Abstract
Taxus is the natural source of the anticancer drug paclitaxel. Although significant progress has been made in elucidating the biosynthetic pathway of paclitaxel, its tissue-specific accumulation and associated regulatory networks in Taxus remains unclear. In this study, we conducted integrated transcriptomic and metabolomic analyses of the root, leaf, shoot, bark, and wood of Taxus chinensis var. mairei to investigate the tissue-specific biosynthesis and accumulation of paclitaxel. We found that paclitaxel, 10-deacetylbaccatin III, and several taxoids were significantly enriched in the leaf, bark, and shoot, while paclitaxel derivatives, such as taxayunnansin A and taxol B, accumulated primarily in the root. Most genes involved in paclitaxel biosynthesis showed the highest expression in the root and the lowest in the wood. Using weighted gene co-expression network analysis (WGCNA), we identified several candidate transcription factors potentially regulating paclitaxel biosynthesis. Further validation using yeast one-hybrid (Y1H) and dual-luciferase reporter assays confirmed that ERF68 activates the expression of taxane-2α-hydroxylase (T2H) gene, a key gene in the paclitaxel biosynthesis pathway. Collectively, our finding provides crucial insights into the transcriptional regulation of paclitaxel biosynthesis in Taxus.
1. Introduction
Taxus spp. (Taxaceae) are evergreen trees or shrubs, predominantly distributed in the northern hemisphere [1]. These species produce a variety of bioactive compounds, including paclitaxel (also named taxol), polysaccharides and flavonoids [2,3,4]. As a vital anticancer drug, paclitaxel was firstly isolated from the bark of T. brevifolia Nutt., and its structure was elucidated by Wall and Wani in 1971 [2]. Its anticancer mechanism involves the disruption of cancer cell mitosis [5], and it has been widely used in the treatment of ovarian, breast, esophageal, lung, and stomach cancers [6]. Due to the slow growth and rarity of many Taxus species, coupled with the extremely low natural yield of paclitaxel, paclitaxel production cannot meet the global demand. Intermediates such as 10-deacetylbaccatin III (10-DAB) and baccatin Ⅲ are extracted from the branches and needles of Taxus and used for the semi-synthesis of paclitaxel [7]. Synthetic biology and metabolic engineering approaches have newly emerged as promising alternatives to explore paclitaxel biosynthesis in recent years [8,9,10,11,12,13].
Paclitaxel is a complex diterpene compound synthesized through a network involving both terpene and phenylalanine pathways. With the development of the sequencing technology, the publications of three sets of Taxus chromosome-level genomes have provided new gene resources for the studies of the paclitaxel biosynthesis and its regulation in Taxus [14,15,16]. The biosynthetic pathway of paclitaxel can be divided into three main stages, including the synthesis of taxa-4(5), 11(12)-diene as a taxane skeleton, the synthesis of intermediate baccatin III, and the assembly of the side chain. The universal precursors of terpenoids, dimethylallyl diphosphate (DMAPP) and isoprenyl diphosphate (IPP), are produced by the mevalonic pathway (MVA) in the cytosol or via the methylerythritol phosphate (MEP) pathway in plastids [17,18]. Geranylgeranyl diphosphate (GGPP), which is synthesized from three IPP molecules and one DMAPP by GGPP synthase (GGPPs), serves as a precursor for paclitaxel [19]. GGPP is then catalyzed by taxadiene synthase (TS) to form taxa-4(5),11(12)-diene and taxa-4(20),11(12)-diene [20]. In the second stage, taxa-4(5),11(12)-diene is hydroxylated at C1,C2, C5, C7, C9, C10, and C13 by taxane-1β-hydroxylase (T1H), taxane-2α-hydroxylase (T2H), taxadiene-5α-hydroxylase (T5H), taxane-7β-hydroxylase (T7H), taxane-9α-hydroxylase (T9H), taxane-10β-hydroxylase (T10H), and taxane-13α-hydroxylase (T13H); acylated at C5 and C10 by taxadiene-5α-ol-O-acetyl transferase (TAT) and 10-deacetyl baccatin III-10β-O-acetyltransferase (DBAT); epoxidized at C4 and C20 by epoxidase or oxetanized by taxaneoxetanase (TOT); oxidized at C9 by taxane-9α-dioxygenase (T9O); and modified at C2 by taxane-2α-O-benzoyltransferase (TBT) to produce the key intermediate baccatin III [21,22,23,24,25,26,27,28,29,30]. In the third stage, the side chain derived from α-phenylalanine is synthesized via phenylalanine aminomutase (PAM) and β-phenylalanine coenzyme A ligase (PCL). Baccatin III-3-amino-3-phenylpropanoyltransferase (BAPT) catalyzed the conjugation of the β-phenylalanoyl-CoA side chain to baccatin III, forming 3′-N-debenzoyl-2′-deoxytaxol. Then, 3′-N-debenzoyl-2′-deoxytaxol is hydroxylated by taxane 2′α-hydroxylase (T2′H). Finally, 3′-N-debenzoyl-taxol was modified by 3′-N-debenzoyl-2′-deoxytaxol-N-benzoyltransferase (DBTNBT), yielding the end product, paclitaxel [31,32,33,34,35]. Due to the complex biosynthetic route and various branching reactions, over 600 taxoids have been isolated from Taxus species to date [36]. Some of these derivatives, like cephalomannine and 7-epi-taxol, share the same antitumor mechanism and can be converted into paclitaxel, representing potential alternative sources of paclitaxel that may help alleviate supply limitations [37,38,39,40].
Although the paclitaxel biosynthesis genes were almost thoroughly studied, some regulators remain unknown. Previous studies have shown that transcription factors (TFs) play significant roles in the regulation of paclitaxel biosynthesis by activating or repressing the expression of structural genes in the paclitaxel biosynthesis pathway. These TFs are often hormone-inducible or tissue/sex-specific. For example, jasmonate-responsive ERFs, such as TcERF12 and TcERF15, act as the repressor and activator of TS, respectively, by binding to the GCC-box in its promoter [41]. TcMYC2a positively regulates the expression of TS and TcERF15 [42]. TcWRKY1 was also induced by MeJA and can activate the transcription of DBAT [43]. Salicylic acid-responsive factor TcWRKY33 promoted the expression of DBAT and TcERF15 [44]. TcMYB29a, an abscisic acid-responsive factor, upregulated T5H [45]. Phloem-specific TmMYB3 positively regulates paclitaxel biosynthesis by activating the expression of TBT and TS [46]. It has been reported that paclitaxel accumulates at a significantly higher level in female T. media. The female-predominant MYB39-bHLH13 complex trans-activates the expression of GGPPS and T10H genes [47]. Endodermal cell-specific MYB47 significantly activates T5H expression in T. mairei stems. Xylem parenchyma cell-specific bHLH68 significantly activates TAT and T10H expression [48]. MYB17 and bHLH46 significantly inhibit the expression of TS and GGPPS in T. mairei leaves, respectively [49]. A regulatory network, including 10 enzyme genes of the paclitaxel pathway and 28 TFs, was reported [50]. However, the TFs involved in the regulation of the expression of the paclitaxel genes in different Taxus tissues require further investigation.
In this study, we performed a comprehensive analysis combining the metabolomic profiling and RNA sequencing of five tissues (root, leaf, shoot, bark, and wood) of T. chinensis var. mairei to investigate tissue-specific paclitaxel accumulation. We identified differentially expressed genes (DEGs) and differentially accumulated metabolites (DAMs) associated with paclitaxel biosynthesis. Through weighted gene co-expression network analysis (WGCNA), we constructed a regulatory network for paclitaxel accumulation and identified ERF68 as a potential regulator of the biosynthetic pathway. Y1H and dual-luciferase reporter assays demonstrated that ERF68 activates the expression of a key paclitaxel biosynthesis gene, T2H. Together, our findings reveal a novel mechanism underlying the regulation of paclitaxel biosynthesis mediated by TF ERF68.
2. Results
2.1. Overview of the Metabolomes
To investigate variations in metabolite accumulation between different tissues, a widely targeted metabolomic analysis was conducted. A total of 1731 metabolites were identified across five different tissues (Table S1). The total ion chromatograms (TICs) of the mixed-sample extracts are shown in Figure S1. Principal component analysis (PCA) was performed to assess the overall metabolic variation between samples. The first two principal components, PC1 and PC2, explained 30.33% and 22.42% of the total variance, respectively. The PCA result shows five distinct sample groupings, indicating clear metabolic differentiation between the tissues (Figure 1A). The identified 1731 metabolites were assigned to 14 categories, including flavonoids (17.62%), lipids (11.38%), alkaloids (10.28%), phenolic acids (9.19%), terpenoids (8.78%), amino acids and derivatives (8.61%), lignans and coumarins (5.37%), organic acids (3.99%), nucleotides and derivatives (3.41%), tannins (1.68%), quinones (1.1%), steroids (0.75%), others (17.74%), and uncategorized metabolites (0.12%) (Figure 1B). The results show the diversity of the metabolites in five tissues.
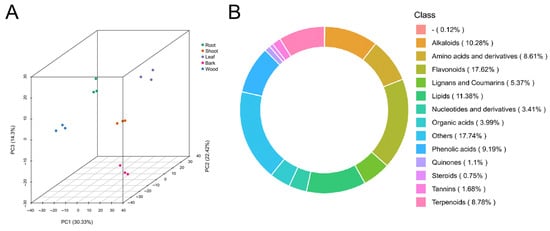
Figure 1.
Summary of metabolome. (A) The principal component analysis of metabolome data from the 15 samples. (B) Class-count ring of metabolome data. “-” represents unknown metabolites.
2.2. Analysis of DAMs in Different Tissues
A total of 1616 DAMs from different comparisons (root/leaf, wood/leaf, bark/leaf, root/shoot, wood/root, wood/shoot, bark/wood, bark/root, shoot/leaf, and bark/shoot) were classified into two distinct clusters (Figure 2). Clusters I comprised 764 metabolites that predominately accumulated in the root and wood tissue, whereas Cluster II (852 metabolites) were enriched in the leaves, shoot, and bark tissues. The Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that the significantly enriched pathways in Cluster I included ‘linoleic acid metabolism’, ‘biosynthesis of flavanols II’, ‘galactose metabolism, aminoacyl-tRNA biosynthesis’, ‘ABC transporters’, ‘biosynthesis of flavanols I’, ‘cyanoamino acid metabolism’, ‘glycerophospholipid metabolism’, ‘glucosinolate biosynthesis’, and ‘biosynthesis of nucleotide sugars’ (Figure 2). In contrast, Cluster II was significantly enriched in ‘flavone and flavonol biosynthesis’, ‘biosynthesis of quercetin aglycones I’, and ‘pyruvate metabolism’. Interestingly, Cluster I contained dimethylallyl diphosphate (DMAPP), a key intermediate in the terpenoid backbone biosynthesis pathway that provides essential precursors for paclitaxel biosynthesis. Meanwhile, Cluster II included 10-deacetylbaccatin III and paclitaxel, both of which are critical intermediates or end products in the diterpenoid biosynthesis pathway.
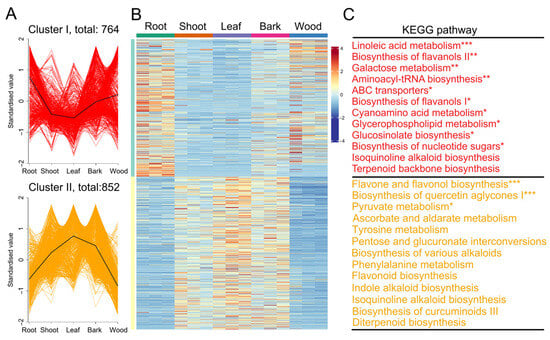
Figure 2.
The differentially accumulated metabolites (DAMs) among five tissues. (A) K-means analysis of DAMs. (B) A heatmap of DAMs. (C) Kyoto Encyclopedia of Genes and Genomes enrichment analysis of DAMs. “*” indicates a significant difference of p < 0.05. “**” indicates a significant difference of p < 0.01. “***” indicates a significant difference of p < 0.001.
2.3. Overview of the Transcriptomes
To investigate the transcription regulation mechanisms of different tissues, RNA sequencing was performed on the same samples used for metabolomic analysis. Each sample generated on average 6.01 Gb of data, and a total of 105.66 Gb clean data was obtained. The Q30 values were above 96.6%, indicating the sequencing data were reliable (Table S2). Clean reads from each sample were aligned with the T. wallichiana genome [14], with the mapping rate ranging from 86.39% to 90.39% (Table S3). In total, 42,657 expressed genes were detected. Among them, 32,631 genes were successfully annotated through the BLAST alignment against six public databases. Specifically, 21,950 (67.27%) genes were annotated in the Gene Ontology (GO) database, 14,034 (43.01%) in KEGG, 28,678 (87.89%) in EggNOG, 31,944 (97.89%) in NR, 25,806 (79.08%) in Swiss-Prot, and 26,098 (79.98%) in Pfam (Figure S2). Principal component analysis (PCA) revealed that the three biological replicates from each tissue group clustered closely together, indicating good reproducibility and reliability of the transcriptomic data (Figure 3A).
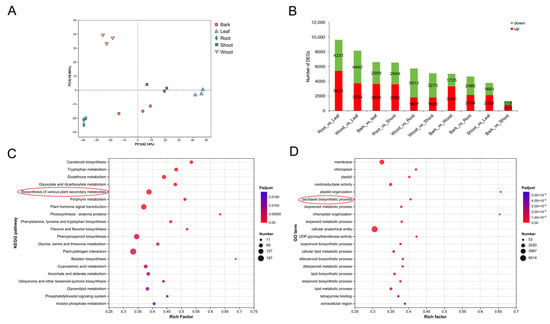
Figure 3.
Analysis of differentially expressed genes (DEGs). (A) PCA of transcriptome data from the 15 samples. (B) The statistics of up- and down-regulated DEGs in the different comparisons. (C) Kyoto Encyclopedia of Genes and Genomes enrichment analysis of DEGs. (D) Gene Ontology enrichment analysis of DEGs.
2.4. Analysis of DEGs in Different Tissues
Based on transcripts per million (TPM) values, gene expression patterns were analyzed across different tissues (Table S4). A total of 13,325 genes were found to be differentially expressed (DEGs) in at least one of the pairwise comparisons, including root/leaf, wood/leaf, bark/leaf, root/shoot, wood/root, wood/shoot, bark/wood, bark/root, shoot/leaf, and bark/shoot. The numbers of upregulated and down-regulated genes in each comparison are presented in Figure 3B.
To better understand the biological functions of these DEGs, KEGG and Gene Ontology (GO) enrichment analyses were performed. The KEGG pathway analysis revealed significant enrichment in pathways related to plant–pathogen interactions, phenylpropanoid biosynthesis, plant hormone signal transduction, and the biosynthesis of various plant secondary metabolites (Figure 3C). GO enrichment analysis identified representative terms, such as cellular anatomical entity, membrane, and oxidoreductase activity. Notably, 53 DEGs were specifically enriched in the paclitaxel biosynthetic process, highlighting the relevance of these genes to the targeted metabolic pathway (Figure 3D).
2.5. Integrated Transcriptome and Metabolome Analyses of Paclitaxel Pathways
A total of 8 upstream genes and 18 downstream genes of paclitaxel biosynthesis pathway genes were identified. Paclitaxel and several taxoids were found to significantly accumulate in the leaves, bark, and shoot. In contrast, paclitaxel derivatives, such as taxayunnansin A, decinnamoltaxinine J, and taxol B, were primarily accumulated in the root (Figure 4B). With the exception of FPP, BAPT, and T2′H (Figure 4A), most of the paclitaxel biosynthesis genes exhibited the lowest expression in wood. Interestingly, paclitaxel, its intermediates, and derivative metabolites correspondingly also had low levels of accumulation in wood (Figure 4B) indicating a strong correlation between gene expression and metabolite distribution across different tissues.
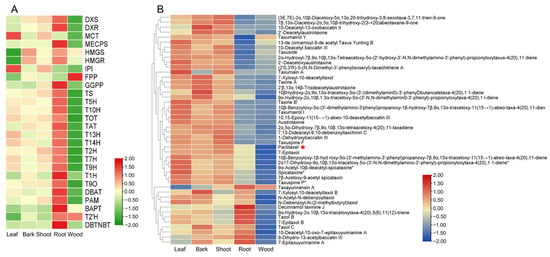
Figure 4.
Integrated metabolomic and transcriptomic analysis of paclitaxel biosynthesis. (A) Differential expression of genes involved in paclitaxel biosynthesis from the five tissues. (B) Differential accumulation of taxoids from the five tissues.
2.6. Gene Co-Expression Network Associated with Paclitaxel Biosynthesis
To identify key TFs that might play important roles in paclitaxel biosynthesis, WGCNA was performed. Based on the expression profiles, WGCNA clustered all DEGs into 11 distinct co-expression modules (Figure 5A, Tables S5 and S6). Modules with similar expression patterns were positively correlated, whereas those with contrasting patterns were negatively correlated (Figure 5A,B). The size of these modules ranged from 4851 eigengenes (turquoise module) to 102 eigengenes (purple). The module–sample relationships showed that the yellow and brown modules were positively correlated with root (Figure 5C). Yellow, brown, and turquoise modules contained 12, 3, and 1 paclitaxel downstream biosynthesis genes, respectively (Table S7). Therefore, the yellow module was selected as the key module for further investigation into the regulatory network of paclitaxel biosynthesis. Within the yellow module, 17 TFs were identified as being co-expressed with paclitaxel biosynthesis genes, including 12 MYB, 3 ERF, and 2 bHLH (Figure S3). Interestingly, two TFs (hds060980_TmMYB3 and hds069200_bHLH46) have been previously reported to regulate paclitaxel biosynthesis [46,49], supporting the reliability of our WGCNA results.
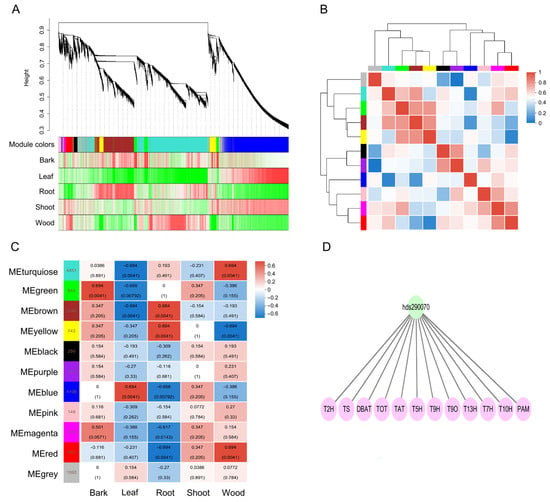
Figure 5.
WGCNA of 13,352 DEGs. (A) Gene dendrogram and module colors in blocks. (B) Correlation between modules. (C) Correlations between modules and samples. (D) Regulatory network involved in paclitaxel biosynthesis.
We identified 293 AP2/ERF family genes in the T. wallichiana genome (Figure 6A and Table S8). A total of 293 AP2/ERF family genes were unevenly distributed across the 12 T. wallichiana chromosomes (Figure S4). An ERF TF, hds290070, was renamed as ERF68 and was selected for further study (Figure 5D). To determine its subcellular localization, the GFP-fused ERF68 was transiently produced in tobacco leaves. The results show that ERF68 is a nucleus-localized protein (Figure 6B).
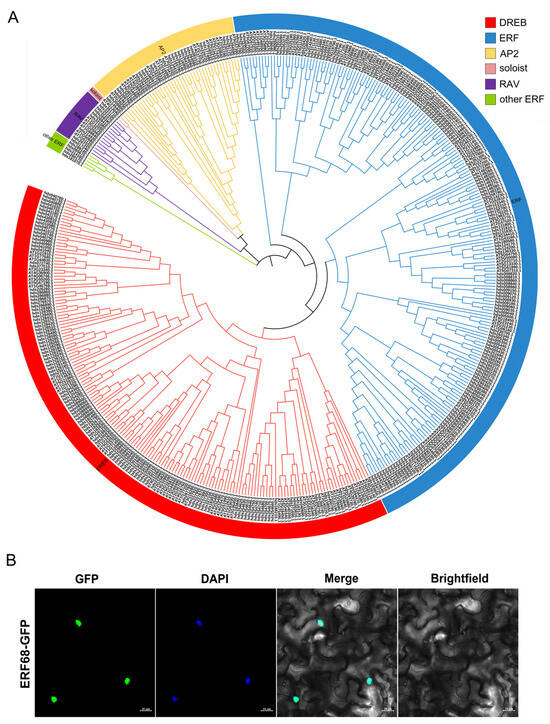
Figure 6.
Phylogenetic analysis of AP2/ERF family genes in the T. wallichiana genome and subcellular localization assay of ERF68. (A) The phylogenetic tree of AP2/ERF transcription factors in T. wallichiana. (B) Subcellular localization of ERF68.
2.7. ERF68 Enhances the Expression of T2H
To evaluate the role of ERF68 in Taxus, the promoter sequences of 12 paclitaxel biosynthesis pathway genes were analyzed (Figure 7A, Table S9). GCC-box and CRT/DRE were identified in the promoter of TS. GCC-box was identified in the promoter of T2H. The Y1H assays confirmed the interaction of ERF68 and T2H, indicating that ERF68 may regulate the expression of T2H by directly binding its promoter (Figure 7B). Furthermore, dual-luciferase reporter assays in tobacco leaves showed that ERF68 significantly activated T2H expression (Figure 7C).
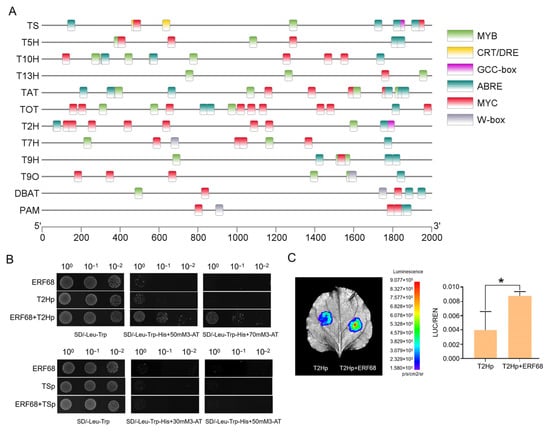
Figure 7.
ERF68 regulates the expression of T2H. (A) Screening of TF-binding elements in the promoter regions of TS, T5H, T13H, T10H, TAT, T2H, T7H, T9H, T9O, DBAT, and PAM genes. (B) Yeast one-hybrid assay analysis of the interaction between ERF68 and the promoters of T2H and TS. (C) Dual-luciferase assays in tobacco leaves show that ERF68 activates the promoter activity of T2H. Each value is the mean ± SD of three biological repeats. “*” indicates a significant difference of p < 0.05.
3. Discussion
Paclitaxel, a well-known anticancer agent, inhibits cancer cell proliferation by stabilizing microtubules [5]. Since its approval for clinical application by the U.S. Food and Drug Administration (FDA), extensive research has been devoted to elucidating its biosynthetic and metabolic regulation in Taxus species [2,51]. With the recent release of Taxus genome sequences, the biosynthetic pathway of paclitaxel has been largely deciphered [13,14,15]. Transcriptome profiling was conducted for different tissues of Taxus [14,15,52,53,54]. However, the tissue-specific accumulation patterns of paclitaxel and related taxoids in Taxus remain incompletely understood [52,53,54].
Over the past 60 years, nearly 600 taxoids—including paclitaxel, cephalomannine, 10-deacetylbaccatin III (10-DAB), baccatin III, and others—have been isolated from the Taxus genus [36]. Previous studies have reported differences in the accumulation of paclitaxel and taxoids between different stem tissues. Paclitaxel and 10-DAB were mainly accumulated in the phloem and bark, whereas baccatin III showed high accumulated in wood tissues [46,55]. In this study, we employed an integrative approach combining widely targeted metabolomics and transcriptomics to investigate paclitaxel biosynthesis and tissue-specific accumulation across five major tissue types—leaf, shoot, bark, wood, and root—of T. chinensis var. mairei. The resulting datasets provide a valuable resource for analyzing the tissue distribution patterns of taxiods and identifying key transcription factors regulating their biosynthesis. This can help to elucidate secondary metabolite regulatory networks and guide breeding strategies aimed at enhancing metabolite content in Taxus.
Using a UPLC-MS/MS-based widely targeted metabolomics approach, we identified 1616 DAMs that were differential accumulated in at least two tissues. These DAMs were grouped into two major clusters, each representing distinct tissue accumulation patterns. Cluster I (mainly enriched in the root, wood, and bark) was involved in terpenoid backbone biosynthesis, while Cluster II (enriched in the leaf, shoot, and bark) was associated with diterpenoid biosynthesis. This suggests that paclitaxel biosynthesis may mainly synthesize in the root or wood, and that its intermediates or end products may be transported to the leaf, shoot, and bark tissues. Referring to the T. wallichiana genome [14], the transcriptional difference of five tissues was analyzed. A total of 13,325 DEGs were identified across tissue comparisons. KEGG and GO enrichment analyses revealed a significant involvement of DEGs in secondary-metabolite biosynthesis pathways, including the paclitaxel biosynthetic process. Most paclitaxel biosynthetic genes showed a high expression in the root, yet paclitaxel and major taxoids were highly accumulated in the leaf, shoot, and bark, while paclitaxel derivatives, such as decinnamoltaxinine J, taxol B, and 7-epitaxol B, were highly accumulated in the roots. The discrepancy between gene expression and metabolite accumulation implies the transport of taxoids across tissues, potentially as a mechanism to mitigate cytotoxicity by relocating paclitaxel and related compounds from the roots to aerial tissues [46,48,55].
Previous studies have identified transcription factors from the MYB, ERF, WRKY, and bHLH families as regulators of paclitaxel biosynthesis [41,42,43,44,45,46,47,48,49]. In this study, we constructed a transcriptional regulatory network using WGCNA. This network not only validated previously reported regulators, but also identified several novel transcription factors potentially involved in paclitaxel biosynthesis. For instance, earlier studies reported that the MYB transcription factor TmMYB3 regulates TS and TBT expression in T. media [46]. In our dataset, we confirmed the co-expression between the homolog of TmMYB3 (hds060980) and TS, further validating the reliability of our data. Moreover, 15 previously unreported TFs were implicated in regulating paclitaxel biosynthesis. Sun et al. reported a regulatory network, including 10 enzyme genes of the paclitaxel pathway and 28 TFs [50]. The regulatory network we constructed involved 12 genes of the paclitaxel pathway, which was more than that reported by Sun et al. Notably, we confirmed the regulatory role of ERF68 on T2H expression using yeast one-hybrid (Y1H) assays and dual-luciferase (LUC) analysis. To date, only two ERF TFs—TcERF12 and TcERF15—have been functionally characterized in paclitaxel biosynthesis regulation, acting as a negative and a positive regulator of TS [36]. The activation effect of our ERF68 on the paclitaxel biosynthesis gene T2H revealed a new mechanism underlying the transcriptional regulation of paclitaxel biosynthesis.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Five-year-old T. chinensis var. mairei seedlings were grown in the greenhouse of the Chinese Academy of Forestry. The leaves, shoots, barks, woods, and roots were collected and immediately flash-frozen in liquid nitrogen from three T. mairei plants with a similar growth status. All samples were stored at −80 °C for subsequent metabolomic analysis and transcriptomic analysis.
4.2. Metabolite Extraction and Detection
Metabolite extraction and detection were provided by Wuhan Maiwei Metabolic Biotechnology Co., Ltd. (Wuhan, China). The tissue samples were freeze-dried and ground into a fine powder. A total of 50 mg of powder was extracted with 1.2 mL of 70% aqueous methanol. The mixture was vortexed every 30 min for 30 s, 6 times in total, and then centrifuged at 12,000 rpm for 3 min. Supernatant was collected and filtered through a microporous filtration membrane (0.22 µm pore size) for ultra-performance liquid chromatography tandem mass spectrometry (UPLC−MS/MS) analysis.
The composition of the extracts was determined using ultra-performance liquid chromatography coupled with tandem mass spectrometry. The liquid chromatography column was Agilent (Santa Clara, CA, USA) SB-C18 1.8 µm, 2.1 mm × 100 mm. The mobile phase consisted of a 0.1% aqueous solution of formic acid (A) and a solution of acetonitrile (B). The gradient program was set according to the following conditions: 0 min, 5% of solvent B; 0~9 min, 5 to 95% of solvent B; 9~10 min, 95% of solvent B; 10~11 min, 5% of solvent B; and 11~14 min, 5% of solvent B. The flow rate was maintained at 0.35 mL/min, the column temperature was set at 40 °C, and the injection volume was 2 μL. MS/MS analysis conditions were as follows: electrospray ionization temperature was set at 500 °C; the ion spray voltages were set at 5500 V (positive ion mode) and 4500 V (negative ion mode); the ion source gas I, gas II, and curtain gas were set to 50, 60, and 25 psi, respectively; and the collision-induced ionization parameter was set high. The triple-quadrupole (QQQ) scans were performed in the multiple-reaction monitoring (MRM) mode with collision gas (nitrogen) set to medium. The declustering potential (DP) and collision energy (CE) for individual MRM transitions were performed based on the optimized DP and CE. A specific set of MRM transitions was monitored for each period according to the metabolites eluted during this period.
4.3. Analysis of Widely Targeted Metabolomic Data
PCA was implemented using the stats package [56] in R (version 4.1.2) after data preprocessing was performed using unit variance scaling (UV) normalization. The cluster heatmap was created using Metware Cloud (https://cloud.metware.cn) (accessed on 11 January 2025). OPLS-DA was generate using the OPLSR Anal function in the R package MetaboAnalystR (version 1.0.1) [57]. Data preprocessing was performed using logarithmic normalization and zero-centered (Ctr) before performing OPLS-DA. A one-way analysis of variance (ANOVA) was carried out to compare the content differences of metabolites between pairwise comparisons (root/leaf, wood/leaf, bark/leaf, root/shoot, wood/root, wood/shoot, bark/wood, bark/root, shoot/leaf, and bark/shoot) using R (version 3.5.1). Multivariate statistical analysis with threshold values of VIP ≥ 1 (VIP based on OPLS-DA) and a p-value < 0.05 was performed to analyze tissue DAMs. The relative content of all DAMs was pretreated with UV, and then K-means clustering analysis was performed using R (version 3.6.2). The metabolites were annotated using the KEGG database (http://www.kegg.jp/kegg/compound/) (accessed on 8 July 2024) and mapped to the KEGG pathway database (http://www.kegg.jp/kegg/pathway.html) (accessed on 8 July 2024).
4.4. RNA Extraction, Library Construction, and RNA-Seq
Total RNA was extracted from five tissues (leaves, shoot, bark, wood, and root) of T. chinensis var. mairei with a total RNA extraction kit (Majorbio, Shanghai, China). Purity and quantity of RNA were assessed by the 5300 Bioanalyser (Agilent Technologies, Santa Clara, CA, USA) and Nano Drop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The sequencing library was constructed following illumina® Stranded mRNA Prep, Ligation (illumina, San Diego, CA, USA) using 1 µg of RNA from each sample. RNA sequencing was performed on the Nova Seq × Plus platform (illumina, San Diego, CA, USA). The raw paired-end reads were trimmed and quality-controlled by fastp (https://github.com/OpenGene/fastp) (accessed on 18 September 2024) with default parameters. The clean reads were mapped to the T. wallichiana reference genome [14] on HISAT2 version 2.2.1 [58].
4.5. Differential Expression Analysis and Functional Enrichment
PCA was conducted on the online tool Majorbio Cloud Platform (https://cloud.majorbio.com/page/tools/) (accessed on 18 September 2024). To identify DEGs between different tissues, the expression level of each transcript was quantified according to the read counts. RSEM [59] was used to quantify gene abundances. Essentially, differential expression analysis was performed using DESeq2 (Version 1.42.0) [60]. The BH method (Benjamini–Hochberg, which is the same as FDR) was used for multiple testing correction. And the genes with |log2FC| ≧ 1 and FDR < 0.05 were identified DEGs. In addition, GO and KEGG functional enrichment analyses were subsequently carried out by Goatools (Version 1.4.4) [61] and Python scipy software (Version1.0.0), respectively.
4.6. WGCNA Analysis
WGCNA analysis was performed on the online tool Majorbio Cloud Platform (https://cloud.majorbio.com/page/tools/) (accessed on 30 October 2024) with the TPM of DEGs as the input [62,63]. The co-expression modules were obtained using the automatic network construction function (blockwise Modules) with a soft threshold power of 5, network type was signed, merge CutHeight was 0.25, and min module size was 500. Eigengene values were calculated for each module based on the Spearman’s correlation. The weighted value of the correlation coefficient was used in the WGCNA analysis, which made the connections between genes in the network obey scale-free networks. The hierarchical clustering tree was constructed according to the correlation coefficient between genes. Genes with similar expression patterns were grouped into the same module. The module containing paclitaxel biosynthesis genes was selected for analysis. The networks were visualized by Cytoscape software (version 3.7.1).
4.7. Identification of the AP2/ERF Family Member
The hidden Markov model (HMM) of the AP2 domain (PF00847) obtained from the Pfam database (http://pfam.xfam.org/) (accessed on 26 May 2024) was used to conduct the putative AP2 proteins search in T. wallichiana by Tbtoolsv1.098696 software. And the Arabidopsis thaliana AP2/ERF protein sequences were downloaded from the PlantTFDB database and used as a query to search against AP2/ERF candidate proteins in T. wallichiana by BLASTp. All putative AP2/ERF proteins were further confirmed using PlantTFDB, SMART (http://smart.embl.de/) (accessed on 24 April 2025), and InterPro tools (http://pfam.xfam.org/) (accessed on 16 April 2025). A phylogenetic tree was constructed by MEGA7 software [64] using the Neighbor-Joining (NJ) method with 1000 bootstraps. Then, the phylogenetic tree was colored by the online website of iTOL (https://itol.embl.de/) (accessed on 23 April 2025). All identifiedAP2/ERF genes were mapped to the T. wallichiana chromosomes using Tbtoolsv1.098696 software.
4.8. Subcellular Localization
The open reading frame of ERF68 was cloned and inserted into the pCAMBIA1300 vector, generating 35S-ERF68:GFP. 35S-ERF68:GFP and 35S:GFP were separately transiently expressed in Nicotiana benthamiana leaves via Agrobacterium tumefaciens strain GV3101. After 48–72 h, the infiltrated leaves were collected and incubated with a phosphate-buffered saline containing 4′,6′-diamidino-2-phenylindole (DAPI). The fluorescence signals were detected using a confocal microscope (LSM 880, Zeiss, Baden Wurttemberg, Germany). The primers used for subcellular location are provided in Table S10.
4.9. Promoter Isolation and Cis-Element Scanning
Genomic DNA was isolated from T. mairei root using the classical cetyl trimethyl ammonium bromide method. The 2000 bp promoter sequences of TS, T2H, T5H, T7H, T9H, T10H, T13H, TAT, T9O, DBAT, PAM, and TOT were extracted according to the T. mairei genome [14]. Then, the promoter sequences were uploaded and scanned using the PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (accessed on 29 March 2025) and visualized using TBtools-II (Toolbox for Biologists) v2.152 software.
4.10. Yeast One-Hybrid Assays
The promoters of T2H and TS were cloned and inserted into the PHIS2 vector, respectively. The coding region of ERF68 was inserted into the pGADT7 vector. The resulting vector was co-transformed into a yeast Y187 cell and grown on SD/-Leu/-Trp medium. The activation activity was examined on the SD/-Leu/-Trp/-His/-3AT medium. The primers are shown in Table S10.
4.11. Dual-Luciferase Reporter Assay
The promoter of T2H was inserted into the pGreen 0800-LUC reporter vector. The coding region of ERF68 was cloned into the pGreenII 62-SK effector vector. The empty pGreenII 62-SK effector vector were used as a control. All completed constructs were co-transformed into N. benthamiana leaves by A. tumefaciens (GV3101)-mediated transient expression. Detection of Firefly Luciferase (LUC) and Renilla (REN) luciferase activities was performed using a Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA). The ratio of LUC to REN was used to analyze the results, and 3 biological repeats were set. The primers used for the dual-luciferase reporter assay are shown in Table S10.
5. Conclusions
In summary, we integrated metabolomic and transcriptomic approaches to investigate the biosynthesis and accumulation patterns of paclitaxel across five major tissue types (leaf, shoot, bark, wood, and root) in T. mairei. Most enzyme genes involved in paclitaxel biosynthesis showed the highest expression in the root. Paclitaxel was significantly enriched in the leaf, bark, and shoot. Paclitaxel derivatives, such as taxayunnansin A and taxol B, accumulated primarily in the root. We constructed a regulatory network associated with paclitaxel biosynthesis. Furthermore, ERF68 was identified as a potential positive regulator of paclitaxel biosynthesis. These findings offer valuable insights into the regulatory mechanisms underlying paclitaxel production and provide a foundation for future efforts in the genetic improvement and metabolic engineering of Taxus species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14121775/s1, Figure S1. The total ion chromatograms (TICs) of the mixed-sample extracts; Figure S2. The number of genes annotated by different databases, including GO, KEGG, EggNOG, NR, Swiss-Prot, and Pfam; Figure S3. Regulatory network involved in paclitaxel biosynthesis; Figure S4. The distribution of AP2/ERF genes on 12 T. wallichiana chromosomes; Table S1. The detailed information of metabolite annotations; Table S2. The detailed information of the RNA-sequencing; Table S3. Mapping rates for each sample; Table S4. The detailed information of all genes; Table S5. Members in modules; Table S6. The connectivity of co-expressed genes in different modules; Table S7. Modules of paclitaxel biosynthesis genes; Table S8. The ERF family in T. wallichiana; Table S9. The promoter sequences of the paclitaxel biosynthesis genes; Table S10. The primer sequences used in the present study.
Author Contributions
Conceptualization, D.Q.; writing—original draft preparation, L.J.; writing—review and editing, F.S., I.W., A.H. and D.Q.; Experiment: L.J., X.J. and W.W.; Data analysis, L.J., Y.L., F.S., F.X., I.W., A.H. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (32171809; 31170628), Natural Science Foundation of Henan (252300420212).
Data Availability Statement
All data supporting the findings of this study are included in the article/Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hao, D.; Xiao, P.; Huang, B.; Ge, G.; Yang, L. Interspecifc relationships and origins of Taxaceae and Cephalotaxaceae revealed by partitioned Bayesian analyses of chloroplast and nuclear DNA sequences. Plant Syst. Evol. 2008, 276, 89–104. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Q.; Zhao, Y.; Xiong, J.; Wang, F.; Zhang, T.; Zhang, C. Extraction, purification, and biological activities of polysaccharides from branches and leaves of Taxus cuspidata S. et Z. Molecules 2019, 24, 2926. [Google Scholar] [CrossRef]
- Zhan, X.; Liang, X.; Lin, W.; Ma, R.; Zang, Y.; Wang, H.; Wang, L.; Yang, Y.; Shen, C. Cell type specific regulation of phenolic acid and flavonoid metabolism in Taxus mairei leaves. Ind. Crops Prod. 2024, 219, 118975. [Google Scholar] [CrossRef]
- Fuchs, D.A.; Johnson, R.K. Crtologic Evidence that Taxol, an antineoplastic agent from Taxus brevifolia, acts as a mitotic spindle. Cancer Treat. Rep. 1978, 62, 1219–1222. [Google Scholar] [PubMed]
- Perez-Matas, E.; Hanano, A.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazon, J. Insights into the control of taxane metabolism: Molecular, cellular, and metabolic changes induced by elicitation in Taxus baccata cell suspensions. Front. Plant Sci. 2022, 13, 942433. [Google Scholar] [CrossRef]
- Wuts, P.G. Semisynthesis of Taxol. Curr. Opin. Drug Discov. Dev. 1998, 1, 329–337. [Google Scholar] [PubMed]
- Ajikumar, P.K.; Xiao, W.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef]
- Zhou, K.; Qiao, K.; Edgar, S.; Stephanopoulos, G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Walls, L.E.; Malcı, K.; Nowrouzi, B.; Li, R.A.; d’Espaux, L.; Wong, J.; Dennis, J.A.; Semião, A.J.C.; Wallace, S.; Martinez, J.L.; et al. Optimizing the biosynthesis of oxygenated and acetylated Taxol precursors in Saccharomyces cerevisiae using advanced bioprocessing strategies. Biotechnol. Bioeng. 2021, 118, 279–293. [Google Scholar] [CrossRef]
- De-La-Peña, R.; Sattely, E.S. Rerouting plant terpene biosynthesis enables momilactone pathway elucidation. Nat. Chem. Biol. 2021, 17, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, W.; Luo, Z.; Li, C.X.; Huang, Q.; Xu, J.H. Improving solubility and copy number of taxadiene synthase to enhance the titer of taxadiene in Yarrowialipolytica. Sythrtic Syst. Biotechnol. 2023, 8, 331–338. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Cheng, J.; Jiang, H. A new era for paclitaxel biosynthesis is coming. Mol. Plant 2024, 17, 370–371. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Liu, X.; Zhu, X.; Li, Z.; Chu, H.; Wang, Q.; Lou, Q.; Cai, B.; Yang, Y.; et al. Chromosome-level genome of Himalayan yew provides insights into the origin and evolution of the paclitaxel biosynthetic pathway. Mol. Plant 2021, 14, 1199–1209. [Google Scholar] [CrossRef]
- Xiong, X.; Guo, J.; Liao, Q.; Li, Y.; Zhou, Q.; Bi, G.; Li, C.; Du, R.; Wang, X.; Sun, T.; et al. The Taxus genome provides insights into paclitaxel biosynthesis. Nat. Plants. 2021, 7, 1026–1103. [Google Scholar] [CrossRef]
- Song, C.; Fu, F.; Yang, L.; Niu, Y.; Tian, Z.; He, X.; Yang, X.; Chen, J.; Sun, W.; Wang, T.; et al. Taxus yunnanensis genome offers insights into gymnosperm phylogeny and taxol production. Commun. Biol. 2021, 4, 1203. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Ketchum, R.E.; Long, R.M.; Kaspera, R.; Wildung, M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006, 5, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Jiang, X.; Lian, J. Intricate metabolic network for paclitaxel biosynthesis. BioDesign Res. 2024, 6, 0035. [Google Scholar] [CrossRef]
- Hefner, J.; Ketchum, R.E.; Croteau, R. Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for taxol production. Arch. Biochem. Biophys. 1998, 360, 62–74. [Google Scholar] [CrossRef]
- Wildung, M.R.; Croteau, R. A cDNA Clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of taxol biosynthesis. J. Biol. Chem. 1996, 271, 9201–9204. [Google Scholar] [CrossRef]
- Escrich, A.; Jonguitud-Borrego, N.; Malcı, K.; Sanchez-Muñoz, R.; Palazon, J.; Rios-Solis, L.; Moyano, E. A novel step towards the heterologous biosynthesis of paclitaxel: Characterization of T1βOH taxane hydroxylase. Metab. Eng. 2024, 85, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wiese, L.; Fang, H.; Alseekh, S.; de-Souza, L.P.; Scossa, F.; Molloy, J.; Christmann, M.; Fernie, A.R. Synthetic biology identifies the minimal gene set required for paclitaxel biosynthesis in a plant chassis. Mol. Plant 2023, 16, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Chau, M. Molecular Cloning and Characterization of Three Enyzmes Involved in Taxol/Taxoid Biosynthesis: Taxoid 2α-Hydroxylase, Taxoid 7β-Hydroxylase, and Taxoid 5α-O-Acetyltransferase; Washington State University: Pullman, WA, USA, 2004; p. 3145784. [Google Scholar]
- Jennewein, S.; Long, R.M.; Williams, R.M.; Croteau, R. Cytochrome P450 taxadiene 5α-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of taxol biosynthesis. Chem. Biol. 2004, 11, 379–387. [Google Scholar] [CrossRef]
- Jiang, B.; Gao, L.; Wang, H.; Sun, Y.; Zhang, X.; Ke, H.; Liu, S.; Ma, P.; Liao, Q.; Wang, H. Characterization and heterologous reconstitution of Taxus biosynthetic enzymes leading to baccatin III. Science 2024, 383, 622–629. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Su, Z.; Xiong, L.; Wang, P.; Lei, W.; Yan, X.; Ma, D.; Zhao, G.; Zhou, Z. Biosynthesis of the highly oxygenated tetracyclic core skeleton of Taxol. Nat. Commun. 2024, 15, 2339. [Google Scholar] [CrossRef]
- Schoendorf, A.; Rithner, C.D.; Williams, R.M.; Croteau, R.B. Molecular cloning of a cytochrome P450 taxane 10β-hydroxylase cDNA from Taxus and functional expression in yeast. Proc. Natl. Acad. Sci. USA 2001, 98, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, S.; Rithner, C.D.; Williams, R.M.; Croteau, R.B. Taxol biosynthesis: Taxane 13α-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc. Natl. Acad. Sci. USA 2001, 98, 13595–13600. [Google Scholar] [CrossRef]
- Walker, K.; Croteau, R. Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 583–587. [Google Scholar] [CrossRef]
- Walker, K.; Croteau, R. Taxol biosynthesis: Molecular cloning of a benzoyl-CoA: Taxane 2α-O-benzoyltransferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 13591–13596. [Google Scholar] [CrossRef]
- Walker, K.D.; Klettke, K.; Akiyama, T.; Croteau, R. Cloning, heterologous expression, and characterization of a phenylalanine aminomutase involved in Taxol biosynthesis. J. Biol. Chem. 2004, 279, 53947–53954. [Google Scholar] [CrossRef]
- Ramírez-Estrada, K.; Altabella, T.; Onrubia, M.; Moyano, E.; Notredame, C.; Osuna, L.; Bossche, R.V.; Goossens, A.; Cusido, R.M.; Palazon, J. Transcript profiling of jasmonate-elicited Taxus cells reveals a β-phenylalanine-CoA ligase. Plant Biotechnol. J. 2016, 14, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Fujisaki, S.; Long, R.; Croteau, R. Molecular cloning and heterologous expression of the C-13 phenylpropanoid side chain-CoA acyltransferase that functions in Taxol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12715–12720. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Muñoz, R.; Perez-Mata, E.; Almagro, L.; Cusido, R.M.; Bonfill, M.; Palazon, J.; Moyano, E. A novel hydroxylation step in the taxane biosynthetic pathway: A new approach to paclitaxel production by synthetic biology. Front. Bioeng. Biotechnol. 2002, 8, 410. [Google Scholar] [CrossRef]
- Walker, K.; Long, R.; Croteau, R. The final acylation step in taxol biosynthesis: Cloning of the taxoid C13-side-chain N-benzoyltransferase from Taxus. Proc. Natl. Acad. Sci. USA 2002, 99, 9166–9171. [Google Scholar] [CrossRef]
- Lange, B.M.; Conner, C.F. Taxanes and taxoids of the genus Taxus—A comprehensive inventory of chemical diversity. Phytochemistry 2021, 190, 112829. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; Chordia, M.D.; Kingston, D.G.I. Efficient conversion of cephalomannine to paclitaxel and 3′-N-acyl-3′-N-debenzoylpaclitaxel analogs. J. Org. Chem. 1997, 62, 3775–3778. [Google Scholar] [CrossRef]
- Gao, F.; Wang, D.; Huang, X. Synthesis, isolation, stereo structure and cytotoxicity of paclitaxel analogs from cephalomannine. Fitoterapia 2013, 90, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, J.L.; Miller, R.W.; Powell, R.G.; Smith, C.R. 19-hydroxybaccatin III, 10-deacetylcephalomannine, and 10-deacetyltaxol: New antitumor taxanes from Taxus wallichiana. J. Nat. Prod. 1981, 44, 312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.; Zhou, M.; Hao, X.; Zhu, X. An effective method to produce 7-epitaxol from taxol in HCO3−. Bioorganic Med. Chem. Lett. 2020, 30, 127285. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.; Nie, L.; Chen, Q.; Xu, X.; Yu, L.; Fu, C. Two jasmonate-responsive factors, TcERF12 and TcERF15, respectively act as repressor and activator of tasy gene of taxol biosynthesis in Taxus chinensis. Plant Mol. Biol. 2015, 89, 463–473. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, X.; Chen, Y.; Wei, M.; Liao, W.; Zhao, S.; Fu, C.; Yu, L. TcMYC2a, a basic helix–loop–helix transcription factor, transduces JA-signals and regulates taxol biosynthesis in Taxus chinensis. Front. Plant Sci. 2018, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Y.; Nie, L.; Jin, X.; Liao, W.; Zhao, S.; Fu, C.; Yu, L. Transcriptome-wide identification and screening of WRKY factors involved in the regulation of taxol biosynthesis in Taxus chinensis. Sci. Rep. 2018, 8, 5197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Zhang, M.; Zhang, W.; Ou, Z.; Peng, Z.; Fu, C.; Zhao, C.; Yu, L. Salicylic acid-responsive factor TcWRKY33 positively regulates taxol biosynthesis in Taxus chinensis in direct and indirect ways. Front. Plant Sci. 2021, 12, 697476. [Google Scholar] [CrossRef]
- Cao, X.; Xu, L.; Li, L.; Wan, W.; Jiang, J. TcMYB29a, an ABA-responsive R2R3-MYB transcriptional factor, upregulates taxol biosynthesis in Taxus chinensis. Front. Plant Sci. 2022, 13, 804593. [Google Scholar] [CrossRef]
- Yu, C.; Luo, X.; Zhang, C.; Xu, X.; Huang, J.; Chen, Y.; Feng, S.; Zhan, X.; Zhang, L.; Yuan, H. Tissue-specific study across the stem of Taxus media identifies a phloem-specific TmMYB3 involved in the transcriptional regulation of paclitaxel biosynthesis. Plant J. 2020, 103, 95–110. [Google Scholar] [CrossRef]
- Yu, C.; Huang, J.; Wu, Q.; Zhang, C.; Li, X.; Xu, X.; Feng, S.; Zhan, X.; Chen, Z.; Wang, H. Role of female-predominant MYB39-bHLH13 complex in sexually dimorphic accumulation of taxol in Taxus media. Hortic. Res. 2022, 9, uhac062. [Google Scholar] [CrossRef]
- Yu, C.; Hou, K.; Zhang, H.; Liang, X.; Chen, C.; Wang, Z.; Wu, Q.; Chen, G.; He, J.; Bai, E. Integrated mass spectrometry imaging and single-cell transcriptome atlas strategies provide novel insights into taxoid biosynthesis and transport in Taxus mairei stems. Plant J. 2023, 115, 1243–1260. [Google Scholar] [CrossRef]
- Zhan, X.; Qiu, T.; Zhang, H.; Hou, K.; Liang, X.; Chen, C.; Wang, Z.; Wu, Q.; Wang, X.; Li, X. Mass spectrometry imaging and single-cell transcriptional profiling reveal the tissue-specific regulation of bioactive ingredient biosynthesis in Taxus leaves. Plant Commun. 2023, 4, 100630. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.S.; Jia, Y.; Chen, X.Y.; Chen, J.S.; Guo, Y.; Fu, F.F.; Xue, L.J. Regulatory microRNAs and phasiRNAs of paclitaxel biosynthesis in Taxus chinensis. Front. Plant Sci. 2024, 15, 11. [Google Scholar] [CrossRef]
- Kham, V.; Susan, C.R. Advancements in the understanding of Paclitaxel metabolism in tissue culture. Curr. Pharm. Biotechnol. 2007, 8, 219–236. [Google Scholar] [CrossRef]
- Kuang, X.; Sun, S.; Wei, J.; Li, Y.; Sun, C. Iso-seq analysis of the Taxus cuspidata transcriptome reveals the complexity of taxol biosynthesis. BMC Plant Biol. 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- He, C.T.; Li, Z.L.; Zhou, Q.; Shen, C.; Huang, Y.Y.; Mubeen, S.; Yang, J.Z.; Yuan, J.G.; Yang, Z.Y. Transcriptome profiling reveals specific patterns of paclitaxel synthesis in a new Taxus yunnanensis cultivar. Plant Physiol. Biochem. 2018, 112, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Li, Z.L.; Huang, Q.M.; He, C.T.; Yang, Z.Y. Comparative transcriptome analysis revealed the tissue-specific accumulations of taxanes among three experimental lines of Taxus yunnanensis. J. Agric. Food Chem. 2018, 66, 10410–10420. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.; Raizada, M.N. Sites of biosynthesis and storage of Taxol in Taxus media (Rehder) plants: Mechanism of accumulation. Phytochemistry 2020, 175, 112369. [Google Scholar] [CrossRef]
- Gentleman, R.; Ihaka, R. R: A Language and Environment for Statistical Computing. Computing 2011, 1, 12–21. [Google Scholar]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A python library for gene ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Shi, C.; Liu, L.; Han, J.; Yang, Q.; Wang, Y.; Li, X.; Fu, W.; Gao, H.; Huang, H.; et al. Majorbio cloud 2024: Update single-cell and multiomics workflows. iMeta 2024, 3, c217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).