Abstract
Gmelina philippensis Cham. (Lamiaceae) is a traditionally valued medicinal plant with unexplored potential for the management of neurodegenerative disorders. In the present study, the phytochemical profile of its methanolic leaf extract was comprehensively characterized using untargeted liquid chromatography–tandem mass spectrometry metabolomics (LC–MS/MS) and molecular networking. In addition, the extract was evaluated for its antioxidant and cholinesterase inhibitory activities relevant to Alzheimer’s disease (AD). Metabolite profiling led to the annotation of 27 compounds, with a predominance of flavonoids and iridoid glycosides unique to the genus Gmelina, along with phenolic acids, lipids, and other minor compounds. The extract exhibited potent in vitro antioxidant activity, with an IC50 of 7.49 ± 0.002 μg/mL in the DPPH assay and 639.63 ± 0.814 μg AAE/mg in the FRAP assay. Notably, the extract showed significant inhibitory activity against acetylcholinesterase and butyrylcholinesterase, with an IC50 of 4.87 ± 0.16 and 40.99 ± 0.03 μg/mL, respectively. Molecular networking further supported the metabolite annotation and highlighted clusters of bioactive iridoids and flavonoids. Overall, these findings highlight that G. philippensis as a rich source of multi-target bioactive compounds, supporting that the extract has good anti-acetylcholinesterase activity comparable to the rivastigmine that used in neurodegenerative disease. This study provides a promising foundation for the development of novel therapeutic approaches targeting neurodegenerative diseases.
1. Introduction
Secondary metabolites derived from natural sources, particularly from plants, have long been recognized as important contributors to the treatment and prevention of various diseases [1]. These compounds play a pivotal role in combating complex health disorders, including oxidative stress, inflammatory conditions, wound healing, diabetes, and neurodegenerative diseases [2]. Among them, plant polyphenols are prominent natural antioxidants capable of mitigating oxidative damage associated with several pathological conditions such as cancer, liver and cardiovascular diseases, neurodegeneration, aging-related disorders, and diabetes [3,4]. This growing evidence reinforces the global interest in exploring natural products for the development of novel and effective therapeutic agents.
The genus Gmelina (Lamiaceae; formerly Verbenaceae) comprises approximately 40 species. Among these, Gmelina philippensis Cham., commonly referred to as Parrot’s Peak, is a perennial shrub widely cultivated for its ornamental value due to its distinctive golden-yellow inflorescence [5]. G. philippensis holds a longstanding history of ethnomedicinal use across several Asian countries, including Cambodia, Pakistan, and the Philippines, where it is traditionally employed to improve maternal and fetal health, treat gastrointestinal disorders, foot eczema, cough, and also used as a purgative and for managing joint and nerve-related conditions [6,7].
Previous phytochemical studies on the aerial parts of G. philippensis have consistently revealed its richness in iridoid glycosides—particularly rhamnopyranosylcatalpol esters—as well as flavonoids, terpenoids and fatty acids [6,7]. Although limited, pharmacological studies have reported antioxidant, antidiabetic, and enzyme inhibitory activities associated with G. philippensis extracts [6,7,8]. Furthermore, the ethanolic extract of its aerial parts has shown notable cytotoxic effects against hepatocellular carcinoma (HepG2) cell lines [7]. Network pharmacology-guided identification of G. arborea suggests that several phytochemicals can modulate various targets associated with AD pathology, such as amyloid-beta aggregation, tau hyperphosphorylation, oxidative stress, and neuroinflammation [9]. Also, G. asiatica showed neuroprotection properties in an in silico study [10].
Building on these findings, the present study aims to comprehensively characterize the phytochemical composition of the methanolic extract of G. philippensis using liquid chromatography–tandem mass spectrometry (LC–MS/MS) and to evaluate its antioxidant capacity and cholinesterase inhibitory potential through in vitro 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) assays.
2. Results and Discussion
2.1. Untargeted Metabolome Analysis
A comprehensive metabolomic profiling of the methanolic extract of Gmelina philippensis leaves was carried out using untargeted liquid chromatography–tandem mass spectrometry (LC–MS/MS) in both positive and negative ionization modes (For chromatograms see Figure S1). A total of 27 metabolites were annotated based on the alignment of retention times, accurate mass values, and MS/MS fragmentation patterns with previously reported data [11,12].
The identified metabolites were grouped into distinct phytochemical classes according to their polarity and elution order. Compounds eluted in the sequence of decreasing polarity, starting with phenolic acids, followed by flavonoid and iridoid glycosides, their aglycones, and finally fatty acids and phospholipids. Among these, flavonoids constituted the most abundant class, accounting for nine identified compounds. Overall, the detected metabolites comprised nine flavonoids, three iridoid glycosides, two phenolic acids, four fatty acids and fatty acid amides, two phospholipids, and miscellaneous compounds, including sugars, a diterpene, and a coumarin derivative (Table 1 and Figure 1).

Table 1.
Annotated metabolites in the methanol extract of G. philippensis using LC–MS/MS analysis in positive and negative modes.

Figure 1.
Different classes of metabolites identified in the methanol extract of G. philippensis leaves.
2.1.1. Flavonoids
Flavonoids were the predominant class of metabolites detected in the methanolic extract of G. philippensis, with a particular abundance of flavone and flavonol glycosides. Notable representatives included derivatives of isovitexin, rhoifolin, quercetin, and kaempferol, which corresponded to nine distinct chromatographic peaks (peaks 8 and 10–17).
The identification of these flavonoid glycosides was primarily based on two characteristic MS/MS fragmentation features: (i) the neutral loss of sugar moieties, indicative of glycosidic cleavage, and (ii) the Retro-Diels–Alder (RDA) fragmentation pattern of the aglycone. The aglycone ion, often observed as the base peak following deglycosylation, was confirmed by its accurate mass corresponding to the expected loss of the sugar unit [13].
Peak 8 (m/z 563.1408; [M−H]−) exhibited a characteristic fragmentation pattern consistent with a flavone di-C-hexoside. Diagnostic fragment ions were observed at m/z 473.1100 ([M−H−90]−) and 443.1005 ([M−H−120]−), corresponding to typical cross-ring cleavages of sugar moieties. Additional fragment ions at m/z 353.0678 and 383.0779, assigned as [Aglycone+83]− and [Aglycone+113]−, respectively, indicated the presence of a di-C-glycoside structure retaining partial sugar residues on the aglycone, as previously described by Farag et al. [14]. Accordingly, peak 8 was identified as apigenin 6-C-arabinoside-8-C-glucoside (isoschaftoside).
Peak 10 (m/z 433.1127; [M+H]+) was annotated as isovitexin, a mono-C-glycosyl flavone. Its MS/MS spectrum showed a prominent fragment at m/z 313.0706 ([M−120]−), indicative of sugar cleavage specific to C-hexosides.
Peak 14 (m/z 577.1559; [M−H]−) was assigned as apigenin 7-O-neohesperidoside (rhoifolin), based on its fragment ions at m/z 413.0839 ([M−H−164]−) and 269.0441 ([M−H−308]−), corresponding to the sequential loss of rhamnose moiety and H2O (−164 Da) and hexosyl and rhamnose moieties (−308 Da), consistent with the formation of apigenin aglycone [15].
Several apigenin glycosides, including vicenin-II, rhoifolin, and isorhoifolin, have been previously isolated from the butanol fraction of the aerial parts of G. philippensis [7].
The presence of a kaempferol aglycone fragment ion (m/z 286; C15H10O6) was observed in peaks 13, 16, and 17, supporting their structural annotation as kaempferol glycosides. Specifically, peak 13 (m/z 447.0931; [M−H]−) showed a prominent fragment ion at m/z 284.0310, corresponding to the neutral loss of a hexose moiety (−162 Da), consistent with its identification of peak 13 as kaempferol 3-O-glucoside (astragalin). Peak 16 (m/z 417.0827; [M−H]−), showed a major fragment ion at m/z 284.0323, indicate of pentose loss (−132 Da). confirming its identity as kaempferol-3-O-arabinoside (juglalin) [16]. In the positive ionization mode, peak 17 (m/z 565.1546; [M+H]+) was identified as kaempferol 3-O-α-l-arabinopyranosyl-7-O-α-l-rhamnopyranoside, based on its molecular formula and characteristic kaempferol-based structure.
Peak 11 (m/z 463.0871; [M−H]−) and peak 12 (m/z 433.0776; [M−H]−) yield a quercetin aglycone ion (m/z 302; C15H10O7), supporting their annotation as quercetin-3-O-galactoside and quercetin-3-O-xyloside, respectively. Both compounds showed base fragment ions at m/z 300.0264 and 300.0262, resulting from the neutral loss of hexose (−162 Da) and pentose (−132 Da) moieties, respectively.
Additionally, peak 15 (m/z 287.0543; [M+H]+) was identified as luteolin, representing a free flavone.
These findings are consistent with previous phytochemical reports on G. arborea, in which kaempferol, quercetin, and luteolin were isolated from various plant organs either in glycosylated or aglycone forms [17].
2.1.2. Iridoid Glycosides
LC–MS/MS analysis enabled the identification of three iridoid glycosides characteristic of the genus Gmelina, namely 3″-O-caffeoyl-6-O-rhamnopyranosylcatalpol (peak 7), saccatoside (peak 9), and gmelinoside N (peak 19).
Peak 7 (m/z 669.2027; [M−H]−) was annotated as 3″-O-caffeoyl-6-O-rhamnopyranosylcatalpol. Its MS/MS spectrum exhibited a diagnostic fragment ion at m/z 179.0334, corresponding to the caffeoyl moiety (dihydroxycinnamoyl group), followed by a secondary fragment at m/z 161.0232, resulting from the neutral loss of a H2O (−18 Da) from the caffeoyl group, supporting the proposed structure.
Peak 9 (m/z 653.2088; [M−H]−) was identified as saccatoside, also known as 6-O-α-(2″-O-p-coumaroyl) rhamnopyranosylcatalpol. The MS/MS fragmentation revealed a prominent fragment ion at m/z 291.0879 attributed to the catalpol moiety, as well as an ion at m/z 163.0404 corresponding to the p-coumaroyl group. A subsequent fragment at m/z 145.0299, resulting from H2O loss (−18 Da) from the coumaroyl residue, further supported the identification. These findings are in agreement with previous reports of saccatoside in the leaves of G. arborea [17,18].
Peak 19 (m/z 799.2465; [M−H]−) was identified as gmelinoside N. This compound is structurally known as 6-O-α-l-(2″,3″-di-O-trans-p-hydroxycinnamoyl)rhamnopyranosylcatalpol. Fragmentation analysis revealed an ion at m/z 653.2079, corresponding to the loss of a rhamnose unit (−146 Da). Additional fragment ions at m/z 163.0401 and 145.0297 were assigned to the p-hydroxycinnamoyl moiety and its H2O loss (−18 Da), respectively. A fragment ion at m/z 437.1234 was also detected, indicating the presence of the catalpol skeleton after cleavage of sugar and cinnamoyl groups. This compound was previously isolated from the flowers of G. arborea [19].
2.1.3. Phenolic Acids
Two phenolic glycosides were identified in the methanolic extract. Peak 5 (m/z 325.0925; [M−H]−) was annotated as a p-coumaric acid glucoside, based on the presence of diagnostic fragment ions at m/z 163.0382 and 119.0491, corresponding to p-coumaric acid. A base peak at m/z 145.0284, resulting from the H2O loss (−18 Da), further supported this identification [20].
Peak 6 (m/z 309.0967; [M−H2O+H]+) was assigned as trans-β-d-glucosyl-2-hydroxycinnamate. Its MS/MS spectrum showed a base peak at m/z 147.0428, arising from the loss of a glucose moiety (−162 Da), a fragmentation pattern commonly observed for hydroxycinnamate glycosides.
These phenolic acids and their glycosylated derivatives have previously been reported in G. arborea [21,22]. Moreover p-coumaric and cinnamic acid derivatives are known to be common structural components of iridoid glycosides in various Gmelina species [17,23].
2.1.4. Lipids
Fatty acids and fatty acid amides were annotated in peaks 20, 22, 26, and 27. Peak 20 (m/z 329.2330; [M−H]−) was identified as 9,12,13-trihydroxy-10-E-octadecenoic acid. peak 22 (m/z 279.2317; [M+H]+) was assigned as α-linolenic acid. Peaks 26 (m/z 322.2730; [M+H]+) and 27 (m/z 324.2887; [M+H]+) were annotated as fatty acid ethanolamides, specifically α-linolenoyl ethanolamide and linoleoyl ethanolamide, respectively, based on accurate mass measurements and characteristic MS/MS fragmentation patterns.
In addition, two phospholipids were characterized in peaks 23 (m/z 452.2784; [M−H]−) and 25 (m/z 496.3392; [M+H]+) based on their exact masses and fragmentation patterns. Peak 24 (m/z 353.2685; [M+H]+) was assigned as 1-monolinolenin.
The presence of similar fatty acids and related lipids has previously been reported in the leaves of G. asiatica L. [24] and in the seeds of G. arborea [17,25,26].
2.1.5. Miscellaneous
LC–MS/MS analysis also revealed the presence of several miscellaneous compounds. Peak 18 (m/z 277.1064; [M+H]+) was identified as the coumarin derivative murrangatin, exhibiting characteristic base fragment ion at m/z 131.0487 [27]. Additional compound classes identified in the extract included terpenoids, sulfonic acids, sugars, as well as carboxylic and amino acids, as summarized in Table 1.
Overall, the LC–MS/MS profiling of the methanolic extract of G. philippensis revealed a chemically diverse and structurally complex metabolite profile that substantiates its traditional medicinal applications. The detection of multiple compound classes—such as iridoid glycosides, flavonoids, terpenoids, phenolic acids, and structurally diverse minor constituents—emphasizes the rich phytochemical composition of this species.
This comprehensive metabolomic characterization provides a solid foundation for future pharmacological investigations and highlights the potential of G. philippensis as a valuable source of bioactive natural products with therapeutic relevance.
2.2. LC–MS/MS Analysis and Molecular Networking
A metabolomics investigation guided by LC–MS/MS analysis was conducted to elucidate the chemical complexity of the methanolic extract of G. philippensis leaves. For metabolite annotation and structural classification, MS/MS-based molecular networking was employed using the Global Natural Products Social Molecular Networking (GNPS) platform. Spectral library matching facilitated dereplication of known metabolites, while clustering based on fragment ion similarity revealed relationships among structurally related compound families. The constructed molecular network—based on data acquired in negative ionization mode—consisted of 2079 nodes, grouped into 157 clusters, including 737 self-looped nodes (Figure 2). In this network, nodes represent precursor ions, and edges indicate the mass differences between connected features. GNPS-based molecular networking enabled the dereplication of several secondary metabolite classes, including iridoid glycosides (e.g., gmelinoside N, saccatoside, and caffeoyl-rhamnopyranosyl catalpol), cinnamic acid derivatives (e.g., p-coumaric acid glucoside), flavonoids (e.g., astragalin, rhoifolin, juglalin, isoschaftoside, luteolin, and quercetin glucoside), as well as benzene sulfonic acids, phospholipids, and unsaturated fatty acids.
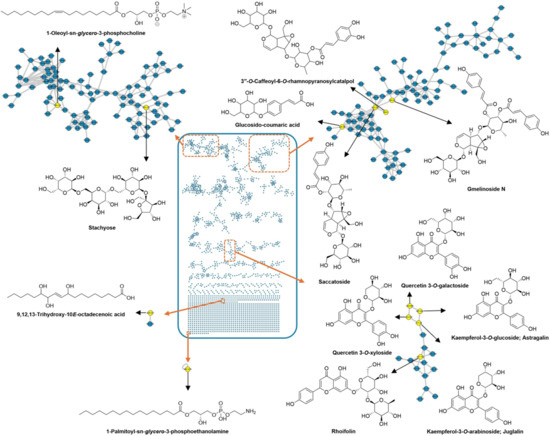
Figure 2.
Untargeted analysis of the methanol extract of G. philippensis leaves using MS/MS molecular networking (negative mode).
2.3. Assessment of the Antioxidant Activity Using DPPH and ABTS Assays
The percentages of DPPH radical scavenging activity of different concentrations of the methanol extract of G. philippensis are illustrated in Figure 3. The results revealed that G. philippensis showed an IC50 of 7.49 ± 0.002 μg/mL as compared to ascorbic acid with an IC50 of 3.6 ± 0.001 μg/mL, revealing a promising antioxidant potential. Moreover, in FRAP assay, the methanol extract of G. philippensis showed 639.63 ± 0.814 µg equivalent ascorbic acid equivalent (AAE) /mg of extract. From the previously reported results, leaves and flowers of G. philippensis from Dhaka, Bangladesh, showed significant variation in antioxidant activity depending on the solvent used for fractionation. DPPH IC50 values ranged from 9.75 to 35.25 μg/mL, indicating varying degrees of free-radical scavenging capacity [8]. Another study revealed the antioxidant activity of the n-hexane extract from G. philippensis leaves by different assays as DPPH and ABTS, cupric reducing antioxidant capacity (CUPRAC) and metal chelating ability (MCA) assays [6]. A major constituent in the extract is the iridoid glycosides which have been reported for their antioxidant potential [28].
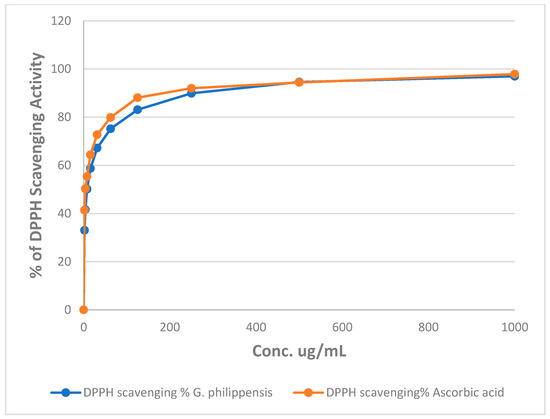
Figure 3.
Changes in the % of DPPH assay of different concentrations of the methanol extract of G. philippensis and ascorbic acid (positive control).
2.4. Assessment of the In Vitro Inhibition of Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Enzyme Activities
The percentage of AChE and BChE enzyme activities after incubation with different concentrations of the methanol extract of G. philippensis are illustrated in Figure 4A,B. The extract exhibited AChE inhibitory activity with an IC50 of 4.869 ± 0.16 μg/mL, compared to the standard rivastigmine (0.73 ± 0.025 μg/mL). It also showed BChE inhibitory effects with an IC50 of 40.985 ± 0.034 μg/mL compared to the standard tacrine (0.044 ± 0.002 μg/mL). At a concentration of 10 μg/mL, G. philippensis methanol extract inhibited butyrylcholinesterase by 69%, compared to tacrine (85%) and inhibited acetylcholinesterase by 51%, compared to rivastigmine (81%), revealing a promising anti-Alzheimer’s potential. This finding is corroborated by a previous study that revealed that the flower extract demonstrated the most promising anti-Alzheimer activity through AChE inhibition with an IC50 of 89.07 ± 5.14 μg/mL, followed by leaves (IC50 = 137.22 ± 7.39 μg/mL). The prevalence of glycosides (gmelinosides) is particularly noteworthy, as these compounds represent a unique chemical class with potential neuroprotection [29,30,31].
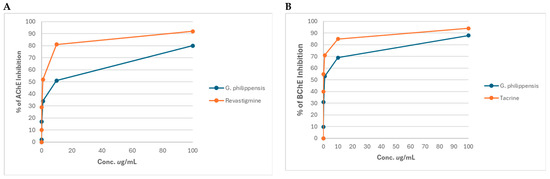
Figure 4.
Acetylcholinesterase (A) and Butyrylcholinesterase (B) inhibitory activity of the methanol extract of G. philippensis leaves.
3. Materials and Methods
3.1. Plant Material Collection and Authentication
Leaves of Gmelina philippensis were collected in March 2021 from El Orman Botanical Garden, Giza, Egypt. Dr. Mohammed El-Gebaly (National Research Centre, Giza, Egypt) authenticated the plant. A voucher specimen (BUC-PHG-GP-12) was deposited at Badr University in Cairo’s Pharmacognosy Department.
3.2. Extraction Procedure
Dried leaves (10 g) of G. philippensis underwent sequential solvent extraction. First, defatting was performed via maceration in n-hexane for three days. After filtration, the solvent was evaporated using a Rotavap (40 °C), yielding 294 mg of hexane extract. The residual plant material was then extracted with 100% methanol, yielding 2.32 g of dried methanolic extract. This extract was stored refrigerated for subsequent analysis [11].
3.3. HPLC-MS Analysis
Separation was performed on a Waters ACQUITY UPLC® BEH C18 column (150 × 2.1 mm i.d., 1.7 μm) maintained at 30 °C [32,33]. A gradient elution program was employed using mobile phase A (water with 0.1% formic acid) and B (acetonitrile) as follows: 15% B (0.00–1.00 min); linear increase to 100% B (1.00–16.00 min); hold at 100% B (16.00–18.00 min); rapid return to 15% B (18.00–18.01 min); hold at 15% B (18.01–20.00 min). The post-time was 2.00 min for column re-equilibration. The extract was first dissolved in 100% methanol, and then diluted with an acetonitrile-water (1:1, v/v) mixture to a final concentration of 2000 ppm, and an aliquot (3 µL) was injected. MS/MS analysis utilized both positive and negative electrospray ionization (ESI) modes. Key parameters included: gas temperature 320 °C; gas flow 8 L/min; nebulizer pressure 35 psi; sheath gas temperature 350 °C; sheath gas flow 11 L/min; capillary voltage 3500 V; nozzle voltage 1000 V; fragmentor voltage 100 V. Full-scan MS spectra (100–1700 m/z) were acquired at 3 spectra/s (333 ms/spectrum), while MS/MS spectra (100–1700 m/z) were acquired at 2 spectra/s (500 ms/spectrum). Collision energy used a linear ramp (offset 16.6 eV; slope 3.3 eV per 100 m/z). Real-time mass calibration employed internal references purine and HP-0921, providing lock masses at m/z 121.0508, 322.0481, 922.0097 (positive mode) and m/z 112.9855, 119.0363, 966.0007, 980.0163 (negative mode).
3.4. GNPS-Based Molecular Networking
The raw mass data files obtained from the untargeted LC-MS analysis of the methanol extract of G. philippensis leaves were converted to mzML using MSConvert tool from ProteoWizard version 3.0.22288 (http://proteowizard.sourceforge.net, accessed on 1 March 2024). The chromatogram was centroided and then uploaded to the GNPS platform (http://gnps.ucsd.edu, accessed on 1 march 2024) to construct a network in positive and negative modes. Clustering and spectral library search were performed based on the similarity in MS/MS fragment ions whose precursor and fragment ion mass tolerance were set at 0.02 Da each. The advanced network options were as follows: Minimum pairs cosine of 0.6, network TopK of 10, maximum connected component size of 100, maximum matched fragment ions of 3, and minimum cluster size of 2. For the library search, a minimum of 3 matching peaks with a score threshold of 0.6 was selected. All other parameters were kept at default values. The molecular networks generated in GNPS were exported to Cytoscape (version 3.9.1) in ‘graphml’ format to enable customized visualization and further analysis [34].
3.5. Evaluation of Antioxidant Activity by DPPH Radical Scavenging Method
The free-radical scavenging activity of the extract was evaluated using the DPPH method. Briefly, different concentrations of the extract (1.95–1000 µg/mL) were prepared in ethanol and were mixed with 0.1 mM ethanolic DPPH solution (1 mL extract + 3 mL DPPH). After vigorous shaking and 30 min incubation at room temperature, absorbance was measured at 517 nm using a UV-Vis spectrophotometer. Ascorbic acid served as the standard reference, and experiments were performed in triplicate. The percentage inhibition was calculated as [(A0 − A1)/A0] × 100, where A0 is the control (DPPH + ethanol) absorbance and A1 is the test/standard absorbance. Lower absorbance indicated higher activity. The IC50 value (concentration inhibiting 50% of DPPH radicals) was determined from a log dose inhibition curve [35].
3.6. Ferric Reducing Antioxidant Power (FRAP) Assay
The total reducing power (TRP, equivalent to FRAP) of the extract was assessed using a modified microplate adaptation [36] of the potassium ferricyanide-trichloroacetic acid method [37]. In brief, 40 µL sample was mixed with 50 µL sodium phosphate buffer (0.2 mol/L), 50 µL potassium ferricyanide (1%), and 50 µL trichloroacetic acid (10%). After centrifugation (3000 rpm, 10 min), 166.66 µL of supernatant was transferred to a 96-well plate, reacted with 33.3 µL ferric chloride (1%), and absorbance was measured at 630 nm using a Biotek ELX800 microplate reader. DMSO and ascorbic acid (1 mg/mL) served as negative and positive controls, respectively. Results are expressed as ascorbic acid equivalents (AAE) per mg of extract.
3.7. In Vitro Assay for Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) inhibition
The acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) inhibitory activity of the extract was assessed using Bio-vision’s AChE Inhibitor Screening Kit (Cat. #K197-100, Bio Vision, Egypt), and BChE Activity Assay Kit (Colorimetric) (LS-K17-100, LSBio, LifeSpan Bio Sciences Inc., Newark, CA, USA) following a published method [38,39]. Briefly, the extract was dissolved in DMSO (Merck), to prepare the extract in different concentrations. The sample mixture was further diluted with the assay buffer then 10 μL of diluted test inhibitors were transferred into the appropriate wells of a 96-well plate. The reference inhibitors used in the assay were rivastigmine and tacrine. Acetylthiocholine iodide served as the colorimetric substrate in the reaction, while 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) was utilized to measure cholinesterase activity. The absorbance (OD) was measured at 412 nm. The concentration of the sample required to inhibit 50% of the enzyme activity (IC50) was determined by plotting the percentage of enzyme inhibition against the sample concentrations.
4. Conclusions
This study provides the first comprehensive phytochemical and biological assessment of G. philippensis leaf methanol extract targeting Alzheimer’s disease (AD) pathology. Untargeted LC–MS/MS analysis identified 27 metabolites, revealing a complex profile rich in flavonoids and iridoid glycosides, alongside phenolic acids, lipids, and phospholipids. Molecular networking further corroborated these annotations, emphasizing structural clusters associated with bioactivity. In vitro evaluations demonstrated remarkable antioxidant capacity, evidenced by low DPPH IC50 (7.49 μg/mL) and high FRAP value (639.63 μg AAE/mg), attributable to the abundance of polyphenols and iridoids. Crucially, the extract exhibited potent dual cholinesterase inhibition, with an IC50 of 4.87 μg/mL (AChE) and 40.99 μg/mL (BChE), suggesting its ability to enhance cholinergic neurotransmission—a key therapeutic target in AD. The presence of iridoids and flavonoids aligns with these activities, as both classes are known for neuroprotective properties. These findings validate G. philippensis’s traditional medicinal uses and position it as a promising source of multi-target agents against AD. However, further in vivo studies and detailed mechanistic investigations are required to validate these results and advance the development of lead compounds for neurodegenerative therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14223494/s1, Figure S1. Total ion chromatogram (TIC) of the methanol extract of G. philippensis leaves in positive ion mode (A) and negative ion mode (B).
Author Contributions
Conceptualization, S.H.A.; methodology, S.H.A., G.S.L. and Y.S.J.; software, S.F., G.S.L. and Y.S.J.; formal analysis, S.H.A., G.S.L., Y.S.J. and S.F.; investigation, S.H.A., G.S.L., Y.S.J. and S.F.; resources, S.H.A., G.S.L. and Y.S.J.; data curation, S.H.A., G.S.L. and Y.S.J.; writing—original draft preparation, S.H.A., G.S.L., Y.S.J. and S.F.; writing—review and editing, K.H.K., C.S.K. and M.E.-S.; visualization, S.H.A. and M.E.-S.; supervision, M.E.-S., C.S.K. and K.H.K.; project administration, S.H.A., M.E.-S., C.S.K. and K.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. RS-2019-NR040057, RS-2021-NR059240, 2021R1C1C1011045, and 2022R1A6A1A03054419). This work was also supported by the Korea Environment Industry & Technology Institute (KEITI) through a project to make multi-ministerial national biological research resources more advanced funded by the Korea Ministry of Environment (MOE; 2021003420003). Additionally, this work was supported by a grant (21153MFDS607) from Ministry of Food and Drug Safety of South Korea in 2021–2025, and by the BK21 FOUR Project. This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00440614).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Elebeedy, D.; Ghanem, A.; Aly, S.H.; Ali, M.A.; Faraag, A.H.I.; El-Ashrey, M.K.; Salem, A.M.; El Hassab, M.A.; El Maksoud, A.I.A. Synergistic Antiviral Activity of Lactobacillus acidophilus and Glycyrrhiza glabra against Herpes Simplex-1 Virus (HSV-1) and Vesicular Stomatitis Virus (VSV): Experimental and In Silico Insights. BMC Microbiol. 2023, 23, 173. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Uba, A.I.; Nilofar, N.; Majrashi, T.A.; El Hassab, M.A.; Eldehna, W.M.; Zengin, G.; Eldahshan, O.A. Chemical Composition and Biological Activity of Lemongrass Volatile Oil and N-Hexane Extract: GC/MS Analysis, in Vitro and Molecular Modelling Studies. PLoS ONE 2025, 20, e0319147. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elissawy, A.M.; Mahmoud, A.M.A.; El-Tokhy, F.S.; Mageed, S.S.A.; Almahli, H.; Al-Rashood, S.T.; Binjubair, F.A.; El Hassab, M.A.; Eldehna, W.M. Synergistic Effect of Sophora japonica and Glycyrrhiza glabra Flavonoid-Rich Fractions on Wound Healing: In Vivo and Molecular Docking Studies. Molecules 2023, 28, 2994. [Google Scholar] [CrossRef]
- Anwar, M.A.; Sayed, G.A.; Hal, D.M.; Hafeez, M.S.; Shatat, A.-A.S.; Salman, A.; Eisa, N.M.; Ramadan, A.; El-Shiekh, R.A.; Hatem, S. Herbal Remedies for Oral and Dental Health: A Comprehensive Review of Their Multifaceted Mechanisms Including Antimicrobial, Anti-Inflammatory, and Antioxidant Pathways. Inflammopharmacology 2025, 33, 1085–1160. [Google Scholar] [CrossRef]
- de Kok, R. A Revision of the Genus Gmelina (Lamiaceae). Kew Bull. 2012, 67, 293–329. [Google Scholar] [CrossRef]
- Eldahshan, O.A.; El Hassab, M.A.; Zengin, G.; Aly, S.H. GC/MS Analysis, In Vitro Antioxidant and Enzyme Inhibitory Activities of the n-Hexane Extract of Gmelina philippensis Cham. and in Silico Molecular Docking of Its Major Bioactive Compounds. Proc. Indian Natl. Sci. Acad. 2025, 1–10. [Google Scholar] [CrossRef]
- Sayed, H.M.; Ahmed, A.S.; Khallaf, I.S.; Qayed, W.S.; Mohammed, A.F.; Farghaly, H.S.M.; Asem, A. Phytochemical Investigation, Molecular Docking Studies and DFT Calculations on the Antidiabetic and Cytotoxic Activities of Gmelina Philippensis CHAM. J. Ethnopharmacol. 2023, 303, 115938. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Islam, F.; Quadery, T.M.; Shihan, M.H.; Rashid, M.A. In Vitro Antioxidant, Total Phenolic Content and Preliminary Toxicity Studies of Gmelina philippensis Chem. Afr. J. Pharm. Pharmacol. 2012, 6, 855–859. [Google Scholar] [CrossRef]
- Gangwal, A.; Ansari, A.; Ansari, I.; Sawale, J.A.; Wong, L.S.; Subramaniyan, V.; Kumarasamy, V. Network Pharmacology-Guided Identification and Molecular Validation of Multi-Target Phytoconstituents from Gmelina Arborea Against Alzheimer’s Disease. 2025. Available online: https://www.researchsquare.com/article/rs-7399280/v1 (accessed on 14 October 2025).
- Andarghiske, K.R.; Bhanukiran, K.; Ksirri, R.; Hemalatha, S. In Silico Exploration of Gmelina asiatica for Multitarget Neuroprotection in Alzheimer’s Disease. Pharmacogn. Res. 2025, 17, 115–125. [Google Scholar] [CrossRef]
- Aly, S.H.; Mahmoud, A.M.A.; Abdel Mageed, S.S.; Khaleel, E.F.; Badi, R.M.; Elkaeed, E.B.; Rasheed, R.A.; El Hassab, M.A.; Eldehna, W.M. Exploring the Phytochemicals, Antioxidant Properties, and Hepatoprotective Potential of Moricandia sinaica Leaves against Paracetamol-Induced Toxicity: Biological Evaluations and in Silico Insights. PLoS ONE 2024, 19, e0307901. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Yagi, S.; Eldahshan, O.A.; Singab, A.N.; Selvi, S.; Rodrigues, M.J.; Custodio, L.; Dall’Acqua, S.; Ponnaiya, S.K.M.; Aly, S.H. Decoding Chemical Profiles and Biological Activities of Aerial Parts and Roots of Eryngium thorifolium Boiss by HPLC-MS/MS, GC-MS and in Vitro Chemical Assays. Food Biosci. 2024, 61, 104556. [Google Scholar] [CrossRef]
- Aly, S.; Elissawy, A.; El Hassab, M.; Majrashi, T.; Hassan, F.; Elkaeed, E.; Eldehna, W.; Singab, A. Comparative Metabolic Study of the Chloroform Fraction of Three Cystoseira species Based on UPLC/ESI/MS Analysis and Biological Activities. J. Enzyme Inhib. Med. Chem. 2023, 39, 2292482. [Google Scholar] [CrossRef]
- Farag, M.A.; Otify, A.; Porzel, A.; Michel, C.G.; Elsayed, A.; Wessjohann, L.A. Comparative Metabolite Profiling and Fingerprinting of Genus Passiflora Leaves Using a Multiplex Approach of UPLC-MS and NMR Analyzed by Chemometric Tools. Anal. Bioanal. Chem. 2016, 408, 3125–3143. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, N.M.; Hifnawy, M.S.; Lotfy, R.A.; Younis, I.Y. LC/MS/MS and GC/MS/MS Metabolic Profiling of Leontodon hispidulus, in Vitro and in Silico Anticancer Activity Evaluation Targeting Hexokinase 2 Enzyme. Sci. Rep. 2024, 14, 6872. [Google Scholar] [CrossRef]
- Aquino, A.J.; Alves, T.D.C.; Oliveira, R.V.; Ferreira, A.G.; Cass, Q.B. Chemical Secondary Metabolite Profiling of Bauhinia longifolia Ethanolic Leaves Extracts. Ind. Crops Prod. 2019, 132, 59–68. [Google Scholar] [CrossRef]
- El Sayed, A.M.; El Hawary, S.; Elimam, H.; Saleh, A.M.; Zokalih, A.H.; Mohyeldin, M.M.; Bassam, S.M. ESI-LC-MS/MS Based Comparative Multivariate Metabolomic and Biological Profiling with Dynamic Molecular Docking of Gmelina arborea Roxb Different Organs. Fitoterapia 2023, 168, 105540. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Yadav, A.K.; Srivastava, P.; Shanker, K.; Verma, R.K.; Gupta, M.M. Iridoid Glycosides from Gmelina arborea. Phytochemistry 2008, 69, 2387–2390. [Google Scholar] [CrossRef]
- Gu, W.; Hao, X.-J.; Liu, H.-X.; Wang, Y.-H.; Long, C.-L. Acylated Iridoid Glycosides and Acylated Rhamnopyranoses from Gmelina arborea Flowers. Phytochem. Lett. 2013, 6, 681–685. [Google Scholar] [CrossRef]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In Vitro Bioaccessibility and Gut Biotransformation of Polyphenols Present in the Water-insoluble Cocoa Fraction. Mol. Nutr. Food Res. 2011, 55, S44–S55. [Google Scholar] [CrossRef]
- Shankar, S.R.; Girish, R.; Karthik, N.; Rajendran, R.; Mahendran, V.S. Allelopathic Effects of Phenolics and Terpenoids Extracted from Gmelina arborea on Germination of Black Gram (Vigna mungo) and Green Gram (Vigna radiata). Allelopath. J. 2009, 23, 323–332. [Google Scholar]
- Shreeda Adhyapak, S.A.; Vidya Dighe, V.D.; Dhanashri Mestry, D.M.; Neeta Shambhu, N.S. High Performance Liquid Chromatographic Method for Quantization of Apigenin from Dried Root Powder of Gmelina arborea Linn. Int. J. Pharma Bio Sci. 2011, 2, 742–749. [Google Scholar]
- Hosny, M.; Rosazza, J.P.N. Gmelinosides A–L, Twelve Acylated Iridoid Glycosides from Gmelina arborea. J. Nat. Prod. 1998, 61, 734–742. [Google Scholar] [CrossRef]
- Florence, A.R.; Jeeva, S. Chemical Composition of Essential Oil from the Leaves of Gmelina asiatica L. J. Med. Plants Stud. 2016, 4, 8–10. [Google Scholar]
- Basumatary, S.; Deka, D.C.; Deka, D.C. Composition of Biodiesel from Gmelina arborea Seed Oil. Adv. Appl. Sci. Res. 2012, 3, 2745–2753. [Google Scholar]
- Deepak Kumar, D.K.; Ashwani Sanghi, A.S.; Raju Chandra, R.C.; Shefali Arora, S.A.; Sharma, A.K. Membrane Stability and Antioxidant Activity of Gmelina arborea Seed Extracts and Their Fatty Acid Composition. Br. J. Pharm. Res. 2015, 6, 261–268. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, S.; Ma, X.; Song, Q.; Song, Y.; Tu, P.; Jiang, Y. A Quantitative Chemomics Strategy for the Comprehensive Comparison of Murraya paniculata and M. exotica Using Liquid Chromatography Coupled with Mass Spectrometry. J. Chromatogr. A 2024, 1718, 464736. [Google Scholar] [CrossRef] [PubMed]
- Falah, S.; Katayama, T.; Suzuki, T. Chemical Constituents from Gmelina arborea Bark and Their Antioxidant Activity. J. Wood Sci. 2008, 54, 483–489. [Google Scholar] [CrossRef]
- Dinda, B. Pharmacology and Applications of Naturally Occurring Iridoids; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 3030055752. [Google Scholar]
- Pasdaran, A.; Hamedi, A. The Genus Scrophularia: A Source of Iridoids and Terpenoids with a Diverse Biological Activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Lee , D.E.; Park , K.H.; Hong, J.H.; Kim, S.H.; Park, K.M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Archives of Pharmacal Research 2023, 46, 771–781. [Google Scholar]
- Lee , S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.K.; Kim , K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Archives of pharmacal research 2024, 47, 272–287. [Google Scholar]
- Thapa, B.B.; Huo, C.; Budhathoki, R.; Chaudhary, P.; Joshi, S.; Poudel, P.B.; Magar, R.T.; Parajuli, N.; Kim, K.H.; Sohng, J.K. Metabolic Comparison and Molecular Networking of Antimicrobials in Streptomyces Species. Int. J. Mol. Sci. 2024, 25, 4193. [Google Scholar] [CrossRef]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the Antioxidant Activity of Aqueous and Methanol Extracts of Pleurotus ostreatus in Different Growth Stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef] [PubMed]
- Athamena, S.; Laroui, S.; Bouzid, W.; Meziti, A. The Antioxidant, Anti-Inflammatory, Analgesic and Antipyretic Activities of Juniperu thurifera. J. Herbs Spices Med. Plants 2019, 25, 271–286. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kivrak, İ.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Antioxidant, Anticholinesterase and Antimicrobial Constituents from the Essential Oil and Ethanol Extract of Salvia potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- Mintjens, N.; Brummans, R.; Soetens, F.; Claes, K.B.M.; Vanlinthout, L.E. Timing of Blood Sampling for Butyrylcholinesterase Phenotyping in Patients with Prolonged Neuromuscular Block after Mivacurium or Suxamethonium. Acta Anaesthesiol. Scand. 2021, 65, 182–187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).