Abstract
Trifolium repens L. (white clover) is a widely distributed perennial legume, which is regarded as one of the most important forages for its high protein content and excellent palatability. Low temperature limits the distribution and productivity of white clover, thereby reducing its economic returns. WRKY transcription factors are key regulators in stress defense and are involved in multiple abiotic stress responses in plants. In this study, a cold inducible gene named TrWRKY41 was cloned from white clover. The TrWRKY41 protein is predominantly localized in the nucleus and functions as a hydrophilic, acidic protein. Under cold stress, the overexpression plants had significantly higher chlorophyll (CHL) and proline (Pro) contents, significantly increased activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD), and malondialdehyde (MDA) content significantly decreased. Compared to wild-type Arabidopsis thaliana, TrWRKY41-overexpressing plants exhibited better cold tolerance. In addition, target genes downstream of the TrWRKY41 transcription factor were predicted utilizing BLAST alignment and AlphaFold2 (version 0.2.0) software, the expression of six genes, including AtCOR47, AtCOR6.6, and AtABI5, was significantly up-regulated under cold stress. It suggests that TrWRKY41 may enhance cold tolerance in Arabidopsis by activating the ICE-CBF-COR cascade. This study provides candidate genes for research on enhancing the cold tolerance of white clover.
1. Introduction
White clover (Trifolium repens L.) is a perennial legume widely utilized as forage and silage due to its high protein content, nutritional value, and palatability, supporting livestock industry development [1]. Due to its short growth cycle and strong regenerative capacity, and incorporating white clover into lawns enhances visual appeal, thus providing distinct landscaping value [2]. White clover is widely distributed in temperate and cool-temperate regions, where its growth is frequently compromised by abiotic stresses particularly cold stress [3]. These limitations severely constrain yield and reduce economic viability.
Cold stress is a key climatic factor affecting crop growth, development and geographical distribution [4]. Low temperature constitutes a major stress factor limiting crop productivity and development. Prolonged exposure to low temperatures triggers physiological and biochemical changes in plant cells. For example, damage to the plasma membrane; changes in membrane lipid composition and alterations in the photosynthetic apparatus and electron flow in plants [5]. In order to survive under cold stress conditions, plants have developed a system of mechanisms to protect themselves from negative environmental attacks [6].
In recent years, an increasing number of studies have focused on the cold tolerance of perennial leguminous plants, such as alfalfa (Medicago sativa L.) and Glycyrrhiza glabra. For instance, the expression of the MsbHLH genes was significantly up-regulated under cold stress, and it is involved in the cold stress signaling transduction network in alfalfa [7]. Similarly, NAC transcription factors widely regulate plant growth and development [8]. Fifteen MsNAC genes were identified to actively respond to cold, salt, and drought stress [9]. Under cold stress, the expression of GgWRKY15, 53, and 54 was up-regulated, indicating a positive response to cold, whereas GgWRKY14 and 40 were down-regulated, suggesting their negative regulatory roles [10]. These findings highlight the significance of TFs in mediating cold adaptation, and provide a foundation for studying TFs function in white clover.
Transcription factors (TFs) play an important role in this complex gene regulatory network [11]. Transcription factors broadly participate in cellular differentiation and developmental processes. By binding to cis-acting elements, TF genes regulate the expression of downstream target genes through signal transduction pathways, enabling adaptation to environmental stresses. Prominent TF families include bZIP, MYB, WRKY, and AP2/ERF [12,13,14,15]. Among the cold response pathways, the Inducer of CBF Expression (ICE), C-repeat Binding Factors (CBF), and Cold-Regulated Genes (CORs) are key genes in the cold stress response [16]. Together, these genes constitute the ICE-CBF-COR signal transduction pathway, which mediates plant cold tolerance by transmitting stress signals and reducing physiological damage [17,18]. ICE positively regulates and induces the expression of CBF (DREB1 genes) [19]. Subsequently, CBF transcription factors bind to the cis-acting elements in the promoter regions of COR genes and induce their expression, thereby directly or indirectly regulating plant cold tolerance [20,21].
The WRKY family is found primarily in plants and constitutes one of the largest TF families in plants. WRKY transcription factors recognize and bind to the W-box cis-element (TTGACC/T) within target gene promoters, this molecular regulatory mechanism is capable of inducing changes at the plant physiological level, which enhances plant tolerance. WRKY proteins are zinc finger-type transcriptional regulators. Their name derives from the highly conserved WRKYGQK amino acid sequence at the N-terminus, and containing a conserved zinc finger motif (CX4–5CX22–23HXH or CX7CX23HXC) at the C-terminus [22]. Previous studies have shown that CsWRKY2 plays an important role in the stress response to cold and drought stresses by participating in the downstream ABA signaling pathway [23]. OsWRKY63 negatively regulates cold tolerance through the OsWRKY63-OsWRKY76-OsDREB1B cascade reaction in rice [24]. Banana fruit WRKY TFs improve crop cold tolerance by directly activating NECD expression [25]. Recent studies have demonstrated that overexpression of the grape WRKY transcription factor VhWRKY44 increased cold tolerance in Arabidopsis plants [26]. These studies collectively demonstrate the critical functions of WRKY TFs in regulating plant growth and stress adaptation.
In recent years, with the continuous advancement of genome sequencing technology, WRKY TFs have been identified in an increasing number of species. In 2023, the WRKY transcription factor family was first identified in white clover [27]. The TrWRKY gene family comprises a total of 145 members. Based on the evolutionary relationship of AtWRKYs, TrWRKY proteins can be divided into three major categories. Following 30 min of cold stress treatment, the expression of most TrWRKY genes was significantly upregulated, indicating a rapid response to cold stress. In addition, qRT-PCR analysis revealed that the expression levels of TrWRKY41, TrWRKY79, and TrWRKY100 initially increased and then decreased after cold treatment, indicating their responsiveness to early cold stress.
Despite the identification of the TrWRKY gene family, the specific functions of individual members, such as TrWRKY41, in cold tolerance remain largely unexplored. Based on previous research results, we selected the TrWRKY41 gene as the subject of our study to further explore its potential functions. To this end, we isolated TrWRKY41 from white clover and then expressed it heterotopically in the model plant Arabidopsis thaliana. This study obtained three transgenic lines and preliminarily explored the mechanism of action of the TrWRKY41 gene under cold stress. Our results provide potential genetic mechanisms for further studies of cold tolerance in white clover, as well as offering insights for molecular breeding.
2. Results
2.1. Cloning and Physicochemical Characterization of the TrWRKY41 Gene
TrWRKY41 was cloned from the Haifa variety white clover. It was amplified from white clover cDNA with the specific primers TrWRKY41-F and TrWRKY41-R (Table A1). The PCR amplified TrWRKY41 gene product was recovered and the product was detected by agarose gel electrophoresis. A specific target band of about 868 bp in length was obtained, and the results were as expected (Figure A1).
The physicochemical properties were further analyzed using the NCBI database as follows, the molecular formula of the protein encoded by TrWRKY41 is C1405H2250N408O453S14; the molecular weight is 32,554.62 kDa, and the theoretical isoelectric point is 6.90; its instability index is 44.24, inferring that the TrWRKY41 protein is an unstable acidic protein. TrWRKY41 is composed of 20 amino acids, with serine (Ser), threonine (Thr), and leucine (Leu) being the three most abundant residues. The protein contains 33 positively charged residues (Arg + Lys) and 34 negatively charged residues (Asp + Glu). Additionally, it exhibits an aliphatic index of 72.47, a theoretical half-life of 100 h, and an average hydrophilicity (GRAVY) value of −0.664. Subcellular localization prediction was performed by WoLF PSORT. The results showed that TrWRKY41 protein accounted for 61.0%, 34.1% and 4.9% in the nucleus, cell membrane and cytoskeleton, respectively. ProtScale analysis of the TrWRKY41-encoded protein revealed a hydrophobicity range of −2.811 to 1.878. Combined with the results in ProtParam, the TrWRKY41 protein was predicted to be hydrophilic.
2.2. Overexpression of TrWRKY41 in Arabidopsis Increases Cold Tolerance
In order to reveal the potential biological functions of TrWRKY41, both pMD18T-TrWRKY41 and pCAMBIA1300 plasmids were double-digested with PstI/BamHI restriction enzymes. Digestion products were gel purified separately. Subsequently, the digested fragments were ligated using T4 DNA ligase and transformed into E. coli DH5α competent cells via heat shock method. Plasmids extracted from positive clones were verified by agarose gel electrophoresis. The observed band sizes were as expected and consistent with the sequencing results (Figure A2). The correctly assembled plasmid was named pCAMBIA1300-TrWRKY41.
The recombinant plasmid was introduced into Agrobacterium tumefaciens GV3101 and then transformed into Arabidopsis. This resulted in six independent T0 transgenic lines (Figure 1). Genomic DNA extracted from leaves was subjected to PCR analysis. All six transgenic lines exhibited target bands matching the expected size, while no amplification was observed in wild-type plants (Figure 1). Three transgenic seedlings (Q1, Q4, and Q6) were finally obtained from TrWRKY41-overexpressing lines after multiple rounds of antibiotic screening (Figure 1). To further identify T3 generation positive seedlings, total leaf RNA was extracted and reverse transcribed to cDNA for qRT-PCR analysis. The results showed that the TrWRKY41 gene was not expressed in the wild-type, whereas the expression of TrWRKY41 in the transgenic lines (Q1, Q4, and Q6) was significantly higher than that in the control group, indicating that the gene was successfully transferred into Arabidopsis (Figure 1). The agarose gel electrophoresis results were further verified.
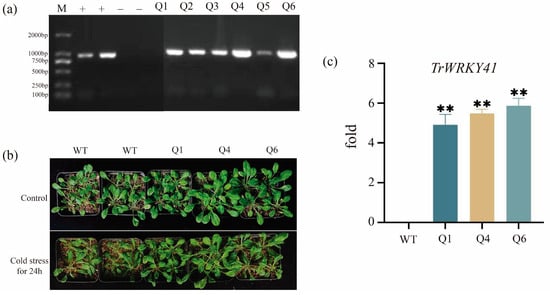
Figure 1.
Phenotypic analysis and validation of T3 generation positive transgenic arabidopsis thaliana under cold stress. (a) Six independent T0 transgenic Arabidopsis lines (Q1–Q6) were identified by PCR using the 2000 bp Marker with negative (−) and positive (+) controls. Target bands were amplified in all transgenic lines but not in wild-type. (b) Phenotypes of wild-type and TrWRKY41-overexpressing homozygous lines (Q1, Q4, Q6) after 24 h cold stress treatment. (c) Expression of TrWRKY41 in wild-type and transgenic lines (Q1, Q4, Q6) under cold stress, asterisks indicates a significant difference between groups (** p ≤ 0.01), and T-tests were used for intergroup comparisons. Expression data were calculated using the 2−ΔΔCT method.
To verify the function of TrWRKY41, four-week-old Arabidopsis seedlings were subjected to cold stress (4 °C) for 24 h. Compared to wild-type controls, T3 generation transgenic lines (Q1, Q4, and Q6) exhibited significantly reduced leaf wilting and sustained growth vigor, whereas WT plants showed severe growth retardation and conspicuous leaf dehydration. These findings suggest that heterologous expression of the TrWRKY41 enhances tolerance to cold stress in Arabidopsis.
2.3. Heterologous Expression of TrWRKY41 Gene Enhances Cold Tolerance in Arabidopsis
Both transgenic homozygous lines and wild-type plants were subjected to cold stress (4 °C). Subsequently, chlorophyll (CHL), malondialdehyde (MDA), and proline (Pro) contents, as well as the enzymatic activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD), were measured (Figure 2). The results showed that the three overexpression lines Q1, Q4, and Q6, exhibited significantly enhanced cold tolerance compared to the wild-type. Specifically, the chlorophyll content was significantly higher than that of the wild-type under cold stress. This indicates that chlorophyll degradation is reduced in the transgenic lines, thereby enhancing plant cold tolerance. The three transgenic lines showed significantly reduced malondialdehyde contents than the wild-type (p < 0.05), indicating less membrane damage and superior cold stress tolerance. Moreover, the proline contents of Q1, Q4, and Q6 were significantly higher than wild-type (p < 0.05), suggesting that TrWRKY41 was able to enhance cold stress tolerance by accumulating more proline. CAT, POD and SOD are crucial antioxidant enzymes in plants, which are capable of removing ROS generated under cold stress, and thus protecting plant cells from oxidative damage. Under cold stress, the activities of all three antioxidant enzymes were significantly increased in the transgenic plants. This suggests that the TrWRKY41 gene removes ROS and reduces oxidative stress by increasing the activity of antioxidant enzymes. In summary, TrWRKY41 enhances cold stress tolerance through changes in physiological and biochemical indicators in Arabidopsis.
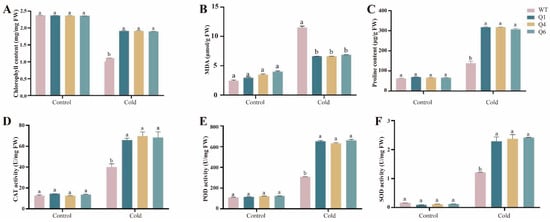
Figure 2.
Physiological and biochemical indicators of transgenic Arabidopsis under cold stress. From left to right: wild-type Arabidopsis, homozygous lines Q1, Q4, and Q6. (A) Chlorophyll (CHL) content; (B) Malondialdehyde (MDA) content; (C) Proline (Pro) content; (D) Catalase (CAT) activity; (E) Peroxidase (POD) activity; (F) Superoxide dismutase (SOD) activity. Each sample was repeated three times. Duncan’s test was used for intergroup comparisons, with different letters indicating significant differences between different groups (p < 0.05).
2.4. TrWRKY41 Transcription Factor Binding Site and Structure Prediction
To investigate the cold tolerance function of TrWRKY41, identifying its downstream target genes is necessary. In this study, we defined the 2000 bp upstream region of the Arabidopsis genomics as the promoter, and identified multiple potential WRKY binding sites by the core motif TTGAC [C/T]. The 20 sites with the highest frequency of occurrence were selected for the next study. (Table A2). Further analysis of the sequence shows that the WRKY core motif TTGAC [C/T] is highly enriched. This indicates that this motif is evolutionarily conserved, which is consistent with the results of previous studies. Subsequently, we performed BLAST alignments of Arabidopsis promoter sequences using the 20 motifs as query sequences. Finally, we randomly selected 30 sequences and used AlphaFold2 software to predict the structure of TrWRKY41 target genes (Table 1). All TrWRKY41 protein-target gene structures had ipTM ≥ 0.8, indicating that the prediction results were highly reliable and credible [28]. Eight of the predicted results were selected for visualization using Chimera X (Figure 3). In summary, we found that TrWRKY41 directly binds to the W-box region of the target gene promoter, forming a stable complex to mediate its regulatory effects in specific biological processes.

Table 1.
Prediction of binding sites between TrWRKY41 and target genes.
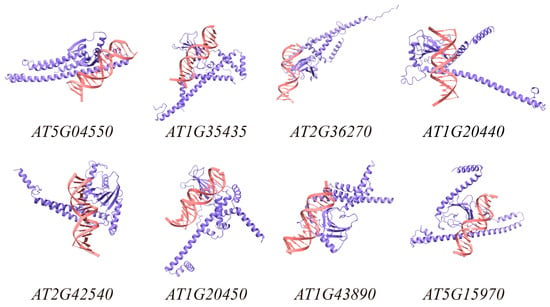
Figure 3.
Structural prediction of TrWRKY41 protein and target genes. Purple represents TrWRKY41 protein, and pink represents the double strand of target gene DNA. The results were visualized with Chimera X (version 1.9) software.
2.5. qRT-PCR Analysis of TrWRKY41 Downstream Target Genes Under Cold Stress
WRKY transcription factors precisely activate or suppress downstream gene expression within complex regulatory networks. AtCOR47, AtCOR6.6, AtABI5, AtRAB18, AtCOR15A and AtERD10 are reported cold stress response genes, and based on the prediction of cis-acting elements in the promoter region of Arabidopsis, we focused on studying these six key WRKY-regulated downstream genes. First, overexpressing TrWRKY41 plants and the wild-type were subjected to cold stress (4 °C) for 24 h. The expression levels of these six key genes were significantly up-regulated in the TrWRKY41 transgenic lines, and were significantly higher than in wild-type Arabidopsis (Figure 4). In summary, WRKY transcription factors respond to cold stress by specifically binding to W-box sequences in target gene promoters, thereby delicately regulating downstream genes expression. In contrast, overexpression of the TrWRKY41 gene was able to significantly up-regulate the expression levels of cold stress-related genes, providing plants with a stronger low-temperature protection mechanism.
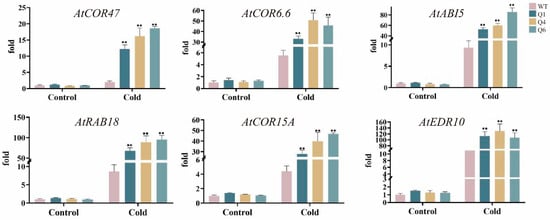
Figure 4.
Expression analysis of downstream target genes in TrWRKY41-overexpressing lines under cold stress. X-axis: Control group and cold stress treatment group (with 3 replicates each). The different colors in the bar graph from left to right represent WT, Q1, Q4, and Q6 plants, respectively. Y-axis: Relative gene expression levels. The asterisk indicates a significant difference between the two groups. Statistical significance was determined using t-tests (** p < 0.01). WT expression was normalized. Expression levels were calculated using the 2−ΔΔCT method.
3. Discussion
During growth and development, plants are subjected to a variety of stresses. In order to survive and reproduce, plants have adapted to adversity through the evolution of their morphology and the regulation of their metabolic levels [29]. WRKY TFs are a key category of regulatory factors, which participate extensively in various physiological and biochemical processes. Previously, numerous studies have demonstrated the broad involvement of WRKY transcription factors in plant secondary metabolism. This involvement occurs in response to abiotic and biotic stresses, senescence, seed dormancy, and germination [30]. Therefore, studying the pivotal role of TrWRKY genes under cold stress is crucial for white clover breeding and crop improvement.
In this study, the TrWRKY41 gene was successfully isolated from the white clover genome by homologous cloning. Its function was analyzed by Agrobacterium-mediated transformation into Arabidopsis plants. After 24 h of low-temperature treatment, the transgenic plants showed insignificant leaf damage and significantly better growth than the controls, and the expression of TrWRKY41 gene was significantly increased, indicating the ability to respond positively to cold stress and less plant stress damage (Figure 1).
Cold stress also causes an influence on the metabolic level of the plant, so the measurement of physiological indicators is an important tool in the study of plant tolerance. Chlorophyll content is used to characterize plant growth [31]. Chlorophyll is the most essential tool for plants to capture light. Photosynthesis is highly susceptible to cold stress [32]. Studies have shown that cold stress inhibits PSII activity and reduces maximum photochemical efficiency in tobacco [33]. Excess malondialdehyde damages cell membranes. The more MDA accumulates, the more severe the membrane damage becomes [34]. Accumulation of osmotic substances contributes to plant tolerance to stress, and proline plays a critical role in regulating osmotic homeostasis [35]. Research has shown that free radicals and reactive oxygen species cause oxidative damage and tissue dysfunction [36]. Therefore, the removal of excess reactive oxygen species is essential to maintain the vital activities of the organism. CAT, POD and SOD are believed to scavenge reactive oxygen species and are considered as key physiological indicators in abiotic stress studies. Previously, the expression of the CsWRKY21 gene in tea tree was significantly increased 6-times under cold stress [37]. Under cold stress conditions, the PmWRKY57 gene overexpressing plants showed significantly higher superoxide dismutase and peroxidase enzyme activities, as well as higher proline content, compared with the wild-type. This significantly improved the cold tolerance in Arabidopsis [38]. Expression of VvWRKY28 significantly increased under cold stress and enhanced cold tolerance in Arabidopsis plants. The activities of SOD, POD and CAT were increased while MDA content was decreased after treatment [39]. In this study, we determined the physiological indexes under cold stress. A number of studies have shown that WRKY genes can increase cold tolerance by increasing chlorophyll content, such as VhWRKY44, BcWRKY46 and so on [26,40]. The results showed that the chlorophyll content of the transgenic lines was higher than the control, indicating that TrWRKY41 may enhance the cold tolerance by regulating the expression of the CHLH genes in Arabidopsis (Figure 2) [41]. We also found that MDA content was reduced and proline content was raised in the transgenic plants, suggesting that the TrWRKY41 gene enhances cold tolerance by enhancing cell membrane stability and regulating osmotic pressure. Moreover, CAT, POD and SOD activities were significantly higher than those of the control group, which was consistent with the results of previous studies (Figure 2). The above results emphasized that TrWRKY41 gene plays an important role in plant response to cold stress.
Different WRKY transcription factors regulate different mechanisms. Certain WRKY transcription factors can bind to a cis-acting element (W-box) in the promoter region of a downstream target gene, thereby activating gene expression [42]. To clarify the regulatory mechanism of TrWRKY41 and identify its downstream target genes, we extracted genome-wide promoters from Arabidopsis. We screened 30 downstream target genes of TrWRKY41 by recognition and alignment of W-box elements in the promoter. Protein-DNA complex structures were predicted using AlphaFold2. Prediction accuracy was evaluated using the interface prediction template modelling (ipTM) score; all models achieved an ipTM ≥ 0.8, demonstrating their reliability [28]. Interestingly, the presence of W-box motifs in target gene sequences promoted interaction with TrWRKY41 (Figure 3).
Studies have shown that WRKY transcription factors are broadly involved in various biological processes. For example, AT1G20450, AT1G20440, AT2G42540, and AT5G15970 are all cold-regulated genes [39]. AT2G36270 participates in ABA signal transduction during seed maturation and germination and positively responds to drought stress [43,44]. AT1G67320, AT5G04550, and AT2G44140 exhibit enzymatic activity [45]. AT2G39350 was identified as an ABC transporter and is involved in substrate transport processes [46]. AtCOR47, AtABI5, AtRAB18, AtCOR15A and AtERD10 are reported cold stress response genes [39]. The ICE-CBF-COR cascade is a critical regulatory pathway in plant cold tolerance. This cascade pathway contains three principal components, which synergistically enhance plant cold tolerance [47]. Phylogenetic analysis indicates that the CBF gene structure is highly conserved across both monocotyledons and dicotyledons [48]. Research indicates that CBF genes regulate cold stress responses by binding to the promoter region of the COR15A gene in Arabidopsis [49]. Overexpression of CBF/DREB enhances cold tolerance in Arabidopsis [50]. Numerous transcription factors can mediate the regulation of the ICE-CBF-COR cascade. For instance, overexpression of MYB15 leads to reduced expression of CBF genes, thereby diminishing Arabidopsis cold tolerance [51]. In wheat, TaMYC2 interacts with TaICE41 to activate the downstream CBF-COR pathway, thereby enhancing the plant’s cold tolerance [47]. This study showed that overexpression of TrWRKY41 significantly upregulated the expression levels of AtCOR47, AtCOR15A and AtERD10, thereby enhancing the cold tolerance of Arabidopsis (Figure 4). It is hypothesized that TrWRKY41 may improve Arabidopsis cold tolerance by activating the ICE-CBF-COR cascade. Based on the above, we have constructed a schematic diagram illustrating the mechanism through which WRKY TFs regulates downstream genes in response to cold stress (Figure 5).
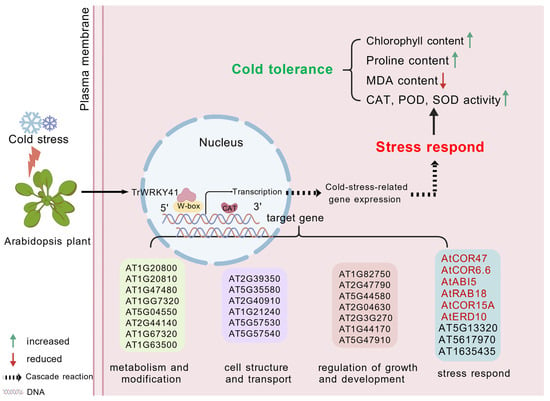
Figure 5.
Mechanism of WRKY transcription factor response to cold stress.
WRKY transcription factors serve as essential components in plant signal transduction pathways. The introduction of TrWRKY41 into Arabidopsis can significantly improve cold tolerance. The results of target gene prediction further improved the mechanism of TrWRKY41 in the model organism Arabidopsis thaliana. This study provides new candidate genes for research on the cold tolerance of white clover, and enrichment of germplasm resources. Although this study investigated the function of TrWRKY41 after its heterologous expression in Arabidopsis plants, due to differences in the genetic background and physiological characteristics between Arabidopsis and white clover, the mechanism of action of TrWRKY41 in its native organism remains to be explored. Furthermore, this study was limited to laboratory conditions, which differ from the complex field environment. Future research will further deepen the functional investigation of the TrWRKY41 gene in white clover.
4. Materials and Methods
4.1. Plant Material and Growing Conditions
Uniformly sized and full grains of white clover (cultivar Haifa, purchased from Barenbrug China Ltd. Com. Beijing, China) were selected as the experimental material. Seeds were vernalized at 4 °C for 2–3 days to enhance germination rates. Before sowing, the seeds were surface-sterilized with 75% (v/v) alcohol and 10% (v/v) sodium hypochlorite. Germinated seeds were sown in a substrate of perlite and sand at a volume ratio of 3:1 [27]. All seedlings were then placed in an environmental growth chamber set to a light intensity of 100 µmol/(m2·s) and a 16 h (24 °C): 8 h (18 °C) cycle. They were irrigated with Hoagland nutrient solution every 2 days until the second leaf was fully expanded.
To validate the effect of WRKY transcription factors on cold tolerance in white clove, four-week-old seedlings were randomly divided into four groups and treated at 4 °C for 0 min, 30 min, 1 h, and 3 h [52]. Leaves were subsequently collected. Each sample comprised five seedlings, and all samples were frozen in liquid nitrogen and stored at −80 °C.
Arabidopsis thaliana Columbia was selected as wild-type growth for genetic transformation. The similar growth protocol was adopted in Arabidopsis growth, such as light intensity and temperature condition. Seedlings were irrigated with distilled water every 3–4 days. Four weeks after germination, switch to watering with Hoagland nutrient solution every two weeks.
4.2. Isolation and Cloning of the TrWRKY41 Gene
Total RNA of white clover was detected from the leaf samples using an RNAprep Pure PlantKit (Tiangen Biotech Co., Ltd., Beijing, China). The RNA was then reverse transcribed into cDNA by PrimeScript RTkit (Dongbao, Shanghai, China), and the obtained cDNA templates were divided and stored at −20 °C in the fridge. Based on the nucleotide sequence of TrWRKY41, a pair of primers TrWRKY41-F: AAACTGCGTCAACAATTTTT and TrWRKY41-R: CTTGTCCACAGTGCAATACAT was designed using Primer3 (version 4.1.0) software. These primers were used to amplify the full-length TrWRKY41 cDNA by PCR. The purified PCR product was ligated into the pMD™18-T Vector using the Cloning Kit and sequenced. NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (accessed on 25 August 2024) software were utilized to compare the homologous amino acid sequences of TrWRKY41 proteins. To exclude the influence of Arabidopsis homologous genes, the MEGA11 (version 11.0) software was used to construct a phylogenetic tree of TrWRKY41 and the Arabidopsis WRKY gene family. The phylogenetic tree was constructed using the neighbor-joining (NJ) method with the bootstrap replicates set to 1000 [53].
4.3. Construction and Transformation of Plant Expression Vectors
Both the pMD18T-TrWRKY41 plasmid and the pCAMBIA1300 plasmid were double-digested with PstI/BamHI. The digested fragments were then separately purified. The digested fragments were ligated using T4 DNA ligase at 16 °C for 4–5 h. The ligation product was then transformed into E. coli DH5α competent cells by the heat-shock method. After overnight incubation in LB medium containing kanamycin antibiotic, single colonies that were successfully identified were picked and incubated on a shaker at 37 °C at 200 rpm for 12 h. The plasmid was extracted and sequenced, and the correct plasmid was named pCAMBIA1300-TrWRKY41. The recombinant plasmid was introduced into Agrobacterium tumefaciens GV3101 by freeze–thaw method.
Four-week-old Arabidopsis plants were selected, and the already-flowering inflorescences and pods were removed with shears. The unflowering but whitish inflorescences were sequentially immersed in the resuspension solution for about 30 s [54]. Following watering, plants were incubated in darkness for 24 h before being transferred to standard light growth conditions. T0 generation seeds were subsequently harvested. Transformant seeds were germinated in MS (0.5×) medium (containing 50 mg/L kanamycin) to identify tolerance plants. After 14 days, the healthy seedlings were transferred to the above greenhouse soil conditions for further cultivation. The DNA of Arabidopsis leaves was used as template for PCR validation. The samples with amplified products of the expected size were sent to BGI Genetics for sequencing. The above methods were used until the T3 generation positive transgenic plants were selected for the experiment. T3 generation positive plants and wild-type Arabidopsis were sown in nutrient soil at a ratio of 1:1 and watered regularly with Hoagland nutrient solution (Solution A: 0.945 g/L, Solution B: 1.240 g/L).
4.4. Measurement of Relevant Physiological Indicators
Wild-type and homozygous T3 lines Arabidopsis seeds were sterilized and cleaned. Seeded in solid MS (0.5×) medium, vernalized for 3 days at 4 °C, then grown in a light chamber for four weeks, see previous described [55]. There were two chambers in Arabidopsis analysis, one chamber was set as control condition, with 24/18 °C across experiments, and another chamber was cold stress condition, with four weeks normal condition, and the temperature was set as 4 °C for simulating cold stress. In each chamber, there were five groups of Arabidopsis in total, three independent transgenic lines and two control groups (all of them were WT Arabidopsis lines). After four-weeks, the control condition was set with normal temperature, while cold stress chamber was set with 4 °C, 24 h later, considering cost of experiments, four groups of Arabidopsis from each chamber, one WT group and three transgenic lines, were harvested for RNA detection and physiological analysis. All samples were then rapidly frozen in liquid nitrogen and stored in a −80 °C freezer. qRT-PCR analysis was used to measure the expression of the TrWRKY41 gene in homozygous lines. The reaction conditions and system for qRT-PCR followed the operating procedures of SYBR PreMix Ex Taq™ II (Toyobo, Shanghai, China), and three biological replicates per group GADPH is used as an internal reference gene. Use the T-test for intergroup comparisons (** p ≤ 0.01). Expression data were calculated using the 2−ΔΔCT method [56].
In addition, the above-mentioned Arabidopsis samples were also used to the determination of chlorophyll (CHL), proline (Pro), malondialdehyde (MDA), catalase (CAT), peroxidase (POD), superoxide dismutase (SOD) content. The Chlorophyll content was determined using the acetone extraction method [57]. The MDA content, an indicator of lipid peroxidation, was measured using the thiobarbituric acid (TBA) method. Briefly, fresh leaf sample was ground in liquid nitrogen and homogenized in 0.9 mL of extraction buffer containing 50 mM phosphate buffer (pH 7.4) and 1% polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 3500 rpm for 10 min at 4 °C. The supernatant was collected, and its absorbance was measured at 532 nm using a UV spectrophotometer (Thermo, Halios Beta, Waltham, MA, USA). The MDA content was calculated according to the standard protocol [58]. For the extraction of SOD and POD, leaf sample was ground in liquid nitrogen using a pre-chilled mortar and pestle, followed by homogenization in 3.0 mL of extraction buffer consisting of 50 mM phosphate buffer (pH 7.8) and 1% PVP. The homogenate was centrifuged at 10,000 rpm for 20 min. The resulting supernatant was used for enzyme activity assays. SOD activity was determined according to the method of Li et al. (2013), and one unit SOD activity (U) was defined as the quantity of SOD required to produce 50% inhibition of the reduction of nitrite in 1 mL reaction solution by measuring the change of absorbance at 550 nm [58]. POD activity was assayed in a reaction mixture containing 50 mM phosphate buffer (pH 7.0), 0.2% guaiacol, 0.3% H2O2, and enzyme extract. The activity was measured by monitoring the increase in absorbance at 470 nm due to guaiacol oxidation. One unit of POD activity was defined as an increase in absorbance of 0.01 per minute [59]. CAT activity was determined by tracking the decrease in absorbance at 405 nm resulting from H2O2 decomposition via the molybate method. One unit of CAT activity was defined as the amount of enzyme that decomposes 1 μmol of H2O2 per second [58]. All enzyme activities were measured using a microplate reader (Thermo, Halios Beta, Waltham, MA, USA). Each experiment was performed with three independent replicates. To compare differences within and between groups, statistical analysis was performed on the data. Analysis of variance (ANOVA) was performed using R (version 4..4.2) software, followed by Duncan’s test (p < 0.05) for assessment. Different letters indicate significant differences.
4.5. Identification of TrWRKY41 Transcription Factor (W-Box) and Target Gene Prediction
To further reveal the regulatory mechanisms of WRKY transcription factors on downstream target genes in Arabidopsis, we performed a whole-gene scan and comparison of Arabidopsis promoter regions. First, the Arabidopsis upstream 2000 bp sequence was downloaded from the TAIR database (https://www.arabidopsis.org/). (accessed on 11 March 2025) These sequences were defined as promoter regions. W-box elements are short and highly conserved. Therefore, the regular expression matching algorithm is used to identify proteins containing the core sequence TTGAC [C/T]. The 20 most frequently occurring motifs were selected as representative W-box elements. To enhance prediction accuracy and match proteins with complete W-box elements, we extended the upstream and downstream regions of the element by 4 bp (with downstream padding as needed) based on miRNA length (20–24 nt), resulting in a 17-bp extended sequence [60]. Subsequently, the extracted Arabidopsis promoter regions were subjected to BLAST alignment, using an E-value threshold of 2 to find downstream target genes [61]. Initial alignments permitted a sequence length variation of 2–6 nt and could tolerate a maximum of two internal mismatches [62]. Based on the comparison results, 30 sequences containing W-box elements were randomly selected, and their potential interactions with TrWRKY41 were predicted using AlphaFold2 (version 0.2.0) software [63]. Results were visualized using Chimera X (version 1.9) software [64]. In addition, to comprehensively analyze TrWRKY41 function, downstream target genes were functionally classified based on annotation data from the TAIR database.
4.6. Expression Analysis of WRKY-Regulated Downstream Target Genes in Arabidopsis
Total RNA was extracted from WT and transgenic lines using the RNA pure Plant Kit (Tiangen, Beijing, China), as Section 4.4 described. Subsequently, cDNA was synthesized using the PrimeScript RT kit (Toyobo, Shanghai, China) and used as a template for quantitative reverse transcription PCR (qRT-PCR). All operations were performed according to the manufacturer’s instructions. Six candidate genes, namely AtCOR47, AtCOR6.6, AtABI5, AtRAB18, AtCOR15A, AtERD10, were selected for further validation, and six pairs of primers were designed based on their nucleic acid sequences (Table A1). Each biological sample performed three technical replicates. The results were normalized. Subsequently, the T-test was used to compare the groups, with asterisks indicating significant differences between groups (** p ≤ 0.01). Three replicates were set for each sample. Expression data were calculated using the 2−ΔΔCT method [56].
5. Conclusions
In this study, we successfully cloned the TrWRKY41 gene of white clover. The biological function and mechanism of this gene in response to low temperature stress in Arabidopsis were revealed. TrWRKY41 protein is an unstable acidic protein, mainly distributed in the nucleus, and is a hydrophilic protein. Compared to WT plants, TrWRKY41-overexpressing plants exhibited significantly elevated chlorophyll and proline content, markedly enhanced antioxidant enzyme activity, and substantially reduced malondialdehyde levels. This indicates that TrWRKY41 enhances Arabidopsis cold tolerance by mitigating cell membrane damage, regulating osmotic pressure, and boosting antioxidant capacity. AlphaFold2 prediction results indicate that the TrWRKY41 gene can interact with the W-box element of target genes, thereby performing specific biological functions. Furthermore, qRT-PCR results indicate that the expression levels of cold-response genes (such as AtCOR15A and AtERD10) were increased in the overexpressing plants. It is hypothesized that the TrWRKY41 gene enhances Arabidopsis cold tolerance by participating in the ICE-CBF-COR cascade. This study fully analyzed the physicochemical properties of the TrWRKY41, aiming to understand its cold tolerance mechanism from structural and functional perspectives. Furthermore, this analysis provides insights enabling deeper exploration of WRKY TFs functions.
Author Contributions
Conceptualization, C.G. and Y.S.; Data curation, M.G., M.L. and X.Z.; Formal analysis, M.G. and S.L.; Funding acquisition, M.G., C.G. and Y.S.; Investigation, M.G., S.L., J.T., M.L. and X.Z.; Methodology, M.G., S.L. and M.L.; Project administration, Y.S.; Resources, X.Z. and C.G.; Software, M.G., S.L., J.T. and X.Z.; Supervision, Y.S.; Validation, M.G., J.T. and M.L.; Visualization, J.T.; Writing—original draft, M.G. and Y.S.; Writing—review and editing, C.G. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Harbin Normal University Postgraduate Innovation Project (grant number HSDSSCX2025-47), Heilongjiang Provincial Natural Science Foundation of China (grant number LH2022C050) and Natural and Science Foundation of China (grant number U21A20182).
Data Availability Statement
The datasets presented in this study can be found in Appendix A and Appendix B.
Acknowledgments
We are grateful to the high-performance computing center of Harbin Normal University for the support with our analysis work.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Appendix A.1

Table A1.
Primers for TrWRKY41 and target genes.
Table A1.
Primers for TrWRKY41 and target genes.
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| TrWRKY41-F | AAACTTGCGTCAACAATTTTT |
| TrWRKY41-R | CTTGTCCACAGTGCAATACAT |
| qTrWRKY41-F | GCTATGCTATTGCTGAGAGC |
| qTrWRKY41-R | CCTAGGAGAGGGGTTATCTC |
| qAtCOR47-F | GAAACCTCAAGAGACAACGA |
| qAtCOR47-R | AGAAGAGCTGTTGGATCGG |
| qAtCOR6.6-F | AGAGACCAACAAGAATGCC |
| qAtCOR6.6-R | TGTCCTTCACGAAGTTAACAC |
| qAtABI5-F qAtABI5-R | TCCAGTGGAGAAAGTAGTGG AGGTGTCTTTACCTGTTGCT |
| qAtRAB18-F | GTCAGGACAACCCGAATTT |
| qAtRAB18-R | ATACGAACTTGTTAGCGTCC |
| qAtCOR15A-F | GAACAAGCCTAGTGTCATCG |
| qAtCOR15A-R | ATCATCCTCTGCTGTCTTGT |
| qAtERD10-F | ACATTCGGTAGAGGATCACA |
| qAtERD10-R | TTCCTCTCCAGTGGTCTTG |
Appendix A.2

Table A2.
Top 20 most frequent W-box extended motifs (17 bp).
Table A2.
Top 20 most frequent W-box extended motifs (17 bp).
| Motif Name | Sequence (5′-3′) |
|---|---|
| W-box 1 | AAAGGATTTTGACCAGA |
| W-box 2 | ATTTATTTGTTTTGTTT |
| W-box 3 | CAAAGTTGACTATCATT |
| W-box 4 | AGTGGATTTTGACCATG |
| W-box 5 | ATTGATTGACTGAGAAT |
| W-box 6 | AGTAGTTGACTATCACA |
| W-box 7 | CTCCATTGACCCAGAGA |
| W-box 8 | GTTACTTGACTCGAAAC |
| W-box 9 | GATGAAGCTTGACTCAA |
| W-box 10 | GTTAGGATTTTGACCAC |
| W-box 11 | TCTTGTTCGATTGACCG |
| W-box 12 | TAACTAGTTATATAATG |
| W-box 13 | ACGTTTTGACCACTGTT |
| W-box 14 | CTATTCGATTGACCAAA |
| W-box 15 | TTGATACATTGACCCAT |
| W-box 16 | GATAACTTGACTCGTCT |
| W-box 17 | TTTTACATTGACCCCAG |
| W-box 18 | TTAATTTTGACCACTCA |
| W-box 19 | GGAAATTTTGACCACTG |
| W-box 20 | TACAATTGACAATCTTT |
Appendix B
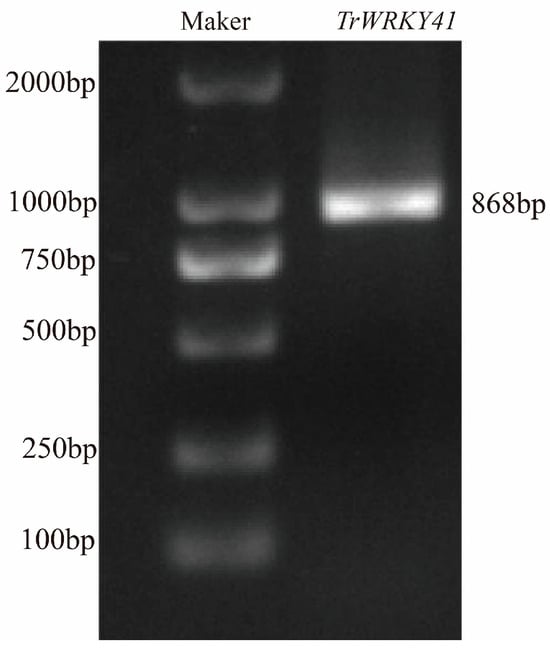
Figure A1.
PCR amplification of the full-length TrWRKY79 from white clover cDNA. Using white clover cDNA as the template, and TrWRKY41 was amplified with gene-specific primers TrWRKY41-F and TrWRKY41-R. The PCR products were separated and verified by agarose gel electrophoresis.
Figure A2.
The purified PCR product was ligated into the pMD18-T Simple vector and transformed into E. coli DH5α competent cells. Single colonies were screened by PCR, and results showed amplicons of the expected size, consistent with sequencing data.
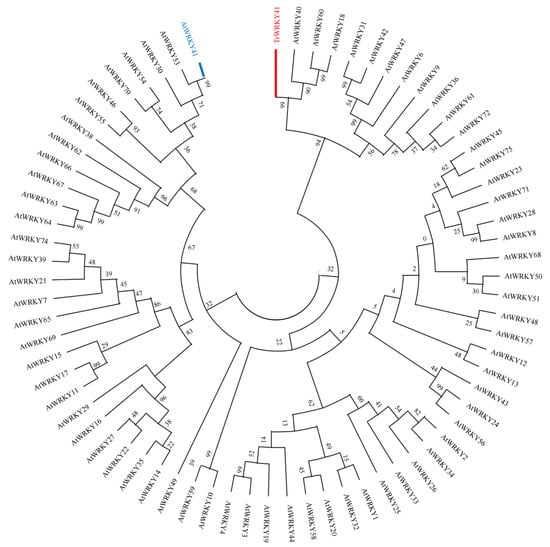
Figure A3.
Phylogenetic analysis of Arabidopsis WRKY gene family members and TrWRKY41. The phylogenetic tree was constructed using the neighbour-joining (NJ) method and subjected to 1000 bootstrap iterations. Among them, the AtWRKY41 and TrWRKY41 proteins are represented by blue and red, respectively.
References
- Vaseva, I.; Akiscan, Y.; Demirevska, K.; Anders, I.; Feller, U. Drought stress tolerance of red and white clover–comparative analysis of some chaperonins and dehydrins. Sci. Hortic. 2011, 130, 653–659. [Google Scholar] [CrossRef]
- Jia, T.; Tang, T.; Cheng, B.; Li, Z.; Peng, Y. Development of two protocols for Agrobacterium-mediated transformation of white clover (Trifolium repens) via the callus system. 3 Biotech 2023, 13, 150. [Google Scholar] [CrossRef]
- Wu, F.; Ma, S.; Zhou, J.; Han, C.; Hu, R.; Yang, X.; Nie, G.; Zhang, X. Genetic diversity and population structure analysis in a large collection of white clover (Trifolium repens L.) germplasm worldwide. PeerJ 2021, 9, e11325. [Google Scholar] [CrossRef] [PubMed]
- Boinot, M.; Karakas, E.; Koehl, K.; Pagter, M.; Zuther, E. Cold stress and freezing tolerance negatively affect the fitness of Arabidopsis thaliana accessions under field and controlled conditions. Planta 2022, 255, 39. [Google Scholar] [CrossRef]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol. Biochem. PPB 2023, 197, 107646. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of plants to overcome abiotic and biotic stresses. Biol. Rev. Camb. Philos. Soc. 2024, 99, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jin, L.; Sheng, S. Genome-Wide Identification of bHLH Transcription Factor in Medicago sativa in Response to Cold Stress. Genes 2022, 13, 2371. [Google Scholar] [CrossRef]
- Marques, D.N.; dos Reis, S.P.; de Souza, C.R.B. Plant NAC transcription factors responsive to abiotic stresses. Plant Gene 2017, 11, 170–179. [Google Scholar] [CrossRef]
- He, F.; Zhang, L.; Zhao, G.; Kang, J.; Long, R.; Li, M.; Yang, Q.; Chen, L. Genome-Wide Identification and Expression Analysis of the NAC Gene Family in Alfalfa Revealed Its Potential Roles in Response to Multiple Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 10015. [Google Scholar] [CrossRef]
- Goyal, P.; Manzoor, M.M.; Vishwakarma, R.A.; Sharma, D.; Dhar, M.K.; Gupta, S. A Comprehensive Transcriptome-Wide Identification and Screening of WRKY Gene Family Engaged in Abiotic Stress in Glycyrrhiza glabra. Sci. Rep. 2020, 10, 373. [Google Scholar] [CrossRef]
- Weidemüller, P.; Kholmatov, M.; Petsalaki, E.; Zaugg, J.B. Transcription factors: Bridge between cell signaling and gene regulation. Proteomics 2021, 21, e2000034. [Google Scholar] [CrossRef] [PubMed]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family-an update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Frangedakis, E.; Yelina, N.E.; Billakurthi, K.; Hua, L.; Schreier, T.; Dickinson, P.J.; Tomaselli, M.; Haseloff, J.; Hibberd, J.M. MYB-related transcription factors control chloroplast biogenesis. Cell 2024, 187, 4859–4876.e4822. [Google Scholar] [CrossRef]
- Javed, T.; Gao, S.J. WRKY transcription factors in plant defense. Trends Genet. TIG 2023, 39, 787–801. [Google Scholar] [CrossRef]
- Feng, K.; Hou, X.L.; Xing, G.M.; Liu, J.X.; Duan, A.Q.; Xu, Z.S.; Li, M.Y.; Zhuang, J.; Xiong, A.S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- Mehrotra, S.; Verma, S.; Kumar, S.; Kumari, S.; Mishra, B.N. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020, 180, 104243. [Google Scholar] [CrossRef]
- Shu, Y.; Li, W.; Zhao, J.; Zhang, S.; Xu, H.; Liu, Y.; Guo, C. Transcriptome sequencing analysis of alfalfa reveals CBF genes potentially playing important roles in response to freezing stress. Genet. Mol. Biol. 2017, 40, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ren, Y.; Tang, Z.; Shi, W.; Zhou, M. Characterization and expression profiling of the ICE-CBF-COR genes in wheat. PeerJ 2019, 7, e8190. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Medina, J.; Catalá, R.; Salinas, J. The CBFs: Three arabidopsis transcription factors to cold acclimate. Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 3–11. [Google Scholar] [CrossRef]
- Song, H.; Guo, Z.; Duan, Z.; Li, M.; Zhang, J. WRKY transcription factors in Arachis hypogaea and its donors: From identification to function prediction. Plant Physiol. Biochem. PPB 2023, 204, 108131. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, Z.; Wang, W.; Jiang, X.; Li, D.; Pan, J.; Li, X. CsWRKY2, a novel WRKY gene from Camellia sinensis, is involved in cold and drought stress responses. Biol. Plant. 2016, 60, 443–451. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Huang, K.; Huang, S.; Wang, H.; Wei, Z.; Li, Z.; Bian, M.; Jiang, W.; Wu, T.; et al. The OsWRKY63–OsWRKY76–OsDREB1B module regulates chilling tolerance in rice. Plant J. 2022, 112, 383–398. [Google Scholar] [CrossRef]
- Luo, D.L.; Ba, L.J.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Involvement of WRKY Transcription Factors in Abscisic-Acid-Induced Cold Tolerance of Banana Fruit. J. Agric. Food Chem. 2017, 65, 3627–3635. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a Grape WRKY Transcription Factor VhWRKY44 Improves the Resistance to Cold and Salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Zhang, T.; Bai, Y.; Chen, C.; Guo, D.; Guo, C.; Shu, Y. Genome-wide analysis of the WRKY genes and their important roles during cold stress in white clover. PeerJ 2023, 11, e15610. [Google Scholar] [CrossRef]
- Varga, J.K.; Ovchinnikov, S.; Schueler-Furman, O. actifpTM: A refined confidence metric of AlphaFold2 predictions involving flexible regions. Bioinformatics 2025, 41, btaf107. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ma, Z.; Hu, L.; Huang, K.; Zhang, M.; Zhang, S.; Jiang, W.; Wu, T.; Du, X. Involvement of rice transcription factor OsERF19 in response to ABA and salt stress responses. Plant Physiol. Biochem. PPB 2021, 167, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef]
- Bakshi, A.; Moin, M.; Madhav, M.S.; Datla, R.; Kirti, P.B. Target of Rapamycin (TOR) negatively regulates chlorophyll degradation and lipid peroxidation and controls responses under abiotic stress in Arabidopsis thaliana. Plant Stress 2021, 2, 100020. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, H.; Wang, L.; Zhao, Q.; Wang, D.; Zhang, T. Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal. Behav. 2022, 17, 2013638. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, W.; Liu, J.; Song, S.; Hou, X.; Jia, C.; Li, J.; Miao, H.; Wang, Z.; Tie, W.; et al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. Biochem. PPB 2020, 147, 66–76. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Tang, M.; Zhu, J.; Shu, M.; Wen, H.; Zhu, J.; Wei, C. Alternative splicing of CsWRKY21 positively regulates cold response in tea plant. Plant Physiol. Biochem. PPB 2024, 208, 108473. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, B.; Wang, N.; Zheng, Z.; Yang, L.; Zhong, S.; Fang, Q.; Xiao, Z.; Zhao, H. A WRKY Transcription Factor PmWRKY57 from Prunus mume Improves Cold Tolerance in Arabidopsis thaliana. Mol. Biotechnol. 2023, 65, 1359–1368. [Google Scholar] [CrossRef]
- Liu, W.; Liang, X.; Cai, W.; Wang, H.; Liu, X.; Cheng, L.; Song, P.; Luo, G.; Han, D. Isolation and Functional Analysis of VvWRKY28, a Vitis vinifera WRKY Transcription Factor Gene, with Functions in Tolerance to Cold and Salt Stress in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 13418. [Google Scholar] [CrossRef]
- Wang, F.; Hou, X.; Tang, J.; Wang, Z.; Wang, S.; Jiang, F.; Li, Y. A novel cold-inducible gene from Pak-choi (Brassica campestris ssp. chinensis), BcWRKY46, enhances the cold, salt and dehydration stress tolerance in transgenic tobacco. Mol. Biol. Rep. 2012, 39, 4553–4564. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, Z.; Yu, Z.; Ding, Q.; Qian, X.; Zhang, C.; Zhu, C.; Wang, Y.; Zhang, C.; Li, Y.; et al. BcWRKY53 promotes chlorophyll biosynthesis and cold tolerance of non-heading Chinese cabbage under cold stress. Plant Physiol. Biochem. PPB 2025, 219, 109398. [Google Scholar] [CrossRef]
- Ciolkowski, I.; Wanke, D.; Birkenbihl, R.P.; Somssich, I.E. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol. Biol. 2008, 68, 81–92. [Google Scholar] [CrossRef]
- Mittal, A.; Gampala, S.S.; Ritchie, G.L.; Payton, P.; Burke, J.J.; Rock, C.D. Related to ABA-Insensitive3(ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol. J. 2014, 12, 578–589. [Google Scholar] [CrossRef]
- Feng, C.Z.; Chen, Y.; Wang, C.; Kong, Y.H.; Wu, W.H.; Chen, Y.F. Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. Plant J. Cell Mol. Biol. 2014, 80, 654–668. [Google Scholar] [CrossRef]
- Tamargo-Gómez, I.; Martínez-García, G.G.; Suárez, M.F.; Mayoral, P.; Bretones, G.; Astudillo, A.; Prieto-Lloret, J.; Sveen, C.; Fueyo, A.; Engedal, N.; et al. Analysis of ATG4C function in vivo. Autophagy 2023, 19, 2912–2933. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Physiological function of ABCG1. Drug News Perspect. 2003, 16, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, M.; Xia, J.; Xing, J.; Fan, X.; Xu, Q.; Cang, J.; Zhang, D. Overexpression of TaMYC2 confers freeze tolerance by ICE-CBF-COR module in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 1042889. [Google Scholar] [CrossRef]
- Campoli, C.; Matus-Cádiz, M.A.; Pozniak, C.J.; Cattivelli, L.; Fowler, D.B. Comparative expression of Cbf genes in the Triticeae under different acclimation induction temperatures. Mol. Genet. Genom. MGG 2009, 282, 141–152. [Google Scholar] [CrossRef]
- Chandler, J.W. Class VIIIb APETALA2 Ethylene Response Factors in Plant Development. Trends Plant Sci. 2018, 23, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Wang, S.; Hao, D.; Xi, J. Proteomics dissection of cold responsive proteins based on PEG fractionation in Arabidopsis. Chem. Res. Chin. Univ. 2014, 30, 272–278. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.S.; Bahk, S.; An, J.; Yoo, Y.; Kim, J.-Y.; Chung, W.S. Phosphorylation of the transcriptional repressor MYB15 by mitogen-activated protein kinase 6 is required for freezing tolerance in Arabidopsis. Nucleic Acids Res. 2017, 45, 6613–6627. [Google Scholar] [CrossRef]
- Duan, Y.; Han, J.; Guo, B.; Zhao, W.; Zhou, S.; Zhou, C.; Zhang, L.; Li, X.; Han, D. MbICE1 Confers Drought and Cold Tolerance through Up-Regulating Antioxidant Capacity and Stress-Resistant Genes in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 16072. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Z. Random local neighbor joining: A new method for reconstructing phylogenetic trees. Mol. Phylogenetics Evol. 2008, 47, 117–128. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Vasil, I.K.; Hildebrandt, A.C. Growth and chlorophyll production in plant callus tissues grown in vitro. Planta 1966, 68, 69–82. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise estimation of chlorophyll a, b and carotenoid content by deconvolution of the absorption spectrum and new simultaneous equations for Chl determination. Plant J. Cell Mol. Biol. 2022, 109, 1630–1648. [Google Scholar] [CrossRef]
- Li, H.X.; Xiao, Y.; Cao, L.L.; Yan, X.; Li, C.; Shi, H.Y.; Wang, J.W.; Ye, Y.H. Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS ONE 2013, 8, e73380. [Google Scholar] [CrossRef]
- Shi, J.; Fu, X.Z.; Peng, T.; Huang, X.S.; Fan, Q.J.; Liu, J.H. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 30, 914–922. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Cui, J.; Wang, H.; Qi, X.; Kuo, L.Y.; Ma, H.; Gao, L.; Mo, B.; Chen, X. Conservation and divergence of small RNA pathways and microRNAs in land plants. Genome Biol. 2017, 18, 158. [Google Scholar] [CrossRef]
- Li, S.; Guo, M.; Hong, W.; Li, M.; Zhu, X.; Guo, C.; Shu, Y. Overexpression of a White Clover WRKY Transcription Factor Improves Cold Tolerance in Arabidopsis. Agronomy 2025, 15, 1700. [Google Scholar] [CrossRef]
- Berruezo, F.; de Souza, F.S.J.; Picca, P.I.; Nemirovsky, S.I.; Martínez Tosar, L.; Rivero, M.; Mentaberry, A.N.; Zelada, A.M. Sequencing of small RNAs of the fern Pleopeltis minima (Polypodiaceae) offers insight into the evolution of the microrna repertoire in land plants. PLoS ONE 2017, 12, e0177573. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, X.; Zhao, Y.; Chen, R. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduct. Target. Ther. 2023, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. A Publ. Protein Soc. 2023, 32, e4792. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).