Phenolic-Rich Extracts of Galenia africana and Tulbaghia violacea Accelerate Keratinocyte Migration and Mitigate Oxidative Stress to Enhance Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Plant Material and Extract Preparation
2.3. Phytochemical Analysis
2.3.1. Total Polyphenolic Content
2.3.2. Determination of Total Flavonoid Content
2.4. Quantitative Analysis of Extracts by High-Performance Liquid Chromatography (HPLC)
2.5. Antioxidant Studies
2.5.1. DPPH Radical Scavenging Assay
2.5.2. Oxygen Radical Absorbance Capacity Assay
2.5.3. Ferric Reducing Antioxidant Power Assay (FRAP) Assay
2.6. Cell Culture Studies
2.6.1. Cell Line
2.6.2. Cell Viability and Proliferation
2.6.3. Scratch Assay
2.6.4. Dichlorofluorescin Diacetate (DCFDA) Cellular Antioxidant Detection Assay
2.7. Data Analysis
3. Results and Discussion
3.1. Phytochemical Analysis and Antioxidant Activity
3.2. HPLC Chromatographic Profiles of the Plant Extracts
3.2.1. The Effects of Ethanolic Extracts on Cell Viability in HaCaT Cells
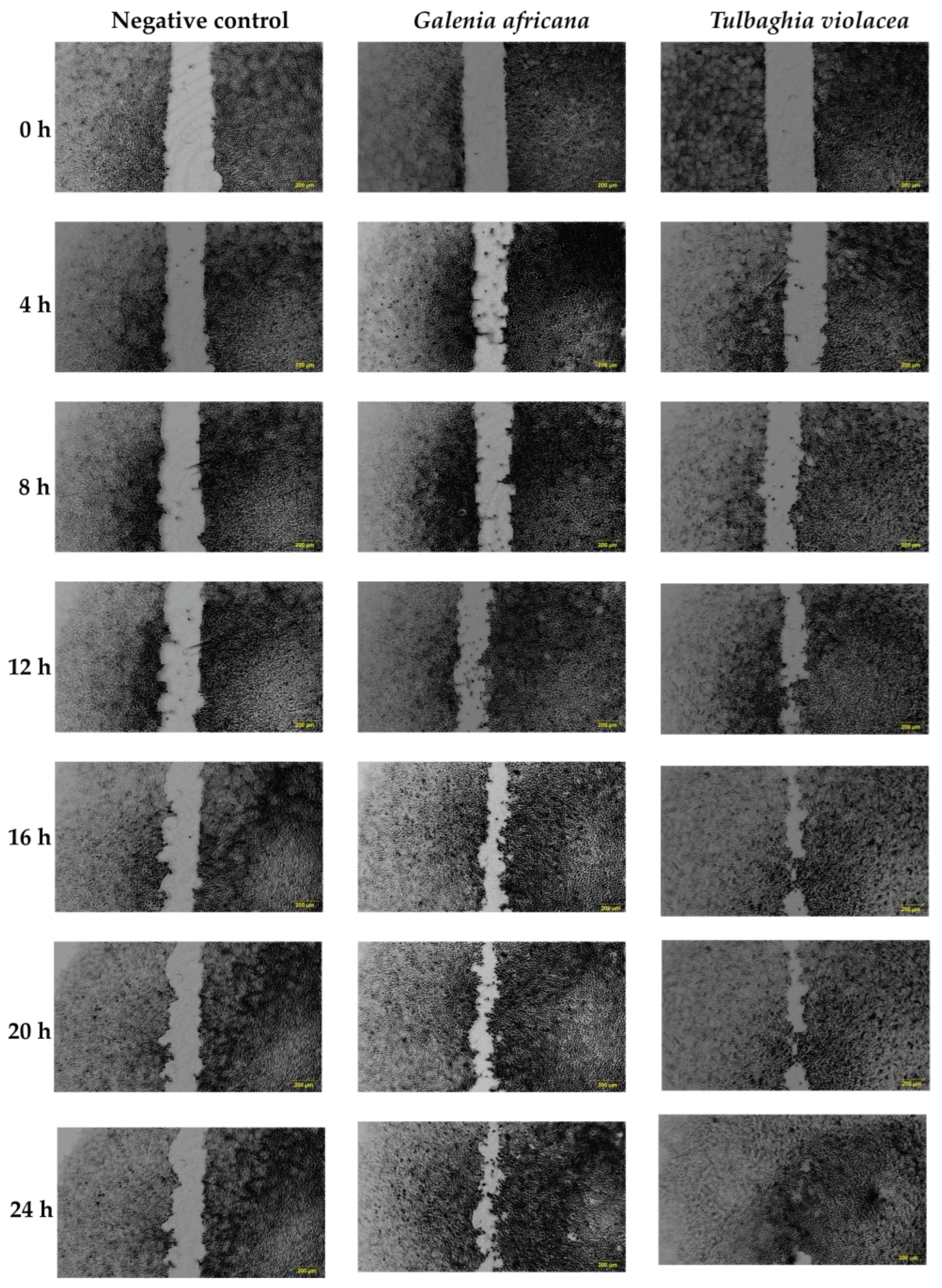
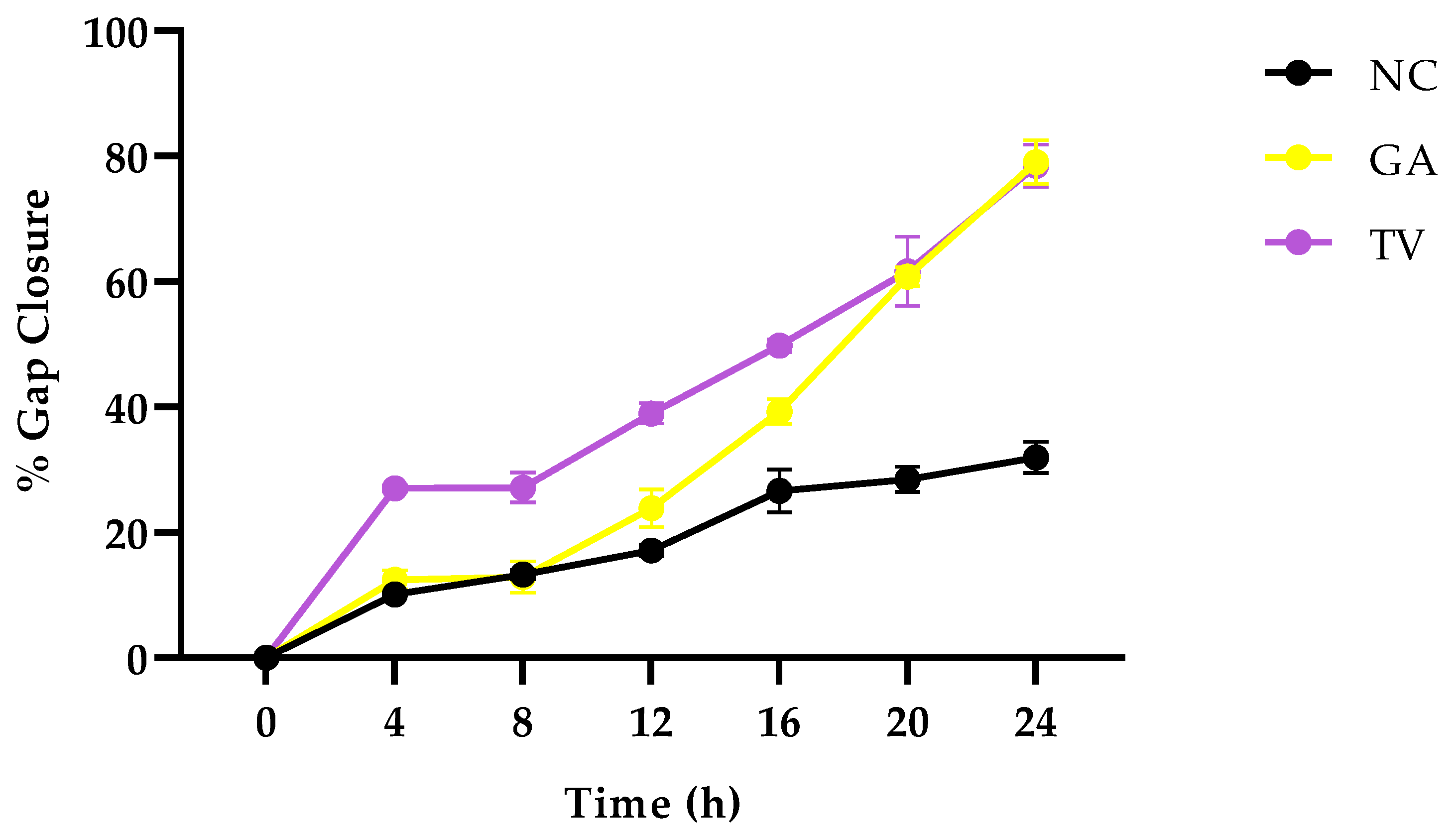
3.2.2. The Effect of Ethanolic Extracts on Cell Migration
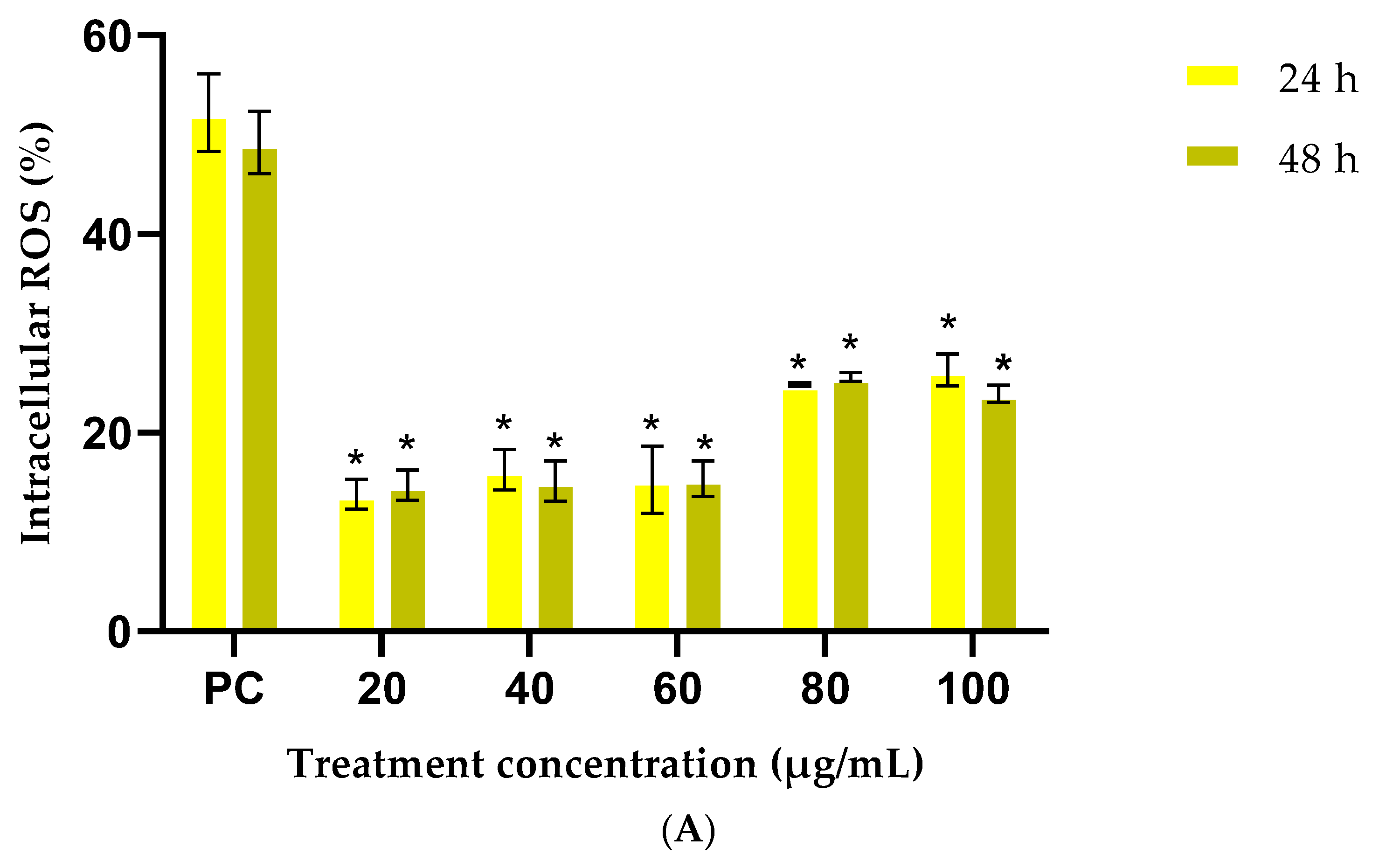
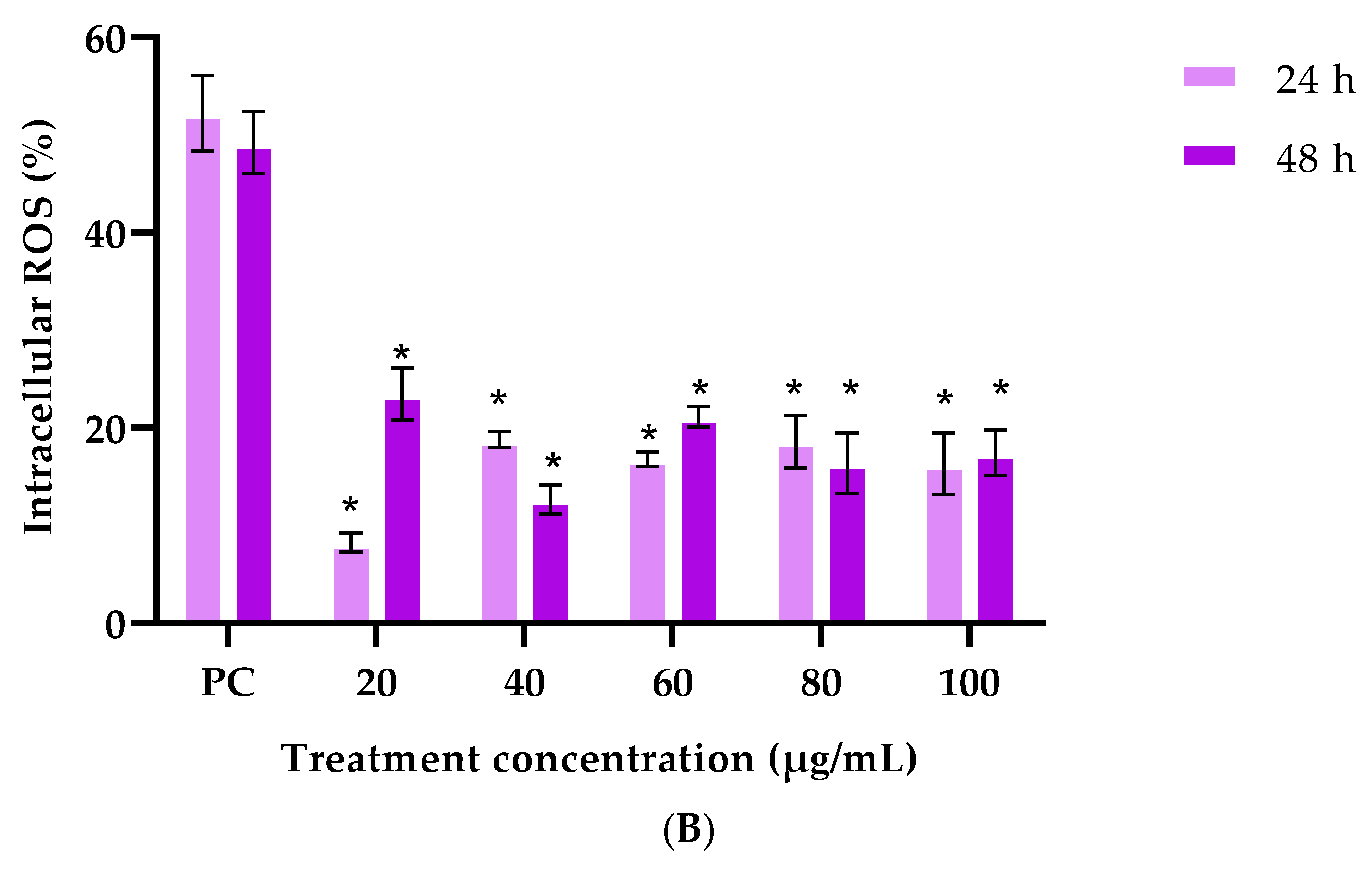
3.2.3. Cellular Antioxidant Assay (CAA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirwan, H.; Pignataro, R. The skin and wound healing. Pathol. Interv. Musculoskelet. Rehabil. 2015, 25, 125–129. [Google Scholar]
- Ferreira, M.C.; Júnior, P.T.; Carvalho, V.F.; Kamamoto, F. Complex wounds. Clinics 2006, 61, 571–578. [Google Scholar] [CrossRef]
- Madubula, N.; van Eeden, E. An analysis of the vulnerability of informal and formal households to disaster risks in the Rand West City region. Jàmbá-J. Disaster Risk Stud. 2024, 16, 1589. [Google Scholar] [CrossRef]
- Davies, B.; du Toit, C.; Hlela, M.B.K.M. Fire deaths in Cape Town, South Africa: A retrospective review of medico-legal and toxicological findings (2006–2018). Burns 2024, 50, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Asong, J.; Ndhlovu, P.; Khosana, N.; Aremu, A.; Otang-Mbeng, W. Medicinal plants used for skin-related diseases among the Batswanas in Ngaka Modiri Molema District Municipality, South Africa. South Afr. J. Bot. 2019, 126, 11–20. [Google Scholar] [CrossRef]
- Karan, A.; Amado, V.; Vitorino, P.; Kulber, D.; Taela, A.; DeUgarte, D.A. Evaluating the socioeconomic and cultural factors associated with pediatric burn injuries in Maputo, Mozambique. Pediatr. Surg. Int. 2015, 31, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Li, M.; Xia, W.; Khoong, Y.M.; Huang, L.; Huang, X.; Liang, H.; Zhao, Y.; Mao, J.; Yu, H.; Zan, T. Smart and versatile biomaterials for cutaneous wound healing. Biomater. Res. 2023, 27, 87. [Google Scholar] [CrossRef]
- Hughes, G.D.; Aboyade, O.M.; Okonji, C.O.; Clark, B.; Mabweazara, S.Z. Comparison of the prevalence of non-communicable diseases and traditional herbal medicine use in urban and rural communities in South Africa. Adv. Integr. Med. 2021, 8, 136–143. [Google Scholar] [CrossRef]
- Goldblatt, P. Floristic diversity in the Cape flora of South Africa. Biodivers. Conserv. 1997, 6, 359–377. [Google Scholar] [CrossRef]
- Philander, L.A. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef]
- Klak, C.; Hanáček2, P.; Bruyns, P.V. Disentangling the Aizooideae: New generic concepts and a new subfamily in Aizoaceae. Taxon 2017, 66, 1147–1170. [Google Scholar] [CrossRef]
- Moodley, K.; Joseph, K.; Naidoo, Y.; Islam, S.; Mackraj, I. Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement. Altern. Med. 2015, 15, 408. [Google Scholar] [CrossRef]
- Van Wyk, B.-E. A review of Khoi-San and Cape Dutch medical ethnobotany. J. Ethnopharmacol. 2008, 119, 331–341. [Google Scholar] [CrossRef]
- Heredia, D.; Green, I.; Klaasen, J.; Rahiman, F. Importance and relevance of phytochemicals present in Galenia africana. Scientifica 2022, 2022, 5793436. [Google Scholar] [CrossRef] [PubMed]
- Ng’uni, T.; Klaasen, J.A.; Fielding, B.C. Acute toxicity studies of the South African medicinal plant Galenia africana. Toxicol. Rep. 2018, 5, 813–818. [Google Scholar] [CrossRef]
- Mohamed, L.; Chakraborty, S.; ArulJothi, K.; Mabasa, L.; Sayah, K.; Costa-Lotufo, L.V.; Jardine, A.; Prince, S. Galenia africana plant extract exhibits cytotoxicity in breast cancer cells by inducing multiple programmed cell death pathways. Saudi Pharm. J. 2020, 28, 1155–1165. [Google Scholar] [CrossRef]
- Ndlovu, B.; De Kock, M.; Klaasen, J.; Rahiman, F. In vitro comparison of the anti-proliferative effects of Galenia africana on human skin cell lines. Sci. Pharm. 2021, 89, 12. [Google Scholar] [CrossRef]
- Styger, G.; Aboyade, O.M.; Gibson, D.; Hughes, G. Tulbaghia—A Southern African Phytomedicine. J. Altern. Complement. Med. 2016, 22, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Tulbaghia violacea and Allium ursinum extracts exhibit anti-parasitic and antimicrobial activities. Molecules 2018, 23, 313. [Google Scholar] [CrossRef]
- Raji, I.; Obikeze, K.; Mugabo, P. Potential beneficial effects of Tulbaghia violacea William Henry Harvey (Alliaceae) on cardiovascular system-A review. Trop. J. Pharm. Res. 2015, 14, 1111–1117. [Google Scholar] [CrossRef]
- Bungu, L.; van de Venter, M.; Frost, C. Evidence for an in vitro anticoagulant and antithrombotic activity in Tulbaghia violacea. Afr. J. Biotechnol. 2008, 7, 681–688. [Google Scholar]
- Iwalewa, E.; McGaw, L.; Naidoo, V.; Eloff, J. Inflammation: The foundation of diseases and disorders. A review of phytomedicines of South African origin used to treat pain and inflammatory conditions. Afr. J. Biotechnol. 2007, 6, 2868–2885. [Google Scholar] [CrossRef]
- Hulley, I.; Van Wyk, B.-E. Quantitative medicinal ethnobotany of Kannaland (western Little Karoo, South Africa): Non-homogeneity amongst villages. South Afr. J. Bot. 2019, 122, 225–265. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; De Wet, H.; Van Heerden, F. An ethnobotanical survey of medicinal plants in the southeastern Karoo, South Africa. South Afr. J. Bot. 2008, 74, 696–704. [Google Scholar] [CrossRef]
- Thring, T.; Weitz, F. Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Tyavambiza, C.; Meyer, M.; Wusu, A.D.; Madiehe, A.M.; Meyer, S. The antioxidant and in vitro wound healing activity of cotyledon orbiculata aqueous extract and the synthesized biogenic silver nanoparticles. Int. J. Mol. Sci. 2022, 23, 16094. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Yo, S.R.; Lee, M.Y.; Seo, C.S.; Shin, H.K.; Jeong, S.J. Potential inhibitory effects of the traditional herbal prescription Hyangso-san against skin inflammation via inhibition of chemokine production and inactivation of STAT1 in HaCaT keratinocytes. Mol. Med. Rep. 2018, 17, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth). J. Taibah Univ. Med. Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Kerebba, N.; Rautenbach, F.; Singh, S.K.; Dua, K.; Oguntibeju, O.O. UPLC-ESI-QTOF-MS phenolic compounds identification and quantification from ethanolic extract of: In vitro antioxidant and antidiabetic potentials. Arab. J. Chem. 2022, 16, 104447. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.-G.; Scheper, T. Identification of major constituents of Hypericum perforatum L. extracts in Syria by development of a rapid, simple, and reproducible HPLC-ESI-Q-TOF MS analysis and their antioxidant activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Kumar, B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar]
- Kumar, G.; Gautam, G.K.; Kumar, R.; Rana, H. The pharmacological and phytochemical study of Adansonia digitata. Curr. Trends Pharm. Pharm. Chem. 2022, 4, 54–58. [Google Scholar] [CrossRef]
- López, V.; Cásedas, G.; Petersen-Ross, K.; Powrie, Y.; Smith, C. Neuroprotective and anxiolytic potential of green rooibos (Aspalathus linearis) polyphenolic extract. Food Funct. 2022, 13, 91–101. [Google Scholar] [CrossRef]
- Gohari, A.; Hajimehdipoor, H.; Saeidnia, S.; Ajani, Y.; Hadjiakhoondi, A. Antioxidant activity of some medicinal species using FRAP assay. J. Med. Plants 2011, 10, 54–60. [Google Scholar]
- Ilaghi, M.; Sharifi, I.; Sharififar, F.; Sharifi, F.; Oliaee, R.T.; Babaei, Z.; Meimamandi, M.S.; Keyhani, A.; Bamorovat, M. The potential role and apoptotic profile of three medicinal plant extracts on Leishmania tropica by MTT assay, macrophage model and flow cytometry analysis. Parasite Epidemiol. Control 2021, 12, e00201. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive materials promote wound healing through modulation of cell behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef]
- Kučera, O.; Endlicher, R.; Roušar, T.; Lotková, H.; Garnol, T.; Drahota, Z.; Červinková, Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxidative Med. Cell. Longev. 2014, 2014, 752506. [Google Scholar] [CrossRef]
- Skóra, B.; Piechowiak, T.; Szychowski, K.A.; Gmiński, J. Entrapment of silver nanoparticles in L-α-phosphatidylcholine/cholesterol-based liposomes mitigates the oxidative stress in human keratinocyte (HaCaT) cells. Eur. J. Pharm. Biopharm. 2021, 166, 163–174. [Google Scholar] [CrossRef]
- Vasarri, M.; Bergonzi, M.C.; Leri, M.; Castellacci, R.; Bucciantini, M.; De Marchi, L.; Degl’Innocenti, D. Protective Effects of Oleanolic Acid on Human Keratinocytes: A Defense Against Exogenous Damage. Pharmaceuticals 2025, 18, 238. [Google Scholar] [CrossRef]
- Lee, B.M.; Woo, H.J.; Jang, B.J.; Shin, J.A.; Ham, Y.M.; Jang, E.B.; Kim, S.C.; Kim, J.M.; Shin, H.S. Assessment of Damnacanthus major Siebold & Zucc callus for antioxidative and moisturizing capacities using an artificial skin alternative. Biotechnol. Bioprocess Eng. 2024, 29, 689–698. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Panou, E.; Tsafantakis, N.; Zengin, G.; Graikou, K.; Ganos, C.; Fokialakis, N.; Chinou, I. Untargeted Metabolomic Profiling and Bioactivity Insights into Alkanna corcyrensis. Sci. Pharm. 2025, 93, 45. [Google Scholar] [CrossRef]
- Takaidza, S.; Mtunzi, F.; Pillay, M. Analysis of the phytochemical contents and antioxidant activities of crude extracts from Tulbaghia species. J. Tradit. Chin. Med. 2018, 38, 272–279. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Saleem, U.; Khalid, S.; Zaib, S.; Anwar, F.; Ahmad, B.; Ullah, I.; Zeb, A.; Ayaz, M. Phytochemical analysis and wound healing studies on ethnomedicinally important plant Malva neglecta Wallr. J. Ethnopharmacol. 2020, 249, 112401. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxidative Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.-N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef]
- Fisher, F.; Africa, C.; Klaasen, J.; Fisher, R. South African Medicinal Plants Traditionally Used for Wound Treatment: An Ethnobotanical Systematic Review. Plants 2025, 14, 818. [Google Scholar] [CrossRef]
- Ndlovu, B.; Klaasen, J.; Hughes, G.; Fisher, F. An ethnobotanical survey investigating medicinal plants used by Cape bush doctors to treat dermatophyte infections. Sci. Afr. 2024, 24, e02156. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of bacteria associated with wound infection by biocompatible green synthesized gold nanoparticles from South African plant extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Coricovac, D.; Pinzaru, I.; Marcovici, I.; Macasoi, I.G.; Semenescu, A.; Lazar, G.; Cinta Pinzaru, S.; Radulov, I.; Alexa, E. Rutin bioconjugates as potential nutraceutical prodrugs: An in vitro and in ovo toxicological screening. Front. Pharmacol. 2022, 13, 1000608. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, Y.; Zhai, H.; Wu, X.; Liu, A. Rutin alleviates psoriasis-related inflammation in keratinocytes by regulating the JAK2/STAT3 signaling. Ski. Res. Technol. 2024, 30, e70011. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The therapeutic wound healing bioactivities of various medicinal plants. Life 2023, 13, 317. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal plants and their effects on diabetic wound healing. Vet. World 2019, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Ali, S.; Khan, N.; Mishra, A.; Mishra, A. Wound healing potential of Cleome viscosa Linn. seeds extract and isolation of active constituent. South Afr. J. Bot. 2017, 112, 460–465. [Google Scholar] [CrossRef]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef]
- Selvakumar, G.; Lonchin, S. A bio-polymeric scaffold incorporated with p-Coumaric acid enhances diabetic wound healing by modulating MMP-9 and TGF-β3 expression. Colloids Surf. B Biointerfaces 2023, 225, 113280. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating inflammatory markers in wound healing: Understanding implications and identifying artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guan, Y.; Yang, L.; Fang, H.; Sun, H.; Sun, Y.; Yan, G.; Kong, L.; Wang, X. Ferulic Acid as an Anti-Inflammatory Agent: Insights into Molecular Mechanisms, Pharmacokinetics and Applications. Pharmaceuticals 2025, 18, 912. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Ridzuan, N.R.A.; Jalil, N.A.A. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef]
- Muzammil, S.; Neves Cruz, J.; Mumtaz, R.; Rasul, I.; Hayat, S.; Khan, M.A.; Khan, A.M.; Ijaz, M.U.; Lima, R.R.; Zubair, M. Effects of drying temperature and solvents on in vitro diabetic wound healing potential of Moringa oleifera leaf extracts. Molecules 2023, 28, 710. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, E.S.; Cordier, W.; Steenkamp, P.; Steenkamp, V. Effect of ethnomedicinal extracts used for wound healing on cellular migration and intracellular reactive oxygen species release in SC-1 fibroblasts. South Afr. J. Bot. 2018, 118, 11–17. [Google Scholar] [CrossRef]
- Lang, G.-P.; Han, Y.-Y. Rutin ameliorates H2O2-induced oxidative stress injury in HaCaT cells via the Nrf2-regulated pathway. J. Evol. Biochem. Physiol. 2022, 58, 1389–1400. [Google Scholar] [CrossRef]



| Treatment (1 mg/mL) | G. africana Ethanolic Extract | T. violacea Ethanolic Extract |
|---|---|---|
| Polyphenols (mg GAE/g) | 106.39 ± 2.03 | 78.57 ± 2.40 |
| Flavonoid content (mg QE/g) | 39.79 ± 0.27 | - |
| DPPH μmol TE/g | 187.42 ± 6.40 | 117.60 ± 6.33 |
| ORAC μmol TE/g | 1247.83 ± 12.53 | 172.82 ± 12.39 |
| FRAP μmol AAE/g | 195.91 ± 4.26 | 116.25 ± 12.57 |
| Plant Extract | Phytochemicals | Quantity (mg/L) | Retention Time (min) | Wavelength (nm) |
|---|---|---|---|---|
| G. africana | Ferulic acid | 1.88 | 22.227 | 320 |
| Rutin | 35.87 | 25.313 | 350 | |
| T. violacea | Rutin | 1.18 | 24.003 | 350 |
| Coumaric acid | 13.58 | 21.008 | 320 | |
| Ferulic acid | 1.88 | 22.185 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndlovu, B.; Fisher, R.; Fisher, F. Phenolic-Rich Extracts of Galenia africana and Tulbaghia violacea Accelerate Keratinocyte Migration and Mitigate Oxidative Stress to Enhance Wound Healing. Plants 2025, 14, 3523. https://doi.org/10.3390/plants14223523
Ndlovu B, Fisher R, Fisher F. Phenolic-Rich Extracts of Galenia africana and Tulbaghia violacea Accelerate Keratinocyte Migration and Mitigate Oxidative Stress to Enhance Wound Healing. Plants. 2025; 14(22):3523. https://doi.org/10.3390/plants14223523
Chicago/Turabian StyleNdlovu, Banele, Randall Fisher, and Farzana Fisher (née Rahiman). 2025. "Phenolic-Rich Extracts of Galenia africana and Tulbaghia violacea Accelerate Keratinocyte Migration and Mitigate Oxidative Stress to Enhance Wound Healing" Plants 14, no. 22: 3523. https://doi.org/10.3390/plants14223523
APA StyleNdlovu, B., Fisher, R., & Fisher, F. (2025). Phenolic-Rich Extracts of Galenia africana and Tulbaghia violacea Accelerate Keratinocyte Migration and Mitigate Oxidative Stress to Enhance Wound Healing. Plants, 14(22), 3523. https://doi.org/10.3390/plants14223523







