Integrated Transcriptomic and Developmental Analyses Provide Insights into the Intrafloral Stamen Differentiation in Cassia fistula L.
Abstract
1. Introduction
2. Results
2.1. Floral Biology of Cassia fistula
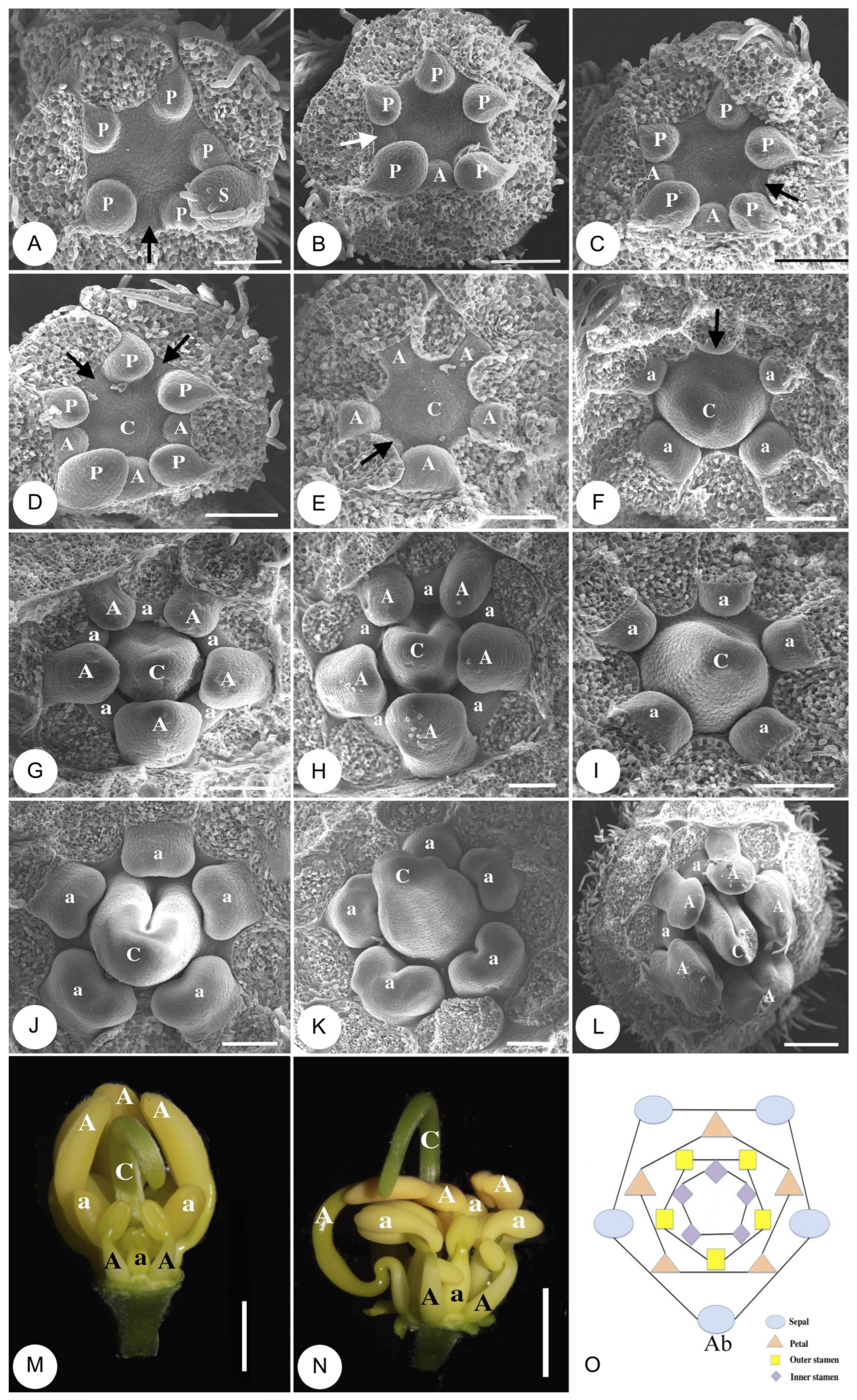
2.2. Morphological Differentiation of Androecium

2.3. Developmental Process of the Three Stamen Sets
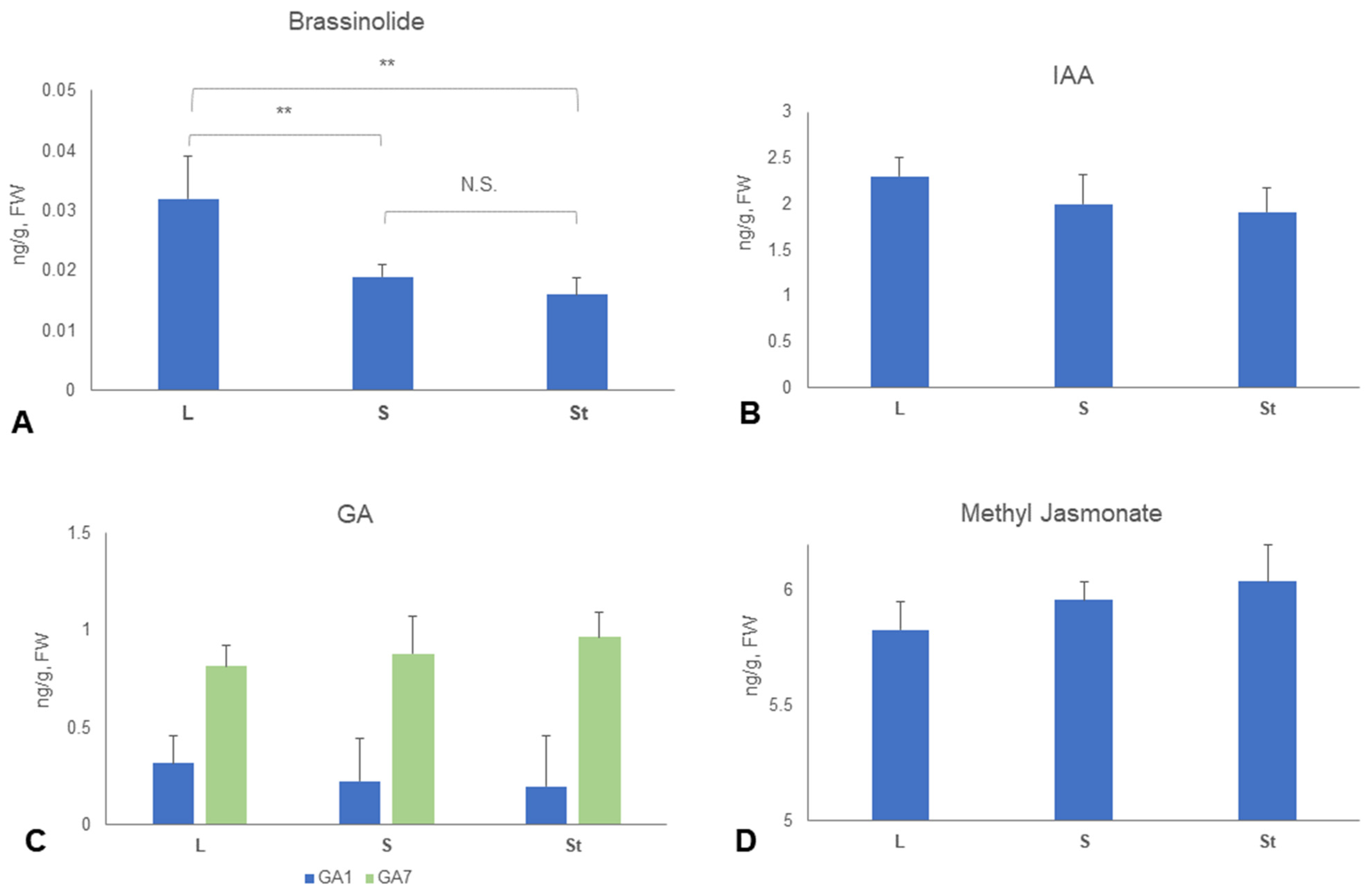
2.4. Phytohormone Quantification and Comparison
2.5. RNA-Sequencing, Functional Annotation, and Classification
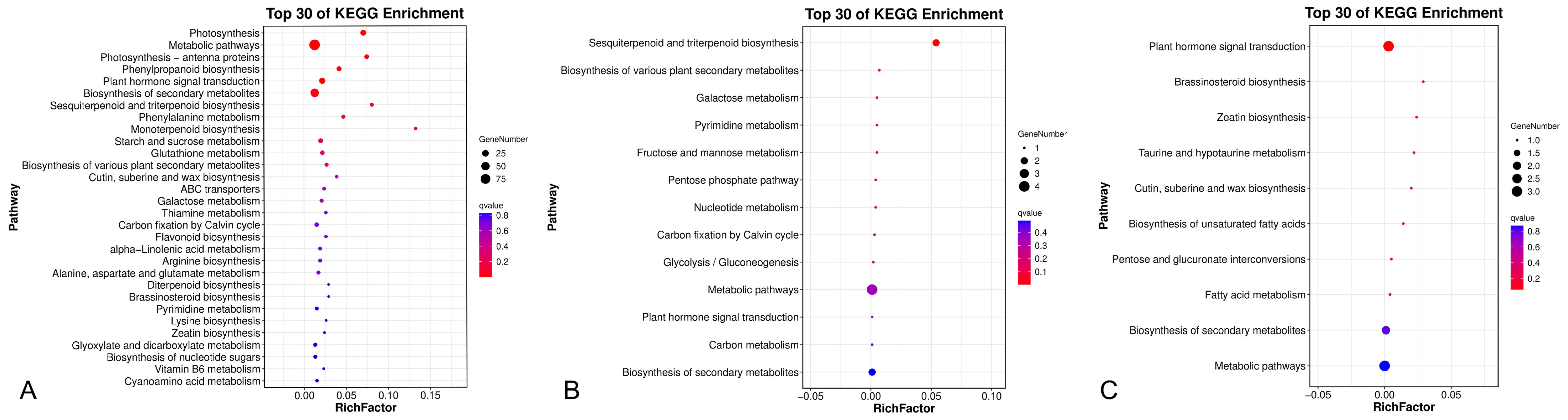
2.6. Differential Expression and Enrichment Analysis of Unigenes
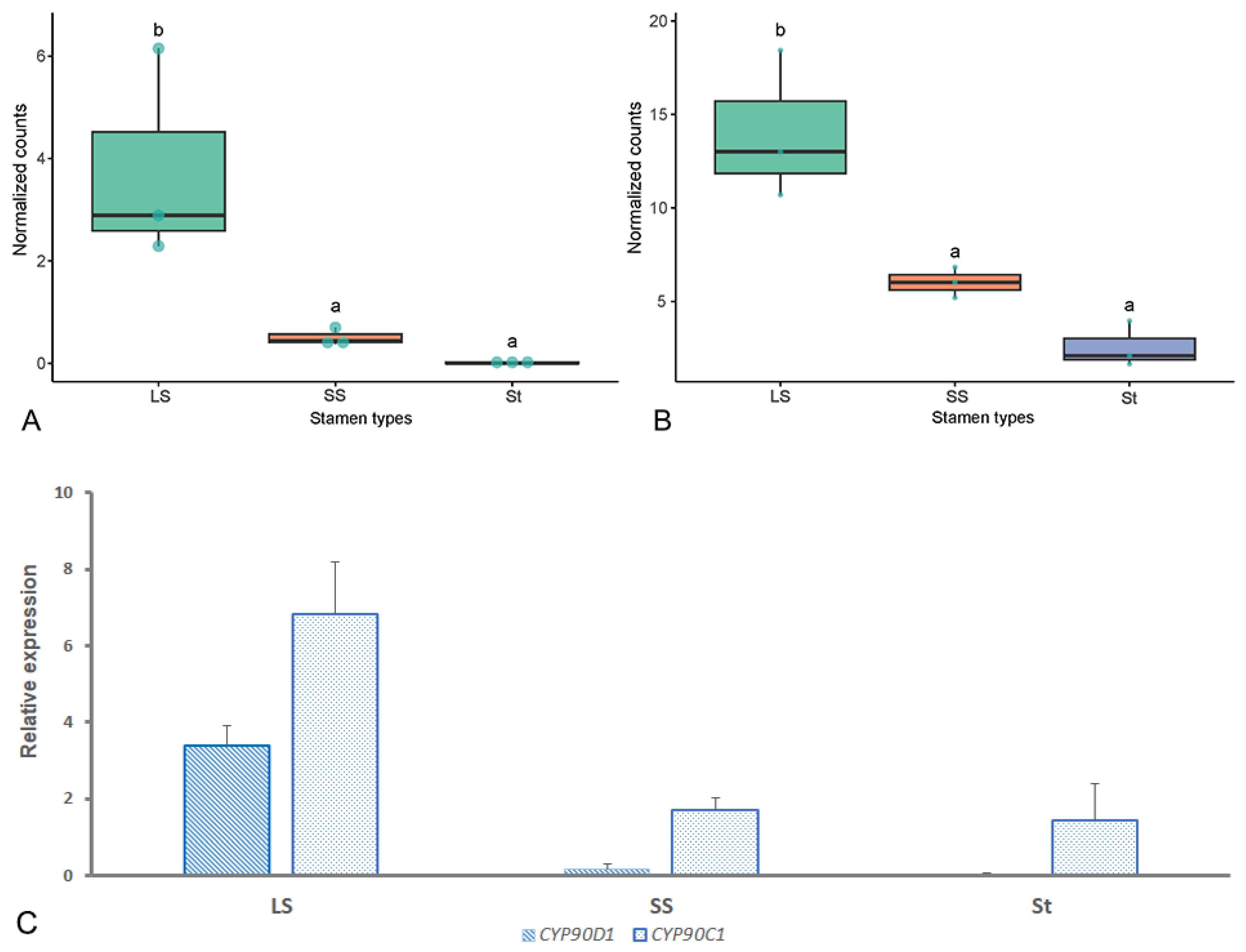
2.7. Screening of Genes Potentially Associated with Heteranthery
2.8. Protein–Protein Interaction Network Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Floral Biology Study
4.2. Stamen Morphogenesis and Development
4.3. Phytohormone Measurements and Analysis
4.4. RNA Extraction, Library Construction, and Sequencing
4.5. Gene Functional Annotation
4.6. Gene Expression and Enrichment Analysis
4.7. Protein–Protein Interaction Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, S.C.H. Mating strategies in flowering plants: The outcrossing-selfing paradigm and beyond. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 991–1004. [Google Scholar] [CrossRef]
- Barrett, S.C.H. Sexual interference of the floral kind. Heredity 2002, 88, 154–159. [Google Scholar] [CrossRef]
- Stanton, M.L.; Snow, A.A.; Handel, S.N. Floral evolution-attractiveness to pollinators increases male fitness. Science 1986, 232, 1625–1627. [Google Scholar] [CrossRef]
- Willson, M.F. Sexual selection in plants. Am. Nat. 1979, 113, 777–790. [Google Scholar] [CrossRef]
- Vallejo-Marín, M.; Da Silva, E.M.; Sargent, R.D.; Barrett, S.C.H. Trait correlates and function of heteranthery. New Phytol. 2010, 188, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H. Heteranthery. Curr. Biol. 2021, 31, R774–R776. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.L.; Gu, L.; Zhang, D.X. Intrafloral differentiation of stamens in heterantherous flowers. J. Syst. Evol. 2009, 47, 43–56. [Google Scholar] [CrossRef]
- Luo, Z.L.; Zhang, D.X.; Renner, S.S. Why two kinds of stamens in buzz-pollinated flowers? Experimental support for Darwin’s division-of-labour hypothesis. Funct. Ecol. 2008, 22, 794–800. [Google Scholar] [CrossRef]
- Vallejo-Marín, M.; Manson, M.; Thomson, J.D.; Barrett, S.C.H. Division of labour within flowers: Heteranthery, a floral strategy to reconcile contrasting pollen fates. J. Evol. Biol. 2009, 22, 828–839. [Google Scholar] [CrossRef]
- Todd, J.E. On the flowers of Solanum rostratum and Cassia chamaecrista. Am. Nat. 1882, 16, 281–287. [Google Scholar] [CrossRef]
- Hooker, J.D. Letter to Darwin C.R. 1 January 1862. Available online: https://www.darwinproject.ac.uk/DCP-LETT-3373 (accessed on 7 November 2024).
- Darwin, C.R. Letter to Asa Gray. 22 January 1862. Available online: https://www.darwinproject.ac.uk/DCP-LETT-3404 (accessed on 7 November 2024).
- Darwin, C.R. The Various Contrivances by Which Orchids Are Fertilised by Insects; John Murray: London, UK, 1899. [Google Scholar]
- Müller, H. Two kinds of stamens with different functions in the same flower. Nature 1882, 24, 307–308. [Google Scholar] [CrossRef]
- Lee, R.E. Pollen dimorphism in Tripogandra grandiflora. Baileya 1961, 9, 53–56. [Google Scholar]
- Dulberger, R. The floral biology of Cassia didymobotrya and C. auriculata (Caesalpiniaceae). Am. J. Bot. 1981, 68, 1350–1360. [Google Scholar] [CrossRef]
- Luo, Z.L.; Chen, S.; Zhang, D.X. Floral reward presentation favored the expression of male function in the pollen-only flower Melastoma malabathricum. J. Syst. Evol. 2012, 50, 488–495. [Google Scholar] [CrossRef]
- Mesquita-Neto, J.N.; Costa, B.K.P.; Schlindwein, C. Heteranthery as a solution to the demand for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biol. 2017, 19, 942–950. [Google Scholar] [CrossRef]
- Dellinger, A.S.; Artuso, S.; Pamperl, S.; Michelangeli, F.A.; Penneys, D.S.; Fernández-Fernández, D.M.; Alvear, M.; Almeda, F.; Armbruster, W.S.; Staedler, Y.; et al. Modularity increases rate of floral evolution and adaptive success for functionally specialized pollination systems. Commun. Biol. 2021, 4, 453. [Google Scholar]
- Tucker, S.C. Trends in evolution of floral ontogeny in Cassia sensu stricto, Senna, and Chamaecrista (Leguminosae: Caesalpinioideae: Cassieae: Cassiinae); A study in convergence. Am. J. Bot. 1996, 83, 687–711. [Google Scholar] [CrossRef]
- Wang, J. A Comparative Study on Pollen Heteromorphism in Heterantherous Plants. Master’s Dissertation, Chinese Academy of Sciences, Guangzhou, China, 2021. [Google Scholar]
- Luo, Z.L.; Barrett, S.C.H.; Tu, T.Y.; Zhao, Z.T.; Jia, S.S.; Gu, S.R.; Duan, T.T.; Zhang, Y.; Xu, B.Q.; Gu, L.; et al. Genetic architecture of the S-locus supergene revealed in a tetraploid distylous species. New Phytol. 2025, 248, 1973–1988. [Google Scholar] [CrossRef]
- Yuan, S.; Barrett, S.C.H.; Tang, C.Q.; Zhang, Y.M.; Sun, Q.L.; Zhao, Z.T.; Zhang, Y.; Zhang, D.X.; Luo, S.X. Genomic evidence unveils the genetic architecture and evolution of the S-locus controlling heterostyly in Rubiaceae. New Phytol. 2025, 247, 1925–1941. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Xue, H.R.; Li, Z.Z.; Zhang, Y.; Shi, T.; He, X.Y.; Barrett, S.C.H.; Wang, Q.F.; Chen, J.M. Haplotype-resolved genome assembly provides insights into the evolution of S-locus supergene in distylous Nymphoides indica. New Phytol. 2023, 240, 2058–2071. [Google Scholar] [CrossRef]
- Shore, J.S.; Hamam, H.J.; Chafe, P.D.J.; Labonne, J.D.J.; Henning, P.M.; McCubbin, A.G. The long and short of the S-locus in Turnera (Passifloraceae). New Phytol. 2019, 224, 1316–1329. [Google Scholar] [CrossRef]
- Huu, C.N.; Kappel, C.; Keller, B.; Sicard, A.; Takebayashi, Y.; Breuninger, H.; Nowak, M.D.; Bäurle, I.; Himmelbach, A.; Burkart, M.; et al. Presence versus absence of CYP734A50 underlies the style-length dimorphism in primroses. eLife 2016, 5, e17956. [Google Scholar] [CrossRef] [PubMed]
- Boualem, A.; Troadec, C.; Kovalski, I.; Sari, M.A.; Perl-Treves, R.; Bendahmane, A. A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science 2016, 353, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Hileman, L.C. Trends in flower symmetry evolution revealed through phylogenetic and developmental genetic advances. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130348. [Google Scholar] [CrossRef]
- Yang, J.S.; Chen, J.M.; He, X.Y.; Wang, G.X.; Barrett, S.C.H.; Li, Z.Z. The Monochoria genome provides insights into the molecular mechanisms underlying floral heteranthery. J. Genet. Genomics. 2025, 52, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Müller, F. Two kinds of stamens with different functions in the same flower. Nature 1883, 27, 364–365. [Google Scholar] [CrossRef]
- Meehan, T. On the Fertilization of Cassia marilandica. Proc. Acad. Nat. Sci. Phila. 1886, 38, 314–318. [Google Scholar]
- Tucker, S.C. Floral development in Legumes. Plant Physio. 2003, 131, 911–926. [Google Scholar] [CrossRef]
- Buchmann, S.L. Buzz pollination of Cassia quiedondilla (Leguminosae) by bees of the genera Centris and Melipona. Bull. South. Calif. Acad. Sci. 1974, 73, 171–173. [Google Scholar]
- Marazzi, B.; Conti, E.; Endress, P.K. Diversity in anthers and stigmas in the buzz-pollinated genus Senna (Leguminosae, Cassiinae). Int. J. Plant Sci. 2007, 168, 371–391. [Google Scholar] [CrossRef]
- Marazzi, B.; Endress, P.K. Patterns and development of floral asymmetry in Senna (Leguminosae, Cassiinae). Am. J. Bot. 2008, 95, 22–40. [Google Scholar] [CrossRef]
- Marazzi, B.; Endress, P.K.; Queiroz, L.P.; Conti, E. Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: Patterns in the evolution of floral symmetry and extrafloral nectaries. Am. J. Bot. 2006, 93, 288–303. [Google Scholar] [CrossRef]
- Ren, H.D. Intrafloral Stamen Differentiation and Its Ddaptive Significance in Cassia fistula L. Master’s Dissertation, Chinese Academy of Sciences, Guangzhou, China, 2017. [Google Scholar]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Nepi, M.; Guarnieri, M.; Pacini, E. “Real” and feed pollen of Lagerstroemia indica: Ecophysiological differences. Plant Biol. 2003, 5, 311–314. [Google Scholar] [CrossRef]
- Endress, P.K. Relationships between floral organization, architecture, and pollination mode in Dillenia (Dilleniaceae). Plant Syst. Evol. 1997, 206, 99–118. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, S. Floral organogenesis in Cassia fistula L. (Caesalpiniaceae). Proc. Indian Acad. Sci. 1978, 87B, 215–221. [Google Scholar] [CrossRef]
- Luo, Z.L.; Hu, J.; Zhao, Z.T.; Zhang, D.X. Transcriptomic analysis of heteromorphic stamens in Cassia biscapsularis L. Sci. Rep. 2016, 6, 31600. [Google Scholar]
- Xie, L.; Yang, C.; Wang, X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 2011, 62, 4495–4506. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, J.; Fracassetti, M.; Berdan, E.L.; Bunikis, I.; Soler, L.; Dainat, J.; Kutschera, V.E.; Losvik, A.; Désamoré, A.; Hughes, P.W.; et al. Genomic analyses of the Linum distyly supergene reveal convergent evolution at the molecular level. Curr. Biol. 2022, 32, 4360–4371. [Google Scholar] [CrossRef]
- Zhao, Z.T.; Zhang, Y.; Shi, M.M.; Liu, Z.Y.; Xu, Y.Q.; Luo, Z.L.; Yuan, S.; Tu, T.Y.; Sun, Z.L.; Zhang, D.X.; et al. Genomic evidence supports the genetic convergence of a supergene controlling the distylous floral syndrome. New Phytol. 2023, 237, 601–614. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Liu, W.; Yang, H.; Wang, C.; Chen, W.; Zhang, X.; Tian, J.; Yu, Y.; Li, J.; et al. Brassinosteroid signals cooperate with katanin-mediated microtubule severing to control stamen filament elongation. EMBO J. 2023, 42, e111883. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Bajguz, A.; Zhou, J.; Bhardwaj, R. Analysis of Brassinosteroids in Plants. J. Plant Growth Regul. 2017, 36, 1002–1030. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Ohnishi, T.; Szatmari, A.M.; Watanabe, B.; Fujita, S.; Bancos, S.; Koncz, C.; Lafos, M.; Shibata, K.; Yokota, T.; Sakata, K.; et al. C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 2006, 18, 3275–3288. [Google Scholar] [CrossRef]
- Zhan, H.; Lu, M.; Luo, Q.; Tan, F.; Zhao, Z.; Liu, M.; He, Y. OsCPD1 and OsCPD2 are functional brassinosteroid biosynthesis genes in rice. Plant Sci. 2022, 325, 111482. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Ohnishi, T.; Fujioka, S.; Watanabe, B.; Mizutani, M. Rice CYP90D2 and CYP90D3 catalyze C-23 hydroxylation of brassinosteroids in vitro. Plant Physiol. Biochem. 2012, 58, 220–226. [Google Scholar] [CrossRef]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S.; The AmiGO Hub; Web Presence Working Group. AmiGO: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Khatri, P.; Sirota, M.; Butte, A.J. Ten years of pathway analysis: Current approaches and outstanding challenges. PLoS Comput. Biol. 2012, 8, e1002375. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [PubMed]

| Anno_Database | Annotated_Number | 300 ≤ Length < 1000 | Length ≥ 1000 |
|---|---|---|---|
| COG | 19,179 | 7519 | 6677 |
| GO | 42,783 | 19,494 | 12,304 |
| KEGG | 27,609 | 12,872 | 7230 |
| KOG | 39,141 | 17,660 | 11,647 |
| Pfam | 38,903 | 16,280 | 14,562 |
| Swissprot | 42,912 | 19,325 | 13,496 |
| eggNOG | 64,952 | 29,132 | 18,657 |
| nr | 70,647 | 32,164 | 19,286 |
| All_Annotated | 72,815 | 32,544 | 19,325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Duan, T.; Li, X.; Zhou, J.; Liu, Q.; Jiang, L. Integrated Transcriptomic and Developmental Analyses Provide Insights into the Intrafloral Stamen Differentiation in Cassia fistula L. Plants 2025, 14, 3490. https://doi.org/10.3390/plants14223490
Luo Z, Duan T, Li X, Zhou J, Liu Q, Jiang L. Integrated Transcriptomic and Developmental Analyses Provide Insights into the Intrafloral Stamen Differentiation in Cassia fistula L. Plants. 2025; 14(22):3490. https://doi.org/10.3390/plants14223490
Chicago/Turabian StyleLuo, Zhonglai, Tingting Duan, Xiaoyuan Li, Jianxuan Zhou, Qiankun Liu, and Libo Jiang. 2025. "Integrated Transcriptomic and Developmental Analyses Provide Insights into the Intrafloral Stamen Differentiation in Cassia fistula L." Plants 14, no. 22: 3490. https://doi.org/10.3390/plants14223490
APA StyleLuo, Z., Duan, T., Li, X., Zhou, J., Liu, Q., & Jiang, L. (2025). Integrated Transcriptomic and Developmental Analyses Provide Insights into the Intrafloral Stamen Differentiation in Cassia fistula L. Plants, 14(22), 3490. https://doi.org/10.3390/plants14223490









