Influence of Microclimatic Variations on Morphological Traits of Ferns in Urban Forests of Central Veracruz, Mexico
Abstract
1. Introduction
2. Results
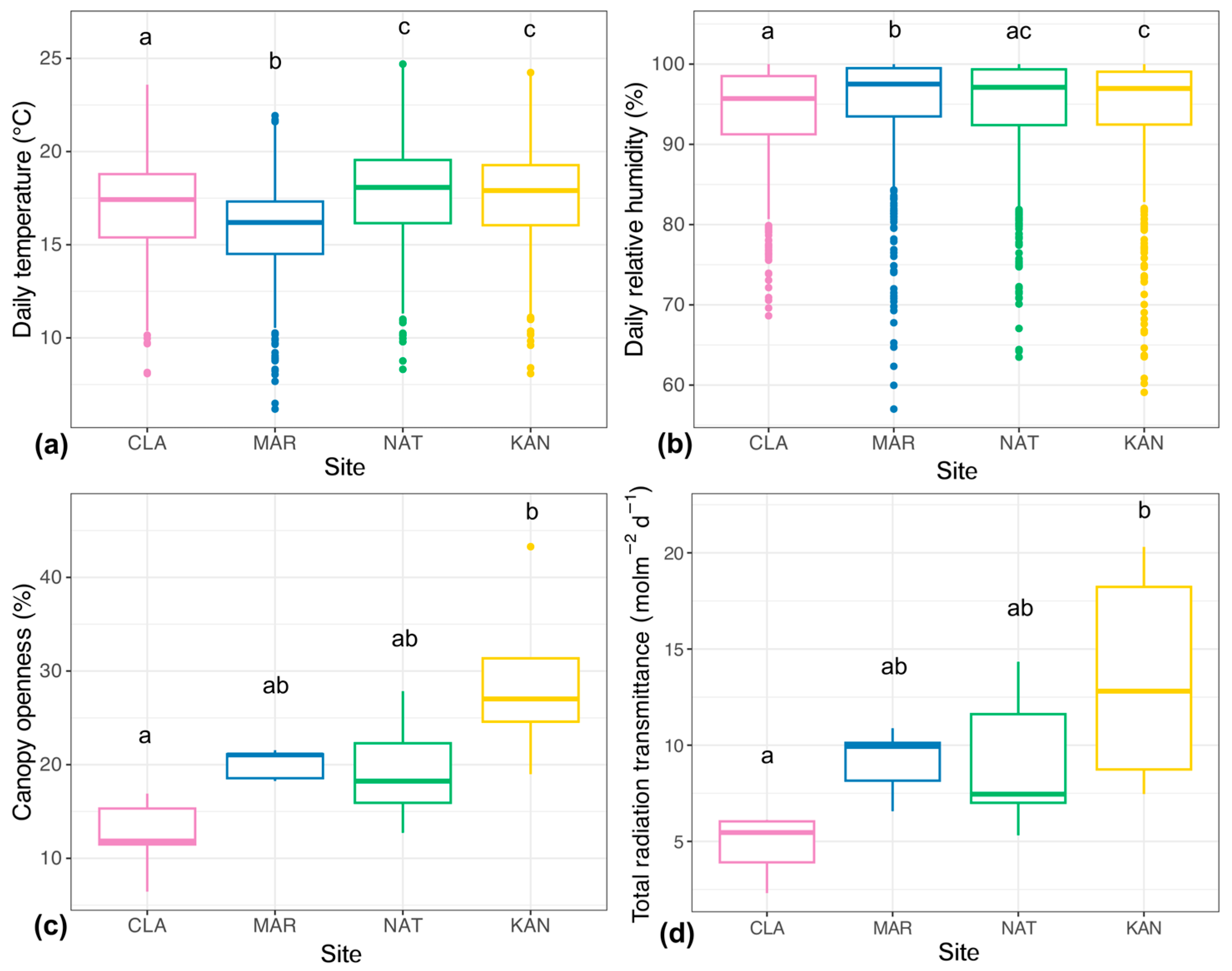
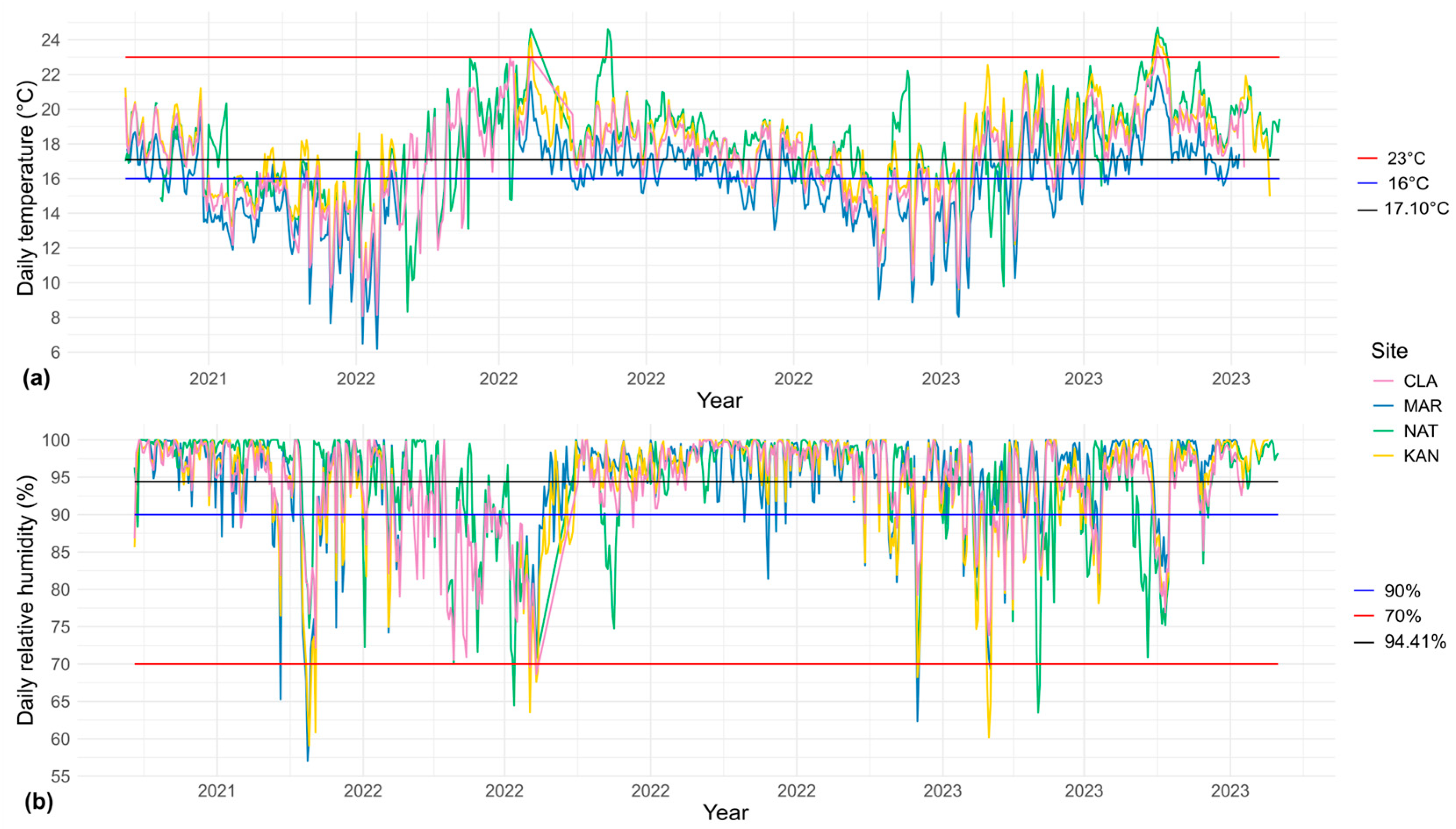
2.1. Microclimate
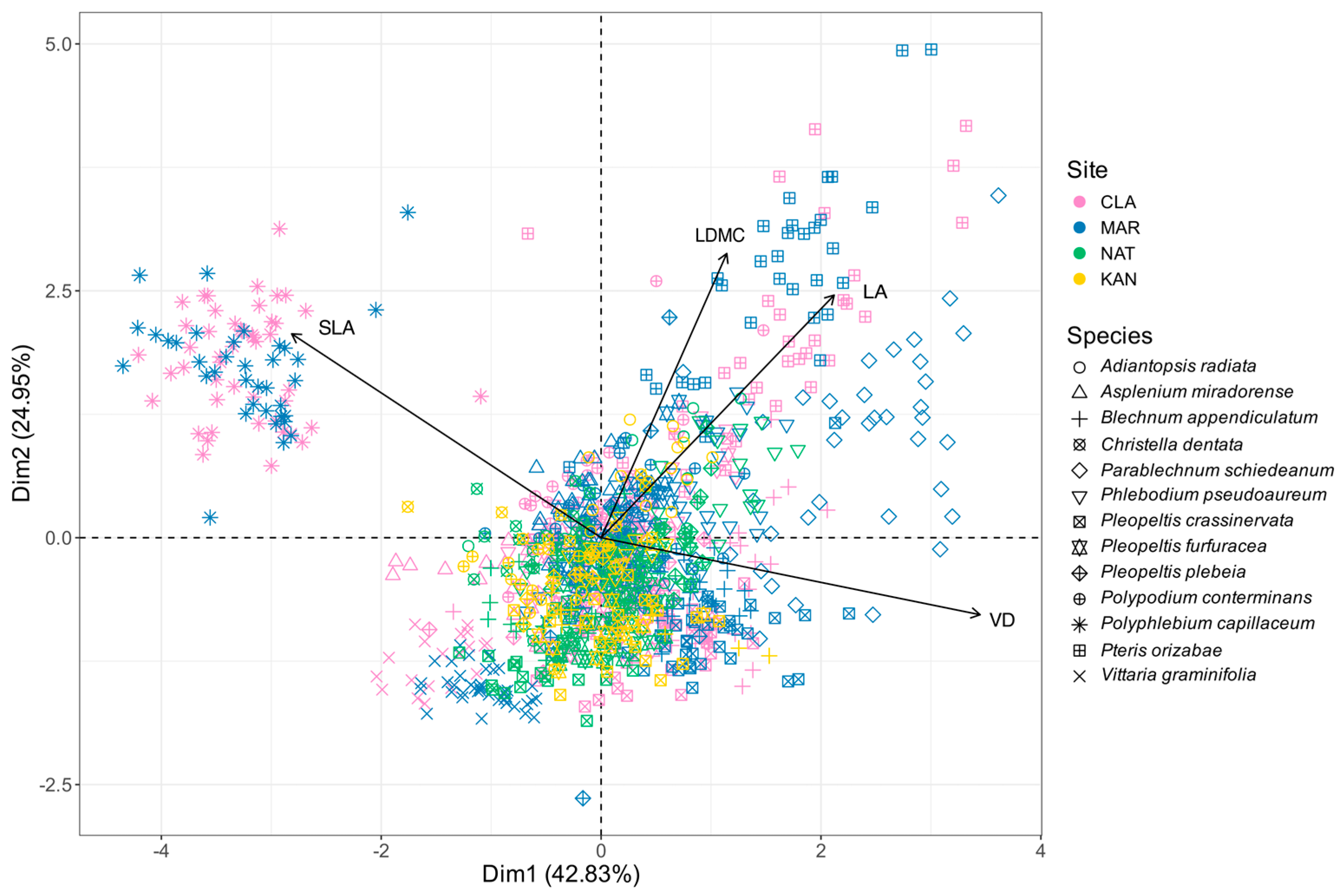
2.2. Principal Component Analysis
2.3. Generalized Linear Mixed Model
3. Discussion
3.1. Microclimatic Variation
3.2. Microclimatic Influence on Morphological Variation
3.3. Morphological Differentiation and Tendency Towards Biotic Homogenization in Urban Forests
4. Materials and Methods
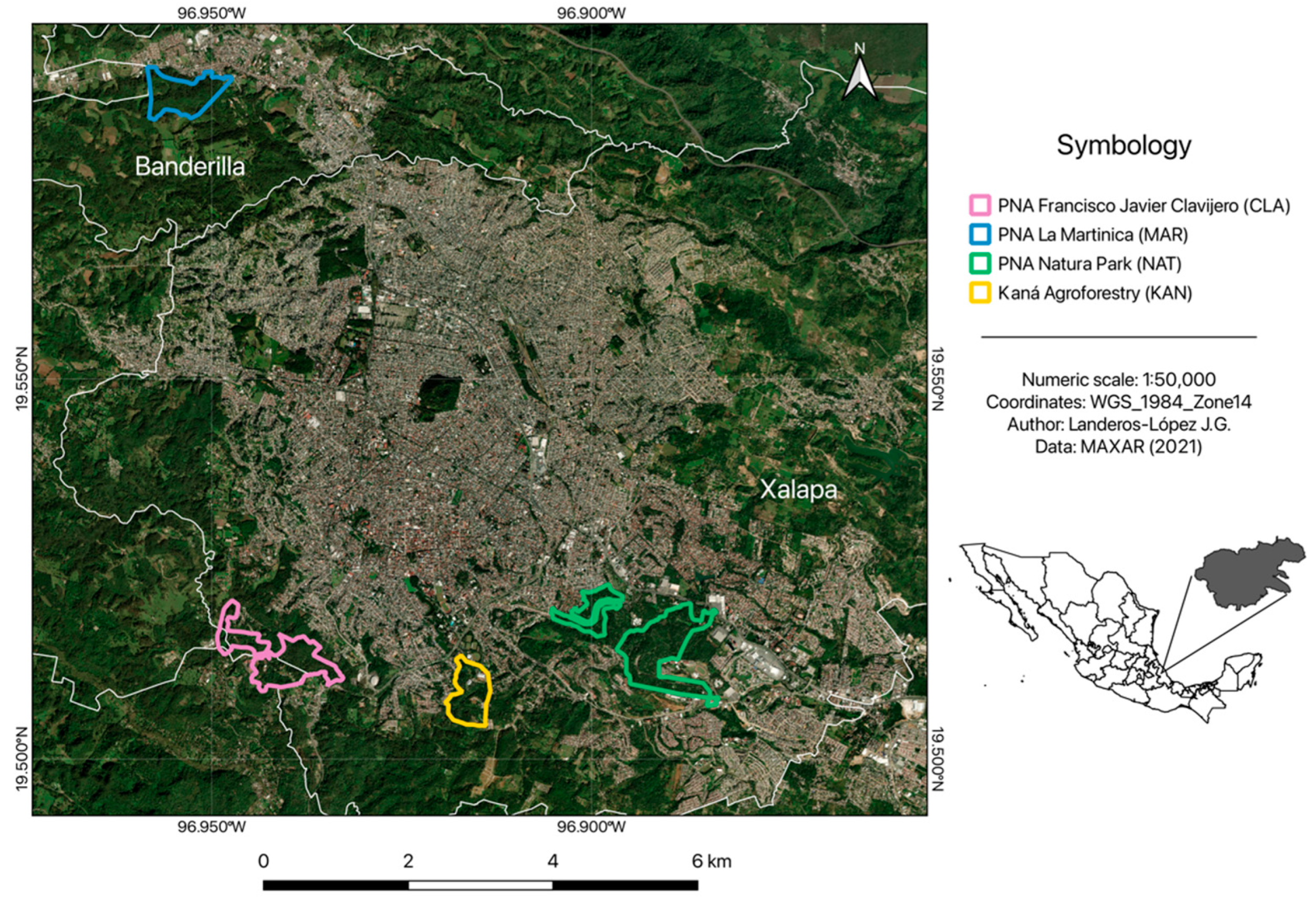
4.1. Study Sites
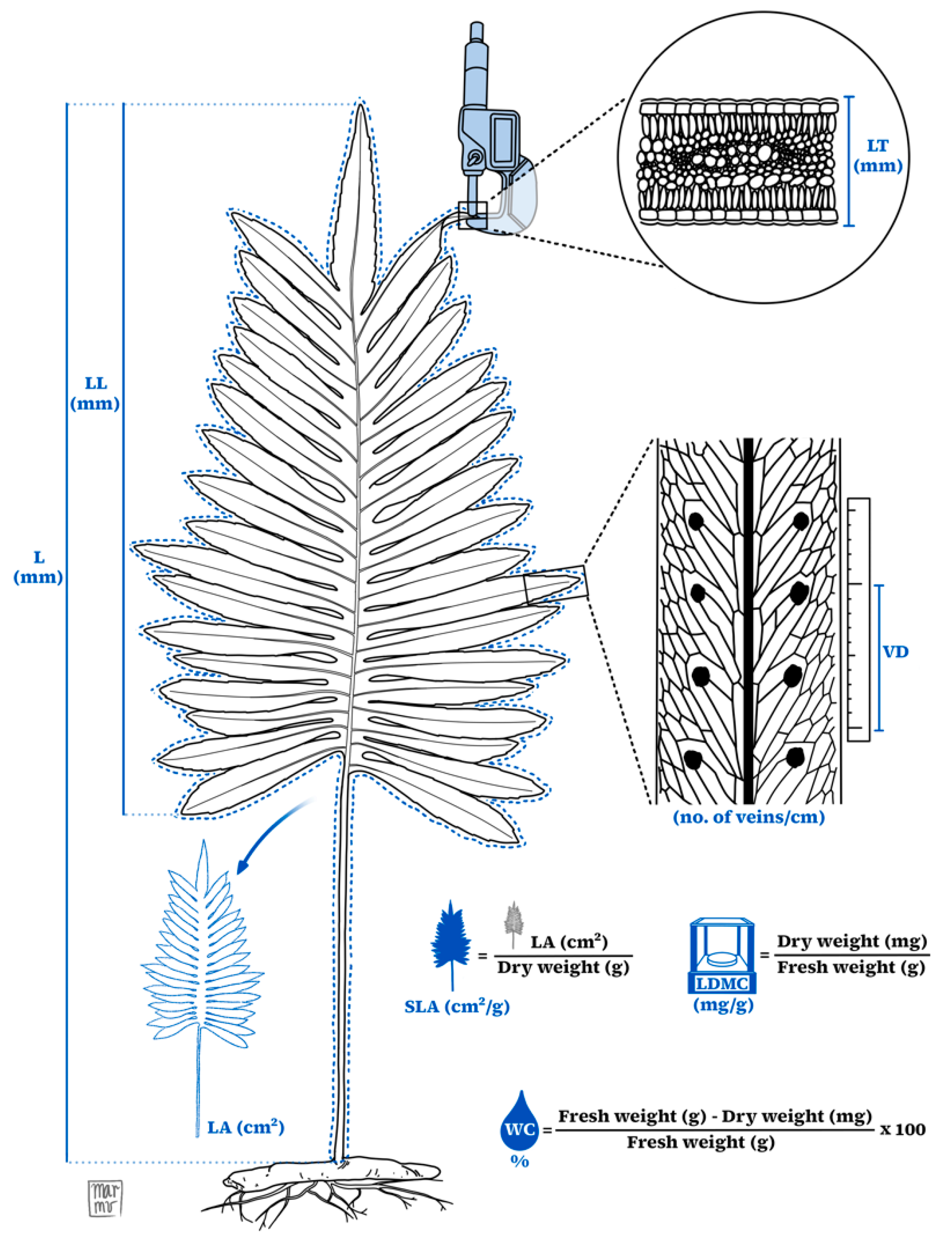
4.2. Data Collection
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvey, A.A. Promoting and Preserving Biodiversity in the Urban Forest. Urban For. Urban Green. 2006, 5, 195–201. [Google Scholar] [CrossRef]
- Jim, C.Y. Conservation and Creation of Urban Woodlands. In Greening Cities: Forms and Functions; Tan, P.Y., Jim, C.Y., Eds.; Springer: Singapore, 2017; pp. 307–330. ISBN 978-981-10-4113-6. [Google Scholar]
- Chacón-Castillo, D. Áreas Potenciales Para la Conservación en Xalapa, Veracruz y Conurbados: Cobertura y Almacenes de Carbono. Master’s Thesis, Universidad Veracruzana, Xalapa, Mexico, 2020. [Google Scholar]
- McKinney, M.L.; Ingo, K.; Kendal, D. The Contribution of Wild Urban Ecosystems to Liveable Cities. Urban For. Urban Green. 2018, 29, 334–335. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Ossola, A.; Hahs, A.K.; Williams, N.S.G.; Wilson, L.; Livesley, S.J. Variation in Vegetation Structure and Composition across Urban Green Space Types. Front. Ecol. Evol. 2016, 4, 66. [Google Scholar] [CrossRef]
- Roeland, S.; Moretti, M.; Amorim, J.H.; Branquinho, C.; Fares, S.; Morelli, F.; Niinemets, Ü.; Paoletti, E.; Pinho, P.; Sgrigna, G.; et al. Towards an Integrative Approach to Evaluate the Environmental Ecosystem Services Provided by Urban Forest. J. For. Res. 2019, 30, 1981–1996. [Google Scholar] [CrossRef]
- Zefferman, E.P.; McKinney, M.L.; Cianciolo, T.; Fritz, B.I. Knoxville’s Urban Wilderness: Moving Toward Sustainable Multifunctional Management. Urban For. Urban Green. 2018, 29, 357–366. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of Changing Biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- de Barros Ruas, R.; Costa, L.M.S.; Bered, F. Urbanization Driving Changes in Plant Species and Communities—A Global View. Glob. Ecol. Conserv. 2022, 38, e02243. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in Forest Ecosystem and Landscape Ecology: Variations in Local Climate Can Be Used to Monitor and Compare the Effects of Different Management Regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Gómez-Sans, V. Forest covers and microclimate response. For. Syst. 2004, 13, S84–S100. [Google Scholar] [CrossRef]
- Laurance, W.F.; Delamônica, P.; Laurance, S.G.; Vasconcelos, H.L.; Lovejoy, T.E. Rainforest Fragmentation Kills Big Trees. Nature 2000, 404, 836. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- McDonnell, M.J.; MacGregor-Fors, I. The Ecological Future of Cities. Science 2016, 352, 936–938. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Effects of Urbanization on Species Richness: A Review of Plants and Animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Alvim, F.S.; Furtado, S.G.; Menini Neto, L. Are Vascular Epiphytes in Urban Green Areas Subject to the Homogenization of Biodiversity? A Case Study in the Brazilian Atlantic Forest. Urban Ecosyst. 2021, 24, 701–713. [Google Scholar] [CrossRef]
- Knapp, S.; Winter, M.; Klotz, S. Increasing Species Richness but Decreasing Phylogenetic Richness and Divergence over a 320-Year Period of Urbanization. J. Appl. Ecol. 2017, 54, 1152–1160. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Mehltreter, K.; Walker, L.R.; Sharpe, J.M. Fern Ecology; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-1-139-48768-9. [Google Scholar]
- Kato, M. Biogeography of Ferns: Dispersal and Vicariance. J. Biogeogr. 1993, 20, 265–274. [Google Scholar] [CrossRef]
- Barrington, D.S. Ecological and Historical Factors in Fern Biogeography. J. Biogeogr. 1993, 20, 275–279. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Zwieniecki, M.A.; Palma, B. Leaf Hydraulic Capacity in Ferns, Conifers and Angiosperms: Impacts on Photosynthetic Maxima. New Phytol. 2005, 165, 839–846. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M. Passive Origins of Stomatal Control in Vascular Plants. Science 2011, 331, 582–585. [Google Scholar] [CrossRef]
- Kawai, H.; Kanegae, T.; Christensen, S.; Kiyosue, T.; Sato, Y.; Imaizumi, T.; Kadota, A.; Wada, M. Responses of Ferns to Red Light are Mediated by an Unconventional Photoreceptor. Nature 2003, 421, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Schuettpelz, E.; Pryer, K.M.; Cranfill, R.; Magallón, S.; Lupia, R. Ferns Diversified in the Shadow of Angiosperms. Nature 2004, 428, 553–557. [Google Scholar] [CrossRef]
- Bergeron, A.; Pellerin, S. Pteridophytes as Indicators of Urban Forest Integrity. Ecol. Indic. 2014, 38, 40–49. [Google Scholar] [CrossRef]
- Carvajal-Hernández, C.I.; Krömer, T.; López-Acosta, J.C.; Gómez-Díaz, J.A.; Kessler, M. Conservation Value of Disturbed and Secondary Forests for Ferns and Lycophytes along an Elevational Gradient in Mexico. Appl. Veg. Sci. 2017, 20, 662–672. [Google Scholar] [CrossRef]
- Krömer, T.; García-Franco, J.G.; Toledo-Aceves, T. Epífitas vasculares como bioindicadores de la calidad forestal: Impacto antrópico sobre su diversidad y composición. In Bioindicadores: Guardianes de Nuestro Futuro Ambiental; Instituto Nacional de Ecología y Cambio Climático/El Colegio de la Frontera Sur.: Versailles, KY, USA, 2014; pp. 606–623. [Google Scholar]
- Silva, V.L.; Mehltreter, K.; Schmitt, J.L. Ferns as Potential Ecological Indicators of Edge Effects in Two Types of Mexican Forests. Ecol. Indic. 2018, 93, 669–676. [Google Scholar] [CrossRef]
- Karst, A.L.; Lechowicz, M.J. Are Correlations among Foliar Traits in Ferns Consistent with Those in the Seed Plants? New Phytol. 2007, 173, 306–312. [Google Scholar] [CrossRef]
- Kessler, M.; Siorak, Y.; Wunderlich, M.; Wegner, C. Patterns of Morphological Leaf Traits among Pteridophytes along Humidity and Temperature Gradients in the Bolivian Andes. Funct. Plant Biol. 2007, 34, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Schellenberger Costa, D.; Zotz, G.; Hemp, A.; Kleyer, M. Trait Patterns of Epiphytes Compared to Other Plant Life-Forms along a Tropical Elevation Gradient. Funct. Ecol. 2018, 32, 2073–2084. [Google Scholar] [CrossRef]
- Guzmán-Jacob, V.; Guerrero-Ramírez, N.R.; Craven, D.; Brant Paterno, G.; Taylor, A.; Krömer, T.; Wanek, W.; Zotz, G.; Kreft, H. Broad- and Small-Scale Environmental Gradients Drive Variation in Chemical, but not Morphological, Leaf Traits of Vascular Epiphytes. Funct. Ecol. 2022, 36, 1858–1872. [Google Scholar] [CrossRef]
- Petter, G.; Wagner, K.; Wanek, W.; Sánchez Delgado, E.J.; Zotz, G.; Cabral, J.S.; Kreft, H. Functional Leaf Traits of Vascular Epiphytes: Vertical Trends Within the Forest, Intra- and Interspecific Trait Variability, and Taxonomic signals. Funct. Ecol. 2016, 30, 188–198. [Google Scholar] [CrossRef]
- Hietz, P.; Briones, O. Correlation Between Water Relations and within-Canopy Distribution of Epiphytic Ferns in a Mexican Cloud Forest. Oecologia 1998, 114, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Hietz, P.; Briones, O. Photosynthesis, Chlorophyll Fluorescence and Within-Canopy Distribution of Epiphytic Ferns in a Mexican Cloud Forest. Plant Biol. 2001, 3, 279–287. [Google Scholar] [CrossRef]
- Kluge, J.; Kessler, M. Morphological Characteristics of Fern Assemblages along an Elevational Gradient: Patterns and Causes. Ecotropica 2007, 13, 27–43. [Google Scholar]
- Susan-Tepetlan, T.M.; Velázquez-Rosas, N.; Krömer, T. Changes in the functional characteristics of vascular epiphytes from montane cloud forest and secondary vegetation in the central region of Veracruz, Mexico. Bot. Sci. 2015, 93, 153–163. [Google Scholar] [CrossRef]
- Castillo-Campos, G. Vegetación y Flora Del Municipio de Xalapa, Veracruz; Instituto de Ecología, A.C., MAB UNESCO, H., Ayuntamiento de Xalapa, Veracruz: Xalapa, Mexico, 1991. [Google Scholar]
- Tejero-Díez, J.D.; Torres-Díaz, A.; Mickel, J.T.; Mehltreter, K.; Krömer, T. Helechos y Licopodios. In La biodiversidad en Veracruz: Estudio de estado; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Gobierno del Estado de Veracruz, Universidad Veracruzana, Instituto de Ecología, A.C.: Xalapa, Mexico, 2011; Volume II, pp. 91–115. [Google Scholar]
- Lemoine-Rodríguez, R.; MacGregor-Fors, I.; Muñoz-Robles, C. Six Decades of Urban Green Change in a Neotropical City: A Case Study of Xalapa, Veracruz, Mexico. Urban Ecosyst. 2019, 22, 609–618. [Google Scholar] [CrossRef]
- Falfán, I.; MacGregor-Fors, I. Woody Neotropical Streetscapes: A Case Study of Tree and Shrub Species Richness and Composition in Xalapa. Madera Bosques 2016, 22, 95–110. [Google Scholar]
- García-Campos, H.M. Las áreas verdes públicas de Xalapa. In Ecología Urbana Aplicada a la Ciudad de Xalapa. Xalapa, México; Instituto de Ecología, A.C., MAB UNESCO, H. Ayuntamiento de Xalapa, Veracruz: Xalapa, Mexico, 1993; pp. 99–132. [Google Scholar]
- Hernández-Rivera, M.G.; Torres-Hernández, L. Analysis of Two Protected Natural Areas in Relation to the Growth of the Metropolitan Area of Xalapa, Veracruz. Investig. Geográficas 2015, 87, 51–61. [Google Scholar] [CrossRef]
- Benítez, G.; Ruelas-Monjardín, L.C.; Von Thaden, J.; Acosta-Rosado, I.; Alvarado-Castillo, G.; Equihua, M. Carbon Storage in a Peri-Urban Neotropical Forest: Assessing Its Potential and Patterns of Change over Half a Century. Urban For. Urban Green. 2023, 86, 128009. [Google Scholar] [CrossRef]
- Lemoine-Rodríguez, R.; Inostroza, L.; Falfán, I.; MacGregor-Fors, I. Too Hot to Handle? On the Cooling Capacity of Urban Green Spaces in a Neotropical Mexican City. Urban For. Urban Green. 2022, 74, 127633. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Avendaño-Reyes, S.; Bandala, V.M.; Chacón-Zapata, S.; Díaz-Toribio, M.H.; González-García, F.; Lorea-Hernández, F.; Martínez-Gómez, J.; Montes de Oca, E.; Montoya, L.; et al. Multi-Taxonomic Diversity Patterns in a Neotropical Green City: A Rapid Biological Assessment. Urban Ecosyst. 2015, 18, 633–647. [Google Scholar] [CrossRef]
- Jara-Toto, E.; Armenta-Montero, S.; Aquino-Zapata, A.M.; Hernández, C.C. Diversidad y estructura de la vegetación leñosa en cuatro bosques urbanos de la zona conurbada Xalapa-Banderilla, Veracruz, Mexico. Acta Bot. Mex. 2023, 130, e2214. [Google Scholar] [CrossRef]
- Vargas-Huipe, N.D.; Rodríguez-Van Gort, M.F. Clima cambiante en la Zona Metropolitana de Xalapa: Factores naturales y antrópicos. Investig. Geográficas 2024, 114, e60855. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Pearcy, R.W. The Importance of Sunflecks for Forest Understory Plants. BioScience 1991, 41, 760–766. [Google Scholar] [CrossRef]
- Jennings, S.B.; Brown, N.D.; Sheil, D. Assessing Forest Canopies and Understorey Illumination: Canopy Closure, Canopy Cover and Other Measures. Forestry 1999, 72, 59–74. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Matlack, G.R. Microenvironment Variation within and among Forest Edge Sites in the Eastern United States. Biol. Conserv. 1993, 66, 185–194. [Google Scholar] [CrossRef]
- Zellweger, F.; Coomes, D.; Lenoir, J.; Depauw, L.; Maes, S.L.; Wulf, M.; Kirby, K.J.; Brunet, J.; Kopecký, M.; Máliš, F.; et al. Seasonal Drivers of Understorey Temperature Buffering in Temperate Deciduous Forests Across Europe. Glob. Ecol. Biogeogr. 2019, 28, 1774–1786. [Google Scholar] [CrossRef]
- Santiago-Pérez, A.L.; Jardel-Peláez, E.J.; Cuevas-Guzmán, R.; Huerta-Martínez, F.M. Vegetación de bordes en un Bosque Mesófilo de Montaña del Occidente de México. Bol. Soc. Botánica México 2009, 85, 31–49. [Google Scholar] [CrossRef][Green Version]
- Ewers, R.M.; Banks-Leite, C. Fragmentation Impairs the Microclimate Buffering Effect of Tropical Forests. PLoS ONE 2013, 8, e58093. [Google Scholar] [CrossRef]
- Geiger, R.; Aron, R.H.; Todhunter, P. The Climate Near the Ground; Rowman & Littlefield: Lanham, MD, USA, 2009. [Google Scholar]
- Bach, K.; Schawe, M.; Beck, S.; Gerold, G.; Gradstein, S.R.; Moraes, R.M. Vegetación, suelos y clima en los diferentes pisos altitudinales de un bosque montano de Yungas, Bolivia: Primeros resultados. Ecol. En Boliv. Rev. Inst. Ecol. 2003, 38, 3–14. [Google Scholar]
- Pérez-Rendón, E.P.; Ramírez-Builes, V.H.; Peña-Quiñones, A.J. Variabilidad espacial y temporal de la temperatura del aire en la zona cafetera colombiana. Investig. Geográficas 2016, 89, 23–40. [Google Scholar] [CrossRef][Green Version]
- Bruijnzeel, L.A.; Proctor, J. Hydrology and Biogeochemistry of Tropical Montane Cloud Forests: What Do We Really Know? In Tropical Montane Cloud Forests; Hamilton, L.S., Juvik, J.O., Scatena, F.N., Eds.; Springer: New York, NY, USA, 1995; pp. 38–78. [Google Scholar]
- Kitayama, K. Biophysical Conditions of the Montane Cloud Forests of Mount Kinabalu, Sabah, Malaysia. In Tropical Montane Cloud Forests; Hamilton, L.S., Juvik, J.O., Scatena, F.N., Eds.; Springer: New York, NY, USA, 1995; pp. 183–197. [Google Scholar]
- Schawe, M.; Gerold, G.; Bach, K.; Gradstein, S.R. Hydrometeorological Patterns in Relation to Montane Forest Types along an Elevational Gradient in the Yungas of Bolivia. In Tropical Montane Cloud Forests: Science for Conservation and Management; Scatena, F.N., Bruijnzeel, L.A., Hamilton, L.S., Eds.; International Hydrology Series; Cambridge University Press: Cambridge, UK, 2011; pp. 199–207. ISBN 978-0-521-76035-5. [Google Scholar]
- Bruijnzeel, L.A.; Mulligan, M.; Scatena, F.N. Hydrometeorology of tropical montane cloud forests: Emerging patterns. Hydrol. Process. 2011, 25, 465–498. [Google Scholar] [CrossRef]
- Foster, P. Changes in mist immersion. In Tropical Montane Cloud Forests: Science for Conservation and Management; Scatena, F.N., Bruijnzeel, L.A., Hamilton, L.S., Eds.; International Hydrology Series; Cambridge University Press: Cambridge, UK, 2010; pp. 57–66. ISBN 978-0-521-76035-5. [Google Scholar]
- Pérez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.; Cornwell, W.; Craine, J.; Gurvich, D.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Benzing, D.H. Vascular Epiphytes: General Biology and Related Biota; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Krömer, T.; Kessler, M.; Gradstein, S.R. Vertical Stratification of Vascular Epiphytes in Submontane and Montane Forest of the Bolivian Andes: The Importance of the Understory. Plant Ecol. 2007, 189, 261–278. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Zotz, G.; Hietz, P. The Physiological Ecology of Vascular Epiphytes: Current Knowledge, Open Questions. J. Exp. Bot. 2001, 52, 2067–2078. [Google Scholar] [CrossRef]
- Hernández-Zamora, D. Riqueza de epífitas vasculares en las Áreas Verdes Urbanas y Periurbanas de Xalapa, Veracruz. Bachelor’s Thesis, Universidad Veracruzana, Xalapa, Mexico, 2022. [Google Scholar]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass Per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to Sun and Shade: A Whole-Plant Perspective. Funct. Plant Biol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, A Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Hietz, P.; Wagner, K.; Nunes Ramos, F.; Cabral, J.S.; Agudelo, C.; Benavides, A.M.; Cach-Pérez, M.J.; Cardelús, C.L.; Chilpa Galván, N.; Erickson Nascimento da Costa, L.; et al. Putting Vascular Epiphytes on the Traits Map. J. Ecol. 2022, 110, 340–358. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, E.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific Leaf Area and Dry Matter Content Estimate Thickness in Laminar Leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- He, N.; Liu, C.; Tian, M.; Li, M.; Yang, H.; Yu, G.; Guo, D.; Smith, M.D.; Yu, Q.; Hou, J. Variation in Leaf Anatomical Traits from Tropical to Cold-Temperate Forests and Linkage to Ecosystem Functions. Funct. Biol. 2018, 32, 10–19. [Google Scholar] [CrossRef]
- Mediavilla, S.; Gallardo-López, V.; González-Zurdo, P.; Escudero, A. Patterns of Leaf Morphology and Leaf N Content in Relation to Winter Temperatures in Three Evergreen Tree Species. Int. J. Biometeorol. 2012, 56, 915–926. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, N.; Wei, T.; Liu, B.; Shen, L.; Liu, Y.; Liu, D. Adaptation Strategies of Leaf Traits and Leaf Economic Spectrum of Two Urban Garden Plants in China. BMC Plant Biol. 2023, 23, 274. [Google Scholar] [CrossRef]
- Körner, C.; Neumayer, M.; Menendez-Riedl, S.P.; Smeets-Scheel, A. Functional Morphology of Mountain Plants. Flora 1989, 182, 353–383. [Google Scholar] [CrossRef]
- Niinemets, Ü. Does the Touch of Cold Make Evergreen Leaves Tougher? Tree Physiol. 2016, 36, 267–272. [Google Scholar] [CrossRef]
- Gehrig-Downie, C.; Marquardt, J.; Obregón, A.; Bendix, J.; Gradstein, S.R. Diversity and Vertical Distribution of Filmy Ferns as a Tool for Identifying the Novel Forest Type “Tropical Lowland Cloud Forest”. Ecotropica 2012, 18, 35–44. [Google Scholar]
- Krömer, T.; Acebey, A.; Kluge, J.; Kessler, M. Effects of Altitude and Climate in Determining Elevational Plant Species Richness Patterns: A Case Study from Los Tuxtlas, Mexico. Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 197–210. [Google Scholar] [CrossRef]
- Dubuisson, J.-Y.; Hennequin, S.; Rakotondrainibe, F.; Schneider, H. Ecological Diversity and Adaptive Tendencies in the Tropical Fern Trichomanes L. (Hymenophyllaceae) with Special Reference to Climbing and Epiphytic Habits. Bot. J. Linn. Soc. 2003, 142, 41–63. [Google Scholar] [CrossRef]
- Saldaña, A.; Parra, M.J.; Flores-Bavestrello, A.; Corcuera, L.J.; Bravo, L.A. Effects of Forest Successional Status on Microenvironmental Conditions, Diversity, and Distribution of Filmy Fern Species in a Temperate Rainforest. Plant Species Biol. 2014, 29, 253–262. [Google Scholar] [CrossRef]
- Larrea, M.L.; Werner, F.A. Response of Vascular Epiphyte Diversity to Different Land-Use Intensities in a Neotropical Montane Wet Forest. For. Ecol. Manag. 2010, 260, 1950–1955. [Google Scholar] [CrossRef]
- Wolf, J.H.D. The Response of Epiphytes to Anthropogenic Disturbance of Pine-Oak Forests in the Highlands of Chiapas, Mexico. For. Ecol. Manag. 2005, 212, 376–393. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, S.; John, R.; Li, R.; Liu, H.; Ye, Q. Habitat Filtering and Exclusion of Weak Competitors Jointly Explain Fern Species Assemblage Along a Light and Water Gradient. Sci. Rep. 2017, 7, 298. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía (INEGI). Compendio de Información Geográfica Municipal de Los Estados Unidos Mexicanos 2010. Xalapa, Veracruz de Ignacio de la Llave. 2010. Available online: https://www.inegi.org.mx (accessed on 16 March 2025).
- Sistema de Información Estadística y Geográfica del Estado de Veracruz de Ignacio de la Llave (SIEGVER). Cuadernillos Municipales 2020: Xalapa. Gobierno del Estado de Veracruz: Veracruz, Mexico. 2020. Available online: https://ceieg.veracruz.gob.mx (accessed on 22 March 2025).
- Miranda, F.; Hernández-X, E. Los tipos de vegetación de México y su clasificación. Bot. Sci. 1963, 28, 29–179. [Google Scholar] [CrossRef]
- Rzedowski, J. La Vegetación de México; Limusa S.A.: Mexico City, Mexico, 1978. [Google Scholar]
- Instituto Nacional de Estadística y Geografía (INEGI). ¿Cuántos Habitantes Tiene Xalapa?—Censo de Población y Vivienda 2020. Available online: https://www.inegi.org.mx/app/cpv/2020/resultadosrapidos/default.html?texto=Xalapa (accessed on 10 February 2025).
- Instituto Nacional de Estadística y Geografía (INEGI). Censo de Población y Vivienda 2020: Población Total de Banderilla. Available online: https://censo2020.mx (accessed on 10 February 2025).
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analizer (GLA), Version 2.0; Simon Fraser University: Burnaby, BC, Canada, 1999.
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R, 1st ed.; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models; SAGE Publications: Thousand Oaks, CA, USA, 2015; ISBN 978-1-4833-2131-8. [Google Scholar]
- O’Hara, R.; Kotze, J. Do not Log-Transform Count Data. Nat. Preced. 2010, 1, 118–122. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A General and Simple Method for Obtaining R2 from Generalized Linear Mixed-Effects Models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R, Version 2024.09.1; RStudio Team: Boston, MA, USA, 2024. [Google Scholar]
- The Jamovi Project. Jamovi, Versión 2.3.28.0; Jamovi: Sydney, Australia, 2023.
- McKinney, M.L. Urbanization, Biodiversity, and Conservation: The Impacts of Urbanization on Native Species Are Poorly Studied, but Educating a Highly Urbanized Human Population about These Impacts Can Greatly Improve Species Conservation in All Ecosystems. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Falfán, I.; García-Arroyo, M.; Lemoine-Rodríguez, R.; Gómez-Martínez, M.A.; Marín-Gómez, O.H.; Pérez-Maqueo, O.; Equihua, M. A Novel Approach for the Assessment of Cities through Ecosystem Integrity. Land 2022, 11, 3. [Google Scholar] [CrossRef]
| Trait | Predictor | Estimate (β) | CI (95%) | t | p | R2 m/R2 c |
|---|---|---|---|---|---|---|
| LA | (Intercept) | 3.59 | 2.55–4.63 | 6.756 | <0.001 | 0.000/0.678 |
| Site KAN | 0.03 | −0.16–0.21 | 0.275 | 0.783 | ||
| Site MAR | −0.05 | −0.17–0.07 | −0.781 | 0.435 | ||
| Site NAT | 0.17 | 0.01–0.33 | 2.142 | 0.032 | ||
| Terrestrial Habit | 2.15 | 0.62–3.69 | 2.748 | 0.006 | ||
| SiteKAN × TerrestrialHabit | −0.49 | −0.83–−0.15 | −2.798 | 0.005 | ||
| SiteMAR × TerrestrialHabit | −0.12 | 0.34–0.09 | −1.158 | 0.247 | ||
| SiteNAT × TerrestrialHabit | −0.75 | −1.05–−0.45 | −4.875 | <0.001 | ||
| SLA | (Intercept) | 5.13 | 4.80–5.46 | 30.661 | <0.001 | 0.000/0.162 |
| Site KAN | 0.17 | 0.09–0.25 | 4.027 | <0.001 | ||
| Site MAR | −0.03 | −0.07–0.00 | −1.823 | 0.069 | ||
| Site NAT | 0.02 | −0.07–0.11 | 0.417 | 0.677 | ||
| TerrestrialHabit | 0.18 | −0.31–0.67 | 0.727 | 0.467 | ||
| SiteKAN × TerrestrialHabit | 0.07 | −0.08–0.23 | −0.950 | 0.342 | ||
| SiteMAR × TerrestrialHabit | −0.10 | −0.19–−0.02 | 2.401 | 0.017 | ||
| SiteNAT × TerrestrialHabit | 0.08 | −0.07–0.23 | 1.030 | 0.303 | ||
| LDMC | (Intercept) | 5.59 | 5.44–5.73 | 75.152 | <0.001 | 0.000/0.036 |
| Site KAN | −0.16 | −0.23–−0.10 | −4.953 | <0.001 | ||
| Site MAR | 0.07 | 0.03–0.11 | 3.818 | <0.001 | ||
| Site NAT | −0.08 | −0.13–−0.03 | −3.006 | 0.003 | ||
| TerrestrialHabit | 0.07 | −0.15–0.29 | 0.634 | 0.526 | ||
| SiteKAN × TerrestrialHabit | 0.16 | 0.04–0.29 | 2.515 | 0.012 | ||
| SiteMAR × TerrestrialHabit | 0.11 | 0.05–0.18 | 3.422 | 0.001 | ||
| SiteNAT × TerrestrialHabit | −0.02 | −0.13–0.09 | −0.281 | 0.779 | ||
| VD | (Intercept) | 1.68 | 1.25–2.11 | 7.649 | <0.001 | 0.050/0.298 |
| Site KAN | 0.03 | −0.04–0.10 | 0.810 | 0.418 | ||
| Site MAR | 0.07 | 0.02–0.12 | 2.746 | 0.006 | ||
| Site NAT | 0.07 | 0.00–0.13 | 2.048 | 0.041 | ||
| TerrestrialHabit | 0.72 | 0.08–1.35 | 2.222 | 0.027 | ||
| SiteKAN × TerrestrialHabit | −0.20 | −0.33–−0.06 | −2.884 | 0.004 | ||
| SiteMAR × TerrestrialHabit | −0.14 | −0.22–−0.05 | −3.137 | 0.002 | ||
| SiteNAT × TerrestrialHabit | −0.43 | −0.55–−0.31 | −7.125 | <0.001 |
| Urban Forest | Area (ha) | Altitude (masl) | Basal Area (m2/ha) | DBH (cm) | Height (m) | Density (tree/ha) | State of Maturity of the Vegetation |
|---|---|---|---|---|---|---|---|
| PNA Francisco Javier Clavijero (CLA) | 22.06 | 1362 | 3.3 ± 1.14 | 19.08 ± 21.89 | 13.48 ± 7.19 | 32 ± 46 | Mature forest with fragments of secondary forest |
| PNA La Martinica (MAR) | 52.30 | 1599 | 1.6 ± 0.72 | 13.57 ± 14.66 | 14.20 ± 6.80 | 49 ± 78 | Mature forest with fragments of secondary forest |
| PNA Natura Park (NAT) | 80 | 1320 | 1.6 ± 0.40 | 14.92 ± 13.38 | 11.20 ± 5.50 | 46 ± 75 | Secondary forest, with few remaining trees of mature forest |
| Kaná Agroforestry (KAN) | 5 | 1366 | 1.9 ± 0.56 | 14.86 ± 16.27 | 9.88 ± 5.73 | 37 ± 35 | Secondary forest with floristic elements of mature forest |
| Family | Species | Habit | CLA | MAR | NAT | KAN |
|---|---|---|---|---|---|---|
| Pteridaceae | Adiantopsis radiata (L.) Fée | T | X | |||
| Aspleniaceae | Asplenium miradorense Liebm. | T | X | X | ||
| Blechnaceae | Blechnum appendiculatum Willd. | T | X | X | X | X |
| Thelypteridaceae | Christella dentata (Forssk.) Brownsey & Jermy | T | X | X | ||
| Blechnaceae | Parablechnum schiedeanum (Schltdl. ex C. Presl) Gasper & Salino | T | X | |||
| Polypodiaceae | Phlebodium pseudoaureum (Cav.) Lellinger | E | X | X | X | X |
| Polypodiaceae | Pleopeltis crassinervata (Fée) T. Moore | E | X | X | X | X |
| Polypodiaceae | Pleopeltis furfuracea (Schltdl. & Cham.) A. R. Sm. & Tejero | E | X | X | X | X |
| Polypodiaceae | Pleopeltis plebeia (Schltdl. & Cham.) A. R. Sm. & Tejero | E | X | X | X | X |
| Hymenophyllaceae | Polyphlebium capillaceum (L.) Ebihara & Dubuisson | E | X | X | ||
| Polypodiaceae | Polypodium conterminans Liebm. | E | X | X | ||
| Pteridaceae | Pteris orizabae Mart. & Galeotti | T | X | X | ||
| Pteridaceae | Vittaria graminifolia Kaulf. | E | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landeros-López, J.G.; Krömer, T.; Gómez-Díaz, J.A.; Velázquez-Rosas, N.; Carvajal-Hernández, C.I. Influence of Microclimatic Variations on Morphological Traits of Ferns in Urban Forests of Central Veracruz, Mexico. Plants 2025, 14, 1732. https://doi.org/10.3390/plants14111732
Landeros-López JG, Krömer T, Gómez-Díaz JA, Velázquez-Rosas N, Carvajal-Hernández CI. Influence of Microclimatic Variations on Morphological Traits of Ferns in Urban Forests of Central Veracruz, Mexico. Plants. 2025; 14(11):1732. https://doi.org/10.3390/plants14111732
Chicago/Turabian StyleLanderos-López, Jessica G., Thorsten Krömer, Jorge A. Gómez-Díaz, Noé Velázquez-Rosas, and César I. Carvajal-Hernández. 2025. "Influence of Microclimatic Variations on Morphological Traits of Ferns in Urban Forests of Central Veracruz, Mexico" Plants 14, no. 11: 1732. https://doi.org/10.3390/plants14111732
APA StyleLanderos-López, J. G., Krömer, T., Gómez-Díaz, J. A., Velázquez-Rosas, N., & Carvajal-Hernández, C. I. (2025). Influence of Microclimatic Variations on Morphological Traits of Ferns in Urban Forests of Central Veracruz, Mexico. Plants, 14(11), 1732. https://doi.org/10.3390/plants14111732









