Abstract
Drought stress is a major limiting factor during the process of plant growth and development, especially in arid and semi-arid regions. MYB transcription factors play vital roles in the regulation of many developmental processes under various stresses. The aim of this study was to determine whether PtrMYB119 enhanced dehydration tolerance in Nicotiana tabacum. PtrMYB119, with a weak transactivation activity, was distributed throughout the cell with no apparent specificity. The transgenic tobacco overexpressing PtrMYB119 might regulate dehydration tolerance through increased ABA content and antioxidant enzyme activities, decreased MDA levels, and up-regulation of antioxidant genes, polyamine biosynthesis genes, and drought-responsive genes. Overall, our results could contribute to the elucidation of drought tolerance underlying PtrMYB119 action in tobacco and indicated that PtrMYB119 could be exploited for engineering drought-enduring plants in the future.
1. Introduction
As the global climate gets warmer and warmer, drought stress is becoming a great threat to future crop production and food security [1,2]. Up to now, more than one-third of the land around the world is arid land, and it is predicted that this will increase by up to two-thirds by 2050, which will cause serious plant growth problems and crop yield reduction [3,4]. Therefore, it is important to understand the mechanisms of drought tolerance in plants.
When plants are subjected to drought stress, many important morphological and physiological parameters undergo changes, such as leaf wilting, antioxidant enzyme system, endogenous abscisic acid (ABA), and so on [5]. To cope with drought conditions, plants undergo morphological adjustments, including modifications like diminished leaf area, augmented leaf thickness, elevated density of epidermal trichomes, enhanced leaf wax layer, decreased stomatal count, more robust palisade tissues, a greater proportion of palisade to spongy parenchyma thickness, and the development of more elaborate vascular sheaths [6]. Drought stress triggers the buildup of reactive oxygen species (ROS) within plants, a process that in turn stimulates the activity of protective enzymes, thereby mitigating the harm inflicted by ROS on cellular components [7]. Additionally, plant hormones such as ABA play crucial roles in perceiving and reacting to drought stress [8]. Drought stress signaling mechanisms can be categorized into ABA-dependent and ABA-independent regulatory pathways. Both pathways are activated when plants are exposed to drought and engage in distinct signal transduction cascades to counteract the stress [9]. Under drought conditions, the transcript levels of transcription factors (TFs) involved in various signaling pathways undergo changes [10]. MYB TFs are extensively present in higher plants and constitute the largest and most functionally diverse TF family, exerting a central role in plant stress tolerance [11]. A significant number of MYB genes are regulated by drought; for instance, in rice, 65% of drought-associated MYB genes are affected, and in Arabidopsis, 51% of AtMYB genes are up-regulated by drought, while 41% are down-regulated [12,13]. Under drought stress, MYB TFs participate in regulation through various mechanisms. For example, the poplar (Populus tomentosa Carr) transcription factor PtoMYB142 can directly bind to the promoters of the wax biosynthesis genes CER4 and KCS6, activating their transcription, which leads to increased leaf wax accumulation and significantly enhances drought tolerance in poplar [14]. In transgenic tomatoes, SlMYB50 negatively regulates drought and high salt resistance by influencing chlorophyll synthesis, flavonoid synthesis, carotenoid synthesis, antioxidant enzymes, and ABA synthesis [15]. Furthermore, MdMYB88 and MdMYB124 in apple regulate root architecture and xylem development by directly binding to the promoters of MdVND6 and MdMYB46, which are key regulators of secondary cell wall biosynthesis. This regulation improves the plant’s adaptation to drought [16], indicating that MYB TFs can contribute to plant drought stress responses by modifying root system architecture. The overexpression of MdoMYB121 in tomato could enhance its drought tolerance partially by reducing the water loss [17]. The ectopic overexpression of OsMYB4, OsMYB3R-2, and OsMYB2 from rice could facilitate its drought resistance through the constitutive activation of several stress-inducible pathways and different kinetics in the accumulation of several metabolites [18,19,20]. The overexpression of TaMYB30-B in Arabidopsis thaliana also improved its drought tolerance through the changes in physiological traits (water loss, proline, MDA, and soluble sugar contents) and the altered expression of drought stress-responsive genes (RD29A and ERD1) [21]. VvMYB60, a gene from grapevine with high similarity to AtMYB60, could increase its drought tolerance through the modulation of guard cells and the reduction in water loss [22]. The overexpression of StMYB1R-1 in potato plants improved plant tolerance to drought stress through reduced rates of water loss, more rapid stomatal closing, and enhanced the expression of drought-regulated genes, such as AtHB-7, RD28, ALDH22a1, and ERD1-like [23]. The expression of CmMYB2 in chrysanthemum leaves was up-regulated in response to drought, and the overexpression of CmMYB2 in Arabidopsis thaliana enhanced their drought tolerance due to their reduced rates of water loss [24]. It has been reported that anthocyanins are important antioxidants, which can protect plants from adversity damage, and plants with high anthocyanin content also have high antioxidant capacity [25,26]. Some R2R3-MYB transcription factors were found to induce anthocyanin accumulation by regulating anthocyanin biosynthesis-related genes [27,28,29]. SbMYB8, a R2R3-MYB gene from Scutellaria baicalensis, was shown to regulate flavonoid biosynthesis, and the overexpression of which enhanced its drought stress resistance in transgenic tobacco [30]. Overexpression of the snapdragon Delila (Del) gene in tobacco enhanced anthocyanin accumulation, which improved their drought tolerance [31]. The aforementioned research demonstrated that many MYB transcription factors (TFs) have been shown to be involved in the responses to abiotic stresses, which could function in drought response and tolerance through the expression level of key components involved in photosynthesis, ABA and/or auxin signaling networks, as well as antioxidant biosynthesis for ROS scavenging and metabolic adjustments, including wax and flavonoid biosynthesis [32,33]. PtrMYB119, a R2R3-MYB transcription factor from Populus trichocarpa, and the overexpression of which could enhance anthocyanin production in hybrid poplar [34]. However, whether PtrMYB119 could enhance drought tolerance in poplar and other plants is still unknown.
In the present study, our goal was to verify the roles of PtrMYB119 in improving drought tolerance and to investigate the underlying mechanisms. Our data provided a comprehensive resource for further molecular research on this species, which could contribute to the elucidation of drought tolerance underlying PtrMYB119 action in tobacco and also provided references for engineering drought-tolerant plants in the future.
2. Results
2.1. PtrMYB119 Was Responsive to the Drought Treatment
To explore the expression patterns of PtrMYB119 in poplar under drought stress, the expression levels of PtrMYB119 in poplar were determined after drought treatment. After 7 days of drought treatment, the expression levels of PtrMYB119 in poplar increased significantly compared with the 0 days of drought treatment (Supplementary Figure S1), which indicated that PtrMYB119 might be involved in the drought response in poplar.
2.2. PtrMYB119 Was Localized to Nucleus and Cytoplasm with a Weak Transactivation Activity
To explore the subcellular localization of PtrMYB119, the full-length ORF of PtrMYB119 was fused to the N-terminal of GFP reporter protein to generate a fusion protein PtrMYB119: GFP, which was driven by the CaMV35S promoter, and the plasmid containing GFP alone was used as a control. The fusion protein (PtrMYB119) and the control (GFP) were analyzed in tobacco leaf epidermis with a confocal laser scanning microscope. The control GFP was uniformly distributed throughout the whole cell (Figure 1A), whereas the PtrMYB119-GFP fusion protein was also observed in the nucleus and cytoplasm (Figure 1B), indicating that PtrMYB119 was distributed throughout the cell with no apparent specificity.
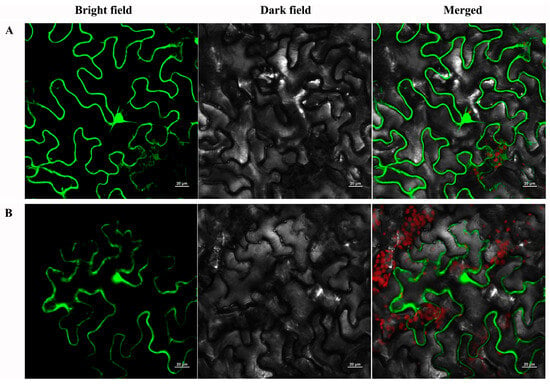
Figure 1.
Subcellular localization analysis of PtrMYB119 protein. (A): p35S-GFP, (B): p35S-PtrMYB119-GFP.
In addition to subcellular localization, transactivation activity is another defining feature of a transcription factor. The Y2H system was used to investigate whether PtrMYB119 functioned as a transcriptional activator. For this purpose, the coding sequence of PtrMYB119 was fused to the coding sequence of GAL4 to generate a fusion plasmid, which was transformed into yeast AH109 to see the growth status of cells on the nutritional selective medium. The positive control was pGADT7-large T/pGBKT7-p53, which showed better growth status of cells on the four nutritional selective medium (SD/-trp, SD/-leu, SD/-trp-leu, SD/-trp-leu-his-ade) and had blue colonies; the negative control was pGADT7-large T/pGBKT7-lamin C, which can grow well on the three nutritional selective media (SD/-trp, SD/-leu, SD/-trp-leu), and cannot grow on the nutritional selective medium of SD/-trp-leu-his-ade (Figure 2). The negative control cannot produce blue colonies compared with the positive control. There were slight blue colonies for the vectors containing PtrMYB119 compared with the positive control, indicating that PtrMYB119 had a weak transactivation activity. On the nutritional selective medium of SD/-trp-leu-his-ade, transgenic yeast cells harboring pGBKT7-MYB119, pGADT7-MYB119, pGADT7/pGBKT7-MYB119, or pGADT7-MYB119/pGBKT7 were able to grow; however, the positive control grew better compared with these transgenic yeast cells (Figure 2). Taken together, these results demonstrated that PtrMYB119 had a weak transactivation activity in yeast cells.
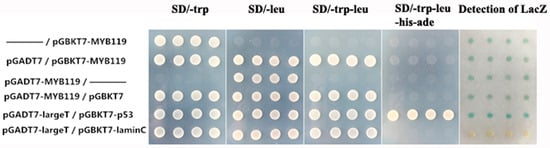
Figure 2.
The transcriptional activation ability of PtrMYB119 was examined using the yeast one-hybrid assay. Yeast cells Y2H expressing the fusion proteins were cultured and adjusted to 2.0 (OD600), and then series diluted and dropped with 2 mL on nutritional selective medium (SD/-trp, SD/-leu, SD/-trp-leu, SD/-trp-leu-his-ade). Yeast cells expressing pGADT7-large T/pGBKT7-lamin C served as the negative control and pGADT7-large T/pGBKT7-p53 as the positive control. The signals of other samples are much weaker compared with those in the p53 positive control, and stronger than those in the negative control. Photos were taken after incubating at 30 °C for 2–4 d.
2.3. The Expression Patterns of PtrMYB119 in Transgenic Tobacco Under Drought Treatments
Transgenic tobacco overexpressing PtrMYB119 was generated to investigate the functions of PtrMYB119 under drought stress. GUS staining and genomic PCR were used to detect whether they were transgenic plants. As shown in Figure 3A, the results of GUS staining indicated that transgenic lines OE-2 and OE-3 were positive. The putative PtrMYB119 fragment (186 bp) with special primers was also amplified from these transgenic lines, OE-2 and OE-3 (Figure 3B).
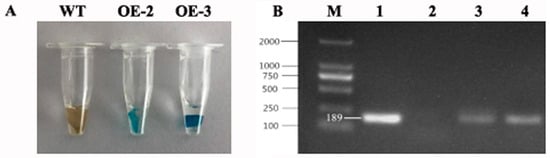
Figure 3.
(A) GUS staining of transgenic plants overexpressing PtrMYB119 (OE-1, transgenic OE-1 line; OE-2, transgenic OE-2 line; OE-3, transgenic OE-3 line). (B) PCR detection of transgenic plants overexpressing PtrMYB119 (M, marker; 1, PtrMYB119 vector; 2, WT; 3, transgenic OE-2 line; 4, transgenic OE-3 line).
The real-time quantitative PCR (qPCR) was performed to evaluate the time-course expression levels of PtrMYB119 in leaves of transgenic tobacco under drought treatment. After 10 days of drought treatment, the expression levels of PtrMYB119 in leaves of transgenic tobacco increased sharply, which indicated that PtrMYB119 in transgenic tobacco might play crucial roles under drought treatment. After 30 days of drought treatment, the expression levels of PtrMYB119 in leaves of transgenic tobacco had a slight decrease, but were still much higher than those on 0 days of drought treatment (Figure 4). Similarly, there was no expression level of PtrMYB119 in WT plants after 10 days and 30 days of drought treatment (Figure 4).
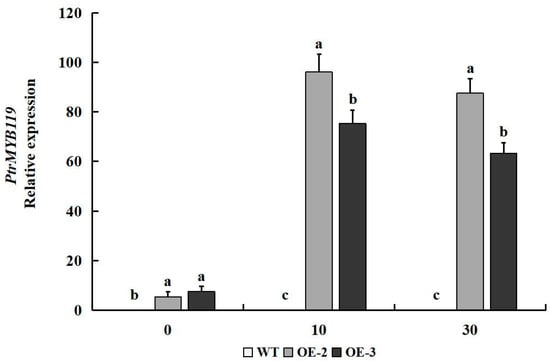
Figure 4.
Expression level of PtrMYB119 in transgenic plants and WT plants after 0, 10, and 30 days of drought treatment. Data are means ± SE of three biological replicates, and means followed by different letters are significantly different (p < 0.05). The X-axis is the days of drought treatment (OE-2, transgenic OE-2 line; OE-3, transgenic OE-3 line).
2.4. Overexpression of PtrMYB119 Affects the Growth of Tobacco Plants Under Drought Stress
The leaves of lines OE-2 and OE-3 appeared wilted first after 10 days of drought treatment, whereas leaves of WT grew normally, which indicated that lines OE-2 and OE-3 responded to drought stress earlier (Figure 5A,B). After 30 days of drought treatment, there were many more withered leaves and a higher ratio of withered leaves to total leaves in WT plants compared with those in lines OE-2 and OE-3 (Figure 5C, Supplementary Figure S2). After the leaves of transgenic and WT plants were removed, the branches of lines OE-2 and OE-3 were much greener, and the degree of wilting and shrinkage in them was less severe compared with those in WT plants (Figure 5D). The cross-sections of the same stem part in transgenic tobacco and WT plants were evaluated, and the results showed that the cross-section of stems in WT plants were more severely wilted and had severe shrinkage compared with those in transgenic tobacco, and the color of the cross-section stem in WT plants was dark compared with those in the transgenic tobacco, indicating that transgenic plants might have better drought tolerance compared with WT plants (Figure 5E). The stem diameters of lines OE-2 and OE-3 were significantly higher than those of WT plants after 30 days of drought treatment (Supplementary Figure S3). However, the plant heights of lines OE-2 and OE-3 were significantly lower than those of WT plants after 30 days of drought treatment (Supplementary Figure S4). To better understand the functions of PtrMYB119 on the growth of transgenic tobacco, the fresh weight and dry weight were evaluated. Although the transgenic lines OE-2 and OE-3 were shorter than WT plants, they had higher fresh weights and dry weights than WT plants after 30 days of drought treatment (Supplementary Figure S5). In addition, the mean root lengths of OE-2 and OE-3 plants were 21.7 ± 1.0 and 24.4 ± 1.5 cm after 30 days of drought treatment, while that of WT plants was 19.5 ± 1.1 cm, which showed that transgenic tobacco lines OE-2 and OE-3 had significantly longer roots (Figure 5F,G), indicating that there was a possible trade-off between growth and stress tolerance in transgenic plants. Overall, transgenic tobacco plants overexpressing PtrMYB119 responded to drought stress more rapidly than WT plants after 10 days of drought treatment, and showed better drought tolerance after 30 days of drought treatment due to their phenotype and physiological indicators.
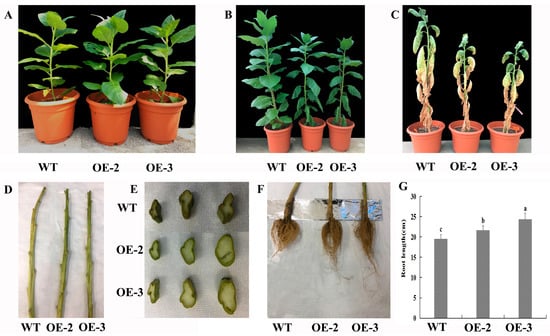
Figure 5.
(A) Transgenic and WT tobacco before drought treatment. (B) Growth of transgenic and WT lines after 10 days of drought treatment. (C) Growth of transgenic and WT lines after 30 days of drought treatment. (D) Stem after 30 days of drought treatment. (E) Cross-section of the same part of the stem of transgenic and WT lines. Data are means ± SE of three biological replicates, and means followed by different letters are significantly different (p < 0.05). (F) Root growth after 30 days of drought treatment. (G) Root length after 30 days of drought treatment (OE-1, transgenic OE-1 line; OE-2, transgenic OE-2 line; OE-3, transgenic OE-3 line).
2.5. The Content of Anthocyanin and Chlorophyll in Tobacco Plants Overexpressing PtrMYB119 Under Drought Stress
The content of anthocyanin in transgenic tobacco overexpressing PtrMYB119 was higher than that in WT plants after 0 days of drought treatment, especially in the transgenic line OE-3. After 10 days and 30 days of drought treatment, the transgenic plants had higher anthocyanin content compared with that in WT plants (Figure 6A). There was no significant difference in chlorophyll content between WT and transgenic tobacco overexpressing PtrMYB119 after 0 days and 10 days of drought treatment. However, chlorophyll content decreased similarly in WT and transgenic plants under prolonged drought (Figure 6B).
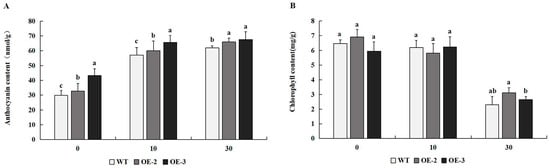
Figure 6.
(A) Anthocyanin content in transgenic and WT plants after drought treatment. (B) Chlorophyll content in transgenic and WT plants after drought treatment. Data are means ± SE of three biological replicates, and means followed by different letters are significantly different (p < 0.05). The X-axis is the days of drought treatment (OE-2, transgenic OE-2 line; OE-3, transgenic OE-3 line).
2.6. Overexpression of PtrMYB119 Affects the ABA Concentration of Tobacco Plants Under Drought Stress
The concentration of ABA in transgenic tobacco overexpressing PtrMYB119 was significantly higher than that in WT plants (Figure 7A). After 10 days of drought treatment, there was a significantly greater increase in ABA concentrations in lines OE-2 and OE-3 than in WT. Compared with the ABA concentration in transgenic tobacco and WT at 10 days of drought treatment, there was less change in ABA concentration in transgenic tobacco and WT at 30 days of drought treatment (Figure 7A). After 10 and 30 days of drought treatment, ABA concentrations in transgenic tobacco and WT plants increased significantly, and ABA concentrations in transgenic tobacco were still much higher than those in WT plants, which indicated that drought stress induced the increase in ABA concentration.
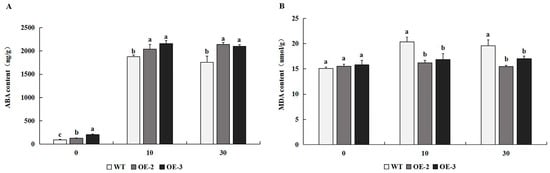
Figure 7.
(A) ABA concentration in transgenic and WT plants after drought treatment. (B) MDA content in transgenic and WT plants after drought treatment. Data are means ± SE of three biological replicates, and means followed by different letters are significantly different (p < 0.05). The X-axis is the days of drought treatment (OE-2, transgenic OE-2 line; OE-3, transgenic OE-3 line).
2.7. Overexpression of PtrMYB119 Decreases MDA Contents of Tobacco Plants Under Drought Stress
MDA played important roles in plants under drought stress. There was no significant difference in MDA content among transgenic plants overexpressing PtrMYB119 and WT plants at 0 days of drought treatment (Figure 7B). After 10 and 30 days of drought treatment, there was a significant difference in MDA content between the transgenic plants and WT plants, and the MDA content in transgenic plants was much lower than that in WT plants, which indicated that transgenic tobacco overexpressing PtrMYB119 might enhance its drought tolerance through a regulatory effect on MDA content.
2.8. Overexpression of PtrMYB119 Enhances Antioxidant Enzyme Activities in Transgenic Tobacco Under Drought Stress
As shown in Figure 8A–C, transgenic tobacco overexpressing PtrMYB119 showed higher POD, SOD, and CAT activities compared with those in WT plants after 0 days of drought treatment. After 10 days of drought treatment, POD and SOD activities increased significantly in both transgenic and WT plants; further, those activities were much higher in transgenic plants than in WT plants. After 30 days of drought treatment, the activities of POD and SOD increased significantly in both transgenic tobacco and WT plants, and they remained much higher in transgenic plants than these in WT plants. CAT activity increased after 10 and 30 days of drought treatment in transgenic plants, while the activity of CAT in WT plants increased after 10 days of drought treatment and decreased after 30 days of drought treatment.
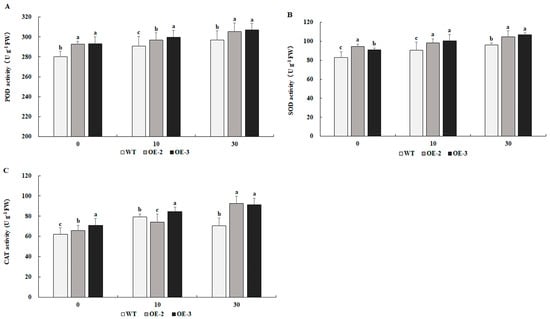
Figure 8.
Antioxidant enzyme activities in transgenic and WT plants under drought treatment. (A) POD, (B) SOD, (C) CAT. Data are means ± SE of three biological replicates, and means followed by different letters are significantly different (p < 0.05). The X-axis is the days of drought treatment (OE-2, transgenic OE-2 line; OE-3, transgenic OE-3 line).
2.9. Expression Levels of Drought Stress-Responsive Genes in Transgenic Tobacco Under Drought Stress
To further investigate the gene expression pattern of transgenic tobacco overexpressing PtrMYB119 under drought stress, transcript abundance of genes involved in drought stress was evaluated in transgenic plants and WT plants under drought conditions. As shown in Figure 9A,B, the relative expression levels of genes associated with the antioxidant enzyme system (SOD and CAT) in the transgenic plants were much higher than those in WT plants, which was consistent with the activity changes in antioxidant enzymes. Arginine decarboxylase 1 (ADC1) and S-adenosylmethionine decarboxylase (SAMDC), involved in the polyamine biosynthesis, can play important roles in various abiotic stresses, including drought stress. There was no significant difference in the expression level of ADC1 between transgenic plants and WT plants at 0 days of drought treatment (Figure 9C). However, there was a significantly greater increase in ADC1 expression level in transgenic plants compared with that in WT plants after 10 days of drought treatment, and the expression level of ADC1 in transgenic plants was maintained at a high level after 30 days of drought treatment (Figure 9C). There is an increase in the wildtype but only a slight one compared to the increase in the transgenic lines after 10 days and 30 days of drought treatment (Figure 9C). Different from the expression level of ADC1, the expression levels of SAMDC in transgenic plants were much higher than those in WT plants after 0 days of drought treatment. After 10 days of drought treatment, the expression level of SAMDC in both transgenic plants and WT plants had a slight increase, and the expression levels of SAMDC in transgenic plants were still much higher than those in WT plants. After 30 days of drought treatment, the expression level of SAMDC in both transgenic plants and WT plants had a slight decrease, and the expression levels of SAMDC in transgenic plants were also much higher than those in WT plants (Figure 9D). These results indicated that the genes SAMDC and ADC1 might play essential roles in the regulation of drought stress in tobacco. To better understand the molecular mechanism of drought tolerance in tobacco mediated by PtrMYB119, the expression levels of drought-responsive genes, including ERD10D, NCED3, and NAC/RD26, were evaluated. There was a slight increase in expression level for NCED3 in both transgenic plants and WT plants after 10 days of drought treatment, and the expression level of this gene in transgenic plants is higher than that in WT plants. After 30 days of drought treatment, the expression level of NCED3 in both transgenic plants and WT plants had a slight decrease, and the expression level of this gene in transgenic plants is still higher than that in WT plants (Figure 9E). The expression level of NAC/RD26 in transgenic plants was much higher than that in WT plants after 0 days of drought treatment, and the increase in expression level for NAC/RD26 in WT plants was much higher than that in transgenic plants after 10 days of drought treatment (Figure 9). After 30 days of drought treatment, the expression level of NAC/RD26 in transgenic lines OE-2 and WT plants had a slight decrease, while the expression level of NAC/RD26 in transgenic lines OE-3 had a slight increase (Figure 9F). For the gene ERD10D, its expression level in transgenic plants had a continued increase after 10 days and 30 days of drought treatment, while there was a low expression level in WT plants after 10 days and 30 days of drought treatment. The expression level of ERD10D in transgenic plants was always much higher than that in WT plants (Figure 9G). Overall, these results indicated that overexpression of PtrMYB119 in transgenic plants enhanced drought tolerance by regulating the expression level of antioxidant genes, polyamine biosynthesis genes, and drought-responsive genes.
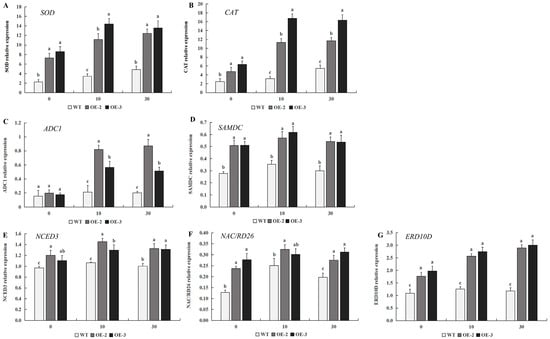
Figure 9.
Expression levels of drought-related genes in transgenic and WT plants under drought stress. (A) Antioxidant enzyme-coding genes SOD. (B) Antioxidant enzyme-coding genes CAT. (C) Polyamine biosynthesis genes ADC1. (D) Polyamine biosynthesis genes SAMDC. (E) Drought-responsive genes NCED3. (F) Drought-responsive genes NAC/RD26. (G) Drought-responsive genes ERD10D. Data are means ± SE of three biological replicates, and means followed by different letters are significantly different (p < 0.05). The X-axis is the days of drought treatment (OE-2, transgenic OE-2 line; OE-3, transgenic OE-3 line).
3. Discussion
Drought is a major abiotic stress that negatively affects vegetative and reproductive development of plants and reduces plant productivity. It is of great significance to discover the candidate genes associated with drought resistance to help plants cope with drought stress. Many MYB family genes have been responsive to drought stress when plants encounter drought stress. Among them, a few MYB genes have been identified and characterized in poplar. Although many reports have described the function of MYB TFs in drought stress of different plant species [35,36,37], it is still unclear and needs to be further explored.
Anthocyanins have been proven to be involved in drought tolerance in many plant species [30,31,38,39,40]. As an antioxidant, anthocyanins containing high contents of enzymatic and non-enzymatic antioxidants can scavenge excess ROS [41], the accumulation of which in the vacuoles prevents the overproduction of ROS due to abiotic stresses [42], which makes them osmo-regulators that maintain water homeostasis in plants [26]. Many researchers indicated that transgenic plants overexpressing MYB TFs increased anthocyanin content [30,32,43], and anthocyanin accumulation had been associated with drought tolerance in many plant species [30,31,38,39,40]. As the previous study indicated that the overexpression of PtrMYB119 in hybrid poplar could promote the production of anthocyanin [34], a substantially increased anthocyanin content in transgenic lines was expected. In our study, the increasing trends of anthocyanin content in WT plants and transgenic plants were similar (Figure 6A), indicating that higher anthocyanin content might not be the main reason why transgenic tobacco overexpressing PtrMYB119 showed higher drought tolerance.
MYB genes functioned with ABA and regulated plant response to drought stress [44,45,46,47]. The higher accumulation of the endogenous ABA content in transgenic plants could enhance their drought tolerance through stress-induced ABA accumulation [44,48]. NCED (9-cis epoxycarotenoid dioxygenase), a rate-limiting enzyme gene, played crucial roles in drought stress-inducible ABA biosynthesis and catabolism [49]. The transgenic Arabidopsis thaliana overexpressing OsNCED3 could increase its ABA content dramatically to cope with various stresses. In accordance with a previous study, transgenic plants overexpressing PtrMYB119 had a higher ABA accumulation compared with these in WT plants under drought treatment, and the expression levels of NCED3 in transgenic tobacco overexpressing PtrMYB119 were higher than those in WT plants (Figure 7A and Figure 9E), which indicated that PtrMYB119 might play a positive role in drought tolerance in tobacco by regulating stress-induced ABA synthesis.
The accumulation of ROS induced by drought could lead to cell toxicity, membrane peroxidation, and even cell death [50,51]. In order to detoxify ROS accumulation, plants have evolved efficient enzymatic antioxidant defense systems, including SOD, POD, CAT, and GST. Changes in antioxidant enzyme activities have been widely reported in plants in response to drought stress [20,52,53], and plants with high levels of these antioxidant enzymes showed tolerance to drought, salinity, or oxidative stress [54,55,56]. Consistent with previous results, transgenic plants overexpressing PtrMYB119 had higher activities of antioxidant enzymes (SOD and CAT) compared with WT plants, which also have higher expression levels of the two genes SOD and CAT (Figure 8 and Figure 9A,B). ROS accumulation can also lead to peroxidation of membrane lipids, which produces a mass of degradation products, such as MDA [57]. As a marker for lipid peroxidation, low MDA content in leaves has been linked to high drought tolerance [39,58]. MDA in OsMYB2-overexpressing plants was markedly lower than that in WT plants under abiotic stress [20]. The overexpression of both GbMYB5 and Rosea1 in tobacco had a lower MDA accumulation compared with WT plants, which contributed to enhanced drought tolerance in transgenic tobacco [59,60]. In this study, the MDA content was lower in the transgenic tobacco plants overexpressing PtrMYB119 than that in WT plants under drought treatment (Figure 7B), which was consistent with previous results [20,59,60]. Therefore, PtrMYB119 might contribute to enhanced drought tolerance in tobacco through scavenging ROS accumulation under drought stress.
Plants adapt to drought stress through the up-regulation of stress-responsive genes, and the overexpression of some MYB TFs in plants can enhance their drought tolerance via this mechanism [21,37,59,60,61,62]. ERD10 (C/D) encoded group 5 LEA proteins, which had crucial roles in withstanding cellular dehydration. The high expression of ERD10 (C/D) can provide more chaperones or protective proteins for maintaining membrane integrity to sustain plant growth during drought [63,64]. NAC/RD26, a drought-inducible gene encoding an NAC transcription factor, had pivotal roles in plants under drought treatment [59]. In this study, the expression of ERD10D and NAC/RD26 was much higher in transgenic plants overexpressing PtrMYB119 under drought stress (Figure 9), indicating that transgenic plants might enhance their drought tolerance through the regulation of drought stress-responsive genes. In addition, the accumulation of osmolytes such as proline and (poly)amine was critical to maintain plant cell turgor and cell structure stabilization during drought stress [8,49,65]. The genes ADC1 and SAMDC participated in the biosynthesis of the osmo-protectants proline and polyamine, which function in resisting adverse environments by adjusting osmotic balance and protecting plasma membrane integrity [8]. There were higher expression levels of ADC1 and SAMDC in transgenic plants overexpressing PtrMYB119 under drought stress (Figure 9C,D), which agreed with previous results [37,59], indicating that the transgenic PtrMYB119 plants might synthesize osmolytes to alleviate cellular damage when they were subjected to drought stress. The above results indicated that the overexpression of PtrMYB119 in tobacco can enhance its drought tolerance by the up-regulation of stress-responsive genes.
4. Materials and Methods
4.1. Plant Materials and Reagents
Tobacco (Nicotiana tabacum L.) cultivar K326 was used as experimental material. E. coli strain DH5α and Agrobacterium tumefaciens EHA105 were purchased from Shanghai Weidi Biotechnology Co., Ltd. (Shanghai, China). The primers were synthesized by Nanjing Genscript Biotech (Nanjing, China).
4.2. Plasmid Construction
Total RNA was extracted from leaves of Populus trichocarpa. First-strand cDNA was synthesized using a first-strand cDNA synthesis kit (Vazyme: R233-01, Nanjing, China). Gene-specific primers of PtrMYB119 were designed using Primer 5 software. The primer sequences are listed in Supplementary Table S1. PCR conditions were: 94 °C for 3 min, 30 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min, and finally 72 °C for 10 min. The PCR product and pCAMBIA2300 vector were digested by Sal I and Xbal, and the target gene fragments and the backbone of the pCAMBIA2300 vector were ligated with T4 DNA ligase to obtain the vector pCAMBIA2300-PtrMYB119, which was confirmed by double digestion with a restriction enzyme and sequencing. The constitutive expression system included the CaMV35S promoter, nopaline synthase (NOS) terminator system, coding sequence of PtrMYB119, β-glucuronidase gene (GUS), and kanamycin-resistant gene in the recombined vector pCAMBIA2300-PtrMYB119 (Supplementary Table S2).
4.3. Transactivitional Activity and Subcellular Localization of PtrMYB119
For the transactivation assay, the complete ORF of PtrMYB119 was amplified by PCR using primers (Supplementary Table S1) containing Sfi I restriction sites, and the amplicon was digested by Sfi I. The resultant fragment containing PtrMYB119 was then fused downstream of the yeast GAL4 DNA-binding domain in pGBKT7 by recombination reactions. The fusion vector and the negative control (pGBKT7) were independently expressed in yeast strain AH109 according to the manufacturer’s instructions.
The full-length cDNA of the PtrMYB119 was synthesized by Nanjing Zoonbio Tech, Co., Ltd. (Nanjing, China) and inserted into a polylinker site of the binary vector pCAMBIA1302 to generate a fusion construct (p35S-PtrMYB119-GFP). After sequence verification, the fusion construct (p35S-PtrMYB119-GFP) and the control vector (pCAMBIA1302) were transferred into Agrobacterium tumefaciens strain GV3101 by heat shock. The abaxial surfaces of 5-week-old N. benthamiana leaves were agroinfiltration with the bacterial suspension (OD600 = 0.5) and then kept in an incubator for 24 h, followed by live cell imaging under an inverted confocal microscope (Zeiss LSM 780, Oberkochen, Germany). The images were presented with bright field, dark field, and a merge of bright field.
4.4. Transformation of Transgenic Tobacco Plant
The pCAMBIA2300-PtrMYB119 was transformed into Agrobacterium tumefaciens EHA105, and the plasmid was also confirmed by double digestion with a restriction enzyme and sequencing. Transgenic tobacco plants were obtained using the Agrobacterium-mediated method as previously described [66]. Transgenic tobacco plants were selected on MS medium containing 50 mg·L−1 Kanamycin (Km) and 100 mg·L−1 Timentin (Tim). Positive transgenic T0 plants from regenerated Km-resistant plants were screened through GUS staining and PCR detection. Wildtype (WT) tobacco plants were used as controls.
4.5. Drought Stress Treatment
For drought treatment, three lines were selected from transgenic plants overexpressing PtrMYB119, and WT plants were used as the control. Each line contained twenty-five plants to conduct the drought treatment. Firstly, tissue culture seedlings were grown for 20 days in the tissue culture room. Secondly, they were placed in a growth chamber for adaptation to grow better. After that, they were transplanted into a peat–perlite mixture (1:5 v/v) in plastic pots (30 × 25 × 22 cm) in the glass greenhouse at 20–25 °C during the day, 10–15 °C at night, and with a 12/12 h (day/night) photoperiod. Plants were irrigated every 3 days until treatment initiation. To conduct the drought treatment, more than eighteen plants of each line and WT plants with a similar phenotype, such as leaf size and plant height, under well-watered conditions were selected. The growth status of tobacco plants was observed and recorded every day. The plants were photographed and sampled every 10 days for up to 30 days.
The one-year Populus sp. Linn. ‘2025’ in the greenhouse was used to conduct the drought treatment. The leaves from five Populus sp. Linn. ‘2025’ were obtained after 0 days of drought treatment and 7 days of drought treatment, separately. Each data point was the average value of three replicates.
4.6. Measurement of Anthocyanin, Chlorophyll, MDA and POD, SOD and CAT Enzyme Activities
To explore the effects of physiological changes caused by the overexpression of PtrMYB119 in transgenic tobacco plants under drought treatment, similar leaves in each biological replicate were collected from three random transgenic and WT tobacco plants under drought treatment to determine the content of anthocyanin, chlorophyll, malondialdehyde (MDA), and the activities of peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT). Total anthocyanin and chlorophyll contents were evaluated with previous methods [67]. The POD, SOD, and CAT activities and MDA content were determined using previous methods [59]. Each data point was the average value of three replicates.
4.7. Measurement of Endogenous ABA Content
All reagents and ABA were obtained from Sigma-Aldrich. HPLC-grade methanol, acetone, glacial acetic acid, formic acid, and acetonitrile were purchased from Merck. Fresh leaves (1 g) of transgenic and WT plants were ground in an ice-cold mortar and extracted twice with acetone/water/acetic acid (80:19:1, v/v/v) at −20 °C. After centrifugation, the extract from the second extraction was reconstituted in 200 μL of acetonitrile/water/acetic acid (90:10:0.05, v/v/v). The final extract was filtered and added to the LC-MS/MS system. Quantification was finished by the standard addition method by spiking control samples with ABA solutions [68]. Each data point was the average value of three replicates.
4.8. Quantitative Real-Time PCR
Total RNA was extracted according to the manufacturer’s instructions, and then the extracted RNA was reverse transcribed using a PrimeScript™1st Strand cDNA Synthesis Kit according to the kit instructions. Quantitative real-time PCR was performed using TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa, Dalian, China). Each 20 µL quantitative real-time PCR contained 10 µL of TB GreenTM PCR master mix, 0.2 mM of each primer and 10 ng of cDNA with the following PCR program, 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, and 62 °C for 1 min in an ABI 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). All results were calculated from three biological replicates with three technical replicates. Drought stress-responsive genes in tobacco plants were analyzed at 10 and 30 d after drought treatment initiation. NtTubulin (N181029A17) was used as a house-keeping gene to investigate gene expression in transgenic tobacco plants overexpressing PtrMYB119 and WT plants. The leaves of Populus sp. Linn. ‘2025’ after 0 days and 7 days of drought treatment were collected. UBQ (Accession number: AF240445) was used to normalize gene expression in poplar [67]. The UBQ primers for qRT-PCR were the following: UBQ-F (5′-TGAACCAAATGATACCATTGATAG-3′) and UBQ-R (5′-GTAGTCGCGAGCTGTCTTG-3′). All gene-specific primers were designed with Primer 5 software and are listed in Supplementary Table S3. The relative abundance of the genes was determined by the method [69].
4.9. Statistical Analysis
The experiments were repeated three times with three technical replicates. All data were expressed as mean ± standard error of three biological replicates. Differences among means of the various treatments were determined by the least significant difference test. Significance analysis was performed using SPSS 17.0 software. Normal distribution was tested with the Shapiro–Wilk test, and homogeneity of variance was tested with Levene’s test. One-way analysis of variance (ANOVA) was carried out to test the significance of treatments at p < 0.05, followed by Duncan’s tests. Means were considered to be significantly different when p ≤ 0.05, as shown in the figures. Microsoft Excel and GraphPad Prism 5.0 software were used for data analysis and charting.
5. Conclusions
In summary, PtrMYB119 overexpression lines showed elevated ABA content, antioxidant enzyme activity, drought stress-responsive gene expression levels, and reduced MDA content following drought treatment, suggesting that PtrMYB119 modulated tolerance to drought in tobacco (Figure 10). The results of this study indicated that PtrMYB119 played an important role in response to drought stress in tobacco and may be a potential gene for improving drought tolerance in other plants. In addition, the drought tolerance function of the PtrMYB119 gene has only been validated in tobacco, and further validation is needed in other woody plants such as poplar trees.
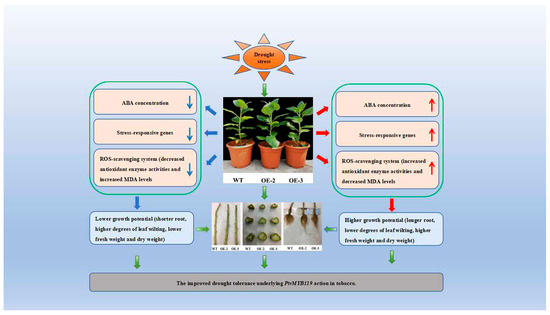
Figure 10.
Proposed mechanisms of PtrMYB119 enhanced drought tolerance in tobacco.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14213251/s1.
Author Contributions
L.S. and J.A.: conceptualization, data curation, and writing—original draft. J.Z. and T.Y.: visualization and investigation. W.Z.: conceptualization, methodology, supervision, writing—original draft and writing—review and editing. Z.W.: writing—review and editing. X.S.: formal analysis and validation. T.W.: software and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Natural Science Foundation of Jiangsu Province, China (BK20242007), the National Natural Science Foundation of China (32271916), and the Jiangsu Agricultural Science and Technology Innovation Fund (CX(24)3048). The funders have no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Data Availability Statement
All data analyzed in this study are included in this published article and Supplementary Materials.
Conflicts of Interest
Author Jie Zhu was employed by the company Lin Yi State Owned Holding Gardens Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Sensing the environment: Key roles of membrane-localized kinases in plant perception and response to abiotic stress. J. Exp. Bot. 2013, 64, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Pessarakli, M. Plant and Crop Stress, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4398-1396-6/978-1-4398-1399-7. [Google Scholar]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; El–Daim, I.A.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant growth promoting bacteria. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Negin, B.; Moshelion, M. The advantages of functional phenotyping in pre–field screening for drought-tolerant crops. Funct. Plant Biol. 2017, 44, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Teng, Y.; Cen, Q.; Fang, Y.; Tian, Q.; Zhang, X.; Wang, H.; Zhang, X.; Xue, D. Genome-wide identification of R2R3-MYB transcription factor and expression analysis under abiotic stress in rice. Plants 2022, 11, 1928. [Google Scholar] [CrossRef]
- Song, Q.; Kong, L.; Yang, X.; Jiao, B.; Hu, J.; Zhang, Z.; Xu, C.; Luo, K. PtoMYB142, a poplar R2R3-MYB transcription factor, contributes to drought tolerance by regulating wax biosynthesis. Tree Physiol. 2022, 42, 2133–2147. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, P.; Zhang, X.; Xie, Q.; Chen, G.; Zhou, S.; Hu, Z. Silencing of SIMYB50 affects tolerance to drought and salt stress in tomato. Plant Physiol. Biochem. 2022, 193, 139–152. [Google Scholar] [CrossRef]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef]
- Cao, Z.H.; Zhang, S.Z.; Wang, R.K.; Zhang, R.F.; Hao, Y.J. Genome wide analysis of the apple MYB transcription factor family allows the identification of MdoMYB121 gene confering abiotic stress tolerance in plants. PLoS ONE 2013, 8, e69955. [Google Scholar] [CrossRef] [PubMed]
- Mattana, M.; Biazzi, E.; Consonni, R.; Locatelli, F.; Vannini, C.; Provera, S.; Coraggio, I. Overexpression of Osmyb4 enhances compatible solute accumulation and increases stress tolerance of Arabidopsis thaliana. Physiol. Plant. 2005, 125, 212–223. [Google Scholar] [CrossRef]
- Dai, X.; Xu, Y.; Ma, Q.; Xu, W.; Wang, T.; Xue, Y.; Chong, K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007, 143, 1739–1751. [Google Scholar] [CrossRef]
- Yang, A.; Dai, X.; Zhang, W.H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.; Xia, C.; Jia, J.; Liu, X.; Kong, X. A wheat R2R3–MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic Arabidopsis. J. Exp. Bot. 2012, 63, 5873–5885. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, M.; Matus, J.T.; Francia, P.; Rusconi, F.; Canon, P.; Medina, C.; Conti, L.; Cominelli, E.; Tonelli, C.; Arce-Johnson, P. The grapevine guard cell-related VvMYB60 transcription factor is involved in the regulation of stomatal activity and is differentially expressed in response to ABA and osmotic stress. BMC Plant Biol. 2011, 11, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Moon, S.J.; Han, S.; Kim, B.G.; Park, S.R.; Lee, S.K.; Yoon, H.J.; Lee, H.E.; Kwon, H.B.; Baek, D.; et al. Expression of StMYB1R-1, a novel potato single MYB–like domain transcription factor, increases drought tolerance. Plant Physiol. 2011, 155, 421–432. [Google Scholar] [CrossRef]
- Shan, H.; Chen, S.; Jiang, J.; Chen, F.; Chen, Y.; Gu, C.; Li, P.; Song, A.; Zhu, X.; Gao, H.; et al. Heterologous expression of the chrysanthemum R2R3–MYB transcription factor CmMYB2 enhances drought and salinity tolerance, increases hypersensitivity to ABA and delays flowering in Arabidopsis thaliana. Mol. Biotech. 2012, 51, 160–173. [Google Scholar] [CrossRef]
- Zhang, Y.; De Stefano, R.; Robine, M.; Butelli, E.; Bulling, K.; Hill, L.; Rejzek, M.; Martin, C.; Schoonbeek, H.J. Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant Physiol. 2015, 169, 1568–1583. [Google Scholar]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Feng, S.Q.; Wang, Y.L.; Yang, S.; Xu, Y.T.; Chen, X.S. Anthocyanin biosynthesis in pears is regulated by a R2R3–MYB transcription factor PyMYB10. Planta 2010, 232, 245–255. [Google Scholar] [CrossRef]
- Albert, N.W.; Griffiths, A.G.; Cousins, G.R.; Verry, I.M.; Williams, W.M. Anthocyanin leaf markings are regulated by a family of R2R3–MYB genes in the genus Trifolium. New Phytol. 2015, 205, 882–893. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, R.; Xu, Z.; Yang, W.; Wang, J.; Cheng, T.; Pan, H.; Zhang, Q. Isolation and functional characterization of a R2R3–MYB regulator of Prunus mume anthocyanin biosynthetic pathway. Plant Cell Tiss. Org. 2017, 131, 417–429. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, L.; Yang, J.; Wu, C.; Liu, Y.; Huang, L. A Scutellaria baicalensis R2R3–MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tiss. Org. 2015, 120, 961–972. [Google Scholar] [CrossRef]
- Naing, A.H.; Park, K.I.; Ai, T.N.; Chung, M.Y.; Han, J.S.; Kang, Y.W.; Lim, K.B.; Kim, C.K. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Zhou, H.P.; Zhang, Y.; Zhao, Y.Q.; Zhang, Y.Y.; Feng, X.X.; Lin, H.H. Diverse roles of MYB transcription factors in plants. J. Interg. Plant Biol. 2025, 67, 539–562. [Google Scholar] [CrossRef]
- Cho, J.S.; Nguyen, V.P.; Jeon, H.W.; Kim, M.H.; Eom, S.H.; Lim, Y.J.; Kim, W.C.; Park, E.J.; Choi, Y.I.; Ko, J.H. Overexpression of PtrMYB119, a R2R3–MYB transcription factor from Populus trichocarpa, promotes anthocyanin production in hybrid poplar. Tree Physiol. 2016, 36, 1162–1176. [Google Scholar] [CrossRef]
- Seo, J.S.; Sohn, H.B.; Noh, K.; Jung, C.; An, J.H.; Donovan, C.M. Expression of the Arabidopsis AtMYB44 gene confers drought/salt–stress tolerance in transgenic soybean. Mol. Breed. 2012, 29, 601–608. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, G.; Zhao, G.; Xia, C.; Jia, J.; Liu, X.; Kong, X. Characterization of a wheat R2R3–MYB transcription factor gene, TaMYB19, involved in enhanced abiotic stresses in Arabidopsis. Plant Cell Physiol. 2014, 55, 1802–1812. [Google Scholar] [CrossRef]
- Wei, Q.H.; Luo, Q.C.; Wang, R.B.; Zhang, F.; He, Y.; Zhang, Y. A wheat R2R3–type MYB transcription factor TaODORANT1 positively regulates drought and salt stress responses in transgenic tobacco plants. Front. Plant Sci. 2017, 8, 1374. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tiss. Org. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Kong, W.; Peng, R.; Liu, Q.; Yao, Q. The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta 2016, 244, 59–73. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Chu, H.D.; Van Ha, C.; Watanabe, Y.; Osakabe, Y. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 2017, 13, e1007076. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Alegre, L.; Van Breusegem, F.; Munne-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.P.; Li, S.J.; Yao, P.F.; Li, C.L.; Chen, H.; Wu, Q.; Zhao, H. The jasmonate–ZIM domain protein FtJAZ2 interacts with the R2R3–MYB transcription factor FtMYB3 to affect anthocyanin biosynthesis in tartary buckwheat. Turk. J. Biol. 2017, 41, 526–534. [Google Scholar] [CrossRef]
- Xiong, H.; Li, J.; Liu, P.; Duan, J.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.L.; Ali, J.; Li, Z.C. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef]
- Cai, H.S.; Tian, S.; Dong, H.S.; Guo, C.H. Pleiotropic effects of TaMYB3R1 on plant development and response to osmotic stress in transgenic Arabidopsis. Gene 2015, 558, 227–234. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.N.; Qiao, M.; Park, J.Y.; Lee, Y.N.; Kim, S.G.; Lee, Y.H.; Park, W.J.; Park, C.M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Cominelli, E.; Galbiati, M.; Vavasseur, A.; Conti, L.; Sala, T.; Vuylsteke, M.; Leonhardt, N.; Dellaporta, S.L.; Tonelli, C. A guard–cell–specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 2005, 15, 1196–1200. [Google Scholar] [CrossRef]
- Jung, C.; Seo, J.S.; Han, S.W.; Koo, Y.J.; Kim, C.H.; Song, S.I.; Nahm, B.H.; Choi, Y.D.; Cheong, J.J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008, 146, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 2006, 1757, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Liu, J.H.; Chen, X.J. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 2010, 10, 230. [Google Scholar] [CrossRef]
- Huang, X.S.; Luo, T.; Fu, X.Z.; Fan, Q.J.; Liu, J.H. Cloning and molecular characterization of a mitogen–activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco. J. Exp. Bot. 2011, 62, 5191–5206. [Google Scholar] [CrossRef]
- Noreen, Z.; Ashraf, M. Assessment of variation in antioxidative defence system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerant markers. J. Plant Physiol. 2009, 166, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef]
- Kim, M.D.; Kim, Y.H.; Kwon, S.Y.; Yun, D.J.; Kwak, S.S. Enhanced tolerance to methyl viologen–induced oxidative stress and high temperature in transgenic potato plants overexpressing the CuZnSOD, APX and NDPK2 genes. Physiol. Plant. 2010, 140, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef]
- Esfandiari, E.O.; Shakiba, M.R.; Mahboob, S.A. Water stress, antioxidant enzyme activity and lipid peroxidation in wheat seedling. J. Food Agric. Environ. 2007, 5, 149–153. [Google Scholar]
- Chen, T.Z.; Li, W.J.; Hu, X.H.; Guo, J.R.; Liu, A.M.; Zhang, B.L. A cotton MYB transcription factor, GbMYB5, is positively involved in plant adaptive response to drought stress. Plant Cell Physiol. 2015, 56, 917–929. [Google Scholar] [CrossRef]
- Naing, A.H.; Ai, T.N.; Lim, K.B.; Lee, I.J.; Kim, C.K. Overexpression of Rosea1 from Snapdragon enhances anthocyanin accumulation and abiotic stress tolerance in transgenic tobacco. Front. Plant Sci. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Su, L.T.; Li, J.W.; Liu, D.Q.; Zhai, Y.; Zhang, H.J.; Li, X.W.; Zhang, Q.L.; Wang, Y.; Wang, Q.Y. A novel MYB transcription factor, GmMYBJ1, from soybean confers drought and cold tolerance in Arabidopsis thaliana. Gene 2014, 538, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.J.; Wang, M.P.; Wei, Y.Y.; Xia, Z.L. Overexpression of the Maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front. Plant Sci. 2015, 6, 1223. [Google Scholar] [CrossRef]
- Hundertmark, M.; Kincha, D.K. LEA (Late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Kovacs, D.; Kalmar, E.; Torok, Z.; Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress–related plant proteins. Plant Physiol. 2008, 147, 381–390. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Takahashi, T.; Michael, A.J.; Kusano, T. A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem. Biophys. Res. Commun. 2007, 352, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, Y.; Meng, Z.; Jiang, J. Optimization of factors affecting Agrobacterium–mediated transformation of Micro–Tom tomatoes. Genet. Mol. Res. 2012, 11, 661–671. [Google Scholar] [CrossRef]
- Noda, Y.; Furukawa, J.; Aohara, T.; Nihei, N.; Hirose, A.; Tanoi, K.; Nakanishi, T.M.; Satoh, S. Short day length-induced decrease of cesium uptake without altering potassium uptake manner in poplar. Sci. Rep. 2016, 6, 38360. [Google Scholar] [CrossRef] [PubMed]
- López-Carbonell, M.; Jáuregui, O. A rapid method for analysis of abscisic acid (ABA) in crude extracts of water stressed Arabidopsis thaliana plants by liquid chromatography–mass spectrometry in tandem mode. Plant Physiol. Biochem. 2005, 43, 407–411. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real–time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).