Abstract
Given the challenges of climate change, effective adaptation strategies for crops like coffee are crucial. This study evaluated twelve 1-aminocyclopropane-1-carboxylate deaminase-producing bacterial strains selectively isolated from the rhizosphere of Coffea arabica L. cv. Costa Rica 95 in a plantation located in Veracruz, Mexico, focusing on their potential to enhance drought resistance. The strains, representing seven genera from the Gamma-proteobacteria and Bacteroidota groups, were characterized for growth-promoting traits, including ACC deaminase activity, indole-3-acetic acid (IAA) synthesis, phosphates solubilization, siderophore production, and nitrogen fixation. Strains of the genus Pantoea exhibited higher ACC deaminase activity, phosphate solubilization, and IAA synthesis, while others, such as Sphingobacterium and Chryseobacterium, showed limited plant growth-promoting traits. A pot experiment was conducted with coffee plants subjected to either full irrigation (soil with 85% volumetric water content) or drought (soil with 55% volumetric water content) conditions, along with inoculation with the isolated strains. Plants inoculated with Pantoea sp. RCa62 demonstrated improved growth metrics and physiological traits under drought, including higher leaf area, relative water content (RWC), biomass, and root development compared to uninoculated controls. Similar results were observed with Serratia sp. RCa28 and Pantoea sp. RCa31 under full irrigation conditions. Pantoea sp. RCa62 exhibited superior root development under stress, contributing to overall plant development. Proline accumulation was significantly higher in drought-stressed, non-inoculated plants compared to those inoculated with Pantoea sp. RCa62. This research highlights the potential of Pantoea sp. RCa62 to enhance coffee plant resilience to drought and underscores the need for field application and further validation of these bioinoculants in sustainable agricultural practices.
1. Introduction
Coffee is a plant in the genus Coffea that belongs to the Rubiaceae family. While many wild species exist, the most commercially significant are C. arabica L. and C. canephora (Pierre) ex A. Froehner. C. arabica, which originated in southern Ethiopia [1], is globally famous for its aroma, sweetness, and variations in acidity and flavor. Coffee is one of the most traded commodities globally [2]. This plant is cultivated in over 70 countries by 25 million farmers, most of them smallholders in developing countries [3]. Furthermore, agroforestry systems, where most coffee is cultivated in many coffee-producing areas, serve as important reservoirs of cultural richness, biodiversity, and ecological processes [4,5,6].
The phenology and, consequently, the yield and quality of C. arabica are strongly dependent on climatic conditions [7]. Prior studies have reported changes in the production and accumulation of several metabolites along with adverse impacts of drought on coffee leaf relative water content (RWC), stomatal conductance, leaf water potential, net carbon assimilation rate, transpiration rate, and, as a result, growth and yield [8,9,10,11]. Hence, climate change presents a significant challenge to the cultivation of coffee in various regions, with considerable reductions anticipated in suitable areas for cultivation [12,13,14,15]. Given this crop’s social, economic, and environmental importance, the potential impact of climate change on coffee production is alarming and requires adaptation and mitigation strategies [16].
A significant limitation of agricultural production in climate change is drought [17], as the lack of water affects plant physiology, development, and crop yield and quality [18,19,20]. The search for new fertilization alternatives that allow production, even under these stressful conditions, becomes more critical. One of these possible strategies is to use microorganisms as biofertilizers, enhancing plant acclimatization, improving soil structure and fertility, and increasing crop yield. Specifically, the beneficial organisms that inhabit the rhizosphere, commonly referred to as plant growth-promoting rhizobacteria (PGPR), possess the capability to preserve or enhance crop growth and production, even in the presence of drought-related environmental stressors, and do not necessarily have to establish endosymbiotic relations with the plant to confer beneficial effects [21,22].
PGPR can improve crop performance through the bacterial synthesis of phytohormones [23]; the increase of nutrient availability through nitrogen fixation, phosphate solubilization, or siderophore production; and the secretion of protective substances such as volatile organic compounds and extracellular polymeric substances [24]. As a result, the interaction with these beneficial microorganisms favors the modulation of the expression profile of several plant genes, either those directly involved in protecting cells from stress (such as enzymes related to osmolyte production or antioxidant activity) or those including regulatory proteins that modulate gene expression (such as transcription factors) [25,26]. In consequence, plants associated with PGPR exhibit, for instance, the regulation of plant hormonal levels, the activation of antioxidant systems along with the consequent reduction in the concentration of malondialdehyde due to damage to cell membranes, and the regulation of plant metabolite synthesis. All these mechanisms contribute to increased growth and better development of the plants [23,24,27]. Among PGPR, those capable of producing ACC deaminase (ACCd-PGPR) are of particular interest, as they degrade the precursor of ethylene, 1-aminocyclopropane-1-carboxylic acid (ACC), into α-ketobutyrate and ammonia. High levels of ethylene over long periods can harm plant health, growth, and productivity [28,29,30]. Accordingly, many studies have documented that inoculation with ACCd-PGPR helps to enhance drought resistance in a wide variety of plants [31,32,33,34,35,36].
To date, insufficient data is available regarding the isolation, characterization, and identification of PGPR from the rhizosphere of coffee plants [37]; even more, no studies explicitly focus on the exploration of ACCd-PGPR. Further, it is essential to consider that, beyond the plant growth-promoting characteristics described for the strains, a significant amount of research has been conducted on seeds and sterilized substrate or soil, reporting positive effects with PGPR inoculation under abiotic stress conditions [38,39,40,41,42,43], still overlooking the bacterial ability to confer tolerance to stressors under conditions that are closer to those found in a productive context. Several strains can promote plant growth and confer resistance to environmental stressors when inoculated onto sterile seeds and planted in sterile soil or substrate. They may also exhibit promising plant growth-promoting characteristics when tested in vitro as axenic cultures. However, the effects of these microorganisms may be less pronounced when the same strains are examined in non-sterile substrates or on older plants [44,45,46]. This is because microorganisms inoculated under sterile conditions do not encounter competitors that might hinder their establishment. Additionally, environmental conditions directly affect the physiological state of plants, which subsequently affects the composition of root exudates and modifies the potential for plant-bacteria interactions [47]. Since root colonization is a multi-step process that depends on species-specific chemical interactions [48], searching for PGPR naturally associated with the crops of interest becomes relevant.
Hence, in this study, we aimed to isolate ACCd-producing rhizobacteria from the rhizosphere of C. arabica cv. Costa Rica 95 in a central Veracruz coffee plantation and determine the impact of these strains on drought resistance strategies on young C. arabica plants exposed to moderate and prolonged drought. This study addresses the knowledge gap regarding ACC deaminase-producing bacteria associated with coffee plants in productive plantations. Even more, we have not found other studies directly assessing the effect of potential plant growth-promoting strains on coffee drought resistance under conditions that more closely resemble real-world agricultural settings (inoculation in young plants and non-sterile soil). This approach offers new insights into the practical application of these bacteria for enhancing coffee plant resilience in actual production environments increasingly threatened by climate change.
2. Results
2.1. Identification and Characterization of ACCd-Producing Rhizobacteria Strains
Twelve strains from seven genera within the bacterial groups Gamma-proteobacteria and Bacteroidota were isolated from the coffee rhizosphere (Table 1; Figure S1). The growth-promoting traits present in these microorganisms vary significantly (Table 1; Figure S2), allowing the strains to form three clusters. The three strains of the genus Pantoea were characterized by moderate to high relative ACCd activity, high PSI, and increased IAA production. In contrast, the group consisting of Sphingobacterium sp. RCa08 and RCa20, Stenotrophomonas sp. RCa07, and Chryseobacterium sp. RCa13 exhibited moderate to low ACCd activity; no other plant growth-promoting characteristics were found. Finally, the third cluster includes Pseudomonas sp. RCa18 and RCa58, Raoultella sp. RCa12, and Serratia sp. RCa01 and RCa28. This cluster showed varying ACCd activity from low to very high, high iron solubilization by siderophores, moderate PSI, and marginal IAA production, particularly by Serratia sp. RCa01. None of the strains fixed atmospheric nitrogen or produced IAA through a tryptophan-independent pathway.

Table 1.
ACCd-producing strains and characterization of plant-growth-promoting attributes.
All the strains exhibited ACCd activity; Pseudomonas sp. RCa18 and Pantoea sp. RCa31 and RCa62 show the highest ACCd activity. Among the twelve strains evaluated, five were found to produce siderophores. Serratia sp. RCa01 is the isolate with the largest solubilization halo after eight days of incubation, compared to Pseudomonas sp. RCa58. On the other hand, eight strains could solubilize phosphates, with the genus Pantoea exhibiting higher PSI than the other genera. Regarding IAA production, five strains were found to produce IAA through a tryptophan-dependent pathway. Notably, the three strains of the genus Pantoea exhibited significant IAA production, with Pantoea sp. RCa62 showing the highest capacity (Table 1). It is important to mention that this characteristic could not be evaluated in strains RCa07 and RCa13, as they did not grow in the Jain and Patriquin medium.
2.2. Effect of ACC Deaminase-Producing Strains on the Growth and Physiological Status of Coffea arabica L.
2.2.1. Differentiation Between Treatments
Based on the pot experiment results, cluster analysis revealed a clear separation among most of the plants under water scarcity (S, 55% VWC, cluster 1), the majority of well-irrigated plants (R, 85% VWC, cluster 2), and plants under full irrigation inoculated with Serratia sp. RCa28 and Pantoea sp. RCa31, as well as drought-stressed plants inoculated with Pantoea sp. RCa62 (cluster 3) (Figure 1). Figure 2 indicates that cluster 3 displayed higher values in leaf area, primary growth, biomass, leaf bud production, and root development. Notably, the cluster with the highest shoot-to-root ratio includes most plants with regular irrigation, whereas plants in cluster 3 have an intermediate shoot-to-root ratio. PCA, in which this cluster analysis was performed, explains 63.1% of the variability. Mean values for each irrigation treatment can be observed in Table S1, again showing a clear differentiation between growth and functional attributes in plants under irrigation and drought.
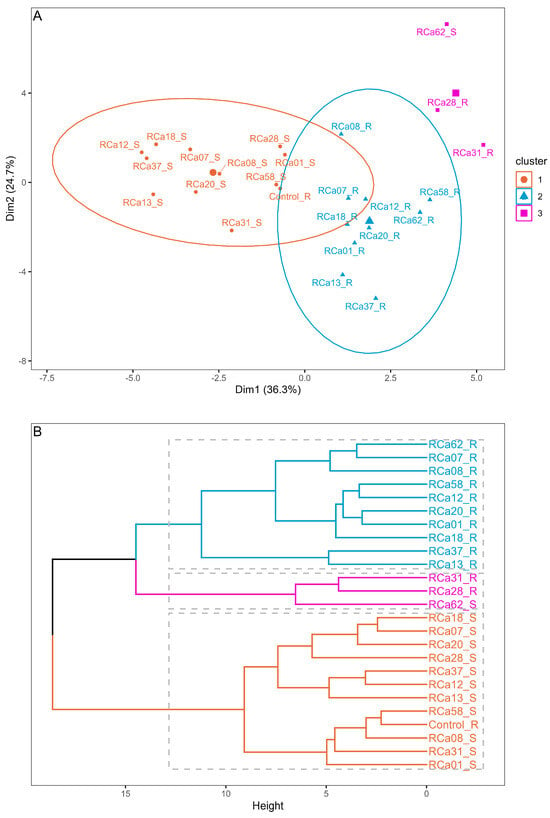
Figure 1.
Clustering (A) and dendrogram (B) of plants under irrigation and inoculation treatments. The cluster calculations were performed using the k-means method. Plants under regular irrigation (85% RWC) are marked with the letter R, while those under drought (55% RWC) are marked with the letter S.
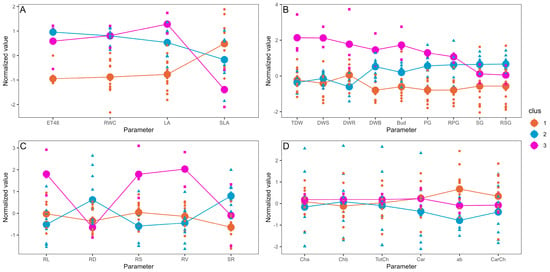
Figure 2.
Normalized mean values for each cluster. The thick points represent the mean normalized value for each parameter. The symbols represent the individual normalized data for each element of the cluster. Cluster calculations were performed using the k-means method. (A) Effects on evapotranspiration and leaf functional attributes. (B) Effects on plant growth and development. (C) Effects on root development. (D) Effects of pigment accumulation. ET48, evapotranspiration over 48 h; LA, leaf area; RWC, relative water content; SLA, specific leaf area; PG, primary growth; RPG, relative primary growth; SG, secondary growth; RSG, relative secondary growth; DWS, dry weight of the shoot; DWR, dry weight of the root; TDW, total dry weight; Bud, number of leaf buds; FWB, fresh weight of leaf buds; DWB, dry weight of leaf buds; RL, total root length; RD, root diameter; RS, total root surface; RV, total root volume; SR, shoot-to-root ratio; Cha, chlorophyll a concentration; Chb, chlorophyll b concentration; TotCh, total chlorophyll concentration; Car, carotenoids concentration; ab, chlorophyll a/b ratio; CarCh, carotenoids/chlorophyll ratio.
2.2.2. Effects on Evapotranspiration
A notable decrease in evapotranspiration was observed in plants exposed to drought conditions (S, 55% VWC). However, in plants inoculated with Pantoea sp. RCa62, this parameter was higher than most of the other drought-stressed groups, except for Serratia sp. RCa01 (Figure 3).
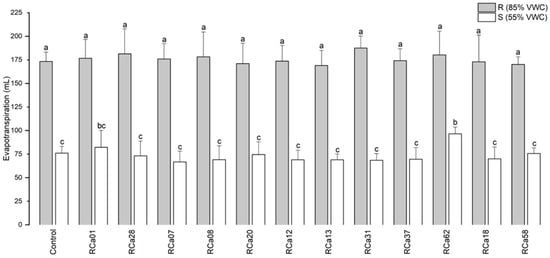
Figure 3.
Mean evapotranspiration of C. arabica L. cv. Costa Rica 95 over 48 h. Plot represents mean ± CI 95% (n = 7) for the plants irrigated at 85% VWC (R) and at 55% VWC (S). Different letters indicate significant differences between groups (p < 0.05; post-hoc Duncan).
2.2.3. Effects on Leaf Functional Traits
The impact of the treatments on leaf relative water content (RWC), leaf area (Figure 4), and specific leaf area (SLA) was analyzed. The R group showed significantly higher values for RWC. Among these, those associated with Pantoea sp. RCa31 exhibited the highest RWC, markedly surpassing plants treated with Chryseobacterium sp. RCa13 and Pseudomonas sp. RCa18. All R groups were statistically similar to uninoculated controls. Likewise, drought-stressed plants inoculated with Chryseobacterium sp. RCa13 and Pseudomonas sp. RCa18 had the lowest RWC, even less than uninoculated controls. Notably, plants associated with Pantoea sp. RCa62 maintained higher RWC levels than drought-stressed uninoculated individuals and was comparable to many well-watered plants. Regarding leaf area, plants inoculated with Pantoea sp. RCa31, under optimal irrigation conditions, had the largest leaves, which were significantly different from those treated with Pseudomonas sp. RCa18 under similar conditions. The smallest leaves were observed in non-inoculated plants under drought stress (Figure 5). On the other hand, Pantoea sp. RCa62, Serratia sp. RCa01, RCa28, Pantoea sp. RCa31, and Pseudomonas sp. RCa58 exhibited positive effects under drought, with leaf areas greater than those of uninoculated controls. Specifically, the leaf areas in RCa01, RCa28, and RCa62 were comparable to those of non-stressed plants across all inoculation treatments. No significant differences in SLA were found among the groups.
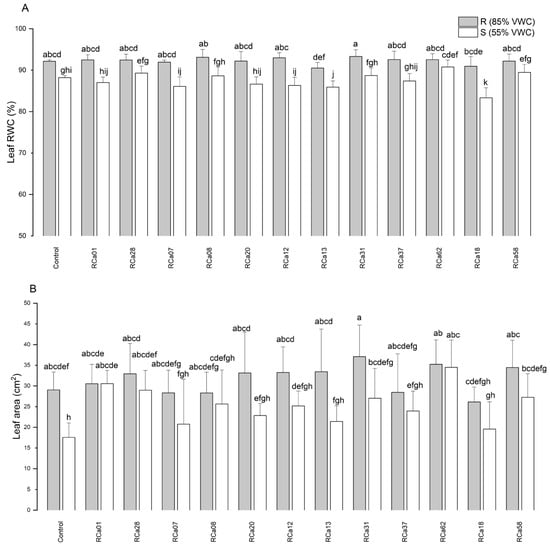
Figure 4.
Leaf relative water content (RWC) (A) and leaf area (B) of C. arabica L. cv. Costa Rica 95. Plot represents mean ± CI 95% (n = 7) for the plants irrigated at 85% VWC (R) and at 55% VWC (S). Different letters indicate significant differences between groups (p < 0.05; post-hoc Duncan).

Figure 5.
Leaf area of uninoculated (Control) and inoculated plants with Pantoea sp. RCa62 under full irrigation (R) and drought stress (S) treatments.
2.2.4. Effect on Plant Growth
The effects of irrigation and inoculation treatments on many parameters, including growth and weight, were analyzed (Supplementary Table S1). These parameters in stressed plants inoculated with Pantoea sp. RCa62 were notably higher than those exhibited by most of the groups under water deficit and even comparable to those of the groups without this stressor.
Absolute and relative primary growth (Table 2) in plants under adequate water conditions inoculated with Pseudomonas sp. RCa58, RCa18, and Serratia sp. RCa28 were elevated but had no significant differences compared to uninoculated well-watered controls.

Table 2.
Effect of ACC deaminase-producing rhizobacteria on primary and secondary growth of Coffea arabica L. cv. Costa Rica 95 after eight weeks of soil humidity regimes near field capacity (R, 85% VWC) and under drought conditions (S, 55% VWC).
The poorest performance was observed on plants under drought conditions inoculated with Pantoea sp. RCa37, although they did not differ from uninoculated plants. It is important to note that the initial length of the plants was homogeneous between all treatments (ANOVA, p > 0.05). In the same way, there is no correlation between the initial plant height and the absolute primary growth (Pearson test, r2 = 0.0128, p > 0.05) and relative primary growth (Pearson test, r2 = 0.0105, p > 0.05). Regarding secondary growth, none of the inoculation treatments differed from the uninoculated controls for each watering group; however, mean secondary growth rates showed that stressed plants inoculated with Stenotrophomonas RCa07, Sphingobacterium sp. RCa20, Raoultella sp. RCa12, Chryseobacterium sp. RCa13, Pseudomonas sp. RCa18, and Pantoea sp. RCa37 had equal or even thinner stems after two months of water stress. The effect of irrigation treatments is evident on the assessed primary and secondary growth parameters.
Regarding the dry weight of the plants after the inoculation and watering treatments, the root weights of well-irrigated plants were lower than expected compared to the stressed groups (Table 3). It is also interesting that plants inoculated with Pantoea sp. RCa62 exhibit the highest value for shoot weight, even when compared to some well-watered plants, including the control group. Furthermore, it was observed that they also had the best root development. No differences in total dry weight were detected by ANOVA (p > 0.05).

Table 3.
Effect of ACC deaminase-producing rhizobacteria after eight weeks of soil humidity regimes on biomass accumulation and partitioning of C. arabica L. cv. Costa Rica 95 under full irrigation (R, 85% VWC) and under drought conditions (S, 55% VWC).
Concerning leaf bud production, plants under abundant watering conditions are in symbiosis with Pantoea sp. RCa31, which stands out, while those with the fewest buds’ production were those subjected to water deficit and inoculated with Pantoea sp. RCa37 (Table 4). When analyzing the fresh weight of the sprouts, the effect of Pantoea sp. RCa31 is again notable compared to other strains under watering conditions (e.g., Stenotrophomonas sp. RCa07, Sphingobacterium sp. RCa08, Chryseobacterium sp. RCa13, and Pseudomonas sp. RCa18); similar trends are observed when exploring the effect on the dry weight of the leaf buds. In the case of drought-stressed plants, the effect of Pantoea sp. RCa62 is again evident, showing fresh and dry sprout weights greater than those of the control group and plants in symbiosis with Stenotrophomonas sp. RCa07, Sphingobacterium sp. RCa08, RCa20, Raoultella sp. RCa12, Pantoea sp. RCa37, Pseudomonas sp. RCa58, and RCa18. Pantoea sp. RCa62 provided a bud production comparable to the plant groups under 85% VWC irrigation conditions.

Table 4.
Effect of ACC deaminase-producing rhizobacteria after eight weeks of soil humidity regimes on bud production of C. arabica L. cv. Costa Rica 95 under full irrigation (R, 85% VWC) and drought conditions (S, 55% VWC).
2.2.5. Effects on Root Development
There is a clear difference between root morphology and development between irrigation treatments since stressed plants show longer and thinner roots with wider surfaces (Table 5). In addition, the image analyses indicated that the percentage of tap roots in well-watered plants is significantly higher than in stressed plants (R = 2.39% ± 0.10, S = 1.91% ± 0.06; U Mann-Whitney, p ˂ 0.001). In contrast, the latter prioritizes the development of thinner secondary roots. Furthermore, the dry-weight shoot-to-root ratio is notoriously lower in stressed plants. Even though there was no statistical significance in the root morphology parameters, the broadest roots in plants were consistently associated with Pantoea sp. RCa62 and were observed under drought conditions (Figure 6). Moreover, it was observed that the groups that exhibited the best performance in the experiment (well-watered and inoculated with Serratia sp. RCa28 and Pantoea sp. RCa31; subjected to drought and inoculated with Pantoea sp. RCa62) had intermediate shoot-to-root ratios compared to the other clusters (Figure 2C).

Table 5.
Effect of ACC deaminase-producing rhizobacteria after eight weeks of soil humidity regimes on root development of C. arabica L. cv. Costa Rica 95 under full irrigation (R, 85% VWC) and drought conditions (S, 55% VWC).
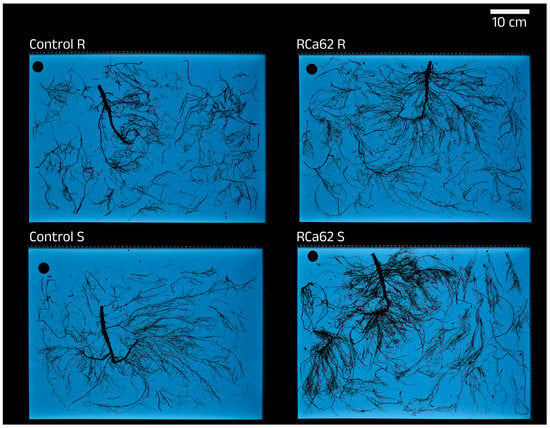
Figure 6.
Root architecture of uninoculated (Control) and inoculated plants with Pantoea sp. RCa62 under full irrigation (R) and drought stress (S) treatments.
2.2.6. Effect on Pigment Concentrations
No differences were found in chlorophyll a, b, or total chlorophylls, nor in carotenoid concentration. However, the chlorophyll a/b ratio was higher in stressed plants. Individuals under drought and associated with Serratia sp. RCa28 exhibited the highest chlorophyll a/b ratio. A higher carotenoids/chlorophyll ratio can also be observed in drought-stressed plants (Table S2).
2.2.7. Effect on Proline Accumulation
When comparing plants inoculated with Pantoea sp. RCa62 to the control group, changes in proline production were observed. Individuals not inoculated and exposed to drought conditions showed increased osmolyte accumulation (Figure 7).
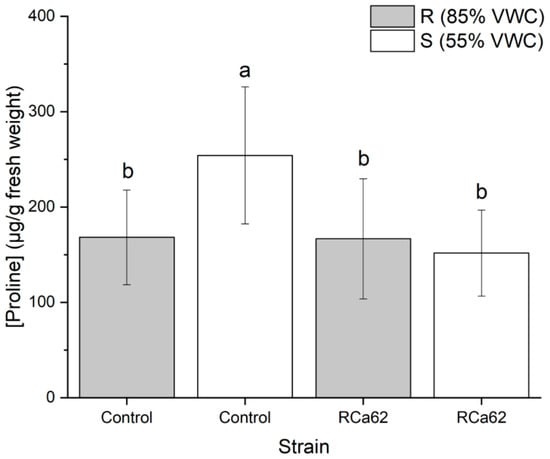
Figure 7.
Proline accumulation on C. arabica L. cv. Costa Rica 95 leaves. Plot represents mean ± CI 95% (n = 7) for the plants irrigated at 85% VWC (R) and 55% VWC (S). Different letters indicate significant differences between groups (p < 0.05; post-hoc Duncan).
3. Discussion
3.1. ACCd-Producing Rhizobacteria Strains
Climate change conditions make it necessary to seek adaptation strategies for agricultural systems to maintain productivity and improve crop quality, particularly for crops with more potential vulnerabilities, such as coffee [11,14]. ACC deaminase-producing bacteria have attracted considerable attention due to their ability to lower ethylene peaks associated with physiological stress, thereby promoting plant growth. This quality makes them appealing as potential ingredients in bioinoculant formulations [29]. This is the first study exploring ACCd-PGPR in C. arabica L. under agricultural conditions. Using a selective medium with ACC as the only nitrogen source, twelve strains were isolated from the rhizospheric soil of C. arabica var. Costa Rica 95. The strains belong to seven genera, all of which are Gram-negative, consistent with previous studies that describe the prevalence of such bacteria associated with coffee [49,50]. All the identified genera have been described as ACC deaminase producers and plant growth promoters when inoculated into various plant species under abiotic stress conditions [38,39,40,41,42,43,51,52,53,54]. In all cases, the strains exhibited more significant ACC deaminase activity than the minimum required for regulating tissue ethylene concentration and promoting plant growth, as reported by Penrose and Glick [55].
Microorganisms found in this research, such as Serratia and Pseudomonas, have been found in the rhizosphere of wild C. arabica in the forests of Ethiopia, as well as bacteria of the genus Erwinia, a member of the Erwiniaceae family [49], to which the genus Pantoea also belongs and shares many phenotypic features [56]. Moreover, some Erwinia species have been reclassified as Pantoea [57], a genus identified in this study as a member of root bacterial communities. Here, Pantoea strains were found displaying traits of ACC deaminase production, IAA, and phosphate solubilization, the latter of which was described by Muleta et al. (2013) [58] for Erwinia strains. Similarly, Stenotrophomonas has been previously reported as an endophytic genus in coffee roots and other tissues as a potential biocontrol agent [50,59]. On the other hand, Sphingobacterium has not been identified as a genus in the coffee rhizosphere; however, it has been found in wastewater from coffee bean processing. Nevertheless, other bacteria from the order Sphingobacteriales have been noted as dominant in the rhizosphere of this crop [60,61].
Regarding the microorganisms isolated in this study, both Serratia strains (RCa01 and RCa28), Raoultella sp. RCa12, and Pseudomonas (RCa18 and RCa58) showed the ability to produce siderophores, previously documented for these genera. For example, in Serratia, a siderophore called serratioquelline, a catecholate, has been identified in various strains of the genus [62,63] and is associated with plant growth promotion under abiotic stress [53]. Similarly, the ability to synthesize siderophores has been identified in Raoultella [64] and Pseudomonas, with the latter also providing antagonistic activity against plant pathogenic fungi [65,66].
The phosphate solubilization capability present in the strains of Serratia, Raoultella, Pseudomonas, and Pantoea becomes particularly relevant as this macronutrient is a frequent limiting factor to plant development [67] and tends to be scarce under drought conditions. In dry soil, the size of the water-filled pores decreases significantly, reducing the mobility of this element [24]. In addition, the diminished water mobility from the soil to the plants through the root system also reduces the movement of dissolved nutrients and, hence, impacts plant nutrition [67]. Among these genera, the PSI of Pantoea strains stands out compared to the others that exhibited activity. This genus has been widely documented as a phosphate solubilizer [68,69,70].
Also remarkable is the production of IAA by Pantoea strains and Serratia sp. RCa28, although the latter produces it in smaller amounts. The synthesis of IAA has been reported for both genera [53,71], and it is particularly significant due to the crucial role this phytohormone plays in regulating plant responses to water stress. It is involved in optimal root system development, regulating gene expression related to protection against oxidative damage caused by water scarcity, and interacting with ABA in controlling stomatal opening and closing.
3.2. Bacterial Effect on Evapotranspiration
Regarding the bacterial effect on C. arabica’s resistance to drought, ANOVA showed a significant effect of irrigation and bacterial inoculation treatments on young C. arabica cv. Costa Rica 95 in many of the parameters assessed. In the same way, the differentiation of the clusters indicates an effective establishment of the irrigation and drought treatments, as well as the distinction of strains with greater plant growth-promoting effects under each type of water availability treatment (full irrigation, plants associated with Serratia sp. RCa28 and Pantoea sp. RCa31; drought stress, plants related to Pantoea sp. RCa62).
When assessing water consumption through evapotranspiration, it’s important to note that water movement from the soil to the atmosphere involves two key components: evaporation, which transfers water directly from the soil to the atmosphere, and transpiration, which occurs through the soil-root-shoot-atmosphere continuum [72]. Evapotranspiration is highly sensitive to soil water availability, particularly in the Coffea genus. Under drought conditions, coffee plants often exhibit stomatal closure as an early response to prevent excessive water loss through transpiration [73,74,75,76]. This explains the significant differences in evapotranspiration observed between drought-stressed groups and those with adequate water availability. These results are consistent with Avila et al. [77] and Naves [78], who found reductions in the evapotranspiration of potted young plants and adult coffee plants under field conditions subjected to water restrictions employing a system of rain exclusion, respectively. However, when water deficiency persists for prolonged periods, as in this study, the decline in stomatal conductance limits CO2 assimilation, ultimately affecting growth and productivity [79,80,81,82,83]. This is especially evident in coffee, which has lower stomatal conductance than other tropical plants [74]. Notably, plants inoculated with Pantoea sp. RCa62 displayed increased evapotranspiration despite moderate water restrictions. This is particularly remarkable given that these plants also exhibited a larger leaf area, a key parameter directly linked to photosynthetic surface area and productivity—especially in coffee under prolonged water stress [84].
3.3. Bacterial Effect on Leaf Functional Traits
Reducing the leaf area in C. arabica under drought conditions is a strategy to evade water stress by minimizing water loss through transpiration [84,85]. This mechanism can be confirmed with the overall means of leaf area of stressed plants compared to fully irrigated plants and becomes especially evident in non-inoculated individuals under drought conditions. In contrast, drought-stressed plants inoculated with Pantoea sp. RCa62 suggest the absence of these evasion mechanisms without compromising their growth.
RWC indicates coffee water status, showing reduced plants subjected to water stress and reflecting the balance between water uptake and transpiration [74,86]. Notably, plants inoculated with Pantoea sp. RCa62, while showing a lower leaf RWC compared to those under full irrigation conditions, do not differ statistically from the plants without stress. In contrast, their RWC is higher than that of control plants and several other groups under similar stress conditions despite their higher evapotranspiration and leaf area.
To date, we have not found other studies exploring the effect of PGPR inoculation on coffee under drought conditions regarding RWC and other functional attributes. However, the impact of an ACC deaminase-producing strain of Pseudomonas sp. and Serratia marcescens on improving the water status of wheat plants, evaluated through RWC, has been highlighted [87]. Similar results were found in plants derived from sterilized seeds inoculated with Bacillus subtilis Rhizo SF 48, also an ACC deaminase-producing strain [88].
3.4. Bacterial Effect on Plant Growth
In this study, regarding the parameters of primary growth, it is noteworthy that although significant differences were not observed concerning uninoculated controls in well-irrigated plants, the inoculation effect becomes clear under drought conditions. Considering that evapotranspiration can influence photosynthetic rate and plant growth, plants’ absolute and relative primary growth inoculated with Pantoea sp. RCa62 under water deficit conditions stands out and is comparable to plants under higher soil volumetric water content (VWC). This could be associated with higher evapotranspiration and leaf area than plants in other drought groups. Irrigation regimes had a notable effect on primary and secondary growth, as one of the main effects of water stress is reduced plant growth, and inoculation with growth-promoting rhizobacteria has proven to be a viable strategy to mitigate crop productivity losses in this and other studies [22,24,89]. Previous studies have evaluated the effect of Kocuria sp., Bacillus subtilis, Sagenomella diversispora, and Penicillium ochrochloron, all phosphate-solubilizing species, on the growth of coffee seedlings from sterilized seeds. These plants showed more significant growth compared to the non-inoculated controls [90]. However, their response under water stress conditions was not assessed. In this study, all plants in the cluster with the highest growth were inoculated with phosphate-solubilizing strains (Serratia RCa28, Pantoea RCa31, and RCa62).
Although no effects of the treatments were observed on total biomass, a significant irrigation effect was identified on dry mass allocation, with root development being prioritized in stressed plants compared to those fully irrigated. It is well established that drought drastically alters resource allocation, as prioritizing root growth enables water and nutrient uptake even under water scarcity conditions [86,91,92]. In this study, plants associated with Pantoea sp. RCa62 had the heaviest roots, likely facilitating the growth of larger shoots. Moreover, those inoculated with this strain showed better leaf bud production among the stressed plants, generating significantly more new foliar tissue than other stressed groups, including uninoculated controls. We propose that enhanced root development supports and enables the growth of the aerial parts of the plants. In this regard, the group of fully irrigated plants associated with RCa31 and RCa28 and stressed plants in symbiosis with RCa62 exhibited more developed roots and an intermediate root-to-shoot ratio. A well-developed root system supports shoot growth, which, in turn, can meet the plant’s metabolic needs and maintain productivity [92,93].
3.5. Bacterial Effect on Root Development
Root architecture plays a significant role in plant resistance to drought in addition to root weight or biomass partitioning [92,94,95]. In this study, it was shown that the proportion in length of taproot is 25% greater in plants without water restrictions since more complex root architectures in stressed plants, reflected as a lower mean root diameter but longer roots, allow a better water and nutrient uptake efficiency under water scarcity conditions [95]. Even more, the longest roots were found in plants under drought and inoculated with RCa62, the bacterial strain with the most significant IAA production. Among the plant growth regulators, auxins play a substantial role in promoting root elongation, and the effect of these phytohormones depends upon their overall concentration [96,97,98]. Many studies have reported the impact of IAA-producing microorganisms on drought stress resistance through root elongation in some plants such as maize, chickpea, pepper, Vigna radiata, wheat, alfalfa, and white clover [21,24,99,100,101,102]. Regarding cuttings on Robusta coffee, IAA-producing Bacillus wiedmannii and Rhodococcus qingshengii effectively increased the number of primary roots [103]. This study does not explore the effects on plants subjected to water restrictions.
Moreover, ACC deaminase activity enhances the effect of IAA through cross-talk communication between ethylene and IAA [104]. While IAA activates plant ACC synthase transcription, leading to increased ethylene levels that inhibit IAA signal transduction, bacterial ACC deaminase reduces ethylene levels in the plant [29]. This reduction also diminishes the feedback inhibition on IAA signaling. Consequently, in the presence of ACC deaminase, bacterial IAA can continue to promote plant growth and increase ACC synthase transcription with minimal ethylene feedback inhibition. Thus, the synergy between IAA and ACC deaminase lowers plant ethylene levels, allowing IAA to stimulate plant growth more effectively [28].
Therefore, a larger photosynthetic surface, which is more susceptible to water loss through transpiration via the stomata, along with the consequent increased evapotranspiration, and together with the absence of detrimental effects on RWC and growth parameters, suggests a mechanism that enhances water uptake. Given that the drought regime was moderate and prolonged, the success of the plants associated with RCa62 may be attributed to the expansion of the root system. Additionally, it is essential to mention that although the evapotranspiration of plants treated with Pantoea sp. RCa62 is higher than that of most drought-affected groups, it remains significantly lower than that of plants without water restrictions, indicating a possible greater water use efficiency (WUE, i.e., the amount of biomass produced per unit of water consumed), a phenomenon observed in other cases of plants with limited water availability [105]. It has previously been reported that the association of crops with PGPR can increase their WUE under water deficit conditions due to various plant growth promotion mechanisms [106,107].
It is worth noting that Stenotrophomonas sp. RCa07, Sphingobacterium sp. RCa08, Raoultella sp. RCa12, Chryseobacterium sp. RCa13, Pantoea sp. RCa37, and Pseudomonas sp. RCa18 demonstrated less favorable performance in one or more parameters related to primary growth, secondary growth, shoot production, leaf area, and relative water content under drought conditions. Therefore, at least in similar situations, they are not recommended as potential bioinoculants.
3.6. Bacterial Effect on Pigment Concentrations
Contrary to what has been documented in other studies conducted on wheat and maize [108,109], the amount of chlorophylls was not sensitive to the plant’s water conditions. However, other research on diverse coffee cultivars determined that total chlorophyll contents only decline until drought stress is intensified, and the decrease is less evident in drought-resistant cultivars [110]. Given that the present study was performed under moderate drought and with a drought-resistant cultivar such as Costa Rica 95, the absence of response in this parameter is explained. On the other hand, other studies have found that the ratio of chlorophyll a/b is a parameter that has not been modified in plants subjected to water stress [109]. Like chlorophyll, the total carotenoid concentration was not different among the groups. However, the carotenoids/chlorophyll ratio differed between the non-inoculated groups under high water availability and drought conditions and when comparing all plants from the irrigation treatments (Supplementary Table S2). Carotenoids are responsible for dissipating excess energy in the leaves and removing free radicals such as singlet oxygen, with an increased carotenoids/chlorophyll ratio potentially serving as a tolerance mechanism under stress conditions, mainly due to the photoprotective effect required in drought-stressed plants [111]. This effect was not promoted by any of the strains used in this research but by the irrigation regimes.
3.7. Bacterial Effect on Proline Accumulation
Proline is a crucial osmolyte that accumulates under osmotic stress and promotes plant growth as a tolerance strategy [112,113]. In well-irrigated control plants and those inoculated with Pantoea sp. RCa62 under both irrigation regimes, proline levels were significantly lower than in non-inoculated drought-stressed plants. The maintenance of lower proline levels in plants associated with Pantoea sp. RCa62, even under drought conditions, indicates low osmotic stress in the leaf tissue. This is likely due to improved water acquisition efficiency from enhanced root system development, which supports drought resistance through avoidance mechanisms rather than tolerance [114,115]. However, plant responses to drought stress may combine both strategies [93]. These findings are consistent with those of Bhise and Dandge [116], who observed reduced proline accumulation in rice plants inoculated with Pantoea agglomerans KL, even under salt stress, suggesting that this symbiosis mitigates stress effects. The results also align with the general trend of increased proline accumulation as water status declines [117], as seen also in coffee plants [118]. The integration of all the effects of Pantoea sp. RCa62 on coffee morphophysiology under moderate and prolonged drought conditions is shown in Figure 8.
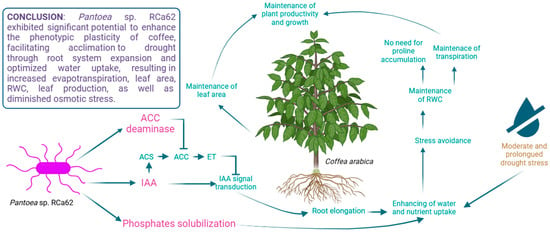
Figure 8.
Pantoea sp. RCa62, a phosphate-solubilizing bacterium that produces IAA and ACC deaminase, promotes root elongation and water and nutrient uptake in young Coffea arabica plants through these synergistic mechanisms. Root development is also notably enhanced under drought stress. These modifications in root architecture serve as a stress avoidance strategy and help maintain morphophysiological parameters that support plant growth and productivity. Bacterial secretions or processes are highlighted in pink, while their effects on the plants are highlighted in green. Arrowheads indicate positive relationships between mechanisms, while barred arrows indicate inhibitory relationships. (ACC: 1-aminocyclopropane-1-carboxylic acid; ACS: ACC synthase; ET: ethylene; IAA: indole-3-acetic acid; RWC: relative water content). Created in BioRender (https://www.biorender.com/, accessed on 27 February 2023).
3.8. Further Remarks
Plant growth promotion traits evaluated in vitro do not necessarily imply success in vivo implementation under controlled conditions, and even less so in field conditions. That is the case with strains of the Pantoea genus (RCa31, RCa37, RCa62), whose plant growth-promoting features were similar, but the results obtained in in vivo assays with non-sterile soil varied drastically. RCa31 showed promising results in promoting plant growth under well-irrigated conditions, RCa62 performed well under water scarcity, and RCa37 showed poor results in primary and secondary growth parameters. The same case is evident in Pseudomonas sp. RCa18, which displayed the highest ACC deaminase activity and other interesting growth-promoting traits but did not show the expected effects on coffee shrubs. Variations in the microbial strain performance under in vitro conditions compared to non-sterile soils can be explained by several factors: interactions with other microorganisms, nutrient competition, and pathogen presence influence their effectiveness [119]. Environmental conditions such as water availability or soil temperature may affect the outcomes; for example, RCa62 could be better adapted to water stress, while RCa31 is more effective with adequate irrigation. Additionally, strains’ action mechanisms, such as hormone production and nutrient solubilization, vary in effectiveness depending on soil context, making growth promotion potential under controlled conditions not always extendible to optimal field performance [37,120].
Moreover, the efficiency of bioinoculants can vary depending on factors such as root exudate composition, presence of organic matter, nutrient availability, organic acids, metals, and phytohormones in the soil [121,122]; conducting experiments closer to field conditions is essential. This approach helps to elucidate the complex biochemical, ecological, and evolutionary factors influencing the bacteria-plant-soil system and leads to more effective biotechnological applications. Consequently, results obtained from experiments using sterile seeds and substrates are necessary but insufficient for accurately determining a strain’s potential as an effective bioinoculant. There is a need for information derived from extensive evaluations under real-world conditions to fully understand the potential of microbial strains to promote plant growth in different environmental contexts [37,121].
In the same way, coffee displays different resistance strategies depending on the severity of stress [25]. In this case, root development was a pivotal trait promoted by Pantoea sp. RCa62, allowing water uptake under progressive and moderate drought conditions, thus enabling the implementation of morphological responses such as leaf area modification. However, coffee’s strategies may vary under more severe drought regimes and repeated drought events [9]. Further assessment of this strain in adult shrubs is also needed, as the bioinoculant effects may extend to yield and quality outcomes.
In this study, coffee phenotypic plasticity is enhanced by Pantoea sp. RCa62, which improves drought resistance and makes this strain a promising potential bioinoculant for reducing the vulnerability of coffee plantations to water scarcity predicted as a consequence of climate change.
4. Materials and Methods
4.1. Sampling
In December 2021, samples of the rhizosphere of Coffea arabica L. var. Costa Rica 95 were collected at the experimental site Teocelo-INIFAP (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias). INIFAP is in Teocelo, Veracruz, Mexico (19°23′33.6″ N, 97°00′03.5″ W) at 1300 m above sea level, with an average annual precipitation of 1972 mm and a mean annual temperature of 18.91 °C. These climatic conditions are considered optimal for coffee cultivation [123].
Nine points were selected along three zigzag lines covering the entire plot for sample collection. Soil was extracted from each point at a diameter of 60 cm around the coffee shrub trunks, extending to the four cardinal points at 0–15 cm depth, where it has been previously documented that most of the coffee roots are found, and the influence of coffee roots is highly evident due to water and soil uptake [124,125,126]. The soil samples were placed in sealable bags, transported, and stored at room temperature in a dark environment, avoiding excessive heat.
The experimental plot includes eight shade tree species arranged in a randomized block design with three replicates for each species. The tree species present in the agronomic trial are Roseodendron donnell-smithii (Rose) Miranda, Juglans pyriformis Liebm., Pinus chiapensis (Martínez) Andresen, Grevillea robusta A. Cunn. ex R. Br., Inga vera Willd., Cordia alliodora (Ruiz and Pav.) Oken, Tapirira mexicana Marchand, and Tabebuia rosea (Bertol.) DC. The soil is described as sandy loam. No pest or weed control practices are implemented. Coffee shrubs receive annual fertilization with 200 g of a 4:2:2 mixture of urea, potassium chloride (KCl), and diammonium phosphate (DAP 18-46-0).
4.2. Isolation and Selection of ACC Deaminase-Producing Rhizobacteria
The collected soil used for isolation was embedded in the root network, so the roots were shaken to remove the attached soil [127]. No sonication or washing protocols were carried out in order to avoid excessive loss of rhizosphere soil, as the influence of plant root presence extends to the soil up to 2 cm from the root surface [128]. To select isolates with the capacity for ACCd production, 1 g of soil (two replicates per sample) was added to vials containing 20 mL of Dworkin and Foster (DF) liquid saline medium [129] with ACC as the sole nitrogen source at a final concentration of 3 mM. The tubes were incubated at 30 °C and 160 rpm for 48 h [55]. Subsequently, subculturing was performed by taking 1 mL of the culture and inoculating it into 19 mL of sterile DF + ACC medium, followed by incubation under the same conditions. Next, DF + ACC medium plates were inoculated. To prepare a solid medium, 30 µmol of ACC per plate was added as the sole nitrogen source and dispersed using sterile glass beads. An amount of 20 µL of the previously cultured DF + ACC broth was inoculated and spread by cross-streaking. Various colonial morphologies on the plates were identified and subcultured onto LB plates until axenic cultures were obtained to isolate microorganisms. A total of 54 purified isolates were obtained and stored at −70 °C in 35% glycerol. The isolates were kept in the collection of the Microbial Biotechnology Laboratory at ENCB-IPN.
A bacterial growth assay was performed in microplates to confirm ACCd production and select the isolates with the highest growth, using only ACC as the nitrogen source. Cryopreserved bacteria were subcultured onto LB plates for 48 h at 30 °C, then inoculated into 5 mL of LB broth and incubated for 24 h at room temperature. The LB cultures were washed twice with 10 mM MgSO4 before inoculation. Microplate wells were prepared with 122 µL of DF medium, 15 µL of a solution containing various nitrogen sources (ACC 0.032 M as the treatment, MgSO4 0.1 M as the negative control, and (NH4)2SO4 0.1 M as the positive control), along with 22 µL of inoculum. Isolates were inoculated in triplicate. Additionally, triplicate wells without inoculum were prepared for each condition, with 22 µL of MgSO4 10 mM instead of bacterial culture [55,130]. Microplates were incubated at room temperature for 72 h with shaking at 150 rpm. Optical density (OD) readings at 600 nm were obtained using a Multiskan FC microplate reader (Thermo Scientific, Woodlands, Singapore). To account for OD contributions from reagents, the mean OD of wells without inoculum was subtracted from the readings of each condition. OD600 values were analyzed using ANOVA to identify isolates with higher growth in DF + ACC compared to the MgSO4 negative control (post-hoc Tukey, p < 0.05).
Isolates that exhibited growth in media with ACC as the only nitrogen source were taxonomically identified using primers 27F (5′-GTGCTGCAGAGACTTTGATCCTGGCTCAG-3′) and 1492R (5′-CACGGATCCTADGGGTACCTTACGACT-3′) to amplify the 16S rRNA gene sequence. The PCR products were sequenced using Sanger sequencing at Macrogen (Seoul, Republic of Korea). The sequences were edited and assembled using Chromas Pro (Technelysium), and the consensus sequences were analyzed with EzBioCloud (https://www.ezbiocloud.net/ (accessed on 27 February 2023) for identification. BOX-PCR analyzed the strains of the same genera for clonal identification using the primer BOXA1R (5′-CTACGGCAAGGCGACGCTGACG-3′) [131]. All rhizobacteria 16S rRNA gene sequences were deposited to NCBI GenBank with accession numbers (PQ449695–PQ449706).
To confirm the identity of the genera of the strains belonging to the order Enterobacteriales (RCa01, RCa28, RCa12, RCa31, RCa37, and RCa2), a phylogenetic analysis was performed based on 16S gene sequences. The analysis incorporated the sequences of the available type strains from EzBioCloud (https://www.ezbiocloud.net/ (accessed on 21 January 2023) that showed high similarity with the study sequences (similarity > 98%). A multiple alignment was performed using MUSCLE (3.8) [132] to conduct a maximum likelihood analysis with IQ-TREE multicore version 1.6.1 [133], using the K2P + I + G4 nucleotide substitution model, based on the BIC values.
4.3. Characterization of Plant Growth-Promoting Traits on ACC Deaminase-Producing Strains
The activity of ACCd was determined based on the induction of ACCd production by the strains and measuring the production of α-ketobutyrate from ACC [55]. Statistical differences in ACCd activity were analyzed using the Kruskal-Wallis test (post-hoc Conover-Iman). Siderophore production and phosphate solubilization were determined by inoculating 2 μL of a culture adjusted to a density of approximately 6 × 108 CFU/mL in quadruplicate onto agar with Chrome Azurol S (CAS medium) [134] and NBRIP medium [135], respectively. Plates were incubated at 30 °C for eight days. Measurements of halo diameter corresponding to iron solubilization in CAS medium were calculated as the total halo diameter minus the colony diameter. The phosphate solubilization index (PSI) in NBRIP medium was determined by the formula [34]. Statistical differences in iron and phosphate solubilization were analyzed using ANOVA (post-hoc Tukey) among the isolates with activity.
Indole-3-acetic acid production was evaluated in Jain and Patriquin medium [136] using the colorimetric method with Salkowski reagent [137]. For this, 100 μL of a culture adjusted to a density of approximately 6 × 108 CFU/mL was inoculated into media both with and without tryptophan to assess the synthesis of this metabolite via tryptophan-dependent and independent pathways at 72 h. All measurements were performed in triplicate and read using a LAMBDA XLS spectrophotometer (Perkin Elmer, Waltham, MA, USA) at 540 nm. Significant differences among IAA-producing isolates were identified using Kruskal-Wallis (post-hoc Conover-Iman) tests.
Similarly, the strains were assessed for nitrogen fixation using the acetylene reduction assay [138,139] in BMGM medium [140]. Gas chromatography analyzed them with a Clarus 580 system (Perkin Elmer, Waltham, Massachusetts, USA) equipped with a flame ionization detector.
4.4. Pot Experiment Design
Ten-month-old C. arabica cv. Costa Rica 95 plants were purchased from a certified nursery in La Estanzuela, Emiliano Zapata, Veracruz. They were transplanted into a substrate composed of 60% commercial black soil (Nutrigarden, Tierra Negra, 30 kg bag), 20% coconut fiber, and 20% perlite. Pest management included using color traps, concentrated neem, and garlic commercial extract (BioNutra, Neem All) at a concentration of 10 mL/L of water and manual removal of ectoparasites. Plants were maintained in a 6-week acclimation period before inoculation. The plants were irrigated during this period to keep the soil moisture close to field capacity (85% VWC). The soil’s volumetric water content (VWC) was monitored using a Yieryi LY-201 soil moisture sensor (Shen Zhen Yage Technology Co., Ltd., Shenzhen, China).
After the acclimation period, each bacterial strain was inoculated in groups of 14 plants. The inoculum was prepared in 100 mL of LB broth in a 250 mL flask, shaking orbitally at 120 rpm at room temperature for 18 h. Bacteria were recovered by centrifugation and resuspended in 0.01 M MgSO4 to achieve a concentration of approximately 108 CFU/mL. A total of 8 mL of the solution was inoculated per plant weekly into the rhizospheric soil for five weeks [101].
After completing the inoculation treatment, each group was divided into two subgroups: one received irrigation to maintain soil moisture near field capacity (R, 85% VWC). Meanwhile, the other subset (S) experienced a two-phase reduction in humidity. During the first phase, soil moisture was kept at 70% VWC for three weeks, and in the second phase, it was lowered to 55% VWC for five weeks. Based on the plants’ needs, irrigation was carried out every 48 to 72 h. This way, groups of seven plants were inoculated with each strain, along with a non-inoculated control group, under moderate and prolonged drought conditions (S) and regular irrigation (R). Evapotranspiration for each plant was recorded gravimetrically between irrigations, and the average was calculated every 48 h.
This experiment was conducted in the greenhouse of the Centro de Estudios Científicos y Tecnológicos “Miguel Othón de Mendizábal”, Instituto Politécnico Nacional. The light and temperature conditions were not directly controlled; ventilation measures and external shading techniques, such as leaf litter, were used to reduce extreme temperatures and simulate light incidence in an agroforestry system. All the plants were distributed uniformly and randomly across the greenhouse area to avoid biases caused by peak irradiance times or minor differences in the microclimate.
4.5. Effects of ACC Deaminase-Producing Strains on the Growth and Physiological Status of Coffea arabica
At the end of each treatment, the functional leaf attributes were measured: the leaf area and specific leaf area (SLA) were observed using ImageJ 1.54d [141]. Relative water content (RWC) was determined gravimetrically [142,143]. Leaf discs with a diameter of 1.5 cm were hydrated by floating them in water at approximately 20 °C for 3 to 4 h, avoiding darkness and maintaining consistent illumination. After hydration, the discs were quickly blotted dry with filter paper and weighed to obtain the turgid weight. They were then dried in an oven at 80 °C for 48 h, cooled in a desiccator, and weighed again to determine the dry weight. Leaf functional attributes were measured between 8.00 and 10.00 AM.
Additionally, the following growth parameters were assessed: height; absolute and relative primary growth; diameter at 5 cm from the ground; absolute and relative secondary growth; number, fresh weight, and dry weight of leaf buds; shoot and root biomass production; and root architecture, with the latter analyzed using RhizoVision Explorer v2.0.2 [144]. Chlorophyll a, b, and carotenoid concentrations were determined using the method of Hiscox and Israelstam [145], which involves fractionating 100 mg of leaf tissue into uniform pieces of 1 to 4 mm2 in 10 mL of dimethyl sulfoxide (DMSO) and extracting the pigments without homogenizing the tissue at 65 °C for 4 h. Absorbances at 665, 649, and 480 nm were measured to determine pigment concentrations using the formulas according to previously standardized formulas [146]. Young and fully expanded leaves from the upper third of the plant, specifically between the second and third pair of branches, were used for all leaf tissue tests, ensuring a clean cut.
4.6. Proline Accumulation
Proline content was determined in uninoculated plants and those associated with Pantoea sp. RCa62 using a colorimetric method with ninhydrin [147].
4.7. Data Analysis
All statistical analyses were conducted using R version 4.3.2. To group the strains according to the previously determined plant growth-promoting traits, a k-means analysis was performed using the heatmaps library version 1.0.12. The obtained values for each characteristic were normalized to a maximum value of 1. Multivariate analyses associated with plant traits in response to inoculation and drought treatments were performed using libraries factoextra version 1.0.7 and NbClust version 3.0.1. To determine the sources of variability and detect differences between groups, ANOVA tests were performed over plant parameters after verifying the assumptions of each test and transforming the data when necessary. Means were compared using Duncan’s test as well as Dunnett’s. All the plots were built with ggplot2 version 4.4.4.
5. Conclusions
Climate change underscores the need to develop agricultural systems adaptation strategies, particularly for vulnerable crops like coffee. This study primarily focused on exploring ACC deaminase-producing rhizobacteria in coffee plantations. Twelve ACC deaminase-producing bacterial strains were identified and characterized from the rhizosphere of Coffea arabica L. cv. Costa Rica 95, all belonging to Gram-negative genera known for their plant growth-promoting abilities under stress. Notably, Pantoea sp. RCa62, RCa31, and Serratia sp. RCa28 demonstrated the ability to enhance plant growth and drought resistance (in the case of RCa62) and promote growth under adequate irrigation (for the latter strains). This study highlights the variability in bioinoculant performance between in vitro and in vivo assays, emphasizing the need for extensive evaluations under real-world conditions to fully assess their effectiveness. Specifically, Pantoea sp. RCa62 exhibited significant potential to enhance the phenotypic plasticity of coffee, facilitating drought acclimation through root system expansion and optimized water uptake. This resulted in increased evapotranspiration, leaf area, relative water content (RWC), leaf production, and reduced osmotic stress. These findings suggest that Pantoea sp. RCa62 is a promising candidate for bioinoculant formulation aimed at reducing the vulnerability of coffee plantations to water scarcity, which is projected to worsen due to climate change. It is still necessary to evaluate the strain’s performance in coffee plants in productive fields and explore the possibility of optimizing inoculation by evaluating various methods and potential matrices to prolong the inoculum’s presence in the soil. Similarly, it is interesting to explore the strain’s mechanisms of action through the modification of gene expression associated with drought resistance in plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14071084/s1, Figure S1: Maximum-likelihood tree based on 16S rRNA gene sequences of the Enterobacteriales strains. Figure S2: Hierarchical clustering heatmap of the strains according to the relative values for each characterized plant growth-promoting trait; Table S1: Mean values and ANOVA for each plant parameter determined; Table S2: Pigment concentrations.
Author Contributions
Conceptualization, Y.J.-A., J.A.I. and P.E.-d.l.S.; methodology, Y.J.-A., J.A.I., F.d.F.R.-C. and P.E.-d.l.S.; validation, Y.J.-A., J.A.I., F.d.F.R.-C. and P.E.-d.l.S.; formal analysis, Y.J.-A.; investigation, Y.J.-A.; resources, Y.J.-A., J.A.I. and P.E.-d.l.S.; data curation, Y.J.-A. and P.E.-d.l.S.; writing—original draft preparation, Y.J.-A. and P.E.-d.l.S.; writing—review and editing, Y.J.-A., J.A.I., F.d.F.R.-C. and P.E.-d.l.S.; visualization, Y.J.-A., F.d.F.R.-C. and P.E.-d.l.S.; supervision, P.E.-d.l.S.; project administration, P.E.-d.l.S.; funding acquisition, Y.J.-A. and P.E.-d.l.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Secretaría de Investigación y Posgrado, Instituto Politécnico Nacional, grant numbers 2022-1936, Innovación 2023-2723, 2024-1040, and 2024-A026.
Data Availability Statement
All the rhizobacteria 16S rRNA gene sequences were deposited to NCBI GenBank with accession numbers (PQ449695–PQ449706).
Acknowledgments
The authors thank the experimental site Teocelo-INIFAP and Rosalío López Morgado for providing facilities for soil sampling, as well as Centro de Estudios Científicos y Tecnológicos 6 “Miguel Othón de Mendizábal” and Sergio Arturo Murillo Jiménez for granting access to the greenhouse facilities and providing material and administrative support, all of which were essential for the completion of this research. The authors also extend their gratitude to Karl Heinrich Marx Sánchez Díaz for his indispensable assistance during soil sampling, to Víctor Sebastián Rosales Sánchez for his invaluable support during the laboratory experiments and plant management, and to Francisco Moreno Ramírez, Alma Alejandra López Zarco, Mónica Rivera Rosas, and Alfredo Jasso Padilla for their significant contributions to plant establishment, management, and measurements. P.E.-d.l.S. thanks Comisión de Operación y Fomento de Actividades Académicas (COFAA-IPN), Programa de Estímulos al Desempeño de los Investigadores (EDI-IPN) and Sistema Nacional de Investigadores e Investigadoras, Secretaría de Ciencia, Humanidades, Tecnología e Innovación for the support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ACCd | ACC deaminase |
| PGPR | Plant growth promoting rhizobacteria |
| R | Full-irrigation treatment |
| RWC | Relative water content |
| S | Drought stress treatment |
| VWC | Volumetric water content |
References
- Beining, A.M. Ecophysiological Diversity of Wild Coffea arabica Populations in Ethiopia: Drought Adaptation Mechanisms. Ph.D. Thesis, Universität Bonn, Bonn, Germany, 2007. [Google Scholar]
- Amrouk, M.; Palmeri, F.; Magrini, E. Global Coffee Market and Recent Price Developments; FAO: Rome, Italy, 2025. [Google Scholar]
- Tucker, C.M. Coffee culture. In Local Experiences, Global Connections, 2nd ed.; Anthropology of Stuff series; Routledge: New York, NY, USA, 2018; ISBN 9781315678795. [Google Scholar]
- Wright, D.R.; Gordon, A.; Bennett, R.E.; Selinske, M.J.; Lentini, P.E.; Garrard, G.E.; Rodewald, A.D.; Bekessy, S.A. Biodiverse coffee plantations provide co-benefits without compromising yield. J. Sustain. Agric. Environ. 2024, 3, 1–12. [Google Scholar] [CrossRef]
- Lugo-Pérez, J.; Hajian-Forooshani, Z.; Perfecto, I.; Vandermeer, J. The importance of shade trees in promoting carbon storage in the coffee agroforest systems. Agric. Ecosyst. Environ. 2023, 355, 108594. [Google Scholar] [CrossRef]
- Wildtruth, F.; Perfecto, I. Effects of canopy connectivity on the arboreal ant community in coffee shade trees. Biotropica 2023, 55, 1106–1113. [Google Scholar] [CrossRef]
- Villers, L.; Arizpe, N.; Orellana, R.; Conde, C.; Hernandez, J. Impactos del cambio climático en la floración y desarrollo del fruto del café en Veracruz, Mexico. Interciencia 2009, 34, 322–329. [Google Scholar]
- Melke, A.; Fetene, M. Eco-physiological basis of drought stress in coffee (Coffea arabica, L.) in Ethiopia. Theor. Exp. Plant Physiol. 2014, 26, 225–239. [Google Scholar] [CrossRef]
- Menezes-Silva, P.E.; Sanglard, L.M.V.P.; Ávila, R.T.; Morais, L.E.; Martins, S.C.V.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araújo, W.L.; Fernie, A.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef]
- Da Matta, F.M.; Ramalho, C. Impacts of drought and temperature stress on coffee physiology and production: A review. Brazilian J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate change and coffee quality: Systematic review on the effects of environmental and management variation on secondary metabolites and sensory attributes of Coffea arabica and Coffea canephora. Front. Plant Sci. 2021, 12, 708013. [Google Scholar] [CrossRef]
- da Silva Tavares, P.; Giarolla, A.; Chou, S.C.; de Paula Silva, A.J.; de Arruda Lyra, A. Climate change impact on the potential yield of Arabica coffee in southeast Brazil. Reg. Environ. Change 2018, 18, 873–883. [Google Scholar] [CrossRef]
- Laderach, P.; Lundy, M.; Jarvis, A.; Ramirez, J.; Portilla, E.P.; Schepp, K.; Eitzinger, A. Predicted impact of climate change on coffee supply chains. In The Economic, Social and Political Elements of Climate Change; Filho, W.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 703–723. ISBN 978-3-642-14775-3. [Google Scholar]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Change 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Ovalle-Rivera, O.; Läderach, P.; Bunn, C.; Obersteiner, M.; Schroth, G. Projected shifts in Coffea arabica suitability among major global producing regions due to climate change. PLoS ONE 2015, 10, e0124155. [Google Scholar] [CrossRef]
- Jawo, T.O.; Kyereh, D.; Lojka, B. The impact of climate change on coffee production of small farmers and their adaptation strategies: A review. Clim. Dev. 2023, 15, 93–109. [Google Scholar] [CrossRef]
- Kim, T.-W.; Jehanzaib, M. Drought risk analysis, forecasting and assessment under climate change. Water 2020, 12, 1862. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Deepak; Singh, S.; Bakshi, M.; Bansal; Sangeeta; Bansal, S.; Thakur, A.; Singh, S.; Bakshi, M.; Bansal, S. Changes in crop physiology under drought stress: A review. J. Pharmacogn. Phytochem. 2019, 8, 1251–1253. [Google Scholar]
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR mediated alterations in root traits: Way toward sustainable crop production. Front. Sustain. Food Syst. 2021, 4, 287. [Google Scholar] [CrossRef]
- Barnawal, D.; Singh, R.; Singh, R.P. Role of plant growth promoting rhizobacteria in drought tolerance. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 107–128. ISBN 9780128158791. [Google Scholar]
- Shaffique, S.; Shah, A.A.; Odongkara, P.; Elansary, H.O.; Khan, A.L.; Adhikari, A.; Kang, S.M.; Lee, I.J. Deciphering the ABA and GA biosynthesis approach of Bacillus pumilus, mechanistic approach, explaining the role of metabolic region as an aid in improving the stress tolerance. Sci. Rep. 2024, 14, 28923. [Google Scholar] [CrossRef]
- Ali, S.; Khan, N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants. Microbiol. Res. 2021, 249, 126771. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the impact of drought in Coffea genotypes: Transcriptomic analysis supports a common high resilience to moderate water deficit but a genotype dependent sensitivity to severe water deficit. Agronomy 2021, 11, 2255. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J. Soil Sci. Plant Nutr. 2023, 23, 106–124. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: A review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Stress control and ACC deaminase. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015; pp. 257–264. ISBN 9783319085753. [Google Scholar]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-Ul-Hye, M.; Hussain, S.; Riaz, M.; Qayyum, M.F. Mitigation of drought stress in maize through inoculation with drought tolerant ACC deaminase containing PGPR under axenic conditions. Pakistan J. Bot. 2020, 52, 49–60. [Google Scholar] [CrossRef]
- Buqori, D.M.A.I.; Sugiharto, B.; Suherman; Siswoyo, T.A.; Hariyono, K. Mitigating drought stress by application of drought-tolerant Bacillus spp. enhanced root architecture, growth, antioxidant and photosynthetic genes expression in sugarcane. Sci. Rep. 2025, 15, 5259. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Gupta, A.; Rai, S.; Bano, A.; Sharma, S.; Kumar, M.; Binsuwaidan, R.; Suhail Khan, M.; Upadhyay, T.K.; Alshammari, N.; Saeed, M.; et al. ACC Deaminase produced by PGPR mitigates the adverse effect of osmotic and salinity stresses in Pisum sativum through modulating the antioxidants activities. Plants 2022, 11, 3419. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, Y.; Liu, Z.; Fan, Y.; Liu, M.; Ningjing, M.; Li, Y. Promotional properties of ACC deaminase-producing bacterial strain DY1-3 and its enhancement of maize resistance to salt and drought stresses. Microorganisms 2023, 11, 2654. [Google Scholar] [CrossRef]
- Urgiles-Gómez, N.; Avila-Salem, M.E.; Loján, P.; Encalada, M.; Hurtado, L.; Araujo, S.; Collahuazo, Y.; Guachanamá, J.; Poma, N.; Granda, K.; et al. Plant growth-promoting microorganisms in coffee production: From isolation to field application. Agronomy 2021, 11, 1531. [Google Scholar] [CrossRef]
- Arshad, M.; Shaharoona, B.; Mahmood, T. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.). Pedosphere 2008, 18, 611–620. [Google Scholar] [CrossRef]
- Bhise, K.K.; Bhagwat, P.K.; Dandge, P.B. Synergistic effect of Chryseobacterium gleum sp. SUK with ACC deaminase activity in alleviation of salt stress and plant growth promotion in Triticum aestivum L. 3 Biotech 2017, 7, 105. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Gulati, A.; Nayyar, H. Indigenous salt-tolerant rhizobacterium Pantoea dispersa (PSB3) reduces sodium uptake and mitigates the effects of salt stress on growth and yield of chickpea. Acta Physiol. Plant. 2016, 38, 278. [Google Scholar] [CrossRef]
- Shaharoona, B.; Arshad, M.; Zahir, Z.A.; Khalid, A. Performance of Pseudomonas spp. containing ACC-deaminase for improving growth and yield of maize (Zea mays L.) in the presence of nitrogenous fertilizer. Soil Biol. Biochem. 2006, 38, 2971–2975. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef]
- Wu, Z.; Yue, H.; Lu, J.; Li, C. Characterization of rhizobacterial strain Rs-2 with ACC deaminase activity and its performance in promoting cotton growth under salinity stress. World J. Microbiol. Biotechnol. 2012, 28, 2383–2393. [Google Scholar] [CrossRef]
- Cardinale, M.; Ratering, S.; Suarez, C.; Zapata Montoya, A.M.; Geissler-Plaum, R.; Schnell, S. Paradox of plant growth promotion potential of rhizobacteria and their actual promotion effect on growth of barley (Hordeum vulgare L.) under salt stress. Microbiol. Res. 2015, 181, 22–32. [Google Scholar] [CrossRef]
- Gupta, G.; Jha, P. Screening of potential PGPR candidates as future biofertilizers-A strategic approach from lab to field. Res. J. Biotechnol. 2015, 10, 48–62. [Google Scholar]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Dunn, M.F.; Becerra-Rivera, V.A. The biosynthesis and functions of polyamines in the interaction of plant growth-promoting rhizobacteria with plants. Plants 2023, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Knights, H.E.; Jorrin, B.; Haskett, T.L.; Poole, P.S. Deciphering bacterial mechanisms of root colonization. Environ. Microbiol. Rep. 2021, 13, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Muleta, D.; Assefa, F.; Hjort, K.; Roos, S.; Granhall, U. Characterization of rhizobacteria isolated from wild Coffea arabica L. Eng. Life Sci. 2009, 9, 100–108. [Google Scholar] [CrossRef]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic bacteria in Coffea arabica L. J. Basic Microbiol. 2005, 45, 371–380. [Google Scholar] [CrossRef]
- Chen, M.; Li, N.; Zhang, X.-F.; Zhou, X.-K.; Shi, R.; Su, Y.-X.; Liu, J.-J.; Cao, Y.; Mo, M.H.; Ma, L. Sphingobacterium faecale sp. nov., a 1-aminocyclopropane-1-carboxylate deaminase producing bacterium isolated from camel faeces. Int. J. Syst. Evol. Microbiol. 2022, 72, 005215. [Google Scholar] [CrossRef]
- Simarmata, R.; Ngadiman, N.; Rohman, M.S.; Simanjuntak, P. Identification of 1-aminocyclopropane-1-carboxilid acid (ACC)-deaminase producing endophytic bacteria from local agricultural plantation based on 16S ribosomal RNA gene as genetic marker. Biotropic J. Trop. Biol. 2019, 3, 13–23. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Palmer, M.; Steenkamp, E.T.; Coetzee, M.P.A.; Chan, W.Y.; van Zyl, E.; De Maayer, P.; Coutinho, T.A.; Blom, J.; Smits, T.H.M.; Duffy, B.; et al. Phylogenomic resolution of the bacterial genus Pantoea and its relationship with Erwinia and Tatumella. Antonie Van Leeuwenhoek 2017, 110, 1287–1309. [Google Scholar] [CrossRef]
- Mergaert, J.; Verdonck, L.; Kersters, K. Transfer of Erwinia ananas (synonym, Erwinia uredovora) and Erwinia stewartii to the genus Pantoea emend. as Pantoea ananas (Serrano 1928) comb. nov. and Pantoea stewartii (Smith 1898) comb. nov., respectively, an. Int. J. Syst. Bacteriol. 1993, 43, 162–173. [Google Scholar] [CrossRef]
- Muleta, D.; Assefa, F.; Börjesson, E.; Granhall, U. Phosphate-solubilising rhizobacteria associated with Coffea arabica L. in natural coffee forests of southwestern Ethiopia. J. Saudi Soc. Agric. Sci. 2013, 12, 73–84. [Google Scholar] [CrossRef]
- Mekete, T.; Hallmann, J.; Kiewnick, S.; Sikora, R. Endophytic bacteria from ethiopian coffee plants and their potential to antagonise meloidogyne incognita. Nematology 2009, 11, 117–127. [Google Scholar] [CrossRef]
- Ferreira Pires, J.; de Souza Cardoso, L.; Freitas Schwan, R.; Ferreira Silva, C. Diversity of microbiota found in coffee processing wastewater treatment plant. World J. Microbiol. Biotechnol. 2017, 33, 211. [Google Scholar] [CrossRef]
- Solis Pino, A.F.; Delgado Espinosa, Z.Y.; Ramos Cabrera, E.V. Characterization of the rhizosphere bacterial microbiome and coffee bean fermentation in the Castillo-Tambo and Bourbon varieties in the Popayán-Colombia plateau. BMC Plant Biol. 2023, 23, 217. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Cleto, S.; Carr, G.; Vlamakis, H.; João Vieira, M.; Kolter, R.; Clardy, J. Mixing and matching siderophore clusters: Structure and biosynthesis of serratiochelins from Serratia sp. V4. J. Am. Chem. Soc. 2012, 134, 13550–13553. [Google Scholar] [CrossRef]
- Weakland, D.R.; Smith, S.N.; Bell, B.; Tripathi, A.; Mobley, H.L.T. The Serratia marcescens siderophore serratiochelin is necessary for full virulence during bloodstream infection. Infect. Immun. 2020, 88, e00117-20. [Google Scholar] [CrossRef]
- Appel, T.M.; Quijano-Martínez, N.; De La Cadena, E.; Mojica, M.F.; Villegas, M.V. Microbiological and clinical aspects of Raoultella spp. Front. Public Health 2021, 9, 686789. [Google Scholar] [CrossRef]
- Santoyo, G.; Valencia-Cantero, E.; Orozco-Mosqueda, M.d.C.; Peña-Cabriales, J.J.; Farías-Rodríguez, R. Role of siderophores in antagonic activity of Pseudomonas fluorescens ZUM80 against plant fungi. Terra Latinoam. 2010, 28, 53–60. [Google Scholar]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)—Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Ciríaco da Silva, E.; Custódio Nogueira, R.J.; Almeida da Silva, M.; Bandeira de Albuquerque, M. Drought stress and plant nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Chen, Q.; Liu, S. Identification and characterization of the phosphate-solubilizing bacterium Pantoea sp. S32 in reclamation soil in Shanxi, China. Front. Microbiol. 2019, 10, 2171. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Zuo, Z.; Lv, Z.; Yang, Z.; Mao, W.; Liu, Y.; Zhou, Y.; Huang, J.; Song, Z. Evaluation and improvement of phosphate solubilization by an isolated bacterium Pantoea agglomerans ZB. World J. Microbiol. Biotechnol. 2020, 36, 27. [Google Scholar] [CrossRef]
- Son, H.J.; Park, G.T.; Cha, M.S.; Heo, M.S. Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour. Technol. 2006, 97, 204–210. [Google Scholar] [CrossRef]
- Apine, O.A.; Jadhav, J.P. Optimization of medium for indole-3-acetic acid production using Pantoea agglomerans strain PVM. J. Appl. Microbiol. 2011, 110, 1235–1244. [Google Scholar] [CrossRef]
- Novák, V. Evapotranspiration in the Soil-Plant-Atmosphere System; Springer Netherlands: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-3839-3. [Google Scholar]
- Martins, S.C.V.; Sanglard, M.L.; Morais, L.E.; Menezes-Silva, P.E.; Mauri, R.; Avila, R.T.; Vital, C.E.; Cardoso, A.A.; DaMatta, F.M. How do coffee trees deal with severe natural droughts? An analysis of hydraulic, diffusive and biochemical components at the leaf level. Trees Struct. Funct. 2019, 33, 1679–1693. [Google Scholar] [CrossRef]
- Carvalho da Silva, P.; Ribeiro Junior, W.Q.; Gerosa Ramos, M.L.; Cruz Rocha, O.; Delly Veiga, A.; Henriques Silva, N.; de Oliveira Brasileiro, L.; Cardoso Santana, C.; Filgueiras Soares, G.; Vitória Malaquias, J.; et al. Physiological changes of arabica coffee under different intensities and durations of water stress in the Brazilian Cerrado. Plants 2022, 11, 2198. [Google Scholar] [CrossRef]
- León-Burgos, A.F.; Unigarro, C.; Balaguera-López, H.E. Can prolonged conditions of water deficit alter photosynthetic performance and water relations of coffee plants in central-west Colombian? S. Afr. J. Bot. 2022, 149, 366–375. [Google Scholar] [CrossRef]
- Da Matta, F.M.; Chaves, A.R.M.; Pinheiro, H.A.; Ducatti, C.; Loureiro, M.E. Drought tolerance of two field-grown clones of Coffea canephora. Plant Sci. 2003, 164, 111–117. [Google Scholar] [CrossRef]
- Avila, R.T.; Cardoso, A.A.; de Almeida, W.L.; Costa, L.C.; Machado, K.L.G.; Barbosa, M.L.; de Souza, R.P.B.; Oliveira, L.A.; Batista, D.S.; Martins, S.C.V.; et al. Coffee plants respond to drought and elevated [CO2] through changes in stomatal function, plant hydraulic conductance, and aquaporin expression. Environ. Exp. Bot. 2020, 177, 104148. [Google Scholar] [CrossRef]
- Naves, V.L. Coffee Trees Under Rainfall Exclusion: Evidences for Canopy Acclimation to Water Shortage. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2018. [Google Scholar]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–16. ISBN 9783319288994. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Hu, W.; Lu, Z.; Meng, F.; Li, X.; Cong, R.; Ren, T.; Sharkey, T.D.; Lu, J. The reduction in leaf area precedes that in photosynthesis under potassium deficiency: The importance of leaf anatomy. New Phytol. 2020, 227, 1749–1763. [Google Scholar] [CrossRef] [PubMed]
- Kathpalia, R.; Bhatla, S.C. Plant water relations. In Plant Physiology, Development and Metabolism; Bhatla, S., Lal, M., Eds.; Springer: Singapore, 2018; pp. 3–36. [Google Scholar]
- Sharma-Natu, P.; Ghildiyal, M.C. Potential targets for improving photosynthesis and crop yield. Curr. Sci. 2005, 88, 1918–1928. [Google Scholar]
- Da Matta, F.M. Exploring drought tolerance in coffee: A physiological approach with some insights for plant breeding. Brazilian J. Plant Physiol. 2004, 16, 1–6. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Chen, Y.J.; Guo, Y.H.; Cao, K.F. Responses of two field-grown coffee species to drought and re-hydration. Photosynthetica 2005, 43, 187–193. [Google Scholar] [CrossRef]
- Worku, M.; Astatkie, T. Dry matter partitioning and physiological responses of Coffea arabica varieties to soil moisture deficit stress at the seedling stage in Southwest Ethiopia. African J. Agric. Res. 2010, 5, 2066–2072. [Google Scholar]
- Khan, A.; Singh, A.V. Multifarious effect of ACC deaminase and EPS producing Pseudomonas sp. and Serratia marcescens to augment drought stress tolerance and nutrient status of wheat. World J. Microbiol. Biotechnol. 2021, 37, 198. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, B.; Murail, M.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.N.; Niranjana, S.R. Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiol. Res. 2020, 234, 126422. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A review on potential plant-basedwater stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Cisneros, C.A.; Franco, J.M.; Realpe Fernández, M.; Fuenmayor, J.C. Influencia de microorganismos en la disponibilidad de fósforo en plántulas de café (Coffea arabica). Biotecnoloía En El Sect. Agropecu. Y Agroindustrial 2017, 15, 19. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Rivas-Ubach, A.; Oravec, M.; Vecerova, K.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.; et al. Opposite metabolic responses of shoots and roots to drought. Sci. Rep. 2014, 4, 6829. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Souchère, V., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. ISBN 9789048126668. [Google Scholar]
- Fonta, J.E.; Giri, J.; Vejchasarn, P.; Lynch, J.P.; Brown, K.M. Spatiotemporal responses of rice root architecture and anatomy to drought. Plant Soil 2022, 479, 443–464. [Google Scholar] [CrossRef]
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Fu, X.; Harberd, N.P. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 2003, 421, 740–743. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef]
- Uzma, M.; Iqbal, A.; Hasnain, S. Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiata. PLoS ONE 2022, 17, e0262932. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Bacterial indole-3-acetic acid: A key regulator for plant growth, plant-microbe interactions, and agricultural adaptive resilience. Microbiol. Res. 2024, 281, 127602. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef]
- Firdaus, N.K.; Oktafiyanto, M.F.; Sasmita, K.D.; Pranowo, D. Efficacy of phytohormones producing isolates on the growth of two stem-cutting robusta coffee (Coffea canephora). IOP Conf. Ser. Earth Environ. Sci. 2023, 1172, 012002. [Google Scholar] [CrossRef]
- Zemlyanskaya, E.V.; Omelyanchuk, N.A.; Ubogoeva, E.V.; Mironova, V.V. Deciphering auxin-ethylene crosstalk at a systems level. Int. J. Mol. Sci. 2018, 19, 4060. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Zhu, M.; Wan, H.; Chen, Z.; Yang, N.; Duan, J.; Wei, Z.; Hu, T.; Liu, F. Effects of plant growth promoting hizobacteria (PGPR) strain Bacillus licheniformis with biochar amendment on potato growth and water use efficiency under reduced irrigation regime. Agronomy 2022, 12, 1031. [Google Scholar] [CrossRef]
- Le, A.T.; Pék, Z.; Takács, S.; Neményi, A.; Helyes, L. The effect of plant growth-promoting rhizobacteria on yield, water use efficiency and Brix degree of processing tomato. Plant, Soil Environ. 2018, 64, 523–529. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef]
- Gholamin, R.; Khayatnezhad, M. Assessment of the correlation between chlorophyll content and drought resistance in corn cultivars (Zea Mays). Helix 2020, 10, 93–97. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Chekol, H.; Bezuayehu, Y.; Warkineh, B.; Shimber, T.; Mierek-Adamska, A.; Dąbrowska, G.B.; Degu, A. Unraveling drought tolerance and sensitivity in coffee genotypes: Insights from seed traits, germination, and growth-physiological responses. Agriculture 2023, 13, 1754. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Barata-Luís, R.; Vitorino, H.S.; Gonçalves, E.R.; Rolim, E.V.; Santos, M.G.; Almeida-Cortez, J.S.; Ferreira, V.M.; Lemos, E.E.; Endres, L. Photosynthesis, photoprotection and antioxidant activity of purging nut under drought deficit and recovery. Biomass Bioenergy 2010, 34, 1207–1215. [Google Scholar] [CrossRef]
- Tamirat, W. Review on role of proline on coffee under drought conditions. J. Environ. Earth Sci. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Basu, S.; Ramegowda, V.; Pereira, A. Mechanisms of drought tolerance in rice. In Achieving Sustainable Cultivation of Rice; Sasaki, T., Ed.; Burleigh Dodds Science Publishing Limited: Sawston, UK, 2016; pp. 131–163. [Google Scholar]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Bhise, K.K.; Dandge, P.B. Alleviation of salinity stress in rice plant by encapsulated salt tolerant plant growth promoting bacteria Pantoea agglomerans strain KL and its root colonization ability. Arch. Agron. Soil Sci. 2019, 65, 1955–1968. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Cavatte, P.C.; Oliveira, Á.A.G.; Morais, L.E.; Martins, S.C.V.; Sanglard, L.M.V.P.; Damatta, F.M. Could shading reduce the negative impacts of drought on coffee? A morphophysiological analysis. Physiol. Plant. 2012, 144, 111–122. [Google Scholar] [CrossRef]
- Barea, J.M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef]
- Alemneh, A.A.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Soil environment influences plant growth-promotion traits of isolated rhizobacteria. Pedobiologia 2022, 90, 150785. [Google Scholar] [CrossRef]
- Pantoja-Guerra, M.; Valero-Valero, N.; Ramírez, C.A. Total auxin level in the soil–plant system as a modulating factor for the effectiveness of PGPR inocula: A review. Chem. Biol. Technol. Agric. 2023, 10, 6. [Google Scholar] [CrossRef]
- Martínez, O.A.; Jorquera, M.A.; Crowley, D.E.; de la Mora, M.L. Influence of nitrogen fertilisation on pasture culturable rhizobacteria occurrence and the role of environmental factors on their potential PGPR activities. Biol. Fertil. Soils 2011, 47, 875–885. [Google Scholar] [CrossRef]
- Descroix, F.; Snoeck, J. Environmental factors suitable for coffee cultivation. In Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers; Wintgens, J.N., Ed.; WILEY-VCH Verlag GMBH & Co. KGaA: Weinheim, Germany, 2004; pp. 164–177. ISBN 3527307311. [Google Scholar]
- Muñoz-Villers, L.E.; Geris, J.; Alvarado-Barrientos, M.S.; Holwerda, F.; Dawson, T. Coffee and shade trees show complementary use of soil water in a traditional agroforestry ecosystem. Hydrol. Earth Syst. Sci. 2020, 24, 1649–1668. [Google Scholar] [CrossRef]
- Padovan, M.P.; Cortez, V.J.; Navarrete, L.F.; Navarrete, E.D.; Deffner, A.C.; Centeno, L.G.; Munguía, R.; Barrios, M.; Vílchez-Mendoza, J.S.; Vega-Jarquín, C.; et al. Root distribution and water use in coffee shaded with Tabebuia rosea Bertol. and Simarouba glauca DC. compared to full sun coffee in sub-optimal environmental conditions. Agrofor. Syst. 2015, 89, 857–868. [Google Scholar] [CrossRef]
- Montoani Silva, B.; César de Oliveira, G.; Evaldo Serafim, M.; Eloize Carducci, C.; Andressa da Silva, É.; Martins Barbosa, S.; Beatriz Batista de Melo, L.; Junior Reis dos Santos, W.; Henrique Pereira Reis, T.; Henrique Caputo de Oliveira, C.; et al. Soil management and water-use efficiency in Brazilian coffee crops. In Coffee—Production and Research; IntechOpen: London, UK, 2020. [Google Scholar]
- Clairmont, L.K.; Stevens, K.J.; Slawson, R.M. Site-specific differences in microbial community structure and function within the rhizosphere and rhizoplane of wetland plants is plant species dependent. Rhizosphere 2019, 9, 56–68. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J.W. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC Deaminase producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef]
- Yang, A.; Yen, C. PCR optimization of BOX-A1R PCR for microbial source tracking of Escherichia coli in waterways. J. Exp. Microbiol. Immunol. 2012, 16, 85–89. [Google Scholar]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Jain, D.K.; Patriquin, D.G. Characterization of a substance produced by Azospirillum which causes branching of wheat root hairs. Can. J. Microbiol. 1985, 31, 206–210. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Morikawa, T.; Wasai, S.; Kasahara, Y.; Koshiba, T.; Yamazaki, K.; Fujiwara, T.; Tokunaga, T.; Minamisawa, K. Identification of nitrogen-fixing Bradyrhizobium associated with roots of field-grown sorghum by metagenome and proteome analyses. Front. Microbiol. 2019, 10, 407. [Google Scholar] [CrossRef]
- Schöllhorn, R.; Burris, R.H. Acetylene as a competitive inhibitor of N-2 fixation. Proc. Natl. Acad. Sci. USA 1967, 58, 213–216. [Google Scholar] [CrossRef]
- Estrada-de los Santos, P.; Bustillos-Cristales, R.; Caballero-Mellado, J. Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl. Environ. Microbiol. 2001, 67, 2790–2798. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Thangappan, S.; Uthandi, S. Plant growth-promoting Bacillus sp. cahoots moisture stress alleviation in rice genotypes by triggering antioxidant defense system. Microbiol. Res. 2020, 239, 126518. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Pardha-Saradhi, P. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma 2016, 253, 1577–1582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).