Water Translocation and Photosynthetic Responses in Clones of Kentucky Bluegrass to Heterogeneous Water Supply
Abstract
1. Introduction
2. Results
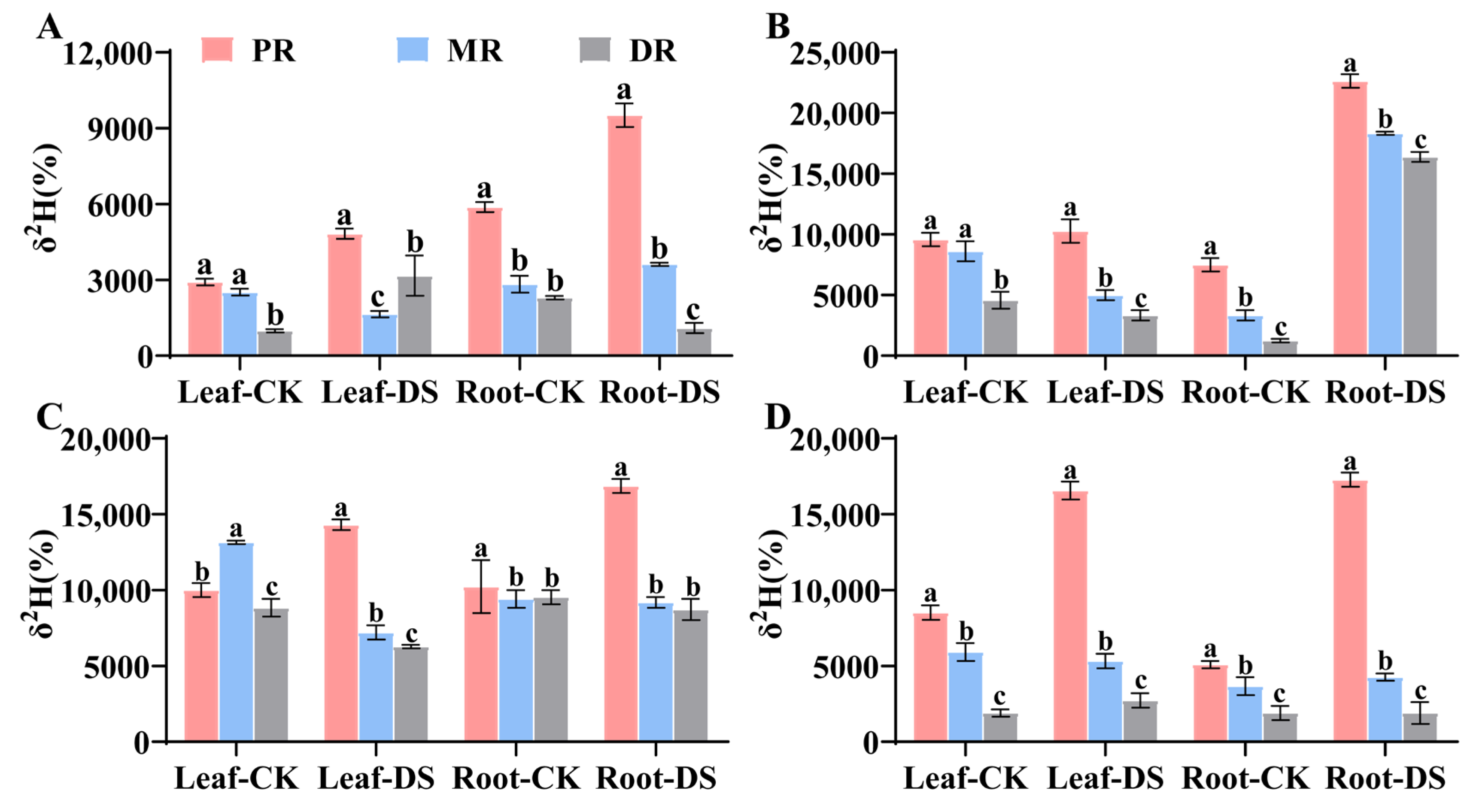
2.1. Water Translocation Characteristics in Clonal Ramets When Proximal Ramet Exposed to Drought Stress
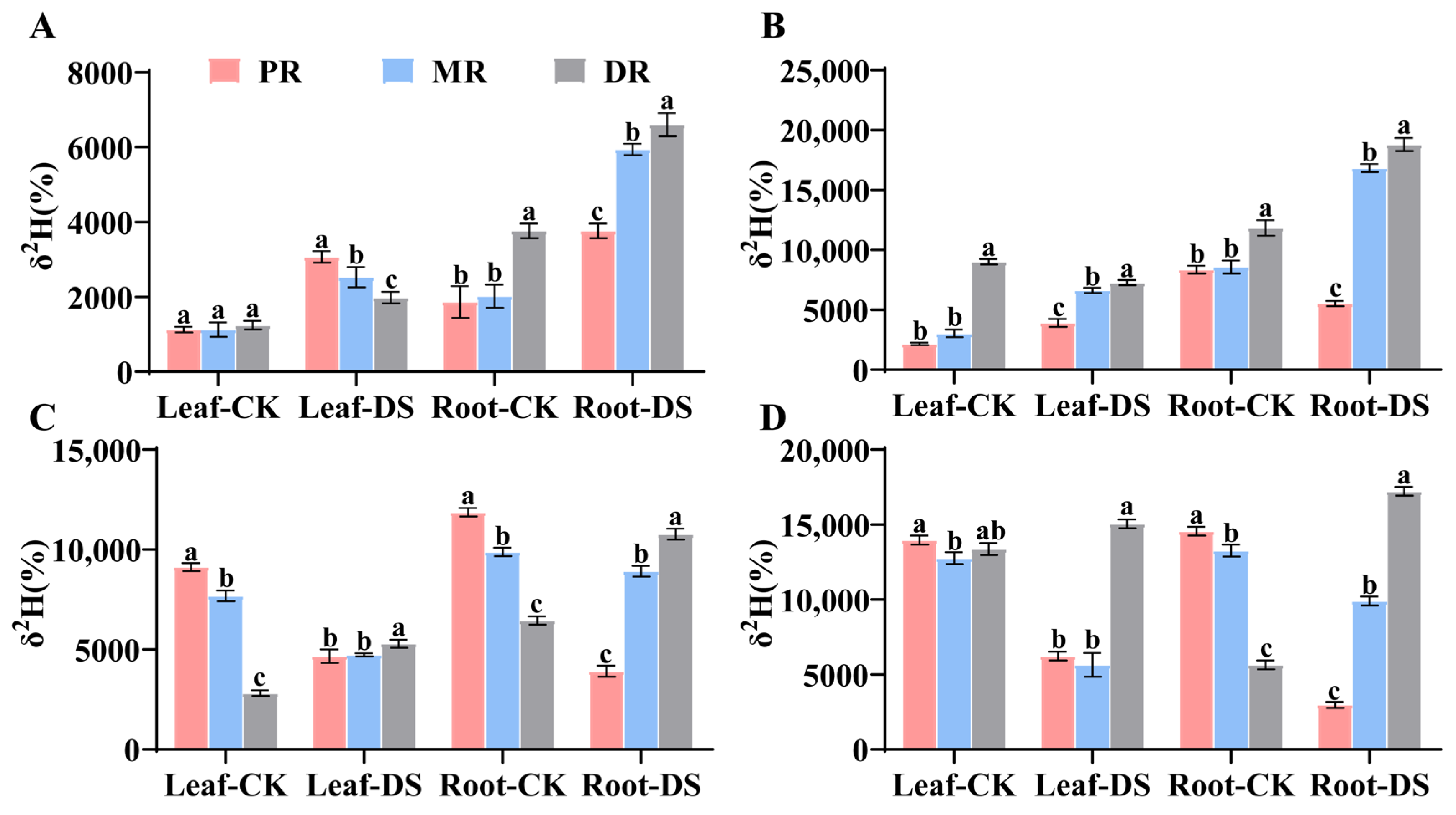
2.2. Water Translocation Characteristics in Clonal Ramets When Middle Ramet Exposed to Drought Stress
2.3. Water Translocation Characteristics in Clonal Ramets When Distal Ramet Exposed to Drought Stress
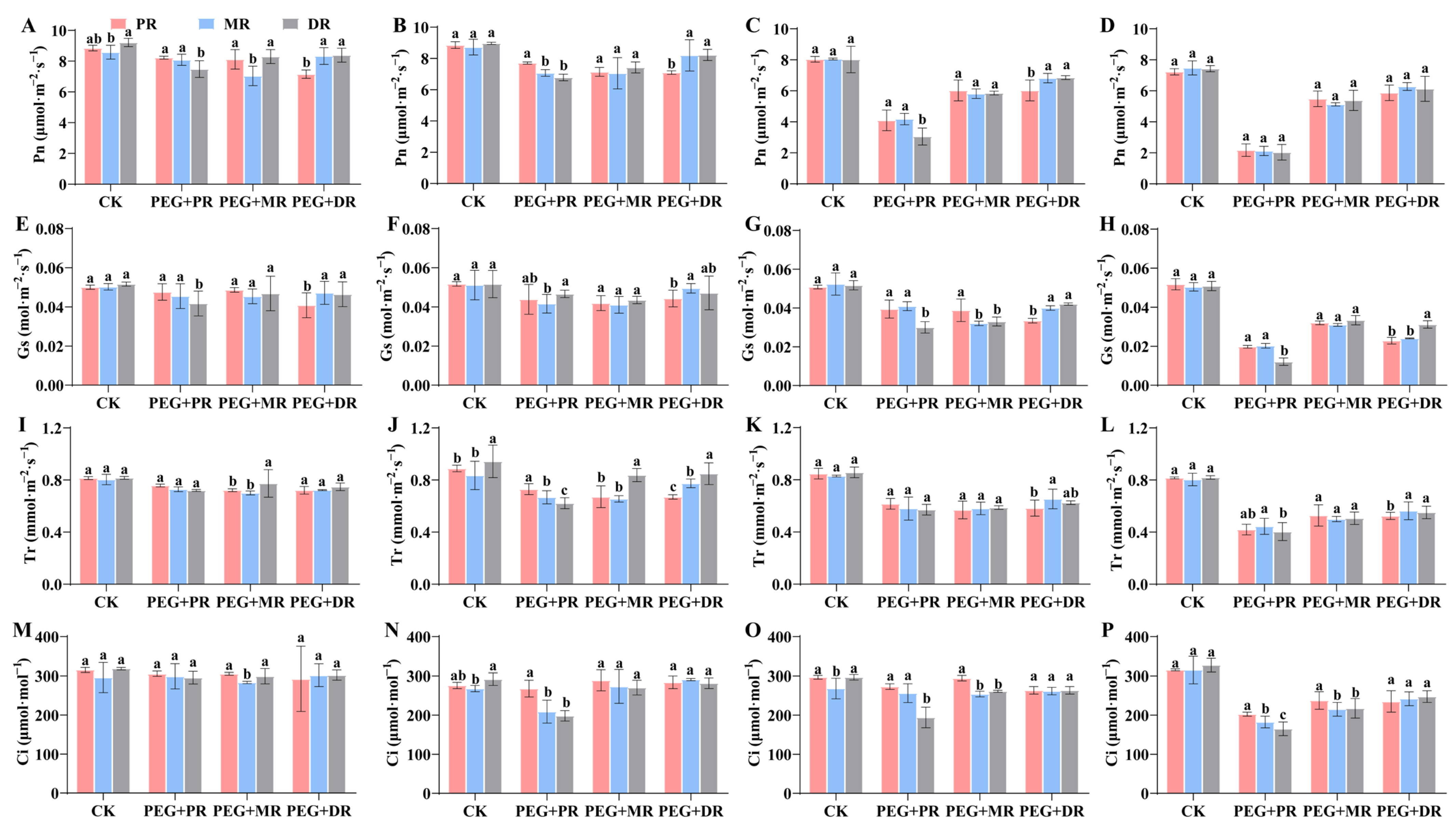
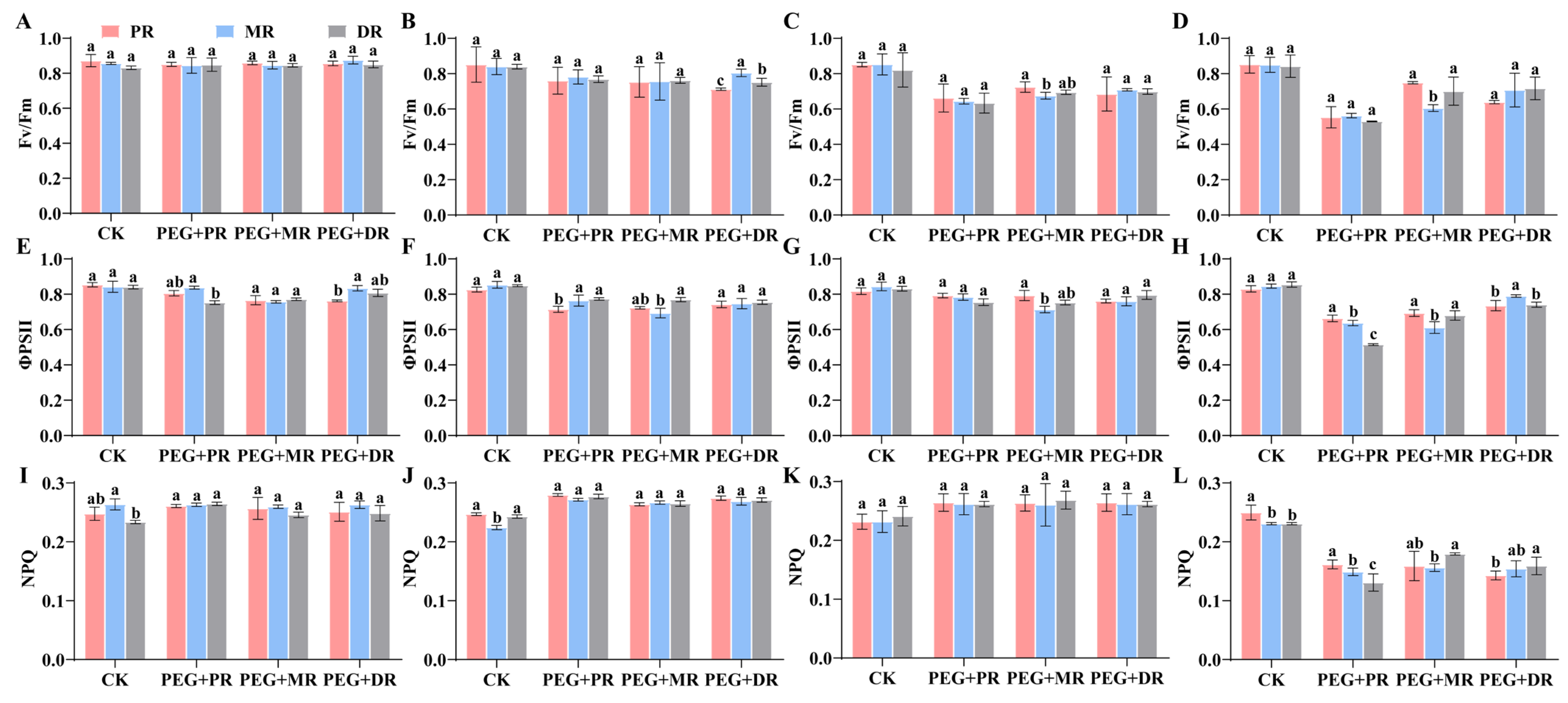
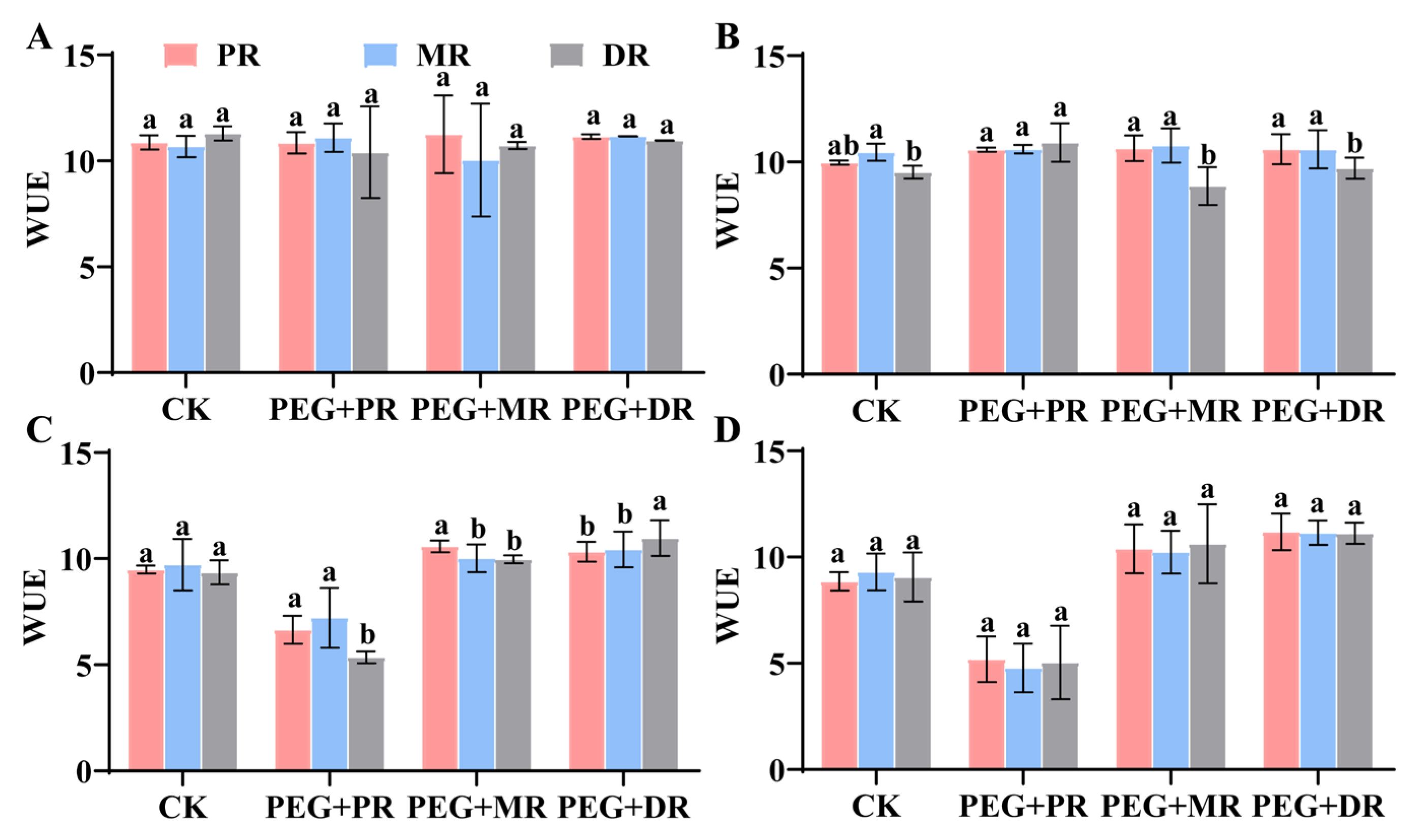
2.4. Changes in Photosynthetic Characteristics and Water Use Efficiency in Clonal Ramets Under Drought Stress
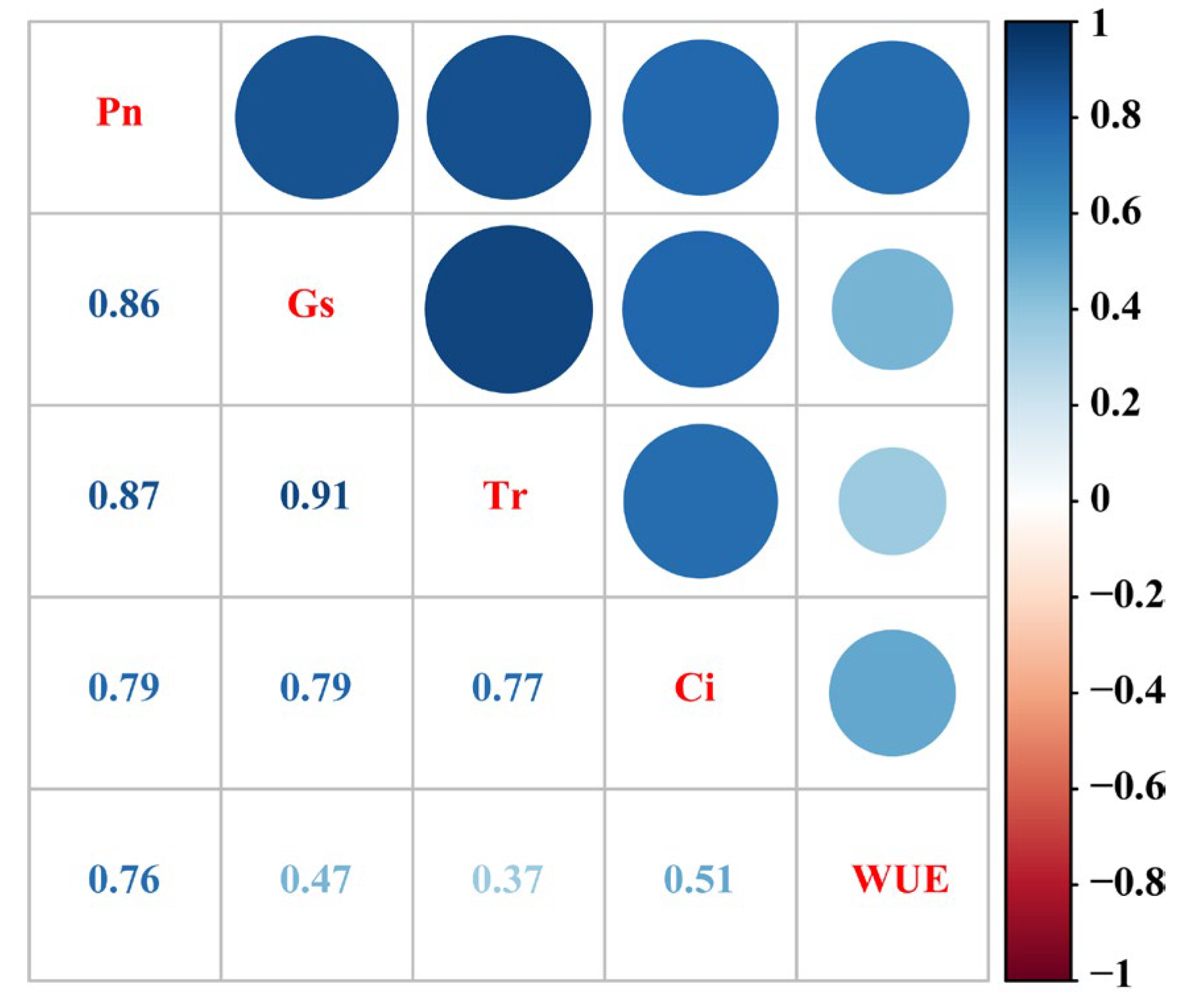
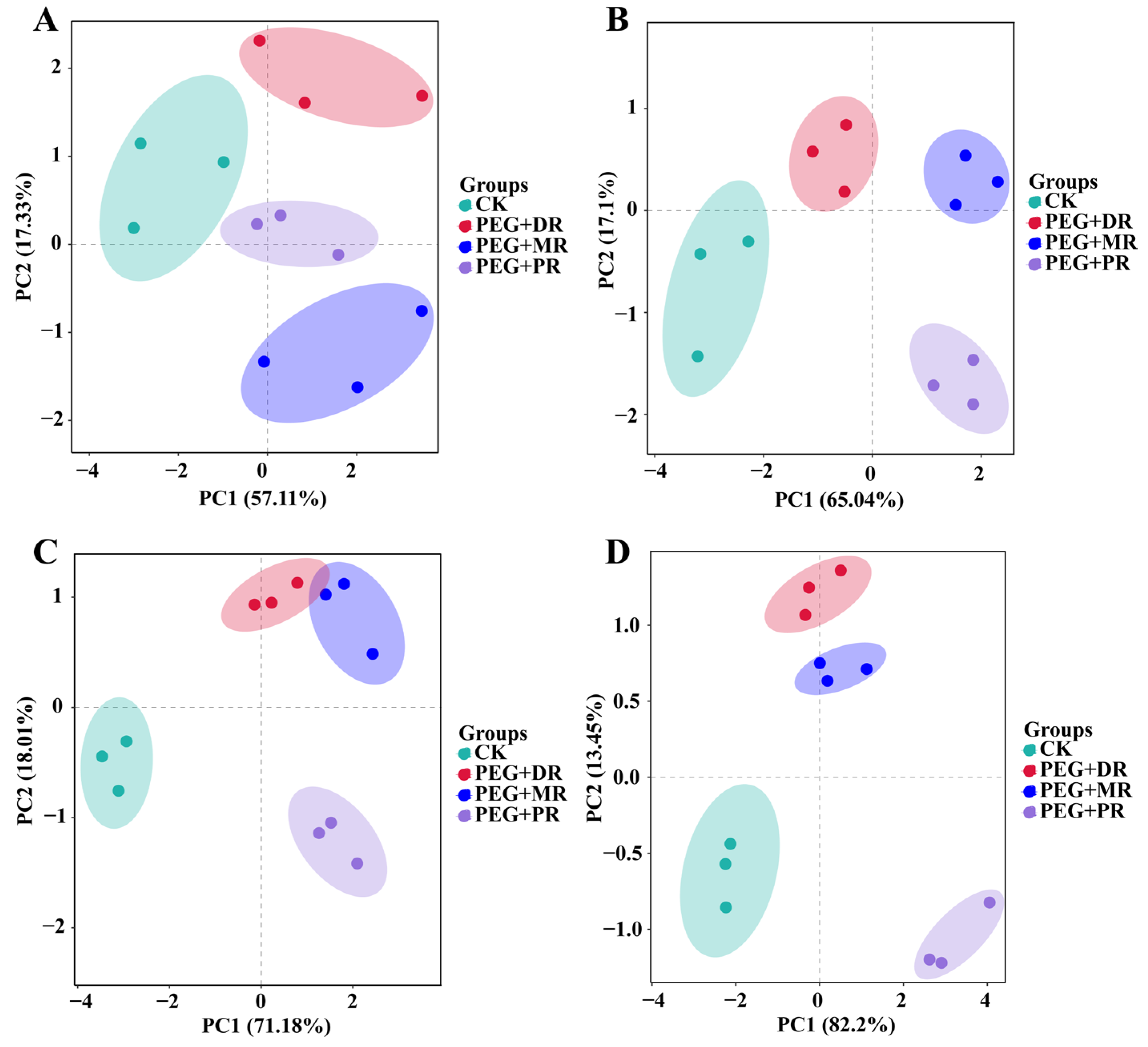
2.5. Principal Component Analysis and Correlation Analysis of Gas Exchange Parameters
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Conditions
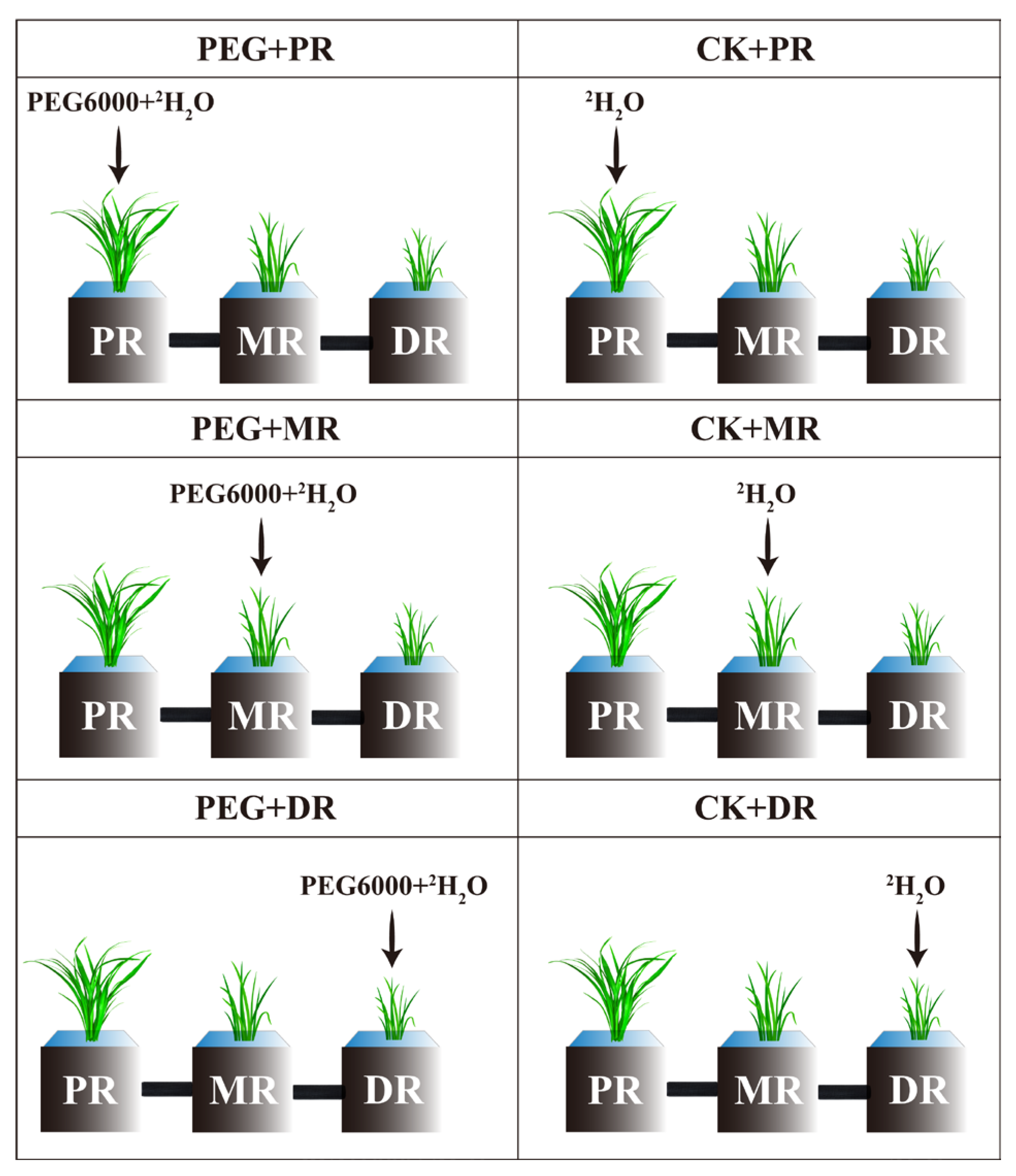
5.2. Treatments
5.3. Analysis of 2H Isotope
5.4. Determination of Leaf Gas Exchange Parameters
5.5. Determination of Chlorophyll Fluorescence
5.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.O.; Siqueira-Silva, A.I.; Pereira, E.G. How does drought affect native grasses’ photosynthesis on the revegetation of iron ore tailings? Environ. Sci. Pollut. Res. Int. 2021, 28, 14797–14811. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.P.; Zeng, Y.; Li, D.M.; Guo, D.J.; Rajput, V.D.; Chen, G.L.; Barakhov, A.; Minkina, T.M.; Li, Y.R. Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef]
- Manuchehri, R.; Salehi, H. Physiological and biochemical changes of common bermudagrass (Cynodon dactylon [L.] Pers.) under combined salinity and deficit irrigation stresses. S. Afr. J. Bot. 2014, 92, 83–88. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Wang, Q.; Chen, M.; Ye, X.; Li, D.; Chen, X.; Li, L.; Gao, D. 24-Epibrassinolide-alleviated drought stress damage influences antioxidant enzymes and autophagy changes in peach (Prunus persicae L.) leaves. Plant Physiol. Biochem. 2019, 135, 30–40. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.; Flexas, J.; Alsina Mdel, M.; Bota, J.; Carambula, C.; de Herralde, F.; Galmés, J.; Lovisolo, C.; Jiménez, M.; Ribas-Carbó, M.; et al. Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri x V. rupestris). Physiol. Plant 2008, 134, 313–323. [Google Scholar] [CrossRef]
- Guo, J.; Li, H.; Yang, Y. Phenotypic plasticity in sexual reproduction based on nutrients supplied from vegetative ramets in a Leymus chinensis population. Front. Plant Sci. 2019, 10, 1681. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Sammul, M. Physiological integration ameliorates negative effects of drought stress in the clonal herb Fragaria orientalis. PLoS ONE 2012, 7, e44221. [Google Scholar] [CrossRef]
- Zhu, C.; Li, W.; Yaning, C.; Chen, Y. Characteristics of water physiological integration and its ecological significance for Populus euphratica young ramets in an extremely drought environment. J. Geophys. Res. Atmos. 2018, 123, 5657–5666. [Google Scholar] [CrossRef]
- Li, Y.; Ning, W.; Xu, S.; Yu, N.; Chen, Z.; Zhang, L. Response of physiological integration in the clonal herb Zoysia japonica to heterogeneous water conditions. Acta Physiol. Plant 2022, 44, 34. [Google Scholar] [CrossRef]
- Roiloa, S.; Retuerto, R. Presence of developing ramets of Fragaria vesca L., increases photochemical efficiency in parent ramets. Int. J. Plant Sci. 2005, 166, 795–803. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Luo, P.; Wu, N. Photosynthetic response of Fragaria orientalis in different water contrast clonal integration. Ecol. Res. 2009, 24, 617–625. [Google Scholar] [CrossRef]
- Fernando, A.; Boléo, S.; Barbosa, B.; Costa, J.; Sidella, S.; Nocentini, A.; Mp, D.; Mendes, B.; Monti, A.; Cosentino, S. Perennial grasses: Environmental benefits and constraints of its cultivation in Europe. In Proceedings of the 20th European Biomass Conference and Exhibition, Milan, Italy, 18–22 June 2012. [Google Scholar]
- Jiang, Y. Application of gamma-aminobutyric acid and nitric oxide on turfgrass stress resistance: Current knowledge and perspectives. Grass Res. 2023, 3, 3. [Google Scholar] [CrossRef]
- Liu, W.; Xie, F.; Chen, Y.; Cui, G. Growth and morphological responses of Kentucky bluegrass to homogeneous and heterogeneous soil water availabilities. Agronomy 2022, 12, 1265. [Google Scholar] [CrossRef]
- Takahashi, H.; Miyazawa, Y.; Fujii, N. Hormonal interactions during root tropic growth: Hydrotropism versus gravitropism. Plant Mol. Biol. 2009, 69, 489–502. [Google Scholar] [CrossRef]
- Ashmun, J.W.; Thomas, R.J.; Pitelka, L.F. Translocation of photoassimilates between sister ramets in two rhizomatous forest herbs. Ann. Bot. 1982, 49, 403–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Yirdaw, E.; Luo, P.; Wu, N. Clonal integration of Fragaria orientalis driven by contrasting water availability between adjacent patches. Bot. Stud. 2008, 49, 373–383. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.R.; Moradi, P.; MohseniFard, E.; Shekari, F.; Kompany-Zareh, M. Effect of drought stress on metabolite adjustments in drought tolerant and sensitive thyme. Plant Physiol. Biochem. 2018, 132, 391–399. [Google Scholar] [CrossRef]
- Lin, H.F.; Alpert, P.; Zhang, Q.; Yu, F.H. Facilitation of amphibious habit by physiological integration in the clonal, perennial, climbing herb Ipomoea aquatica. Sci. Total Environ. 2018, 618, 262–268. [Google Scholar] [CrossRef]
- Wang, Y.J.; Bai, Y.F.; Zeng, S.Q.; Yao, B.; Wang, W.; Luo, F.L. Heterogeneous water supply affects growth and benefits of clonal integration between co-existing invasive and native Hydrocotyle species. Sci. Rep. 2016, 6, 29420. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Fahad, S.; Cui, G.; Yajun, C.; Anwar, S. Determining nitrogen isotopes discrimination under drought stress on enzymatic activities, nitrogen isotope abundance and water contents of Kentucky bluegrass. Sci. Rep. 2020, 10, 6415. [Google Scholar] [CrossRef] [PubMed]
- You, W.H.; Han, C.M.; Liu, C.H.; Yu, D. Effects of clonal integration on the invasive clonal plant Alternanthera philoxeroides under heterogeneous and homogeneous water availability. Sci. Rep. 2016, 6, 29767. [Google Scholar] [CrossRef]
- Alpert, P.; Mooney, H.A. Resource sharing among ramets in the clonal herb, Fragaria chiloensis. Oecologia 1986, 70, 227–233. [Google Scholar] [CrossRef]
- Netto, L.A.; Jayaram, K.M.; Puthur, J.T. Clonal variation of tea [Camellia sinensis (L.) O. Kuntze] in countering water deficiency. Physiol. Mol. Biol. Plants 2010, 16, 359–367. [Google Scholar] [CrossRef]
- Sun, X.; Niu, J.; Xu, Y.; Zhou, H. Long term water integration in interconnected ramets of stoloniferous grass, buffalograss. Afr. J. Biotechnol. 2010, 9, 5503–5510. [Google Scholar]
- Zhang, X.; Xu, B.; Günther, F.; Gleixner, G. Seasonal variation of leaf wax n-alkane δ2H values: Differences between Quercus aquifolioides (an evergreen tree) and Stipa bungeana (a perennial grass) from the southeastern Tibetan Plateau. Glob. Planet. Change 2021, 207, 103674. [Google Scholar] [CrossRef]
- Luo, C.; Wang, R.; Li, C.; Zheng, C.; Dou, X. Photosynthetic characteristics, soil nutrients, and their interspecific competitions in an apple–soybean alley cropping system subjected to different drip fertilizer regimes on the Loess Plateau, China. Agric. Water Manag. 2023, 275, 108001. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Xu, Y.; Li, S.; Zhang, S.; Yuan, Z.; Li, J.; Ni, Y. Overexpression of BnKCS1-1, BnKCS1-2, and BnCER1-2 promotes cuticular wax production and increases drought tolerance in Brassica napus. Crop J. 2020, 8, 26–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, Z.; Han, Y.; Chen, X.; Kong, X.; Sun, W.; Chen, C.; Chen, M. Plasticity of the cuticular transpiration barrier in response to water shortage and resupply in Camellia sinensis: A role of cuticular waxes. Front. Plant Sci. 2020, 11, 600069. [Google Scholar] [CrossRef]
- Li, Q.M.; Liu, B.B.; Wu, Y.; Zou, Z.R. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J. Integr. Plant Biol. 2008, 50, 1307–1317. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Wang, C.; Chen, A.; Xie, F.; Chen, Y. Water Translocation and Photosynthetic Responses in Clones of Kentucky Bluegrass to Heterogeneous Water Supply. Plants 2025, 14, 826. https://doi.org/10.3390/plants14050826
Jiang J, Wang C, Chen A, Xie F, Chen Y. Water Translocation and Photosynthetic Responses in Clones of Kentucky Bluegrass to Heterogeneous Water Supply. Plants. 2025; 14(5):826. https://doi.org/10.3390/plants14050826
Chicago/Turabian StyleJiang, Jia, Chen Wang, Along Chen, Fuchun Xie, and Yajun Chen. 2025. "Water Translocation and Photosynthetic Responses in Clones of Kentucky Bluegrass to Heterogeneous Water Supply" Plants 14, no. 5: 826. https://doi.org/10.3390/plants14050826
APA StyleJiang, J., Wang, C., Chen, A., Xie, F., & Chen, Y. (2025). Water Translocation and Photosynthetic Responses in Clones of Kentucky Bluegrass to Heterogeneous Water Supply. Plants, 14(5), 826. https://doi.org/10.3390/plants14050826




