1. Introduction
Cowpea (
Vigna unguiculata (L.) Walp) is a crop of significant socioeconomic and nutritional importance in several regions of the world, particularly in tropical and subtropical areas, with notable relevance in Brazil. Predominantly cultivated in the North and Northeast regions of the country, especially by small-scale farmers, cowpea is a crucial source of low-cost protein and carbohydrates. It plays a strategic role in food security and provides essential nutrients to vulnerable populations, particularly those in the Brazilian Northeast region [
1,
2].
In 2020, global cowpea production reached approximately 7.4 million tons, cultivated over 12.6 million hectares, resulting in an average yield of 0.587 t ha
−1 [
3]. Brazil is the third-largest cowpea producer worldwide, with the North and Northeast regions accounting for 94.5% of the cultivated area. In the 2023/2024 growing season, the national average yield was 0.449 t ha
−1. Despite having a smaller cultivated area—around 5.4 thousand hectares—the Central-West region led in productivity, reaching 1.1 t ha
−1, particularly in the state of Mato Grosso [
4]. During the same period, the North region achieved an average yield of 0.869 t ha
−1, with the states of Amazonas and Tocantins standing out, cultivating a combined area of 9.4 thousand hectares. In the Northeast, the average yield was 0.425 t ha
−1, with Maranhão representing the leading producer (0.541 t ha
−1) and Piauí having the largest cultivated area (186 thousand hectares) [
4]. Yields are even lower in the semi-arid region of the Northeast, reaching only 0.289 t ha
−1 [
5].
The main factors contributing to the low cowpea productivity in the Northeast—particularly in the semi-arid region—include limited adoption of research-based technologies, abiotic stresses, and the cultivation of low-yielding varieties [
6,
7]. Nonetheless, cowpea possesses considerable genetic diversity, especially among traditional varieties, which have been cultivated for generations and exhibit greater adaptation to local environmental conditions compared to improved cultivars.
These varieties are preserved by seed custodians in traditional rainfed farming systems, such as those in the Brazilian semi-arid region, and possess genes that confer tolerance to local environmental constraints [
7]. They are often preferred by farmers who contend with the challenges of the semi-arid environment [
8].
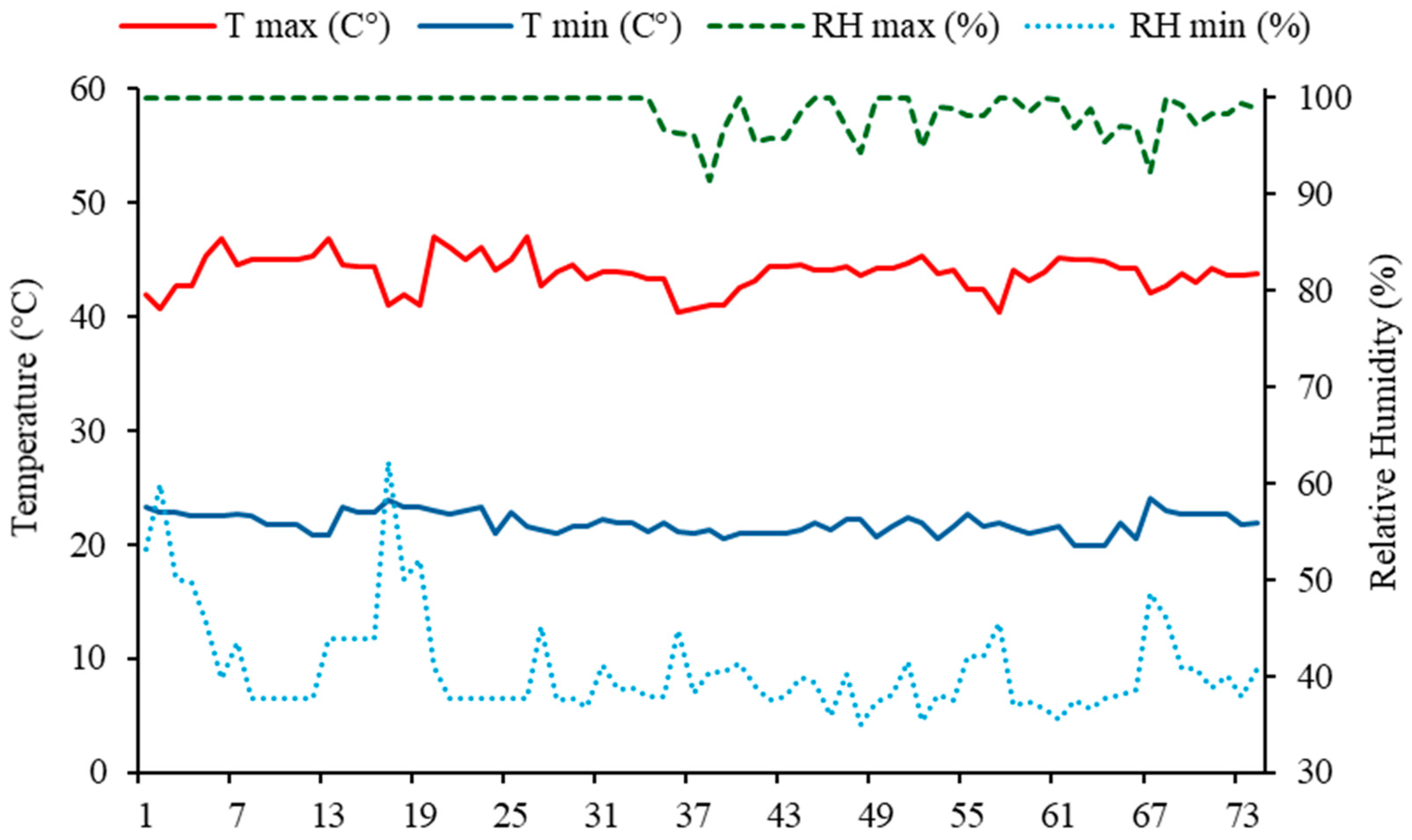
The semi-arid region encompasses a vast portion of northeastern Brazil and is characterized by challenging edaphoclimatic conditions. Rainfall is poorly distributed, with precipitation concentrated in short, high-intensity periods, contributing to regional water scarcity. These conditions are compounded by high temperatures and low relative humidity [
9,
10,
11]. Under such limitations, irrigation becomes an essential practice for agriculture. However, much of the water available for irrigation contains high concentrations of soluble salts—e.g., desalination by-products—which, although they expand water availability, pose risks of soil salinization and osmotic stress for plants [
12,
13].
The reuse of saline effluent from desalination systems is particularly relevant in semi-arid communities due to the scarcity of freshwater. In addition to minimizing the environmental impacts associated with the improper disposal of these effluents, their use in irrigation may represent a sustainable strategy when combined with appropriate irrigation and drainage management practices, thereby reducing excessive salt accumulation in the soil. Addressing this approach is essential, as the reuse of desalination brine contributes to regional water sustainability, while simultaneously mitigating the negative effects of direct environmental discharge and promoting social benefits, such as increased access to water for agricultural activities. Thus, management strategies that reconcile agricultural productivity with natural resource conservation are indispensable in semi-arid areas.
Cowpea is moderately tolerant to salinity, with irrigation water salinity thresholds of 3.3 dS m
−1 and 4.9 dS m
−1 for the soil saturation extract [
14]. Under salt stress, cowpea plants exhibit stomatal closure, reduced transpiration, internal CO
2 concentration, photosynthesis, dry matter accumulation, nodulation, and leaf area [
15,
16]. Nevertheless, saline water is commonly used in semi-arid agriculture due to the lack of better-quality water sources.
This reality poses a significant challenge for local agriculture, making saline water often the only available option. The most critical changes induced by salinity in irrigation water include alterations in osmotic potential and ionic toxicity, which result in overall reductions in plant growth [
15,
16,
17]. To optimize the use of saline water and minimize its adverse effects, it is necessary to adopt sustainable management practices and technologies that can mitigate its harmful impacts.
Irrigation water salinity above the tolerance threshold of cowpea reduces plant height and leaf number, significantly affecting plant physiology, yield, and biomass production [
18]. Salt stress leads to osmotic imbalance and water-related disorders in the plant, limiting gas exchange and resulting in decreased efficiency of the photosynthetic apparatus. Therefore, it is essential to implement strategies that enhance these physiological parameters, such as the use of stress-mitigating substances and fertilization practices that promote healthy plant growth even under high electrical conductivity conditions.
Although several strategies have been proposed to mitigate the effects of salt stress in plants—including the application of compounds such as salicylic acid [
19,
20,
21] and silicon [
22]—other approaches have focused on fertilization with macronutrients such as nitrogen, potassium [
23], and phosphorus [
17]. However, the specific role of magnesium (Mg) in this context remains unexplored. Magnesium plays critical roles in plant biochemical processes, being essential for photosynthesis, enzyme activation, and the stability of cellular membranes [
24,
25,
26,
27].
There is a growing interest in understanding how foliar magnesium fertilization can help cowpea plants combat the challenges posed by salinity, yet research on this topic remains limited. Unveiling the potential benefits of magnesium could be key to enhancing cowpea resilience under salt stress. Thus, we hypothesize that foliar Mg application may be a viable strategy to mitigate the detrimental effects of high salt concentrations in irrigation water on cowpea cultivation. Therefore, we evaluated the potential of foliar Mg fertilization to alleviate salt stress effects on growth, yield components, and leaf gas exchange in two traditional cowpea varieties, ‘Pingo de Ouro’ and ‘Costela de Vaca’.
3. Discussion
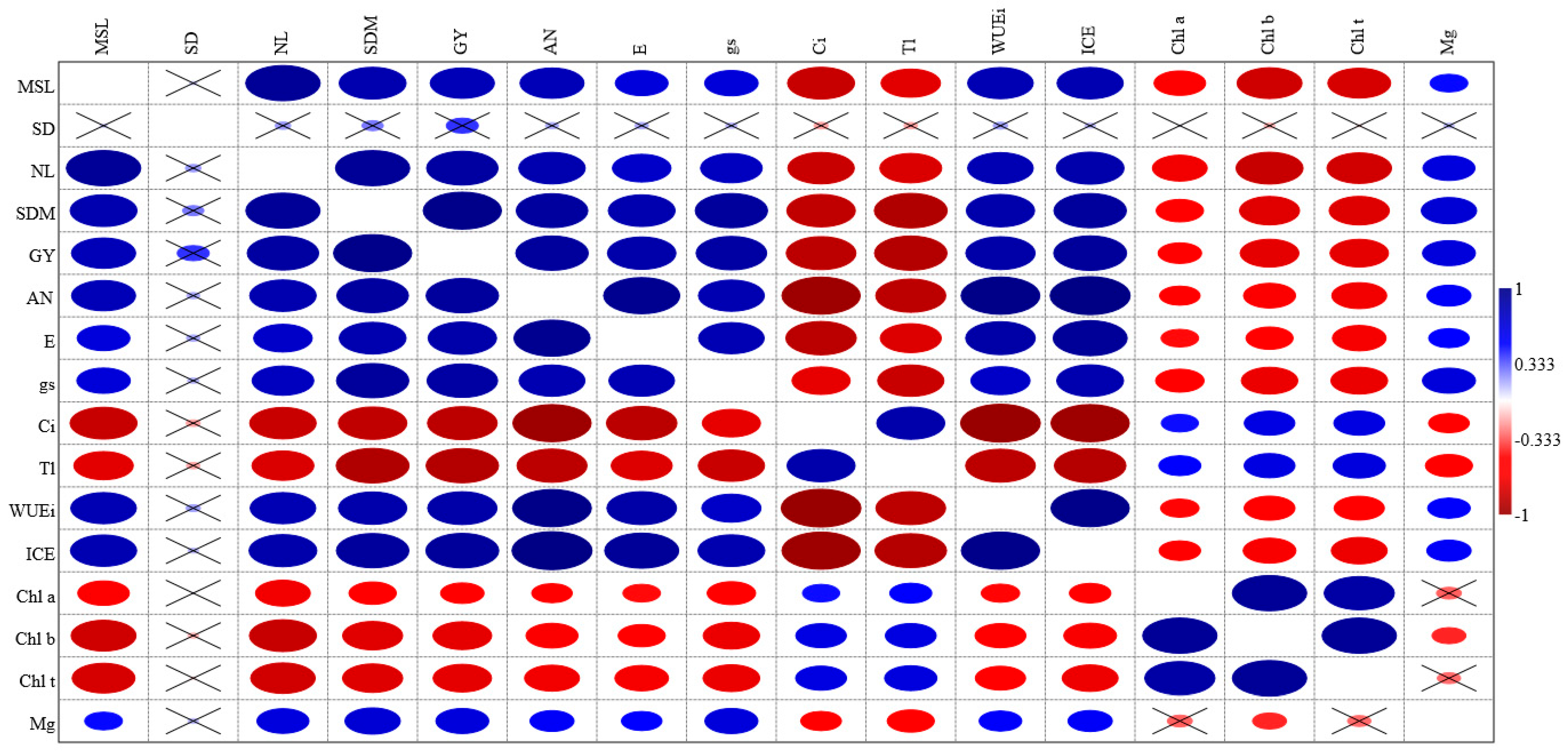
Identifying effective alternatives for irrigating crops with water that have high salt concentrations is vital for addressing food insecurity, particularly in semi-arid regions where fresh, high-quality water is increasingly scarce. Innovative research focused on enhancing crop resilience and performance in saline water irrigation—particularly through the application of substances that alleviate salt stress —plays a crucial role in developing sustainable agricultural practices. These strategies maximize crop yields in challenging conditions and contribute to the overall stability of food supplies in vulnerable communities, especially regarding cowpea production. This research evaluated the potential of foliar magnesium (Mg) fertilization to alleviate the effects of salt stress on growth, yield components, and leaf gas exchange in two traditional cowpea varieties: V1—‘Pingo de Ouro’ and V2—‘Costela de Vaca’. We found that at a salinity level of 3.50 dS m−1, a foliar application of 1 mL L−1 resulted in the highest yield per plant. For the ‘Costela de Vaca’ variety, CO2 assimilation was highest with 2 mL L−1 of Mg at the same salinity level (3.50 dS m−1), while the ‘Pingo de Ouro’ variety showed the greatest CO2 assimilation with 1 mL L−1 of Mg at a higher salinity of 5.00 dS m−1. The ‘Pingo de Ouro’ variety demonstrated greater tolerance to salt stress compared to ‘Costela de Vaca’. Overall, foliar Mg fertilization appears to be a promising strategy for mitigating the effects of salt stress in cowpea, particularly for the ‘Pingo de Ouro’ variety. While magnesium plays a vital role in alleviating salt stress in plants; however, its effectiveness can differ significantly depending on the specific plant variety and the salinity in the irrigation water. This variability highlights the need to customize magnesium dosage for each crop, emphasizing their optimal growing conditions.
Our findings show that the doses of Mg influenced plant yields differently depending on the salinity levels. Under salinity S1 (0.54 dS m
−1), Mg application had minimal impact on yield, suggesting that under low-salinity conditions, Mg supplementation does not confer significant benefits to yield. The lack of response in this scenario may be attributed to the plant’s ability to maintain nutritional and osmotic balance without additional Mg. In low-salinity environments, osmotic stress is less pronounced, allowing for adequate nutrient uptake. Additionally, photosynthesis remains relatively unaffected, as stomatal conductance and water-use efficiency are preserved, supporting energy production and carbon assimilation [
28]. Therefore, Mg supplementation becomes less critical under such conditions, as photosynthetic and metabolic functions remain stable. However, the 3 mL L
−1 dose exhibited a slight reduction in yield, which may indicate that, in the absence of salt stress, higher Mg doses could be detrimental, potentially due to nutritional imbalance or mild toxicity. Under salinity level S2 (3.50 dS m
−1), the 1 mL L
−1 dose resulted in the highest yield, indicating that this dose was most effective in mitigating moderate salt stress. This effect may be linked to Mg’s role in photosynthesis and enzyme activation, helping maintain metabolic processes under adverse conditions [
29]. In contrast, the 2 mL L
−1 dose yielded the lowest production, suggesting that higher Mg concentrations under moderate salinity may exacerbate osmotic stress, thus reducing yield. Under S3 (5.00 dS m
−1), the yield was drastically reduced at all Mg doses, with no significant differences between them. This indicates that under high salinity, osmotic stress overrides the beneficial effects of foliar Mg application. Elevated salt concentrations in the soil likely impaired Mg uptake, limiting its contribution to yield improvement. Additionally, sodium-induced ionic toxicity may have disrupted key physiological processes such as nutrient transport and photosynthesis, rendering Mg application ineffective.
Regarding the two varieties studied, Praxedes et al. [
30] reported higher yields, particularly in variety V2, with values substantially above those observed in the present study. A likely explanation is the difference in fertilization regimes: the referenced study employed the full recommended dose of fertilizers [
31], whereas this study used only 50% of that dosage. Reduced fertilization may have limited the availability of essential nutrients such as nitrogen, phosphorus, and potassium—critical for vegetative and reproductive growth [
32]. This limitation likely constrained photosynthetic capacity, reducing biomass production and, consequently, yield. In contrast, full fertilization in the previous study ensured greater nutrient availability, promoting plant growth and significantly enhancing yield.
Salinity exposure significantly affected plant development, as evidenced by reduced main stem length (MSL). In variety V2, the reduction was more pronounced, indicating greater sensitivity to high salinity levels and a consequent impairment in growth. Conversely, variety V1 also exhibited reduced MSL under the same conditions, but to a lesser extent, suggesting slightly greater salt stress tolerance compared to V2. These results align with those of Andrade et al. [
33], who found that MSL decreased by an average of 46.25% under salinity of 5.1 dS m
−1 compared to a control level of 0.6 dS m
−1 when evaluating salt-tolerant cowpea varieties. These findings reinforce that V1 is more tolerant to salt stress in terms of stem length than V2. The reduction in MSL is primarily attributed to the decreased soil osmotic potential, which increases water retention forces, impeding water uptake and cell turgor, thereby negatively affecting cell elongation and division rates [
34]. With regard to Mg doses, although no significant effect on MSL was observed, variety V1 consistently showed higher means across nearly all treatments. This suggests a potential advantage of Mg foliar application in managing salinity stress for this variety.
Salt stress led to an average reduction of 10% in stem diameter (SD), consistent with observations by Oliveira et al. [
35], who reported a similar decrease in variety V1 under 4.0 dS m
−1 salinity compared to the control (0.35 dS m
−1). This reduction is likely a plant response to the limited water uptake caused by osmotic stress, which diminishes sap flow through conductive tissues [
36]. Moreover, sodium-induced ionic toxicity can inhibit the uptake of several nutrients, including magnesium, which plays a key role in the transport of photoassimilates via the plant’s vascular system. Reduced Mg absorption may have contributed to the decline in SD. This is supported by the physiological effects of Mg deficiency, which disrupts carbon metabolism and, secondarily, reduces stem thickness [
37]. Under salinity level S2, foliar application of 1 mL L
−1 Mg led to an 11.82% increase in SD for variety V1 compared to the untreated control, suggesting efficient Mg uptake and utilization by this genotype. In contrast, none of the Mg doses significantly affected SD in variety V2, implying that lower doses may be more appropriate for this variety or that additional strategies beyond Mg supplementation are required to mitigate salt stress under moderate salinity. At the highest salinity level (S3), SD tended to decline across all Mg treatments, particularly for variety V2. These findings underscore the importance of considering the interaction between salinity level, genotype, and Mg dose when evaluating stem growth in cowpea.
Salinity at S2 caused a 22.76% reduction in the number of leaves (NL) compared to S1, while salinity at S3 led to a more pronounced decline of 36.82%. Similarly, Oliveira et al. [
35] observed a 17.1% reduction in NL in cowpea variety V1 under 4.0 dS m
−1 salinity relative to the control (0.35 dS m
−1). In another study, Praxedes et al. [
30] reported an average 50% reduction in the NL of cowpea under 4.5 dS m
−1 compared to 0.5 dS m
−1. Such variation in NL response among studies is likely due to the high genetic diversity of traditional cowpea varieties, which results in different salinity tolerance levels. Regarding foliar Mg doses, no significant differences were observed between varieties. However, when analyzed individually, variety V1 exhibited higher NL values than V2, primarily due to its greater main stem length (MSL), which correlates with higher leaf production.
Overall, Mg doses had no significant effect on instantaneous water use efficiency (WUE
i). However, under high salinity, the 3 mL L
−1 dose resulted in the lowest WUE
i mean. This finding suggests that high Mg concentrations under salt stress may have adverse effects, intensifying osmotic stress and ionic toxicity, which compromise stomatal regulation and photosynthesis. Thus, selecting an appropriate Mg dose is critical to optimizing WUE
i under saline conditions and avoiding excess application that could impair plant performance. In both varieties, salinity level S2 caused an average reduction of 31.6% in net photosynthesis compared to the control (S1). This decline may be attributed to multiple salt stress-related factors. High salt concentrations induce osmotic stress, leading to stomatal closure and reduced CO
2 uptake. Additionally, salinity can damage chloroplasts, affecting photosystem II efficiency and compromising the photosynthetic capacity of the plant [
38].
Regarding CO
2 assimilation (A
N) in variety V1 under a salinity level of S2, a foliar application of 2 mL L
−1 magnesium (Mg) resulted in an 11.8% increase in A
N compared to the 0 mL L
−1 treatment. These findings indicate that applying 2 mL L
−1 of Mg through foliar fertilization can help alleviate salt stress in variety V1. Other doses of magnesium did not show significant differences in effects. Magnesium deficiency, often induced by sodium toxicity, can reduce the activity of the RuBisCO enzyme, impairing CO
2 assimilation [
39,
40]. Mg also plays a key role in regulating stomatal opening and closing [
41] directly influencing gas exchange and transpiration. Foliar Mg application circumvents ionic competition in the rhizosphere by allowing direct absorption through leaves, improving ionic balance and osmotic adjustment, and consequently enhancing photosynthetic efficiency [
42,
43]. For variety V2, the 1 mL L
−1 Mg dose under salinity level S3 resulted in the highest Aₙ. While the 2 mL L
−1 dose may have supplied enough Mg to compensate for ionic and osmotic stress in V1, variety V2 may exhibit higher Mg-use efficiency for photosynthesis, making the lower dose (1 mL L
−1) sufficient to optimize A
N even under severe salinity (S3). These findings indicate that excess Mg may not be beneficial and could cause nutritional imbalances. In V2, 1 mL L
−1 appears to be the optimal concentration, offering the benefits of Mg without adverse effects. Thus, photosynthetic efficiency varies with genotype and salt stress intensity, leading to different responses to foliar Mg application.
With regard to internal CO
2 concentration (Ci), the V1 variety showed that applying 3 mL L
−1 of magnesium at salinity level S3 resulted in a higher internal CO
2 concentration compared to other doses at this salinity level. This outcome can be attributed to various factors related to the role of magnesium in photosynthesis and the plant’s responses to salt stress. Salt stress can reduce photosynthetic efficiency, leading to CO
2 accumulation in the leaves due to the decline in carbon assimilation rates. As magnesium is essential for the activation of RuBisCO—the enzyme responsible for CO
2 fixation in the Calvin cycle—ensuring an adequate Mg supply can help maintain the enzyme’s efficient functioning, even under salt stress conditions [
29]. Although salinity level S3 represents a condition of severe stress for plants, the application of 3 mL L
−1 of Mg may have significantly alleviated the osmotic and ionic stress, resulting in a higher
Ci. This suggests better maintenance of stomatal opening and increased availability of CO
2 for photosynthesis. The application of 3 mL L
−1 of Mg in variety V1 under S3 salinity conditions led to a higher
Ci compared to other doses, indicating that this specific Mg concentration may be effective in mitigating the negative effects of salt stress and in improving the plant’s ability to sustain gas exchange and internal CO
2 availability. However, an increase in
Ci may also indicate that photosynthesis is not occurring efficiently, possibly due to reduced enzymatic activity [
44]. This rise in
Ci may reflect that, despite the higher availability of CO
2, photosynthesis is still limited due to the severity of the salt stress, which may impair other components of the photosynthetic process—even with Mg supplementation. Therefore, adjusting Mg doses appropriately according to salinity levels is essential to optimize growth and photosynthetic efficiency in cowpea.
The Instantaneous Carboxylation Efficiency (ICE) for the variety V2, when treated with a 1 mL L
−1 Mg dose under salinity S2, was higher than for the other doses. This may be attributed to V2’s superior ability to utilize magnesium, making the lower dose sufficient to optimize ICE. Higher magnesium doses could lead to nutritional imbalances or even toxicity in V2, while the 1 mL L
−1 dose seems to strike an ideal balance, providing benefits without any adverse effects. For variety V1, the 2 mL L
−1 Mg dose was the most effective under salinity S2, likely due to this variety’s need for a greater amount of Mg to counteract the negative effects of salinity and maximize carboxylation. Magnesium is essential for activating the RuBisCO enzyme, which plays a key role in CO
2 fixation in the Calvin cycle, thereby increasing photosynthetic efficiency and ICE. Although an increase in internal CO
2 concentration may initially seem unfavorable, when associated with improved ICE, it indicates a beneficial adjustment. By activating RuBisCO and enhancing CO
2 assimilation, Mg helps stabilize cell membranes and optimize photosynthetic processes under salt stress [
45,
46]. Thus, even with higher CO
2 concentration, the plant efficiently uses the gas, favoring carbohydrate production and growth [
47], mitigating the adverse effects of salt stress and maintaining photosynthetic efficiency [
48]. These findings show that the response of instantaneous carboxylation efficiency to Mg varies between cowpea varieties under saline conditions. While variety V1 benefits more from the 2 mL L
−1 Mg dose under S2 salinity, variety V2 achieved higher ICE with the 1 mL L
−1 dose. These findings highlight the importance of adjusting Mg supplementation according to plant variety and stress conditions to optimize photosynthesis and carboxylation.
In summary, our findings indicate that foliar application of magnesium is a promising strategy for enhancing salt stress tolerance in cowpea. This is particularly relevant for farmers in semi-arid regions where salinity is a persistent challenge. This practice can help mitigate the harmful effects of salinity, allowing plants to maintain essential physiological functions such as photosynthesis and pod formation, even in adverse conditions. However, the effectiveness of magnesium application depends on several factors, including the dosage used, the specific variety of cowpea cultivated, and the level of salinity present. For farmers, this means that management practices should be tailored to local conditions and the unique characteristics of the planted varieties. We emphasize that while magnesium application does not completely eliminate the impacts of salinity, it can enhance plant performance, making it a viable option for improving production in sustainable agricultural systems within semi-arid regions.