Abstract
Non-structural carbohydrates (NSCs), comprising soluble sugars (SS) and starch (ST), are essential for plant growth and development. The distribution of SS and ST concentration across various organs fluctuates throughout time due to the changes in root morphology in plants, ultimately demonstrating multiple strategies for adapting to seasonal environmental variations. The purpose of this investigation was to explore the seasonal dynamic patterns of root morphology in Pinus yunnanensis, with particular emphasis on specific root length (SRL), specific root surface area (SRA), root tissue density (RTD), and average diameter (AD). This study also aimed to investigate the seasonal fluctuation patterns of NSC. The SRL, SRA, RTD, and AD in both first-order and second-order seedlings had analogous fluctuation patterns from March to December. Although the SRL, SRA, RTD, and AD of third-order seedlings exhibited minor differences from the preceding orders, the overall variance patterns corresponded with those of the first two seedling groups. Consequently, the seasonal fluctuations in SS, ST, and NSC levels in various seedling orders exhibited patterns similar to root morphological characteristics. The SRL, SRA, and AD of three seedling orders exhibited a significant correlation with SS, ST, and NSC, confirming the link between NSC concentration and root morphology. The responses of SS, ST, and NSC in various organs of P. yunnanensis seedlings to root morphological characteristics further substantiated the correlation between the variations in NSC across different organs and root morphological traits.
1. Introduction
Biomass allocation to different organs could contribute to plant growth [1,2]. The allocation of biomass between aboveground and belowground components significantly influences several physiological processes in individual plants [3,4,5,6]. According to the optimal partitioning theory [7,8,9], plant organs that absorb the most limiting resources are likely to obtain a comparatively bigger allotment of biomass [10,11,12]. In woody plants, differential growth rates resulted in a discrepancy in wood accumulation, causing a progressive rise in aboveground biomass relative to belowground biomass [5]. These observations validated the trade-offs between aboveground and belowground biomass.
The concentrations and accumulations of non-structural carbohydrates (NSCs) differed among organs, resulting in differences in biomass within those organs. Root development may fluctuate on a monthly basis [13], and plants may rapidly mobilize and allocate NSC (e.g., starch and soluble sugars) stored in roots to aerial organs [14]. The allocation of NSC affects biomass distribution, indicating the specific growth circumstances of plant organs.
NSC is a crucial component participating in physiological processes. NSC comprises soluble sugars (SS) and starch (ST) [15], functioning as markers of the carbon supply status in plants, which ultimately influences their development [16]. In perennial woody species, despite variations in the quantities and collections of NSC across stems and roots, these organs serve as the primary reservoirs of NSC in plants [17]. These preservatives function as essential compounds that support the growth of woody plants [16,18]. In recent decades, researchers have extensively investigated the correlation between NSC and root absorption ability. Researchers anticipate that global temperature change will influence carbon trade-offs and physiological functions in plants [19,20]. Consequently, several researchers have focused on elucidating the impact of NSC on root absorption ability during drought stress. To endure drought stress, plants modify SS concentration to regulate physiological processes [21,22]. Yang et al. [23] showed that thick roots have increased NSC accumulation under drought circumstances. Blumstein et al. [24], utilizing a database of NSC measurements, noted that while environmental stress influenced the total NSC stored by a plant, the capacity of its roots to absorb carbon may exert a more significant impact.
Root traits can modify the absorption and transport efficiency of water and nutrients, significantly influencing plant growth and survival strategies [25,26,27]. Specific root area (SRA, root surface area per mass), specific root length (SRL, root length per mass), and root tissue density (RTD, mass per root volume) are critical root morphological characteristics that influence resource absorption rates, root respiration, and rhizodeposition [28,29,30,31]. Weigelt et al. [32] discovered that an increased RTD resulted in greater tissue investment. Weemstra et al. [33] showed that the SRL and SRA typically indicated the root uptake area at a certain biomass cost and nutrient absorption capability. Roots can modify their morphological, chemical, and physiological characteristics, which are functional traits, to respond to environmental changes [34]. Plants often enhance resource acquisition by extending total root length (RL) to optimize soil use and efficiently exploit available resource patches [35,36] or by modifying root morphological traits (e.g., higher SRL) to enhance nutrient absorption efficiency [36,37,38,39].
Previous studies indicate that increased carbon uptake correlates with an enhancement in root surface area and root length [40,41]. NSC plays a crucial role in regulating plant physiological processes across various environmental conditions. Water [42,43,44,45], CO2 content [46], and shade [45,47] are the primary factors considered in most studies when assessing NSC dynamics at the tissue or organ level. In addition to aboveground reserves, sufficient belowground reserves in woody plants contribute to their resistance and resprouting capacity [48]. Resprouting is a prevalent strategy among conifers [49] and is considered a derived state [50]. Starch reserves in woody underground organs, particularly roots, are essential for re-sprouting [51,52]. Subsequently, additional studies began to examine the correlation between root traits and NSC. The results for Fraxinus mandshurica indicate that elevated root length is facilitated by reduced tissue density [53]. Additionally, the observed negative correlation between root tissue density and root length is a prevalent trend across various tree species [54]. Mei et al. [55] conducted a study that determined the biomass and seasonal dynamics of NSC in leaves, stems, and roots. The results indicated that non-absorptive thick roots mobilized stored NSC to support absorptive thin roots. Additionally, species with smaller belowground NSC reserves exhibited a more significant decline in root NSC storage following stem girdling. Recent studies have concentrated on root traits, particularly the correlation between root morphology and NSC content in woody plants [22,56], which have demonstrated that NSC dynamics are linked to belowground reserves. These studies have examined the relationship between woody plant root morphology and NSC reserves in influencing plant resistance capacity to drought stress. The results of current and previous studies indicate the potential to investigate the relationship between root morphology and NSC storage in relation to plant survivability.
Pinus yunnanensis is one of the abundant tree species in southwest China, accounting for approximately 52% [57] of the forest area in Yunnan Province. P. yunnanensis is a species within the forest community that prefers high light conditions. Its tolerance for barren areas and low temperatures provides substantial ecological, economic, social, and research benefits to the region [58]. Seedling order is an indicator to evaluate the quality of P. yunnanensis seedlings based on plant height. P. yunnanensis seedlings with a higher plant height would be classified as high quality and graded into a lower order. Accordingly, the P. yunnanensis seedlings were arbitrarily divided into three height-based orders. During the growth stage, the mortality rate of P. yunnanensis seedlings rises exponentially as rainfall decreases, due to the underdeveloped root system’s sensitivity to surface soil moisture [59]. Consequently, studying the role of NSC content in influencing root uptake in P. yunnanensis seedlings is essential. A number of investigations have examined the absorption issues associated with the underdeveloped root systems of P. yunnanensis seedlings, focusing on morphological and growth changes as well as metabolic–physiological regulation [60,61]. Additional studies have validated that NSC serves as a connection among allocation, metabolism, and uptake stresses [62,63]. Numerous studies have investigated the effects of nutrient concentrations and stoichiometric characterization in P. yunnanensis [64,65,66,67,68,69]. Recent research has shifted to examining the relationship between NSC content and the stoichiometry of carbon, nitrogen, and phosphorus, along with the implications of NSC storage and root morphology [22,70]. To our knowledge, limited research has examined the response of NSC concentration in P. yunnanensis to variations in root morphological traits across different grading orders.
This study aimed to investigate the influence of root morphological traits across various seedling orders on the variation patterns of NSC concentration, ultimately affecting the seasonal growth dynamics of P. yunnanensis. The hypotheses proposed were as follows: (1) seedlings with thinner roots will modify their root morphological traits to enhance water and nutrient absorption; (2) roots from different seedling orders will utilize SS and primarily store ST to improve water uptake during winter drought conditions; and (3) variations in NSC across different organs may be closely associated with root morphological characteristics.
2. Materials and Methods
2.1. Description of the Experimental Zone
The experiment was conducted at the greenhouse of Southwest Forestry University in the Panlong District of Kunming, Yunnan Province (25°04′00″ N, 102°45′41″ E). The experimental site, situated at an altitude of approximately 1945 m, experiences a subtropical semi-humid plateau monsoon climate. The mean annual temperature is 14.7 °C, with an absolute maximum of 32.5 °C and an absolute minimum of −9 °C. The average annual relative humidity is 68.2%, with annual precipitation ranging from 700 to 1100 mm. Precipitation is primarily concentrated from May to October, featuring a maximum monthly rainfall of 208.3 mm and a maximum daily rainfall of 153.3 mm. The average annual sunshine duration is 2445.6 h, with a sunshine rate of 56%. The experimental site exhibits a significant solar projection angle year-round, with an average annual total radiation of 543.36 kJ/cm2 and an average annual evaporation of 1856.4 mm.
2.2. Source of Sapling Materials
The sapling seeds were collected from a P. yunnanensis asexual seed orchard in Maidu County, Yunnan Province, and the mother tree was in good growth condition. The seeds with full and uniform grains were selected. Seeds were uniformly sterilized with a 0.5% potassium permanganate solution for 1 h, removed and rinsed with water, and then soaked in warm water at 50 °C for 24 h. Similarly, the soil and seedling trays were sterilized with potassium permanganate before sowing, and seeds were planted at the end of January 2022, with 2–3 seeds in each hole. After sowing, the pine needles were spread on seedling trays, and the arch was used to build the greenhouse skeleton, which was then covered with a film to fix it. Greenhouse management included the regular implementation of tasks such as watering, weeding, and other necessary measures in accordance with prevailing weather conditions and the developmental stages of the seedlings. After the seeds had fully sprouted, transplant the seedlings into nursery pots (container size 18 cm base diameter × 32 cm height) in May 2022, with a soil substrate ratio of humus/laterite = 2:1. Individual seedlings of uniform size were selected for the late stage of the experiment, during which the seedlings were managed uniformly, weeding every two weeks, watering every 3–5 days, and ensuring that the soil was watered thoroughly each time.
2.3. Field Deployment and Seedling Grading
All seedlings were cultivated in the pots under uniform environmental circumstances in the nursery garden of the greenhouse at Southwest Forestry University, and over 1900 seedlings were assessed for grading post-cultivation in March 2023. The grading criteria were determined by the development status of seedlings, utilizing the mean ± 1/2 standard deviation approach as the average growth type, which was classified into three categories (1st-order seedlings, 2nd-order seedlings, and 3rd-order seedlings). The calculation formula is as follows:
where is the order of P. yunnanensis seedlings, is the average height of the seedlings, and is the standard deviation of the population of seedling heights.
θ = ω ± 1/2σ,
Seedling order measures the quality of seedling growth based on plant height. National standards (LY/T 1950-2011) for the development of seedling quality indicators as the basis for grading [71], the range of sizes of the 1st-order seedlings (Figure 1) was calculated by using the standard formula for the division as follows:
I > ω + 1/2 σ,
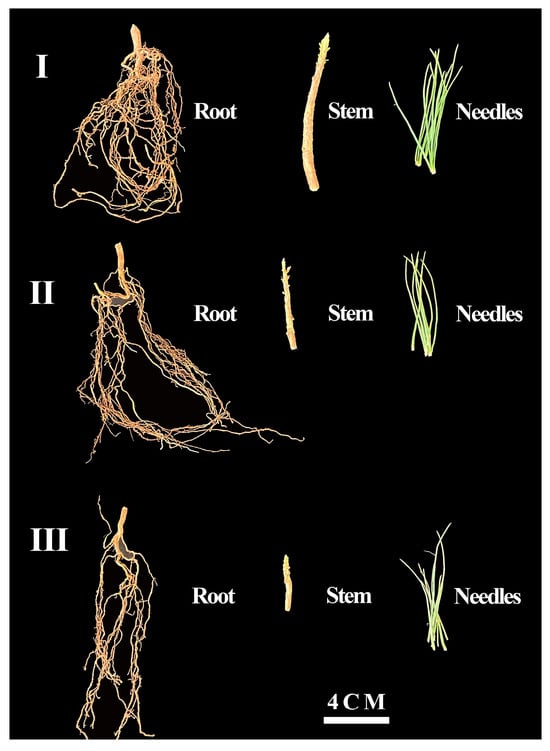
Figure 1.
The Pinus yunnanensis seedlings of different orders. I: The first-order seedlings; II: the second-order seedlings; and III: the third-order seedlings.
The 2nd-order seedlings (Figure 1) were calculated as follows:
ω − 1/2 σ ≤ II < ω + 1/2 σ,
And 3rd-order seedlings (Figure 1) were calculated as follows:
III < ω − 1/2 σ
After grading, all the different orders of seedlings were used for planting incubated under uniform circumstances for further experiments.
2.4. Analysis and Measurement
2.4.1. Growth Measurement
Measurement of data related to basic indicators (seedling height and stem diameter at ground level) began in March 2023 (when the seedlings were 420 days old), and up to December 2023 (when the seedlings were 690 days old), measurements were taken once a month for a total of 10 measurements. Seedling height was measured by straightedges (accuracy 0.1 cm), while stem diameter at ground level was measured by electronic vernier calipers (accuracy 0.01 mm) to determine phenotypic data for one growth cycle of the seedlings. The growth changes in seedling height, stem diameter at ground level, and other relevant indexes of P. yunnanensis seedlings were analyzed using SPSS Statistics 26 analysis software of IBM to evaluate the differences in growth patterns among seedlings of different orders.
2.4.2. Individual Biomass Measurement
Seedlings were assessed to ascertain individual biomass, and a fully randomized block design was applied to collect experimental samples. Three seedlings were selected as biomass-measuring specimens, with three biological replicates in each of three blocks for each order, resulting in a total of 27 seedlings throughout the three orders per timepoint. These seedlings were measured once a month during March (420 days), June (510 days), September (600 days), and December (690 days) of 2023, for a total of four measurements. The experiments were conducted using the whole plant sampling method (without destroying the seedling tissue) for digging the seedlings, carefully rinsing the root soil with water to keep the root system intact, and then bringing them back to the laboratory to air-dry in a cool place. The sampling plants were divided into three parts: roots, stems, and needles. The fresh weight of each component was weighed and recorded. The measured samples were placed in paper bags and labeled, after which they were put into an oven at 105 °C for 30 min, and then the temperature was adjusted to 80 °C for drying treatment until the mass was constant. The dry weight of each component, which was the biomass of each component, was weighed with an electronic balance with an accuracy of 0.001 g, and the data were recorded.
The aboveground biomass was calculated as follows:
And the individual biomass was calculated as follows:
The biomass allocation ratio refers to the ratio of the biomass of a component to the total biomass of a single plant. A differential analysis of P. yunnanensis biomass was carried out to observe the differences between biomass allocation of different orders of P. yunnanensis seedlings.
2.4.3. Soil Chemical Characteristics Measurement
In September 2022 (240 days), three uniformly grown seedlings with three biological replicates were randomly selected from each order to obtain soil samples. Soil was extracted from each of the nine seedling pots of identical order. The collected soil was thoroughly blended and utilized as a sample for that order. Soil samples were dried and ground through a sieve. For subsequent determinations, 0.2 g of each soil sample from each order was used. The H2SO4-H2O2 decoction method was used to decoct the collected samples to obtain the solution to be measured. N and P contents were determined using an auto discrete analyzer provided by Shenzhen E-Zheng Tech Co., Ltd. (Shenzhen, China). C content was determined by the potassium dichromate titration method, and K content was determined by the flame photometric method [72].
2.4.4. Root System Measurement
A fully randomized block design was applied to collect experimental samples. Three seedlings were selected as biomass-measuring specimens, with three biological replicates in each of three blocks for each order, resulting in a total of 27 seedlings throughout the three orders per timepoint. After digging out the seedlings for the biomass determinations as described above, and before proceeding to dry, the root systems were scanned with an LA-S root scanner. After scanning, the root images were analyzed with LA-S plant image analysis software (ver. 2000) provided by Wanshen Detection Technology Co., Ltd., Hangzhou, China. The data were derived from a table of root morphology indexes for the total root length, root surface area, average root diameter, and root volumes, respectively.
2.4.5. Measurement of Soluble Sugar and Starch Content
Saccharides represent a fundamental component of plant bodies and function as the primary storage substrate for metabolic processes. The anthrone colorimetric method was used for the measurement, and the detection kit was provided by Michy Biomedical Technology Co., Ltd., Suzhou, China (M1503A). The experimental steps were carried out according to the experimental instructions of the detection kit. P. yunnanensis seedlings with good growth conditions under each treatment were randomly selected at 420 days, 510 days, 600 days, and 690 days, and their dry samples were used for the measurement. Three seedlings with three biological replicates for each order were dug out as measuring plants, resulting in a total of 27 seedlings for the three orders. The method of Buysse and Merckx [73] was referred to for determination and calculation.
Starch is a nutrient stored in the plant body, and determining its content is important for investigating sugar metabolism in plants. The content of starch was determined by a detection kit (M1101A, Michy Biomedical Technology Co., Ltd., Suzhou, China), which is based on anthrone–sulfuric acid colorimetry. The experimental steps were carried out according to the experimental instructions. P. yunnanensis seedlings with good growth conditions under each treatment were randomly selected from 420 days to 690 days, and their dry samples were used for measurement. Three seedlings with three biological replicates for each order were dug out as measuring plants, resulting in a total of 27 seedlings for the three orders. The method of Xie et al. [74] was referred to for determination and calculation.
The calculated soluble sugar content and starch concentration were used to calculate the total NSC concentration according to the following formula:
2.4.6. Measurements of Root Morphological Traits
The phenotypic plasticity index (PI) for each root morphological trait was determined using the approach outlined by Valladares et al. [75] with the subsequent formula:
Max(i) denotes the maximum value of the functional trait under a treatment, whereas min(i) signifies the minimum value of the functional trait under a treatment.
The coefficient of variation (CV) for root morphological traits was calculated according to Zhang et al. [76] using the following formula:
RL denotes root length, RA signifies root surface area, RV indicates root volume, and RDW refers to the mass of the root system desiccated to a constant weight and subsequently measured dry.
2.4.7. Statistical Analysis
SPSS Statistics 26 analysis software of IBM was used to analyze the statistics of this study. The statistical methods used were two-way ANOVA, redundancy analysis (RDA), and Pearson correlation analysis. Duncan’s multiple comparison method was used for significance tests (α = 0.05).
3. Results
3.1. Soil Chemical Characteristics of Different Seedling Orders
The C, N, P, and K contents in soil samples from different orders showed similar absorption patterns (Table 1). The K contents among three seedling orders were higher than C, N, and P contents. The C contents were the lowest compared to other nutrient elements. The N contents and P contents of the same seedling order were generally at a consistent level.

Table 1.
Carbon, nitrogen, phosphorus, and potassium contents of the soil in which the seedlings were growing.
The C and P contents in the soil from different orders exhibited analogous absorption trends. The concentrations of C and P in the first-order soil samples were the highest. The N and K contents of the first-order soil samples showed lower trends compared to other orders.
3.2. Allocation of Biomass to Different Organs Among Different Seedling Orders
Time interval and seedling orders significantly correlated with the individual biomass of P. yunnanensi seedlings (Table 2). With the increase in seedling orders, the individual biomass of P. yunnanensis seedlings showed progressive decreases during different sampling dates. In contrast, the biomass of different organs generally showed significant increasing trends under the same seedling order (Figure 2A–C). During the same month, the biomass with all seedling orders was the highest in needles and the lowest in stems. On the other hand, this difference in biomass allocation between stems and needles is meaningful, and the relationships of biomass allocations over time are important data. The biomass in needles of the first two seedling orders increased significantly in June (Figure 2A,B), whereas the third-order seedlings increased markedly in September (Figure 2C). The stem and root biomass in the first two orders increased greatly in December, while third-order seedlings increased significantly in September. The present study examined the biomass of the first-order, second-order, and third-order seedlings in various organs, with a focus on the timing of peak biomass accumulation. The results indicated that the biomass of the first-order, second-order, and third-order seedlings exhibited a progressive increase, reaching its peak in December. The analysis further revealed that, among the seedlings of the three distinct orders, the biomass allocated to needles was the most substantial, followed by roots (Figure 2A–C).

Table 2.
Results of two-way (Time intervals × Seedling orders) ANOVA of Pinus yunnanensis roots biomass and root morphological traits.
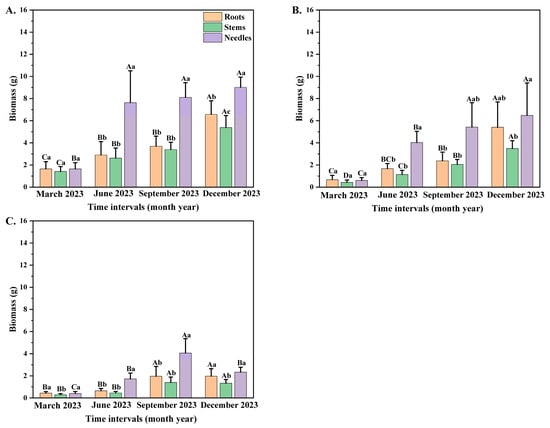
Figure 2.
The biomass allocation of different P. yunnanensis seedling orders among different months. (A) First-order seedlings; (B) second-order seedlings; and (C) third-order seedlings. The different uppercase letters indicate significant differences among time intervals (p < 0.05). The different lowercase letters indicate significant differences among different organs (p < 0.05).
The biomass of second-order seedlings had the most significant coefficient of variation among distinct periods of time, with values of 89.63, 39.06, and 35.67 in roots, stems, and needles. With the growth in seedling orders, the coefficient of variation of the second-order seedling biomass among different months was the most significant, with values of 53% (in March), 63% (in June), 62% (in September), and 48% (in December) (Figure 2B).
3.3. Root Morphological Traits Observed Among Different Seedling Orders
Time intervals and seedling orders have a significant correlation with the specific root length (SRL), specific root surface area (SRA), root tissue density (RTD), and average diameter (AD) of P. yunnanensis seedlings (Table 2). As orders for P. yunnanensis seedlings rise, both SRL and SRA exhibited significant upward trends at the same timepoint (Figure 3A,B). As dates of measurement progressed, both SRL and SRA had notable declining tendencies. Overall, the RTD of three different orders demonstrated initial increases followed by subsequent declines as dates of measurement progressed. The AD displayed general upward trends as dates of measurement progressed (Figure 3C,D).
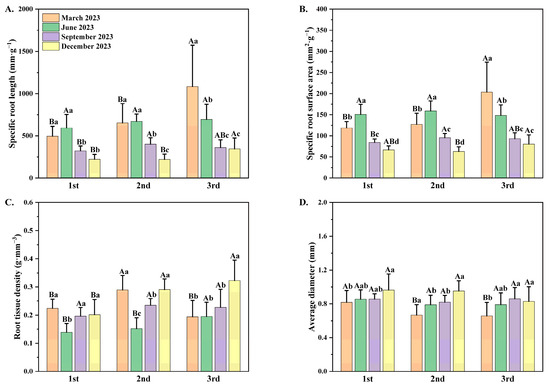
Figure 3.
(A) The specific root length (SRL); (B) specific root surface area (SRA); (C) root tissue density (RTD); and (D) the average diameter (A,D) of P. yunnanensis seedlings in different seedling orders. The different uppercase letters indicate significant differences among seedling orders (p < 0.05). The different lowercase letters indicate significant differences among time intervals (p < 0.05).
The plasticity indices (PI) for RTD, AD, SRA, and SRL indicated that, as seedling orders increased, the PI generally exhibited a decreasing trend (Figure 4A–C). The average values for PI across different orders were 0.587, 0.501, and 0.562, respectively. Among the three seedling orders, the PI of AD had the highest value.
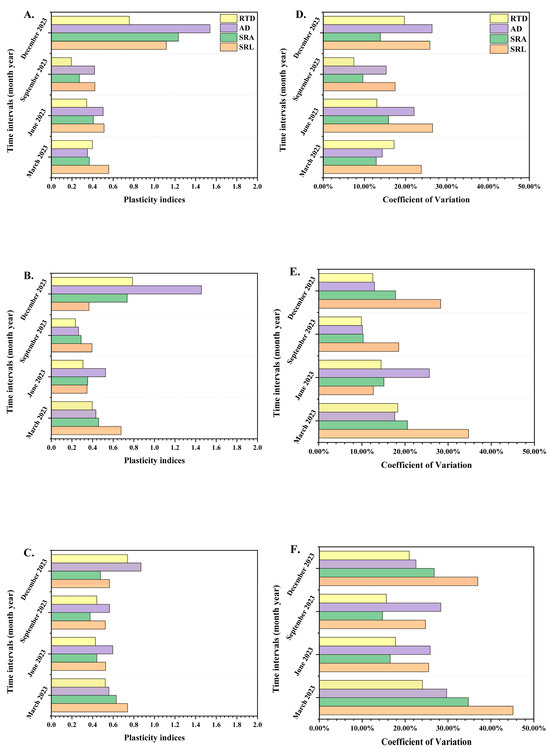
Figure 4.
The plasticity indices of different P. yunnanensis seedling orders among different months (A–C) and the coefficient of variation of different seedling orders among different months (D–F). First-order seedlings (A,D); second-order seedlings (B,E); and third-order seedlings (C,F). SRL: specific root length; SRA: specific root surface area; RTD: root tissue density; AD: average diameter.
With the rise in seedling orders, the coefficient of variation (CV) showed increasing trends (Figure 4B,D,F). The average values of CV from the first-order to the third-order seedlings were 18%, 17%, and 26%, and the average values of CV in the first-order and the second-order were lower than 20%, which demonstrated the first-order and the second-order seedlings showed internal homeostasis in root morphological traits [23,76].
Under the identical period, the CV of SRL, SRA, RTD, and AD in the third-order seedlings were the highest, which were 45%, 35%, 30%, and 24%. Among the three seedling orders, the CV of SRL had the highest values, which were 27%, 35%, and 45%.
3.4. Seasonal Variation of Root Morphological Traits Among Different Seedling Orders
The SRL and SRA of the first-order and the second-order seedlings had the same seasonal variation patterns (Figure 3A,B). The SRL and SRA in the first two seedling orders increased from March to a maximum in June and then decreased to a minimum in December, while the SRL and the SRA in the third-order seedlings showed increasing tendencies and peaked in December. The RTD of all seedling orders decreased from March to a minimum in June and then showed increasing trends (Figure 3C), whereas the AD of three seedling orders increased from March until peaking in September or December (Figure 3D). The seasonal variation patterns in SRL and SRA of P. yunnanensis seedlings among different orders were similar: first-order and second-order seedlings had the same seasonal variation trends, whereas the seasonal dynamic of third-order seedlings differed from those of the first two orders of seedlings. The first two orders of seedlings showed an increasing and then a decreasing trend. Third-order seedlings exhibited a decreasing tendency from March to December. Seasonal variation in RTD and AD of P. yunnanensis seedlings also showed consistency: seedlings of all three orders displayed the same dynamic patterns among different months (Figure 3C,D).
3.5. Allocation of NSC Concentration to Different Organs of Seedlings Among Three Orders
Time interval and seedling orders have significant correlation with soluble sugar (SS), starch (ST), and NSC of P. yunnanensis seedlings (Table 3). With the increasing seedling orders, the SS and ST concentrations per unit dry mass in roots and stems, respectively, showed decreasing tendencies in general (Figure 5A,B). Within the same seedling order, SS concentration in roots and stems of seedlings was generally higher than that of needles. With ascending seedling orders, the ST concentration of first-order and second-order seedlings in roots and stems were 30%, 37%, 15%, and 27% greater than those of needles, respectively (Figure 5A–C). The total NSC concentration per unit dry mass of all three seedling orders in stems was 38%, 82%, 52%, 75%, 37%, and 49% higher than those of roots and needles, respectively. The ST and total NSC concentration of lower-order seedlings between stems and roots varied considerably (Figure 5A,B). Third-order seedlings exhibited the highest SS-ST ratio in roots compared with the first two seedling orders, and the SS-ST ratio in stems showed progressively increased trends with increased seedling orders (Table 4).

Table 3.
Results of two-way (Time intervals × Seedling orders) ANOVA of P. yunnanensis soluble sugar (SS), starch (ST), and total NSC.
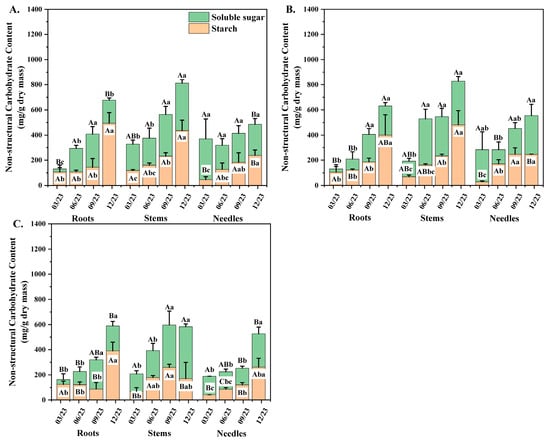
Figure 5.
The NSC concentration of different P. yunnanensis seedling orders in organs among different sampling dates. (A) First-order seedlings; (B) second-order seedlings; (C) and third-order seedlings. The different uppercase letters indicate significant differences among different organs (p < 0.05). The different lowercase letters indicate significant differences among time intervals (p < 0.05).

Table 4.
The SS-ST ratio across different P. yunnanensis seedling orders under different time intervals.
3.6. Seasonal Variation of NSC Concentration in Different Organs Among Different Seedling Orders
In the needles of P. yunnanensis, the SS concentration of second-order and third-order seedlings peaked in December, whereas the ST concentration showed increasing trends until peaking in September or December (Figure 5A–C). In stems, the SS concentration of three seedling orders increased from March to a maximum in December, and the ST concentration of the first two orders showed the same increasing patterns. In contrast, third-order seedlings increased from March to a maximum in September and then decreased (Figure 5A–C). The total NSC concentrations of aboveground organs showed similar seasonal variation in the first two seedling orders. The NSC concentration in the needles of first-order and second-order seedlings decreased from March to a minimum in June and then increased until peaking in December, while third-order seedlings showed an increasing trend and peaked in December. The NSC concentration in stems of first-order and second-order seedlings showed increasing trends and peaked in December, whereas third-order seedlings increased from March to a maximum in September and then decreased. Unlike the aboveground organs, the root NSC concentrations of three seedling orders showed increasing trends during different time intervals (Figure 5A–C). The SS concentration of all seedling orders increased in March and peaked in December. The minimum ST concentrations of third-order seedlings occurred in September, and the maximum was found in December, while the NSC concentrations of the first two orders increased from March and peaked in December.
3.7. Relationship Among the Individual Biomass, Root Morphology, and NSC Concentration of Three Seedling Orders
RDA was used to assess the root morphological characteristics among the first three orders of P. yunnanensis roots under different months. The findings revealed that the first two axes of the RDA represented roughly 65% of the overall variance among all seedling orders (Figure 6A–C and Figure S1A–C). The first and second ordination axes correspondingly indicated the variation in individual biomass, root morphological traits, and NSC concentration. Under the same seedling order, the SRL, SRA, SS, ST, and NSC concentrations in plots had a decent extent of separation (Figure 6A–C and Figure S1A–C). The relationship among SRL, SRA, SS, ST, and NSC concentrations in three seedling orders showed the same results (Figure 6D and Figure S1D).
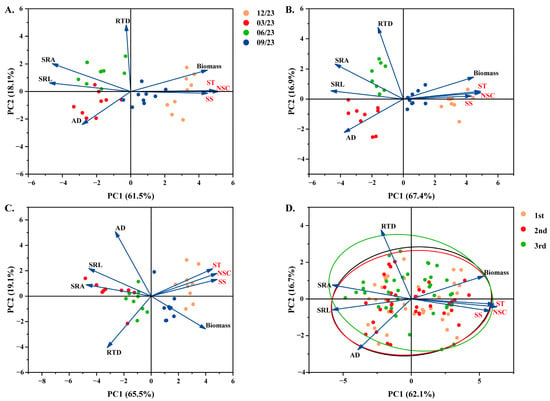
Figure 6.
Redundancy analysis of root morphology traits and NSC in different P. yunnanensis seedling orders among different time intervals. (A) First-order seedlings; (B) second-order seedlings; (C) and third-order seedlings; (D) Redundancy analysis of three seedling orders during the whole experiment period. SRL: specific root length and the ellipses in this figure represent the area of distribution of the samples of different orders of seedlings; SRA: specific root surface area; RTD: root tissue density; AD: average diameter; SS: soluble sugar; ST: starch; NSC: non-structural carbohydrates.
The individual seedling biomass and root morphology of the three orders of roots of P. yunnanensis seedlings on different sampling dates were analyzed by using the Pearson correlation coefficient. The results showed that among different seedling orders, the individual biomass of P. yunnanensis seedlings was positively correlated with SS, ST, and NSC. The SRL and SRA had an adverse association with SS, ST, and NSC concentration. In contrast, the RTD in three seedling orders had no significant correlation with SS, ST, and NSC (Figure 7A–C). The AD in second-order and third-order seedlings was positively correlated with SS, ST, and NSC, whereas the AD of first-order seedlings was only significantly correlated with ST (Figure 7A).
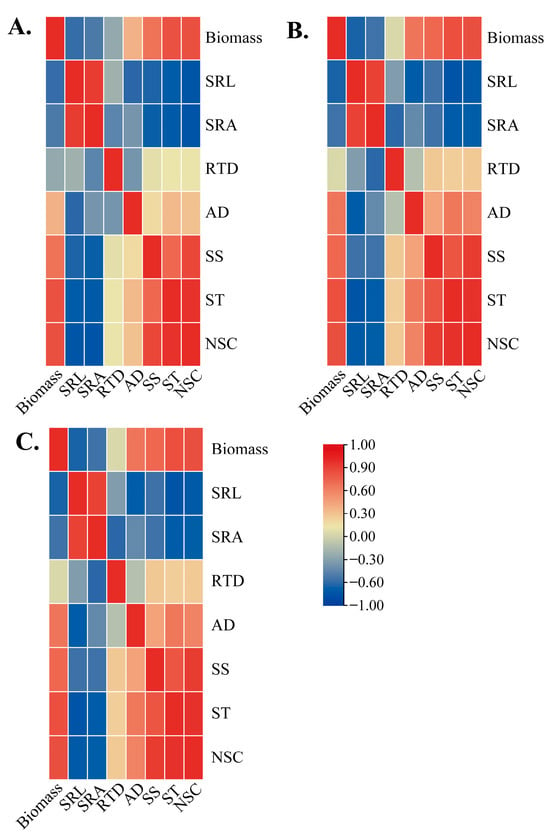
Figure 7.
Relationships among biomass, root morphological traits, soluble sugar, and starch concentration in different P. yunnanensis seedling orders during the whole experiment period. (A) First-order seedlings; (B) second-order seedlings; (C) and third-order seedlings. SRL: specific root length; SRA: specific root surface area; RTD: root tissue density; AD: average diameter; SS: soluble sugar; ST: starch; NSC: non-structural carbohydrates.
4. Discussion
4.1. Relationship Between Biomass Allocation and Root Morphology of Different Seedling Orders
In this study, the biomass of P. yunnanensis in needles was significantly higher than that in roots and stems. Recent research has indicated that the biomass allocation is influenced by both the development of the plant and its leaf characteristics. Furthermore, the distribution of biomass to different organs (such as leaves, stems, and roots) is frequently utilized to gain a more comprehensive understanding of a plant’s survival tactics [77,78]. The findings of these research studies correspond with this study’s outcomes and offer significant insights into the physiological and ecological processes of the plant population. He et al. [79] concluded that biomass allocation to leaves and roots increased with the increasing height and height–diameter ratio. In this study, the lower-order seedlings with a higher height and height–diameter ratio showed similar variation patterns.
The SRL, SRA, and other root morphological traits are capable of effectively influencing their water uptake capability [79]. Previous studies have demonstrated that plant roots exhibit greater water absorption efficiency with longer root lengths and smaller root diameters [80,81,82]. Ostonen et al. [41] proposed the following two strategies describing how plants boosted the root system’s absorbing capability to adapt to arid environments: (1) the acquisition strategy, which was to maximize root production while preserving a higher intake surface area; and (2) the conservative strategy, which was to maximize efficiency by altering the morphological traits of the root system. In this research, with the rise of seedling orders, the SRL and SRA increased significantly, which demonstrated that the higher-order seedlings with thinner roots changed their root morphological traits progressively to enhance their absorption capacity. These results confirmed the first hypothesis and were consistent with previous studies: the efficiency of water or nutrient absorption in roots increases when the SRL or SRA increases and the AD decreases [80,81]. Generally, the findings of this investigation highlighted the adaptive strategies of P. yunnanensis roots in three different seedling orders.
4.2. Effects of Different Seedling Orders on NSC
The shift in NSC with the seasons suggested that the roots and stems were the main storage site of NSC in P. yunnanensis, consistent with that of other woody plants [83], supporting that NSC provides essential nutrients for plants [84]. In this study, the SS-to-ST ratio of roots and stems generally increased with the increasing seedling orders. This result showed that plants might change the SS-to-ST ratio to balance the trade-off between needles and roots and improve water transport efficiency, which was consistent with previous studies [85]. This study showed that the total NSC concentration of lower-order seedlings was higher than that of higher-order seedlings, which was similar to the results in Pinus tabuliformis Carr. [86] that implied that thick roots transport nutrients to support the entire root, whereas thin roots (absorptive roots) might obtain more significant carbon investment because the primary function was to absorb water and nutrients. These results, along with those of the present study, revealed the interaction of different seedling orders, SS concentration, SS-to-ST, and NSC concentration of P. yunnanensis seedlings.
4.3. Linking the Root Morphological Traits to the NSC Level
The root system serves as the primary organ in charge of intaking water and was the initial indicator of various stresses [22]. Although Yang et al. [23] have pointed out that thick roots increase NSC accumulation, the premise of this conclusion is that roots build up their unique response strategies under drought conditions. The relationship between root morphological traits and NSC allocation is still open to discussion. In this study, with the increase in the seedling orders, the biomass of P. yunnanensis seedlings declined, and the root morphological traits changed to facilitate the intake of water and nutrients (Figure 2A–C and Figure 3A–D).
NSC played a crucial role in root physiological regulation and could promote root growth [87]. In the present study, SRL and SRA of all seedling orders were significantly negatively correlated with the SS, ST, and NSC concentrations. AD was positively correlated with the SS, ST, and NSC concentrations in general (Figure 6A–D and Figure 7A–C). The results confirmed that compared with thicker roots, when the roots were narrower, the thinner ones had more negligible NSC accumulations to sustain normal physiological activities [88]. The finding in this study (Figure 5A–C) supported the anterior result: with a thicker root diameter, plants could easily distribute more carbon to the roots to obtain a more remarkable absorptive ability. Consequently, our findings indicated that the difference in NSC resulting from distinct seedling orders may be associated with variations in root morphological traits.
4.4. Seasonal Variation Patterns of NSC Caused by Seasonal Variation of Root Morphological Traits
The ST and SS concentrations in coniferous needles vary in seasons [77,78]. The seasonal variation of the soluble sugar and starch concentration in P. yunnanensis is apparent, and the starch and NSC concentration in needles are significant indicators of physiological strategies for coping with seasonal variation [70]. The SS, ST, and NSC concentrations of P. yunnanensis in needles were essential indicators of phenotypic plasticity, and the degree of variation in the concentration reflected the degree of the P. yunnanensis seedlings’ responses to seasonal variation [70]. The SS concentration in needles of three orders in this study had accumulated, causing the highest needle SS concentration in P. yunnanensis in March. During the growing seasons, the SS concentration in needles and ST concentration hydrolyzed into SS concentration were utilized for growth and development. Therefore, the needle SS concentration tended to decrease to the minimum levels in June or September, consistent with those of the previous results [89].
Large NSC reserves in stems could be utilized seasonally, which, as a survival strategy, ensured the resprouting of woody plants after drought, fire, or other disasters [90,91]. NSC storages in roots were crucial for basal resprouting in ecosystems [92,93]. The findings of this study, which were consistent with the observations of the previous study [94], denoted that the ST concentration in roots generally reached the minimum level before June. The seasonal variation patterns of the ST concentration showed a synchrony with that of the SS concentration, demonstrating that root starch in P. yunnanensis seedlings serves as a rapid and easily accessible intra-seasonal reserve during growth. The SS concentration in the roots of all the seedling orders declined between September and December, while the ST accumulation of roots in December reached its maximum. The consumption of SS and the preservation of ST might be the survival strategy of P. yunnanensis to deal with the drought in the winter season; this finding, in accordance with the second hypothesis, was previously confirmed [22].
In the previous discussions of this present study, the results confirmed the relationship between root morphology variation and the variation of SS, ST, and NSC. Based on this conclusion, it can be assumed that the seasonal variation of NSC is related to the seasonal variation of root morphology, which supported the third hypothesis. Though seasonal variation in total NSC concentration of different seedling orders in different organs generally showed consistency, as for the slight difference in root ST concentration between third-order and the first two-order seedlings, it probably was caused by seasonal variation of root morphology among third-order seedlings.
This study still exists with the limitation of not further exploring the relationship between fine roots and NSC concentration. Therefore, the specific mode of interaction between the fine root system and NSC concentration of P. yunnanensis seedlings needs to be further investigated in future research.
5. Conclusions
This study investigated the interactive response of root morphological traits and NSC concentration of P. yunnanensis seedlings to seedling orders. The P. yunnanensis seedlings of different orders exhibited consistent adaptive strategies to seasonal changes in environmental factors, particularly regarding root morphological traits and NSC allocation.
The SRL and SRA of three seedling orders presented significant similarity in responses to seasonal variation, demonstrating a comparable reflection of root water and nutrient absorption. The SRL and SRA values for all seedling orders reached their maximum in March or June, assuming that P. yunnanensis altered their root morphology to enhance root absorptive capacity, thereby supporting seedling growth. The morphological characteristics of the roots led to a progressive accumulation of NSC concentration in various organs starting in March. These NSC reserves are partially utilized by P. yunnanensis seedlings throughout the growing seasons to adapt to seasonal variation. The total NSC accumulation in roots, stems, and needles across three seedling orders all peaked in December; it would contribute to the growth potential of P. yunnanensis seedlings in the next year. The variation patterns of SS and ST concentration in roots across three orders might reveal the adaptive strategy of P. yunnanensis. Correspondingly, a significant correlation occurred between root morphology and NSC concentration in principal component analysis and Pearson correlation analysis, and these conclusions revealed that root morphological traits, SS, ST, and NSC concentration were prominent indicators of physiological strategies to cope with seasonal variation.
The results provide information on the seasonal variation of root morphological traits and NSC concentration, which improves our comprehension of the relationship between root morphology and NSC concentration in different orders and provides information useful for the breeding of P. yunnanensis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14050825/s1, Supplementary Figure S1: Redundancy analysis of biomass of different organs, root morphological traits, and NSC in different seedling orders among different time intervals.
Author Contributions
Conceptualization, L.C., S.C., N.C., D.W. and Y.X.; funding acquisition, Y.X.; investigation, Z.P., Z.L., S.L. and J.L.; methodology, Z.P., Z.L. and C.Z.; writing—original draft, Z.P.; writing—review and editing, L.C., S.C., N.C., D.W. and Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was co-supported by the Key Project of Joint Funds of the Basic Agricultural Research of Yunnan Province (202401BD070001-023), Yunnan Young and Elite Talents Projects (YNWRQNBJ-2019-075), Joint Funds of the Basic Agricultural Research of Yunnan Province (202301BD070001-035), and the Key Project of Joint Funds of the Basic Agricultural Research of Yunnan Province (202301BD070001-152).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Felton, A.J.; Slette, I.J.; Smith, M.D.; Knapp, A.K. Precipitation amount and event size interact to reduce ecosystem functioning during dry years in a mesic grassland. Glob. Change Biol. 2020, 26, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, M.; Li, G.; Wang, M.; Li, Z.; De Boeck, H.J. Changes of Aboveground and Belowground Biomass Allocation in Four Dominant Grassland Species Across a Precipitation Gradient. Front. Plant Sci. 2021, 12, 650802. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 1191. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G. Thinking about efficiency of resource use in forests. For. Ecol. Manag. 2004, 193, 5–16. [Google Scholar] [CrossRef]
- Niklas, K.J. Modelling below-and above-ground biomass for non-woody and woody plants. Ann. Bot. 2005, 95, 315–321. [Google Scholar] [CrossRef]
- Hui, D.; Jackson, R.B. Geographical and interannual variability in biomass partitioning in grassland ecosystems: A synthesis of field data. New Phytol. 2006, 169, 85–93. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S., III; Mooney, H.A. Resource limitation in plants-an economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Gedroc, J.J.; McConnaughay, K.; Coleman, J.S. Plasticity in root/shoot partitioning: Optimal, ontogenetic, or both? Funct. Ecol. 1996, 10, 44–50. [Google Scholar] [CrossRef]
- Mao, W.; Allington, G.; Li, Y.; Zhang, T.-H.; Zhao, X.-Y.; Wang, S.-K. Life history strategy influences biomass allocation in response to limiting nutrients and water in an arid system. Pol. J. Ecol. 2012, 60, 545–557. [Google Scholar]
- Villar, R.; Veneklaas, E.J.; Jordano, P.; Lambers, H. Relative growth rate and biomass allocation in 20 Aegilops (Poaceae) species. New Phytol. 1998, 140, 425–437. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.; Chen, X.; Tian, X.; Wang, X.; Luo, G. Biomass allocation patterns across China’s terrestrial biomes. PLoS ONE 2014, 9, e93566. [Google Scholar] [CrossRef]
- Baker, T.T., III; Conner, W.H.; Lockaby, B.G.; Stanturf, J.A.; Burke, M.K. Fine root productivity and dynamics on a forested floodplain in South Carolina. Soil Sci. Soc. Am. J. 2001, 65, 545–556. [Google Scholar] [CrossRef]
- Retzlaff, W.A.; Handest, J.A.; O’Malley, D.M.; McKeand, S.E.; Topa, M.A. Whole-tree biomass and carbon allocation of juvenile trees of loblolly pine (Pinus taeda): Influence of genetics and fertilization. Can. J. For. Res. 2001, 31, 960–970. [Google Scholar] [CrossRef]
- Shi, S.; Shi, T.; Zhou, S.; Gao, S.; Zhao, Y.; Shi, G. Non-structural carbohydrates accumulation in seedlings improved flowering quality of tree peony under forcing culture conditions, with roots playing a crucial role. Plants 2024, 13, 2837. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Li, Q.; Hu, Y.; Yang, J.; Song, Z. Effects of NSC in different organs and at different growth stages on the yield of oil peony Fengdan with different ages. Front. Plant Sci. 2023, 14, 1108668. [Google Scholar] [CrossRef]
- Ziemer, R.R. Translocation of 14C in ponderosa pine seedlings. Can. J. Bot. 1971, 49, 167–171. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Field, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- Quentin, A.G.; Pinkard, E.A.; Ryan, M.G.; Tissue, D.T.; Baggett, L.S.; Adams, H.D.; Millard, P.; Marchand, J.; Landhausser, S.M.; Lacointe, A.; et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol. 2015, 35, 1146–1165. [Google Scholar] [CrossRef]
- Ji, L.; Liu, Y.; Wang, J.; Lu, Z.; Zhang, L.; Yang, Y. Differential variation in non-structural carbohydrates in root branch orders of Fraxinus mandshurica Rupr. seedlings across different drought intensities and soil substrates. Front. Plant Sci. 2021, 12, 692715. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, W.; Li, R.; Xu, M.; Wang, S. Different responses of non-structural carbohydrates in above-ground tissues/organs and root to extreme drought and re-watering in Chinese fir (Cunninghamia lanceolata) saplings. Trees 2016, 30, 1863–1871. [Google Scholar] [CrossRef]
- Blumstein, M.; Gersony, J.; Martínez-Vilalta, J.; Sala, A. Global variation in nonstructural carbohydrate stores in response to climate. Glob. Change Biol. 2023, 29, 1854–1869. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.M.; McCormack, M.L.; Powell, A.S.; Blackwood, C.B.; Freschet, G.T.; Kattge, J.; Roumet, C.; Stover, D.B.; Soudzilovskaia, N.A.; Valverde-Barrantes, O.J.; et al. A global Fine-Root Ecology Database to address below-ground challenges in plant ecology. New Phytol. 2017, 215, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Raich, J.W. Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 1992, 73, 1139–1147. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Laskowski, M.J.; Burton, A.J.; Lessard, V.C.; Zak, D.R. Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol. 1998, 18, 665–670. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Tjoelker, M.G.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]
- Weigelt, A.; Mommer, L.; Andraczek, K.; Iversen, C.M.; Bergmann, J.; Bruelheide, H.; Fan, Y.; Freschet, G.T.; Guerrero Ramirez, N.R.; Kattge, J. An integrated framework of plant form and function: The belowground perspective. New Phytol. 2021, 232, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Weemstra, M.; Mommer, L.; Visser, E.J.; van Rijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef] [PubMed]
- Lõhmus, K.; Truu, M.; Truu, J.; Ostonen, I.; Kaar, E.; Vares, A.; Uri, V.; Alama, S.; Kanal, A. Functional diversity of culturable bacterial communities in the rhizosphere in relation to fine-root and soil parameters in alder stands on forest, abandoned agricultural, and oil-shale mining areas. Plant Soil 2006, 283, 1–10. [Google Scholar] [CrossRef]
- Ostonen, I.; Helmisaari, H.S.; Borken, W.; Tedersoo, L.; Kukumägi, M.; Bahram, M.; Lindroos, A.J.; Nöjd, P.; Uri, V.; Merilä, P. Fine root foraging strategies in N orway spruce forests across a European climate gradient. Glob. Change Biol. 2011, 17, 3620–3632. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Ostonen, I.; Truu, M.; Helmisaari, H.S.; Lukac, M.; Borken, W.; Vanguelova, E.; Godbold, D.L.; Lõhmus, K.; Zang, U.; Tedersoo, L. Adaptive root foraging strategies along a boreal–temperate forest gradient. New Phytol. 2017, 215, 977–991. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Wells, C.E.; Yanai, R.D.; Whitbeck, J.L. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Helmisaari, H.; Truu, J.; Meel, S. Fine root morphological adaptations in Scots pine, Norway spruce and silver birch along a latitudinal gradient in boreal forests. Tree Physiol. 2007, 27, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A. Carbon dynamics of Acer pseudoplatanus seedlings under drought and complete darkness. Tree Physiol. 2016, 36, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Asshoff, R.; Bignucolo, O.; Hättenschwiler, S.; Keel, S.G.; Peláez-Riedl, S.; Pepin, S.; Siegwolf, R.T.W.; Zotz, G. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science 2005, 309, 1360–1362. [Google Scholar] [CrossRef]
- Wiley, E.; Hoch, G.; Landhäusser, S.M. Dying piece by piece: Carbohydrate dynamics in aspen (Populus tremuloides) seedlings under severe carbon stress. J. Exp. Bot. 2017, 68, 5221–5232. [Google Scholar] [CrossRef]
- Nzunda, E.F.; Griffiths, M.E.; Lawes, M.J. Sprouting by remobilization of above-ground resources ensures persistence after disturbance of coastal dune forest trees. Funct. Ecol. 2008, 22, 577–582. [Google Scholar] [CrossRef]
- Dietze, M.C.; Clark, J.S. Changing the gap dynamics paradigm: Vegetative regeneration control on forest response to disturbance. Ecol. Monogr. 2008, 78, 331–347. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Bell, T.L.; Ojeda, F. Underground starch storage in Erica species of the Cape Floristic Region–differences between seeders and resprouters. New Phytol. 1999, 144, 143–152. [Google Scholar] [CrossRef]
- Verdaguer, D.; Ojeda, F. Root starch storage and allocation patterns in seeder and resprouter seedlings of two Cape Erica (Ericaceae) species. Am. J. Bot. 2002, 89, 1189–1196. [Google Scholar] [CrossRef]
- Ryser, P. The importance of tissue density for growth and life span of leaves and roots: A comparison of five ecologically contrasting grasses. Funct. Ecol. 1996, 10, 717–723. [Google Scholar] [CrossRef]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Root structure and lifespan are largely independent of leaf structure and lifespan in a common garden comparison of eleven tree species. Ecol. Monogr. 2006, 76. [Google Scholar]
- Mei, L.; Xiong, Y.; Gu, J.; Wang, Z.; Guo, D. Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 2015, 177, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Attaullah, K.; Wang, J.; Yu, D.; Yang, Y.; Yang, L.; Lu, Z. Root traits determine variation in nonstructural carbohydrates (NSCs) under different drought intensities and soil substrates in three temperate tree species. Forests 2020, 11, 415. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. Announcement on the Nomination of Yunnan Provincial Science and Technology Awards for 2023; National Forestry and Grassland Administration: Beijing, China, 2023. [Google Scholar]
- Liu, Y.; Wu, J.; Wu, D.; Li, S.; Wang, L. Seasonal variation in δ13C of Pinus. yunnanensis and Pinus. armandii at different stand ages. Sci. Rep. 2023, 13, 7938. [Google Scholar] [CrossRef]
- Marod, D.; Kutintara, U.; Tanaka, H.; Nakashizuka, T. Effects of drought and fire on seedling survival and growth under contrasting light conditions in a seasonal tropical forest. J. Veg. Sci. 2004, 15, 691–700. [Google Scholar] [CrossRef]
- Yukun, F.; Qinying, L.; Linlin, H.; Zengquan, L. Research progress of seed germination characteristics and seedling drought resistance of Pinus yunnanensis Franch. Seed Sci. Res. 2018, 37, 47–51. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Li, L.; Sun, A.; Wang, W.; Su, N.; Yu, G.; Zhang, W.; Bao, X.; Zheng, S.; et al. Effects of watering control on physiological and biochemical traits of Pinus yunnanensis seedlings. J. Northwest For. Univ. 2016, 31, 78–84. [Google Scholar] [CrossRef]
- Aguadé, D.; Poyatos, R.; Rosas, T.; Martínez-Vilalta, J. Comparative drought responses of Quercus ilex L. and Pinus sylvestris L. in a montane forest undergoing a vegetation shift. Forests 2015, 6, 2505–2529. [Google Scholar] [CrossRef]
- Lin, T.; Zheng, H.; Huang, Z.; Wang, J.; Zhu, J. Non-structural carbohydrate dynamics in leaves and branches of Pinus massoniana (Lamb.) following 3-year rainfall exclusion. Forests 2018, 9, 315. [Google Scholar] [CrossRef]
- Cai, N.; Tang, J.; Che, F.; Chen, S.; Wang, J.; Xu, Y.; Li, G. Effects of stumping height on carbon, nitrogen, and phosphorus stoichiometry in different organs of Pinus yunnanensis seedlings. Chin. J. Ecol. 2022, 41, 849–857. [Google Scholar] [CrossRef]
- Cai, N.; Tang, J.; Che, F.; Chen, S.; Wang, J.; Xu, Y.; Li, G. Effects of stumping treatments on C, N, and P stoichiometry in different organs of Pinus yunnanensis Franch. J. Northeast For. Univ. 2022, 50, 35–42. [Google Scholar] [CrossRef]
- Cai, N.; Hu, Z.; He, B.; Cheng, S.; Chen, L.; Tang, J.; Chen, S.; Xu, Y. Variation in the allometric relationship between the stoichiometric ratio of nitrogen, phosphorus, potassium and plant size for Pinus yunnanensis seedlings. Chin. J. Ecol. 2023, 43, 1300–1306. [Google Scholar] [CrossRef]
- Liang, S.; Tan, T.; Wu, D.; Li, C.; Jing, H.; Wu, J. Seasonal variations in carbon, nitrogen, and phosphorus of Pinus yunnanenis at different stand ages. Front. Plant Sci. 2023, 14, 1107961. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, M.; He, B.; Wang, Q.; Yu-lan, X.; Wei, L.; Nian-hui, C. Response of allometric relationship among N, P and K contents of Pinus yunnanensis seedlings to combined to nitrogen and phosphorus addition. J. Northeast For. Univ. 2023, 39, 26–34. [Google Scholar]
- Li, Y.; Chen, L.; Tang, J.; Chen, S.; Yulan, X.; Nianhui, C. Effects of fertilization on stoichiometric ratio of nitrogen, phosphorus and potassium in Pinus yunnanensis seedling and soil. J. Northwest A F Univ. (Nat. Sci. Ed.) 2024, 52, 40. [Google Scholar]
- Liu, Y.; Xiao, J.; Sun, J.; Zhao, Z.; Deng, X.; Wu, J.; Zhang, D.; Bao, Y. Seasonal variation in C:N:P stoichiometry, nonstructural carbohydrates, and carbon isotopes of two coniferous pioneer tree species in subtropical China. Front. Plant Sci. 2023, 14, 1225436. [Google Scholar] [CrossRef]
- LY/T 1950-2011; Fast-Growing and High-Yielding Plantation of Pinus yunnanensis. National Forestry and Grassland Administration: Beijing, China, 2011.
- Zhang, Z. Effect of Biochar and Reduced Fertilizer on Soil Physichemical Properties and Nutrient Utilization in Continuous Cropping. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Xie, H.; Yu, M.; Cheng, X. Leaf non-structural carbohydrate allocation and C:N:P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physiol. Biochem. 2018, 124, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, C.; Chen, J.; Shi, Z.; Xiao, W.; Zhao, G.; Yuan, X.; Wu, J. Leaf and fine root functional traits response of 10-year-old Cunninghamia lanceolate plantations to soil phosphorus addition. For. Res. 2022, 35, 23–32. (In Chinese) [Google Scholar] [CrossRef]
- Song, S.; Leng, H.; Feng, S.; Meng, C.; Luo, B.; Zhao, L.; Zhang, C. Biomass allocation pattern of urban shrubs in the Yangtze River Delta region, China—A field observation of 13 shrub species. Urban For. Urban Green. 2021, 63, 127228. [Google Scholar] [CrossRef]
- Umaña, M.N.; Cao, M.; Lin, L.; Swenson, N.G.; Zhang, C. Trade-offs in above- and below-ground biomass allocation influencing seedling growth in a tropical forest. J. Ecol. 2021, 109, 1184–1193. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Wang, X.; Wen, X.; Li, T.; Ye, M.; Chen, G.; Zhao, K.; Hou, G.; Li, X.; et al. Stand structure adjustment influences the biomass allocation in naturally generated Pinus massoniana seedlings through environmental factors. Front. Plant Sci. 2022, 13, 997795. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C. Sampling roots to capture plant and soil functions. Funct. Ecol. 2017, 31, 1506–1518. [Google Scholar] [CrossRef]
- Dhiman, I.; Bilheux, H.; DeCarlo, K.; Painter, S.L.; Santodonato, K.; Warren, J.M. Quantifying root water extraction after drought recovery using sub-mm in situ empirical data. Plant Soil 2018, 424, 73–89. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Peng, L.L.; Jiang, W.B.; Han, J. Effects of source-sink relationship change on yield and quality in fruit tree. Nonwood For. Res. 2012, 30, 134–140. [Google Scholar]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Xiao, L.; Wang, W.; Yu, K.; Shi, P. Heterogeneity in short-term allocation of carbon to roots of Pinus tabuliformis seedlings and root respiration under drought stress. Plant Soil 2020, 452, 359–378. [Google Scholar] [CrossRef]
- Xu, X.; Kuzyakov, Y.; Wanek, W.; Richter, A. Root-derived respiration and non-structural carbon of rice seedlings. Eur. J. Soil Biol. 2008, 44, 22–29. [Google Scholar] [CrossRef]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Briones, E.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; Martínez-Gallardo, N.; Tiessen, A.; Molina-Torres, J.; Délano-Frier, J.P.; Zañudo-Hernández, J. Seasonal variation in non-structural carbohydrates, sucrolytic activity and secondary metabolites in deciduous and perennial Diospyros species sampled in Western Mexico. PLoS ONE 2017, 12, e0187235. [Google Scholar] [CrossRef]
- Bazot, S.; Barthes, L.; Blanot, D.; Fresneau, C. Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees 2013, 27, 1023–1034. [Google Scholar] [CrossRef]
- Scartazza, A.; Moscatello, S.; Matteucci, G.; Battistelli, A.; Brugnoli, E. Seasonal and inter-annual dynamics of growth, non-structural carbohydrates and C stable isotopes in a Mediterranean beech forest. Tree Physiol. 2013, 33, 730–742. [Google Scholar] [CrossRef]
- Canadell, J.; López-Soria, L. Lignotuber reserves support regrowth following clipping of two Mediterranean shrubs. Funct. Ecol. 1998, 12, 31–38. [Google Scholar] [CrossRef]
- Wildy, D.T.; Pate, J.S. Quantifying above- and below-ground growth responses of the western Australian oil mallee, Eucalyptus Kochii subsp. plenissima, to contrasting decapitation regimes. Ann. Bot. 2002, 90, 185–197. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.; Schiestl-Aalto, P.; Saurer, M.; Sahlstedt, E.; Kulmala, L.; Kolari, P.; Ryhti, K.; Salmon, Y.; Jyske, T.; Ding, Y.; et al. Tree organ growth and carbon allocation dynamics impact the magnitude and δ13C signal of stem and soil CO2 fluxes. Tree Physiol. 2022, 42, 2404–2418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).