Ascorbic Acid Priming Boosts Cotton Seed Chilling Tolerance via Membrane Stability and Antioxidant Cycles
Abstract
1. Introduction
2. Result
2.1. Effects of AsA Priming with Different Concentrations and Times on Cotton Seed Germination
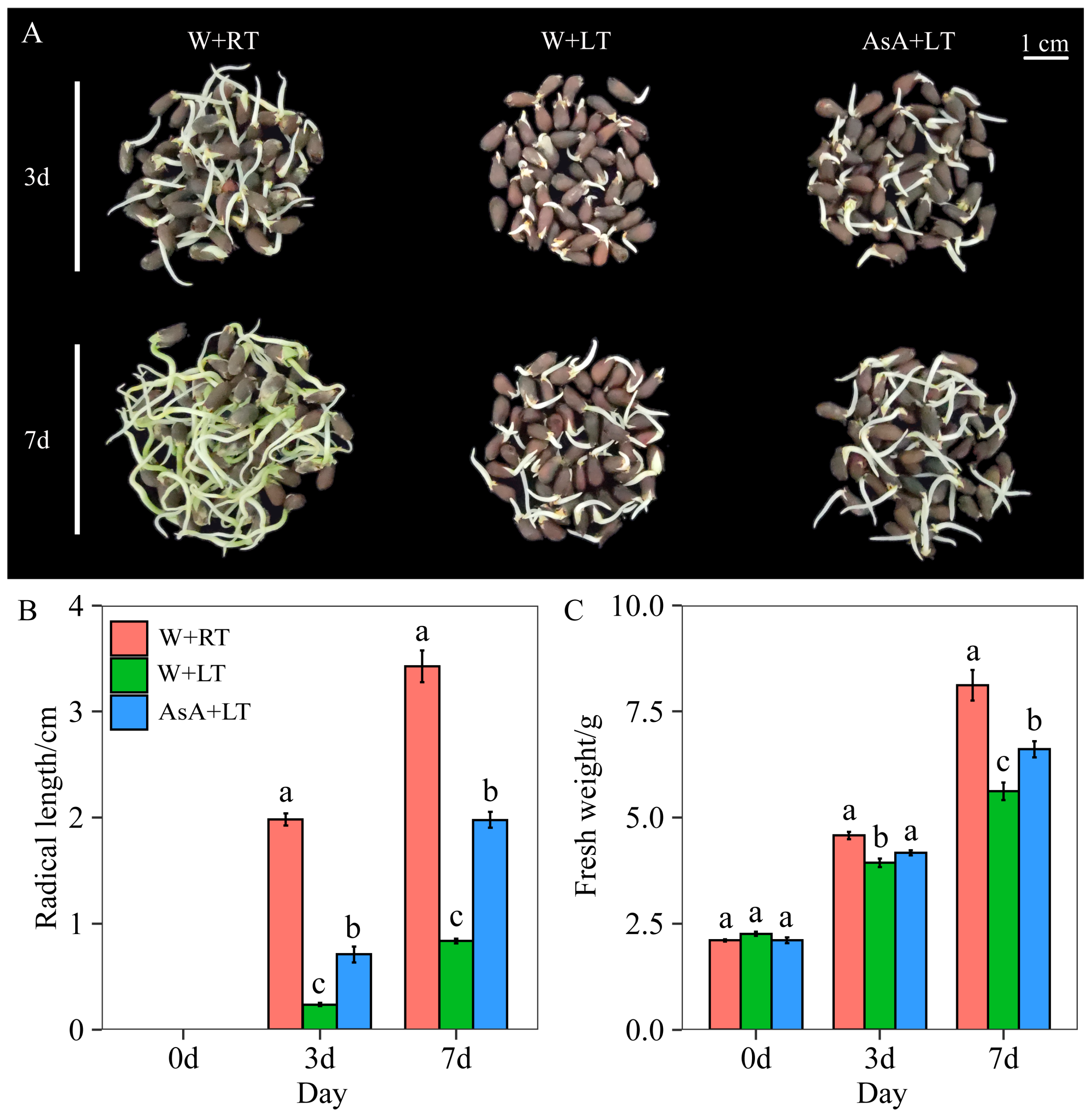
2.2. Effects of Appropriate AsA Priming on Seed Growth Under Low-Temperature (15 °C) Stress
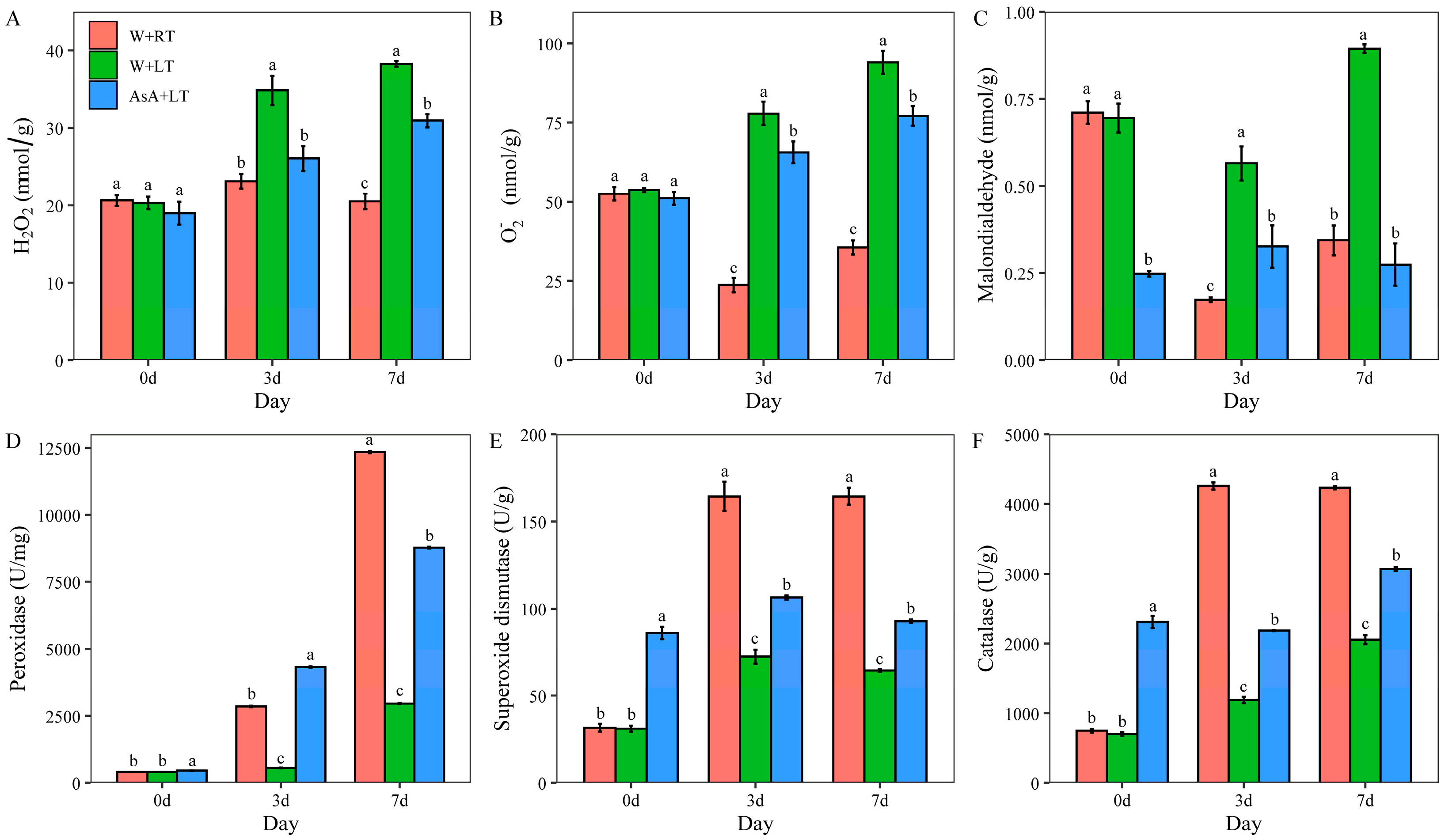
2.3. Impact of Appropriate AsA Priming on Cell Membrane Stability, and Antioxidant Enzyme Activities Under Low-Temperature (15 °C) Stress
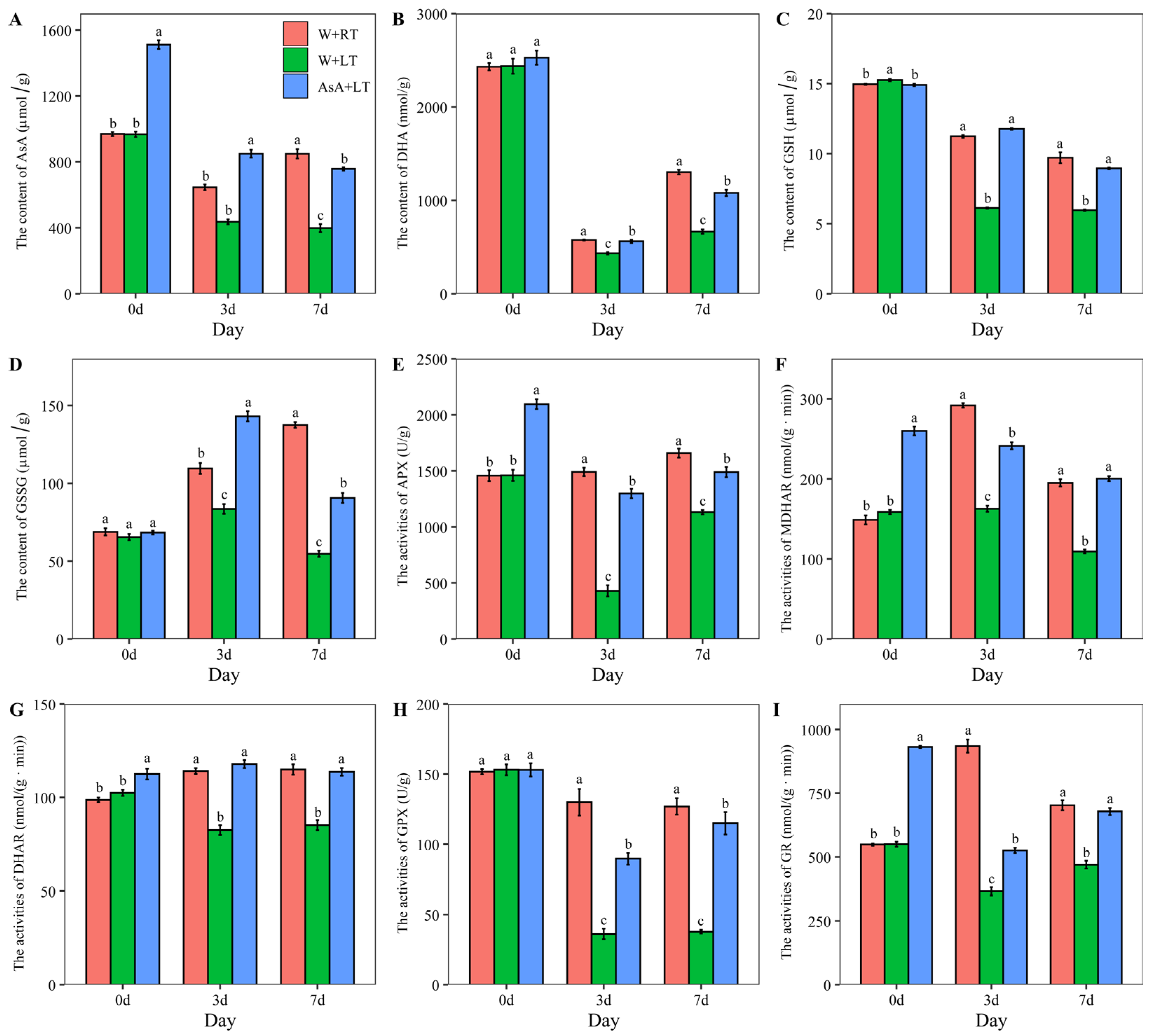
2.4. Effects of Suitable AsA Priming on Enzyme Activities and Metabolite Content in the Ascorbate-Glutathione Cycle Under Low-Temperature (15 °C) Stress
2.5. Synergistic Effects and Trade-Off Mechanisms of Antioxidant Metabolism in Cold-Resistant Germination
3. Discussion
3.1. AsA Priming to Enhance Cotton Seed Germination at Low Temperatures
3.2. AsA Priming Boosts Cotton Seed Germination Under Low-Temperature Stress by Enhancing Antioxidant Defense
3.3. Unraveling the Complex Interactions Among Oxidative Stress, Antioxidant Defense, and Seed Development
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Seed Cultivation
4.4. Determination of Relevant Indicators of Seed Germination
4.5. Determination of the Seed Growth Indicators
4.6. Determination of the Enzyme Activities of the Antioxidant System
4.7. Determination of MDA and Reactive Oxygen Species Contents in Seeds
4.8. Determination of the Enzyme’s Activities and Substance Content of Ascorbate-Glutathione Cycle
4.9. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NBSPRC. Announcement of National Bureau of Statistics on Cotton Output in 2024. Available online: https://www.stats.gov.cn/sj/zxfb/202412/t20241225_1957879.html (accessed on 24 August 2025).
- Majeed, S.; Rana, I.A.; Mubarik, M.S.; Atif, R.M.; Yang, S.-H.; Chung, G.; Jia, Y.; Du, X.; Hinze, L.; Azhar, M.T. Heat Stress in Cotton: A Review on Predicted and Unpredicted Growth-Yield Anomalies and Mitigating Breeding Strategies. Agronomy 2021, 11, 1825. [Google Scholar] [CrossRef]
- Dev, W.; Sultana, F.; He, S.; Waqas, M.; Hu, D.; Aminu, I.M.; Geng, X.; Du, X. An insight into heat stress response and adaptive mechanism in cotton. J. Plant Physiol. 2024, 302, 154324. [Google Scholar] [CrossRef]
- Abro, A.A.; Qasim, M.; Abbas, M.; Muhammad, N.; Ali, I.; Khalid, S.; Ahmed, J.; Waqas, M.; Ercisli, S.; Iqbal, R.; et al. Integrating physiological and molecular insights in cotton under cold stress conditions. Genet. Resour. Crop Evol. 2025, 72, 2561–2591. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Xu, J.; Zhang, X.; Xie, Z.; Li, Z. Effect of cold stress on photosynthetic physiological characteristics and molecular mechanism analysis in cold-resistant cotton (ZM36) seedlings. Front. Plant Sci. 2024, 15, 1396666. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Liu, D.; He, P.; Li, Z.; Shi, M.; Xu, J. Spatiotemporal variability characteristics of extreme climate events in Xinjiang during 1960–2019. Environ. Sci. Pollut. Res. 2023, 30, 57316–57330. [Google Scholar] [CrossRef]
- Guan, J.; Yao, J.; Li, M.; Li, D.; Zheng, J. Historical changes and projected trends of extreme climate events in Xinjiang, China. Clim. Dyn. 2022, 59, 1753–1774. [Google Scholar] [CrossRef]
- Abro, A.A.; Qasim, M.; Younas, M.U.; Ali, I.; Abbas, M.; Muhammad, N.; Khalid, S.; Ahmed, J.; Bibi, U.; Waqas, M.; et al. Impact of elevated temperatures on the genetic and morpho-physiological traits of cotton genotypes cultivation. Genet. Resour. Crop Evol. 2025, 72, 2533–2560. [Google Scholar] [CrossRef]
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.S.; Rout, G.R. Overview of Cold Stress Regulation in Plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zhang, H.F.; Liu, S.Y.; Ma, J.H.; Wang, X.K.; Haq, S.U.; Meng, Y.C.; Zhang, Y.M.; Chen, R.G. CaDHN4, a Salt and Cold Stress-Responsive Dehydrin Gene from Pepper Decreases Abscisic Acid Sensitivity in Arabidopsis. Int. J. Mol. Sci. 2019, 21, 26. [Google Scholar] [CrossRef]
- Considine, M.J.; Sandalio, L.M.; Foyer, C.H. Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann. Bot. 2015, 116, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous Melatonin Improves Antioxidant Defense in Cucumber Seeds (Cucumis sativus L.) Germinated under Chilling Stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Dhaliwal, L.K.; Angeles-Shim, R.B. Cell Membrane Features as Potential Breeding Targets to Improve Cold Germination Ability of Seeds. Plants 2022, 11, 3400. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Romdhane, W.; Baazaoui, N.; Bouteraa, M.T.; Chouaibi, Y.; Mnif, W.; Ben Hsouna, A.; Kačániová, M. Functional Characterization of Lobularia maritima LmTrxh2 Gene Involved in Cold Tolerance in Tobacco through Alleviation of ROS Damage to the Plasma Membrane. Int. J. Mol. Sci. 2023, 24, 3030. [Google Scholar] [CrossRef]
- Nayyar, H.; Bains, T.S.; Kumar, S. Chilling stressed chickpea seedlings: Effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ. Exp. Bot. 2005, 54, 275–285. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, H.; Yan, C.; Zhang, W.; Li, Z. Exogenous Melatonin Enhances Cold Tolerance by Regulating the Expression of Photosynthetic Performance, Antioxidant System, and Related Genes in Cotton. Plants 2024, 13, 2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Dong, Y.; Zhang, F.; He, Q.; Chen, J.; Zhu, S.; Zhao, T. Seed priming with melatonin improves salt tolerance in cotton through regulating photosynthesis, scavenging reactive oxygen species and coordinating with phytohormone signal pathways. Ind. Crops Prod. 2021, 169, 113671. [Google Scholar] [CrossRef]
- Xia, J.; Hao, X.; Wang, T.; Li, H.; Shi, X.; Liu, Y.; Luo, H. Seed Priming with Gibberellin Regulates the Germination of Cotton Seeds Under Low-Temperature Conditions. J. Plant Growth Regul. 2023, 42, 319–334. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J. Plant Growth Regul. 2021, 40, 464–485. [Google Scholar] [CrossRef]
- Ansary, M.W.R.; Sakib, M.H.; Islam, T. Application of Selenium and Nano-selenium in Abiotic Stress Management, Crop Improvement, and Agro-biotechnology. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 271–310. [Google Scholar]
- Fatima, M.; Maqbool, A.; Sardar, R.; Maqsood, M.F.; Zulfiqar, U. Nano-Selenium: A Green Promising Approach against Abiotic Stresses in Plants. J. Soil Sci. Plant Nutr. 2024, 24, 6000–6023. [Google Scholar] [CrossRef]
- Qian, H.F.; Peng, X.F.; Han, X.; Ren, J.; Zhan, K.Y.; Zhu, M. The stress factor, exogenous ascorbic acid, affects plant growth and the antioxidant system in Arabidopsis thaliana. Russ. J. Plant Physiol. 2014, 61, 467–475. [Google Scholar] [CrossRef]
- Mi, C.; Hong, L.; Sun, S.; Zhao, S.; Dou, L.; Mao, P. Ascorbic acid priming restores the seed vigor by enhancing the mitochondrial AsA-GSH cycle and related gene expression in the aged oat seeds. Physiol. Plant. 2025, 177, e70190. [Google Scholar] [CrossRef]
- Wu, P.; Li, B.; Liu, Y.; Bian, Z.; Xiong, J.; Wang, Y.; Zhu, B. Multiple Physiological and Biochemical Functions of Ascorbic Acid in Plant Growth, Development, and Abiotic Stress Response. Int. J. Mol. Sci. 2024, 25, 1832. [Google Scholar] [CrossRef]
- Feng, Y.; Fu, X.; Han, L.; Xu, C.; Liu, C.; Bi, H.; Ai, X. Nitric Oxide Functions as a Downstream Signal for Melatonin-Induced Cold Tolerance in Cucumber Seedlings. Front. Plant Sci. 2021, 12, 686545. [Google Scholar] [CrossRef]
- Rasul, F.; Gupta, S.; Olas, J.J.; Gechev, T.; Sujeeth, N.; Mueller-Roeber, B. Priming with a Seaweed Extract Strongly Improves Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1469. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, H.; Dai, X.; Yu, M.; Yu, Z. Effect of methyl jasmonate on the quality and antioxidant capacity by modulating ascorbate-glutathione cycle in peach fruit. Sci. Hortic. 2022, 303, 111216. [Google Scholar] [CrossRef]
- Wang, H.; Lu, T.; Yan, W.; Yu, P.; Fu, W.; Li, J.; Su, X.; Chen, T.; Fu, G.; Wu, Z.; et al. Transcriptome and Metabolome Analyses Reveal Ascorbic Acid Ameliorates Cold Tolerance in Rice Seedling Plants. Agronomy 2024, 14, 659. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Chashmi, N.A. The Effects of Foliar Application of Ascorbic Acid (Vitamin C) on Antioxidant Enzymes Activities, Lipid Peroxidation and Proline Accumulation of Canola (Brassica napus L.) under Conditions of Salt Stress. J. Agron. Crop Sci. 2008, 194, 206–213. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Al-Huqail, A.A.; Al-Harbi, M.S.; Ali, E.F.; Wang, J.; Ding, Z.; Rekaby, S.A.; Ghoneim, A.M.; Eissa, M.A. Mechanisms of Chitosan Nanoparticles in the Regulation of Cold Stress Resistance in Banana Plants. Nanomaterials 2021, 11, 2670. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Geng, S.; Du, H.; Chen, B.; Sun, L.; Wang, G.; Sha, M.; Dong, T.; Zhang, X.; et al. Identification of the GDP-L-Galactose Phosphorylase Gene as a Candidate for the Regulation of Ascorbic Acid Content in Fruits of Capsicum annuum L. Int. J. Mol. Sci. 2023, 24, 7529. [Google Scholar] [CrossRef]
- Bulley, S.M.; Cooney, J.M.; Laing, W. Elevating Ascorbate in Arabidopsis Stimulates the Production of Abscisic Acid, Phaseic Acid, and to a Lesser Extent Auxin (IAA) and Jasmonates, Resulting in Increased Expression of DHAR1 and Multiple Transcription Factors Associated with Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 6743. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Li, W.; Li, J.; Li, S.; Zhang, W.; Zhang, P.; Cui, K.; Huo, J.; Gang, H.; et al. Transcriptome profiling reveals the regulatory mechanisms of AsA (ascorbic acid) and flavonoid synthesis and metabolic processes in fruit development of Ribes nigrum L. Mol. Genet. Genom. 2025, 300, 62. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed Hydropriming Enhances Osmotic Stress Tolerance Potential in Vigna radiata. Agric. Res. 2018, 7, 145–151. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Obata, T.; Van Der Kelen, K.; Harokopos, V.; Kanetis, L.; Aidinis, V.; Van Breusegem, F.; Fernie, A.R.; Fotopoulos, V. Kresoxim-methyl primes Medicago truncatula plants against abiotic stress factors via altered reactive oxygen and nitrogen species signalling leading to downstream transcriptional and metabolic readjustment. J. Exp. Bot. 2016, 67, 1259–1274. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Han, G.; Sun, X.; Yang, X. Seed osmopriming with polyethylene glycol (PEG) enhances seed germination and seedling physiological traits of Coronilla varia L. under water stress. PLoS ONE 2024, 19, e0303145. [Google Scholar] [CrossRef] [PubMed]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Nafees, M.; Chen, J.; Darras, A.; Ferrante, A.; Hancock, J.T.; Ashraf, M.; Zaid, A.; Latif, N.; Corpas, F.J.; et al. Chemical priming enhances plant tolerance to salt stress. Front. Plant Sci. 2022, 13, 946922. [Google Scholar] [CrossRef] [PubMed]
- Zrig, A.; Yousif Sidahmed Elsheikh, S.; Hamouda, F.; Najar, B.; Alsherif, E.A.; Magdy Korany, S.; Hassan, A.H.A.; AbdElgawad, H. Potassium Nitrate and Ascorbic Acid Priming Improved Tissue Chemical Composition and Antioxidant and Antimicrobial Activities of Linseed (Linum usitatissimum L.) Sprouts. ACS Omega 2023, 8, 35975–35987. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Hussain, S.; Khan, S.M.; Ejaz, S.; Ahmad, S. Abiotic Stress Tolerance in Plants by Priming and Pretreatments with Ascorbic Acid. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 459–493. [Google Scholar]
- Baig, Z.; Khan, N.; Sahar, S.; Sattar, S.; Zehra, R. Effects of seed priming with ascorbic acid to mitigate salinity stress on three wheat (Triticum aestivum L.) cultivars. Acta Ecol. Sin. 2021, 41, 491–498. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Caverzan, A.; Balbinott, N.; Menguer, P.K.; Paiva, A.L.S.; Lemos, M.; Cunha, J.R.; Gaeta, M.L.; Costa, M.; Zamocky, M.; et al. Stromal Ascorbate Peroxidase (OsAPX7) Modulates Drought Stress Tolerance in Rice (Oryza sativa). Antioxidants 2023, 12, 387. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Vanacker, H.; Guichard, M.; Bohrer, A.S.; Issakidis-Bourguet, E. Redox Regulation of Monodehydroascorbate Reductase by Thioredoxin y in Plastids Revealed in the Context of Water Stress. Antioxidants 2018, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Javad, S.; Iqbal, S.; Shahzadi, K.; Gatasheh, M.K.; Javed, T. Alleviation potential of green-synthesized selenium nanoparticles for cadmium stress in Solanum lycopersicum L.: Modulation of secondary metabolites and physiochemical attributes. Plant Cell Rep. 2024, 43, 113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Magwanga, R.O.; Cai, X.; Zhou, Z.; Wang, X.; Wang, Y.; Zhang, Z.; Jin, D.; Guo, X.; Wei, Y.; et al. Deep Transcriptome Analysis Reveals Reactive Oxygen Species (ROS) Network Evolution, Response to Abiotic Stress, and Regulation of Fiber Development in Cotton. Int. J. Mol. Sci. 2019, 20, 1863. [Google Scholar] [CrossRef]
- Berlanga, D.J.; Molina, A.; Torres, M.Á. Mitogen-activated protein kinase phosphatase 1 controls broad spectrum disease resistance in Arabidopsis thaliana through diverse mechanisms of immune activation. Front. Plant Sci. 2024, 15, 1374194. [Google Scholar] [CrossRef]
- Mesa, J.M.; Paige, K.N. Molecular constraints on tolerance-resistance trade-offs: Is there a cost? Plant-Environ. Interact. 2023, 4, 317–323. [Google Scholar] [CrossRef]
- Scheibe, R. Maintaining homeostasis by controlled alternatives for energy distribution in plant cells under changing conditions of supply and demand. Photosynth. Res. 2019, 139, 81–91. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Jia, J.; Wang, C.; Fu, Y. Flavonoid-Lignin Crosstalk: Engineering Metabolic Flux for Optimised Plant Growth and Stress Resilience. Plant Cell Environ. 2025, 48, 8141–8160. [Google Scholar] [CrossRef]
- Shahzad, R.; Koerniati, S.; Harlina, P.W.; Hastilestari, B.R.; Djalovic, I.; Prasad, P.V.V. Iron oxide nanoparticles enhance alkaline stress resilience in bell pepper by modulating photosynthetic capacity, membrane integrity, carbohydrate metabolism, and cellular antioxidant defense. BMC Plant Biol. 2025, 25, 170. [Google Scholar] [CrossRef]
- Wang, L.; Ju, C.; Han, C.; Yu, Z.; Bai, M.-Y.; Wang, C. The interaction of nutrient uptake with biotic and abiotic stresses in plants. J. Integr. Plant Biol. 2025, 67, 455–487. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Van Breusegem, F. Improving oxidative stress resilience in plants. Plant J. 2022, 109, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Huang, R.; Zhang, Z.; Shi, S. Seed-Borne Erwinia persicina Affects the Growth and Physiology of Alfalfa (Medicago sativa L.). Front. Microbiol. 2022, 13, 891188. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X. Plant Physiology Experiment Guidance; South China University of Technology Press: Guangzhou, China, 2006; pp. 119–121. [Google Scholar]
- Svara, A.; Tarkowski, Ł.P.; Janse van Rensburg, H.C.; Deleye, E.; Vaerten, J.; De Storme, N.; Keulemans, W.; Van den Ende, W. Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis. Int. J. Mol. Sci. 2020, 21, 5885. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chatterjee, S.; Jiang, J.; Sinha, B.K.; Mason, R.P. Detection and imaging of the free radical DNA in cells–site-specific radical formation induced by Fenton chemistry and its repair in cellular DNA as seen by electron spin resonance, immuno-spin trapping and confocal microscopy. Nucleic Acids Res. 2012, 40, 5477–5486. [Google Scholar] [CrossRef]
- Liang, B.; Wan, S.; Ma, Q.; Yang, L.; Hu, W.; Kuang, L.; Xie, J.; Liu, D.; Liu, Y. Transcriptome and Physiological Analyses of a Navel Orange Mutant with Improved Drought Tolerance and Water Use Efficiency Caused by Increases of Cuticular Wax Accumulation and ROS Scavenging Capacity. Int. J. Mol. Sci. 2022, 23, 5660. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of Hydrogen Peroxide in the Regulation of Senescence in Pear 1. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.; Ma, H.; Lu, L.; Zhu, J.; Nie, X.; Xu, J.; Li, Z. Ascorbic Acid Priming Boosts Cotton Seed Chilling Tolerance via Membrane Stability and Antioxidant Cycles. Plants 2025, 14, 3122. https://doi.org/10.3390/plants14203122
Han P, Ma H, Lu L, Zhu J, Nie X, Xu J, Li Z. Ascorbic Acid Priming Boosts Cotton Seed Chilling Tolerance via Membrane Stability and Antioxidant Cycles. Plants. 2025; 14(20):3122. https://doi.org/10.3390/plants14203122
Chicago/Turabian StyleHan, Peng, Haixia Ma, Lu Lu, Jincheng Zhu, Xinhui Nie, Jianwei Xu, and Zhibo Li. 2025. "Ascorbic Acid Priming Boosts Cotton Seed Chilling Tolerance via Membrane Stability and Antioxidant Cycles" Plants 14, no. 20: 3122. https://doi.org/10.3390/plants14203122
APA StyleHan, P., Ma, H., Lu, L., Zhu, J., Nie, X., Xu, J., & Li, Z. (2025). Ascorbic Acid Priming Boosts Cotton Seed Chilling Tolerance via Membrane Stability and Antioxidant Cycles. Plants, 14(20), 3122. https://doi.org/10.3390/plants14203122






