Nutritional Characterization of Fruits from Three African Plant Species: Dialium guineense Willd, Parkia biglobosa Jacq. and Andansonia digitata L.
Abstract
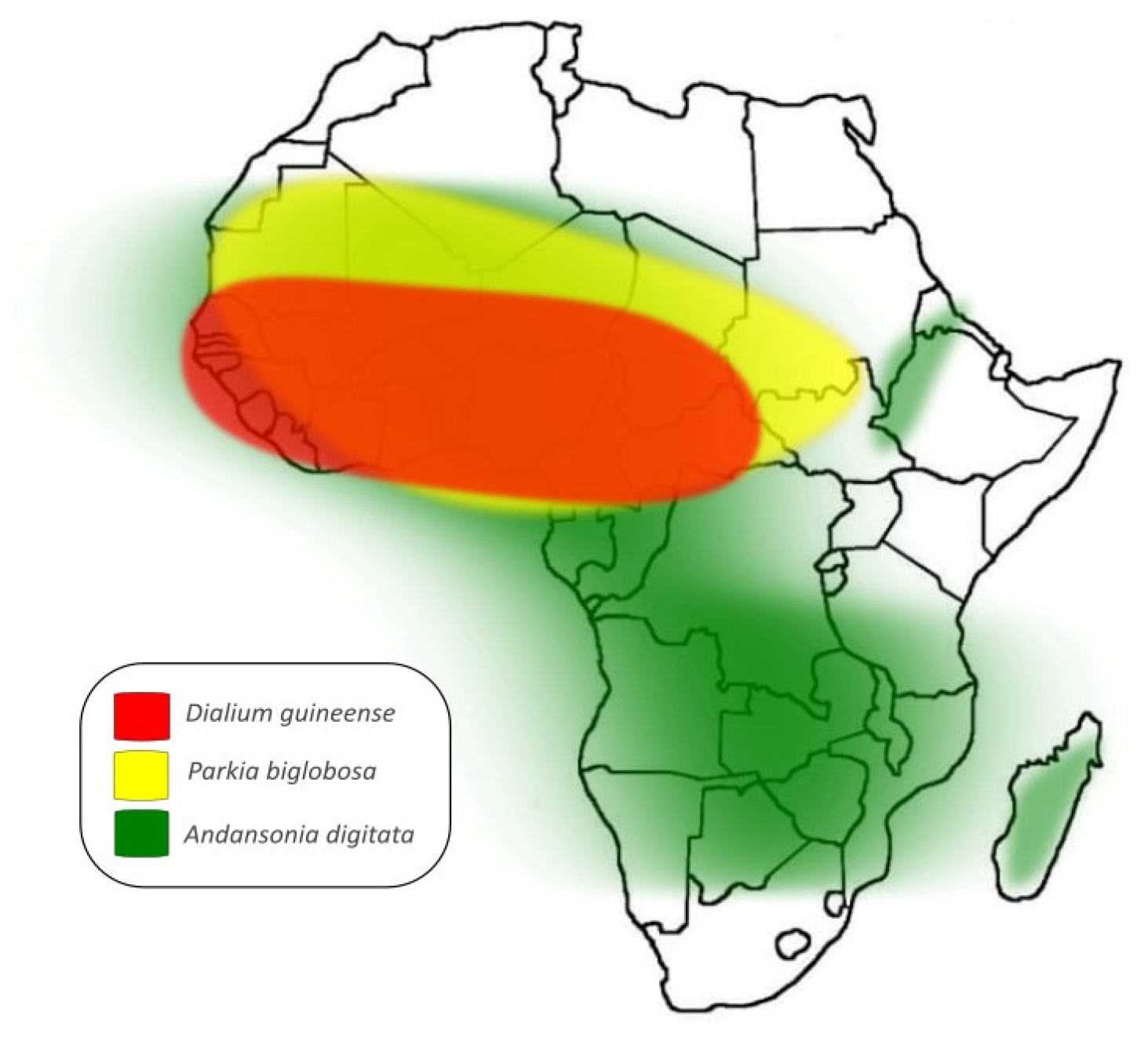
1. Introduction
2. Results
2.1. Colourimetric Analysis
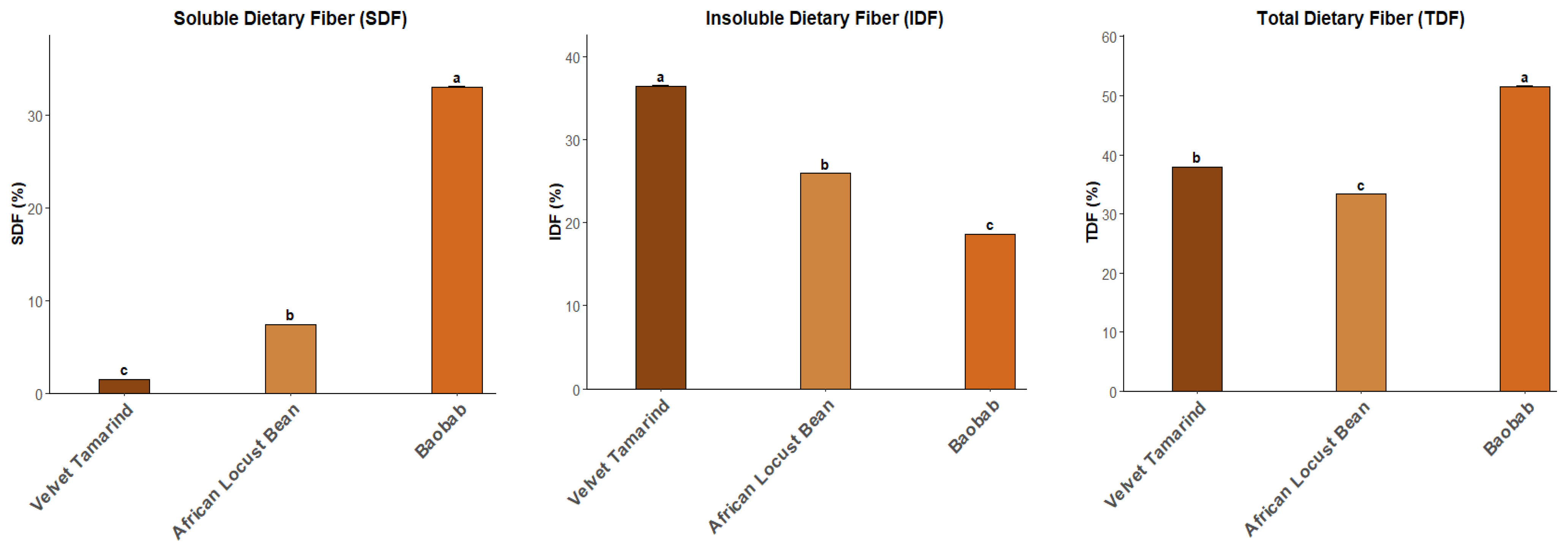
2.2. Moisture, Ash, Protein, Fat and Dietary Fibre Content
2.3. Organic Acid Profile
2.4. Free Sugars Profile
2.5. Carbohydrates and Energy
2.6. Element Contents
2.7. Toxicological Analysis
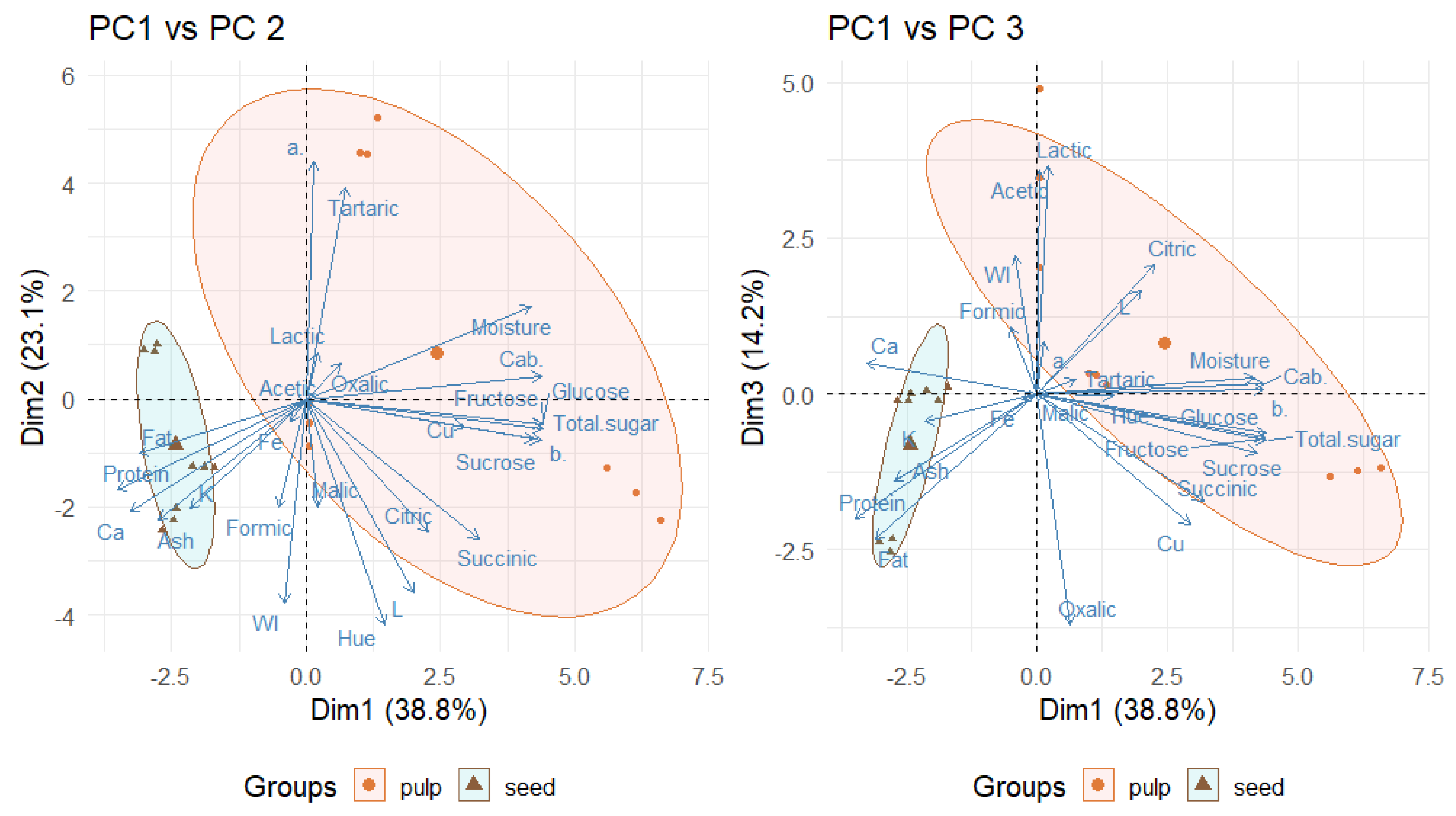
2.8. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Physicochemical Characterisation
4.2.1. Colourimetric Properties
4.2.2. Macro Characterization
4.2.3. Toxic Amygdaline Content
4.2.4. Elemental Analysis
4.3. Statistical Analysis and Principal Component Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, G.; Wang, Z.; Maundu, P.; Hunter, D. The Role of Traditional Knowledge and Food Biodiversity to Transform Modern Food Systems. Trends Food Sci. Technol. 2022, 130, 32–41. [Google Scholar] [CrossRef]
- Kumar, N.; Devi, R.; Kumar, S.; Kurbah, I.; Chandel, A. Empowering Food Security Through Indigenous Biodiversity Management. In Ecologically Mediated Development: Promoting Biodiversity Conservation and Food Security; Jatav, H.S., Raiput, V.D., Minkina, T., Eds.; Springer Nature: Singapore, 2025; pp. 3–25. ISBN 978-981-96-2413-3. [Google Scholar]
- Leakey, R.R.B.; Tientcheu Avana, M.-L.; Awazi, N.P.; Assogbadjo, A.E.; Mabhaudhi, T.; Hendre, P.S.; Degrande, A.; Hlahla, S.; Manda, L. The Future of Food: Domestication and Commercialization of Indigenous Food Crops in Africa over the Third Decade (2012–2021). Sustainability 2022, 14, 2355. [Google Scholar] [CrossRef]
- Ndhlovu, P.T.; Mokgehle, S.N.; Kola, M.E.; Chauke, S.; Falemara, B.C.; Otang-Mbeng, W. The Contribution of Underutilized Fruits and Vegetables to Enhanced Food and Nutrition Security: A Review. In Food Security and Nutrition; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-46976-6. [Google Scholar]
- Arogba, S.S.; Ajiboro, A.A.; Odukwe, I.J. A Physico-Chemical Study of Nigerian Velvet Tamarind (Dialium guineense L) Fruit. J. Sci. Food Agric. 1994, 66, 533–534. [Google Scholar] [CrossRef]
- Aburime, L.C.; Nneoyi-Egbe, A.F.; Jeremiah, J.C.O. Vitamins and Phytochemical Compositions of African Velvet Tamarind (Dialium guineense) Fruit Pulp. J. Home Econ. Res. 2024, 31, 112. [Google Scholar]
- Asoiro, F.U.; Ezeoha, S.L.; Ezenne, G.I.; Ugwu, C.B. Chemical and Mechanical Properties of Velvet Tamarind Fruit (Dialium guineense). Niger. J. Technol. 2017, 36, 252–260. [Google Scholar] [CrossRef]
- Termote, C.; Odongo, N.O.; Dreyer, B.S.; Parkouda, C.; Vinceti, B. Nutrient Composition of Parkia Biglobosa Pulp, Raw and Fermented Seeds: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 119–144. [Google Scholar] [CrossRef]
- Koura, K.; Ganglo, J.C.; Assogbadjo, A.E.; Agbangla, C. Ethnic Differences in Use Values and Use Patterns of Parkia Biglobosa in Northern Benin. J. Ethnobiol. Ethnomedicine 2011, 7, 42. [Google Scholar] [CrossRef]
- Houndonougbo, J.S.H.; Kassa, B.; Mensah, S.; Salako, V.K.; Glèlè Kakaï, R.; Assogbadjo, A.E. A Global Systematic Review on Conservation Nd Domestication of Parkia biglobosa (Jacq.) R. Br. Ex G. Don, an Indigenous Fruit Tree Species in Sub-Sahara African Traditional Parklands: Current Knowledge and Future Directions. Genet. Resour. Crop Evol. 2020, 67, 1051–1066. [Google Scholar] [CrossRef]
- Obafemi, Y.D.; Ajayi, A.A.; Adebayo, H.A.; Oyewole, O.A.; Olumuyiwa, E.O. The Role of Indigenous Nigerian Fermented Agrifoods in Enhancing Good Health and Well˗being. Discov. Food 2024, 4, 133. [Google Scholar] [CrossRef]
- Thompson, P.T.; Boamah, V.E.; Badu, M. In-Vitro Antioxidant, Antimicrobial and Phytochemical Properties of Extracts from the Pulp and Seeds of the African Baobab Fruit (Adansonia digitata L.). Heliyon 2024, 10, e29660. [Google Scholar] [CrossRef]
- Monteiro, S.; Reboredo, F.H.; Lageiro, M.M.; Lourenço, V.M.; Dias, J.; Lidon, F.; Abreu, M.; Martins, A.P.L.; Alvarenga, N. Nutritional Properties of Baobab Pulp from Different Angolan Origins. Plants 2022, 11, 2272. [Google Scholar] [CrossRef]
- Vinha, A.F.; Costa, A.S.G.; Pimentel, F.B.; Espírito Santo, L.; Sousa, C.; Freitas, M.; Fernandes, E.; Oliveira, M.B.P.P. Bioactive Compounds and Scavenging Capacity of Adansonia digitata L. (Baobab Fruit) Pulp Extracts against ROS and RNS of Physiological Relevance. Appl. Sci. 2024, 14, 3408. [Google Scholar] [CrossRef]
- OJEU. Commission Decision of Authorising the Placing on the Market of Baobab Dried Fruit Pulp as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (Notified under Document Number C (2008/575/EC). Off. J. Eur. Union 2008, L183, 38–39. [Google Scholar]
- Barakat, H. Nutritional and Rheological Characteristics of Composite Flour Substituted with Baobab (Adansonia digitata L.) Pulp Flour for Cake Manufacturing and Organoleptic Properties of Their Prepared Cakes. Foods 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.; Dias, J.; Lourenço, V.; Partidário, A.; Lageiro, M.; Lampreia, C.; Fernandes, J.; Lidon, F.; Reboredo, F.; Alvarenga, N. Development of a Functional Dark Chocolate with Baobab Pulp. Foods 2023, 12, 1711. [Google Scholar] [CrossRef]
- Lageiro, M.M.; Fernandes, J.; Marques, A.C.; Soares, A.; Partidário, A.M.C.; Coelho, A.R.F. Nutritional, Elemental and Toxicity Assessment of Three Tropical Fruits’ Pulps and Seeds. Biol. Life Sci. Forum 2024, 40, 22. [Google Scholar] [CrossRef]
- Achoba, I.I.; Lori, J.A.; Elegbede, J.A.; Kagbu, J.A. Nutrient Composition of Black (African) Velvet Tamarind (Dialium guineense Wild) Seed and Pulp from Nigeria. J. Food Biochem. 1992, 16, 229–233. [Google Scholar] [CrossRef]
- Gernmah, D.I.; Atolagbe, M.O.; Echegwo, C.C. Nutritional Composition of the African Locust Bean (Parkia biglobosa) Fruit Pulp. Niger. Food J. 2007, 25, 190–196. [Google Scholar] [CrossRef]
- Abdulwaliyu, I.; Arekemase, S.O.; Batari, M.L.; Oshodin, J.O.; Mustapha, R.A.; Ibrahim, D.; Ekere, A.T.; Olusina, O.S. Nutritional and Pharmacological Attributes of Baobab Fruit Pulp. Food Prod. Process Nutr. 2024, 6, 98. [Google Scholar] [CrossRef]
- Adeleye, A.O.; Ajiboye, T.O.; Iliasu, G.A.; Abdussalam, F.A.; Balogun, A.; Ojewuyi, O.B.; Yakubu, M.T. Phenolic Extract of Dialium guineense Pulp Enhances Reactive Oxygen Species Detoxification in Aflatoxin B1 Hepatocarcinogenesis. J. Med. Food 2014, 17, 875–885. [Google Scholar] [CrossRef]
- Khashayar, G.; Bain, P.A.; Salari, S.; Dozic, A.; Kleverlaan, C.J.; Feilzer, A.J. Perceptibility and Acceptability Thresholds for Colour Differences in Dentistry. J. Dent. 2014, 42, 637–644. [Google Scholar] [CrossRef]
- Touré, O.; Cissé, O.I.K.; Faye, M.; Faye, P.G.; Ayessou, N.C.; Cissé, M. Nutritional Potential of Parkia Biglobosa Fruits Pulp Harvested in Senegal. Food Nutr. Sci. 2024, 15, 1288–1298. [Google Scholar] [CrossRef]
- Abiodun, O.A.; Dauda, A.O.; Adebisi, T.T.; Alonge, C.D. Physico-Chemical, Microbial and Sensory Properties of Kunu Zaki Beverage Sweetened with Black Velvet Tamarind (Dialium guineense). Croat. J. Food Sci. Technol. 2017, 9, 46–56. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) Carbohydrate Intake for Adults and Children: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-007359-3. [Google Scholar]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Gouveia-Nhanca, M.; Rolim Bezerra, M.L.; Batista, K.S.; Pinheiro, R.O.; Soares, N.L.; De Paiva Sousa, M.C.; Alves, A.F.; Ribeiro, M.D.; Silva, A.S.; Magnani, M.; et al. The Non-Conventional Edible Plant Foroba (Parkia biglobosa) Has Anti-Obesity Effect, Improves Lipid Peroxidation and Reverses Colon and Hippocampal Lesions in Healthy and Obese Rats. J. Funct. Foods 2023, 108, 105745. [Google Scholar] [CrossRef]
- Stadlmayr, B.; Wanangwe, J.; Waruhiu, C.G.; Jamnadass, R.; Kehlenbeck, K. Nutritional Composition of Baobab (Adansonia digitata L.) Fruit Pulp Sampled at Different Geographical Locations in Kenya. J. Food Compos. Anal. 2020, 94, 103617. [Google Scholar] [CrossRef]
- De Caluwé, E.; De Smedt, S.; Assogbadjo, A.E.; Samson, R.; Sinsin, B.; Van Damme, P. Ethnic Differences in Use Value and Use Patterns of Baobab (Adansonia digitata L.) in Northern Benin. Afr. J. Ecol. 2009, 47, 433–440. [Google Scholar] [CrossRef]
- Kouassi, K.A.; Beugré, G.A.M.; Kouassi, K.N.; N’Dri, Y.D.; Amani, N.G.; Gnakri, D. Biochemical Characterization of Juices from Three Wild Fruit Species Consumed in Côte d’Ivoire “Adansonia digitata, Parkia Biglobosa and Tamarindus Indica”. Open Sci. J. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Osman, M.A. Chemical and Nutrient Analysis of Baobab (Adansonia digitata) Fruit and Seed Protein Solubility. Plant Foods Hum. Nutr. 2004, 59, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Aborisade, C.A.; Bakare, L.M.; Balogun, F.O. Elemental Characterization of African Locust Beans (Parkia biglobosa) in Igbope, Oyo State of Nigeria. Asian J. Appl. Sci. 2021, 9, 127–132. [Google Scholar] [CrossRef]
- Shragg, T.A.; Albertson, T.E.; Fisher, C.J. Cyanide Poisoning after Bitter Almond Ingestion. West. J. Med. 1982, 136, 65–69. [Google Scholar]
- Dang, T.; Nguyen, C.; Tran, P.N. Physician Beware: Severe Cyanide Toxicity from Amygdalin Tablets Ingestion. Case Rep. Emerg. Med. 2017, 2017, 4289527. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Pereira, N.; Silva, M.; Alvarenga, N.; Ramos, A.C.; Alegria, C.; Abreu, M. Influence of Air-Drying Conditions on Quality, Bioactive Composition and Sensorial Attributes of Sweet Potato Chips. Foods 2023, 12, 1198. [Google Scholar] [CrossRef]
- Pereira, P.; Palma, M.L.; Palma, C.; Borges, C.; Maurício, E.; Fernando, A.L.; Duarte, M.P.; Lageiro, M.; Fernandes, A.; Mateus, N.; et al. Exploring the Benefits of Nutritional and Chemical Characteristics of Touriga Nacional and Arinto Varieties (Vitis vinifera L.). Foods 2024, 13, 1535. [Google Scholar] [CrossRef]
- Nicolai, M.; Palma, M.L.; Reis, R.; Amaro, R.; Fernandes, J.; Gonçalves, E.M.; Silva, M.; Lageiro, M.; Charmier, A.; Maurício, E.; et al. Assessing the Potential of Brewer’s Spent Grain to Enhance Cookie Physicochemical and Nutritional Profiles. Foods 2025, 14, 95. [Google Scholar] [CrossRef]
- Pereira, N.; Farrokhi, M.; Vida, M.; Lageiro, M.; Ramos, A.C.; Vieira, M.C.; Alegria, C.; Gonçalves, E.M.; Abreu, M. Valorisation of Wasted Immature Tomato to Innovative Fermented Functional Foods. Foods 2023, 12, 1532. [Google Scholar] [CrossRef]
- Cartas, J.; Alvarenga, N.; Partidário, A.; Lageiro, M.; Roseiro, C.; Gonçalves, H.; Leitão, A.E.; Ribeiro, C.M.; Dias, J. Influence of Geographical Origin in the Physical and Bioactive Parameters of Single Origin Dark Chocolate. Eur. Food Res. Technol. 2024, 250, 2569–2580. [Google Scholar] [CrossRef]
- Panda, A.; Alvarenga, N.; da Silva, J.L.; Partidário, A.; Lageiro, M.; Roseiro, C.; Dias, J. Influence of Cocoa Origin on the Nutritional Characterization of Chocolate. Eur. Food Res. Technol. 2022, 248, 2569–2577. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Determination of Amygdalin in Apple Seeds, Fresh Apples and Processed Apple Juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef]
- Bolarinwa, I.; Orfila, C.; Morgan, M. Amygdalin Content of Seeds, Kernels and Food Products Commercially-Available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef]
- Fernandes, J.; Reboredo, F.H.; Luis, I.; Silva, M.M.; Simões, M.M.; Lidon, F.C.; Ramalho, J.C. Elemental Composition of Commercial Herbal Tea Plants and Respective Infusions. Plants 2022, 11, 1412. [Google Scholar] [CrossRef] [PubMed]

| Type | Fruit | L* | a* | b* | WI | Cab* | Hue Angle |
|---|---|---|---|---|---|---|---|
| Pulp | Velvet tamarind | 48.81 ± 0.48 cd | 18.6 ± 0.39 a | 22.97 ± 0.17 b | 40.89 ± 0.52 c | 29.56 ± 0.27 b | 51.02 ± 0.64 f |
| African locust bean | 79.87 ± 0.11 a | 3.08 ± 0.03 d | 38.4 ± 0.1 a | 56.54 ± 0.06 ab | 38.52 ± 0.1 a | 85.41 ± 0.05 a | |
| Baobab | 73.09 ± 0.08 ab | 7.82 ± 0.03 b | 21.14 ± 0.03 c | 64.9 ± 0.07 a | 22.54 ± 0.03 c | 69.69 ± 0.07 d | |
| Seed | Velvet tamarind | 60.35 ± 6.81 bc | 5.09 ± 0.13 c | 15.51 ± 0.08 e | 56.88 ± 6.42 ab | 16.32 ± 0.11 e | 71.85 ± 0.34 c |
| African locust bean | 72.81 ± 0.18 ab | 2.94 ± 0.09 d | 19.71 ± 0.13 d | 66.29 ± 0.08 a | 19.93 ± 0.12 d | 81.51 ± 0.27 b | |
| Baobab | 45.2 ± 0.3 d | 6.94 ± 0.09 b | 11.88 ± 0.21 f | 43.5 ± 0.25 bc | 13.76 ± 0.19 f | 59.67 ± 0.53 e |
| ΔE | ΔE Pulp | ΔE Seed | ΔE Pulp/Seed |
|---|---|---|---|
| Velvet tamarind/African locust bean | 37.99 | 13.33 | 28.84 |
| Velvet tamarind/Baobab | 26.63 | 15.69 | 16.49 |
| African locust bean/Baobab | 19.13 | 28.98 | 5.10 |
| Type | Fruit | Moisture (%) | Ash (%) | Protein (%) | Fat (%) |
|---|---|---|---|---|---|
| Pulp | Velvet tamarind | 12.14 ± 0.32 b | 2.04 ± 0.07 c | 2.62 ± 0.11 d | 0.27 ± 0.00 d |
| African locust bean | 13.04 ± 0.15 a | 2.21 ± 0.01 b | 2.29 ± 0.03 d | 0.80 ± 0.00 d | |
| Baobab | 9.60 ± 0.24 c | 2.24 ± 0.12 b | 1.54 ± 0.07 d | 0.85 ± 0.00 d | |
| Seed | Velvet tamarind | 7.52 ± 0.30 d | 2.38 ± 0.10 b | 19.89 ± 1.00 c | 4.53 ± 0.00 c |
| African locust bean | 7.09 ± 0.22 d | 2.41 ± 0.10 b | 28.41 ± 0.07 a | 18.85 ± 0.00 b | |
| Baobab | 7.54 ± 0.04 d | 2.52 ± 0.07 a | 23.36 ± 0.35 b | 21.49 ± 0.00 a |
| Organic Acid (%) | Velvet Tamarind | African Locust Bean | Baobab | |||
|---|---|---|---|---|---|---|
| Pulp | Seed | Pulp | Seed | Pulp | Seed | |
| Acetic | 0.038 ± 0.01 b | <0.016 | <0.016 | <0.016 | 2.501 ± 0.00 a | <0.016 |
| Citric | <0.007 | 0.939 ± 0.12 b | 2.826 ± 0.88 a | 1.000 ± 0.12 b | 3.243 ± 0.41 a | 0.776 ± 0.01 b |
| Formic | <0.076 | <0.076 | 0.326 ± 0.33 a | 1.038 ± 0.11 a | 0.995 ± 0.00 a | 0.230 ± 0.09 a |
| Lactic | 0.113 ± 0.02 a | <0.014 | <0.014 | <0.014 | 0.351 ± 0.00 a | <0.014 |
| Malic | 0.579 ± 0.37 c | 4.321 ± 0.18 a | 1.961 ± 0.59 b | 0.954 ± 0.08 c | 0.970 ± 0.23 bc | 0.349 ± 0.01 c |
| Oxalic | 0.233 ± 0.11 c | 0.203 ± 0.01 c | 0.477 ± 0.08 b | 0.078 ± 0.01 c | <0.004 | 0.696 ± 0.01 a |
| Succinic | <0.050 | 1.520 ± 0.02 b | 3.937 ± 0.52 a | 0.866 ± 0.09 bc | 0.487 ± 0.00 c | 0.754 ± 0.02 bc |
| Tartaric | 3.43 ± 1.42 a | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Type | Fruit | Carbohydrates (%) | Energy (Kcal per 100 g of Dry Weight) |
|---|---|---|---|
| Pulp | Velvet tamarind | 45.0 ± 0.48 b | 282.0 ± 7.3 b |
| African locust bean | 48.4 ± 0.14 a | 305.0 ± 6.6 a | |
| Baobab | 34.2 ± 0.31 c | 279.3 ± 3.2 c |
| Type | Fruit | Ca | K | P | S | Fe | Cu |
|---|---|---|---|---|---|---|---|
| Pulp | Velvet tamarind | 66.6 ± 4.8 e | 857.9 ± 13.0 f | 32.2 ± 4 d | 15.7 ± 4.6 e | 3.1 ± 0.3 d | 1.8 ± 0.3 b |
| African locust bean | 107.2 ± 5.6 d | 3263 ± 9.3 a | 79.7 ± 2 b | 26.6 ± 0.7 de | 5.5 ± 0.4 bc | 3.7 ± 0.8 a | |
| Baobab | 310.4 ± 6.6 c | 2777 ± 14.2 b | 25.5 ± 5.5 d | 30 ± 1.1 d | 4.4 ± 1.0 cd | 1.8 ± 0.1 b | |
| Seed | Velvet tamarind | 406.4 ± 3.7 a | 1305 ± 6.6 e | 171.9 ± 8.5 c | 280.9 ± 2.9 c | 6.3 ± 0.7 b | 1.6 ± 0.4 b |
| African locust bean | 257.2 ± 3.1 b | 1737 ± 16.4 d | 183.5 ± 5.6 c | 2138 ± 6.6 a | <3.5 | 1.5 ± 0.2 b | |
| Baobab | 327.5 ± 8.3 c | 2265 ± 11.3 c | 768.7 ± 7 a | 314.7 ± 5.3 b | 10.9 ± 0.2 a | 2.7 ± 0.3 ab |
| Variable | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Fat | −0.685 | −0.222 | −0.516 | 0.399 |
| Protein | −0.772 | −0.370 | −0.443 | −0.042 |
| Total sugar | 0.970 | −0.121 | −0.166 | 0.018 |
| Sucrose | 0.930 | −0.165 | −0.209 | −0.009 |
| Glucose | 0.968 | −0.105 | −0.141 | 0.060 |
| Fructose | 0.965 | −0.105 | −0.156 | −0.001 |
| Oxalic | 0.142 | 0.143 | −0.820 | 0.313 |
| Citric | 0.498 | −0.542 | 0.459 | 0.365 |
| Tartaric | 0.162 | 0.858 | 0.051 | −0.245 |
| Lactic | 0.046 | 0.189 | 0.809 | 0.352 |
| Succinic | 0.705 | −0.569 | −0.378 | −0.100 |
| Formic | −0.111 | −0.443 | 0.236 | 0.239 |
| Acetic | 0.007 | −0.020 | 0.792 | 0.408 |
| Malic | 0.049 | −0.437 | 0.000 | −0.775 |
| Moisture | 0.919 | 0.373 | 0.058 | 0.023 |
| Ash | −0.605 | −0.495 | −0.308 | 0.295 |
| Ca | −0.721 | −0.461 | 0.111 | −0.079 |
| K | −0.474 | −0.446 | −0.100 | 0.034 |
| Fe | −0.065 | −0.093 | −0.018 | −0.920 |
| Cu | 0.648 | −0.114 | −0.463 | 0.341 |
| L* | 0.438 | −0.790 | 0.367 | −0.123 |
| a* | 0.031 | 0.965 | 0.184 | −0.113 |
| b* | 0.959 | −0.169 | 0.016 | 0.008 |
| WI | −0.092 | −0.833 | 0.489 | −0.193 |
| Cab* | 0.961 | 0.095 | 0.036 | −0.024 |
| Hue angle | 0.321 | −0.920 | −0.007 | −0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lageiro, M.; Fernandes, J.; Marques, A.C.; Simões, M.; Coelho, A.R.F. Nutritional Characterization of Fruits from Three African Plant Species: Dialium guineense Willd, Parkia biglobosa Jacq. and Andansonia digitata L. Plants 2025, 14, 2344. https://doi.org/10.3390/plants14152344
Lageiro M, Fernandes J, Marques AC, Simões M, Coelho ARF. Nutritional Characterization of Fruits from Three African Plant Species: Dialium guineense Willd, Parkia biglobosa Jacq. and Andansonia digitata L. Plants. 2025; 14(15):2344. https://doi.org/10.3390/plants14152344
Chicago/Turabian StyleLageiro, Manuela, Jaime Fernandes, Ana C. Marques, Manuela Simões, and Ana Rita F. Coelho. 2025. "Nutritional Characterization of Fruits from Three African Plant Species: Dialium guineense Willd, Parkia biglobosa Jacq. and Andansonia digitata L." Plants 14, no. 15: 2344. https://doi.org/10.3390/plants14152344
APA StyleLageiro, M., Fernandes, J., Marques, A. C., Simões, M., & Coelho, A. R. F. (2025). Nutritional Characterization of Fruits from Three African Plant Species: Dialium guineense Willd, Parkia biglobosa Jacq. and Andansonia digitata L. Plants, 14(15), 2344. https://doi.org/10.3390/plants14152344









