Abstract
Studies on how SlPR5 genes are involved in Pst DC3000 disease resistance response are lacking. Here, 27 members of the tomato SlPR5 gene family were identified and analyzed. Analysis of conserved structural domains and promoter structure revealed that SlPR5 family members are structurally conserved and contain a variety of antidisease and antistress response elements. We screened out SlPR5-3, which was significantly upregulated, through an analysis of the expression pattern of Pst DC3000 in tomato after inoculation. We generated SlPR5-3 mutants via CRISPR/Cas9 gene editing and SlPR5-3-overexpressing tomato plants to elucidate the function of this gene. The results showed that the SlPR5-3 overexpression lines had reduced lesions, significantly lower pathogen counts, and significantly higher activity indexes of defense-related enzymes, while the mutant lines showed the opposite, indicating that the SlPR5-3 gene positively regulates the immune response against Pst DC3000 in tomato. In this study, we systematically mined and analyzed the tomato SlPR5 family genes, screened out the important genes in this family for the regulation of Pst DC3000 disease resistance, and verified the disease resistance regulatory function of SlPR5-3, laying the foundation for the theoretical study of tomato Pst DC3000 disease resistance and providing a new molecular target for the future breeding of tomato disease resistance.
1. Introduction
As a valuable horticultural cash crop and an important part of the human diet, tomato (Solanum lycopersicum) is one of the main vegetable crops in China. A variety of factors affect the yield and quality of tomato [1], and disease is among the major constraints to tomato production. Bacterial leaf spot of tomato, also known as spot or blotch disease, is a very serious bacterial disease caused by Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) [2]. Bacterial leaf spot may occur throughout the reproductive period of tomato, and it mainly affects the leaves, stems, flowers, fruit stalks, and fruits [3]. In addition to fungal and viral diseases, bacterial diseases have a major impact on plants. The prevalence of bacterial diseases of vegetables is increasing annually, and these diseases occur more frequently in cruciferous, cucurbit, lycopene and leguminous crops, which are common economic crops, resulting in substantial economic losses. Bacterial diseases are widespread and can easily spread over a large area when conditions are favorable, resulting in massive yield loss and field destruction; thus, bacterial control needs to be emphasized during production. Currently, disease control relies mainly on chemical pesticides, but the frequent use of pesticides poses a serious threat to food safety and the ecological environment.
Typical tomato disease resistance responses to Pst DC3000 include PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI). When Pst DC3000 infects tomato leaves [4], the 22-amino-acid peptide of the N-terminal end of the bacterial flagellin flg22 and its derivatives flgII-28 activate the pattern recognition receptors (PRRs) Fls2 and Fls3, respectively [5,6,7], triggering early PTI responses such as reactive oxygen species (ROS) production, activation of the mitogen-activated protein kinase (MAPK) cascade, and transcriptional reprogramming of a subset of defense genes [8,9,10]. Two Pst effector proteins, AvrPto and AvrPtoB [11,12], subsequently bind and interfere with the binding of the intracellular protein kinase structural domains of Fls2, Fls3, and the coreceptor Bak1, thereby disrupting the host response to these microbe-associated molecular patterns (MAMPs) [13]. Moreover, these two effector proteins are recognized by the host Pto kinase and activate ETI via the NLR protein Prf [14,15].
The regulatory network involved in plant disease resistance to Pst DC3000 is very complex, and recent studies have shown that a variety of biological processes and different types of regulatory factors are involved in this network. Transcription factors, such as the Pto interaction protein, Pti4 and Pti5 [16], which belong to the ERF (ethylene-response factor) family, play important roles and can improve resistance to bacterial leaf spot in Arabidopsis and tomato [17]. AtWRKY1 can bind to the promoter of the disease resistance-associated protein AtPR1 to repress its transcription and thus negatively regulate Arabidopsis resistance to Pst DC3000 [18]. The expression of the tomato bHLH transcription factor SlNrd1 protein increases tomato susceptibility to Pst DC3000 by repressing the expression of the defense gene SlAgp1 [19]. Mutation of the Arabidopsis transcription factor AtREPLUMLESS (AtRPL) decreases the expression of the AtGH3 promoter, resulting in the accumulation of indole-3-acetic acid aspartate (IAA-Asp) and the activation of the transcription of virulence effector genes. In addition to transcription factors, many other types of proteins and regulatory pathways are involved in the regulation of Pst DC3000 resistance; e.g., the tomato sugar transporter protein SlSTP2 is induced by Pst DC3000 and positively regulates tomato resistance to bacterial leaf spot, and SlSTP2 interacts with the positive regulator SlAGB1 [20]. The histone deacetylase HDA6 reduces Arabidopsis resistance to Pst DC3000 by inhibiting the biosynthesis of salicylic acid [21]. As research continues to expand and deepen, more details of the regulation of the plant response to Pst DC3000 disease resistance are being revealed, but the regulatory processes still need to be explored in depth to elucidate the basic regulatory network.
Disease course-associated proteins compose a general class of proteins whose production is induced by biotic or abiotic stresses in plants [22]. A protein associated with allergic reactions was detected for the first time in tobacco leaves infected with tobacco mosaic virus (TMV) and referred to as a disease process-related protein, abbreviated as PR or PRP [23]. With the in-depth study and development of disease course-related proteins, PR proteins were categorized into 17 families, distributed as PR-1~17 [24]. Studies on the role of PR proteins in plant fruiting and stress tolerance have focused on PR-1, PR-2, PR-5, and PR-10. These classes of proteins have antimicrobial activity, with PR-5 exhibiting antifungal activity and resistance to abiotic stresses [25]. SlPR5 family proteins are called thaumatin-like proteins (TLPs) because of their high amino acid sequence homology to thaumatin of the African species Thaumatococcus daniellii L. The TLPs are also known as “sweet taste proteins” [26]. SlPR5 proteins are unique proteins with multiple functions, such as antimicrobial activity; they are also associated with plant stress tolerance, such as increased tolerance to frost [27] and enhanced tolerance to salt stress and drought stress [25]. The SlPR5 family is an important family of disease process-associated proteins in the plant kingdom that are associated with responses to biotic stresses. Twenty-seven SlPR5 family members have been identified in the tomato genome, and PR5 genes have been characterized in many species other than tomato; 43 PR5 members have been identified in the Setaria italica genome [28], 32 in Allium sativum [29], 42 in Glycine max [30], and 31 in Camellia sinensis [31], and some PR5 members exhibit broad-spectrum resistance to a variety of pathogens. For example, the PR5-x gene plays a role in tomato resistance to Fusarium oxysporum, and the accumulation of the PR-P2 protein is associated with disease resistance [32]. NP24 I and NP24 II were found to inhibit the growth of Fusarium beetles and Trichoderma ferruginea in ripe tomato fruits [33]. Similarly, exudin-like PR-5 proteins not only are tolerant to abiotic stresses in plants but also have a wide range of antifungal activities [34]. In addition, thaumatin-like protein increased the resistance of tomato to five fungal pathogens and bacterial pathogens by increasing the activity of β-1,3-glucanase [35]. Pst DC3000 infestation of tomato induced a significant up-regulation of SlPR5 gene expression through activation of the salicylic acid-mediated pathway [36,37,38]. Up-regulation of PR5 expression in Arabidopsis improves resistance to Bacterial Leaf Spot [39]. Although the roles of SlPR5 genes in disease resistance in tomato have been described, little has been reported on their roles in bacterial leaf spot resistance in tomato.
In view of the important role of the SlPR5 family of genes in plant disease resistance, this study utilized bioinformatics techniques for the genome-wide identification and functional prediction of the SlPR5 gene family in tomato. A preliminary analysis of the motif composition and promoter cis-elements was carried out to clarify the structural basis of the disease resistance of this family of genes. Further functional prediction was carried out on the basis of the RNA-seq data via expression pattern analysis, after which the SlPR5 genes were determined to have significant differential expression patterns under biotic stress (Pst DC3000) and in specific tissues. Finally, the SlPR5-3 gene with the most important role was predicted at the genome level and transcript level, and its function was verified by experimental analysis. This study provides a molecular target (SlPR5-3) for the genetic improvement of tomato resistance to bacterial leaf spot.
2. Materials and Methods
2.1. Identification and Evolutionary Tree Construction of SlPR5 Gene Family Members in Tomato
Tomato genome and gene annotation files were downloaded from the Solanaceae Genomics Network (https://solgenomics.net/, accessed on 3 September 2023). website [40]. A hidden Markov model (HMM E-value < 1 × 10−5) (http://hmmer.janelia.org/, accessed on 5 September 2023) of the conserved structural domain of SlPR5 (No. PF 00314) was constructed from the Pfam database (http://pfam.xfam.org/family, accessed on 11 September 2023) for the identification of all possible SlPR5 members of the tomato genome in the transcription factor family [41]. Further screening of tomato SlPR5 family members was carried out using NCBI-BLASTP (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 16 September 2023) (BLAST E-value < 1 × 10−5~1 × 10−10). Finally, sequence analysis of tomato SlPR5 family members was carried out using NCBI-CDD (https://www.ncbi.nlm.nih.gov/cdd/, accessed on 20 September 2023) to confirm the members of the tomato SlPR5 gene family. Nucleotide sequences (https://sgn.cornell.edu/help/index.pl/, accessed on 23 September 2023) and amino acid sequences of 27 tomato SlPR5 candidate genes were downloaded from the Ensembl Plants database (http://plants.ensembl.org/index.html/, accessed on 24 September 2023) for subsequent analysis [42]. The 27 conserved structural domains of the tomato SlPR5 protein were validated by the SMART database (http://smart.embl-heidelberg.de/, accessed on 27 September 2023). The Arabidopsis SlPR5 protein sequence was obtained from The Arabidopsis Information Resource (TAIR) (http://www.arabidopsis.org/, accessed on 28 September 2023) [43]. SlPR5 protein sequences from tomato and Arabidopsis were subjected to multiple sequence comparisons by ClusterX. On the basis of the comparison results, a rootless developmental tree was constructed using MEGA 7 with the neighbor-joining (NJ) method.
2.2. Analysis of the Physicochemical Properties of the SlPR5 Family Members in Tomato
The amino acid number, relative molecular weight and theoretical isoelectric point of SlPR5 family members were predicted by ExPASy’s ProtParam tool (https://web.expasy.org/protparam/, accessed on 30 September 2023) [44]. Signal peptides of 27 tomato SlPR5 proteins were predicted using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/, accessed on 8 October 2023) [45] and WoLF PSORT (https://wolfpsort.hgc.jp/, accessed on 11 October 2023) to predict their subcellular localization [46].
2.3. Chromosomal Localization Prediction, Gene Structure and Conserved Structural Domain Analysis of SlPR5 in Tomato
The annotation information of 27 tomato SlPR5 genomes was retrieved from SGN (https://sgn.cornell.edu/help/index.pl, accessed on 13 October 2023), and the chromosomal position of tomato SlPR5 was obtained from MG2C (http://mg2c.iask.in/mg2c_v2.0/, accessed on 15 October 2023). The structure and conserved structural domains of the tomato SlPR5 genes were analyzed via NCBI-CDD and visualization in TBtools [47]. To further clarify the functions of SlPR5 genes, the protein sequence was submitted to MEME (http://meme-suite.org/tools/meme, accessed on 18 October 2023) [48] to characterize the order of the SlPR5 genes in tomato. The maximum number of motifs was set to 10, and the other parameters were set to default values.
2.4. Analysis of Cis-Regulatory Elements
The 2000 bp upstream promoter sequences of 27 candidate genes were downloaded from the Tomato Genome Database (https://solgenomics.net/, accessed on 19 October 2023) and submitted to Plant-CARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 21 October 2023) for the prediction of cis-acting elements in the promoter regions.
2.5. Subcellular Localization of the SlPR5-3 Protein
Primer Premier 5.0 software was used to design primers for SlPR5-3-EGFP (Table S1) to amplify SlPR5-3 with the stop codon removed. The pCAMBIA2300-EGFP vector was enzymatically digested with BamHI and EcoRI, the target fragment was recombined with the plasmid, the recombinant plasmid was transfected into Escherichia coli, and the plasmid was transfected into Agrobacterium for the infection of tobacco plants. After 3 days of incubation in the dark, fluorescence imaging was performed by laser coaggregation microscopy.
2.6. Plant Material and Treatment
Ailsa Craig (AC) tomato material was used as the test material, which is susceptible to disease. All seedlings were grown in a thermostat incubator at a diurnal temperature of 20–25 °C with 16 h of light and 8 h of darkness, a light intensity of 35,000 lx, and a relative humidity of 45%. When the tomato seedlings reached the five-leaf stage, they were subjected to foliar sprays of Pst DC3000 and AC plant stress treatments, followed by qRT–PCR. Specifically, Pst DC3000 was cultured on King’s B (KB) medium supplemented with 50 mg/L rifampicin overnight until the OD600 was 0.6 to 0.8 (OD 0.1 = 108 cfu mL−1). Then, Pst DC3000 was diluted with 10 mM MgCl2 to a concentration of 107 cfu mL−1. Before infiltration, Pst DC3000 was resuspended in 10 mM MgCl2 to an OD600 of 0.0002 [4]. Ten seedlings were selected for each treatment, and all the treatments were repeated three times. Leaves from SlPR5-3 overexpression and CRISPR mutant plants were collected at 0, 12, 24, 48, and 72 h after pathogen stress for subsequent determination of physiological indices, immediately frozen in liquid nitrogen and stored at −80 °C.
2.7. Transcriptome Data Analysis
The expression of SlPR5 family members after inoculation with Pst DC3000 was queried through the Tomato Gene Function Database (http://ted.bti.cornell.edu/, accessed on 22 December 2023). A tissue-specific analysis of SlPR5 family members in tomato was performed through Plant Biology’s Bioanalytical Resources (http://bar.utoronto.ca/eplant/, accessed on 24 December 2023). An expression heatmap was constructed using the TBtools heatmap.
2.8. Real-Time Quantitative PCR (qRT–PCR) Analysis
Total RNA was extracted from the samples using TRIzol. cDNA synthesis was performed using an M-MLVRTase kit (TaKaRa, Dalian, China). qRT–PCR was performed using the iQ 5 system. The actin-7 gene (Solyc11g005330.1.1) was used as an internal control (Table S2). For each qRT–PCR, the mixture consisted of 10 μL of SYBR® Green Master Mix, 0.5 μL of forward and reverse primers, 1 μL of diluted cDNA, and 8 μL of ddH2O. In a 20 μL reaction, the reaction was carried out as follows: 95 °C for 5 min, followed by 40 cycles of 94 °C for 5 s, 60 °C for 15 s, and 72 °C for 10 s; three biological replicates were included.
2.9. Measurement of Physiological Indicators
Leaf samples of WT, OE-SlPR5-3, and Cr-SlPR5-3 plants were collected before and after pathogen infection, and each sample consisted of three replicates. Superoxide dismutase (SOD) and peroxidase (POD) activities as well as catalase (CAT) activity and malondialdehyde (MDA) content were determined according to the instructions provided with the kit used (Suzhou Grace Biotechnolgy Co., Ltd, Suzhou, China).
2.10. Construction and Validation of SlPR5-3-Overexpressing and CRISPR Mutant Lines
Primer Premier 5.0 software was used to design primers for the SlPR5-3 gene, clone the CDS of the SlPR5-3 gene, insert the CDS into the pCAMBIA2300-HA vector, and construct the overexpression plasmid. Overexpressing plants were obtained by Agrobacterium-mediated transformation, and then the overexpressing lines were screened with kanamycin and verified by genomic PCR and qRT–PCR. CRISPR mutant vectors were constructed, and targets were designed using the TargetDesign program on the CRISPR-GE web page (http://skl.scau.edu.cn/, accessed on 3 January 2024). The sgRNA expression cassettes for the two targets of each gene were constructed by using pYLgRNA-AtU3b and pYLgRNA-AtU3d as templates, amplified by the overlap method, and then ligated into the pYLCRISPR/Cas9P35S-H binary vector, the details of which are referenced from the operation published by Zeng, Dong-chang et al. [49].
2.11. Reactive Oxygen Species Staining
Reactive oxygen species (ROS) were detected using nitrogen blue tetrazolium (NBT) [50] and 3,3′-diaminobenzidine (DAB) staining [51]. Leaves of wild-type plants, SlPR5-3-overexpressing plants, and SlPR5-3 CRISPR mutant plants 3 days after inoculation with Pst DC3000 were immersed in 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) dye solutions. After incubation in the dark for 12 h, the dye solutions were discarded, 30 mL of anhydrous ethanol was added, and the material was heated in a water bath at 100 °C for 12 min while shaking every 3 min. If the color of the leaves did not fade completely, they were washed again several times with anhydrous ethanol. The fully discolored leaves were placed on slides to observe the staining and collect images.
2.12. Statistical Analysis
SPSS 24.0 (IBM) and prism 8.0 (GraphPad) software were used for statistical analysis. Student’s t-test (p < 0.05) and least significant difference (LSD) tests (p < 0.05) were performed to analyze the significant difference. All measurements were taken from the average of at least three independent biological replicates.
3. Results
3.1. Identification and Physical Characterization of the SlPR5 Family in Tomato
On the basis of HMM and conserved structural domain analysis, a total of 27 SlPR5 family members were identified in tomato, and their basic information is listed in Table 1. The predicted relative molecular weights of the proteins ranged from 8266.26 Da (SlPR5-21) to 97,494.25 Da (SlPR5-8); SlPR5-21 (75 aa) had the shortest protein sequence, and SlPR5-8 (878 aa) had the longest protein sequence. By predicting the physicochemical properties, we found that eleven of them were basic proteins (PI > 7) and the remaining 16 were acidic proteins (PI < 7). In addition, nine proteins of the SlPR5 family have a GRAVY index higher than 0, and the other SlPR5 proteins were hydrophilic. Among them, the minimum GRAVY index was 46.29 (SlPR5-19), and the maximum was 79.96 (SlPR5-15). Seventeen of the 27 SlPR5 family members were stable proteins (instability coefficient < 40), and the rest were unstable. As shown in Table 1, most members of the SlPR5 family are located in the cell wall and chloroplasts, with a few in other locations. The exact locations of these proteins require further experimental verification. The locus IDs for the tomato SlPR5 family are in Table S4.

Table 1.
Physicochemical properties of the tomato SlPR5 family.
3.2. Analysis of Protein Conserved Motifs and Structural Domains
A phylogenetic tree containing only the SlPR5 family was constructed using MEGA-X, which classified the SlPR5 family into three groups (Figure 1A), and conserved motifs and structural domains were analyzed for SlPR5 family members (Figure 1B,C). The results of the motif analysis revealed a total of 10 conserved motifs in the tomato SlPR5 family proteins. Among them, motif 2 and motif 8 were conserved in every SlPR5 family protein (Figure 1B). Analysis of the conserved structural domains of SlPR5 family proteins revealed that nine SlPR5 contained a conserved thaumatin structural domain, 10 SlPR5 contained a conserved TLP-PA structural domain, and seven SlPR5 contained a conserved GH64-TLP-SF structural domain (Figure 1C). All of these structural domains are associated with pathogen defense, and the SlPR5 family may play a regulatory role in the disease resistance pathway in tomato.
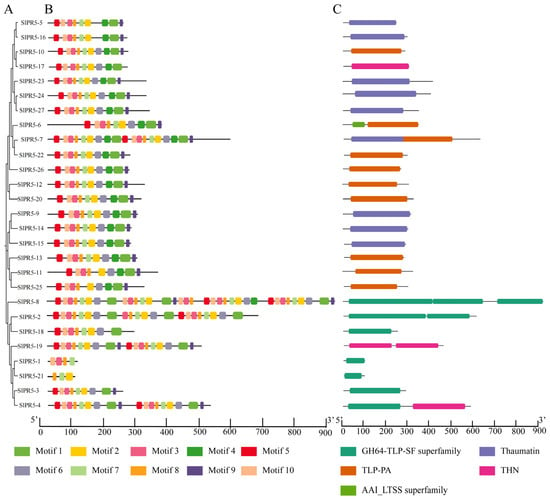
Figure 1.
NJ tree, motif and domain of SlPR5 in tomato. (A), NJ tree constructed by MEGA 7.0 software using SlPR5 family members. (B), Motif positions of tomato SlPR5 family members. The ten motifs are displayed in different colors. (C), Domain positions of tomato SlPR5 family members. The scale at the bottom shows the length of the motif and domain.
3.3. Chromosome Localization Prediction and Cis-Acting Element Analysis
Using tomato genome annotation information and TBtools software (v2.034), we visualized the chromosomal distribution of family members of the tomato SlPR5 gene family (Figure 2A). We found that tomato SlPR5 genes were unevenly distributed on the chromosomes, with chromosome 8 containing the greatest number of genes, five in total, and showing coaggregation, which may be related to tandem replication of the chromosomes.
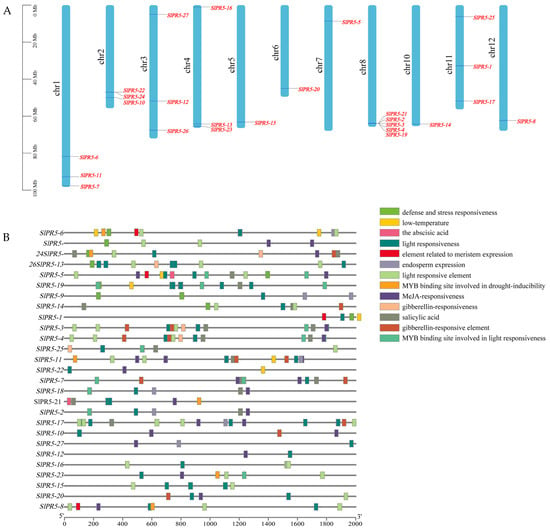
Figure 2.
Chromosomal mapping and cis-acting elements of the SlPR5 genes in tomato. (A) The chromosome number is located to the left of each chromosome, and the scale value on the left corresponds to the chromosome length. (B) The different colored squares represent different cis-acting elements.
To investigate the function of the tomato SlPR5 gene, the promoter sequence (2000 bp upstream of the CDS) of the tomato SlPR5 gene was analyzed to detect cis-elements (Figure 2B) using Plant-CARE. A total of 13 major classes of cis-acting elements were detected in the promoter regions of members of the tomato SlPR5 gene family. These include abiotic stress response elements in response to light, salt stress, and drought and low-temperature stress and hormone (ABA, GA, MeJA, and SA) response elements associated with disease resistance. These results suggest that tomato SlPR5 genes may play roles in tomato growth and development as well as in biotic and abiotic stress responses.
3.4. Analysis of SlPR5 Family Gene Expression Patterns in Tomato on the Basis of Transcriptome Data
To investigate the role of the SlPR5 gene family in various tomato tissues at different growth stages, we analyzed the expression levels of these genes in six tomato tissues, through Plant Biology’s Bioanalytical Resources (http://bar.utoronto.ca/eplant/, accessed on 15 October 2023) (Figure 3A). Among them, 14 genes had the highest expression in roots; 9 genes had the highest expression in leaves. Compared with the other genes, SlPR5-3 and SlPR5-4 had the highest expression in tomato fruits at the turn-color stage (Figure 3A). It is hypothesized that this gene may be closely related to plant resistance and growth and development. The expression of SlPR5 family members after inoculation with Pst DC3000 was queried through the Tomato Gene Function Database, and the expression of SlPR5-3, SlPR5-4, SlPR5-10, SlPR5-17, SlPR5-19, and SlPR5-23 significantly increased in disease-resistant tomato materials 6 h after inoculation (Figure 3B); these genes might be involved in the regulation of disease resistance in tomato.
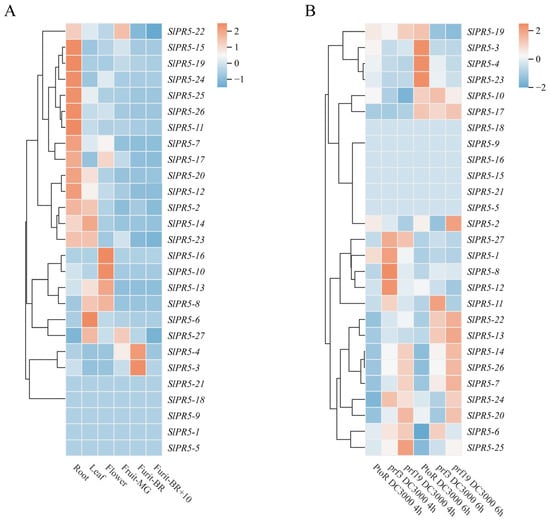
Figure 3.
Expression of SlPR5 genes. (A) The chromaticity on the right side of the heatmap shows the relative expression, and the color gradient from blue to red corresponds to an increase in expression. Fruit_MG indicates mature green (MG), Fruit_BR indicates a breaker (early ripening), and Fruit_BR +10 indicates 10 days post-B (red ripe). (B) Number of SlPR5 family members after Pst DC3000 inoculation. The chromaticity on the right side of the heatmap represents the relative expression, and the color gradient from green to red corresponds to an increase in expression. Note: PtoR is a disease resistant variety, prf is a susceptible variety.
3.5. Analysis of the Disease Resistance Response of SlPR5 Genes to Pst DC3000 and the Tissue Specificity of SlPR5-3
Based on the transcriptome data, it was observed that the expression of six genes was significantly up-regulated in the disease-resistant varieties. To explore the response patterns of the above six SlPR5 genes in susceptible tomato varieties inoculated with Pst DC3000, we used ‘Ailsa Craig (AC)’ tomato as a material and determined the expression of SlPR5 members in plants inoculated with Pst DC3000 at 0 h and 24 h (Figure 4A). The expression levels of the SlPR5-10, SlPR5-17, and SlPR5-22 genes were not significantly upregulated at 24 h compared with those at 0 h, whereas the expression levels of SlPR5-3, SlPR5-4, and SlPR5-23 were significantly upregulated, with the greatest upregulation occurring in SlPR5-3. These results suggest that SlPR5-3 gene expression is induced by Pst DC3000 and that this gene may play an important role in tomato resistance to bacterial leaf spot. Therefore, We chose the SlPR5-3 gene with the highest expression for functional validation, and the others may continue to be analyzed in future studies. A phylogenetic comparison of SlPR5-3 with other Solanaceae species was also made (Figure S1).
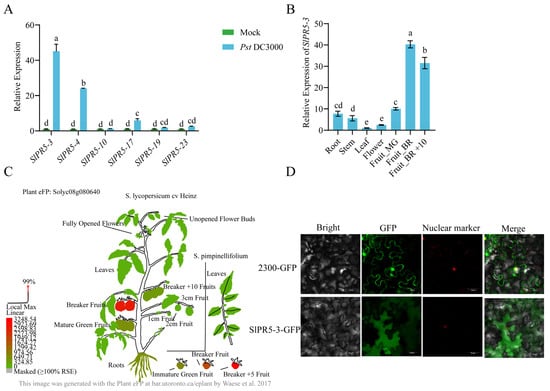
Figure 4.
Analysis of tissue expression patterns of SlPR5-3. (A) Number of SlPR5 family members in ‘Ailsa Craig’ tomato after Pst DC3000 inoculation. (B) Tissue-specific expression. Fruit_MG indicates mature green (MG), Fruit_BR indicates breaker fruits (early ripening), and Fruit_BR +10 indicates 10 days post-B (red ripe). Letters (a–e) indicate significant differences (p ≤ 0.05). (C) Graphical representation of the SlPR5-3 gene content in various tissues of tomato plants [52]. (D) Results of the subcellular localization of SlPR5-3.
To investigate the transcript levels of SlPR5-3 in different tissues, the expression of SlPR5-3 in different tissues of tomato was predicted using an expression prediction website and verified by qRT–PCR (Figure 4B,C). The results revealed that the SlPR5-3 gene was expressed in all the tissues, with higher and gradually increasing expression in Fruit_MG, Fruit_B, and Fruit_B +10, with the highest expression in Fruit-B and lower expression in the leaves and flowers (Figure 4B,C). The above results indicated that SlPR5-3 gene expression differed significantly among the different tissues and was most highly expressed in the BR_+10 fruits. Thus, the SlPR5-3 gene may play an important role in tomato fruit ripening.
To clarify the site of action of SlPR5-3 in plant cells, the subcellular localization of SlPR5-3 was determined. As shown in Figure 4D, the SlPR5-3-GFP fusion protein was distributed in the cytoplasm when expressed, indicating that the SlPR5-3 protein is localized in the cytoplasm.
3.6. Generation of SlPR5-3 Mutants and Overexpression Plants
In the identification of SlPR5-3 mutants and overexpression plants, the genome is amplified with primers flanking the target site, and the amplified PCR product is sequenced. From the sequencing results, the Cr-12, Cr-16, Cr-17, Cr-23, and Cr-25 lines produced 3 bp, 5 bp, 7 bp, 6 bp, and 1 bp fragment deletions, respectively (Figure 5A), and three lines, Cr-16, Cr-17, and Cr-23, were selected for the subsequent experiments. The expression of SlPR5-3 in these five lines was examined by qRT–PCR of the positive plants, and the results revealed that the expression of the SlPR5-3 gene was approximately 10-fold, 17-fold, 36-fold, and 44-fold greater in the four SlPR5-3-overexpressing lines than in the wild-type line (Figure 5B). All the above four lines were available for subsequent experiments, and full progeny seeds from the four lines OE-3, OE-6 and OE-8 were selected for subsequent studies. All the overexpression plants tested were T2 generation plants.
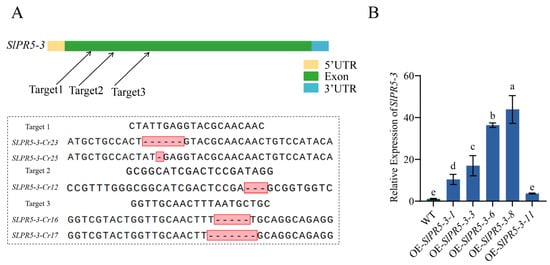
Figure 5.
SlPR5-3 identification in positive plants of the overexpression and CRISPR mutation lines. (A) Sequencing results of the T1 generation of SlPR5-3 gene CRISPR mutant lines; (B) Identification of SlPR5-3-overexpressing lines. Letters (a–e) indicate significant differences (p ≤ 0.05).
3.7. Phenotypic Analysis of Disease Resistance in SlPR5-3-Overexpressing and Mutant Lines
To investigate the function and role of SlPR5-3, which is strongly induced and expressed by Pst DC3000, in tomato resistance to bacterial leaf spot, Pst DC3000 inoculation assays were performed on one-month-old seedling-sized WT, OE-SlPR5-3, and Cr-SlPR5-3 lines. Compared with the WT strain, the SlPR5-3 CRISPR mutant strain showed a significant increase in the number of bacteria on the leaves (Figure 6B) and increased accumulation of H2O2 and O2▪− compared with the WT (Figure 6A), whereas the overexpression strain showed a lower number of bacteria on the leaves, a decreased accumulation of H2O2 and O2▪− and increased resistance to disease. These results suggest that SlPR5-3 positively regulates disease resistance by either attenuating ROS damage or promoting ROS scavenging.
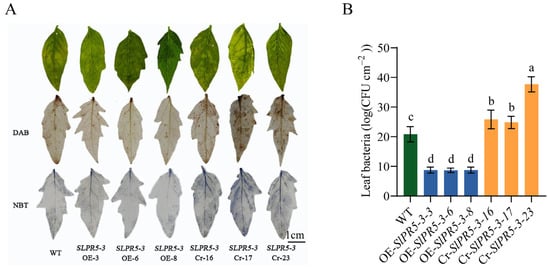
Figure 6.
Changes in disease symptoms in SlPR5-3-overexpressing and CRISPR mutant tomato plants inoculated with Pst DC3000. (A) Leaf H2O2 and O2▪− accumulation was observed by DAB and NBT staining 3 days after inoculation with Pst DC3000. (B) The number of bacteria on the leaves. Letters (a–d) indicate significant differences (p ≤ 0.05).
3.8. Measurement of Physiological Indicators of the Disease Resistance Response in SlPR5-3-Overexpressing and Mutant Lines
Physiological indices of three overexpression lines, three CRISPR-mutant lines and wild-type plants inoculated with the Pst DC3000 pathogen for 3 d were determined, as shown in Figure 4. The disease resistance of the SlPR5-3 gene was determined by evaluating the SOD, POD, and CAT activities as well as the MDA content (Figure 7). The numerical data are shown in Table S3. Under the stress of bacterial leaf spot disease in tomato, the SOD, POD and CAT activities in the overexpression-treated plants were significantly higher than in the control, whereas they were decreased in the mutant lines (Figure 7). Compared with that in the WT strain, the MDA content in the overexpression strain was reduced and that in the CRISPR mutant strain was increased (Figure 7). The results revealed an increase in disease resistance in SlPR5-3-overexpressing plants and a decrease in disease resistance in CRISPR-mutant plants.
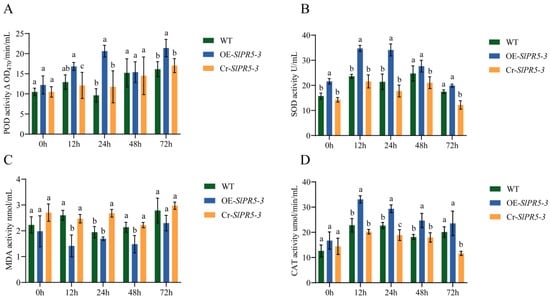
Figure 7.
Changes in (A) POD, (B) SOD and (D) CAT activities and the (C) MDA content at different time points under Pst DC3000 stress conditions. Letters (a–c) indicate significant differences (p ≤ 0.05).
4. Discussion
In this study, all 27 SlPR5 genes contained conserved structural domains for pathogen defense functions, and nine of the SlPR5 genes expressed the thaumatin structural domain in tomato. The functions of these genes include three-dimensional structural stability, substrate binding and catalytic activity, and signal sensing and stress response [53], and 10 SlPR5 genes contain a conserved TLP-PA structural domain. The functions of these genes are realized through four main types of mechanisms: structural stability, antipathogenic activity, the integration of stress signals and developmental regulation [54]. Structural domains associated with antifungal activity (TLP-PA) are present in some sequences of wild olive trees and have a potential role in pathogen resistance [55]. Seven SlPR5 genes contain a conserved GH64-TLP-SF structural domain. The rice OsTLP2 gene [56], the sugarcane ScTLP2 and ScTLP3 genes [57], and the Arabidopsis AtTLP6 gene [58] all contain the GH64-TLP-SF structural domain, which plays a central role in pathogen defense and stress response [59,60]. These results suggest that SlPR5 is involved in plant defense systems against biotic and abiotic stresses and in the regulation of physiological processes in many plant species.
By participating in transcriptional regulation, cis-acting elements play important roles in plant growth and development and the abiotic stress response [61], and the disease resistance functions of SlPR5 family members are regulated by cis-acting elements in their promoter regions. These elements activate SlPR5 gene expression in response to pathogen infestation, hormone signaling, and environmental stress through interactions with transcription factors. By analyzing the sequence structure of the internal and promoter regions of the genes, we found that all 27 SlPR5 gene family members contain several different cis-acting elements and that W-box cis-acting elements are present in 11 SlPR5 genes. The W-box responds directly to pathogenic effector molecules, activates the immune cascade response [62], and directly activates SlPR5 expression. The W-box in the walnut JrPR5L promoter, which is bound and activated for expression by JrWRKY21, is involved in resistance to anthracnose [63]. The expression of W-box in the tobacco NbTLP1 promoter is rapidly induced by Mycobacterium avium infection [64]. MYB is predicted to be present in 24 SlPR5 genes, and both rice R2R3-MYB [65] and Arabidopsis AtMYB41 [66] contain MYB cis-elements and play regulatory roles in antiretroviral activity. Moreover, the MYB transcription factor plays a crucial role in the defense response of plants [67]. SlPR5 also includes 13 response elements related to abiotic stresses, hormones and other inputs. The presence of promoter elements such as W-boxes and MYBs provides a structural basis for SlPR5 family members to perform stress resistance regulatory functions, elucidating the possible modes of regulation of this gene family in the regulation of the stress response.
In this study, SlPR5 proteins were distributed in a variety of plant organs and were predominant in flower buds, the fruit epidermis, and roots, suggesting that the function of SlPR5 genes may play an irreplaceable role in plant growth and development [68]. However, SlPR5 genes may be localized differently and are predicted to be in extracellular regions [69] or to accumulate in vesicles in response to pathogen attack [70], depending on their specific function [71]. A similar report revealed that most of the PR5 expression in Glycine max is predicted to be in the extracellular region [72].
To explore the response pattern of the tomato SlPR5 gene family to pathogen stress, we analyzed the expression of SlPR5 gene family members under Pst DC3000 stress. The results revealed that most of the genes responded differently under Pst DC3000 stress conditions, while SlPR5-3 responded most strongly. NbTLP1 is a homolog of PR5, and overexpression of NbTLP1 significantly enhanced chitin and flg22-induced ROS accumulation and strengthened the PTI response. Directly interacts with NbPR1 and stabilizes its protein level to enhance resistance to Phytophthora capsici through the SA pathway [64]. Potato StPR-5 may be involved in ROS burst and enhance resistance to late blight (Phytophthora infestans) by regulating NADPH oxidase (RBOH) activity [73]. Fls2 activates PTI, induces SA synthesis, and directly upregulates SlPR5-3 expression [74,75]. Pto recognition of AvrPto triggers ETI and indirectly regulates SlPR5-3 through the SA and ethylene pathways [76,77]. To further explore the role of SlPR5-3 in resistance to Pst DC3000, reactive oxygen species and defense-related enzyme activities were measured in tomato plants infected with the pathogen. The accumulation of reactive oxygen species is considered one of the most direct antimicrobial effects and initiates signal activation of downstream defense responses [78]. Reactive oxygen species, including H2O2 and O2▪-, are the most important signaling molecules in plant immunity. The moderate accumulation of reactive oxygen species in plants is favorable for plant growth and development, but pathogenic bacteria increase the accumulation of reactive oxygen species in plants. Excessive accumulation of reactive oxygen species can exacerbate damage to plants and aggravate pathogen damage [79]. In soybean, GmSTOP1-3 reduces ROS accumulation and increases aluminum tolerance [80]. The transcription of TaNOX10 by TaWRKY19 inhibited ROS production and enhanced wheat susceptibility to stripe rust [81]. In this study, H2O2 and O2▪− accumulation were detected by DAB and NBT staining and quantification, respectively, in tomato inoculated with Pst DC3000 in both the wild-type and overexpressing materials, with the lowest amount of reactive oxygen species accumulation in the SlPR5-3-overexpression strain and significantly greater reactive oxygen species accumulation in the CRISPR-mutant SlPR5-3 strain than in the wild-type strain.
In addition, we examined the activities of defense-related enzymes, the CAT, POD and SOD activities. The core function of defensive enzymes is the specific removal of ROS; the higher the enzyme activity, the more ROS are removed. The results showed that except for MDA, were significantly higher in SlPR5-3-overexpression plants than in the wild-type and CRISPR mutant lines after inoculation with Pst DC3000. After wheat was inoculated with Pseudomonas syringae, the damage caused by the bacteria to wheat leaves was delayed by the increased activity of defense enzymes, such as POD and SOD, and a decrease in the level of reactive oxygen species in the leaves [82]. The rice ubiquitin ligase RBRL enhances peroxidase (POD) and polyphenol oxidase (PPO) activity and inhibits viral replication [83]. High levels of defense-related enzyme activities and low levels of reactive oxygen species accumulation are detected in plant materials that are resistant to biotic stresses such as pathogens [84]. In summary, we hypothesize that SlPR5-3 enhances tomato resistance to tomato bacterial leaf spot by decreasing ROS accumulation and increasing the activity of defense enzymes.
5. Conclusions
In this study, the tomato SlPR5 gene family was analyzed at the genome-wide level, 27 SlPR5 genes were obtained, and their phylogenetic relationships, physicochemical properties, gene structures, chromosomal locations, cis-acting elements, and conserved motifs were analyzed using bioinformatics methods. Disease resistance response was also analyzed on the basis of transcriptomic data, which revealed that most of the genes were able to respond to Pst DC3000 stress. The function of the SlPR5-3 gene was verified by overexpression and knockdown treatments of the gene. Disease resistance analysis showed that SlPR5-3 overexpression lines had reduced spots, lower pathogen counts, less ROS accumulation, and indicators such as SOD, POD, and CAT showed that plant disease resistance was improved compared with the wild type, whereas the gene-edited lines behaved in the opposite direction, with a significant decrease in disease resistance. SlPR5-3 was found to have a positive regulatory effect under Pst DC3000 stress. These results deepen the research on the regulatory mechanism of the biotic stress response of the tomato SlPR5 gene family and provide support for future innovative breakthroughs in tomato resistance breeding and the application of gene editing technology and are of great significance to the development of tomato disease resistance legacy breeding.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14213389/s1, Table S1: GFP-SlPR5-3 gene PCR primers; Table S2: Primers for fluorescent quantitative qRT-PCR of tomato SlPR5 gene family; Table S3: SOD, POD, CAT activities and MDA content; Table S4: Locus ID of the tomato SlPR5 family; Figure S1: Comparative phylogeny of SlPR5-3 with other Solanaceae.
Author Contributions
Conceptualization, X.P., Y.W., X.X. and T.Z.; methodology, X.P., Y.W. and B.J.; software, X.P., Y.W., H.Z. and B.J.; validation, X.P., Y.W., B.J. and D.L. (Dalong Li); formal analysis, X.P., B.J. and H.Z.; investigation, X.P., Y.W. and B.J.; resources, X.P., D.L. (Dalong Li) and H.Z.; data curation, X.P., Y.W., D.L. (Dalong Li) and H.Z.; writing—original draft preparation, X.P. and Y.W.; writing—review and editing, X.P., X.X., T.Z. and D.L. (Dong Liu); visualization, X.P., Y.W. and B.J.; supervision, X.X., T.Z. and D.L. (Dong Liu); project administration, X.X. and T.Z.; funding acquisition, X.X. and T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for expert positions in the national vegetable industry technology system (CARS-23-A11); Regional Innovation and Development Joint Fund of the National Natural Science Foundation of China (U22A20496).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We appreciate the support of Heilongjiang Province’s modern agricultural industrial technology collaborative innovation and promotion system.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, Z.Y.; Zhao, M.S.; Ma, H.Y.; Liu, L.Z.; Yang, G. Biological and molecular characterization oftomato brown rugose fruit virus and development of quadruplex RT-PCR detection. J. Integr. Agric. 2021, 20, 1871–1879. [Google Scholar] [CrossRef]
- Lindeberg, M.; Cartinhour, S.; Myers, C.R.; Schechter, L.M.; Schneider, D.J.; Collmer, A. Closing the circle on the discovery of genes encoding Hrp regulon members and type III secretion system effectors in the genomes of three model Pseudomonas syringae strains. Mol. Plant-Microbe Interact. 2006, 19, 1151–1158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yunis, H.; Bashan, Y.; Okon, Y.; Henis, Y. Weather dependence, yield losses and control of bacterial speck of tomato caused by pseudomonas tomato. Plant Dis. 1980, 64, 937–939. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Zhou, J.; Han, X.; Song, K.; Gu, H.; Sun, L. Comprehensive analysis of NAC genes reveals differential expression patterns in response to Pst DC3000 and their overlap expression pattern during PTI and ETI in tomato. Genes 2022, 13, 2015. [Google Scholar] [CrossRef] [PubMed]
- Hind, S.R.; Strickler, S.R.; Boyle, P.C.; Dunham, D.M.; Bao, Z.; O’Doherty, I.M.; Baccile, J.A.; Hoki, J.S.; Viox, E.G.; Clarke, C.R.; et al. Tomato receptor Flagellin-Sensing 3 binds flgII-28 and activates the plant immune system. Nat. Plants 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Roberts, R.; Liu, A.E.; Wan, L.; Geiger, A.M.; Hind, S.R.; Rosli, H.G.; Martin, G.B. Molecular characterization of differences between the tomato immune receptors flagellin sensing 3 and flagellin sensing 2. Plant Physiol. 2020, 183, 1825–1837. [Google Scholar] [CrossRef]
- Zhang, N.; Pombo, M.A.; Rosli, H.G.; Martin, G.B. Tomato wall-associated kinase SlWak1 depends on Fls2/Fls3 to promote apoplastic immune responses to Pseudomonas syringae. Plant Physiol. 2020, 183, 1869–1882. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Daudi, A.; Shah, J. Transcriptional reprogramming during establishment of systemic acquired resistance in Arabidopsis. Plant Physiol. 2010, 154, 708–721. [Google Scholar]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Zhou, Z.; Zhou, J.M. Plant pattern—Recognition receptors controlling innate immunity. Sci. China Life Sci. 2016, 59, 878–888. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, F.; Fan, F.; Gu, L.; Cang, H.; Martin, G.B.; Chai, J. Crystal structure of the complex between Pseudomonas effector AvrPtoB and the tomato Pto kinase reveals both a shared and a unique interface compared with AvrPto-Pto. Plant Cell 2009, 21, 1846–1859. [Google Scholar] [CrossRef]
- Göhre, V.; Spallek, T.; Häweker, H.; Mersmann, S.; Mentzel, T.; Boller, T.; de Torres, M.; Robatzek, S. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 2008, 18, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; He, P.; Li, J.; Heese, A.; Peck, S.C.; Nürnberger, T.; Martin, G.; Sheen, J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 2008, 4, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lin, N.C.; Martin, G.B. Two distinct Pseudomonas effector proteins interact with the Pto kinase and activate plant immunity. Cell 2002, 109, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Nabi, Z.; Manzoor, S.; Nabi, S.U.; Wani, T.A.; Gulzar, H.; Farooq, M.; Vlădulescu, C.; Mansoor, S. Pattern-Triggered Immunity and Effector-Triggered Immunity: Crosstalk and cooperation of PRR and NLR-mediated plant defense pathways during host–pathogen interactions. Physiol. Mol. Biol. Plants 2024, 30, 587–604. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Wildermuth, M.C.; Chakravarthy, S.; Loh, Y.T.; Yang, C.; He, X.; Han, Y.; Martin, G.B. Tomato transcription factors Pti4, Pti5, and Pti6 activate defense responses when expressed in Arabidopsis. Plant Cell 2002, 14, 817–831. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattarai, K. Progress in developing bacterial spot resistance in tomato. Agronomy 2019, 9, 26. [Google Scholar] [CrossRef]
- Fang, X.; Meng, X.; Zhang, J.; Cao, S.; Tang, X.; Fan, T. AtWRKY1 negatively regulates the response of Arabidopsis thaliana to Pst. DC3000. Plant Physiol. Biochem. 2021, 166, 799–806. [Google Scholar] [CrossRef]
- Zhang, N.; Hecht, C.; Sun, X.; Fei, Z.; Martin, G.B. Loss of function of the bHLH transcription factor Nrd1 in tomato enhances resistance to Pseudomonas syringae. Plant Physiol. 2022, 190, 1334–1348. [Google Scholar] [CrossRef]
- Li, Y.M.; Wang, J.; Wang, P.; Shi, K. Function of sugar transport protein SlSTP2 in tomato defense against bacterial leaf spot. Sci. Agric. Sin. 2022, 55, 3144–3154. [Google Scholar]
- Wu, Z.; He, L.; Jin, Y.; Chen, J.; Shi, H.; Wang, Y.; Yang, W. Histone Deacetylase 6 suppresses salicylic acid biosynthesis to repress autoimmunity. Plant Physiol. 2021, 187, 2592–2607. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Piggott, N.; Ekramoddoullah, A.K.; Liu, J.J.; Yu, X. Gene cloning of a thaumatin-like (PR-5) protein of western white pine (Pinus monticola, D. Don) and expression studies of members of the PR-5 group. Physiol. Mol. Plant Pathol. 2004, 64, 1–8. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.C.; Sandeep; Kamthan, M.; Kumar, S.; Ghosh, S. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci. Rep. 2016, 6, 25340. [Google Scholar] [CrossRef] [PubMed]
- Eden, D.; Matthew, J.B.; Rosa, J.J.; Richards, F.M. Increase in apparent compressibility of cytochrome c upon oxidation. Proc. Natl. Acad. Sci. USA 1982, 79, 815–819. [Google Scholar] [CrossRef]
- Dalen, L.S.; Johnsen, Ø.; Lönneborg, A.; Yaish, M.W. Freezing tolerance in Norway spruce, the potential role of pathogenesis-related proteins. Acta Physiol. Plant. 2015, 37, 1717. [Google Scholar] [CrossRef]
- Liu, C.; Han, L.H.; Wang, H.B. Identification and codon bias analysis of the sweet protein gene family in cereals. Northwest J. Agric. 2018, 27, 52–61. [Google Scholar]
- Anisimova, O.K.; Kochieva, E.Z.; Shchennikova, A.V.; Filyushin, M.A. Thaumatin-like protein (TLP) genes in garlic (Allium sativum L.): Genome-wide identification, characterization, and expression in response to Fusarium proliferatum infection. Plants 2022, 11, 748. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, L.; Wang, W.W.; Yu, Z.Y.; Yu, D.Y.; Liu, L.J. Bioinformatics analysis of soybean disease course-related protein PR-5 and its homologous protein TPLs. Soybean Sci. 2016, 35, 380–387. [Google Scholar]
- Xi, Z.; Jia, H.; Li, Y.; Ma, J.; Lu, M.; Wang, Z.; Kong, D.X.; Deng, W.W. Identification and functional analysis of PR genes in leaves from variegated tea plant (Camellia sinensis). Agronomy 2024, 14, 156. [Google Scholar] [CrossRef]
- Šimkovicová, M.; Kramer, G.; Rep, M.; Takken, F.L. Tomato R-gene-mediated resistance against Fusarium wilt originates in roots and extends to shoots via xylem to limit pathogen colonization. Front. Plant Sci. 2024, 15, 1384431. [Google Scholar] [CrossRef] [PubMed]
- Pressey, R. Two isoforms of NP24: A thaumatin-like protein in tomato fruit. Phytochemistry 1997, 44, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Anžlovar, S.; Dermastia, M. The comparative analysis of osmotins and osmotin-like PR-5 proteins. Plant Biol. 2003, 5, 116–124. [Google Scholar] [CrossRef]
- Li, X.; Xu, B.; Xu, J.; Li, Z.; Jiang, C.; Zhou, Y.; Zhao, K. Tomato-thaumatin-like protein genes Solyc08g080660 and Solyc08g080670 confer resistance to five soil-borne diseases by enhancing β-1, 3-glucanase activity. Genes 2023, 14, 1622. [Google Scholar] [CrossRef]
- Jia, X.; Zeng, H.; Wang, W.; Zhang, F.; Yin, H. Chitosan oligosaccharide induces resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana by activating both salicylic acid–and jasmonic acid–mediated pathways. Mol. Plant-Microbe Interact. 2018, 31, 1271–1279. [Google Scholar] [CrossRef]
- Weng, Q.Y.; Song, J.H.; Zhao, Y.T.; Zheng, X.; Huang, C.C.; Wang, G.Y.; Dong, J.G. T1N6_22 positively regulates Botrytis cinerea resistance but negatively regulates Pseudomonas syringae pv. tomato DC3000 resistance in Arabidopsis thaliana. Biotechnol. Biotechnol. Equip. 2017, 31, 690–697. [Google Scholar] [CrossRef]
- Khare, E.; Kim, K.; Lee, K.J. Rice OsPBL1 (ORYZA SATIVA ARABIDOPSIS PBS1-LIKE 1) enhanced defense of Arabidopsis against Pseudomonas syringae DC3000. Eur. J. Plant Pathol. 2016, 146, 901–910. [Google Scholar] [CrossRef]
- Jiang, C.H.; Fan, Z.H.; Xie, P.; Guo, J.H. Bacillus cereus AR156 extracellular polysaccharides served as a novel micro-associated molecular pattern to induced systemic immunity to Pst DC3000 in Arabidopsis. Front. Microbiol. 2016, 7, 664. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Mueller, L.A. The Sol Genomics Network (SGN)—From genotype to phenotype to breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Finn, R.D. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Turkoglu, M.; Yanikoğlu, B.; Hanbay, D. PlantDiseaseNet: Convolutional neural network ensemble for plant disease and pest detection. Signal Image Video Process. 2021, 16, 301–309. [Google Scholar] [CrossRef]
- Swarbreck, D.; Wilks, C.; Lamesch, P.; Berardini, T.Z.; Garcia-Hernandez, M.; Foerster, H.; Huala, E. The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 2007, 36 (Suppl. S1), D1009–D1014. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, P.T.; Søren, B.; Gunnar, V.H.; Henrik, N. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; AdamsCollier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Zeng, D.C.; Ma, X.L.; Xie, X.R.; Zhu, Q.L.; Liu, Y.G. Operational methods for plant CRISPR/Cas9 multi-gene editing vector construction and mutation analysis, Science in China. Life Sci. 2018, 48, 783–794. [Google Scholar]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Bogard, M.; Couée, I.; Gouesbet, G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Waese, J.; Fan, J.; Pasha, A.; Yu, H.; Fucile, G.; Shi, R.; Cumming, M.; Kelley, L.A.; Sternberg, M.J.; Krishnakumar, V. ePlant: Visualizing and exploring multiple levels of data for hypothesis generation in plant biology. Plant Cell 2017, 29, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Yuan, Z.; Qian, X. Expression Profile Analysis of Thionin-like Gene Family in Barley. Biotechnol. Bull. 2022, 38, 140–147. [Google Scholar]
- Wang, T.; Hu, J.; Ma, X.; Li, C.; Yang, Q.; Feng, S.; Li, M.; Li, N.; Song, X. Identification, evolution and expression analyses of whole genome-wide TLP gene family in Brassica napus. BMC Genom. 2020, 21, 264. [Google Scholar] [CrossRef]
- Alves, M.C.S.; de Souza, R.S.; da Silva, R.C.C. Genome-Wide Identification and Characterization Thaumatin-like Protein (TLP) Genes in Wild Olive (Olea europaea var. sylvestris). Sci. Plena 2024, 20. [Google Scholar]
- Singh, S.; Tripathi, R.K.; Lemaux, P.G.; Buchanan, B.B.; Singh, J. Redox-dependent interaction between thaumatin-like protein and β-glucan influences malting quality of barley. Proc. Natl. Acad. Sci. USA 2017, 114, 7725–7730. [Google Scholar] [CrossRef]
- Li, C.N.; Liu, F.; Zhang, X.; Ren, Y.J.; Tang, H.C.; Que, Y.X. Bioinformatics and Expression Analysis of Thaumatin-like Protein Genes ScTLP2 and ScTLP3 from Sugarcane. Res. Gate 2020, 18, 65–73. [Google Scholar]
- Zhang, Y.; He, X.; Su, D.; Feng, Y.; Zhao, H.; Deng, H.; Liu, M. Comprehensive profiling of tubby-like protein expression uncovers ripening-related TLP genes in tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2020, 21, 1000. [Google Scholar] [CrossRef]
- Sun, L.; Yu, G.; Han, X.; Xin, S.; Qiang, X.; Jiang, L.; Zhang, S.; Cheng, X. TsMIP6 enhances the tolerance of transgenic rice to salt stress and interacts with target proteins. J. Plant Biol. 2015, 58, 285–292. [Google Scholar] [CrossRef]
- Liu, C.; Han, L.; Wang, H.; Gao, Y.; Tang, L. Research Advances on Plant Thaumatin-like Protein Family. Biotechnol. Bull. 2018, 34, 9–17. [Google Scholar]
- Baker, S.S.; Wilhelm, K.S.; Thomashow, M.F. The 5′-region of Arabidopsis thaliana corl5a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol. Biol. 1994, 24, 701–713. [Google Scholar] [CrossRef]
- Gao, Y.; Zan, X.-L.; Wu, X.-F.; Yao, L.; Chen, Y.-L.; Jia, S.-W.; Zhao, K.-J. Identification of fungus-responsive cis-acting element in the promoter of Brassica juncea chitinase gene, BjCHI1. Plant Sci. 2014, 215, 190–198. [Google Scholar] [CrossRef]
- Zhou, R.; Dong, Y.; Liu, X.; Feng, S.; Wang, C.; Ma, X.; Liu, J.; Liang, Q.; Bao, Y.; Xu, S. JrWRKY21 interacts with JrPTI5L to activate the expression of JrPR5L for resistance to Colletotrichum gloeosporioides in walnut. Plant J. 2022, 111, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, G.; Liu, C.; Tian, S.; Qiao, G.; Sun, H.; Luo, X.; Wang, S.; Cai, L.; Sun, X. NbTLP1 stabilizes NbPR1 to enhance resistance against Phytophthora capsici via salicylic acid signalling pathway in Nicotiana benthamiana. Plant Biotechnol. J. 2025, 23, 3748–3750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-C.; Gong, Y.-H.; Tao, T.; Lu, S.; Zhou, W.-Y.; Xia, H.; Zhang, X.-Y.; Yang, Q.-Q.; Zhang, M.-Q.; Hong, L.-M. Genome-wide identification of R2R3-MYB transcription factor subfamily genes involved in salt stress in rice (Oryza sativa L.). BMC Genom. 2024, 25, 797. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Sala, T.; Calvi, D.; Gusmaroli, G.; Tonelli, C. Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. Plant J. 2008, 53, 53–64. [Google Scholar] [CrossRef]
- Liu, Z.; Luan, Y.; Li, J.; Yin, Y. Expression of a tomato MYB gene in transgenic tobacco increases resistance to Fusarium oxysporum and Botrytis cinerea. Eur. J. Plant Pathol. 2016, 144, 607–617. [Google Scholar] [CrossRef]
- Hu, Z.L.; Deng, L.; Yao, N. Analysis of expression of the PR-1 and PR-5 genes in tomato. J. Southwest Agric. Univ. 2009, 31, 67–72. [Google Scholar]
- Samac, D.A.; Penuela, S.; Schnurr, J.A.; Hunt, E.N.; Foster-Hartnett, D.; Vandenbosch, K.A.; Gantt, J.S. Expression of coordinately regulated defence response genes and analysis of their role in disease resistance in Medicago truncatula. Mol. Plant Pathol. 2011, 12, 786–798. [Google Scholar] [CrossRef]
- Jami, S.K.; Anuradha, T.S.; Guruprasad, L. Molecular, biochemical and structural characterization of osmotin-like protein from black nightshade (Solanum nigrum). J. Plant Physiol. 2007, 164, 238–252. [Google Scholar] [CrossRef]
- Melchers, L.S.; Sela-Buurlage, M.B.; Vloemans, S.A.; Woloshuk, C.P.; Van Roekel, J.S.; Pen, J.; van den Elzen, P.J.; Cornelissen, M.J. Extracellular targeting of the vacuolar tobacco proteins AP24, chitinase and β-1, 3-glucanase in transgenic plants. Plant Mol. Biol. 1993, 21, 583–593. [Google Scholar] [CrossRef]
- Onishi, M.; Tachi, H.; Kojima, T.; Shiraiwa, M.; Takahara, H. Molecular cloning and characterization of a novel salt-inducible gene encoding an acidic isoform of PR-5 protein in soybean (Glycine max [L.] Merr.). Plant Physiol. Biochem. 2006, 44, 574–580. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Lu, X.; Xu, J.; Wang, H.; Tang, W.; Li, C. The role of ribosomal protein StRPS5 in mediating resistance of Solanum tuberosum plants to Phytophthora infestans. Plant Sci. 2025, 357, 112539. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, J.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Liu, Y.; Ru, L.; Yan, G.; Xu, Y.; Yu, Y.; Zhu, Z.; He, Y. miR398-SlCSD1 module participates in the SA-H2O2 amplifying feedback loop in Solanum lycopersicum. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Brommonschenkel, S.H.; Chunwongse, J.; Frary, A.; Ganal, M.W.; Spivey, R.; Wu, T.; Earle, E.D.; Tanksley, S.D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 1993, 262, 1432–1436. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Craig, A.; Ewan, R.; Mesmar, J.; Gudipati, V.; Sadanandom, A. E3 ubiquitin ligases and plant innate immunity. J. Exp. Bot. 2009, 60, 1123–1132. [Google Scholar] [CrossRef]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef]
- Liu, G.; Li, D.; Mai, H.; Lin, X.; Lu, X.; Chen, K.; Wang, R.; Riaz, M.; Tian, J.; Liang, C. GmSTOP1-3 regulates flavonoid synthesis to reduce ROS accumulation and enhance aluminum tolerance in soybean. J. Hazard. Mater. 2024, 480, 136074. [Google Scholar] [CrossRef]
- Wang, N.; Fan, X.; He, M.; Hu, Z.; Tang, C.; Zhang, S.; Lin, D.; Gan, P.; Wang, J.; Huang, X. Transcriptional repression of TaNOX10 by TaWRKY19 compromises ROS generation and enhances wheat susceptibility to stripe rust. Plant Cell 2022, 34, 1784–1803. [Google Scholar] [CrossRef]
- Zhang, M.; Kang, H.; Zhang, G.; Chen, Y.; Kong, X.; Guo, Q.; Wang, W.J.P. Overexpression of TaUb2 enhances disease resistance to Pseudomonas syringae pv. tomato DC3000 in tobacco. Physiol. Mol. Plant Pathol. 2015, 90, 98–104. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Sun, X.; Li, J.; Cao, X.; Yao, S.; Han, Y.; Chen, C.; Du, L.; Li, S. Perception of viral infections and initiation of antiviral defence in rice. Nature 2025, 641, 173–181. [Google Scholar] [CrossRef]
- De Gara, L.; de Pinto, M.C.; Tommasi, F. The antioxidant systems vis-à-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol. Biochem. 2003, 41, 863–870. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).