Heterologous Overexpression of Magnaporthe oryzae Effector PWL2 Enhances Rice Blast Resistance via SA-Mediated and PWL2-Derived siRNA Defense
Abstract
1. Introduction
2. Results
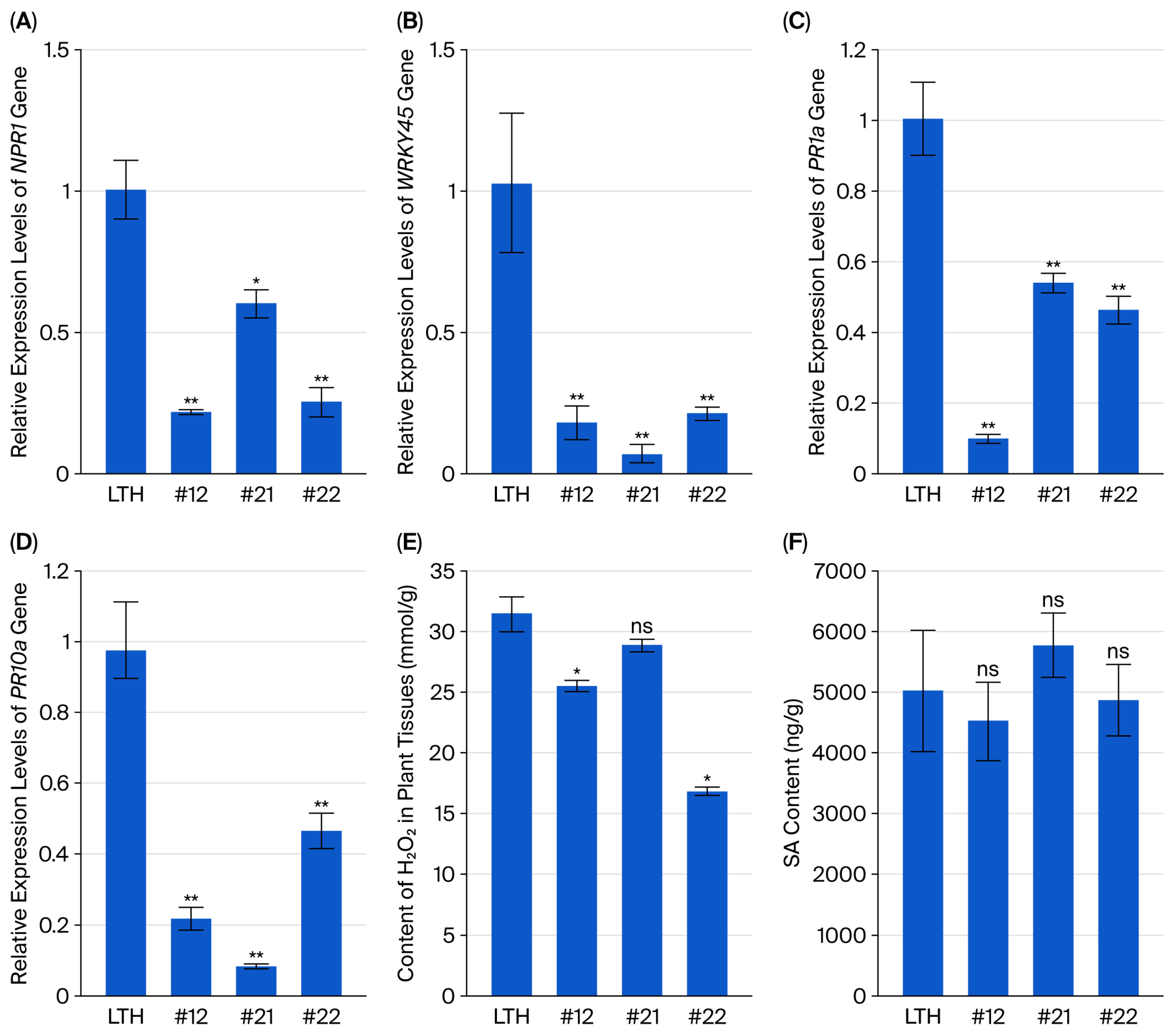
2.1. Transgenic Rice Lines Enhanced Resistance to Rice Blast
2.2. SA Treatment Induced Rice Resistance to Blast Disease
2.3. PWL2-Derived siRNA Was Detected in the PWL2-Overexpressing Transgenic Rice Lines Inoculated with Fungus GUY11
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction and Rice Transformation and Screening
4.2. RNA Isolation, cDNA Biosynthesis and RNA Sequencing
4.3. Real-Time PCR Analysis
4.4. Subcellular Localization Assay
4.5. M. oryzae Inoculated Rice Seedlings
4.6. Hydrogen Peroxide (H2O2) and SA Measurement in Rice Leaves
4.7. Small RNA Gel Blot
4.8. Protein Extraction and Immunoblotting
4.9. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Liu, J.; Ning, Y.; Ding, B.; Wang, X.; Wang, Z.; Wang, G.-L. Recent progress in understanding PAMP- and effector-triggered immunity against the rice blast fungus Magnaporthe oryzae. Mol. Plant 2013, 6, 605–620. [Google Scholar] [CrossRef]
- Azizi, P.; Rafii, M.Y.; Abdullah, S.N.A.; Nejat, N.; Maziah, M.; Hanafi, M.M.; Latif, M.A.; Sahebi, M. Toward understanding of rice innate immunity against Magnaporthe oryzae. Crit. Rev. Biotechnol. 2016, 36, 165–174. [Google Scholar] [CrossRef]
- Valent, B.; Khang, C.H. Recent advances in rice blast effector research. Curr. Opin. Plant Biol. 2010, 13, 434–441. [Google Scholar] [CrossRef]
- Martin-Urdiroz, M.; Oses-Ruiz, M.; Ryder, L.S.; Talbot, N.J. Investigating the biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genet. Biol. 2016, 90, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sweigard, J.A.; Carroll, A.M.; Kang, S.; Farrall, L.; Chumley, F.G.; Valent, B. Identification, cloning, and characterization of PWLP, a gene for host species specificity in the rice blast fungus. Plant Cell 1995, 7, 1221–1233. [Google Scholar] [PubMed]
- Laugé, R.; De Wit, P.J.G.M. Fungal avirulence genes: Structure and possible functions. Fungal Genet. Biol. 1998, 24, 285–297. [Google Scholar] [CrossRef]
- Kang, S. The PWL host specificity gene family in the blast fungus Magnaporthe grisea. MPMI 1995, 8, 939. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Fu, Z.; Gu, X.; Wang, Y.; Jin, X.; Yang, Y.; Wu, W.; Zhang, Y. Distribution and variation of PWL gene family in rice Magnaporthe oryzae from Heilongjiang province and Hainan province. Sci. Agric. Sin. 2023, 56, 264–274. [Google Scholar]
- Oliveira-Garcia, E.; Hamilton, A.J. A pharmacological approach to investigating effector translocation in rice-Magnaporthe oryzae interactions. Plant Signal. Behav. 2024, 19, 2350869. [Google Scholar] [CrossRef]
- Khang, C.H.; Berruyer, R.; Giraldo, M.C.; Kankanala, P.; Park, S.-Y.; Czymmek, K.; Kang, S.; Valent, B. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 2010, 22, 1388–1403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, X.; Zhao, Y.; Zhu, H.; Fu, Q.; Lu, X.; Huang, W.; Yang, X.; Zhou, X.; Wu, L.; et al. Phytoalexin sakuranetin attenuates endocytosis and enhances resistance to rice blast. Nat. Commun. 2024, 15, 3437. [Google Scholar] [CrossRef]
- Were, V.M.; Yan, X.; Foster, A.J.; Sklenar, J.; Langner, T.; Gentle, A.; Sahu, N.; Bentham, A.; Zdrzałek, R.; Ryder, L.S.; et al. The Magnaporthe oryzae effector PWL2 alters HIPP43 localization to suppress host immunity. Plant Cell 2025, 37, koaf116. [Google Scholar] [CrossRef]
- Zhu, J.; Jeong, J.S.; Khang, C.H. Tandem DNA repeats contain cis-regulatory sequences that activate biotrophy-specific expression of Magnaporthe effector gene PWL2. Mol. Plant Pathol. 2021, 22, 508–521. [Google Scholar] [CrossRef]
- Xie, X.-Z.; Xue, Y.-J.; Zhou, J.-J.; Zhang, B.; Chang, H.; Takano, M. Phytochromes regulate SA and JA signaling pathways in rice and are required for developmentally controlled resistance to Magnaporthe grisea. Mol. Plant 2011, 4, 688–696. [Google Scholar] [CrossRef]
- Duan, L.; Liu, H.; Li, X.; Xiao, J.; Wang, S. Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiol. Plant. 2014, 152, 486–500. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, Q.; Cao, Y.; Zhang, Y.; Chen, D.; Lou, X.; Cheng, S.; Cao, L. The OsMPK15 negatively regulates Magnaporthe oryza and Xoo disease resistance via SA and JA signaling pathway in rice. Front. Plant Sci. 2019, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hwarari, D.; Zhang, Y.; Mo, X.; Luo, Y.; Ma, H. Proteomic analysis reveals salicylic acid as a pivotal signal molecule in rice response to blast disease infection. Plants 2022, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Shimono, M.; Koga, H.; Akagi, A.; Hayashi, N.; Goto, S.; Sawada, M.; Kurihara, T.; Matsushita, A.; Sugano, S.; Jiang, C.; et al. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 2012, 13, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Shimono, M.; Sugano, S.; Nakayama, A.; Jiang, C.-J.; Ono, K.; Toki, S.; Takatsuji, H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 2007, 19, 2064–2076. [Google Scholar] [CrossRef]
- Takatsuji, H. Development of disease-resistant rice using regulatory components of induced disease resistance. Front. Plant Sci. 2014, 5, 630. [Google Scholar] [CrossRef]
- Chen, P.; Lan, Y.; Ding, S.; Du, R.; Hu, X.; Zhang, H.; Yu, H.; Xu, L.; Li, C.; Lin, F.; et al. RNA interference-based dsRNA application confers prolonged protection against rice blast and viral diseases, offering a scalable solution for enhanced crop disease management. J. Integr. Plant Biol. 2025, 67, 1633–1648. [Google Scholar] [CrossRef]
- Campo, S.; Sánchez-Sanuy, F.; Camargo-Ramírez, R.; Gómez-Ariza, J.; Baldrich, P.; Campos-Soriano, L.; Soto-Suárez, M.; San Segundo, B. A novel transposable element-derived microRNA participates in plant immunity to rice blast disease. Plant Biotechnol. J. 2021, 19, 1798–1811. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Y.; Shan, D.; Wang, Z.; Song, X.; Wang, Z.; Wang, J.; He, L.; Wu, L.; Zhang, Z.; et al. Magnaporthe oryzae induces the expression of a microRNA to suppress the immune response in rice. Plant Physiol. 2018, 177, 352–368. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.-G.; Shi, Y.; Wu, L.; Xu, Y.-J.; Huang, F.; Guo, X.-Y.; Zhang, Y.; Fan, J.; Zhao, J.-Q.; et al. Multiple rice microRNAs are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiol. 2014, 164, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dean, R.A. Host induced gene silencing of Magnaporthe oryzae by targeting pathogenicity and development genes to control rice blast disease. Front. Plant Sci. 2022, 13, 959641. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, U.; Munir, F.; Salum, Y.M.; He, W. Function and regulation of plant ARGONAUTE proteins in response to environmental challenges: A Review. PeerJ 2024, 12, e17115. [Google Scholar]
- Liang, C.; Wang, X.; He, H.; Xu, C.; Cui, J. Beyond Loading: Functions of plant ARGONAUTE proteins. Int. J. Mol. Sci. 2023, 24, 16054. [Google Scholar] [CrossRef] [PubMed]
- Agorio, A.; Vera, P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 2007, 19, 3778–3790. [Google Scholar] [CrossRef]
- Incarbone, M.; Bradamante, G.; Pruckner, F.; Wegscheider, T.; Rozhon, W.; Nguyen, V.; Gutzat, R.; Mérai, Z.; Lendl, T.; MacFarlane, S.; et al. Salicylic acid and RNA interference mediate antiviral immunity of plant stem cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2302069120. [Google Scholar] [CrossRef]
- Alamillo, J.M.; Saénz, P.; García, J.A. Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco. Plant J. 2006, 48, 217–227. [Google Scholar] [CrossRef]
- Oliveira-Garcia, E.; Tamang, T.M.; Park, J.; Dalby, M.; Martin-Urdiroz, M.; Rodriguez Herrero, C.; Vu, A.H.; Park, S.; Talbot, N.J.; Valent, B. Clathrin-mediated endocytosis facilitates the internalization of Magnaporthe oryzae effectors into rice cells. Plant Cell 2023, 35, 2527–2551. [Google Scholar] [CrossRef]
- Sesma, A.; Osbourn, A.E. The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 2004, 431, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, P.; Sakvarelidze-Achard, L.; Bruun-Rasmussen, M.; Dunoyer, P.; Yamamoto, Y.Y.; Sieburth, L.; Voinnet, O. Widespread translational inhibition by plant miRNAs and siRNAs. Science 2008, 320, 1185–1190. [Google Scholar] [CrossRef]
- Wu, H.; Li, B.; Iwakawa, H.; Pan, Y.; Tang, X.; Ling-hu, Q.; Liu, Y.; Sheng, S.; Feng, L.; Zhang, H.; et al. Plant 22-Nt siRNAs mediate translational repression and stress adaptation. Nature 2020, 581, 89–93. [Google Scholar] [CrossRef]
- Ravet, A.; Zervudacki, J.; Singla-Rastogi, M.; Charvin, M.; Thiebeauld, O.; Perez-Quintero, A.L.; Courgeon, L.; Candat, A.; Lebeau, L.; Fortunato, A.E.; et al. Vesicular and non-vesicular extracellular small RNAs direct gene silencing in a plant-interacting bacterium. Nat. Commun. 2025, 16, 3533. [Google Scholar] [CrossRef]
- Wu, C.-H.; Abd-El-Haliem, A.; Bozkurt, T.O.; Belhaj, K.; Terauchi, R.; Vossen, J.H.; Kamoun, S. NLR network mediates immunity to diverse plant pathogens. Proc. Natl. Acad. Sci. USA 2017, 114, 8113–8118. [Google Scholar] [CrossRef]
- Shepherd, S.; Yuen, E.L.H.; Carella, P.; Bozkurt, T.O. The wheels of destruction: Plant NLR immune receptors are mobile and structurally dynamic disease resistance proteins. Curr. Opin. Plant Biol. 2023, 74, 102372. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Aichi, I.; Matsuoka, M. A protocol for agrobacterium-mediated transformation in rice. Nat. Protoc. 2006, 1, 2796–2802. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, B.; Zhang, X.; Wu, L.; Fu, Q.; Zhao, Y.; Cao, Y.; Zhu, R.; Lu, X.; Huang, W.; et al. Salicylic acid inhibits rice endocytic protein trafficking mediated by OsPIN3t and clathrin to affect root growth. Plant J. 2023, 115, 155–174. [Google Scholar] [CrossRef]
- Shi, X.; Long, Y.; He, F.; Zhang, C.; Wang, R.; Zhang, T.; Wu, W.; Hao, Z.; Wang, Y.; Wang, G.-L.; et al. The fungal pathogen Magnaporthe oryzae suppresses innate immunity by modulating a host potassium channel. PLoS Pathog. 2018, 14, e1006878. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yu, J.; Yu, M.; Yin, X.; Liu, Y. Dry flowable formulations of antagonistic bacillus subtilis strain T429 by spray drying to control rice blast disease. Biol. Control 2015, 85, 46–51. [Google Scholar] [CrossRef]
- Yashaswini, C.; Reddy, P.N.; Pushpavati, B.; Rao, C.S.; Madhav, M.S. Prevalence of rice blast (Magnaporthe oryzae) incidence in south india. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 370–373. [Google Scholar]
- Setotaw, Y.B.; Li, J.; Qi, J.; Ma, C.; Zhang, M.; Huang, C.; Wang, L.; Wu, J. Salicylic acid positively regulates maize defenses against lepidopteran insects. Plant Divers. 2024, 46, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Si, F.; Feng, Z.; Li, S.; Liang, D.; Yang, P.; Yang, C.; Yan, B.; Tang, J.; Yang, Y.; et al. The OsSGS3-tasiRNA-OsARF3 module orchestrates abiotic-biotic stress response trade-off in rice. Nat. Commun. 2023, 14, 4441. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Fu, Q.; Wu, L.; Yang, Y.; Luo, H.; Dong, Q.; Li, S.; Zhao, Y.; Zhou, X.; Xiao, S.; et al. Heterologous Overexpression of Magnaporthe oryzae Effector PWL2 Enhances Rice Blast Resistance via SA-Mediated and PWL2-Derived siRNA Defense. Plants 2025, 14, 3312. https://doi.org/10.3390/plants14213312
Sun X, Fu Q, Wu L, Yang Y, Luo H, Dong Q, Li S, Zhao Y, Zhou X, Xiao S, et al. Heterologous Overexpression of Magnaporthe oryzae Effector PWL2 Enhances Rice Blast Resistance via SA-Mediated and PWL2-Derived siRNA Defense. Plants. 2025; 14(21):3312. https://doi.org/10.3390/plants14213312
Chicago/Turabian StyleSun, Xiaoqian, Qijing Fu, Lixia Wu, Yu Yang, Hao Luo, Qian Dong, Saijie Li, Yiting Zhao, Xuan Zhou, Suqin Xiao, and et al. 2025. "Heterologous Overexpression of Magnaporthe oryzae Effector PWL2 Enhances Rice Blast Resistance via SA-Mediated and PWL2-Derived siRNA Defense" Plants 14, no. 21: 3312. https://doi.org/10.3390/plants14213312
APA StyleSun, X., Fu, Q., Wu, L., Yang, Y., Luo, H., Dong, Q., Li, S., Zhao, Y., Zhou, X., Xiao, S., Li, J., Cheng, Z., Peng, S., Zhong, Q., & Du, Y. (2025). Heterologous Overexpression of Magnaporthe oryzae Effector PWL2 Enhances Rice Blast Resistance via SA-Mediated and PWL2-Derived siRNA Defense. Plants, 14(21), 3312. https://doi.org/10.3390/plants14213312





