Integrated Transcriptomic and Metabolomic Profiling of Paclobutrazol-Induced Dwarfism in Tomato Epicotyls
Abstract
1. Introduction
2. Results
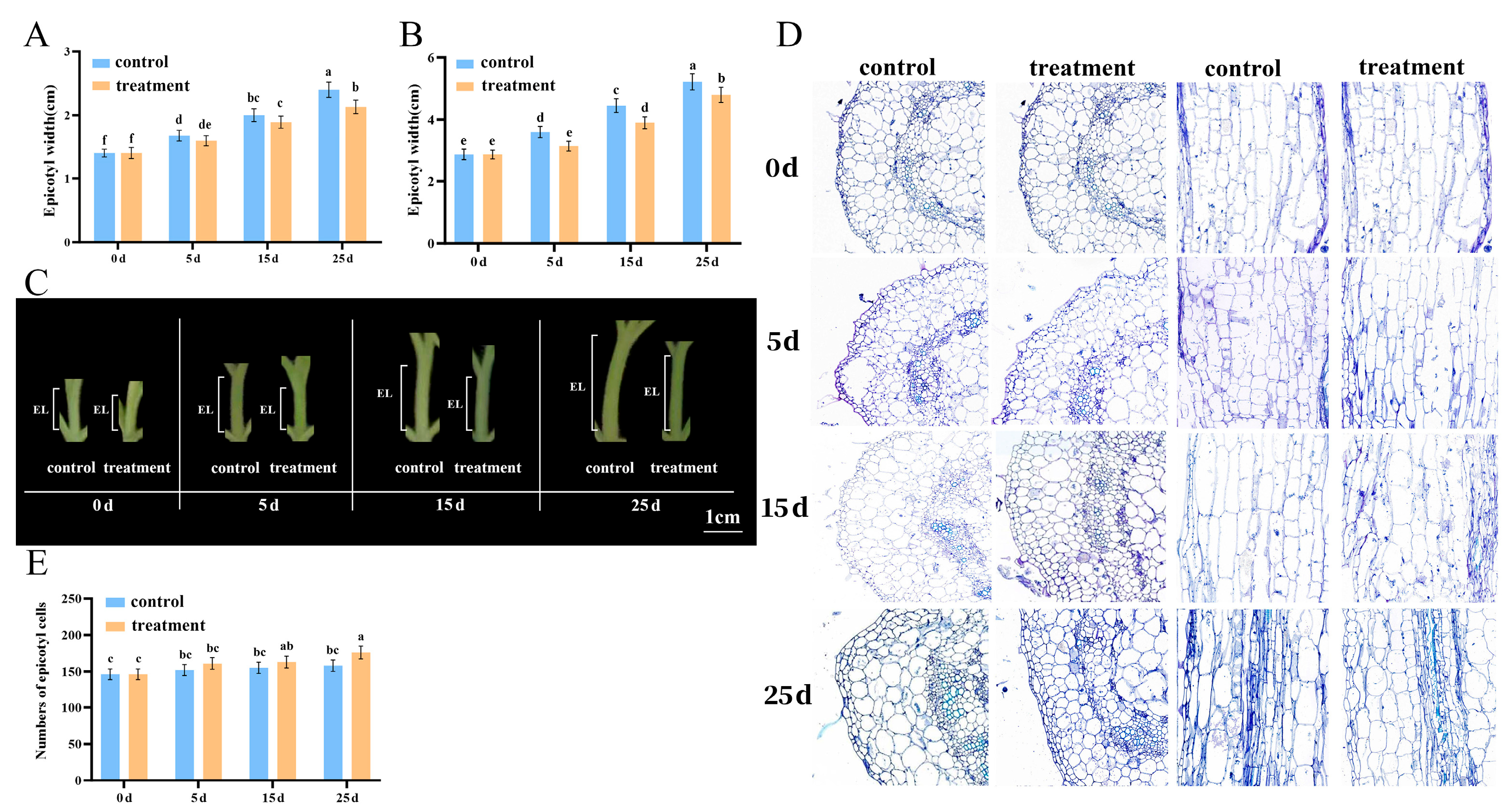
2.1. PBZ-Dwarfed Epicotyls in Tomato Seedlings
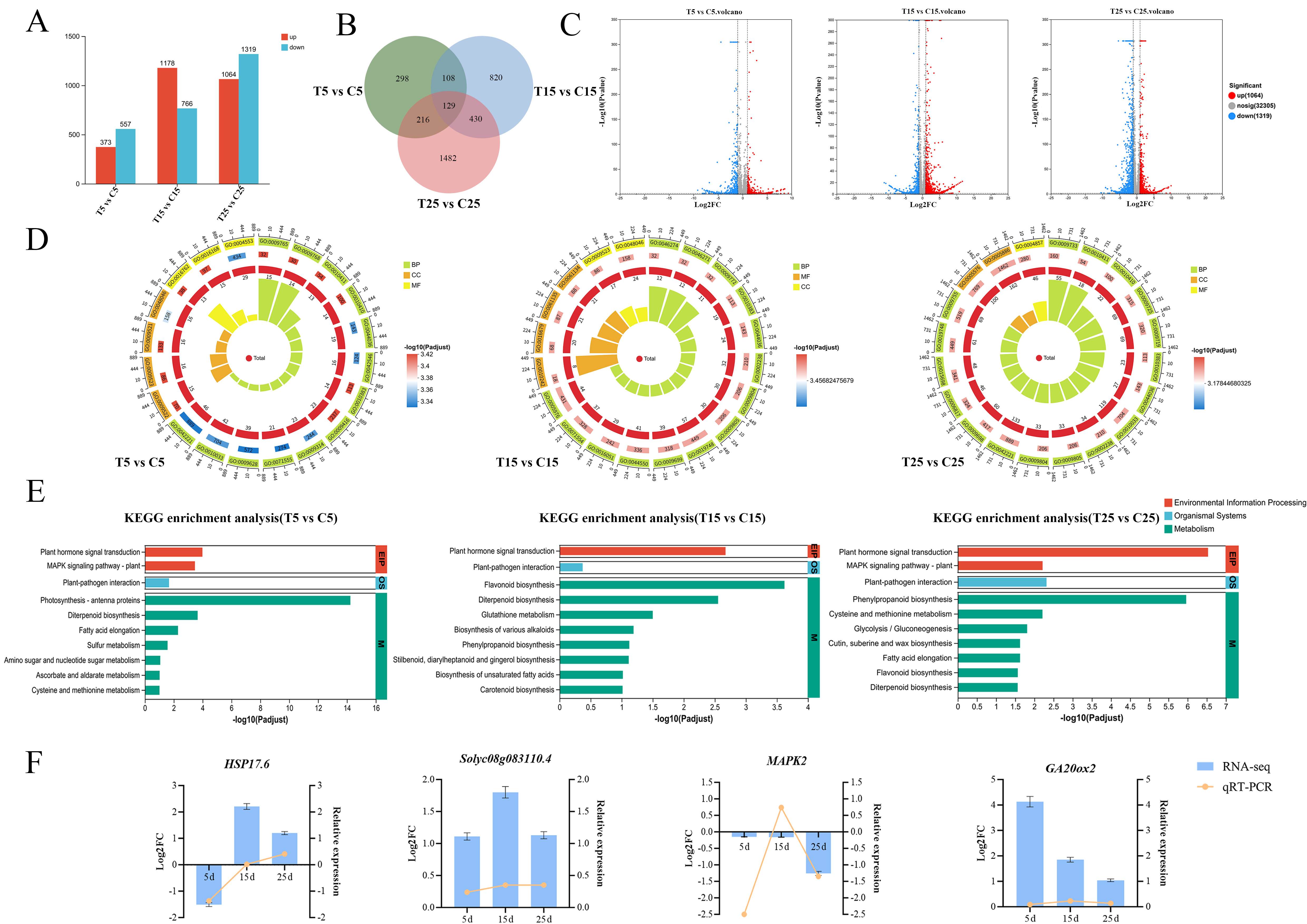
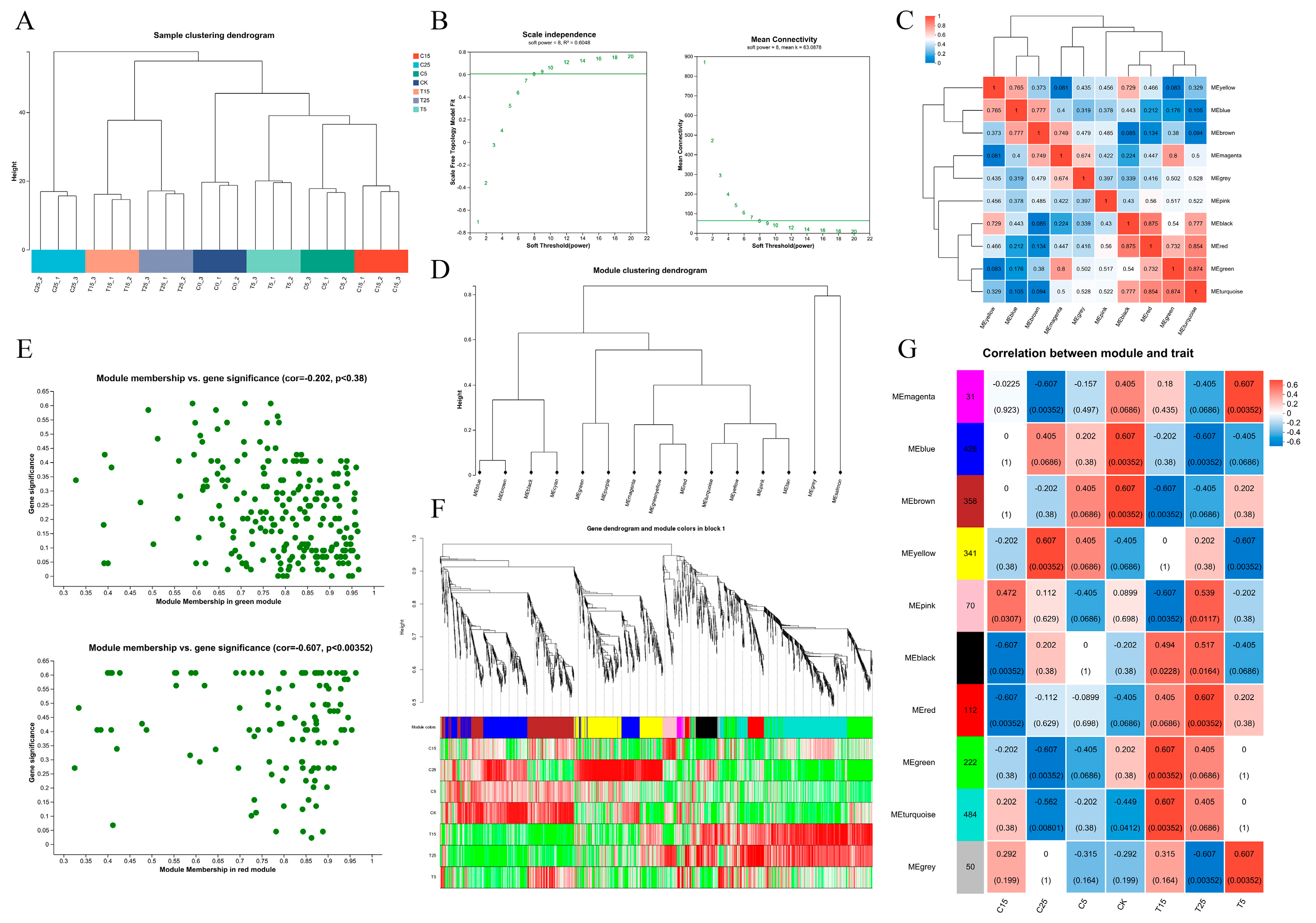
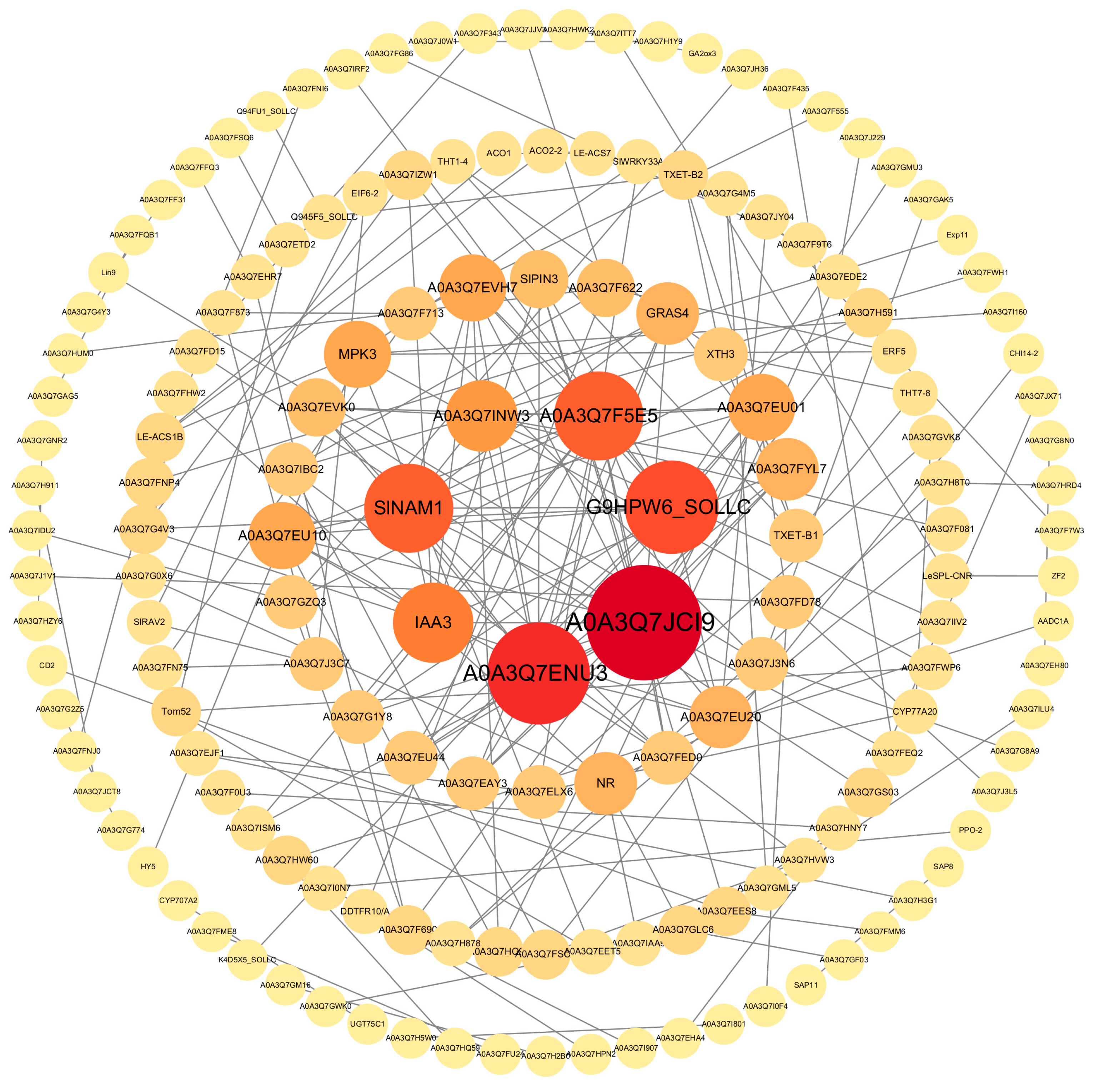
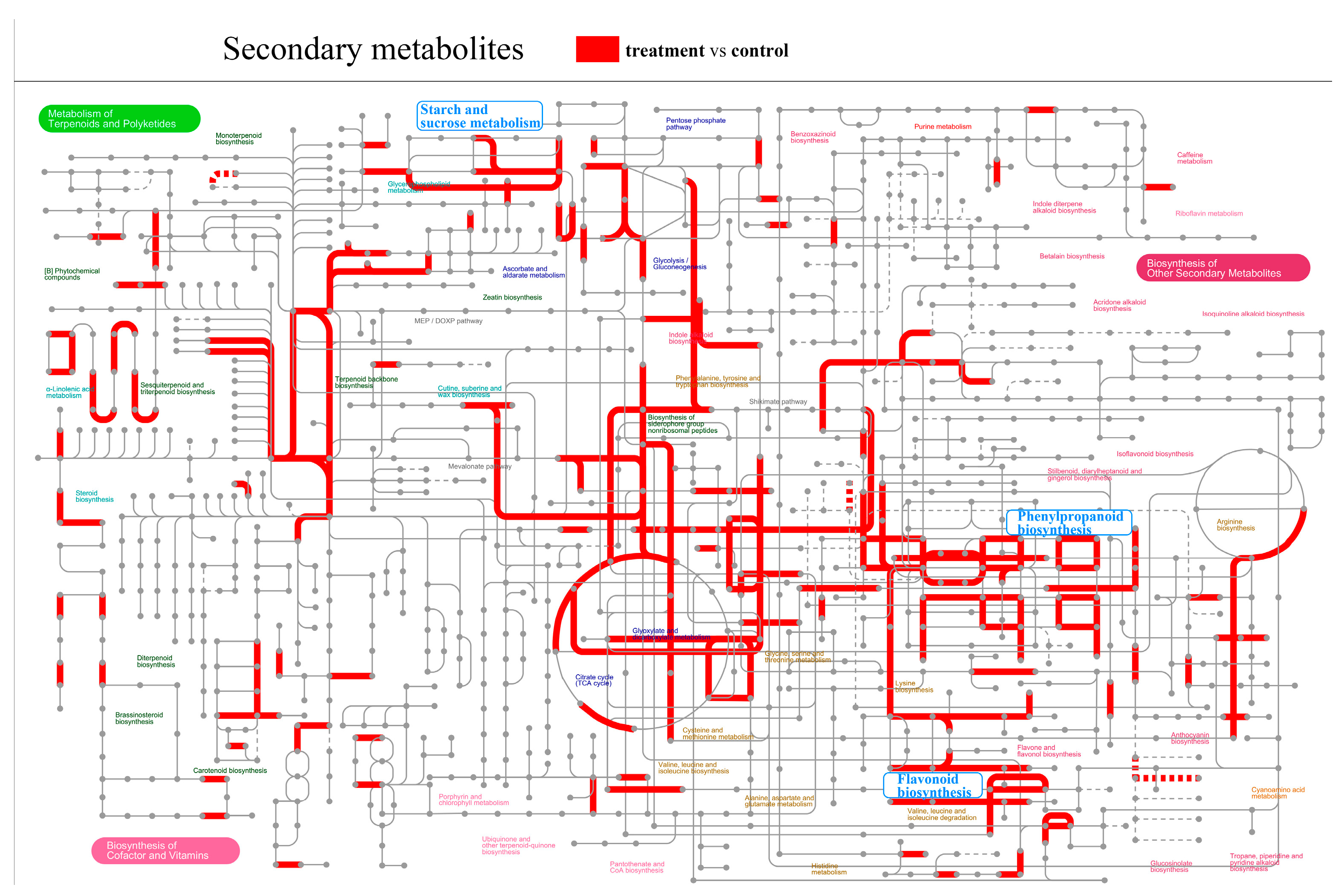
2.2. Transcriptomic Analysis of Epicotyls Dwarfed by PBZ Treatment
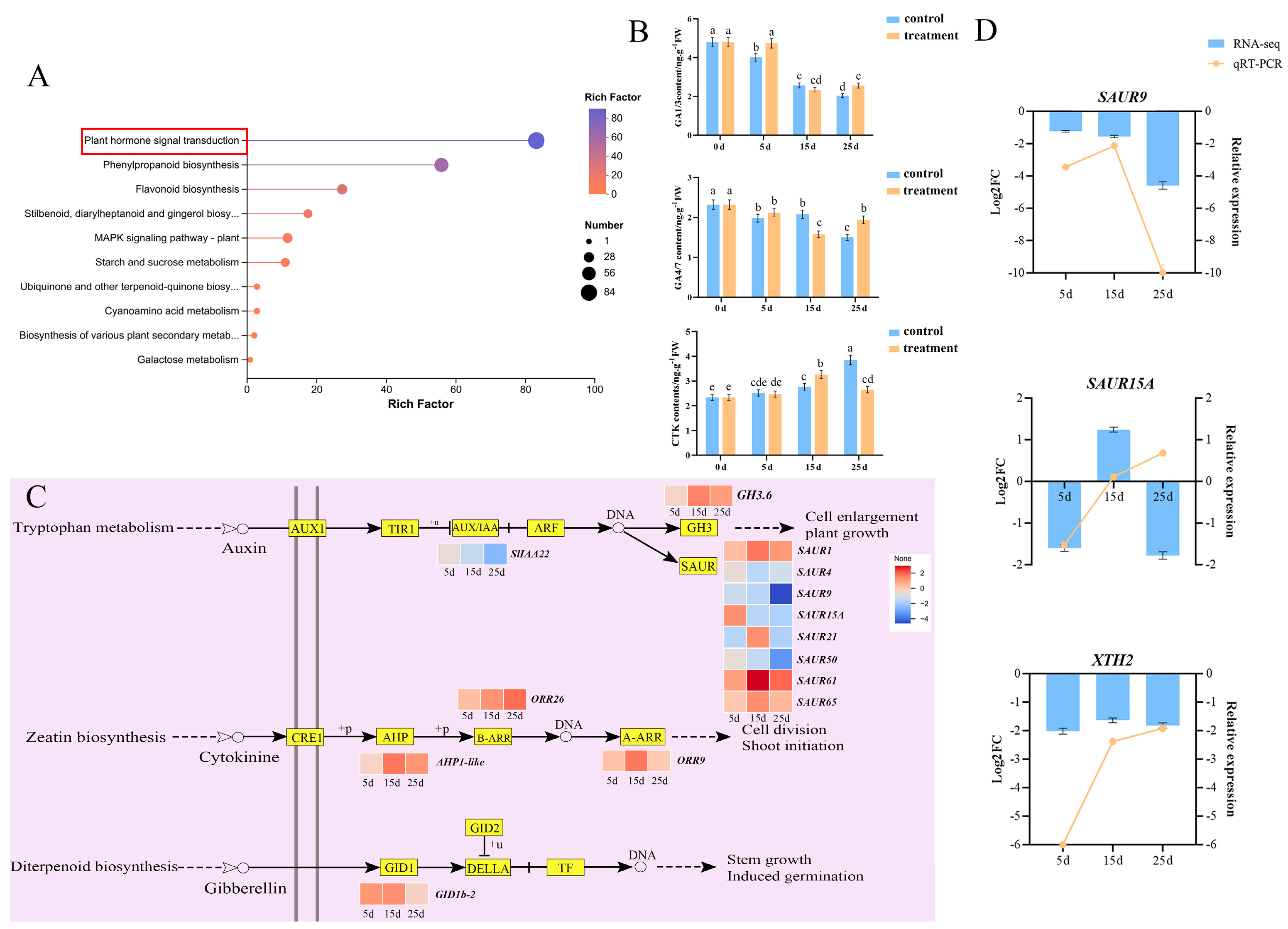
2.3. Effect of Plant Hormones on Epicotyl Dwarfing
2.4. Effect of Sucrose Metabolism on Epicotyl Dwarfing
2.5. Effect of the Phenylpropanoid and Flavonoid Biosynthesis Pathway on Epicotyl Dwarfing
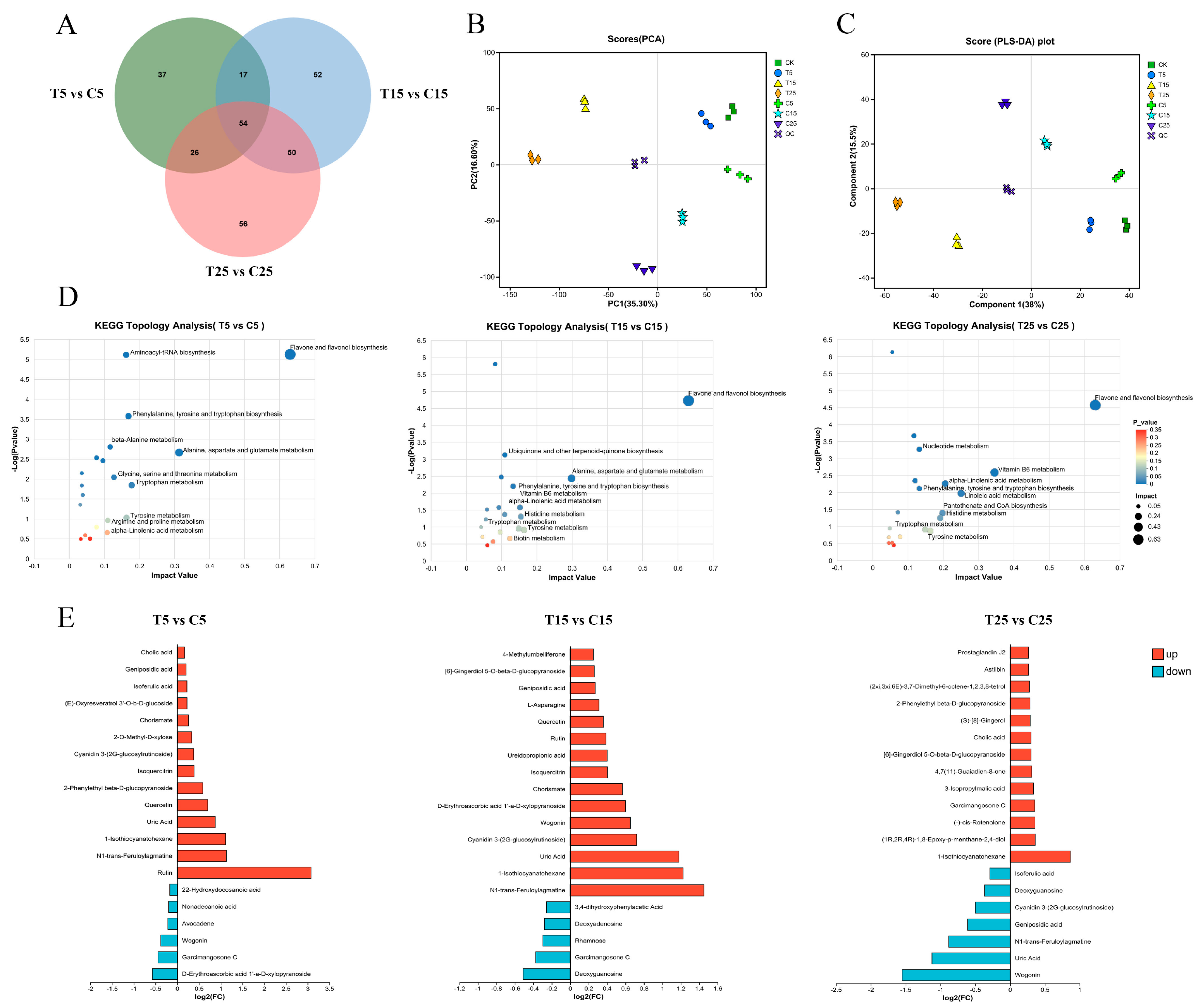
2.6. Metabolomic Analysis of Epicotyls Dwarfed by PBZ Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Microscopic Observation of Epicotyls
4.3. Plant Hormone Quantification
4.4. RNA Extraction and Transcriptome Analysis
4.5. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT–qPCR)
4.6. Metabolomic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.H.; Yang, H.C.; Bae, Y.H.; Hyeon, S.J.; Hwang, S.J.; Kim, D.H.; Jang, D.C. Preventing overgrowth of cucumber and tomato seedlings using difference between day and night temperature in a plant factory with artificial lighting. Plants 2023, 12, 3164. [Google Scholar] [CrossRef]
- Kaneta, T.; Kakimoto, T.; Shibaoka, H. Actinomycin D inhibits the GA3-induced elongation of azuki bean epicotyls and the reorientation of cortical microtubules. Plant Cell Physiol. 1993, 34, 1125–1132. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, Z.; Tian, Z. ERECTA regulates cell elongation by activating auxin biosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1688. [Google Scholar] [CrossRef]
- Yamoune, A.; Zdarska, M.; Depaepe, T.; Rudolfova, A.; Skalak, J.; Berendzen, K.W.; Hejatko, J. Cytokinins regulate spatially specific ethylene production to control root growth in Arabidopsis. Plant Commun. 2024, 5, 101013. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Tekalign, T.; Hammes, P.S. Growth and biomass production in potato grown in the hot tropics as influenced by paclobutrazol. Plant Growth Regul. 2005, 45, 37–46. [Google Scholar] [CrossRef]
- Rahman, H.; Khan, M.; Khokhar, M. Effect of paclobutrazol on growth and yield of tomato. Pak. J. Agric. Res. 1989, 10, 49–51. [Google Scholar]
- García-González, J.; Lacek, J.; Weckwerth, W.; Retzer, K. Exogenous carbon source supplementation counteracts root and hypocotyl growth limitations under increased cotyledon shading, with glucose and sucrose differentially modulating growth curves. Plant Signal. Behav. 2021, 16, 1969818. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Ma, C.; Zhang, D.; Zhou, D.; Zhang, J.; Yan, M. Metabolome and transcriptome analyses reveal changes of rapeseed in response to ABA signal during early seedling development. BMC Plant Biol. 2024, 24, 245. [Google Scholar] [CrossRef]
- Su, T.; Liu, H.; Wu, Y.; Wang, J.; He, F.; Li, H.; Li, S.; Wang, L.; Li, L.; Cao, J.; et al. Soybean hypocotyl elongation is regulated by a MYB33-SWEET11/21-GA2ox8c module involving long-distance sucrose transport. Plant Biotechnol. J. 2024, 22, 2859–2872. [Google Scholar] [CrossRef]
- Soga-Morimoto, A.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Suppression of sugar accumulation in coleoptile and mesocotyl cells by light irradiation to etiolated maize seedlings. J. Plant Physiol. 2021, 260, 153409. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Muhammad, S.; Jin, W.; Zhong, W.; Zhang, S.; Lin, Y.; Zhou, Y.; Liu, J.; Liu, H.; Munir, R.; et al. Modulating root system architecture: Cross-talk between auxin and phytohormones. Front. Plant Sci. 2024, 15, 1343928. [Google Scholar] [CrossRef]
- Qi, X.; Zhuang, Z.; Ji, X.; Bian, J.; Peng, Y. The mechanism of exogenous salicylic acid and 6-benzylaminopurine regulating the elongation of maize mesocotyl. Int. J. Mol. Sci. 2024, 25, 6150. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. J. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef]
- Ohtaka, K.; Yoshida, A.; Kakei, Y.; Fukui, K.; Kojima, M.; Takebayashi, Y.; Yano, K.; Imanishi, S.; Sakakibara, H. Difference between day and night temperatures affects stem elongation in tomato (Solanum lycopersicum) seedlings via regulation of gibberellin and auxin synthesis. Front. Plant Sci. 2020, 11, 577235. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Z.; Lu, Q.; Li, M.; Dai, X.; Shan, M.; Liu, Z.; Bai, M.; Xiang, F. miR394 modulates brassinosteroid signaling to regulate hypocotyl elongation in Arabidopsis. Plant J. 2024, 119, 645–657. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, L.; Peng, F.; Song, C.; Manzoor, M.A.; Cai, Y.; Jin, Q. Transcriptomics and metabolomics changes triggered by exogenous 6-benzylaminopurine in relieving epicotyl dormancy of Polygonatum cyrtonema Hua seeds. Front. Plant Sci. 2022, 13, 961899. [Google Scholar] [CrossRef]
- Liu, W.; Li, D.; Bai, B.; Mao, X.; Xie, Y.; Wu, C.; Zhang, N. Effects of paclobutrazole on epicotyl dwarfing in tomato. Plant Physiol. J. 2022, 58, 2151–2162. [Google Scholar]
- Deng, B.; Wang, X.; Long, X.; Fang, R.; Zhou, S.; Zhang, J.; Peng, X.; An, Z.; Huang, W.; Tang, W.; et al. Plant hormone metabolome and transcriptome analysis of dwarf and wild-type banana. J. Plant Growth Regul. 2022, 41, 2386–2405. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Fu, C.; Ahmad, N.; Zhao, C.; Hou, L.; Naeem, M.; Pan, J.; Wang, X.; Zhao, S. Genome-wide identification and expression analysis of GA20ox and GA3ox genes during pod development in peanut. PeerJ 2023, 11, e16279. [Google Scholar] [CrossRef]
- Miedes, E.; Suslov, D.; Vandenbussche, F.; Kenobi, K.; Ivakov, A.; Van Der Straeten, D.P.; Lorences, E.J.; Mellerowicz, E.; Verbelen, J.-P.; Vissenberg, K. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. J. Exp. Bot. 2013, 64, 2481–2497. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, V.; Fornalé, S.; Fry, S.C.; Ruel, K.; Ferrer, P.; Encina, A.; Sonbol, F.-M.; Bosch, J.; Puigdomènech, P.; Rigau, J.; et al. ZmXTH1, a new xyloglucan endotransglucosylase/hydrolase in maize, affects cell wall structure and composition in Arabidopsis thaliana. J. Exp. Bot. 2008, 59, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yokoyama, R. Reconsidering the function of the xyloglucan endotransglucosylase/hydrolase family. J. Plant Res. 2022, 135, 145–156. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L. Wood of trees: Cellular structure, molecular formation, and genetic engineering. J. Integr. Plant Biol. 2024, 66, 443–467. [Google Scholar] [CrossRef]
- Shin, Y.-K.; Yum, H.; Kim, E.-S.; Cho, H.; Gothandam, K.M.; Hyun, J.; Chung, Y.-Y. BcXTH1, a Brassica campestris homologue of Arabidopsis XTH9, is associated with cell expansion. Planta 2006, 224, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, X.; Pang, W.; Jiang, J. GA3-Induced SlXTH19 expression enhances cell wall remodeling and plant height in tomatoes. Plants 2024, 13, 3578. [Google Scholar] [CrossRef]
- Henschel, J.M.; Brito, F.A.; Pimenta, T.M.; Picoli, E.A.; Zsögön, A.; Ribeiro, D.M. Irradiance-regulated biomass allocation in Raphanus sativus plants depends on gibberellin biosynthesis. Plant Physiol. Biochem. 2021, 168, 43–52. [Google Scholar] [CrossRef]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Dewitte, W.; Scofield, S.; Alcasabas, A.A.; Maughan, S.C.; Menges, M.; Braun, N.; Collins, C.; Nieuwland, J.; Prinsen, E.; Sundaresan, V.; et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. USA 2007, 104, 14537–14542. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Ruan, M.; Ye, Q.; Wang, R.; Yao, Z.; Wan, H. Roles and regulations of acid invertases in plants: Current knowledge and future perspectives. Plants 2025, 14, 320. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the study of the function and mechanism of the action of flavonoids in plants under environmental stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.S.; Thomas, M.; Clarke, D.J. The gene stlA encodes a phenylalanine ammonia-lyase that is involved in the production of a stilbene antibiotic in Photorhabdus luminescens TT01. Microbiology 2005, 151, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Dixon, R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, K.; Pawar, S.V.; Sembi, J.K. Genome-wide characterization of PAL, C4H, and 4CL genes regulating the phenylpropanoid pathway in Vanilla planifolia. Sci. Rep. 2025, 15, 10714. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Bashandy, H.; Ainasoja, M.; Kontturi, J.; Pietiainen, M.; Laitinen, R.A.; Albert, V.A.; Valkonen, J.P.; Elomaa, P.; Teeri, T.H. Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of Gerbera hybrida. New Phytol. 2013, 201, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Abrahan, C.; Colquhoun, T.A.; Liu, C.J. A proteolytic regulator controlling chalcone synthase stability and flavonoid biosynthesis in Arabidopsis. Plant Cell 2017, 29, 1157–1174. [Google Scholar] [CrossRef]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, X.; Sun, B.Y.; Peng, C.L.; Chow, W.S. Over-expression of the CHS gene enhances resistance of Arabidopsis leaves to high light. Environ. Exp. Bot. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Schijlen, E.G.; de Vos, C.H.; Martens, S.; Jonker, H.H.; Rosin, F.M.; Molthoff, J.W.; Tikunov, Y.M.; Angenent, G.C.; van Tunen, A.J.; Bovy, A.G. RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol. 2007, 144, 1520–1530. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)-From Genotype to Phenotype to Breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, H.; Shi, J.; Wu, Y.; Jiang, J. Functional characterization of class I SlHSP17.7 gene responsible for tomato cold-stress tolerance. Plant Sci. 2020, 298, 110568. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The genome sequence archive family: Toward explosive data growth and diverse data types. Genom. Proteom. Bioinf. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, J.; Xiao, C.; Qi, Y.; Bai, B.; Cao, X.; Mao, X.; Wu, C.; Liu, Q.; Tang, M.; et al. Integrated Transcriptomic and Metabolomic Profiling of Paclobutrazol-Induced Dwarfism in Tomato Epicotyls. Plants 2025, 14, 3311. https://doi.org/10.3390/plants14213311
Wang J, Li J, Xiao C, Qi Y, Bai B, Cao X, Mao X, Wu C, Liu Q, Tang M, et al. Integrated Transcriptomic and Metabolomic Profiling of Paclobutrazol-Induced Dwarfism in Tomato Epicotyls. Plants. 2025; 14(21):3311. https://doi.org/10.3390/plants14213311
Chicago/Turabian StyleWang, Junqi, Jinzhe Li, Changxin Xiao, Yingbin Qi, Bing Bai, Xia Cao, Xiujie Mao, Chuncheng Wu, Qun Liu, Mingjia Tang, and et al. 2025. "Integrated Transcriptomic and Metabolomic Profiling of Paclobutrazol-Induced Dwarfism in Tomato Epicotyls" Plants 14, no. 21: 3311. https://doi.org/10.3390/plants14213311
APA StyleWang, J., Li, J., Xiao, C., Qi, Y., Bai, B., Cao, X., Mao, X., Wu, C., Liu, Q., Tang, M., & Zhang, N. (2025). Integrated Transcriptomic and Metabolomic Profiling of Paclobutrazol-Induced Dwarfism in Tomato Epicotyls. Plants, 14(21), 3311. https://doi.org/10.3390/plants14213311





