Abstract
Seed deterioration is an inevitable process during storage, characterized by a gradual loss of germination capacity and eventual seed death, which poses challenges to seed longevity and the preservation of genetic resources. Understanding the molecular mechanisms driving seed aging and inherent resistance pathways, alongside developing innovative rejuvenation strategies for deteriorated seeds, is crucial for agricultural sustainability and germplasm banking. This review systematically examines (1) redox-regulated deterioration pathways involving reactive oxygen species (ROS) and macromolecular damage cascades, (2) anti-deterioration mechanisms mediated by the antioxidant system and macromolecular repair mechanisms, (3) genetic–epigenetic networks governing seed aging resistance, particularly ABA- and IAA-mediated signaling through ABI3/ABI5/LEC1 regulons, and (4) technological advances in seed priming that restore aged seeds via metabolic resetting and repair potentiation. By integrating multi-omics insights with physiological evidence, we propose a hierarchical model of seed deterioration and establish mechanistic links between priming interventions and longevity enhancement. These insights offer a theoretical framework for cultivating anti-deterioration crop varieties and developing seed longevity-enhancement technologies.
1. Introduction
Seeds, as the fundamental reproductive units of plants, play a pivotal role in the evolution and survival of higher plants [1]. Maintaining germination capacity serves as the foundation for fulfilling their reproductive function. Seed germination capacity peaks at physiological maturity or when dormant seeds attain maximum viability through after-ripening and/or stratification treatments. However, after this peak, seeds inevitably undergo progressive viability loss until eventual death—a natural process termed seed deterioration or aging. In agricultural production, deteriorated seeds not only exhibit compromised germination performance (e.g., delayed germination and reduced uniformity) but also impair seedling development through low establishment rates, uneven growth patterns, and heightened susceptibility to abiotic/biotic stresses [2]. Consequently, seed deterioration directly undermines reproductive success and diminishes agricultural value.
Although seed deterioration is inevitable, the rate of the process is determined by both intrinsic seed properties and extrinsic environmental factors. Seed deterioration is intricately associated with morphological, physiological, and biochemical alterations in stored seeds, including compromised seed coat structure, excessive reactive oxygen species (ROS) generation, lipid peroxidation, depletion of storage reserves, aberrant protein modifications, dysregulated gene expression and accumulated genetic damage [3]. Storage conditions—particularly ambient temperature, relative humidity, atmospheric composition, and microbial activity—are critical determinants of deterioration kinetics [4,5,6].
Current strategies to delay deterioration (e.g., low-temperature preservation, ultra-dry storage, anaerobic storage) focus on environmental modulation [4,5,6], yet these methods merely decelerate aging and prove ineffective for already deteriorated or low-vigor seeds. In contrast, seed priming has emerged as a robust intervention that reactivates repair mechanisms (e.g., DNA damage repair, protein refolding) and potentiates antioxidant responses in aged seeds [7]. Priming treatment significantly enhances germination synchrony, improves seedling stress tolerance and disease resistance, and elevates crop yield with enriched grain micronutrient profiles [8,9,10]. Notably, its efficacy in revitalizing deteriorated seeds has been validated across staple crops like maize and rice [11,12].
2. Determinants of Seed Deterioration
2.1. External Factors
Seed storage longevity is critically influenced by storage conditions, including temperature, relative humidity (RH), environmental gas composition, and biotic factors [13]. Based on desiccation sensitivity, seeds are categorized into three types: orthodox, intermediate, and recalcitrant [14]. Orthodox seeds can be desiccated to internal moisture contents below 12% (on a fresh weight basis) and survive long-term storage at subzero temperatures. Recalcitrant seeds cannot be stored in a conventional freezer, as they are unable to survive after drying and/or freezing at −20 °C. Intermediate seeds tend to age faster than orthodox seeds and may have a lifespan of only 5 years when stored at −20 °C. They achieve their greatest longevity when dried to equilibrium with 45–65% RH [15]. Notably, orthodox seeds are the most prevalent, particularly among cultivated plants, and have been the most extensively studied.
For orthodox seeds, seed moisture content (or equilibrium RH) and temperature are key factors influencing seed storage longevity. Based on the constant relationships among storage temperature, moisture content, and longevity, several general rules for seed storage have been proposed. Two simple and commonly applied rules include (1) James’ Rule: Ideal storage conditions require that the temperature (°F) plus the ambient RH (%) remain below 100. For example, at 50% RH, the storage temperature should be below 50 °F (10 °C) to achieve medium-term seed storage; (2) Harrington’s Rule: Within temperature ranges of 0–40 °C and moisture content ranges of 5–14%, the seed storage longevity approximately doubles for every 10 °F (5.6 °C) reduction in temperature and every 1% reduction in seed moisture content. Both rules emphasize the critical importance of low seed moisture content and low temperature for prolonging seed longevity, particularly highlighting the need to avoid the synergistic effects of high temperature and high moisture content [16]. It is noteworthy that the relationships among storage temperature, moisture content, and longevity are not necessarily constant or even linear at the scales on which these properties are measured. Based on the observed patterns of crop seed longevity under varying temperature and moisture conditions during storage, Ellis & Roberts developed a predictive log-linear regression equation for orthodox seeds using statistical methodologies [17,18]. This equation provides a more accurate representation of the quantitative relationships among storage temperature, seed moisture content, and longevity, indicating that longevity increases as both storage temperature and moisture content decrease [19,20]. Therefore, to facilitate research on seed longevity, accelerated seed deterioration (artificial aging, AA) methods have been developed. These techniques utilize high-temperature and high-humidity conditions to accelerate the deterioration process and shorten seed storage longevity for experimental purposes.
For recalcitrant and intermediate seeds, cryopreservation technologies represent the most efficient method to preserve viability. Cryopreservation involves storage at ultralow temperatures, often in liquid nitrogen (−196 °C). Rapidly advancing methods can be used to essentially stop water from freezing within recalcitrant seed cells and obviate lethal freezing damage. The technology requires careful dehydration of tissues and extremely rapid cooling [21].
RH serves as the primary driver of deterioration by mediating enzymatic reactivation and non-enzymatic oxidation. Elevated seed moisture accelerates deterioration through oxidative modifications (e.g., lipid peroxidation, protein carbonylation, DNA strand breaks) and microbial proliferation [22]. Under desiccated conditions (<5% moisture), molecular oxygen interacts with intracellular transition metals (e.g., Fe2+, Cu+) via Fenton/Haber–Weiss reactions, generating hydroxyl radicals (•OH) that oxidize macromolecules, thereby exacerbating cellular dysfunction [22].
The gas composition of seed storage containers has received considerable attention for its role in modulating longevity. Oxygen is hypothesized to accelerate seed deterioration through oxidative reactions. In general, compared to storage in air, seed survival is prolonged in nitrogen (N2), carbon dioxide (CO2), or vacuum environments, whereas elevated oxygen partial pressures accelerate the loss of viability [16].
Biotic stressors—including rodents (Rattus spp.), insects (Sitophilus zeamais), and fungal pathogens (Aspergillus spp.)—threaten stored seeds through distinct mechanisms: rodents and insects cause physical damage via consumption, while pathogens secrete hydrolases to degrade storage reserves (e.g., starch, lipids), depleting germination energy and inducing mycotoxin-mediated deterioration [23].
Additionally, seed processing technologies, including seed harvesting, drying, cleaning, packaging, and transportation, influence seed deterioration. For instance, mechanical injuries incurred during these processes not only directly accelerate seed aging but also enhance microbial invasion risks by compromising seed coat integrity. Furthermore, excessive, or overly rapid drying rates can compromise embryo cell integrity through membrane lipid peroxidation and protein denaturation, while insufficient drying predisposes seeds to metabolic reactivation and fungal proliferation.
2.2. Internal Factors
The seed coat (testa) serves as a critical protective barrier between the embryo and the external environment, regulating water and gas exchange primarily through the hilum—a specialized structure that facilitates photosynthetic product transport to the developing embryo during seed maturation. The structural integrity of the hilum directly modulates seed moisture content, with high-moisture seeds exhibiting accelerated deterioration rates due to enhanced enzymatic and oxidative reactions, rendering them unsuitable for long-term storage. Compromised seed coat integrity predisposes seeds to microbial colonization and accelerates deterioration by promoting premature mobilization of storage reserves (e.g., lipid hydrolysis, starch degradation). Hydrophobic substances in the seed coat, such as cuticular wax—a hydrophobic barrier synthesized during late seed maturation—restrict external water infiltration through the testa, thereby maintaining low seed moisture content at levels optimal for storage stability. Furthermore, the physical characteristics of the seed coat, including cuticular cracks, micropyle aperture size, and testa-cotyledon interfacial gaps, collectively govern seed permeability, biotic stress resilience, and long-term storage potential [24,25]. In summary, these factors collectively influence the protective functions of the seed coat, thereby regulating internal physiological activities within the seed.
Seed chemical composition significantly influences deterioration dynamics, particularly through the content and composition of storage reserves. Oils are prone to oxidation and rancidity, generating harmful aldehydes (e.g., malondialdehyde) and free fatty acids that induce cellular damage via membrane lipid peroxidation. Comparative studies reveal that low-lipid seeds (<10% lipid content) exhibit extended longevity compared to high-lipid counterparts (>20%), attributed to reduced oxidative substrate availability [26]. Non-storage reserve components—including chlorophyll, polysaccharides, and phenolic compounds—also modulate deterioration rates. Chlorophyll-retaining seeds demonstrate accelerated aging due to photooxidative stress from retained chloroplasts, which generate singlet oxygen (1O2) under light exposure [27,28]. Conversely, phenolic antioxidants like flavonoids mitigate oxidative damage by scavenging ROS and chelating transition metals.
The physiological state of seeds influences seed deterioration. During seed development, the dry weight of seeds increases significantly, reaching its maximum at the end of the grain-filling phase when seeds attain physiological maturity and their germination capacity peaks [29,30,31]. Premature harvest (before physiological maturity) often results in underdeveloped seed structures (e.g., incomplete testa lignification) and insufficient accumulation of storage reserves (e.g., proteins, lipids), leading to reduced germination rates and increased susceptibility to oxidative deterioration. Conversely, delayed harvest exposes seeds to field risks such as fungal infection (Aspergillus spp.), mechanical damage (e.g., threshing-induced fractures), and desiccation-induced fissures, which collectively contribute to poor germination performance, accelerated aging, and shortened storage longevity [32]. Therefore, seed maturity not only determines the initial germination vigor of stored seeds but also modulates their deterioration kinetics during storage.
The intracellular biochemical reactions of seeds critically influence seed deterioration. Two predominant hypotheses explain the relationship between embryonic cell biochemistry and aging: (1) Membrane lipid peroxidation hypothesis: Free radicals generated through autoxidation or enzymatic oxidation in seeds initiate oxidative attacks on polyunsaturated fatty acids (PUFAs) in membrane lipids. This process generates lipid hydroperoxides (LOOHs) that compromise plasma membrane integrity (e.g., increased electrolyte leakage), thereby accelerating deterioration [2]; (2) Mitochondrial theory of aging: In seed cells lacking chloroplasts, mitochondrial electron transport chains (ETCs) become the primary source of ROS (e.g., superoxide anion, H2O2). Cumulative ROS accumulation induces oxidative damage to mitochondrial macromolecules—including mtDNA deletions, cytochrome C oxidase inactivation, and cardiolipin peroxidation—which disrupts energy metabolism and accelerates senescence [33].
The genetic background of seeds constitutes the fundamental regulator of deterioration processes, as it governs seed structure, chemical composition, physiological state, and biochemical reactions. Hormonal signaling pathways—particularly abscisic acid (ABA) and indole-3-acetic acid (IAA)—orchestrate seed development and maturation by modulating expression of key regulatory genes (e.g., TRANSPARENT TESTA family for seed coat development), controlling enzymatic activity in secondary metabolism (e.g., chalcone synthase for flavonoid biosynthesis), and activating storage compound synthesis genes (e.g., oleosins for lipid body formation). Concurrently, molecular chaperones (heat shock proteins [HSPs]) and late embryogenesis abundant proteins (LEAs) are synthesized during seed development to stabilize proteomes and enhance desiccation tolerance, while antioxidant enzymes (e.g., superoxide dismutase [SOD], catalase [CAT]) are produced to bolster redox homeostasis [34]. These intrinsic factors collectively determine seed deterioration susceptibility through integrated genetic, biochemical, and biophysical mechanisms.
3. Morphological, Anatomical, and Physiological-Biochemical Changes in Aged Seeds
3.1. Morphological Changes
Seeds undergo significant morphological changes during storage. For example, rice seeds develop reddish husk pigmentation after long-term storage [1]. Structural modifications also occur internally with prolonged storage duration. Freshly harvested lentil (Lens culinaris) seeds exhibit olive-green cotyledons that gradually fade to light yellow and eventually turn dark brown after two years of storage, a phenomenon attributed to chlorophyll degradation in their cotyledons [35]. In watermelon (Citrullus lanatus) and melon (Cucumis melo L.) seeds stored for five years, non-viable seeds show pronounced separation between the seed coat and embryo. These deteriorated embryos appear shrunken and irregularly shaped, with structural alterations resulting from substrate depletion and cellular damage [36]. Therefore, the seed coat’s protective capacity becomes compromised.
3.2. Ultrastructural Changes
During the process of seed deterioration, ultrastructural changes occur in the organelles of embryo cells, including mitochondria, endoplasmic reticulum, and Golgi apparatus. Mitochondria, as the most important organelles for cellular energy metabolism, serve as the primary source of ROS. Seed deterioration is closely related to mitochondrial degradation. In this process, mitochondria in aged embryo cells become significantly enlarged and exhibit distorted outer membranes, while cristae become disorganized or even absent. These structurally compromised mitochondria render cells incapable of carrying out respiratory metabolism and producing ATP required for life activities, ultimately leading to cell dysfunction and apoptosis [37].
The Golgi apparatus and endoplasmic reticulum, which are critical sites for cell metabolism and protein synthesis, also undergo fragmentation or degradation in deteriorated embryo cells. Concurrently, lipid droplets fuse to form larger irregular bodies. These alterations disrupt material metabolism and protein synthesis, resulting in cellular dysfunction. Other ultrastructural changes include the dissolution of membrane-bound structures (e.g., protein bodies, vacuoles, and plasma membranes), cell wall contraction, and nuclear chromatin condensation with lobulation [38]. Collectively, these pathological changes induce cell death and progressive loss of embryo viability.
3.3. Physiological and Biochemical Changes
The physiological and biochemical changes associated with seed deterioration primarily include oxidative damage, stored-reserve consumption, and changes in plant hormone levels (Figure 1a). ROS chemically reacts with antioxidant enzymes, structural proteins, and genetic material, leading to the inactivation, instability, or even degradation of these substances. Damage to the antioxidant enzyme system arises from an imbalance between oxidants and antioxidants, with excessive ROS accumulation in mitochondria disrupting redox homeostasis [39]. In pigeon pea (Cajanus cajan) seeds subjected to accelerated deterioration treatment, the activities of SOD, CAT, and ascorbate peroxidase (APX) are significantly reduced [40]. Similarly, aged black pine (Pinus thunbergia) seeds exhibit diminished SOD, glutathione reductase (GR), and CAT activities, severely compromising their antioxidant defense system [41].
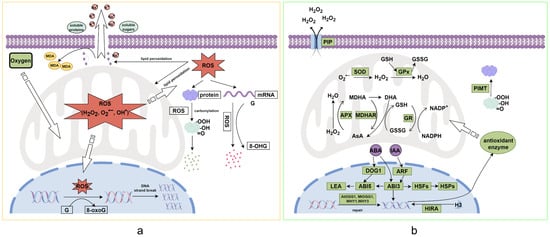
Figure 1.
Mechanisms of Seed Deterioration (a) and Anti-Deterioration (b) (By Figdraw 2.0). (a) Cellular Oxidative Damage: Oxygen molecules entering mitochondria undergo respiratory metabolism, concurrently generating reactive oxygen species (ROS: H2O2, O2·−, OH·). These ROS mediate multilevel oxidative damage: (i) In the nucleus, ROS oxidize guanine (G) to 8-oxo-7,8-dihydroguanine (8-oxoG), inducing DNA strand breaks and replication errors; (ii) In the cytoplasm, ROS catalyzes protein carbonylation through metal-catalyzed oxidation, rendering modified proteins prone to ubiquitin-proteasomal degradation; (iii) Cytosolic mRNA undergoes guanine oxidation to 8-hydroxyguanosine (8-OHG), destabilizing transcript integrity; (iv) Membrane phospholipids undergo peroxidation chain reactions, generating malondialdehyde (MDA) as a byproduct while compromising membrane semi-permeability, leading to electrolyte leakage and organelle dysfunction. (b) Cellular Anti-Deterioration Mechanisms: Phytohormone signaling networks (e.g., ABA, IAA) orchestrate counteractive responses by (i) Upregulating DNA repair enzymes (e.g., OGG1 glycosylase) and antioxidant genes (e.g., SOD, CAT, APX), enhancing ROS-scavenging capacity via mitochondrial Mn-SOD activation; (ii) Protein-L-isoaspartyl methyltransferase (PIMT) catalyzes the repair of isoaspartyl residues in damaged proteins through methylation-demethylation cycles, restoring structural conformation; (iii) Aquaporin-mediated ROS efflux channels facilitate H2O2 extrusion across plasma membranes, while glutathione-ascorbate redox cycles maintain cytoplasmic redox homeostasis. Abbreviations: 8-OHG: 8-hydroxyguanosine, 8-oxoG: 8-oxoguanine, ABA: abscisic acid, APX: ascorbate peroxidase, AsA: ascorbic acid, CAT: catalase, GPx: glutathione peroxidase, GR: glutathione reductase, GSH: glutathione, HSP: heat shock protein, IAA: auxin, LEA: late embryogenesis abundant protein, MDA: malondialdehyde, PIP: plasma membrane intrinsic protein, RFO: raffinose family oligosaccharide, ROS: reactive oxygen species, SOD: superoxide dismutase. The black arrow indicates the activation reaction, and the white wide arrow indicates the substance transportation.
ROS are the primary cause of DNA damage in aged seeds and can induce the hydroxylation modification of the eighth carbon of guanine, resulting in 8-oxoguanine (8-oxoG), which is the most common form of guanine modification and is highly prone to causing base mispairing and DNA single-strand breaks [42]. The accumulation of DNA damage in aged seeds leads to an increased frequency of chromosomal structural variations, such as the formation of bridges due to the chromosomal breakage-fusion-bridge cycle [43,44]. Due to the lack of specialized repair mechanisms, RNA is more sensitive to oxidative damage; the oxidation modification of guanine results in 8-hydroxyguanosine (8-OHG), which reduces mRNA stability and causes codon mismatch during translation, thereby affecting protein synthesis [45]. Oxidative damage to mRNA can cause the fragmentation observed in soybean seeds over 20 years of storage [46,47]. Previous studies have shown that seeds accumulate mRNAs during late seed maturation. Unlike the majority of RNAs in metabolically active cells, seed-stored mRNAs are long-lived, being able to survive for many years [40,41]. These long-lived mRNAs display the ability to survive desiccation and remain translatable in dry quiescent embryos. For seeds to germinate, translation of seed-stored mRNA into proteins is essential. The stored mRNA in dry seeds can be immediately utilized for translation to proteins required by the embryo cells in the pre-germination stage [48]. However, due to the poor stability of mRNA, it gradually becomes damaged and degraded during seed storage, which in turn affects the embryo cells’ vital activities. Therefore, the total amount and integrity of stored mRNA in seeds are positively correlated with seed longevity and can serve as predictive factors for the degree of deterioration in dried stored seeds [49,50,51,52].
Lipid peroxidation is an initial event in the seed deterioration process and is closely associated with the loss of seed viability [53]. Malondialdehyde (MDA), the product of lipid peroxidation, reflects the degree of oxidative damage to membranes and their integrity. Thus, MDA serves as a critical biochemical marker for evaluating seed deterioration progression [54]. Compared to freshly harvested beech (Fagus sylvatica) seeds, the MDA content in 17-year-stored seeds significantly increased 5–6-fold in cotyledons and hypocotyls [55]. Similarly, MDA levels in naturally aged (NA) and AA seeds of physic nut (Jatropha curcas) were significantly higher than in fresh seeds [56]. Lipid peroxidation disrupts plasma membrane integrity, leading to leakage of intracellular solutes (primarily charged ions like Na+ and K+). Therefore, the electrical conductivity (EC) of seed-soaking solutions correlates with membrane damage severity [57]. After 14 days of AA treatment, elm (Ulmus parvifolia) seeds showed significantly reduced germination ability and higher EC compared to untreated controls [58]. The EC of physic nut seed soaking solutions also significantly increased after NA and AA treatments [56].
ROS generated during the seed deterioration process can enter protein carbonylation reactions, affecting their function [37]. ROS-mediated protein damage primarily encompasses three categories: (1) direct metal-catalyzed oxidation of sulfur-containing amino acid residues. For example, cysteine (Cys) forms cystine (wherein two molecules of cysteine are linked by a disulfide bond), which can undergo further oxidation to cysteine sulfinic acid and, irreversibly, to cysteic acid [59,60,61,62]; (2) methionine (Met) oxidation, in which Met is converted to methionine sulfoxide (a reversible modification) and subsequently to methionine sulfone, an irreversible product [59]; (3) carbonylation reactions involving amino acid side chains—particularly those of arginine, lysine, proline, and threonine—which yield stable aldehydes or ketones as a result of oxidative modifications [59,63,64].
The storage reserves are fundamental to the formation of seed viability and are also significant factors for the preservation of seed viability during storage. During the seed storage phase, the content of carbohydrates, proteins, and lipids within seeds tends to decrease, leading to seed deterioration and a decline in vitality. Soluble sugars, which serve as the primary respiratory substrates, are progressively consumed during storage. For example, the contents of fructose and glucose in brazilwood (Caesalpinia echinata) seeds stored at room temperature decreased by 71.7% and 72.2%, respectively [65]. Under both NA and AA treatments, oligosaccharide content in the hypocotyls and cotyledons of Melanoxylon brauna seeds significantly decreased, while glucose content increased [66]. Proteomic analyses have revealed that soluble protein content markedly declines during seed deterioration, accompanied by alterations in protein composition [67,68]. The oxidation and carbonylation of proteins during deterioration reduce enzymatic activity or induce functional loss, thereby impairing metabolic processes in embryonic and storage tissues. Notably, some studies have demonstrated that seed deterioration enhances intracellular proteolytic enzyme activity, promoting the hydrolysis of storage proteins and accelerating the depletion of reserves, which further exacerbates the deterioration process [69].
4. Seed Anti-Deterioration Mechanisms
Seeds have evolved complex anti-deterioration systems to slow down the deterioration process and repair damage caused by deterioration. The seed anti-deterioration system primarily consists of protective systems, repair systems, and detoxifying agents. The protective system includes the seed coat’s protection of internal structures, antioxidant capacity, and protective proteins; the repair system mainly involves the repair of DNA and RNA, protein damage repair, and membrane system repair; detoxifying agents are primarily responsible for clearing toxic metabolites from the cells, such as ROS scavenging systems, antioxidant enzymes, and vitamin E. The various components of the anti-deterioration system work in coordination and are regulated by upstream plant hormones (Figure 1b).
4.1. Protective and Detoxification Mechanisms
There are two types of antioxidant systems present within seeds: one is the enzymatic system, composed of enzymes such as peroxiredoxins (Prxs), CAT, GR, SOD, ascorbate peroxidase, monodehydroascorbate reductase (MDHAR), and glutathione S-transferase (GST); the other is the non-enzymatic system, primarily consisting of ascorbic acid (AsA, also known as vitamin C), glutathione (GSH), α-tocopherol (also known as vitamin E), and carotenoids. The antioxidant protection mechanism depends on the moisture status of the seeds. In dry seeds, the scavenging of ROS mainly occurs through the non-enzymatic system involving vitamin E and GSH, while the antioxidant enzyme system, such as CAT, plays a crucial role in imbibed seeds [70]. Vitamin E is an important antioxidant in seeds, existing in two forms: tocopherols and tocotrienols. Tocopherols protect embryo cells from ROS damage, while tocotrienols may inhibit seed metabolism during deterioration processes. Tocopherols can scavenge lipid peroxide (i.e., free radicals), thereby blocking lipid peroxidation [71,72]. GSH and AsA can protect lipids by converting peroxyl free radicals generated during lipid peroxidation into tocopherol radicals [71,73,74]. Reduced germination rate of aged onion (Allium cepa L.) seeds is closely associated with down-regulated expression of genes encoding CAT and SOD, such as AMY1, BMY1, CTR1, and NPR1 [75]. The expression levels of MDHAR, GR, and GST are significantly downregulated in aged safflower (Carthamus tinctorius L.) seeds, leading to excessive accumulation of ROS, and ultimately resulting in loss of seed viability [76].
Reactive nitrogen species (RNS), like ROS, exhibit dual roles in seed physiology. Excessive RNS induces lipid nitration, protein tyrosine nitration, and S-nitrosylation, accelerating seed deterioration [77,78]. RNS is categorized into free radicals (e.g., nitric oxide [NO], nitrogen dioxide [•NO2]) and non-radicals (e.g., peroxynitrite [ONOO−], nitrosonium [NO+]) [79]. In deteriorating seeds, RNS (particularly NO and its derivatives) modulate oxidative stress, mitochondrial function, and posttranslational protein modifications, influencing the progression of seed aging. Whereas, NO mitigates oxidative damage by stimulating glutathione (GSH, reduced glutathione) synthesis in Arabidopsis seeds [80], activating ethylene biosynthesis to suppress stress-induced cell death, and enhancing antioxidant enzyme activity (e.g., GR, GPX) in recalcitrant Antiaris toxicaria seeds through protein S-nitrosylation/carbonylation balance [81]. Additionally, NO restores metabolic homeostasis in aged apple embryos by elevating phenylalanine and tyrosine levels [82]. The interplay between RNS and ROS, particularly NO and H2O2, plays a central role in regulating the hypersensitive response—a programmed cell death mechanism critical for pathogen defense. NO fine-tunes ROS production by S-nitrosylating NADPH oxidases (e.g., AtRBOHD), while H2O2 reciprocally stimulates NO synthesis via nitrate reductase activation [83]. These findings highlight NO as a key redox modulator for enhancing seed longevity and stress resilience. However, under specific conditions, the presence of RNS can exacerbate deterioration processes, depending on their concentration and the physiological context.
Antioxidant defense systems reduce ROS-induced protein damage, while chaperone proteins play a similar protective role by interacting with substrate proteins to enhance their stability. This dual mechanism mitigates oxidative stress and maintains proteostasis, thereby preserving seed viability during storage. LEAs, HSPs, and soluble sugars (such as raffinose family oligosaccharides, RFOs) are important components of the protective system and are closely related to dehydration tolerance and seed deterioration. SBP65 is a type of LEA protein; sbp65 mutants of pea (Pisum sativum L.) seeds show short longevity even under dry conditions [84]. In Arabidopsis, overexpression of heat stress transcription factors (HSFs) enhances the accumulation of HSPs in mature seeds, thereby improving seed vigor [85]. LEAs and HSPs act as chaperones and molecular shields during drying to prevent protein denaturation and membrane instability [86,87]. The synthesis of protective compounds is regulated by plant hormones, for example, ABA regulates the synthesis of HSPs, LEAs, and RFOs [31,88,89,90]. The major transcription factor ABI3 of the ABA signaling pathway directly binds to the promoter of HSFA9, activating its expression and enhancing seed dehydration tolerance [30,91]. ABI5 regulates seed longevity in legumes by controlling RFO synthesis and LEA expression levels [92]. The expression of HSFs is closely related to auxin signaling, for instance, IAA signaling induces ABI3 expression, thereby activating HSFA9 and promoting HSP synthesis, consistent with findings that auxin extends seed longevity [93,94]. The ability of small heat shock proteins (sHSPs) to form large oligomeric complexes underpins their molecular chaperone activity [95]. Therefore, sHSPs enhance the folding of newly synthesized proteins and the refolding of damaged peptides, while combating reactive oxygen species in seeds [28,31,95,96].
4.2. Repair Mechanisms
In addition to protective and detoxification mechanisms, repair mechanisms represent another critical strategy for embryonic cells to counteract deterioration, ensuring genomic and proteomic integrity during seed aging and storage. The repair system addresses the damage caused by seed deterioration through a series of biochemical reactions that restore the structural or functional integrity of damaged DNA, RNA, proteins, and membrane systems. ROS induces DNA damage such as base mismatches or double-strand breaks, while DNA integrity is essential for cell division. Seed germination capacity requires intact DNA in embryonic cells to sustain their cell division capability; thus, repairing damaged DNA can enhance the germination ability of deteriorated seeds by restoring mitotic activity in the embryo [97]. OGG1 is a bifunctional DNA glycosylase/AP lyase that removes 8-oxoG through a β-elimination reaction and cleaves DNA at the 3′ side of the resulting AP site. Overexpression of AtOGG1 reduces DNA damage during the storage-aging process of dry seeds and enhances DNA repair during germination, thereby promoting seed longevity in Arabidopsis [42]. The expression of MtOGG1 is involved in oxidative stress responses during seed imbibition, activating DNA repair to counteract ROS damage [98]. The DNA-binding protein family WHIRLY (WHY) plays a crucial role in maintaining genomic stability in both the nucleus and organelles. Mutants of why1 and why3 exhibit reduced longevity after deterioration compared to wild-type plants, and the double mutant why1why3 is highly sensitive to deterioration in Arabidopsis [99].
There is a close relationship between protein repair and seed longevity [100]. L-isoaspartate methyltransferase (PIMT), an S-adenosylmethionine (SAM)-dependent enzyme, converts abnormal L-isoaspartate residues (isoAsp) into the typical L-aspartate form (Asp). Overexpression of PIMT in rice and chickpea seeds enhances seed viability and longevity [101,102].
5. Genetic Regulation of Seed Deterioration
Many genes regulating the seed deterioration process have been identified in different plants. Some of these genes influence seed deterioration by regulating various stress proteins (such as LEAs and HSPs), while others interact to regulate dehydration tolerance and dormancy in deteriorating seeds. ABI3, ABI5, and LEC1 form a synergistic transcriptional regulatory network, where LEC1 acts as an upstream regulator that directly activates the expression of ABI3 and ABI5. Together, they integrate abscisic acid (ABA) signaling with embryo development programs to control seed maturation, dormancy, and storage protein synthesis (e.g., 2S albumin and CRA1) [103]. Furthermore, they enhance coordinated repression of downstream stress-responsive genes through protein–protein interactions, ensuring precise temporal control of germination timing. Additionally, some genes improve the antioxidant enzyme system’s activity, thereby mitigating seed deterioration. Table 1 summarizes the reported regulatory genes and their effects on seed deterioration and longevity.
Epigenetics involves the control of gene expression without altering the DNA sequence, primarily including DNA methylation, histone modifications, and microRNAs. Cytosine methylation plays a crucial role in regulating gene expression, and DNA methylation mediates plant responses to environmental stresses. In seeds stored for up to three years, a significant influence of storage time on DNA methylation was observed. DNA methylation, especially the level of 5-methylcytosine (m5C) in seeds, can be used as a truly universal viability marker regardless of the postharvest category of seeds and their composition. Maintaining high levels of DNA methylation delays seed deterioration and extends longevity [104]. Histones, as nucleosome core components, stabilize chromatin structure and maintain genome integrity. In Arabidopsis, mutations in the HISTONE REGULATOR A (HIRA) gene reduce H3 histone levels and increase chromatin accessibility, hence hira mutant seeds exhibit increased dormancy and shortened lifespan after aging treatment, likely due to accumulated DNA damage [105]. In Arabidopsis histone H3.3 plays a significant role in coordinating the expression of genes involved in seed maturation and germination. This protein binds the 5′ region of related genes and opens the DNA helix to make it available for expression. On the other hand, it has also an affinity for the 3′ end of related genes, thereby preventing their improper expression, i.e., cryptic transcription starts sites. The latter function is achieved by stimulating DNA methylation at the end of the gene body. So, the loss of H3.3 results in severely impaired germination and post-embryonic development [106]. Small RNAs, including microRNAs (miRNAs) and small interfering RNAs (siRNAs), post-transcriptionally regulate stress-responsive genes. Downregulation of miR164, miR6260, miR5929, miR6214, miR3946, and miR3446 activates stress responses and antioxidant systems, enhancing seed viability [107].
In summary, epigenetic regulation is pivotal in the seed deterioration process. However, the relationship between epigenetic markers (such as DNA methylation or histone modifications) and seed longevity remains an area of emerging research. While numerous associations have been identified in agricultural species, direct causal evidence is still limited.

Table 1.
Candidate genes regulating seed longevity and deterioration.
Table 1.
Candidate genes regulating seed longevity and deterioration.
| Candidate Genes | Function | Plant | References |
|---|---|---|---|
| HSF9A | Controls the process of seed aging | Medicago truncatula | [34] |
| ABI3 | Activation of HSFA9 expression and conferral of desiccation tolerance | Medicago truncatula | [30] |
| LEC1 | Affect multiple processes of maturation and consequently seed longevity | Arabidopsis | [108] |
| ABI5 | Regulation of RFOs and LEAs levels, as well as the expression of photosynthesis-related nuclear genes | Arabidopsis | [92] |
| HIRA | Reduction in histone H3 levels leading to shortened seed life | Arabidopsis | [105] |
| OGG1 | DNA damage repair | Arabidopsis | [42] |
| RSL1 | Enhance GA-induced response dynamics | Arabidopsis | [109] |
| TIP3 | Prolonged seed longevity via ABA pathway mediation | Arabidopsis | [109] |
| ASPG1 | Seed longevity, dormancy, and germination through mechanisms involving SSP degradation and GA signaling modulation | Arabidopsis | [109] |
| VTE1 VTE2 | Regulation of tocopherol biosynthesis | Arabidopsis | [110] |
| RAP2-12 | Control oxidative stress situations | Arabidopsis | [111] |
| Rc | Results in accumulation of proanthocyanidins | Rice (Oryza sativa L.) | [112] |
| bZIP23 PER1A | Clear ROS | Rice (Oryza sativa L.) | [113] |
| miR156 | Regulation of IPA1 expression to inhibit the GA biosynthesis pathway, enhancing seed dormancy and resistance to deterioration | Rice (Oryza sativa L.) | [114] |
| miR164 miR6260 miR5929 | Activation of stress responses and the antioxidant system | Orange (Citrus sinensis L.) | [107] |
| α-GAL, RAFS | Provide energy and reduce excessive reactive oxygen species | Maize (Zea mays L.) | [115] |
| ZmAGA1 | Hydrolyzing RFOs as well as a precursor, galactinol | Maize (Zea mays L.) | [116] |
| LOX | Modulate lipid peroxidation | Maize (Zea mays L.) | [117] |
| P5CS1 | Catalyzed Proline Biosynthesis | Oat (Avena sativa L.) | [118] |
| AsDMP1 AsDMP19 | Maintain redox steady state | Oat (Avena sativa L.) | [119] |
| AMY1, BMY1 CTR1, NPR1 | Regulation of CAT, SOD, and GPx activities | Onion (Allium cepa L.) | [75] |
| GolS1_A, GolS2_B | RFOs Biosynthesis | Soybean (Glycine max L.) | [120] |
| RS1, RS3 | RFOs Biosynthesis | Soybean (Glycine max L.) | [121] |
| RS2_B | RFOs Biosynthesis | Soybean (Glycine max L.) | [122] |
| SS | Stachyose Biosynthesis | Soybean (Glycine max L.) | [123] |
| RS2 | RFOs and Stachyose Biosynthesis | Soybean (Glycine max L.) | [124] |
| MDH | Organic acid synthesis | Carthamus tinctorius L. | [125] |
| Clpb1 Clpb4 | Regulating Chaperonins | Apple (Malus domestica Borkh.) | [126] |
| PLD | Membrane lipid degradation and damage | Dawn Redwood (Metasequoia glyptostroboides Hu.) | [127] |
| BnLIP1 | Participate in lipid metabolism | Cabbage (Brassica napus L.) | [128] |
| SSIIIb | Starch synthesis | Wheat (Triticum aestivum L.) | [129] |
| PsAKR1 | Detoxifies reactive carbonyl compounds | Tobacco (Nicotiana tabacum L.) | [130] |
| AhSOD | Clear ROS | Peanut (Arachis hypogaea L.) | [131] |
6. Seed Priming Enhances the Vigor of Deteriorated Seeds
The storage of orthodox seeds under low-temperature, dry, and oxygen-free conditions can effectively delay seed deterioration, while seed priming technology can restore the vigor of aged seeds. Seed priming enables seeds to complete preparatory processes for germination, such as enzyme activation, DNA repair, and membrane system restoration, promoting rapid and synchronized germination under favorable conditions. This enhances seedling emergence and establishment rates [132].
6.1. The Principle of Seed Priming Technology
Seed priming, also referred to as controlled prehydration, involves hydrating seeds to advance germination-related metabolic processes followed by dehydration before sowing. This technique exploits the relationship between imbibition dynamics and water potential regulation. By using osmotic solutes or restricting water availability, radicle protrusion is prevented. However, critical metabolic activities during germination Phase II—such as DNA repair, protein turnover, membrane system restoration, antioxidant system activation, and enhanced respiratory and energy metabolism—continue to progress (Figure 2) [16]. Importantly, DNA synthesis, cell division, and embryonic expansion remain arrested, preserving desiccation tolerance and allowing primed seeds to be dehydrated for storage and planting.
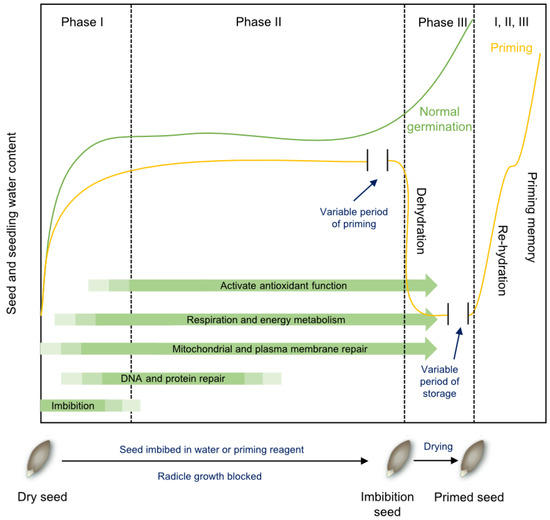
Figure 2.
The principle of seed priming to enhance germination (Modified from [16]). Seeds placed in water progress through three phases of imbibition, including imbibition (Phase I), lag phase (Phase II), and radicle emergence and growth (Phase III). During priming, water uptake is restricted by imbibition in osmotic solutions or by limiting the amount of water provided. This extends Phase II while preventing seeds from entering Phase III. Seeds retain desiccation tolerance and can be dried, stored, and distributed for planting. When subsequently imbibed, the priming treatment shortens Phase II, resulting in a faster transition to Phase III and germination completion.
As shown in Figure 2, Phase II of imbibition can be extended by reducing water potential. Generally, seeds retain desiccation tolerance if radicle emergence is suppressed, enabling safe post-priming dehydration. However, prolonged priming (over-priming) may cause radicle tip damage and subsequent poor seedling growth. After storage, primed seeds exhibit accelerated water uptake under adequate moisture, shortening Phase II duration and facilitating rapid radicle emergence and growth, thereby significantly reducing the time from sowing to seedling establishment [8,16].
Seed priming is a complex process whose efficacy is influenced by multiple factors, including the osmotic potential of the priming environment, priming duration, temperature, aeration conditions, light exposure, seed quality, as well as post-priming re-drying protocols and storage conditions [133]. These factors often interact synergistically or antagonistically. In liquid priming, precise control of osmotic potential is critical. An optimal osmotic potential allows seeds to achieve moderate hydration without triggering visible germination (i.e., radicle protrusion). Post-priming re-drying conditions also significantly affect priming outcomes. After priming, gradual re-drying methods are generally employed to avoid rapid dehydration, which could otherwise shorten seed longevity. The hydration–dehydration cycling method has proven effective in maintaining priming benefits by minimizing desiccation stress and preserving seed viability.
6.2. Vigor-Enhancing Priming Technologies for Deteriorated Seeds
Current seed priming methodologies predominantly include hydro-priming, osmo-priming, chemo-priming, solid-matrix priming, phytohormonal priming, bio-priming, nutrition-priming, nano-priming, and physical priming. Each method offers specific advantages: Water priming is cost-effective and pollution-free, though it may result in uneven germination; Osmotic priming effectively prevents premature germination in highly deteriorated seeds; Solid-matrix priming controls the growth environment while providing nutrients; Plant hormone priming enhances antioxidant defenses, surpassing water priming in repair efficacy; Biological priming utilizes microbial metabolites to repair damaged membranes, maintain integrity, and reduce electrolyte leakage; Nano-priming employs nanoparticles to penetrate seed coats, deliver trace elements to embryos, neutralize ROS through surface-active sites, reduce lipid peroxidation, and activate antioxidant systems. These techniques have been applied to rejuvenate aged seeds in crops such as wheat, maize, soybean, and onion [133,134,135,136]. Table 2 summarizes recent advancements in seed priming for vigor restoration. Notably, the effectiveness of these techniques is highly dependent on the species, degree of deterioration, and environmental conditions.
Recent advancements in seed priming technologies have revolutionized seed enhancement strategies, offering precision solutions to improve germination efficiency, stress resilience, and crop productivity. Nano-priming, utilizing engineered nanoparticles (e.g., ZnO, TiO2, and chitosan-based NPs), enables targeted delivery of micronutrients and reactive oxygen species (ROS) scavenging. For instance, tomato seeds treated with 50 ppm TiO2 nanoparticles exhibited 32% faster germination under saline conditions by enhancing aquaporin-mediated water uptake and antioxidant enzyme activity [137]. Bio-priming, which integrates beneficial microbes like the novel strain SH-8, activates systemic resistance and nutrient solubilization, improving drought tolerance by 20% in wheat [138]. Hybrid approaches such as physio-chemical priming combine cold plasma treatment with phytohormones (e.g., salicylic acid) in rice, enhancing plant growth and uptake of the nutrients under salinity stress while decreasing ROS production by increasing antioxidant enzyme activities [139].
Future trends emphasize multi-modal priming systems that synergize nanotechnology, biotechnology, and data-driven precision. Smart hydrogel-based priming with stimuli-responsive materials (e.g., pH/temperature-sensitive cellulose matrices) enables controlled release of growth regulators and real-time moisture monitoring, enhancing storage stability. In rice, Song et al. identified and genetically confirmed that DNA hypomethylation in the acquired cold tolerance 1 (ACT1) gene, induced by multigenerational cold stress, promotes both cold tolerance acquisition and its stable inheritance [140]. Although this study did not employ priming treatments, the findings imply that epigenetic modifications induced by priming could be stably inherited by subsequent seed generations. Therefore, epigenetic priming (e.g., targeted DNA methylation or histone acetylation) may represent a novel breakthrough in seed priming technologies, enabling heritable enhancements in stress tolerance and longevity. CRISPR-mediated epigenetic priming, using transient gene activation via dCas9 systems, promises non-transgenic enhancement of stress tolerance genes (OsDREB2A, AtHSFA2) [141,142]. Additionally, green priming technologies focusing on eco-friendly nanoparticles (biosynthesized NPs) and biodegradable carriers are gaining traction to address environmental concerns.

Table 2.
Priming techniques for rejuvenating deteriorated seed vigor documented in recent scientific investigations.
Table 2.
Priming techniques for rejuvenating deteriorated seed vigor documented in recent scientific investigations.
| Method | Agent | Description | Crop | Aging Test | References |
|---|---|---|---|---|---|
| Hydro-priming | Water | Priming 10 h, improve germination rate | Napa cabbage (Brassica rapa var. glabra Regel) | NA * (3 years) | [143] |
| Priming 12 h, improve germination rate | Maize (Zea mays L.) | AA * (57 °C, 24 h) | [134] | ||
| Osmo-priming | PEG | Priming 3 days, improve antioxidant enzyme activity and germination rate | Pinus thunbergii | AA | [41] |
| KNO3 | Priming 12 h, improve seedling length, dry weight, and germination rate | Milk Thistle (Silybum marianum (L.) Gaertn) | AA (45 °C, 2 days) | [144] | |
| CaCl2 | Priming 6 h, improve antioxidant enzyme activity and germination rate | Safflower (Carthamus tinctorius L.) | AA (43 ± 1 °C, 100% RH *, 12 h) | [145] | |
| Hormone priming | GA | Priming 12 h, improve germination rate | Maize (Zea mays L.) | AA (45 °C, 95% RH for 4, 7 days) | [12] |
| Improve germination rate, promote seedling growth | Wheat (Triticum aestivum L.) | AA (40 °C, 100% RH, 72 h) | [133] | ||
| SA | Priming 12 h, improved seed quality | Lentil (Lablab purpureus L.) | AA (35 °C, 90% RH, 2 days) | [7] | |
| Priming 8 h, increasing enzyme activity and reducing malondialdehyde (MDA) content | Soybean (Glycine max L.) | AA (40 °C, 100% RH, 2 days) | [146] | ||
| Solid matrix priming | Vermiculite | Priming 36 h, improve antioxidant enzymes and seed quality | Bitter gourd (Momordica charantia L.) | AA (40 °C, 100% RH for 3, 6, 9 days) | [147] |
| Priming 16 h, improved the germination rate | Cabbage (Brassica oleracea var. capitata L.) | NA | [148] | ||
| Micro-Cel E | Priming 5 days, improve germination rate and decreased mean germination time | Switchgrass (Panicum virgatum L.) | AA (42 °C, 95% RH for 10, 20 days) | [149] | |
| Bio-priming | Azotobacter | Priming 16 h, improve germination rate and vigor | Onion (Allium cepa L.) | NA (1, 2, 3 year) | [136] |
| Bacillus megaterium | Priming 5 h, improve shoot length, root length, and seedling vigor index | Soybean (Glycine max L.) | AA (42 °C, 95% RH, 72 h) | [150] | |
| Caffeic acid | Caffeic acid | Priming 6 h, improve germination rate | Soybean (Glycine max L.) | AA (45 °C, 95% RH, 24 h) | [135] |
| Melatonin | Melatonin | Priming 24 h, improve antioxidant enzymes | Oat (Avena sativa L.) | NA (48 days) | [118] |
| Spermidine | Spermidine | Priming 24 h, improve antioxidant enzymes activity and GA content | Rice (Oryza sativa L.) | AA (43 °C, 98% RH, 4 days) | [151] |
| Nano | AgNPs | Priming 24 h, improve germination rate | Rice (Oryza sativa L.) | NA (25–30 °C, 3 years) | [11] |
| Priming 24 h, increased the total phenolic content of seedlings and CAT activity | Broad bean (Vicia faba L.) | NA (5 ± 1.5 °C, 50% RH, 7 years) | [152] | ||
| Plant-derived smoke | Karrikinolide | Priming 16 h, improve germination rate, fresh and dry weight | Marrow (Cucurbita pepo L.), Cabbage (Brassica oleracea var. capitata L.) and Pepper (Piper nigrum L.) | AA (40 °C,100% RH, 48 h) | [153] |
| Union priming | PEG+GA3, PEG+ABA | Priming 6 h, improve germination rate | Moench (Abelmoschus esculentus L.) | AA (40 ± 10 °C, 100% RH, 72 h) | [154] |
| KH2PO4 + KNO3 | Priming 12 h, promote germination | Muskmelon (Cucumis sativus L.) | NA (2 years) | [155] | |
| Physical priming | HVEF (High-voltage electrostatic field) | Priming 55 min, improve antioxidant enzyme activity, reduce leakage rate | Rice (Oryza sativa L.) | AA (40 °C, 100% RH, 8 days) | [156] |
| PAW (Plasma activated water) | Improve germination rate | Pepper (Capsicum annuum L.) | NA (2 years) | [157] |
* NA means natural aged; AA means accelerated aged; RH means relative humidity.
6.3. Mechanisms Underlying Seed Priming-Mediated Enhancement of Vigor in Deteriorated Seeds
Primed seeds exhibit significantly enhanced vigor, improved seedling emergence and establishment rates, and elevated stress tolerance. However, the underlying mechanisms of priming are not fully understood. The relevant literature on seed priming mechanisms reveals two predominant pathways: (1) initiation of germination-associated preparatory processes that facilitate the transition from a quiescent desiccated state to a full-germination state, thereby augmenting germination potential; (2) imposition of controlled abiotic stress during priming, which activates stress-responsive signaling cascades and induces systemic tolerance enhancement (Figure 3).
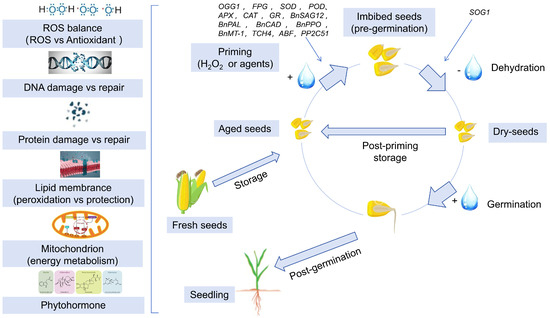
Figure 3.
Mechanisms of seed priming in enhancing the vigor of aged seeds. Seed priming mechanism: During storage, seeds undergo deterioration primarily through oxidative damage to DNA, proteins, and lipids. Priming treatments counteract these effects by enhancing antioxidant defenses, activating repair systems, and ultimately restoring germination vigor.
During seed priming, numerous enzymes associated with the metabolism of storage reserves (carbohydrates, proteins, and lipids) are activated, such as amylases, proteases, and isocitrate lyase, thereby promoting the mobilization of reserves [158,159]. The activity of α-amylase, which facilitates starch hydrolysis, is positively correlated with soluble sugar content during germination. Comparative studies demonstrate that hydro-primed deteriorated wheat (Triticum aestivum) seeds exhibit significantly enhanced α-amylase activity, soluble sugars, and soluble protein levels compared to non-primed controls [160].
Seeds scavenge excess intracellular ROS through antioxidant enzymes and non-enzymatic antioxidants to counteract oxidative stress and enhance viability. The antioxidant function of seeds is activated during priming treatments, thereby improving germination rates, and increasing seedling vigor at sowing [132]. Ulhassan et al. applied silicon nanoparticle (SiNP) priming treatment to Brassica napus seeds, which upregulated the expression of oxidative stress-related genes (BnPAL, BnCAD, BnPPO, BnMT-1) and chlorophyll synthesis-related genes (BnCAO, BnCHLG, BnPOR), while suppressing the senescence-associated gene BnSAG12, thereby enhancing resistance to the deterioration [161]. Polyethylene glycol-6000 (PEG-6000) priming of soybean seeds significantly upregulated antioxidant enzyme-encoding genes (GST, SOD, POD), reduced intracellular ROS levels, and improved germination and seedling establishment rates in deteriorated seeds [162]. Thioredoxin (TRX), a conserved protein in plant oxidative stress responses, directly scavenges H2O2 and ROS radicals [163]. Melatonin priming of aged oat (Avena sativa) seeds elevated intracellular antioxidants TRX and GST, improving germination rates [118].
DNA repair is a critical event in seed germination, initiated during the seed imbibition process. Following the hydro-priming treatment of eggplant (Solanum melongena) seeds, the expression levels of key DNA repair-related genes OGG1 and FPG were upregulated to facilitate DNA repair during germination [164]. In water-treated Medicago truncatula seeds during redrying, the SOG1 gene—encoding a key regulatory factor in plant DNA damage repair—exhibited significantly elevated expression [165]. As DNA repair serves as a critical rate-limiting step during DNA replication, primed cabbage (Brassica oleracea) seeds displayed higher DNA synthesis rates and elevated cell division levels, demonstrating that priming enhances DNA repair processes [166,167].
Damaged proteins caused by dehydration–rehydration cycles require repair or removal. It has been reported that proteins involved in the translation process are primary targets of seed protection and repair mechanisms. Except for those integral to the translational machinery, other proteins may be removed or replaced in imbibing seeds [168]. Translation-associated proteins, such as ribosomal proteins, can reassemble ribosomes to initiate the synthesis of new proteins (replacing oxidatively damaged ones), making these proteins essential for sustaining translation. Meanwhile, LEAs rapidly accumulate during early priming stages, reducing further protein damage through physical shielding and antioxidant activity, thereby stabilizing cellular homeostasis [169,170]. LEAs have been reported to protect proteins from dehydration-induced damage, and their accumulation has been observed in primed sugar beet (Beta vulgaris) [171] and rapeseed (Brassica napus) seeds, facilitating repair of dehydration-related injuries [172]. Beyond translation-associated proteins, other critical proteins also require repair or removal. Silver nanoparticle (AgNP) priming of deteriorated rice (Oryza sativa) seeds triggered the restoration of functional proteomes by upregulating aquaporin genes PIP1;1 and PIP2;1 [11]. L-isoaspartyl methyltransferase, a repair enzyme activated under oxidative stress in seeds, plays a vital role in repairing proteins involved in ribosomal RNA (rRNA) processing within the nucleus [173,174]. Osmopriming of deteriorated tomato (Solanum lycopersicum) seeds restored L-isoaspartyl methyltransferase activity, confirming its role in repairing damaged cellular proteins during priming [171].
The regulation of plant hormone signaling is a central mechanism in seed priming. This process involves promoting the expression of gibberellin (GA)- and cytokinin (CTK)-related genes while reducing ABA levels, thereby modulating the ABA-GA balance [147]. In polyethylene glycol-6000 (PEG-6000)-primed deteriorated soybean (Glycine max) seeds, the expression of seven IAA-coding genes (e.g., Glyma.02G142500, Glyma.03G158700) was upregulated. Additionally, Glyma.13G095200 (encoding TCH4, a brassinosteroid (BR)-responsive transcription factor within the xyloglucan endotransglucosylase/hydrolase (XTH) family that regulates BR- and IAA-mediated cell elongation) exhibited upregulated expression. Conversely, Glyma.14G162100 (encoding PP2C51, a component of the ABA signaling pathway) and Glyma.10G071700/Glyma.19G194500 (encoding ABA-responsive element-binding factors, ABFs) showed downregulated expression [162]. This treatment enhanced BR and IAA signaling pathways while suppressing ABA signaling, thereby antagonizing ABA-mediated inhibition, and promoting germination in aged seeds. In a study where deteriorated wheat seeds were primed with GA, cell membrane integrity improved, leading to reduced electrolyte leakage. Concurrently, proline and soluble sugar levels increased, while MDA content decreased [133].
7. Conclusions and Future Outlook
Seed deterioration is a complex process influenced by both environmental factors and genetic regulation. Understanding its mechanisms is critical for enhancing seed viability and facilitating long-term conservation of germplasm resources. Recent advances in multi-omics technologies (e.g., genomics, transcriptomics, metabolomics, proteomics, epigenomics) and gene-editing tools (e.g., CRISPR/Cas9) have revolutionized mechanistic studies of seed deterioration. Integrated approaches, including genome-wide association studies (GWAS), enable the identification of key regulatory genes, mapping of deterioration-related networks, and clarification of molecular pathways driving seed aging. There is an urgent need for functional validation of molecular mechanisms in non-model species.
Current research reveals that oxidative damage to genetic material, proteins, and membrane systems constitutes the core mechanisms driving seed deterioration. Such damage disrupts cellular functions or induces cell death in embryo cells, ultimately leading to the loss of germination capacity. However, seeds possess intrinsic anti-deterioration mechanisms, including antioxidant systems (e.g., enzymatic scavengers such as SOD and CAT), detoxification systems (e.g., GSTs), and repair systems (e.g., DNA ligases and chaperone proteins), which collectively delay the deterioration process. Seed priming treatments counteract aging effects by reactivating these defense responses, thereby restoring vigor and enhancing germination capacity in aged seeds.
To achieve long-term germplasm conservation and sustainable agricultural productivity, genetic improvement and seed treatment innovations are complementary strategies. Future research should focus on employing genomic/genetic engineering strategies to identify critical regulatory genes for breeding deterioration-resistant crop varieties with enhanced storage tolerance, developing optimized storage protocols and precision seed priming methods based on seed deterioration mechanisms to delay deterioration processes and extend seed longevity, and addressing the challenges in scaling up priming technologies for large-scale agricultural applications. Through these approaches, postharvest economic losses caused by seed deterioration can be significantly reduced, thereby safeguarding global food security.
Author Contributions
B.T. designed the manuscript; W.X., Y.L. (Yi Li), L.Z., H.H., Y.L. (Yuan Liu), S.L., J.Z., Y.Z. and Y.Y. wrote the manuscript; B.T. and W.X. drew the figures; B.T., Z.X. and W.X. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture and Rural Affairs of China (H20230603) and the Tewen Plant Detection Technology Service (Guangzhou) Co., Ltd. (H231249).
Acknowledgments
We apologize in advance for those contributions that might have been left out due to space limitations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of Orthodox Seeds during Ageing: Influencing Factors, Physiological Alterations and the Role of Reactive Oxygen Species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.B. Seed Quality Assessment. Seed Sci. Res. 1998, 8, 265–276. [Google Scholar] [CrossRef]
- Han, P.; Li, Y.; Liu, Z.; Zhou, W.; Yang, F.; Wang, J.; Yan, X.; Lin, J. The Physiology of Plant Seed Aging: A Review. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2022, 38, 77–88. [Google Scholar]
- Pérez-García, F.; González-Benito, M.E.; Gómez-Campo, C. High Viability Recorded in Ultra-Dry Seeds of 37 Species of Brassicaceae after Almost 40 Years of Storage. Seed Sci. Technol. 2007, 35, 143–153. [Google Scholar] [CrossRef]
- Deepa, G.T.; Chetti, M.B.; Khetagoudar, M.C.; Adavirao, G.M. Influence of Vacuum Packaging on Seed Quality and Mineral Contents in Chilli (Capsicum annuum L.). J. Food Sci. Technol. 2011, 50, 153–158. [Google Scholar] [CrossRef]
- Zanotti, R.F.; Motta, L.B.; Bragatto, J.; Labate, C.A.; Salomão, A.N.; Vendrame, W.A.; Cuzzuol, G.R.F. Germination, Carbohydrate Composition and Vigor of Cryopreserved Caesalpinia echinata Seeds. Braz. Arch. Biol. Technol. 2012, 55, 661–669. [Google Scholar] [CrossRef]
- Kumari, N.; Mishra, S.N.; Chaurasia, A.K. Impact of Salicylic and Gibberellic Acid Seed Invigoration on Seed Health Status under (Artificial Ageing) Accelerated Ageing Test in Lentil (Lens culinaris Medik.). Legume Res. 2022, 47, 952–957. [Google Scholar] [CrossRef]
- Rakshit, A.; Singh, H.B. Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-981-13-0031-8. [Google Scholar]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-Priming as Emerging Seed Priming Technology for Sustainable Agriculture—Recent Developments and Future Perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. New Chall. Seed Biol. Basic Transl. Res. Driv. Seed Technol. 2016, 46, 64420. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming Technology for Enhancing Germination and Starch Metabolism of Aged Rice Seeds Using Phytosynthesized Silver Nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Siadat, S.A.; Moosavi, S.A.; Zadeh, M.S.; Fotouhi, F.; Zirezadeh, M. Effects of Halo and Phytohormone Seed Priming on Germination and Seedling Growth of Maize under Different Duration of Accelerated Ageing Treatment. Afr. J. Agric. Res. 2011, 6, 6453–6462. [Google Scholar] [CrossRef]
- Pirredda, M.; Fañanás-Pueyo, I.; Oñate-Sánchez, L.; Mira, S. Seed Longevity and Ageing: A Review on Physiological and Genetic Factors with an Emphasis on Hormonal Regulation. Plants 2023, 13, 41. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H.; Soetisna, U. Seed Storage Behaviour in Elaeis guineensis. Seed Sci. Res. 1991, 1, 99–104. [Google Scholar] [CrossRef]
- Li, L.; Meng, Z.; Long, G.; Zhang, G.; Yang, S.; Chen, J. Advances on Recalcitrant Seeds of Plants. J. Trop. Subtrop. Bot. 2016, 24, 106–118. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development and Germination; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H.; Tao, K.-L. Low Moisture Content Limits to Relations Between Seed Longevity and Moisture. Ann. Bot. 1990, 65, 493–504. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Seed Moisture Content, Storage, Viability and Vigour. Seed Sci. Res. 1991, 1, 275–279. [Google Scholar] [CrossRef]
- Corbineau, F. The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity. Seeds 2024, 3, 56–75. [Google Scholar] [CrossRef]
- Hay, F.R.; Davies, R.M.; Dickie, J.B.; Merritt, D.J.; Wolkis, D.M. More on Seed Longevity Phenotyping. Seed Sci. Res. 2022, 32, 144–149. [Google Scholar] [CrossRef]
- Walters, C.; Patricia, B.; Norman, P.; Kennedy, K.; Raven, P. Preservation of Recalcitrant Seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Ranganathan, U.; Groot, S.P.C. Seed Longevity and Deterioration. In Seed Science and Technology: Biology, Production, Quality; Dadlani, M., Yadava, D.K., Eds.; Springer Nature: Singapore, 2023; pp. 91–108. [Google Scholar]
- Bueso, E.; Serrano, R.; Pallás, V.; Sánchez-Navarro, J.A. Seed Tolerance to Deterioration in Arabidopsis Is Affected by Virus Infection. Plant Physiol. Biochem. 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Ranathunge, K.; Shao, S.; Qutob, D.; Gijzen, M.; Peterson, C.A.; Bernards, M.A. Properties of the Soybean Seed Coat Cuticle Change during Development. Planta 2010, 231, 1171–1188. [Google Scholar] [CrossRef] [PubMed]
- Kuchlan, M.K.; Kuchlan, P.; Onkar, M.; Ramesh, A.; Husain, S.M. Influence of Seed Coat Compactness around Cotyledons, Protein and Mineral Composition on Mechanical Strength of Soybean [Glycine max (L.) Merrill] Seed Coat. Legume Res. Int. J. 2018, 41, 246–252. [Google Scholar]
- Ramtekey, V.; Cherukuri, S.; Kumar, S.; Kudekallu, V.S.; Sheoran, S.; Bhaskar, K.U.; Naik, K.B.; Kumar, S.; Singh, A.N.; Singh, H.V. Seed Longevity in Legumes: Deeper Insights into Mechanisms and Molecular Perspectives. Front. Plant Sci. 2022, 13, 918206. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry Architecture: Towards the Understanding of the Variation of Longevity in Desiccation-Tolerant Germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2015, 57, 660–674. [Google Scholar] [CrossRef]
- Zanakis, G.N.; Ellis, R.H.; Summerfield, R.J. Seed Quality in Relation to Seed Development and Maturation in Three Genotypes of Soyabean (Glycine max). Exp. Agric. 1994, 30, 139–156. [Google Scholar] [CrossRef]
- Verdier, J.; Lalanne, D.; Pelletier, S.; Torres-Jerez, I.; Righetti, K.; Bandyopadhyay, K.; Leprince, O.; Chatelain, E.; Vu, B.L.; Gouzy, J.; et al. A Regulatory Network-Based Approach Dissects Late Maturation Processes Related to the Acquisition of Desiccation Tolerance and Longevity of Medicago truncatula Seeds. Plant Physiol. 2013, 163, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late Seed Maturation: Drying without Dying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar] [CrossRef]
- Ekpong, B.; Sukprakarn, S. Seed Physiological Maturity in Dill (Anethum graveolens L.). Agric. Nat. Resour. 2008, 42, 1–6. [Google Scholar]
- Miquel, J. An Integrated Theory of Aging as the Result of Mitochondrial-DNA Mutation in Differentiated Cells. Arch. Gerontol. Geriatr. 1991, 12, 99–117. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef]
- Copeland, L.O.; Mcdonald, M.B. Principles of Seed Science and Technology; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 978-1-4613-5644-8. [Google Scholar]
- Ahmed, M.R.; Yasmin, J.; Collins, W.; Lohumi, S.; Cho, B.-K. Assessment of the Morphological Structure of Watermelon and Muskmelon Seeds as Related to Viability. J. Biosyst. Eng. 2019, 44, 77–86. [Google Scholar] [CrossRef]
- Bailly, C. Active Oxygen Species and Antioxidants in Seed Biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Wesley-Smith, J.; Berjak, P.; Pammenter, N.W.; Vertucci, C.W. Ultrastructural Evidence for the Effects of Freezing in Embryonic Axes of Pisum sativum L. at Various Water Contents. Ann. Bot. 1995, 76, 59–64. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Kaur, R.; Yadu, B.; Chauhan, N.S.; Parihar, A.S.; Keshavkant, S. Nano Zinc Oxide Mediated Resuscitation of Aged Cajanus cajan via Modulating Aquaporin, Cell Cycle Regulatory Genes and Hormonal Responses. Plant Cell Rep. 2024, 43, 110. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Han, S.; Lee, J. Germination and Biochemical Changes in Accelerated Aged and Osmoprimed Pinus thunbergii Seeds. J. Korean Soc. For. Sci. 2010, 99, 244–250. [Google Scholar]
- Chen, H.; Chu, P.; Zhou, Y.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.T.; Jiang, L.; Wu, K.; Huang, S. Overexpression of AtOGG1, a DNA Glycosylase/AP Lyase, Enhances Seed Longevity and Abiotic Stress Tolerance in Arabidopsis. J. Exp. Bot. 2012, 63, 4107–4121. [Google Scholar] [CrossRef]
- Dourado, A.M.; Roberts, E.H. Chromosome Aberrations Induced during Storage in Barley and Pea Seeds. Ann. Bot. 1984, 54, 767–779. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Footitt, S.; Bray, C.M.; Finch-Savage, W.E.; West, C.E. DNA Damage Checkpoint Kinase ATM Regulates Germination and Maintains Genome Stability in Seeds. Proc. Natl. Acad. Sci. USA 2016, 113, 9647–9652. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Meimoun, P.; Job, C.; Job, D.; Bailly, C. Role of Protein and mRNA Oxidation in Seed Dormancy and Germination. Front. Plant Sci. 2013, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Gallardo, K.; Debeaujon, I.; Vandekerckhove, J.; Job, C.; Job, D. The Effect of α-Amanitin on the Arabidopsis Seed Proteome Highlights the Distinct Roles of Stored and Neosynthesized mRNAs during Germination. Plant Physiol. 2004, 134, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Rajjou, L.; North, H.M. Lost in Translation: Physiological Roles of Stored mRNAs in Seed Germination. Plants 2020, 9, 347. [Google Scholar] [CrossRef]
- Bai, B.; van der Horst, S.; Cordewener, J.H.G.; America, T.A.H.P.; Hanson, J.; Bentsink, L. Seed-Stored mRNAs that Are Specifically Associated to Monosomes Are Translationally Regulated during Germination. Plant Physiol. 2019, 182, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Bray, C.M.; Dasgupta, J. Ribonucleic Acid Synthesis and Loss of Viability in Pea Seed. Planta 1976, 132, 103–108. [Google Scholar] [CrossRef]
- Kranner, I.; Chen, H.; Pritchard, H.W.; Pearce, S.R.; Birtić, S. Inter-Nucleosomal DNA Fragmentation and Loss of RNA Integrity during Seed Ageing. Plant Growth Regul. 2010, 63, 63–72. [Google Scholar] [CrossRef]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the Fate of mRNA in Aging Seeds: Protection, Destruction, or Slow Decay? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef]
- Tetreault, H.; Fleming, M.; Hill, L.; Dorr, E.; Yeater, K.; Richards, C.; Walters, C. A Power Analysis for Detecting Aging of Dry-stored Soybean Seeds: Germination versus RNA Integrity Assessments. Crop Sci. 2023, 63, 1481–1493. [Google Scholar] [CrossRef]
- Fu, Y.-B.; Ahmed, Z.; Diederichsen, A. Towards a Better Monitoring of Seed Ageing under Ex Situ Seed Conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef]
- Yao, R.; Liu, H.; Zhao, G.; Wang, J.; Wang, Q.; Dong, K.; Zhang, R. Effects of Seed Storage Time on Seed Germination and Cytological Structure of Covered Oats and Naked Oats. Acta Prataculturae Sin. 2024, 33, 154. [Google Scholar]
- Małecka, A.; Ciszewska, L.; Staszak, A.; Ratajczak, E. Relationship Between Mitochondrial Changes and Seed Aging as a Limitation of Viability for the Storage of Beech Seed (Fagus sylvatica L.). PeerJ 2021, 9, e10569. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Shah, N.; Kotecha, M.; Robin, P. Evaluation of Biochemical and Physiological Changes in Seeds of Jatropha Curcas L. Under Natural Aging, Accelerated Aging and Saturated Salt Accelerated Aging. Sci. Hortic. 2019, 255, 21–29. [Google Scholar] [CrossRef]
- Matthews, S.; Powell, A. Electrical Conductivity Vigour Test: Physiological Basis and Use. Seed Test. Int. 2006, 131, 32–35. [Google Scholar]
- Kim, D.H.; Han, S.H.; Song, J.H. Evaluation of the Inorganic Compound Leakage and Carbohydrates as Indicator of Physiological Potential of Ulmus Parvifolia Seeds. New For. 2010, 41, 3–11. [Google Scholar] [CrossRef]
- Ghezzi, P.; Bonetto, V. Redox Proteomics: Identification of Oxidatively Modified Proteins. Proteom. Int. Ed. 2003, 3, 1145–1153. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Rinalducci, S.; Murgiano, L.; Zolla, L. Redox Proteomics: Basic Principles and Future Perspectives for the Detection of Protein Oxidation in Plants. J. Exp. Bot. 2008, 59, 3781–3801. [Google Scholar] [CrossRef]
- Demidchik, V. Reactive Oxygen Species and Their Role in Plant Oxidative Stress. In Plant Stress Physiology; CABI: Wallingford, UK, 2017; pp. 64–96. [Google Scholar]
- Shacter, E. Quantification and Significance of Protein Oxidation in Biological Samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and Proteins—Major Targets of Oxidative Modifications in Abiotic Stressed Plants. Environ. Sci. Pollut. Res. 2014, 22, 4099–4121. [Google Scholar] [CrossRef]
- Garcia, I.S.; Souza, A.; Barbedo, C.J.; Dietrich, S.M.C.; Figueiredo-Ribeiro, R.C.L. Changes in Soluble Carbohydrates During Storage of Caesalpinia echinata LAM. (Brazilwood) Seeds, an Endangered Leguminous Tree from the Brazilian Atlantic Forest. Braz. J. Biol. 2006, 66, 739–745. [Google Scholar] [CrossRef]
- Corte, V.B.; Borges, E.E.D.L.; Gonçalves, J.F.D.C.; Silva, M.S. Alterations in the Lipid and Soluble Sugar Content of Melanoxylon brauna Seeds during Natural and Accelerated Ageing. Rev. Bras. Sementes 2010, 32, 152–162. [Google Scholar] [CrossRef]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of Protein Oxidation in Arabidopsis Seeds and during Germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Arc, E.; Galland, M.; Cueff, G.; Godin, B.; Lounifi, I.; Job, D.; Rajjou, L. Reboot the System Thanks to Protein Post-translational Modifications and Proteome Diversity: How Quiescent Seeds Restart Their Metabolism to Prepare Seedling Establishment. Proteomics 2011, 11, 1606–1618. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Lin, X.; Zhou, Y.; Chen, X.; Liu, X.; Lu, X. Proteome Analysis of Maize Seeds: The Effect of Artificial Ageing. Physiol. Plant. 2011, 143, 126–138. [Google Scholar] [CrossRef]
- Gerna, D.; Ballesteros, D.; Arc, E.; Stöggl, W.; Seal, C.E.; Marami-Zonouz, N.; Na, C.S.; Kranner, I.; Roach, T. Does Oxygen Affect Ageing Mechanisms of Pinus densiflora Seeds? A Matter of Cytoplasmic Physical State. J. Exp. Bot. 2022, 73, 2631–2649. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Mène-Saffrané, L.; Jones, A.D.; DellaPenna, D. Plastochromanol-8 and Tocopherols Are Essential Lipid-Soluble Antioxidants during Seed Desiccation and Quiescence in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 17815–17820. [Google Scholar] [CrossRef]
- Colville, L.; Kranner, I. Desiccation Tolerant Plants as Model Systems to Study Redox Regulation of Protein Thiols. Plant Growth Regul. 2010, 62, 241–255. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic Acid in Plants: Biosynthesis and Function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Kamaei, R.; Kafi, M.; Afshari, R.T.; Shafaroudi, S.M.; Nabati, J. Physiological and Molecular Changes of Onion (Allium cepa L.) Seeds Under Different Aging Conditions. BMC Plant Biol. 2024, 24, 85. [Google Scholar] [CrossRef]
- Lv, T.; Li, J.; Zhou, L.; Zhou, T.; Pritchard, H.W.; Ren, C.; Chen, J.; Yan, J.; Pei, J. Aging-Induced Reduction in Safflower Seed Germination via Impaired Energy Metabolism and Genetic Integrity Is Partially Restored by Sucrose and DA-6 Treatment. Plants 2024, 13, 659. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Arnaud, D. Hydrogen Peroxide Metabolism and Functions in Plants. New Phytol. 2018, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-Dependent Signalling Pathways in Plants and Algae Exposed to High Light: Comparisons with Other Eukaryotes. Free Radic. Biol. Med. 2018, 122, 52–64. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Mohammad, F.; Corpas, F.J. Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ciacka, K.; Krasuska, U.; Otulak-Kozieł, K.; Gniazdowska, A. Dormancy Removal by Cold Stratification Increases Glutathione and S-Nitrosoglutathione Content in Apple Seeds. Plant Physiol. Biochem. 2019, 138, 112–120. [Google Scholar] [CrossRef]
- Krasuska, U.; Gniazdowska, A. Nitric Oxide and Hydrogen Cyanide as Regulating Factors of Enzymatic Antioxidant System in Germinating Apple Embryos. Acta Physiol. Plant 2011, 34, 683–692. [Google Scholar] [CrossRef]
- Ciacka, K.; Tyminski, M.; Gniazdowska, A.; Krasuska, U. Nitric Oxide as a Remedy against Oxidative Damages in Apple Seeds Undergoing Accelerated Ageing. Antioxidants 2021, 11, 70. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant–Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Boudet, J.; Buitink, J.; Hoekstra, F.A.; Rogniaux, H.; Larré, C.; Satour, P.; Leprince, O. Comparative Analysis of the Heat Stable Proteome of Radicles of Medicago truncatula Seeds during Germination Identifies Late Embryogenesis Abundant Proteins Associated with Desiccation Tolerance. Plant Physiol. 2006, 140, 1418–1436. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Dapena, P.; Castaño, R.; Almoguera, C.; Jordano, J. Improved Resistance to Controlled Deterioration in Transgenic Seeds. Plant Physiol. 2006, 142, 1102–1112. [Google Scholar] [CrossRef]
- Tejedor-Cano, J.; Prieto-Dapena, P.; Almoguera, C.; Carranco, R.; Hiratsu, K.; Ohme-Takagi, M.; Jordano, J. Loss of Function of the HSFA9 Seed Longevity Program. Plant Cell Amp. Environ. 2010, 33, 1408–1417. [Google Scholar] [CrossRef]
- Chatelain, E.; Hundertmark, M.; Leprince, O.; Gall, S.L.; Satour, P.; Deligny-Penninck, S.; Rogniaux, H.; Buitink, J. Temporal Profiling of the Heat-stable Proteome during Late Maturation of Medicago truncatula Seeds Identifies a Restricted Subset of Late Embryogenesis Abundant Proteins Associated with Longevity. Plant Cell Amp. Environ. 2012, 35, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- Busk, P.K.; Pagès, M. Regulation of Abscisic Acid-Induced Transcription. Plant Mol. Biol. 1998, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic Acid Signaling in Seeds and Seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef] [PubMed]
- Bizouerne, E.; Buitink, J.; Vu, B.L.; Vu, J.L.; Esteban, E.; Pasha, A.; Provart, N.; Verdier, J.; Leprince, O. Gene Co-Expression Analysis of Tomato Seed Maturation Reveals Tissue-Specific Regulatory Networks and Hubs Associated with the Acquisition of Desiccation Tolerance and Seed Vigour. BMC Plant Biol. 2021, 21, 124. [Google Scholar] [CrossRef]
- Kotak, S.; Vierling, E.; Bäumlein, H.; Koskull-Döring, P. A Novel Transcriptional Cascade Regulating Expression of Heat Stress Proteins during Seed Development of Arabidopsis. Plant Cell 2007, 19, 182–195. [Google Scholar] [CrossRef]
- Dekkers, B.J.W.; He, H.; Hanson, J.; Willems, L.A.J.; Jamar, D.C.L.; Cueff, G.; Rajjou, L.; Hilhorst, H.W.M.; Bentsink, L. The Arabidopsis DELAY OF GERMINATION 1 Gene Affects ABSCISIC ACID INSENSITIVE 5 (ABI5) Expression and Genetically Interacts with ABI3 during Arabidopsis Seed Development. Plant J. 2016, 85, 451–465. [Google Scholar] [CrossRef]
- Righetti, K.; Vu, J.L.; Pelletier, S.; Vu, B.L.; Glaab, E.; Lalanne, D.; Pasha, A.; Patel, R.V.; Provart, N.J.; Verdier, J. Inference of Longevity-Related Genes from a Robust Coexpression Network of Seed Maturation Identifies Regulators Linking Seed Storability to Biotic Defense-Related Pathways. Plant Cell 2015, 27, 2692–2708. [Google Scholar] [CrossRef]
- Carranco, R.; Espinosa, J.M.; Prieto-Dapena, P.; Almoguera, C.; Jordano, J. Repression by an Auxin/Indole Acetic Acid Protein Connects Auxin Signaling with Heat Shock Factor-Mediated Seed Longevity. Proc. Natl. Acad. Sci. USA 2010, 107, 21908–21913. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Pukacka, S. Possible Roles of LEA Proteins and sHSPs in Seed Protection: A Short Review. Biol. Lett. 2007, 44, 3–16. [Google Scholar]
- Kaur, H.; Petla, B.P.; Kamble, N.U.; Singh, A.; Rao, V.; Salvi, P.; Ghosh, S.; Majee, M. Differentially Expressed Seed Aging Responsive Heat Shock Protein OsHSP18.2 Implicates in Seed Vigor, Longevity and Improves Germination and Seedling Establishment under Abiotic Stress. Front. Plant Sci. 2015, 6, 713. [Google Scholar] [CrossRef]
- Ventura, L.; Donà, M.; Macovei, A.; Carbonera, D.; Buttafava, A.; Mondoni, A.; Rossi, G.; Balestrazzi, A. Understanding the Molecular Pathways Associated with Seed Vigor. Plant Physiol. Biochem. 2012, 60, 196–206. [Google Scholar] [CrossRef]
- Macovei, A.; Balestrazzi, A.; Confalonieri, M.; Faé, M.; Carbonera, D. New Insights on the Barrel Medic MtOGG1 and MtFPG Functions in Relation to Oxidative Stress Response in Planta and during Seed Imbibition. Plant Physiol. Biochem. 2011, 49, 1040–1050. [Google Scholar] [CrossRef]
- Taylor, R.E.; Waterworth, W.; West, C.E.; Foyer, C.H. WHIRLY Proteins Maintain Seed Longevity by Effects on Seed Oxygen Signalling during Imbibition. Biochem. J. 2023, 480, 941–956. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed Longevity: Survival and Maintenance of High Germination Ability of Dry Seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Verma, P.; Kaur, H.; Petla, B.P.; Rao, V.; Saxena, S.C.; Majee, M. PROTEIN L-ISOASPARTYL METHYLTRANSFERASE2 Is Differentially Expressed in Chickpea and Enhances Seed Vigor and Longevity by Reducing Abnormal Isoaspartyl Accumulation Predominantly in Seed Nuclear Proteins. Plant Physiol. 2013, 161, 1141–1157. [Google Scholar] [CrossRef]
- Petla, B.P.; Kamble, N.U.; Kumar, M.; Verma, P.; Ghosh, S.; Singh, A.; Rao, V.; Salvi, P.; Kaur, H.; Saxena, S.C.; et al. Rice PROTEIN L-ISOASPARTYL METHYLTRANSFERASE Isoforms Differentially Accumulate during Seed Maturation to Restrict Deleterious isoAsp and Reactive Oxygen Species Accumulation and Are Implicated in Seed Vigor and Longevity. New Phytol. 2016, 211, 627–645. [Google Scholar] [CrossRef]
- Gazzarrini, S.; Song, L. LAFL Factors in Seed Development and Phase Transitions. Annu. Rev. Plant Biol. 2024, 75, 459–488. [Google Scholar] [CrossRef]
- Michalak, M.; Plitta-Michalak, B.P.; Suszka, J.; Naskręt-Barciszewska, M.Z.; Kotlarski, S.; Barciszewski, J.; Chmielarz, P. Identification of DNA Methylation Changes in European Beech Seeds during Desiccation and Storage. Int. J. Mol. Sci. 2023, 24, 3557. [Google Scholar] [CrossRef]
- Layat, E.; Bourcy, M.; Cotterell, S.; Zdzieszyńska, J.; Desset, S.; Duc, C.; Tatout, C.; Bailly, C.; Probst, A.V. The Histone Chaperone HIRA Is a Positive Regulator of Seed Germination. Int. J. Mol. Sci. 2021, 22, 4031. [Google Scholar] [CrossRef]
- Zhao, T.; Lu, J.; Zhang, H.; Xue, M.; Pan, J.; Ma, L.; Berger, F.; Jiang, D. Histone H3.3 Deposition in Seed Is Essential for the Post-Embryonic Developmental Competence in Arabidopsis. Nat. Commun. 2022, 13, 7728. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Qi, Y.-P.; Yang, L.-T.; Peng, G.; Yan, L.; Chen, L.-S. Boron-Deficiency-Responsive microRNAs and Their Targets in Citrus sinensis Leaves. BMC Plant Biol. 2015, 15, 271. [Google Scholar] [CrossRef]
- Sugliani, M.; Rajjou, L.; Clerkx, E.J.M.; Koornneef, M.; Soppe, W.J.J. Natural Modifiers of Seed Longevity in the Arabidopsis Mutants Abscisic Acid. Insensitive3-5 (Abi3-5) and Leafy Cotyledon1-3 (Lec1-3). N. Phytol. 2009, 184, 898–908. [Google Scholar] [CrossRef]
- Arif, M.A.R.; Afzal, I.; Börner, A. Genetic Aspects and Molecular Causes of Seed Longevity in Plants—A Review. Plants 2022, 11, 598. [Google Scholar] [CrossRef]
- Tan, S.; Cao, J.; Li, S.; Li, Z. Unraveling the Mechanistic Basis for Control of Seed Longevity. Plants 2025, 14, 805. [Google Scholar] [CrossRef]
- Paul, M.V.; Iyer, S.; Amerhauser, C.; Lehmann, M.; van Dongen, J.T.; Geigenberger, P. Oxygen Sensing via the Ethylene Response Transcription Factor RAP2.12 Affects Plant Metabolism and Performance under Both Normoxia and Hypoxia. Plant Physiol. 2016, 172, 141–153. [Google Scholar] [CrossRef]
- Prasad, C.T.M.; Kodde, J.; Angenent, G.C.; Hay, F.R.; McNally, K.L.; Groot, S.P.C. Identification of the Rice Rc Gene as a Main Regulator of Seed Survival under Dry Storage Conditions. Plant Cell Amp. Environ. 2023, 46, 1962–1980. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Sui, Y.; Ding, X.; Song, X. A Multiomic Study Uncovers a bZIP23-PER1A–Mediated Detoxification Pathway to Enhance Seed Vigor in Rice. Proc. Natl. Acad. Sci. USA 2022, 119, e2026355119. [Google Scholar] [CrossRef]
- Miao, C.; Wang, Z.; Zhang, L.; Yao, J.; Hua, K.; Liu, X.; Shi, H.; Zhu, J.-K. The Grain Yield Modulator miR156 Regulates Seed Dormancy through the Gibberellin Pathway in Rice. Nat. Commun. 2019, 10, 3822. [Google Scholar] [CrossRef]
- Zhu, M.; Xiao, R.; Yu, T.; Guo, T.; Zhong, X.; Qu, J.; Du, W.; Xue, W. Raffinose Priming Improves Seed Vigor by ROS Scavenging, RAFS, and α-GAL Activity in Aged Waxy Corn. Agronomy 2024, 14, 2843. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. ZmAGA1 Hydrolyzes RFOs Late during the Lag Phase of Seed Germination, Shifting Sugar Metabolism toward Seed Germination Over Seed Aging Tolerance. J. Agric. Food Chem. 2021, 69, 11606–11615. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yu, Z.; Wang, Y.; Yang, Y.; Liu, Z.; Jiang, J.; Song, M.; Wu, Y. Superior Storage Stability in Low Lipoxygenase Maize Varieties. J. Stored Prod. Res. 2007, 43, 530–534. [Google Scholar] [CrossRef]
- Yan, H.; Jia, S.; Mao, P. Melatonin Priming Alleviates Aging-Induced Germination Inhibition by Regulating β-Oxidation, Protein Translation, and Antioxidant Metabolism in Oat (Avena sativa L.) Seeds. Int. J. Mol. Sci. 2020, 21, 1898. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wang, J.; Zhao, G.; Niu, K.; Zhou, X.; Zhang, R.; Yao, R. Genomic Identification and Expression Profiling of DMP Genes in Oat (Avena sativa) Elucidate Their Responsiveness to Seed Aging. BMC Genom. 2024, 25, 863. [Google Scholar] [CrossRef]
- Le, H.; Nguyen, N.H.; Ta, D.T.; Le, T.N.T.; Bui, T.P.; Le, N.T.; Nguyen, C.X.; Rolletschek, H.; Stacey, G.; Stacey, M.G.; et al. CRISPR/Cas9-Mediated Knockout of Galactinol Synthase-Encoding Genes Reduces Raffinose Family Oligosaccharide Levels in Soybean Seeds. Front. Plant Sci. 2020, 11, 612942. [Google Scholar] [CrossRef]
- Dierking, E.C.; Bilyeu, K.D. Association of a Soybean Raffinose Synthase Gene with Low Raffinose and Stachyose Seed Phenotype. Plant Genome 2008, 1, 135–145. [Google Scholar] [CrossRef]
- de Koning, R.; Kiekens, R.; Toili, M.E.M.; Angenon, G. Identification and Expression Analysis of the Genes Involved in the Raffinose Family Oligosaccharides Pathway of Phaseolus vulgaris and Glycine max. Plants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Qiu, D.; Vuong, T.; Valliyodan, B.; Shi, H.; Guo, B.; Shannon, J.G.; Nguyen, H.T. Identification and Characterization of a Stachyose Synthase Gene Controlling Reduced Stachyose Content in Soybean. Theor. Appl. Genet. 2015, 128, 2167–2176. [Google Scholar] [CrossRef]
- Valentine, M.F.; De Tar, J.R.; Mookkan, M.; Firman, J.D.; Zhang, Z.J. Silencing of Soybean Raffinose Synthase Gene Reduced Raffinose Family Oligosaccharides and Increased True Metabolizable Energy of Poultry Feed. Front. Plant Sci. 2017, 8, 692. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Chowdhury, A.K.; Kato, H.; Macha, M.M. Cultivars Identification and Their Genetic Relationships in Dimocarpus longan Subspecies Based on RAPD Markers. Sci. Hortic. 2006, 109, 147–152. [Google Scholar] [CrossRef]
- Ciacka, K.; Tyminski, M.; Wal, A.; Gniazdowska, A.; Krasuska, U. Nitric Oxide–an Antidote to Seed Aging Modifies Meta-Tyrosine Content and Expression of Aging-Linked Genes in Apple Embryos. Front. Plant Sci. 2022, 13, 929245. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, Y.; Le, J.; Li, Q.; Mou, J.; Deng, S.; Li, J.; Wang, R.; Deng, Z.; Liu, J. Full-Length Transcriptome Sequencing Reveals the Molecular Mechanism of Metasequoia glyptostroboides Seed Responding to Aging. Antioxidants 2023, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Zuo, K.; Zhao, J.; Qin, J.; Qiu, C.; Sun, X.; Tang, K. Isolation and Characterization of a Homologous to Lipase Gene from Brassica napus. Russ. J. Plant Physiol. 2006, 53, 366–372. [Google Scholar] [CrossRef]
- Zuo, J.; Liu, J.; Gao, F.; Yin, G.; Wang, Z.; Chen, F.; Li, X.; Xu, J.; Chen, T.; Li, L.; et al. Genome-Wide Linkage Mapping Reveals QTLs for Seed Vigor-Related Traits Under Artificial Aging in Common Wheat (Triticum aestivum). Front. Plant Sci. 2018, 9, 1101. [Google Scholar] [CrossRef]
- Nisarga, K.N.; Vemanna, R.S.; Kodekallu Chandrashekar, B.; Rao, H.; Vennapusa, A.R.; Narasimaha, A.; Makarla, U.; Basavaiah, M.R. Aldo-Ketoreductase 1 (AKR1) Improves Seed Longevity in Tobacco and Rice by Detoxifying Reactive Cytotoxic Compounds Generated during Ageing. Rice 2017, 10, 11. [Google Scholar] [CrossRef]
- Yu, S.; Wang, C.; Wang, Q.; Sun, Q.; Zhang, Y.; Dong, J.; Yin, Y.; Zhang, S.; Yu, G. Identification and Analysis of SOD Family Genes in Peanut (Arachis hypogaea L.) and Their Potential. Roles in Stress Responses. Agronomy 2023, 13, 1959. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Moori, S.; Eisvand, H.R. Plant Growth Regulators and Ascorbic Acid Effects on Physiological Quality of Wheat Seedlings Obtained from Deteriorated Seeds. Pak. J. Bot. 2017, 49, 1811–1819. [Google Scholar]
- Colombo, F.; Pagano, A.; Sangiorgio, S.; Macovei, A.; Balestrazzi, A.; Araniti, F.; Pilu, R. Study of Seed Ageing in Lpa1-1 Maize Mutant and Two Possible Approaches to Restore Seed Germination. Int. J. Mol. Sci. 2023, 24, 732. [Google Scholar] [CrossRef]
- Li, G.; Xie, J.; Zhang, W.; Meng, F.; Yang, M.; Fan, X.; Sun, X.; Zheng, Y.; Zhang, Y.; Wang, M.; et al. Integrated Examination of the Transcriptome and Metabolome of the Gene Expression Response and Metabolite Accumulation in Soybean Seeds for Seed Storability under Aging Stress. Front. Plant Sci. 2024, 15, 1437107. [Google Scholar] [CrossRef]
- Brar, N.S.; Kaushik, P.; Dudi, B.S. Effect of Seed Priming Treatment on the Physiological Quality of Naturally Aged Onion (Allium cepa L.) Seeds. Appl. Ecol. Environ. Res. 2020, 18, 849–862. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Wang, X.; Li, T.; Zhang, X. Electrochemical Sensor for Sensitive Detection of Bisphenol a Based on Molecularly Imprinted TiO2 with Oxygen Vacancy. Biosens. Bioelectron. 2023, 237, 115520. [Google Scholar] [CrossRef]
- Shaffique, S.; Imran, M.; Kang, S.-M.; Khan, M.A.; Asaf, S.; Kim, W.-C.; Lee, I.-J. Seed Bio-Priming of Wheat with a Novel Bacterial Strain to Modulate Drought Stress in Daegu, South Korea. Front. Plant Sci. 2023, 14, 1118941. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold Plasma Treatment and Exogenous Salicylic Acid Priming Enhances Salinity Tolerance of Oryza sativa Seedlings. Protoplasma 2018, 256, 79–99. [Google Scholar] [CrossRef]
- Song, X.; Tang, S.; Liu, H.; Meng, Y.; Luo, H.; Wang, B.; Hou, X.-L.; Yan, B.; Yang, C.; Guo, Z.; et al. Inheritance of Acquired Adaptive Cold Tolerance in Rice through DNA Methylation. Cell 2025, 188, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Louis, N.; Dhankher, O.P.; Puthur, J.T. Seed Priming Can Enhance and Retain Stress Tolerance in Ensuing Generations by Inducing Epigenetic Changes and Trans-generational Memory. Physiol. Plant. 2023, 175, e13881. [Google Scholar] [CrossRef]
- Turgut-Kara, N.; Arikan, B.; Celik, H. Epigenetic Memory and Priming in Plants. Genetica 2020, 148, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Yan, M. Hydropriming Promotes Germination of Aged Napa Cabbage Seeds. Seed Sci. Technol. 2015, 43, 303–307. [Google Scholar] [CrossRef]
- Parmoon, G.; Ebadi, A.; Janbakhsh, S.; Moosav, S.A. Effects of Seed Priming on Catalase Activity and Storage Reservoirs of Aged Milk Thistle Seeds (Silybum marianum (L.) Gaertn). J. Agric. Sci. 2015, 21, 363–372. [Google Scholar] [CrossRef]
- Tonguç, M.; Güler, M.; Önder, S. Germination, Reserve Metabolism and Antioxidant Enzyme Activities in Safflower as Affected by Seed Treatments after Accelerated Aging. S. Afr. J. Bot. 2023, 153, 209–218. [Google Scholar] [CrossRef]
- Nazari, R.; Parsa, S.; Tavakkol Afshari, R.; Mahmoodi, S.; Seyyedi, S.M. Salicylic Acid Priming Before and After Accelerated Aging Process Increases Seedling Vigor in Aged Soybean Seed. J. Crop Improv. 2020, 34, 218–237. [Google Scholar] [CrossRef]
- Xia, F.; Cheng, H.; Chen, L.; Zhu, H.; Mao, P.; Wang, M. Influence of Exogenous Ascorbic Acid and Glutathione Priming on Mitochondrial Structural and Functional Systems to Alleviate Aging Damage in Oat Seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.M.; Sung, J.M. Priming Slows Deterioration of Artificially Aged Bitter Gourd Seeds by Enhancing Anti-Oxidative Activities. Seed Sci. Technol. 2008, 36, 350–359. [Google Scholar] [CrossRef]
- Sıtkı; Kara, E. Solid Matrix Priming of Cabbage Seed Lots: Repair of Ageing and Increasing Seed Quality. J. Agric. Sci. 2016, 22, 588–595. [Google Scholar] [CrossRef]
- Hacisalihoglu, G. Responses of Three Switchgrass (Panicum virgatum L.) Cultivars to Seed Priming and Differential Aging Conditions. Acta Agric. Scand. Soil Plant Sci. 2008, 58, 280–284. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, M.; Song, T.; Cheng, C.; Tian, Y.; Hu, J.; Zhang, J. Spermidine Enhanced the Antioxidant Capacity of Rice Seeds during Seed Aging. Plant Growth Regul. 2020, 91, 397–406. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-Priming of Soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to Improve Seed Germination and the Initial Seedling Growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.E.; Abdel-Aziz, H.M.M.; Heikal, Y.M. Nanopriming Technology Enhances Vigor and Mitotic Index of Aged Vicia faba Seeds Using Chemically Synthesized Silver Nanoparticles. S. Afr. J. Bot. 2019, 125, 393–401. [Google Scholar] [CrossRef]
- Gokdas, Z.; Yildirim, E.; Gupta, S.; Demir, I. Karrikinolide Stimulated Seed Germination of Artificially Aged Marrow, Cabbage and Pepper Seeds through Repair of Cell Structure and Enzyme Activity. S. Afr. J. Bot. 2022, 151, 208–213. [Google Scholar] [CrossRef]
- Raj, D.; Dahiya, O.S.; Arya, R.K.; Yadav, A.K.; Kumar, K. Improvement in Germination Characteristics in Artificially Aged Seeds of Okra (Abelmoschus esculentus) by Osmoconditioning. Indian J. Agric. Sci. 2013, 83, 699–702. [Google Scholar]
- Kalia, A.; Sharma, S.P.; Devi, S. Effect of Surface Microbiome and Osmo-Conditioning on Restoration of Storage-Induced Losses of Seed Viability in Muskmelon (Cucumis melo L.). J. Agric. Sci. Technol. 2020, 22, 221–233. [Google Scholar]
- Wang, G.; Huang, J.; Gao, W.; Lu, J.; Li, J.; Liao, R.; Jaleel, C.A. The Effect of High-Voltage Electrostatic Field (HVEF) on Aged Rice (Oryza sativa L.) Seeds Vigor and Lipid Peroxidation of Seedlings. J. Electrost. 2009, 67, 759–764. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhang, X.; Xiong, C. Plasma Activated-water Stimulates Aged Pepper Seeds and Promotes Seedling Growth. Plasma Process. Polym. 2024, 21, 2300173. [Google Scholar] [CrossRef]
- Gamboa-deBuen, A.; Cruz-Ortega, R.; Martínez-Barajas, E.; Sánchez-Coronado, M.E.; Orozco-Segovia, A. Natural Priming as an Important Metabolic Event in the Life History of Wigandia urens (Hydrophyllaceae) Seeds. Physiol. Plant. 2006, 128, 520–530. [Google Scholar] [CrossRef]
- Varier, A.; Vari, A.K.; Dadlani, M. The Subcellular Basis of Seed Priming. Curr. Sci. 2010, 99, 450–456. [Google Scholar]
- Jiang, B.; Wang, L.; Xu, C.; Yan, M. Hydropriming Enhances the Germination of Aged Ultra-Dry Wheat Seeds. Seed Sci. Technol. 2020, 48, 57–63. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Yang, S.; He, D.; Khan, A.R.; Salam, A.; Azhar, W.; Muhammad, S.; Ali, S.; Hamid, Y.; Khan, I.; et al. Seed Priming with Nano-Silica Effectively Ameliorates Chromium Toxicity in Brassica Napus. J. Hazard. Mater. 2023, 458, 131906. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, E.; Yao, M.; Xue, D.; Zhao, N.; Zhou, Y.; Li, B.; Wang, K.; Miao, Y.; Gu, C.; et al. PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation. Agronomy 2023, 13, 3021. [Google Scholar] [CrossRef]
- Dos Santos, C.V.; Rey, P. Plant Thioredoxins Are Key Actors in the Oxidative Stress Response. Trends Plant Sci. 2006, 11, 329–334. [Google Scholar] [CrossRef]
- Córdoba-Cañero, D.; Roldán-Arjona, T.; Ariza, R.R. Arabidopsis ZDP DNA 3′-phosphatase and ARP Endonuclease Function in 8-oxoG Repair Initiated by FPG and OGG1 DNA Glycosylases. Plant J. 2014, 79, 824–834. [Google Scholar] [CrossRef]
- Forti, C.; Ottobrino, V.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular Dynamics of Pre-Germinative Metabolism in Primed Eggplant (Solanum melongena L.) Seeds. Hortic. Res. 2020, 7, 87. [Google Scholar] [CrossRef]
- Pagano, A.; Folini, G.; Pagano, P.; Sincinelli, F.; Rossetto, A.; Macovei, A.; Balestrazzi, A. ROS Accumulation as a Hallmark of Dehydration Stress in Primed and Overprimed Medicago Truncatula Seeds. Agronomy 2022, 12, 268. [Google Scholar] [CrossRef]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-Wide Characterization of Seed Aging in Arabidopsis: A Comparison between Artificial and Natural Aging Protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.M.; Collins, A.R.S.; Powell, A.A. The Effect of Aerated Hydration on DNA Synthesis in Embryos of Brassica oleracea L. Seed Sci. Res. 1993, 3, 195–199. [Google Scholar] [CrossRef]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The Enigmatic LEA Proteins and Other Hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef]
- Dirk, L.M.A.; Downie, A.B. An Examination of Job’s Rule: Protection and Repair of the Proteins of the Translational Apparatus in Seeds. Seed Sci. Res. 2018, 28, 168–181. [Google Scholar] [CrossRef]
- Capron, I.; Corbineau, F.; Dacher, F.; Job, C.; Côme, D.; Job, D. Sugarbeet Seed Priming: Effects of Priming Conditions on Germination, Solubilization of 11-S Globulin and Accumulation of LEA Proteins. Seed Sci. Res. 2000, 10, 243–254. [Google Scholar] [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced Expression of the Proline Synthesis Gene P5CSA in Relation to Seed Osmopriming Improvement of Brassica napus Germination under Salinity Stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Galland, M.; Rajjou, L. Regulation of mRNA Translation Controls Seed Germination and Is Critical for Seedling Vigor. Front. Plant Sci. 2015, 6, 284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).