were performed, as shown in Table 5. The extraction yield of the crude chlorogenic acid obtained was 3.51–3.56%, with no significant difference from the predicted value.

Table 5.
Validation of response surface results.
3.2.3. Determination of CGA Crude Extract in Sweet Potato Leaves
The CGA crude extract content in sweet potato leaves was quantified by HPLC, and the results are shown in Figure 3. Using the regression equation from the standard curve (y = 2.8155x − 16.8910), the concentration was calculated to be 479.48 μg·mL−1. This high CGA content confirmed the presence of the target compound in the extract and validated its suitability for subsequent experiments.

Figure 3.
Content of crude extract of chlorogenic acid in sweet potato leaves. (A) HPLC of crude extract of chlorogenic acid from sweet potato leaves; (B) HPLC of chlorogenic acid standard.
3.3. Comparison of CGA Crude Extracts in Different Parts and Varieties
CGA was extracted from three different parts of sweet potato (younger leaves, older leaves, and stems) and four different varieties (Hongyao 10, Yanshu 25, purple sweet potato, and Shangshu) (Figure 4), and it was found that the CGA crude extract in sweet potato was the highest in the old leaves, and the CGA crude extract in Hongyao 10 was the largest. Sweet potato leaves are rich in CGA, which requires further processing and comprehensive evaluation as a by-product.

Figure 4.
Chlorogenic acid in different parts (younger leaves, older leaves, and stems) and varieties (Hongyao 10, Yanshu 25, purple sweet potato, and Shangshu) of sweet potato.
3.4. Analysis of Network Pharmacology Results
3.4.1. Screening of Active Components, Targets of Sweet Potato Leaves, and Disease Targets
The common chemical components of sweet potato leaves were screened through the TCMSP platform based on OB ≥ 30% or DL ≥ 0.18, resulting in nine chemical components. The first nine chemical components are shown in Table 6. From the disease target database (GeneCards and OMIM databases), 1823 disease-related targets were identified, while 298 target predictions were associated with the active compounds based on the drug target database. Among these, 90 overlapping targets were identified as potential therapeutic targets for the treatment (Figure 5).

Table 6.
Main active components of sweet potatoes leaves.
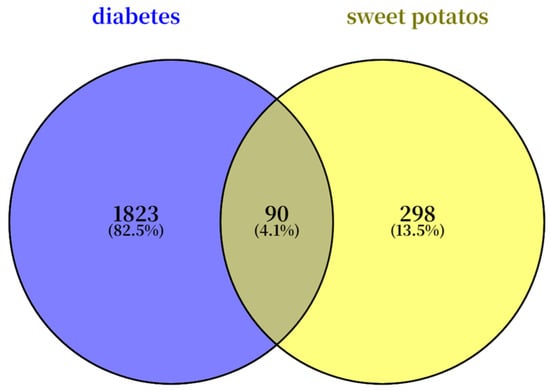
Figure 5.
Venn diagram of overlapping genes between sweet potato leaves and diabetes.
3.4.2. Results of the “Drug-Active Component-Target-Disease” Network Analysis
Using these overlapping targets from Section 3.4.1, a PPI network comprising 101 nodes and 429 edges was constructed (Figure 6A). Multiple active compounds of the sweet potato leaf can act on multiple different targets, reflecting its characteristic of multi-component, multi-target synergistic therapy. Visualize the core targets in the PPI network according to their degree values (Figure 6B). The larger the degree value, the higher the corresponding node, and the darker color indicates that the target protein is more important. Core targets GAPDH (108), AKT1 (100), TNF (92), EGFR (84), and STAT3 (74) are prominent nodes in the network and may be potential targets for sweet potato leaf treatment of diabetes.

Figure 6.
Sweet potato leaves–active ingredients–target genes–diabetes network diagram (A) Squares: active compounds of the sweet potato leaf; circles: targets; diamonds: the sweet potato leaf; arrows: diseases; visualization of the interaction network between sweet potato leaves and diabetes-related proteins (B).
3.4.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Functional Enrichment Analysis
A total of 156 GO terms were significantly enriched with a significance level of p < 0.05. It mainly included 135 biological processes (BP, Figure 7A) such as myelination, negative regulation of autophagy, MAPK cascade, and positive regulation of gene expression. The cellular component (CC, Figure 7B) process was mainly related to functions such as the synapse, endoplasmic reticulum membrane, and vesicle. It mainly included molecular functions (MFs, Figure 7C) such as NADP binding, protein kinase activator activity, α-glucoside transmembrane transporter activity, and steroid binding. Sweet potato leaves may exert their effects by regulating these biological processes. KEGG signaling pathways arranged by p-value are shown in Figure 7D. Lipid and atherosclerosis, tyrosine metabolism, cAMP signaling pathway, JAK-STAT, and other signaling pathways were associated with diabetes. These results indicated that sweet potato leaves treat diabetes through the synergistic effects of multiple pathways.

Figure 7.
Results of GO (A–C) and KEGG (D) functional enrichment analysis.
3.5. Molecular Docking Results
Docking simulation and visualization analysis were performed between chlorogenic acid and the five targets (GAPDH, AKT1, TNF, EGFR, and STAT3) screened in Section 3.4.2. The results showed that chlorogenic acid had binding energies of less than −6.3 kcal·mol−1 with GAPDH, TNF, and AKT1, indicating good binding interactions. CGA could act on multiple core targets simultaneously (Figure 8). It bound to the active pockets of these targets through mechanisms such as hydrogen bonds and hydrophobic interactions, suggesting that its hypoglycemic effect might be achieved through multi-target synergy. Specifically, CGA forms a stable complex with AKT1, where its hydroxyl groups interact with residues such as ASN-279, ASP-274, LYS-276, and THR-160. This study provides a theoretical basis for developing value-added products from sweet potato leaves based on the hypoglycemic properties of CGA. However, further experimental studies are essential to verify its translational potential.

Figure 8.
Visualization results of molecular docking between CGA and AKT1 (A), EGFR (B), STAT3 (C), TNF (D), and GAPDH (E). [H-bond (yellow dotted line)].
3.6. Effect of Crude Extract of Chlorogenic Acid from Sweet Potato on Survival Rate and Glucose Production of Primary Mouse Hepatocytes
3.6.1. Effect of CGA Crude Extract from Sweet Potato Leaves on Survival and Gluconeogenesis of Primary Mouse Hepatocytes
The effect of the CGA crude extract on primary hepatocyte viability was assessed using the MTT assay. As shown in Figure 9A, the extract, at concentrations ranging from 0.1 to 10,000 µg·mL−1, showed no significant impact of sweet potato CGA crude extract on cell proliferation. This confirmed the non-cytotoxic nature of the extract within this concentration range, allowing its use in subsequent experiments. At the cellular level, we further explored the inhibitory effect of CGA crude extract on the hepatic glucose output of primary cells, using the hepatic glucose output rate of primary mouse hepatocytes in the blank group as 100%. As shown in Figure 9B, CGA crude extract exhibited a significant inhibitory effect at a concentration of 10 µg·mL−1, and this effect increased in a concentration-dependent manner. These results suggest that CGA crude extract can reduce hepatic gluconeogenesis induced by glucagon.

Figure 9.
Crude extract of chlorogenic acid from sweet potato inhibited hepatic glucose output in primary mouse hepatocytes. (A) MTT cell proliferation to assess the number of viable cells; (B) hepatic glucose output results. Data were expressed as the mean ± SD (n = 6). * p < 0.05 vs. glucagon, # p < 0.05 vs. blank. CGA, chlorogenic acid; MET, metformin.
3.6.2. Effects of Sweet Potato CGA Crude Extract on Pyruvate Tolerance in Mice
Pyruvate is a substrate for gluconeogenesis in the body, which raises blood glucose. As fasting blood glucose is mainly regulated by endogenous gluconeogenesis, the pyruvate tolerance test serves as an indicator of hepatic glucose production. Blood glucose changes were monitored for 2 h, and the area under the curve (AUC) was calculated (Figure 10A,B). In all groups, the blood glucose level of mice reached its maximum at 0.5 h and returned to baseline approximately 2 h after pyruvate administration. The model group was always higher than that of the other three groups, and the blood glucose value could fall back to the normal level after the administration of chlorogenic acid; i.e., the crude extract of CGA could significantly reduce glucose produced by hepatic gluconeogenesis caused by sodium pyruvate.

Figure 10.
Crude extract of CGA from sweet potato leaves inhibits endogenous glucose production in acute animal mice. (A) Blood glucose change value in mice; (B) pyruvate tolerance in mice. Data were expressed as the mean ± SD (n = 6). * p < 0.05 vs. pyruvate, # p < 0.05 vs. blank. CGA, chlorogenic acid (150 mg/kg); MET, metformin (200 mg/kg).
4. Discussion
The methanol extract of sweet potato leaves contains the highest phenolic acid content, followed by the peel, whole root, and fleshy tissue [35]. Based on a previous report indicating that September offers the highest chlorogenic acid (CGA) content [36], sweet potato leaves harvested in this month were selected for the study. Common methods for extracting CGA include water decoction [37], complete enzymatic hydrolysis [38], supercritical carbon dioxide extraction [39], and ultrasonic-assisted extraction (UAE) [40]. The decoction extraction method is characterized by low cost, minimal equipment requirements, and simplicity, but it has a long duration, produces many impurities, and the effectiveness of extraction is influenced by the inherent properties of the plant. The cost of the supercritical carbon dioxide extraction and complete enzymatic hydrolysis is too high, and the enzyme activity is easily affected. UAE is simple, fast, time-efficient, and has a high yield, so this method was selected for extracting CGA from sweet potato leaves.
Studies have shown that the polyphenols, anthocyanins, and flavonoids in sweet potato leaves have blood sugar-lowering effects [41,42]. CGA is an active ingredient in many medicinal herbs [43] and has hypoglycemic effects. Although chlorogenic acid does not directly affect the digestion of carbohydrates, it may affect the absorption and subsequent utilization of glucose [44] through the effect of metabolites produced by endogenous pathways or intestinal flora, and chlorogenic acid [45] increases the expression of CPT1a, ACOX1, ATGL, and HSL and decreases the expression of MGAT1, DGAT1, DGAT2, CD36, and FATP4 to reduce liver lipid content. In addition, CGA can reduce body weight, improve glucose tolerance and insulin resistance, and has a prominent hypoglycemic effect [46,47]; thus, a network pharmacology approach proved to be a feasible and valuable strategy for systematically analyzing the potential active ingredients, key targets, and signaling pathways of sweet potato leaves in lowering blood glucose. This study, based on KEGG pathway enrichment analysis, further confirmed that the main pathways through which diabetes is intervened involve lipid and atherosclerosis, the PI3K-Akt signaling pathway, and the TNF signaling pathway, which is consistent with previous mechanistic understandings [48]. It was found that nine active ingredients in sweet potato leaves act on 90 targets related to diabetes, with the main targets for their blood glucose-lowering effects being AKT1, GAPDH, STAT3, TNF, and EGFR. AKT is a key mediator of insulin signaling, promoting cellular uptake and utilization of glucose. Accordingly, studies have demonstrated that supplementation of an HFD with CGA improves glucose and lipid metabolism disorders by regulating the AMPK/Akt pathway [49]. TNF-α is associated with diabetic vascular complications [50], and Arctii Fructus lignans alleviate diabetic complications by reducing oxidative stress, regulating TGF-β/VEGF signaling, and restoring autophagy balance [51].
The existing literature on the hypoglycemic mechanisms of CGA has predominantly focused on insulin signaling pathways [52], with comparatively limited investigation into its modulatory effects on glucagon-mediated regulation. Consequently, this study is designed to elucidate the protective role and underlying molecular mechanisms of chlorogenic acid in counteracting glucagon-induced hepatic glucose production. The results showed that CGA could significantly inhibit the production of glucose in primary mouse hepatocytes at 10.00 μg·mL−1. In acute animal tests, mice administered CGA or a crude extract of CGA improved the increase in blood glucose caused by glucagon or sodium pyruvate, and in agreement with the study of Abdollahi Milad et al., CGA supplementation can improve prediabetic complications [28], including weight gain and elevated fasting blood glucose and plasma insulin levels, and these effects are related to changes in mRNA levels of important genes such as hepatic glycogen synthesis, glycogenesis, and glycolysis, suggesting that chlorogenic acid has important significance in inhibiting diabetes. Despite China’s long history of using plants rich in CGA and its derivatives, key challenges persist. These include not only the insufficient mechanistic exploration but also the lack of effective strategies to maximize plant utilization and minimize waste. Addressing these issues is crucial for future research and application.
5. Conclusions
In this study, the optimal process conditions for extracting CGA from sweet potato leaves was determined using a single-factor approach combined with a Box–Behnken response surface methodology, with ethanol as the solvent. Our findings indicate that CGA from sweet potato leaves exerts hypoglycemic effects through multiple targets and pathways. CGA crude extract was non-toxic to primary mouse hepatocytes within the tested concentration and significantly suppressed glucagon-induced glucose production in hepatocytes. This inhibitory effect was confirmed in an acute mouse model, where the extract effectively reduced the glucose production. This study contributes to reducing the waste of sweet potato leaf resources and provides a theoretical basis for the development of sweet potato leaf resources as hypoglycemic health products or medicines.
Author Contributions
Conceptualization, N.X. and M.H.; methodology, X.W., J.Z. and M.H.; validation, N.X.; formal analysis, M.H., N.X., C.Y., X.Y., J.Z. and X.W.; investigation, N.X., M.H., X.W., X.Y., D.T. and J.Z.; resources, N.X., M.H., X.W., C.Y., D.T. and J.Z.; data curation, M.H.; writing—original draft preparation, X.W. and M.H.; writing—review and editing, M.H. and N.X.; visualization, X.Y. and M.H.; project administration, N.X.; funding acquisition, N.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (82004014).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors have declared no conflicts of interest.
References
- Wang, Z.; Zhang, Y.; Deng, J. Exploring the integration and development path of sweet potato industry in yuzhou, Henan Province. Rural. Agric. Farmers (B Ed.) 2022, 8, 7–12. [Google Scholar]
- Hou, F.; Chen, G.; Dong, S.; Xie, B.; Qin, Z.; Li, A.; Zhang, L.; Wang, Q. Comparative study on starch composition, physicochemical properties and noodle quality of different sweet potato varieties. Acta Agric. Nucl. Sin. 2022, 36, 392–401. [Google Scholar]
- Wang, Z.; Li, Z. Biological activity of chlorogenic acid and progress of sweet potato chlorogenic acid research. J. Jiangsu Norm. Univ. (Nat. Sci. Ed.) 2017, 35, 30–34+48. [Google Scholar]
- Shi, Y.; Hu, J.; Li, L. Research progress on nutritional functions and processing utilization of sweet potato. Food Res. Dev. 2022, 43, 205–211. [Google Scholar]
- Miranda, L.; Deußer, H.; Evers, D. The impact of in vitro digestion on bioaccessibility of polyphenols from potatoes and sweet potatoes and their influence on iron absorption by human intestinal cells. Food Funct. 2013, 4, 1595–1601. [Google Scholar] [CrossRef]
- Dai, J.; Li, H.; Gou, L.; Tian, Y.; Cheng, C.; Yuan, F.; Xie, J.; Zhang, L.; Ji, J.; Zhang, L.; et al. Mechanistic study of purple sweet potato anthocyanins: Multifaceted anti-fibrotic effects and targeting of PDGFRβ in liver fibrosis. J. Agric. Food Chem. 2024, 72, 27861–27875. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD—RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population—Based studies with 4.41million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Soderling, S.; Beavo, J. Regulation of cAMP and cGMP signaling: New phospho diesterases and new functions. Curr. Opin. Cell Biol. 2000, 12, 174–179. [Google Scholar] [CrossRef]
- Su, W.; Li, A.; Zhang, Y.; Zhang, Y.; Jiao, X. Regulation of gluconeogenesis in liver, kidney and intestine by glucagon. Chin. Pharmac. Bulletin. 2023, 39, 1332–1338. [Google Scholar]
- Capozzi, M.; D’Alessio, D.; Campbell, J. The past, present, and future physiology and pharmacology of glucagon. Cell Metab. 2022, 34, 1654–1674. [Google Scholar] [CrossRef]
- Han, B.; Jiang, P.; Jiang, L.; Li, X.; Ye, X. Three phytosterols from sweet potato inhibit MCF7-xenograft-tumor growth through modulating gut microbiota homeostasis and SCFAs secretion. Food Res. Int. 2021, 141, 110147. [Google Scholar] [CrossRef]
- Liu, C.; Miao, Y.; Zhou, W.; Ma, Y.; Guo, W.; Li, A. Impact of thermal processing on the structure, antioxidant properties and hypoglycemic activities of sweet potato polysaccharides. Foods 2024, 13, 3082. [Google Scholar] [CrossRef]
- Lee, C.; Yoon, Y.; Yoon, Y.; Chung, K.; Kim, M.; Park, G.; Choi, M.; Jang, Y.; Lee, K. Protective effects of a standardized water extract from the stem of Ipomoea batatas L. against high-fat diet-induced obesity. Nutrient 2025, 17, 1643. [Google Scholar] [CrossRef]
- Ammara, A.; Hira, I.; Ayesha, S.; Zulfiqar, T.; Tareen, M.; Amna, D.; Shakir, M.; Hazafa, A.; Naeem, M.; Lorenzo, J.; et al. Comparative study of potato (Solanum tuberosum L.) and sweet potato (Ipomoea batatas L.): Evaluation of proximate composition, polyphenol content, mineral and sntioxidant sctivities. Appl. Sci. 2021, 11, 11844. [Google Scholar]
- Moraga-Babiano, L.; Lucas-González, R.; Domínguez-Valencia, R.; Gaona-Ruiz, M.; Carrillo, C.; Echegaray, N.; Pateiro, M.; Lorenzo, J. Encapsulated purple sweet potato peel extract as antioxidant and sustainable colourant to preserve the quality of beef burgers during the shelf life. Food Chem. 2025, 487, 144657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bian, S.; Hu, J.; Liu, G.; Peng, S.; Chen, H.; Jiang, Z.; Wang, T.; Ye, Q.; Zhu, H. Natural deep eutectic solvent-based microwave-assisted extraction of total flavonoid compounds from spent sweet potato (Ipomoea batatas L.) leaves: Optimization and antioxidant and bacteriostatic Activity. Molecules 2022, 27, 5985. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.; Chen, C.; Varga, V.; Shih, L.; Chen, P.; Lo, S.; Shyur, L.; Li, S. White sweet potato ameliorates hyperglycemia and regenerates pancreatic islets in diabetic mice. Food Nutr. Res. 2020, 64, 10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yang, X.; Kou, M.; Lu, C.; Wang, Y.; Peng, J.; Chen, P.; Jiang, J. Selenylation of polysaccharide from the sweet potato and evaluation of antioxidant, antitumor, and antidiabetic activities. J. Agric. Food Chem. 2017, 65, 605–617. [Google Scholar] [CrossRef]
- Luo, D. Hypoglycemic Effect and Mechanism of Polyphenols from Stems and Leaves of Sweet Potato ‘Ximeng 1’. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2020. [Google Scholar]
- Hu, Z.; Lv, G.; Dong, W.; Zheng, F.; Yan, P.; Yu, S.; He, D.; Liu, B.; Gao, Y.; Su, D.; et al. Network pharmacology and molecular docking elucidate the mechanism of phillyrin in colorectal cancer. Food Sci. Nutr. 2025, 13, e71069. [Google Scholar] [CrossRef]
- Bae, J.; Park, W.; Kim, H.; Kim, H.; Kang, K.; Kwak, S.; Ahn, M. Protective effect of carotenoid extract from orange-fleshed sweet potato on gastric ulcer in mice by inhibition of NO, IL-6 and PGE2 Production. Pharmaceuticals 2021, 14, 1320. [Google Scholar] [CrossRef]
- Huang, Q.; Shan, Q.; Ma, F.; Li, S.; Sun, P. Chlorogenic acid mitigates heat stress-induced oxidative damage in bovine mammary epithelial cells by inhibiting NF-κB-mediated NLRP3 inflammasome activation via upregulating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2025, 301, 140133. [Google Scholar] [CrossRef]
- Tang, S.; Zhong, W.; Li, T.; Li, Y.; Song, G. Isochlorogenic acid A alleviates dextran sulfate sodium-induced ulcerative colitis in mice through STAT3/NF-κB pathway. Int. Immunopharmacol. 2023, 118, 109989. [Google Scholar] [CrossRef]
- Jackson, K.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural constraints and importance of caffeic acid moiety for anti-hyperglycemic effects of caffeoylquinic acids from chicory. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef]
- Henry-Vitrac, C.; Ibarra, A.; Roller, M.; Mérillon, J.; Vitrac, X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. J. Agric. Food Chem. 2010, 58, 4141–4144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Zhang, T.; Han, M.; Tian, D.; Liu, J.; Li, S.; Yang, L.; Pan, G. Chlorogenic acid inhibits ceramide accumulation to restrain hepatic glucagon response. Nutrients 2023, 15, 3173. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Vázquez, R. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. J. Ethnopharmacol. 2010, 130, 93–97. [Google Scholar] [CrossRef]
- Abdollahi, M.; Marandi, S.; Ghaedi, K.; Safaeinejad, Z.; Kazeminasab, F.; Shirkhani, S.; Sanei, M.H.; Rezvanian, P.; Nasr-Esfahani, M.H. Insulin-related liver pathways and the therapeutic effects of aerobic training, green coffee, and chlorogenic acid supplementation in prediabetic mice. Oxid. Med. Cell. Longev. 2022, 2022, 5318245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Miao, Q.; He, M. Optimization of extraction process and stability study of chlorogenic acid from dandelion. China Feed 2023, 23, 54–60. [Google Scholar]
- Mangiapelo, L.; Blasi, F.; Ianni, F.; Barola, C.; Galarini, R.; Abualzulof, G.; Sardella, R.; Volpi, C.; Cossignani, L. Optimization of ultrasound-assisted extraction of chlorogenic acid from potato sprout waste and enhancement of the in vitro total antioxidant capacity. Antioxidants 2023, 12, 348. [Google Scholar] [CrossRef]
- Chen, M.; Liu, B.; Luan, Y.; Zhou, L.; Huang, Y. Optimization of chlorogenic acid extraction from the leaves of Sambucus chinensis Lindl. by response surface methodology. Hunan Agric. Sci. 2022, 7, 84–87+91. [Google Scholar]
- Liu, J.; Duan, H.; Wang, H.; Gao, Q.; Liu, L.; Liang, Y.; He, M.; Xu, L.; Guo, X. Deep eutectic solvent extraction of chlorogenic acid from dandelion with ultrasonic-assisted: Process optimization, purification, and bioactivity. Ultrason. Sonochem. 2025, 122, 107579. [Google Scholar] [CrossRef]
- Gan, J.; Ji, C.; Mao, X.; Wang, J.; Lv, C.; Shi, Y.; Liao, Y.; He, Y.; Shu, L.; Li, L.; et al. Synchronization isolation method for multiple types of cells from mouse liver. Zhonghua Gan Zang Bing Za Zhi 2023, 31, 532–537. [Google Scholar]
- Wang, Y. Extraction and Genotypic Varietion of Antioxidant Functional Components from Sweet Potato Vines. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2019. [Google Scholar]
- Chiu, C.; Liu, K.; Lin, H.; Chu, W.; Lai, Y.; Chao, P. Analysis of Chlorogenic Acid in Sweet Potato Leaf Extracts. Plants 2022, 11, 2063. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, J.; Li, X. Determination and analysis of polysaccharide content in stem and leaves of different sweet potato varieties. J. Shanxi Agric. Sci. 2018, 46, 182–186+206. [Google Scholar]
- Wang, W. Study on the Preparation Process Andquality Standards of Senecio cannabifolius Extract; CCUCM: Changchun, China, 2013. [Google Scholar]
- Zhang, X.; Xie, J.; Chen, Y.; Zhang, X. Extraction of chlorogenic acid from folium cortex eucommiae by enzymatic hydrolysis method and determination of its content. China Brew. 2016, 35, 149–152. [Google Scholar]
- Li, J.; Yu, L.; Yang, X.; Guo, J. Extraction process of chlorogenic acid from wild chrysanthemum flower via supercritical fluid extraction. Food Res. Dev. 2012, 33, 30–32. [Google Scholar]
- Shi, J.; Fang, D.; Sui, Y.; Xiong, T.; Chen, X.; Fan, C.; Zhou, D.; Cai, F.; Mei, X. Polyphenol content, antioxidant capacity, and composition in different varieties of sweet potato (Ipomoea batatas L.) leaves during growth stages. Sci. Hortic. 2025, 342, 113925. [Google Scholar] [CrossRef]
- Jahurul, M.; Islam, S. Emerging techniques for the recovery of bioactive compounds from sweet potato leaves [Ipomoea batatas (L.) Lam] and their functional health benefits. Food Bioeng. 2025, 4, 318–339. [Google Scholar] [CrossRef]
- Dewi, N.K.S.M.; Ramona, Y.; Saraswati, M.; Wihandani, D.; Wirasuta, I. The potential of the flavonoid content of Ipomoea batatas L. as an alternative analog GLP-1 for diabetes type 2 treatment-systematic review. Metabolites 2023, 14, 29. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.; Khan, G.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Faezeh, M.; et al. Chlorogenic acid(CGA): Apharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Gary, W. Protection against developing type 2 diabetes by coffee consumption: Assessment of the role of chlorogenic acid and metabolites on glycaemic responses. Food Funct. 2020, 11, 4826–4833. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Tan, B.; Chen, H.; Lu, Y. Inhibitory effects of chlorogenic acid and isochlorogenic acid from purple sweet potato leaves on α-glucosidase. Mod. Food Sci. Tech. 2014, 30, 103–107. [Google Scholar]
- Siddiqui, S.; Ahmad, R.; Aziz, T.; Khan, A.; Ashraf, H.; Moin, S. (-)-Epigallocatechin-3-gallate and chlorogenic acid in combination with vitamin D as a therapeutic approach for letrozole-induced polycystic ovary syndrome (PCOS) rats: Biochemical and hormonal modulation. J. Steroid Biochem. Mol. Biol. 2025, 252, 106772. [Google Scholar] [CrossRef]
- Han, M.; Tian, D.; Che, X.; Yu, J.; Xiao, A.; Pan, G.; Xiao, N. Mechanism of Lonicerae japonicae Flos in the treatment of diabetes based on netwwork pharmacology. J. Shandong First Med. Uni. (Shandong Acad. Med. Sci.) 2022, 3, 172–177. [Google Scholar]
- Mubarak, A.; Hodgson, J.; Considine, M.; Croft, K.; Matthews, V. Supplementation of a high-fat diet with chlorogenic acid is associated with insulin resistance and hepatic lipid accumulation in mice. J. Agric. Food Chem. 2013, 61, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Shao, J.; Lai, C.; Huang, E.; Cai, J.; Behrmann, A.; Cheng, S.; Towler, D. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arter. Thromb. Vasc. Biol. 2007, 27, 2589–2596. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, S.; Li, X.; Chen, J.; Jiang, Y.; Ye, L. Effect of glucose-control and N-Acetylcysteine on PARP and GAPDH activities in diabetic patients with chronic kidney disease in diabetes. Chin. J. Diabetes 2013, 5, 48–50. [Google Scholar]
- Karthikesan, K.; Pari, L.; Menon, V.P. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem.-Biol. Interact. 2010, 188, 643–650. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.