Screening and Identification of Cadmium-Tolerant, Plant Growth-Promoting Rhizobacteria Strain KM25, and Its Effects on the Growth of Soybean and Endophytic Bacterial Community in Roots
Abstract
1. Introduction
2. Results
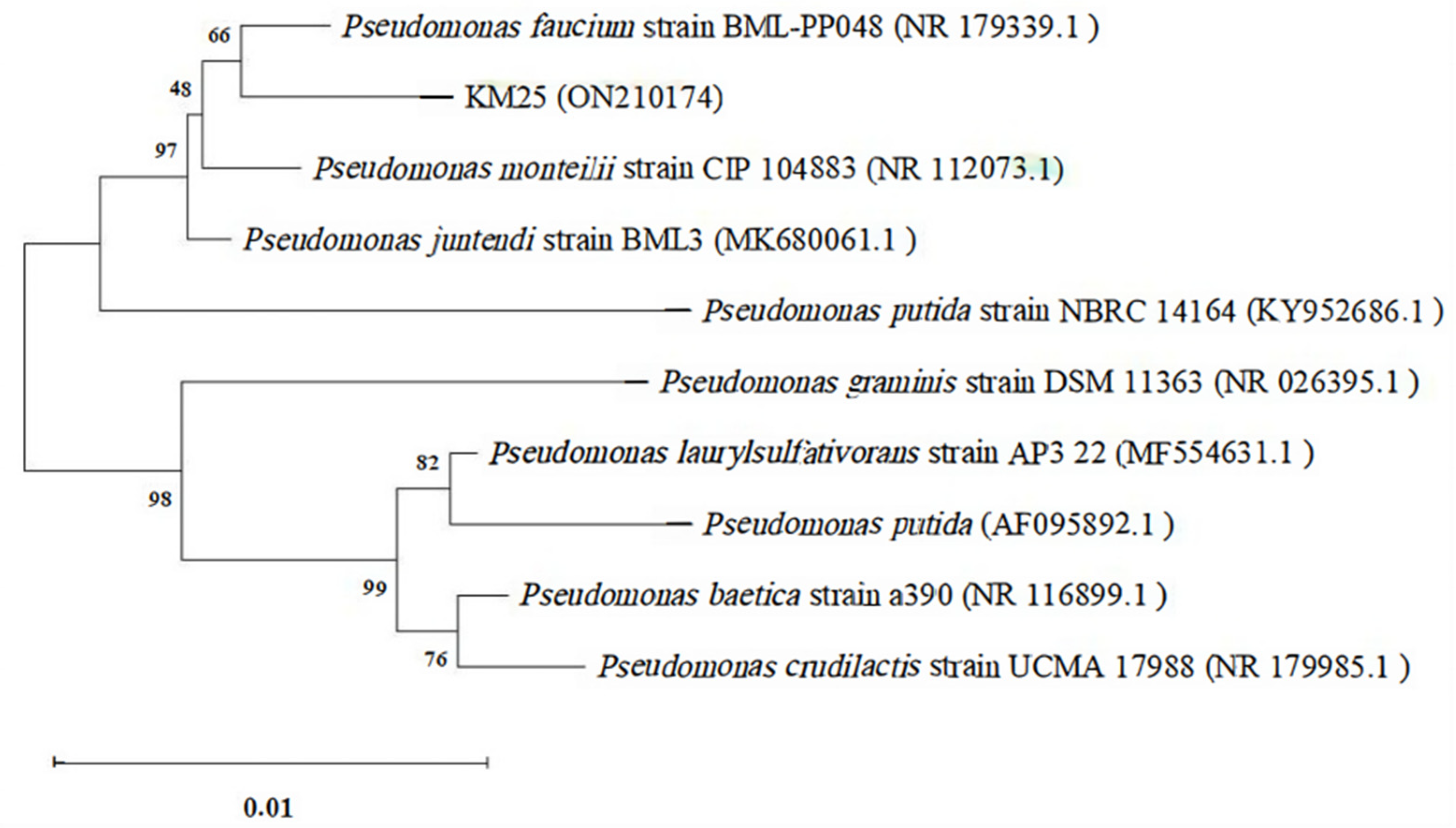
2.1. Isolation of Cadmium-Tolerant Bacterial Strains from Semi-Wild Soybean Root Nodules and Identification of Strain KM25
2.2. PGP Traits
2.3. Biomass and Chlorophyll Content
2.4. Antioxidant Systems and Lipid Peroxidation
2.5. Cd Content
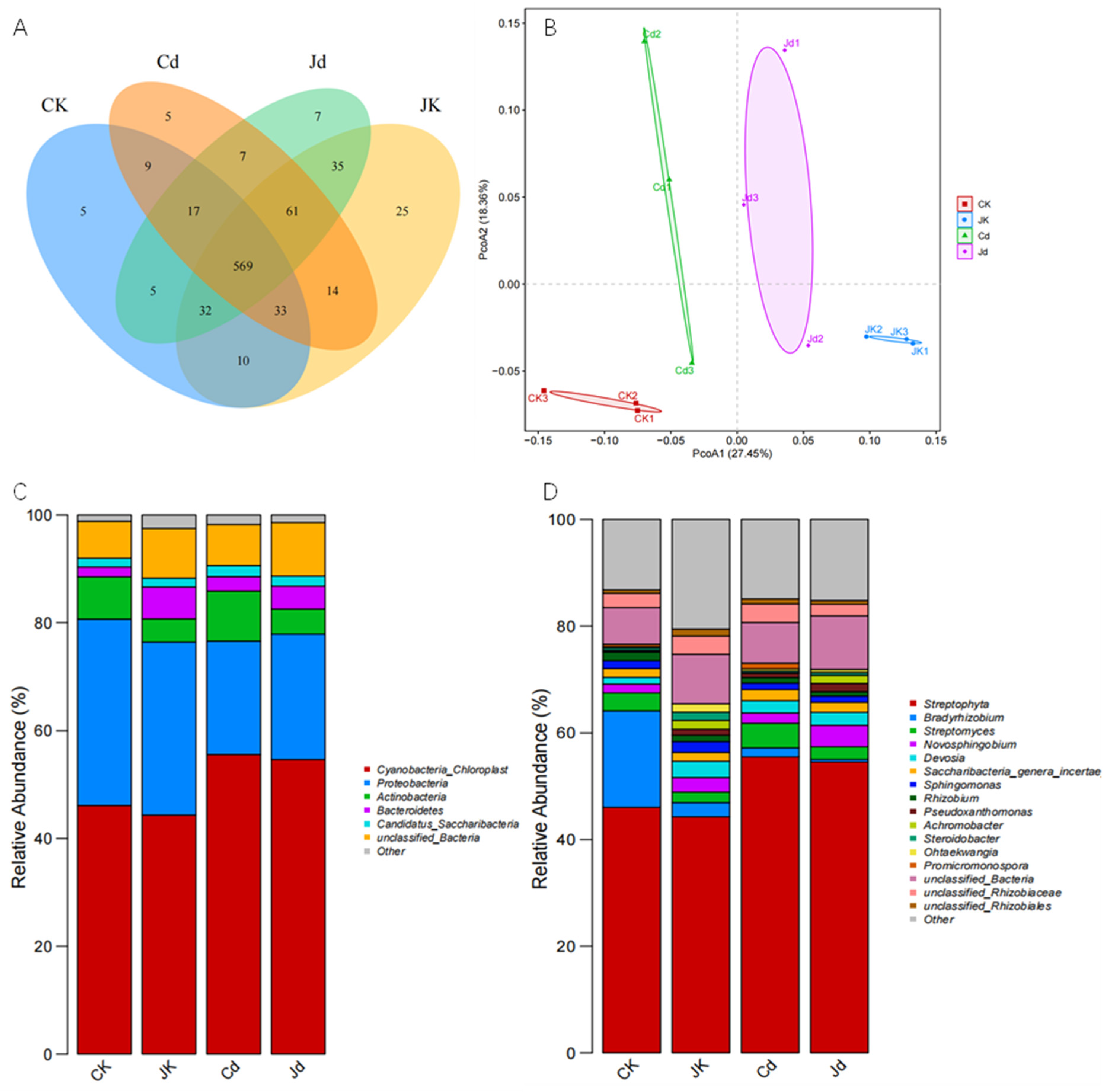
2.6. Diversity of Endophytic Bacterial Communities
2.7. Taxonomic Composition of Endophytic Bacterial Communities
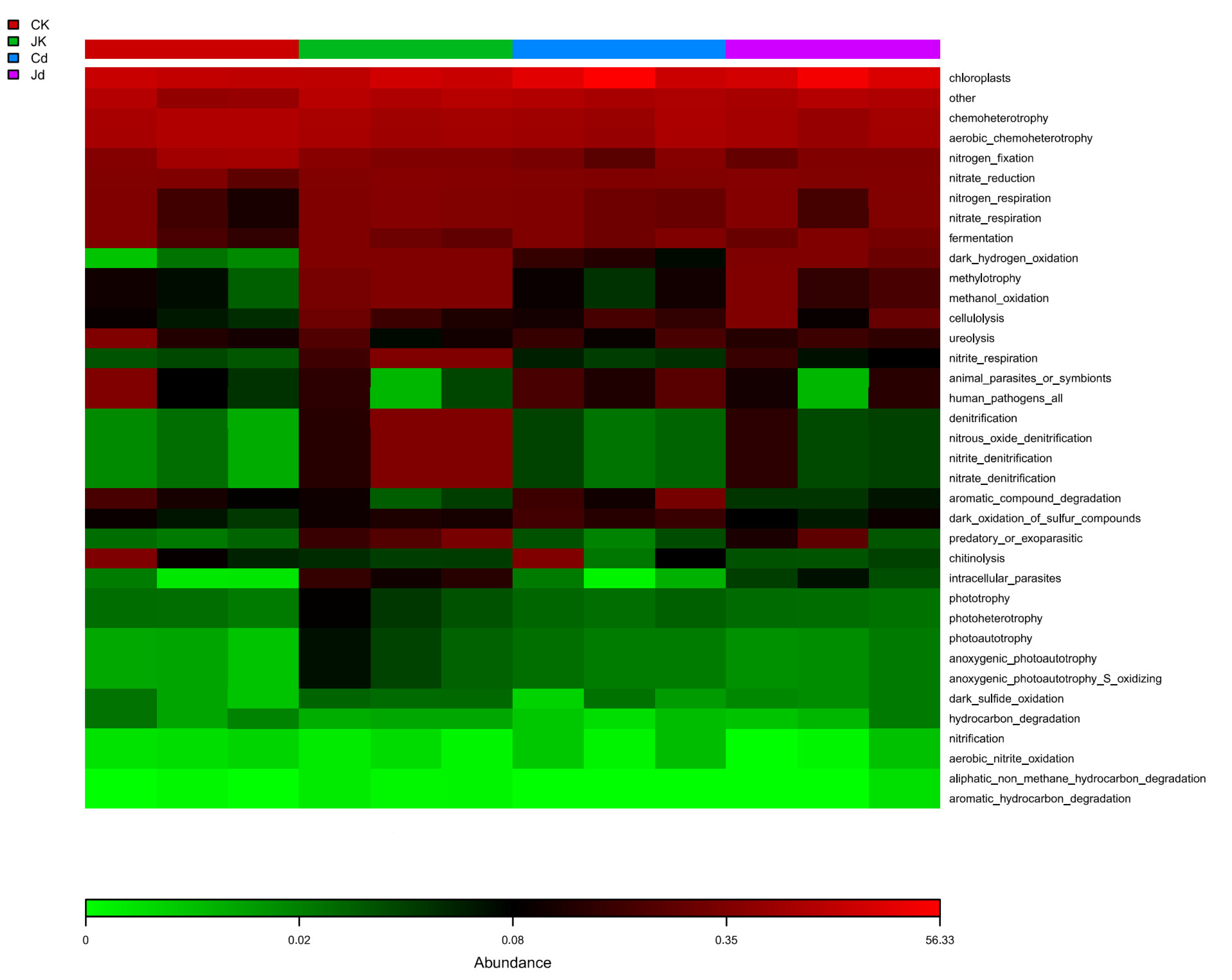
2.8. Functional Prediction Analysis of Endogenous Bacterial Communities
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of Strain KM25
4.2. Screening Cadmium-Tolerant Strains
4.3. DNA Extraction and Bacterial Identification
4.4. Determination of PGP Traits of PGPR
4.5. Hydroponic Experiment
4.5.1. Determination of Biomass and Chlorophyll Content of Soybean Seedlings
4.5.2. Determination of Antioxidant Enzymes and Lipid Peroxidation
4.5.3. Determination of Cd Content
4.6. Analysis of the Community Structure of Endophytic Bacteria in Soybean Roots
4.6.1. Samples Treatment
4.6.2. DNA Extraction
4.6.3. PCR Amplification
4.6.4. High-Throughput Sequencing of the V3–V4 Region of Bacterial 16S rDNA
4.7. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X.; Li, S.; Chen, J.; Zeng, T.; Hou, H. Passivation of multiple heavy metals in lead–zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dou, Y.; Gao, C.; He, C.; Gao, J.; Zhao, S.; Deng, L. Removal of Cd (II) by modified maifanite coated with Mg-layered double hydroxides in constructed rapid infiltration systems. Sci. Total Environ. 2019, 685, 951–962. [Google Scholar] [CrossRef]
- Xian, P.; Yang, Y.; Xiong, C.; Guo, Z.; Alam, I.; He, Z.; Zhang, Y.; Cai, Z.; Nian, H. Overexpression of GmWRKY172 enhances cadmium tolerance in plants and reduces cadmium accumulation in soybean seeds. Front. Plant Sci. 2023, 14, 1133892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, J.; Wu, L.; Chen, Z. Worldwide cadmium accumulation in soybean grains and feasibility of food production on contaminated calcareous soils. Environ. Pollut. 2021, 269, 116153. [Google Scholar] [CrossRef] [PubMed]
- Sahile, A.A.; Khan, M.A.; Hamayun, M.; Imran, M.; Kang, S.M.; Lee, I.J. Novel Bacillus cereus strain, ALT1, enhance growth and strengthens the antioxidant system of soybean under cadmium stress. Agronomy 2021, 11, 404. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.; Ng, H.S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- He, X.; Xu, M.; Wei, Q.; Tang, M.; Guan, L.; Lou, L.; Xu, X.; Hu, Z.; Chen, Y.; Shen, Z. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2020, 205, 111333. [Google Scholar] [CrossRef]
- Meneguzzi, R.V.; Fernandez, M.; Cappellari, L.R.; Giordano, W.; Banchio, E. Isolation and characterization of plant growth-promoting bacteria from the rhizosphere of medicinal and aromatic plant Minthostachys verticillata. Plants 2024, 13, 2062. [Google Scholar] [CrossRef]
- Sharma, M.; Saleh, D.; Charron, J.B.; Jabaji, S. A crosstalk between Brachypodium root exudates, organic acids, and Bacillus velezensis B26, a growth promoting bacterium. Front. Microbiol. 2020, 11, 575578. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, P.; Ma, S.; Yu, Q.; Wang, H.; Liu, Y.; Yang, S.; Chen, Y. Enhancing carrot (Daucus carota var. sativa Hoffm.) plant productivity with combined rhizosphere microbial consortium. Front. Microbiol. 2024, 15, 1466300. [Google Scholar] [CrossRef]
- Yuan, Y.; Zu, M.; Sun, L.; Zuo, J.; Tao, J. Isolation and Screening of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing PGPR from Paeonia lactiflora rhizosphere and enhancement of plant growth. Sci. Hortic. 2022, 297, 110956. [Google Scholar] [CrossRef]
- Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O.; Lee, I.J. Phytohormones producing rhizobacteria alleviate heavy metals stress in soybean through multilayered response. Microbiol. Res. 2023, 266, 127237. [Google Scholar]
- Hu, Z.; Zhao, C.; Li, Q.; Feng, Y.; Zhang, X.; Lu, Y.; Ying, R.; Yin, A.; Ji, W. Heavy metals can affect plant morphology and limit plant growth and photosynthesis processes. Agronomy 2023, 13, 2601. [Google Scholar] [CrossRef]
- Dvorak, M.; Schnegg, R.; Niederwanger, M.; Pedrini-Martha, V.; Ladyrner, P.; Lindner, H.; Kremser, L.; Lackner, R.; Dallinger, R. Cadmium pathways in snails follow a complementary strategy between metallothionein detoxification and auxiliary inactivation by phytochelatins. Int. J. Mol. Sci. 2019, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Castaings, L.; Alcon, C.; Kosuth, T.; Correia, D.; Curie, C. Manganese triggers phosphorylation-mediated endocytosis of the Arabidopsis metal transporter NRAMP1. Plant J. 2021, 106, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Pramanik, K.; Sarkar, A.; Ghosh, P.K.; Soren, T.; Maiti, T.K. Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol. Environ. Saf. 2018, 156, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J. Hazard. Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef]
- Li, X.; Zheng, X.; Yadav, N.; Saha, S.; Salama, E.S.; Li, X.; Wang, L.; Jeon, B.H. Rational management of the plant microbiome for the Second Green Revolution. Plant Commun. 2024, 5, 100812. [Google Scholar] [CrossRef]
- Świątczak, J.; Kalwasińska, A.; Szabó, A.; Brzezinska, M.S. The effect of seed bacterization with Bacillus paralicheniformis 2R5 on bacterial and fungal communities in the canola rhizosphere. Microbiol. Res. 2023, 275, 127448. [Google Scholar] [CrossRef]
- Chi, Y.; Ma, X.; Wu, J.; Wang, R.; Zhang, X.; Chu, S.; Zhang, D.; Zhou, P. Plant growth promoting endophyte promotes cadmium accumulation in Solanum nigrum L. by regulating plant homeostasis. J. Hazard. Mater. 2023, 457, 131866. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, N.; Singh, A.; Kumar, D.; Yadav, D.K.; Varshney, A.; Sharma, N. The effect of cadmium tolerant plant growth promoting rhizobacteria on plant growth promotion and phytoremediation: A review. Curr. Microbiol. 2023, 80, 153. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Huang, Y.; Yao, Y.; Wang, X.; Liu, C.; Liang, Y.; Liu, K.; Yu, F. Bioaugmentation-assisted phytoremediation of manganese and cadmium co-contaminated soil by Polygonaceae plants (Polygonum hydropiper L. and Polygonum lapathifolium L.) and Enterobacter sp. FM-1. Plant Soil 2020, 448, 439–453. [Google Scholar] [CrossRef]
- Shahid, S.; Dar, A.; Hussain, A.; Khalid, I.; Latif, M.; Ahmad, H.T.; Mehmood, T.; Aloud, S.S. Enhancing cauliflower growth under cadmium stress: Synergistic effects of Cd-tolerant Klebsiella strains and jasmonic acid foliar application. Front. Microbiol. 2024, 15, 1444374. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Fu, S.; Sarkodie, E.K.; Zhang, S.; Jiang, L.; Liang, Y.; Yin, H.; Bai, L.; Liu, X.; Liu, H.; et al. Ecological responses of bacterial assembly and functions to steep Cd gradient in a typical Cd-contaminated farmland ecosystem. Ecotoxicol. Environ. Saf. 2022, 229, 113067. [Google Scholar] [CrossRef] [PubMed]
- Fakhar, A.; Gul, B.; Gurmani, A.R.; Khan, S.M.; Ali, S.; Sultan, T.; Chaudhary, H.J.; Rafique, M.; Rizwan, M. Heavy metal remediation and resistance mechanism of Aeromonas, Bacillus, and Pseudomonas: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1868–1914. [Google Scholar] [CrossRef]
- Adhikary, A.; Kumar, R.; Pandir, R.; Bhardwaj, P.; Wusirika, R.; Kumar, S. Pseudomonas citronellolis; a multi-metal resistant and potential plant growth promoter against arsenic (V) stress in chickpea. Plant Physiol. Biochem. 2019, 142, 179–192. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 5855. [Google Scholar] [CrossRef]
- Rojas-Sánchez, B.; Castelán-Sánchez, H.; Santoyo, G. Inoculation with Pseudomonas fluorescens UM270 alters the maize root-associated endobiome and interacting networks in a milpa model. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Kulkova, I.; Jakubowska, Z.; Naziębło, A.; Wróbel, B. Pseudomonas sp. G31 and Azotobacter sp. PBC2 Changed Structure of Bacterial Community and Modestly Promoted Growth of Oilseed Rape. Int. J. Mol. Sci. 2024, 25, 13168. [Google Scholar] [CrossRef]
- Kang, S.M.; Shahzad, R.; Khan, M.A.; Hasnain, Z.; Lee, K.E.; Park, H.S.; Kim, L.R.; Lee, I.J. Ameliorative effect of indole-3-acetic acid-and siderophore-producing Leclercia adecarboxylata MO1 on cucumber plants under zinc stress. J. Plant Interact. 2021, 16, 30–41. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, P.K.; Upadhyay, S.K.; Singh, R.K.; Dwivedi, P.; Wang, J.; Jain, D.; Jiang, M. Mechanistic insights and potential use of siderophores producing microbes in rhizosphere for mitigation of stress in plants grown in degraded land. Front. Microbiol. 2022, 13, 898979. [Google Scholar] [CrossRef]
- Murali, M.; Singh, S.B.; Gowtham, H.G.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.N. Induction of drought tolerance in Pennisetum glaucum by ACC deaminase producing PGPR-Bacillus amyloliquefaciens through Antioxidant defense system. Microbiol. Res. 2021, 253, 126891. [Google Scholar] [CrossRef] [PubMed]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant growth promotion using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, M.; Yang, H.; Pian, R.; Wang, J.; Wu, A. The uptake, transfer, and detoxification of cadmium in plants and its exogenous effects. Cells 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Patel, K.; Al-Keridis, L.A.; Alshammari, N.; Badraoui, R.; Elasbali, A.M.; Al-Soud, W.A.; Hassan, M.I.; Yadav, D.K.; Adnan, M. Cadmium-tolerant plant growth-promoting bacteria Curtobacterium oceanosedimentum improves growth attributes and strengthens antioxidant system in chili (Capsicum frutescens). Sustainability 2022, 14, 4335. [Google Scholar] [CrossRef]
- Martins, L.L.; Mourato, M.P.; Cardoso, A.I.; Pinto, A.P.; Mota, A.M.; Gonçalves, M.D.L.S.; Varennes, A.D. Oxidative stress induced by cadmium in Nicotiana tabacum L.: Effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiol. Plant. 2011, 33, 1375–1383. [Google Scholar] [CrossRef]
- He, L.; Li, J. Integrated transcriptome and physiological analysis of rice seedlings reveals different cadmium response mechanisms between indica and japonica varieties. Environ. Exp. Bot. 2022, 204, 105097. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Ren, Y.; Jiang, Z.; Chen, H.; Hu, W.; Tang, M. The alleviation mechanisms of cadmium toxicity in Broussonetia papyrifera by arbuscular mycorrhizal symbiosis varied with different levels of cadmium stress. J. Hazard. Mater. 2023, 459, 132076. [Google Scholar] [CrossRef]
- Wei, T.; Sun, Y.; Yashir, N.; Li, X.; Guo, J.; Liu, X.; Jia, H.; Ren, X.; Hua, L. Inoculation with rhizobacteria enhanced tolerance of tomato (Solanum lycopersicum L.) plants in response to cadmium stress. J. Plant Growth Regul. 2021, 41, 445–460. [Google Scholar] [CrossRef]
- Wu, B.; He, T.; Wang, Z.; Qiao, S.; Wang, Y.; Xu, F.; Xu, H. Insight into the mechanisms of plant growth promoting strain SNB6 on enhancing the phytoextraction in cadmium contaminated soil. J. Hazard. Mater. 2020, 385, 121587. [Google Scholar] [CrossRef]
- Husna Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Murad, W.; Irshad, M.; Qadir, M.; Kim, H.Y. Pseudocitrobacter anthropi reduces heavy metal uptake and improves phytohormones and antioxidant system in Glycine max L. World J. Microbiol. Biotechnol. 2021, 37, 195. [Google Scholar]
- Bayat, S.; Dalir, N.; Mokhtassi-Bidgoli, A.; Malakouti, M.J.; Shahbazi, K. Selenium alleviates cadmium-induced stress in durum wheat (Triticum durum) by enhancing the accumulation of cadmium in the roots and by modulating of photosynthesis parameters. J. Plant Nutr. 2023, 46, 1903–1919. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, F.; Liu, S.; Du, Y.; Li, F.; Du, R.; Wen, D.; Zhao, J. Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentation in roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2016, 131, 173–180. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Zheng, H.X.; Wang, G.B.; Liu, W.S.; Cao, Y.; Tang, Y.T.; Qiu, R.L. The limited exclusion and efficient translocation mediated by organic acids contribute to rare earth element hyperaccumulation in Phytolacca americana. Sci. Total Environ. 2022, 805, 150335. [Google Scholar] [CrossRef]
- Ru, S.; Xing, J.; Su, D. Rhizosphere cadmium speciation and mechanisms of cadmium tolerance in different oilseed rape species. J. Plant Nutr. 2006, 29, 921–932. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, Z.; Liu, C.; Liu, J.; Zhuang, J. Enhancing remediation potential of heavy metal contaminated soils through synergistic application of microbial inoculants and legumes. Front. Microbiol. 2023, 14, 1272591. [Google Scholar] [CrossRef]
- Gupta, A.; Dutta, A.; Sarkar, J.; Paul, D.; Panigrahi, M.K.; Sar, P. Metagenomic exploration of microbial community in mine tailings of Malanjkhand copper project, India. Genom. Data 2017, 12, 11–13. [Google Scholar] [CrossRef]
- Narendrula-Kotha, R.; Nkongolo, K.K. Bacterial and fungal community structure and diversity in a mining region under long-term metal exposure revealed by metagenomics sequencing. Ecol. Genet. Genom. 2017, 2, 13–24. [Google Scholar] [CrossRef]
- Saghaï, A.; Wittorf, L.; Philippot, L.; Hallin, S. Loss in soil microbial diversity constrains microbiome selection and alters the abundance of N-cycling guilds in barley rhizosphere. Appl. Soil Ecol. 2022, 169, 104224. [Google Scholar] [CrossRef]
- Pacheco-Insausti, M.C.; Ponce, I.T.; Quiñones, M.A.; Pedranzani, H.E.; Pueyo, J.J. Effects of Inoculation with Stress-Tolerant Rhizobia on the Response of Alfalfa (Medicago sativa L.) to Combined Salinity and Cadmium Stress. Plants 2023, 12, 3972. [Google Scholar] [CrossRef]
- Hu, W.; Li, Z.; Ou, H.; Wang, X.; Wang, Q.; Tao, Z.; Huang, S.; Huang, Y.; Wang, G.; Pan, X. Novosphingobium album sp. nov., Novosphingobium organovorum sp. nov. and Novosphingobium mangrovi sp. nov. with the organophosphorus pesticides degrading ability isolated from mangrove sediments. Int. J. Syst. Evol. Microbiol. 2023, 73, 005843. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Ye, Y.; He, Q.; Zhang, S. Isolation and characterization of Pseudoxanthomonas sp. strain YP1 capable of denitrifying phosphorus removal (DPR). Geomicrobiol. J. 2018, 35, 537–543. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; Wang, W.; An, T. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl River Estuary and their exposure risk to human. Environ. Int. 2020, 143, 105934. [Google Scholar] [CrossRef] [PubMed]
- Qarni, A.; Muhammad, K.; Wahab, A.; Ali, A.; Khizar, C.; Ullah, I.; Kazmi, A.; Sultana, T.; Hameed, A.; Younas, M. Molecular characterization of wild and cultivated strawberry (Fragaria × ananassa) through DNA barcode markers. Genet. Res. 2022, 2022, 9249561. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Xu, Q.; Wei, Q.; Kong, Y.; Zhu, L.; Tian, W.; Yan, Y.; Wang, H.; Chi, C.; Zhang, J.; et al. Isolation of the inorganic phosphorus-solubilizing bacteria Lysinibacillus sphaericus and assessing its role in promoting rice growth. Int. Microbiol. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Cheng, S.; Jiang, J.W.; Tan, L.T.; Deng, J.X.; Liang, P.Y.; Su, H.; Sun, Z.X.; Zhou, Y. Plant growth-promoting ability of mycorrhizal Fusarium strain kb-3 enhanced by its IAA producing endohyphal bacterium, Klebsiella aerogenes. Front. Microbiol. 2022, 13, 855399. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.Y.; Cho, K.S. Effect of Novosphingobium sp. CuT1 inoculation on the rhizoremediation of heavy metal-and diesel-contaminated soil planted with tall fescue. Environ. Sci. Pollut. Res. 2023, 30, 16612–16625. [Google Scholar] [CrossRef]
- Kavian, S.; Safarzadeh, S.; Yasrebi, J. Zinc improves growth and antioxidant enzyme activity in Aloe vera plant under salt stress. S. Afr. J. Bot. 2022, 147, 1221–1229. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, Y.; Lü, Q.; Wen, H.; Han, B.; Chen, S.; Zheng, X.; Lin, R. Responses of glutathione and phytochelatins biosysthesis in a cadmium accumulator of Perilla frutescens (L.) Britt. under cadmium contaminated conditions. Ecotoxicol. Environ. Saf. 2020, 201, 110805. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, C.; Cao, H.; Song, J.; Gong, B.; Li, L.; Wang, L.; He, Y.; Liang, M.; Lin, J.; et al. Microbial functional assemblages predicted by the FAPROTAX analysis are impacted by physicochemical properties, but C, N and S cycling genes are not in mangrove soil in the Beibu Gulf, China. Ecol. Indic. 2022, 139, 108887. [Google Scholar] [CrossRef]


| Strain | CdCl2 Concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| 0 | 50 | 150 | 250 | 300 | |
| KM01 | 0.827 ± 0.054 a | 0.449 ± 0.077 b | 0.151 ± 0.041 c | 0.091 ± 0.006 c | 0.051 ± 0.017 c |
| KM04 | 0.661 ± 0.020 a | 0.390 ± 0.036 b | 0.141 ± 0.050 c | 0.074 ± 0.004 d | 0.035 ± 0.020 d |
| KM06 | 0.792 ± 0.072 b | 0.884 ± 0.005 a | 0.411 ± 0.049 c | 0.114 ± 0.022 d | 0.097 ± 0.006 d |
| KM10 | 0.739 ± 0.011 a | 0.627 ± 0.055 b | 0.220 ± 0.062 c | 0.086 ± 0.009 d | 0.058 ± 0.027 d |
| KM14 | 0.859 ± 0.073 a | 0.640 ± 0.126 b | 0.296 ± 0.101 c | 0.082 ± 0.007 d | 0.067 ± 0.009 d |
| KM15 | 0.744 ± 0.036 a | 0.625 ± 0.026 b | 0.308 ± 0.030 c | 0.076 ± 0.006 d | 0.057 ± 0.018 d |
| KM18 | 0.746 ± 0.044 a | 0.849 ± 0.027 b | 0.386 ± 0.097 c | 0.076 ± 0.006 d | 0.078 ± 0.020 d |
| KM19 | 0.793 ± 0.066 a | 0.673 ± 0.026 b | 0.323 ± 0.028 c | 0.090 ± 0.001 d | 0.075 ± 0.015 d |
| KM25 | 0.754 ± 0.025 b | 0.926 ± 0.034 a | 0.692 ± 0.039 c | 0.376 ± 0.041 d | 0.248 ± 0.044 e |
| KM32 | 0.754 ± 0.022 b | 0.842 ± 0.017 a | 0.469 ± 0.051 c | 0.205 ± 0.007 d | 0.121 ± 0.060 e |
| KM34 | 0.745 ± 0.009 b | 0.825 ± 0.014 a | 0.486 ± 0.033 c | 0.129 ± 0.035 d | 0.128 ± 0.054 d |
| KM38 | 0.775 ± 0.021 a | 0.645 ± 0.124 b | 0.085 ± 0.001 c | 0.078 ± 0.001 c | 0.069 ± 0.006 c |
| SH (cm) | RL (cm) | SFW (g) | SDW (g) | RFW (g) | RDW (g) | CC (SPAD) | |
|---|---|---|---|---|---|---|---|
| Cd0 | 16.9 ± 1.237 bc | 12.67 ± 0.15 b | 3.22 ± 0.05 b | 1.04 ± 0.02 b | 3.31 ± 0.06 b | 1.66 ± 0.1 b | 34.47 ± 0.15 b |
| Cd2 | 14.32 ± 1.2 de | 15.5 ± 0.2 a | 2.52 ± 0.12 d | 0.63 ± 0.01 c | 2.6 ± 0.09 d | 1.27 ± 0.03 cd | 32.23 ± 1.09 c |
| Cd5 | 12.84 ± 0.563 e | 10.67 ± 0.21 c | 1.79 ± 0.06 e | 0.54 ± 0.01 cd | 2.43 ± 0.02 e | 0.93 ± 0.04 e | 29.43 ± 0.15 e |
| Cd10 | 9.86 ± 0.736 f | 9.3 ± 0.61 d | 1.33 ± 0.07 f | 0.31 ± 0.01 d | 2.07 ± 0.06 f | 0.74 ± 0.11 e | 28.87 ± 0.87 e |
| Jd0 | 21.26 ± 2.018 a | 10.47 ± 0.31 c | 3.48 ± 0.03 a | 1.47 ± 0.35 a | 3.51 ± 0.06 a | 1.97 ± 0.17 a | 38.23 ± 0.21 a |
| Jd2 | 17.68 ± 1.003 b | 12.37 ± 0.12 b | 2.78 ± 0.08 c | 0.69 ± 0.01 c | 2.74 ± 0.02 c | 1.39 ± 0.11 c | 34.33 ± 1.01 b |
| Jd5 | 15.15 ± 1.089 cd | 9.27 ± 0.15 d | 2.39 ± 0.28 d | 0.61 ± 0.01 c | 2.58 ± 0.01 d | 1.15 ± 0.08 d | 30.87 ± 0.61 d |
| Jd10 | 12.485 ± 1.903 e | 6.57 ± 1.81 e | 1.63 ± 0.13 e | 0.35 ± 0.01 d | 2.46 ± 0.06 e | 0.82 ± 0.06 e | 29.4 ± 0.36 e |
| Shannon | Chao | Ace | Simpson | Shannon even | |
|---|---|---|---|---|---|
| CK | 2.47 ± 0.47 a | 633.72 ± 25.15 a | 636.78 ± 30.55 a | 0.27 ± 0.05 a | 0.39 ± 0.07 a |
| Cd | 2.56 ± 0.58 a | 653.41 ± 52.66 a | 648.79 ± 49.26 a | 0.32 ± 0.11 a | 0.40 ± 0.08 a |
| JK | 3.18 ± 0.36 a | 718.19 ± 23.27 a | 724.25 ± 14.63 a | 0.21 ± 0.05 a | 0.49 ± 0.01 a |
| Jd | 2.53 ± 0.26 a | 675.02 ± 46.27 a | 665.62 ± 42.93 a | 0.31 ± 0.06 a | 0.41 ± 0.04 a |
| Component | Volume |
|---|---|
| 2×Hieff® Robust PCR Master Mix | 15 µL |
| 10 μM Primer R | 1 µL |
| Bar-PCR primer F | 1 µL |
| ddH2O | 9~12 µL |
| PCR products | 10~20 ng |
| Total volume | 30 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yi, E.; Jiang, Y.; Li, X.; Wang, L.; Dong, Y.; Xu, F.; Yu, C.; Ma, L. Screening and Identification of Cadmium-Tolerant, Plant Growth-Promoting Rhizobacteria Strain KM25, and Its Effects on the Growth of Soybean and Endophytic Bacterial Community in Roots. Plants 2025, 14, 2343. https://doi.org/10.3390/plants14152343
Zhang J, Yi E, Jiang Y, Li X, Wang L, Dong Y, Xu F, Yu C, Ma L. Screening and Identification of Cadmium-Tolerant, Plant Growth-Promoting Rhizobacteria Strain KM25, and Its Effects on the Growth of Soybean and Endophytic Bacterial Community in Roots. Plants. 2025; 14(15):2343. https://doi.org/10.3390/plants14152343
Chicago/Turabian StyleZhang, Jing, Enjing Yi, Yuping Jiang, Xuemei Li, Lanlan Wang, Yuzhu Dong, Fangxu Xu, Cuimei Yu, and Lianju Ma. 2025. "Screening and Identification of Cadmium-Tolerant, Plant Growth-Promoting Rhizobacteria Strain KM25, and Its Effects on the Growth of Soybean and Endophytic Bacterial Community in Roots" Plants 14, no. 15: 2343. https://doi.org/10.3390/plants14152343
APA StyleZhang, J., Yi, E., Jiang, Y., Li, X., Wang, L., Dong, Y., Xu, F., Yu, C., & Ma, L. (2025). Screening and Identification of Cadmium-Tolerant, Plant Growth-Promoting Rhizobacteria Strain KM25, and Its Effects on the Growth of Soybean and Endophytic Bacterial Community in Roots. Plants, 14(15), 2343. https://doi.org/10.3390/plants14152343






