Abstract
Vitex L. is the largest genus of the Lamiaceae family, and most of its species are used in the traditional medicinal systems of different countries. A systematic review was conducted, according to the PRISMA methodology, to determine the potential of Vitex plants as sources of antimicrobial agents, resulting in 2610 scientific publications from which 141 articles were selected. Data analysis confirmed that Vitex species are used in traditional medicine for symptoms of possible infectious diseases. Conducted studies showed that these medicinal plants exhibited in vitro antimicrobial activity against Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus. Vitex agnus-castus L. and Vitex negundo L. have been the most studied species, not only against bacterial strains but also against fungi such as Aspergillus niger and Candida albicans, viruses such as HIV-1, and parasites such as Plasmodium falciparum. Natural products like agnucastoside, negundol, negundoside, and vitegnoside have been identified in Vitex extracts and their antimicrobial activity against a wide range of microbial strains has been determined. Negundoside showed significant antimicrobial activity against Staphylococcus aureus (MIC 12.5 µg/mL). Our results show that Vitex species are potential sources of new natural antimicrobial agents. However, further experimental studies need to be conducted.
1. Introduction
Bacterial resistance to clinically available antibiotics is a global phenomenon whose impact has increased significantly in recent years. Multidrug-resistant infections are a very common problem, greatly affecting mortality and morbidity in populations around the world and leading to greatly increased economic burdens. The Organization for Economic Cooperation and Development (OECD) estimates that the increase in multidrug-resistant bacterial infections will result in a total expense of approximately USD 20 to USD 35 trillion by 2050 [1,2]. Resistance mechanisms developed by bacteria to circumvent the effects of antibiotics are very diverse. Enzyme-based bacterial processes can directly inactivate antibiotics; efflux pumps can expel antibiotics from inside bacterial cells, reducing their concentration to subtoxic levels; mechanistic bacterial targets of antibiotics, such as ribosome subunits, DNA gyrase or RNA polymerase, can undergo conformational changes that prevent drugs from binding to them. Such mutations can be either spontaneous or adaptive, and some of them can undergo horizontal gene transfer, which can eventually lead to new naturally resistant bacteria. For example, Staphylococcus aureus, one of the most common etiological agents of infections in hospital and non-hospital contexts, shows a huge increase in resistance patterns to antibiotics of different classes, which means that this species can develop different resistance mechanisms to available drugs [2,3]. Medicinal plants have long been used in several traditional healing systems to treat many infectious symptoms and infectious diseases. Studies have shown that plant extracts (and/or natural products isolated from them) not only exert antimicrobial activity against various bacteria but can also modulate bacterial resistance mechanisms and increase the activity of concurrently administered antibiotics, or, in some cases, even reverse established resistance mechanisms. For example, several flavonoids, which constitute one of the most common classes of natural products, have demonstrated the ability to reverse bacterial multidrug resistance by inhibiting efflux pumps [4,5]. Research has shown that many plants’ secondary metabolites can exhibit antimicrobial activity, which can be exerted through a wide variety of mechanisms. Plants thus serve as direct antimicrobial agents and reservoirs of diverse bioactive compounds capable of inhibiting the growth and spread of harmful microorganisms. Vitex L., also known as the chaste tree genus, is the largest genus in the family Lamiaceae and comprises about 230 species distributed worldwide [6]. Most Vitex species are deciduous shrubs or small trees [7]. These species are scattered and mostly distributed in temperate regions of Asia and warm regions of Europe, being substantially distributed through Southeast Asia [8,9]. However, most species that belong to this genus are used in traditional medicine in southwest Asian countries like India, China, Nepal, Sri Lanka, Bangladesh, Malaysia, and other countries, namely Indonesia, Egypt, Iran, Morocco, Brazil, and Mexico. In India, Vitex agnus-castus L., Vitex negundo L., Vitex peduncularis W., Vitex pinnata L., and Vitex trifolia L. are frequently found throughout the country [10]. Vitex species are well recognized as sources of useful medicines in different geographic areas and have already been the subject of different research studies, mainly referring to V. agnus-castus and V. negundo [11].
Traditionally, Vitex plants have long been used for different types of treatment of menstrual disorders, fertility problems, menopausal symptoms, diarrhea, asthma, fever, cold, headache, migraine, gastrointestinal infections, and breast pain [12,13]. Recent studies have revealed that this genus has a wide range of biological properties, especially antimicrobial activities [14]. It has been utilized in various traditional medicinal systems around the world to address health concerns beyond its antimicrobial applications. Traditional practitioners usually prepare herbal medicines to treat and prevent diseases [15]. They use plant parts of Vitex species for the treatment of various infectious diseases such as bacterial, viral, and protozoal infections [16]. Several studies have examined the antimicrobial properties of Vitex L., and the results have shown that different parts of Vitex such as the leaf, bark, root, stem, flower, fruit, and seed exhibit antimicrobial activity against a wide range of microorganisms. Phytochemical analysis of the Vitex species has found several previously known compounds, mainly terpenoids, flavonoids, and alkaloids. This review has explored the potential antibacterial effects of Vitex extracts and their isolated natural products [17].
Herein, a concise and original review of the literature concerning the ethnomedicinal use of medicinal plants from the Vitex genus and their potential, as both antimicrobial herbal medicines as well as sources of new antimicrobial natural products, was made. This state-of-the-art paper will provide a comprehensive understanding of the potential of this genus as a source of antimicrobial agents.
2. Results
2.1. Selection of the Information
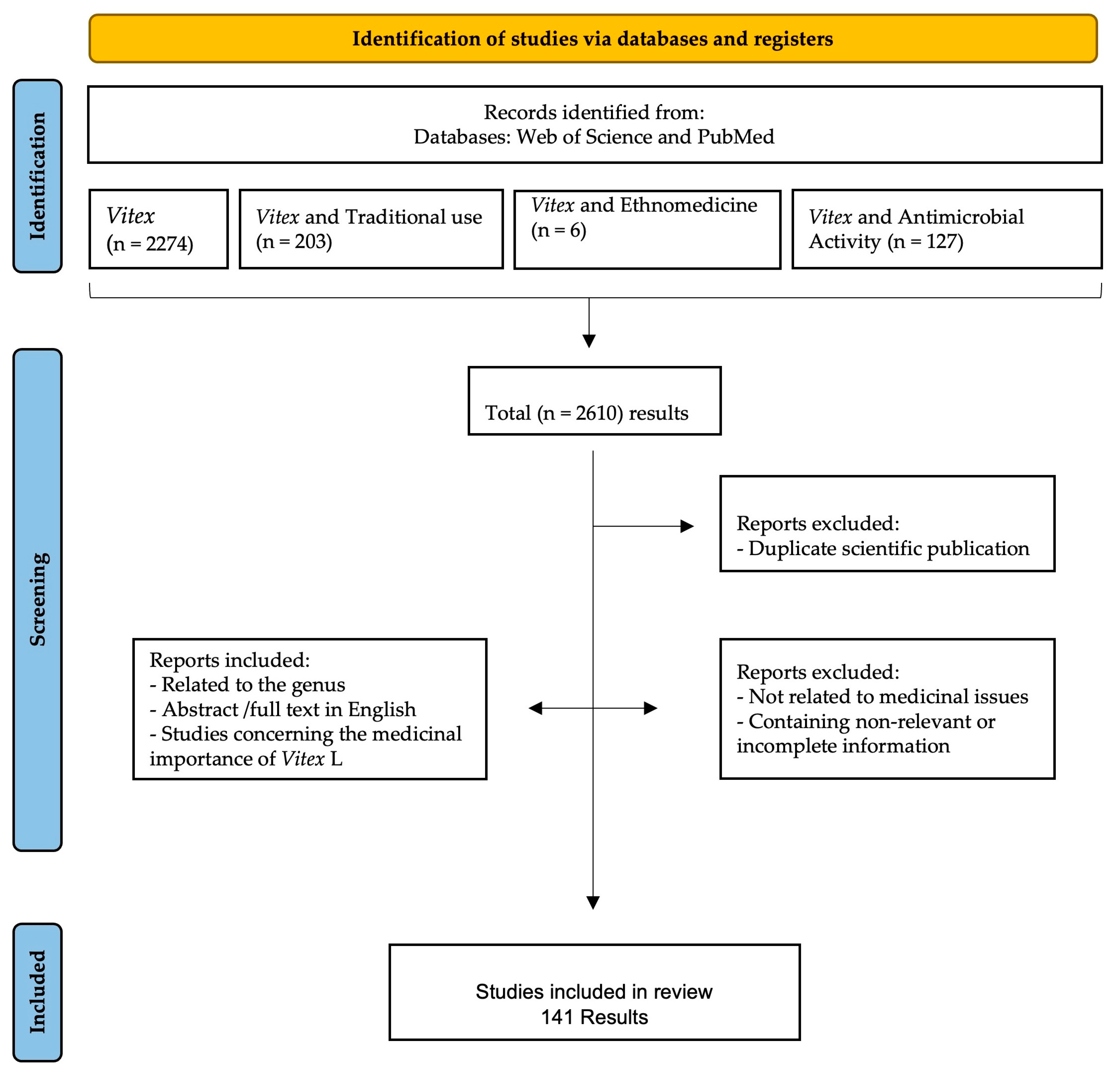
Details of data collection and selection are given in Figure 1. The initial title and abstract search yielded 2610 results. Of those, 2610 scientific publications were considered, and many articles were removed for the following reasons: repeated results, no relation to medicinal issues, and the inclusion of irrelevant or incomplete information. Finally, a total of 141 scientific publications were considered eligible to be included in this review as they were related to the use of Vitex species in traditional medicine, were abstracts or full texts written in English, and the studies conducted focused on the Vitex species and their antimicrobial activity against different microorganisms.
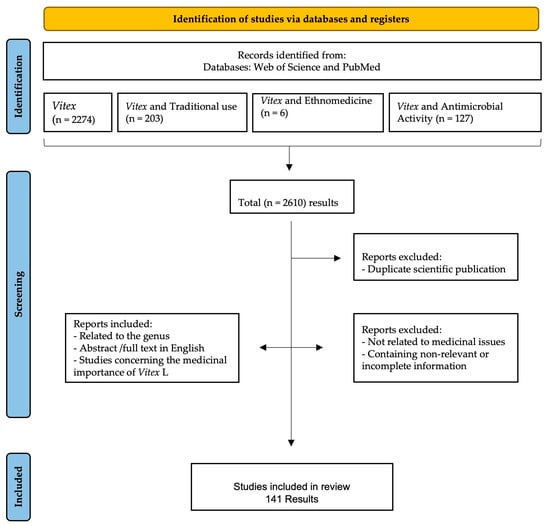
Figure 1.
Data screening based on PRISMA methodology.
2.2. Traditional Uses
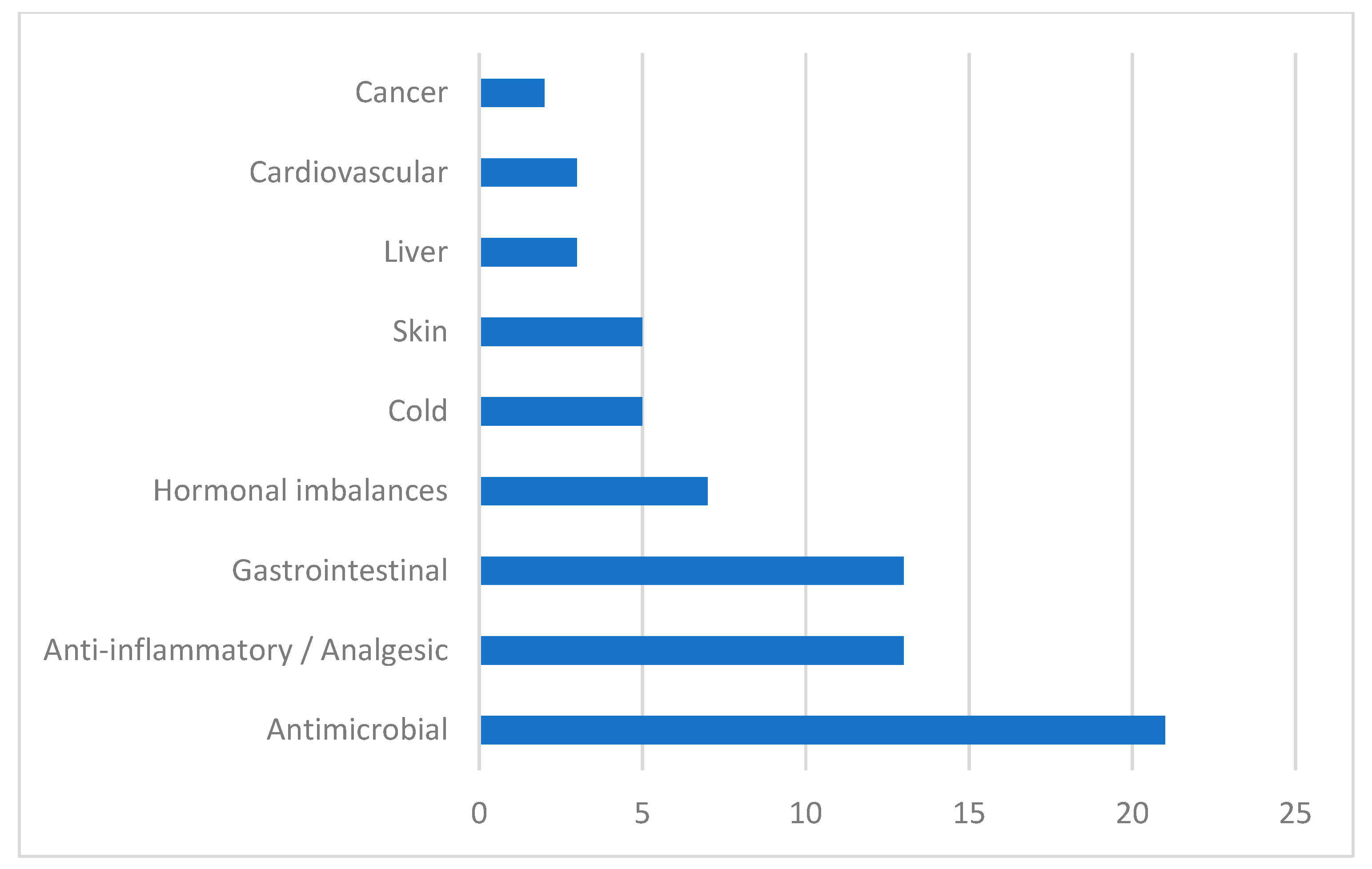
Obtained results concerning the traditional use of the Vitex species are summarized in Table 1 and classified according to the symptoms they were used against (Figure 2). From the recognized 230 species of Vitex, only 13 species have been reported as being used in traditional medicine, namely Vitex agnus-castus L., Vitex doniana L., Vitex gardneriana Schauer., Vitex mollis L. Vitex negundo L., Vitex obovata ssp. wilmsii (Gürke) Bredenkamp & Botha, Vitex peduncularis W., Vitex peduncularis L., Vitex pinnata L., Vitex polygama L., Vitex pseudo-negundo L., Vitex rehmannii sp., Vitex rotundifolia L., and Vitex trifolia L. These species are traditionally used for the treatment of menstrual disorders and hormonal imbalance, increasing breast milk production, and hypertension [18,19,20,21]. Vitex species are also used for infectious diseases treatment such as cavity infections, dysentery, diarrhea, asthma, cholera, and malaria [22,23,24,25,26]. The leaf of Vitex is the most frequently used plant part for medicinal purposes, but other parts like the bark, root, and flower are also referred to in the literature.

Table 1.
Ethnomedicinal use of Vitex species.
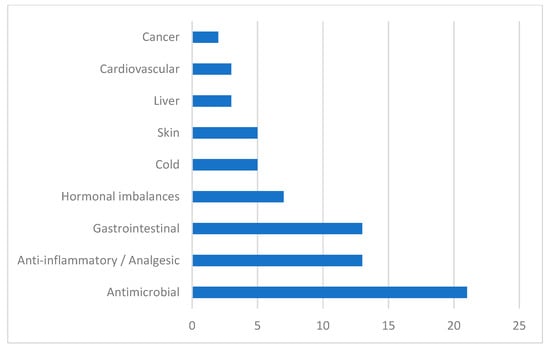
Figure 2.
Symptoms of disease treated with Vitex plants.
In Figure 2, we can see the major symptoms that are treated with Vitex species, grouped according to the physiological systems impaired. Results showed that these species are mostly used by traditional medical practitioners as antimicrobial agents. Vitex plants are also used in inflammatory diseases, as analgesics, as hormonal regulators, and in infectious and non-infectious gastrointestinal diseases.
2.3. In Vitro Antibacterial Activity Studies
Reviewed articles were screened for information regarding plant species and corresponding origin, plant parts and solvents used for extract preparation, antimicrobial activity essay performed, bacteria species used to evaluate antimicrobial activity, and substances used as control. Results were expressed as minimum inhibitory concentrations, minimum bactericidal concentrations, and inhibition zones exhibited according to the type of essay performed. The gathered information is summarized in Table 2. Our analysis showed that different essays were used to study antimicrobial activity against a wide variety of bacterial species and strains. Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus were the most frequent subjects studied, mainly through disk diffusion and broth dilution methodology. Among the controls used in antimicrobial activity essays were known antibiotics like amoxicillin, chloramphenicol, ciprofloxacin, and gentamicin, and results showed that Vitex species plant extracts often exhibited significant activity against tested strains.

Table 2.
In vitro antibacterial activity studies on Vitex species.
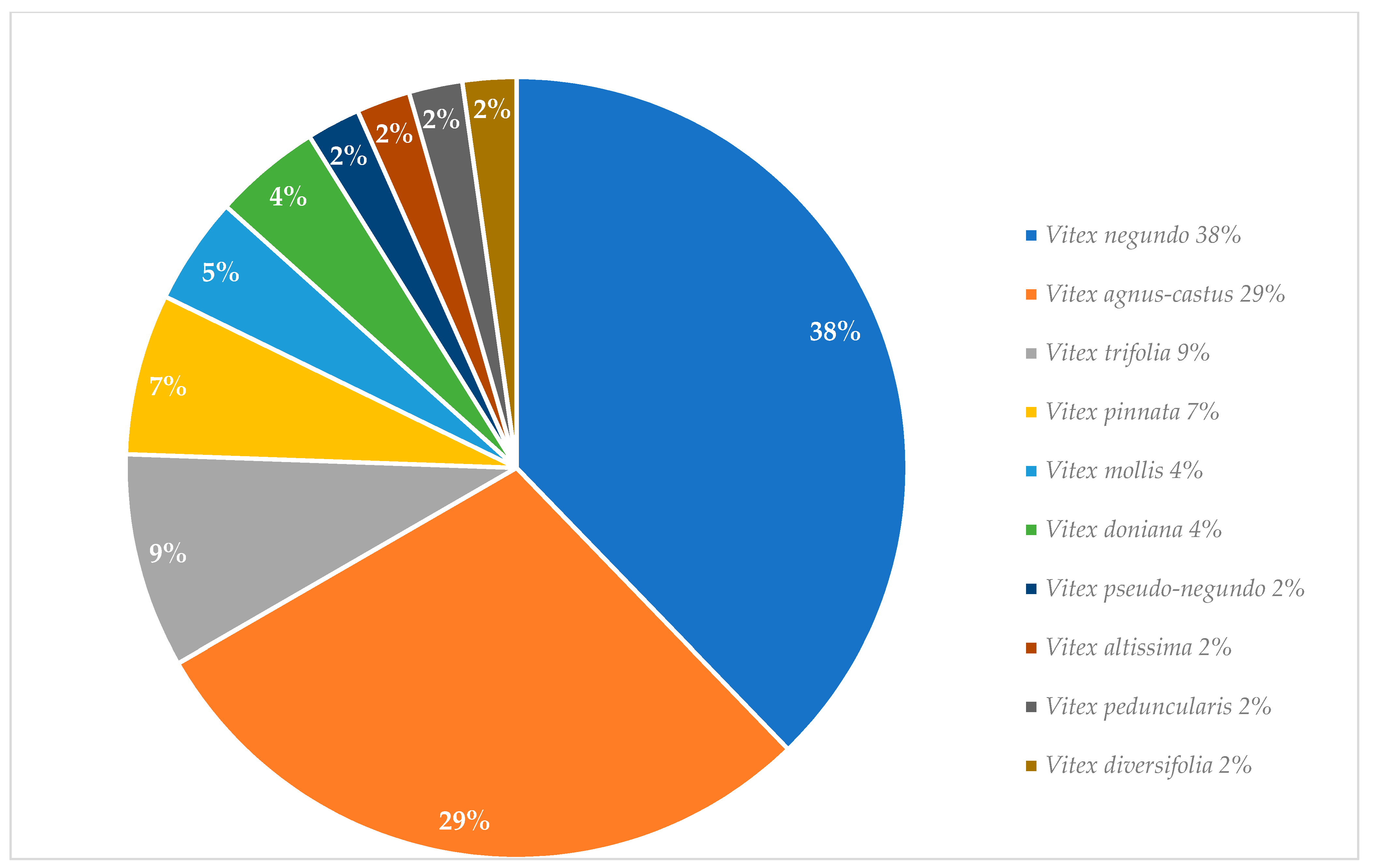
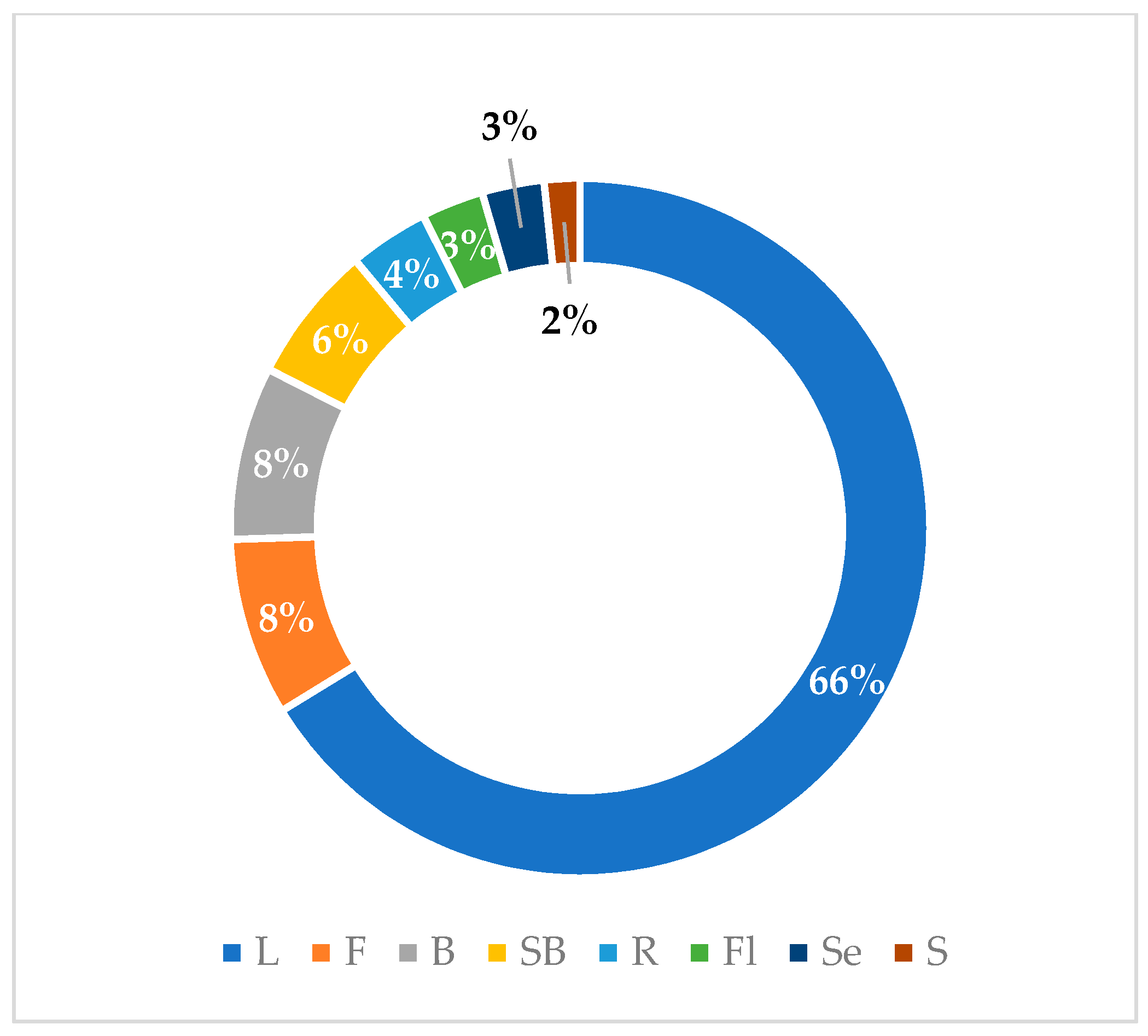
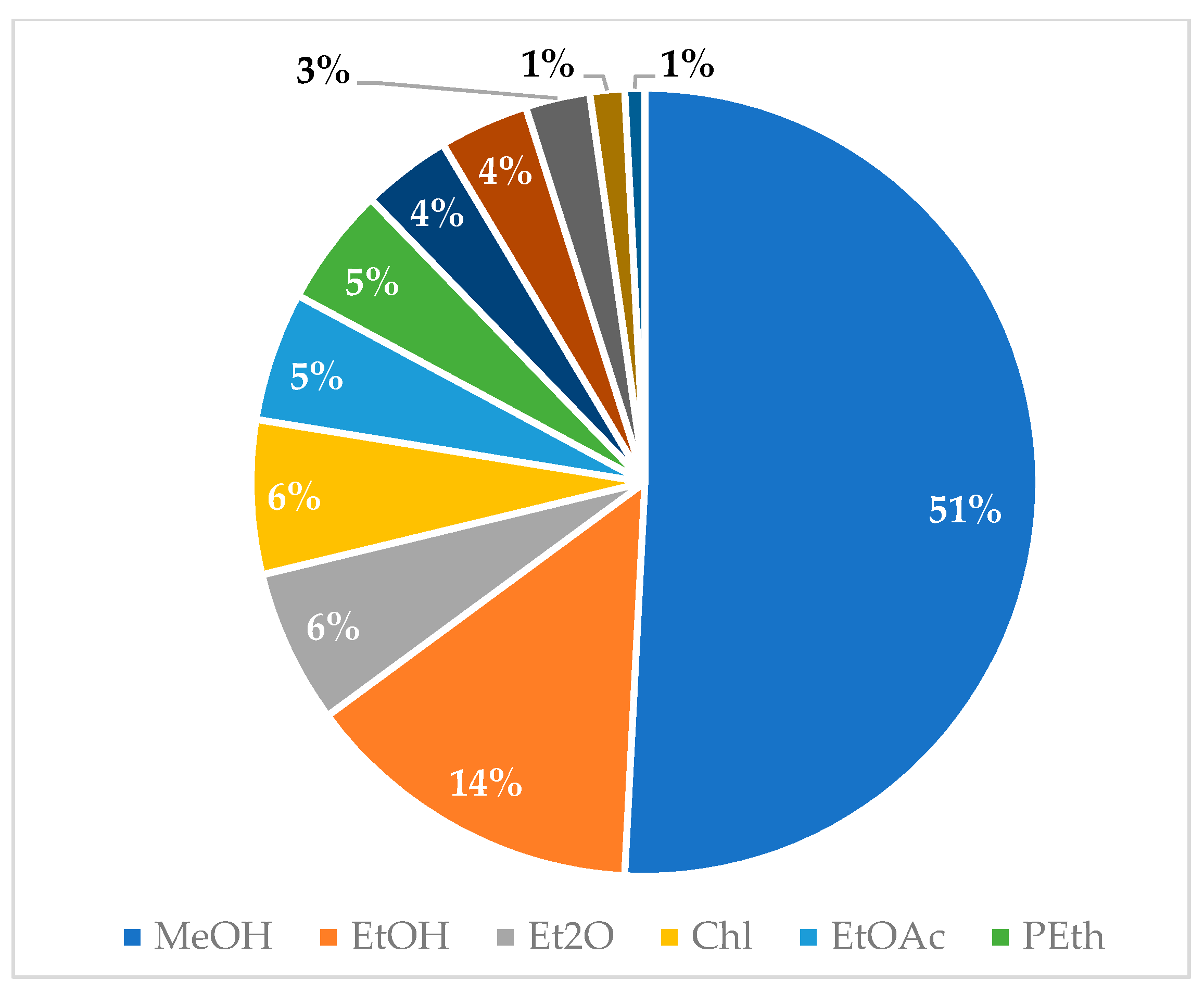
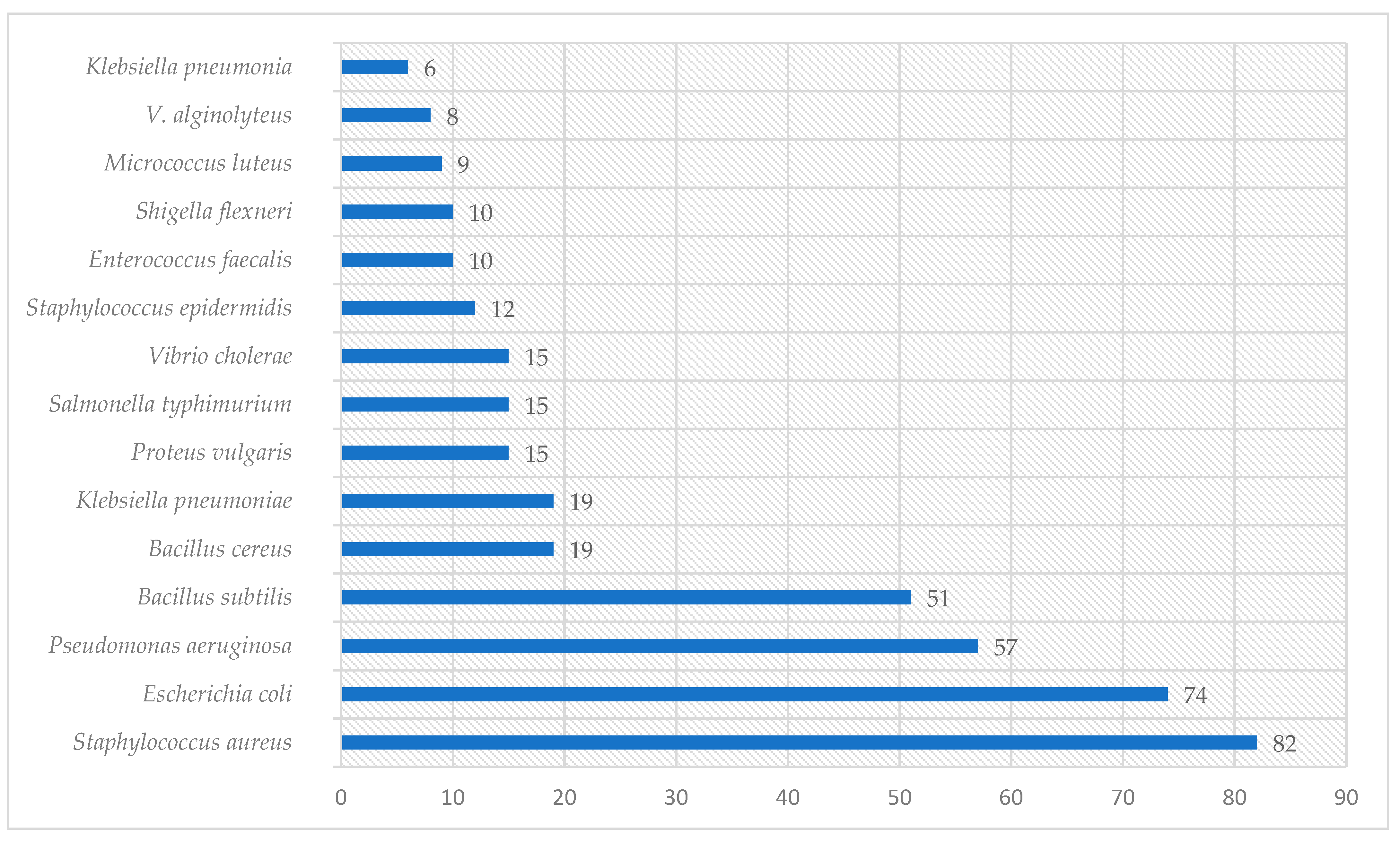
Graphical interpretations of these results can be seen in Figure 3, Figure 4, Figure 5 and Figure 6. Figure 3 shows that V. negundo is the most studied Vitex species (38%), followed by V. agnus-castus (29%). Other species do not have the same expression in terms of scientific research focus. In Figure 4 and Figure 5, we can see that most of the studies focused on leaf plant parts and methanolic and ethanolic extracts of plant material. An analysis of Figure 6 shows the main bacterial strains that Vitex species have been tested on. These strains are widely known to be responsible for infections in humans, which makes the antibacterial activity exhibited by Vitex species an important focus of research for the development of new drugs.
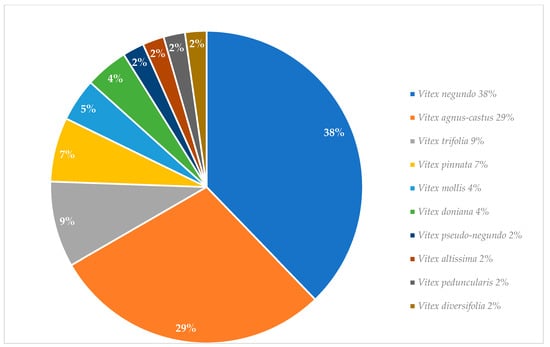
Figure 3.
Vitex species studied for their in vitro antibacterial activity.
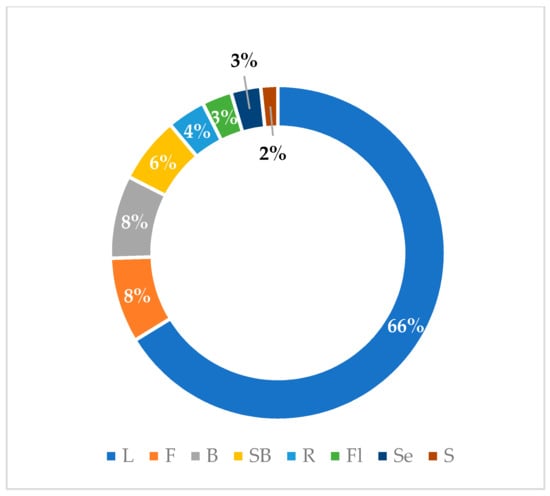
Figure 4.
Plant parts used in antibacterial studies.
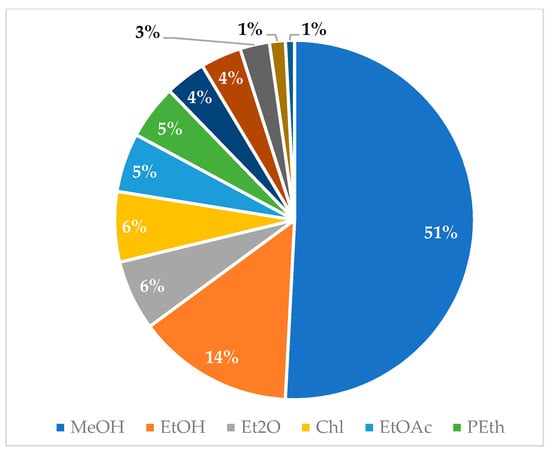
Figure 5.
Solvents used for plant extraction for antibacterial activity essays.
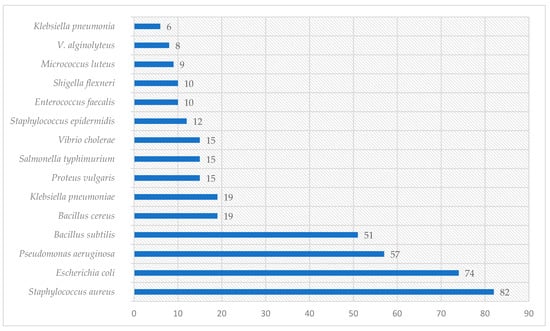
Figure 6.
The microorganisms were extensively studied for their interactions with Vitex species.
Collected data show that all Vitex species used in traditional medicine to treat symptoms of infectious diseases exhibit in vitro antimicrobial activity against several bacterial strains, which can justify their use in traditional medicine to treat symptoms of infectious diseases.
2.4. In Vitro Antifungal, Antiviral, and Antiprotozoal Activity
Results showed that Vitex species exhibit biological activity, such as through antifungal, antiprotozoal, and antiviral activities. This information is summarized in Table 3 and Table 4. V. negundo and V. agnus-castus were the most studied plant species against a wider variety of these types of microorganisms. Methanolic extracts of leaf and root were the most frequent types of extract and plant parts used. Microbial agents tested were mostly fungal, namely C. albicans and A. niger, but viruses like HIV-1 and parasites like Plasmodium falciparum were also tested. Nevertheless, the most noteworthy significant value was observed in terms of antifungal activity against C. albicans. For example, an antifungal activity evaluation of ethanolic, methanolic, and aqueous extracts of the V. agnus-castus leaf showed that all had the ability to inhibit Candida species growth. Minimum inhibitory concentrations of studied extracts ranged from 25 µg/mL to 12.5 µg/mL against C. tropicalis, C. albicans, and C. ciferri while minimum fungicidal concentrations ranged from 100 µg/mL to 25 µg/mL [118].

Table 3.
In vitro antifungal activity studies on Vitex species.

Table 4.
In vitro antiviral and antiprotozoal activity studies on Vitex species.
2.5. Characteristic Vitex Secondary Metabolites with Antimicrobial Activity
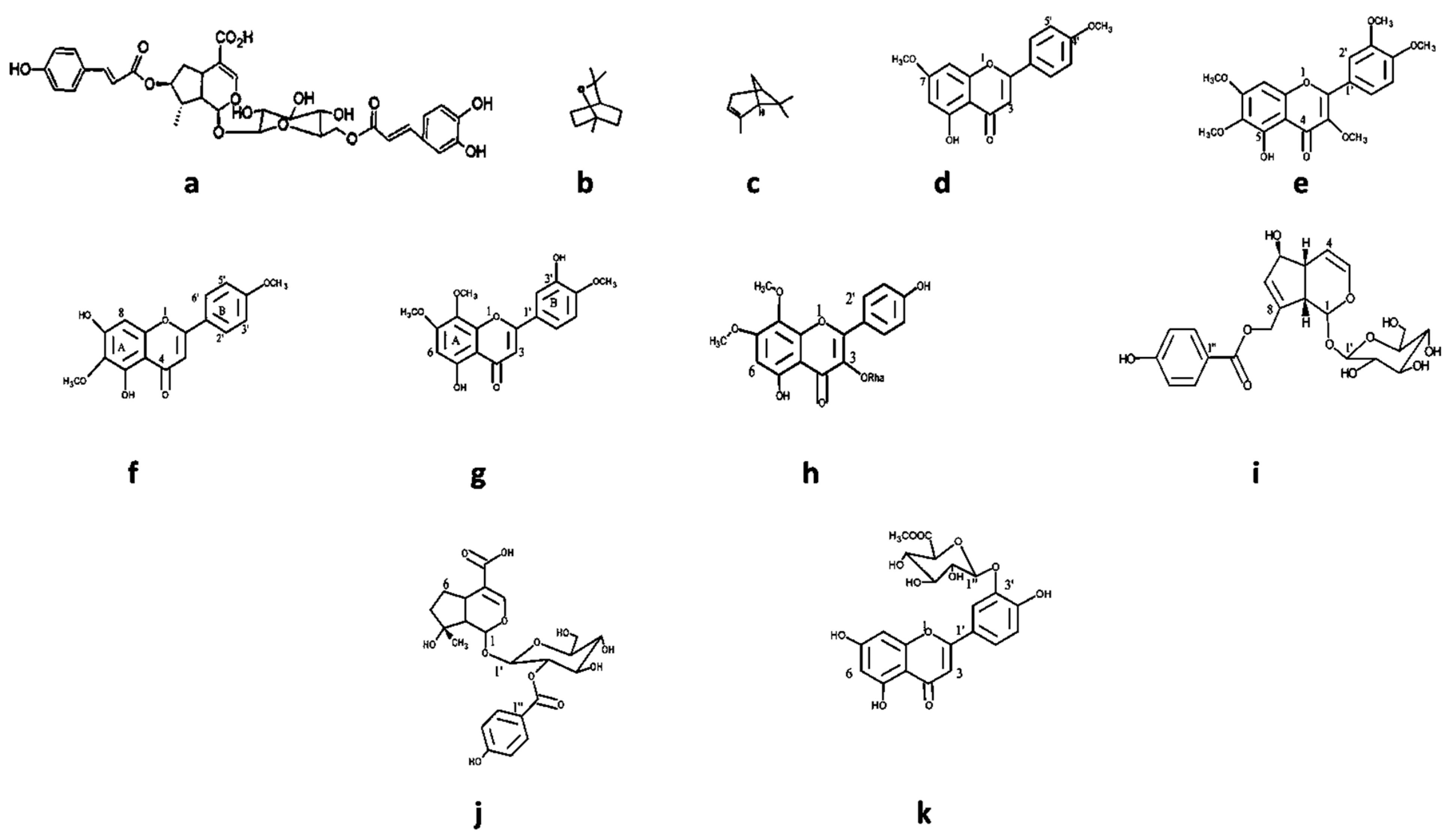
Specific compounds have been isolated from Vitex species (Figure 7 and Table 5) and described for their antimicrobial activity. Most natural products were isolated from V. negundo. Different chemical classes such as phenolic compounds (like 5-hydroxy-7,4′ dimethoxy flavone) and terpenoids (like agnuside, negundoside, and vitegnoside) have been isolated from Vitex species [126,127]. Concerning the isolated natural products’ antimicrobial activity, negundoside is the most active one, showing significant antibacterial activity against B. subtilis, E. coli, M. pyogenes, P. aeruginosa, and S. aureus. Agnuside and vitegnoside, also isolated from V. negundo, showed a similar range of antibacterial activity against the same bacterial strains as negundoside. All these natural products were isolated from the methanolic leaf extract of the plant.
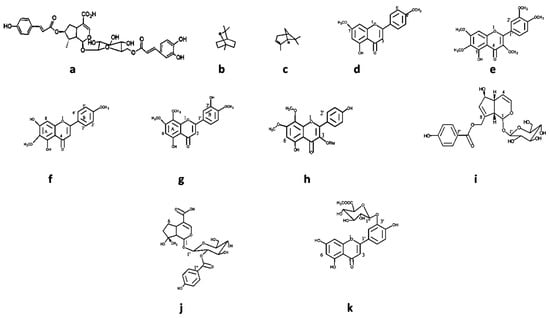
Figure 7.
Some chemical structure of compounds: (a) agnucastoside, (b) 1,8-cineole, (c) α-pinene, (d) 5-hydroxy-7,4′dimethoxy flavone, (e) 5-hydroxy-3,6,7,3′,4′-pentamethoxy flavone, (f) 5,7 dihydroxy-6,4′ dimethoxy flavanone, (g) 5,3′ dihydroxy—7,8,4′-trimethoxy flavone, (h) 7,8 dimethyl herbacetin 3-rhamnoside, (i) agnuside, (j) negundoside, and (k) vitegnuside isolated from Vitex species.

Table 5.
Isolated chemical compounds from Vitex species and their antimicrobial activity.
Isolated from V. agnus-castus fruit and leaf essential oils, α-pinene and 1,8-cineole exhibited significant in vitro antibacterial activity against B. subtilis, E. coli, M. flavus, S. aureus, and other strains. Both compounds were active in preventing A. niger-induced rotting in an in vivo apple fruit assay.
3. Materials and Methods
This review was performed following the criteria described in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 2020 (http://www.prisma-statement.org/PRISMAStatement/FlowDiagram; accessed on 1 January 2022).
3.1. Search Strategy
The scientific data was collected from Web of Science and PubMed scientific publications that were published between 1 January 1980 and 23 May 2023, applying several keywords: Vitex, Vitex AND Traditional Use, Vitex AND Ethnomedicine, Vitex AND Biology, and Vitex AND Antimicrobial activity.
3.2. Data Inclusion and Exclusion Criteria
3.2.1. Inclusion Criteria
- -
- Related to the Vitex genus.
- -
- Abstract or full text in English.
- -
- Studies on Vitex species concerning antimicrobial activity.
3.2.2. Exclusion Criteria
- -
- Duplicate scientific publications.
- -
- Not directly related to medicinal issues.
- -
- Containing irrelevant or incomplete information.
4. General Discussion
Results of our study confirm that Vitex species have been used in traditional medicinal systems to approach several disease symptoms, the most frequent ones being related to infectious diseases, inflammatory states, gastrointestinal disorders, hormonal imbalances, cold symptoms, skin conditions, and liver and cardiovascular symptoms [18].
From more than 200 different Vitex species, 13 species were referred to in this review as the most used in traditional medicine; however, only 10 of them have been studied in vitro for their antimicrobial activity. V. negundo and V. agnus-castus are, by far, the two species that most frequently have been the focus of scientific research. The observed antimicrobial activity against a wide variety of microbial strains in vitro essays contributes to useful scientific validation for the main utilization of Vitex plants in traditional medicinal systems against infectious diseases. Even though specific mechanistic pathways that lead to bacterial death or growth inhibition are still unknown, the published literature indicates that these can be related to major constituents’ classes of natural products present in the corresponding tested extracts. [6].
In 2011, Kannathasan et al. conducted a study focusing on the antibacterial activity of several Vitex species. Leaves of V. altissima, V. diversifolia, V. negundo, V. peduncularis, and V. trifolia were used to prepare methanolic extracts, which were then evaluated for their antibacterial activity using the disc diffusion method. Results showed that Vitex extracts under analysis exhibited a wide range of activity against all tested microorganisms, like S. aureus, E. coli, K. pneumoniae, P. aeruginosa, and P. mirabilis. Mean zones of inhibition observed showed that the antibacterial activity of the extracts was selective for the microorganisms. Generally, V. peduncularis and V. trifolia extracts exhibited larger zones of inhibition than extracts of the other species against all microorganisms, being significantly active when compared to ciprofloxacin used as the control [137].
Research conducted by Berrani et al. on the phytochemical composition and biological activity of V. negundo showed that phenolic compounds were amongst the major constituents of the methanolic extracts analyzed. These extracts were prepared using leaf, root, stem, flower, and seed samples and tested separately against a panel of microbial agents commonly responsible for pathogenic infections in humans. Results showed that all plant extracts had selective antibacterial activity against all tested strains [74].
In a study conducted in 2010 by Nagarsekar et al., leaves of V. negundo were used to prepare different extracts. These extracts were studied for their antimicrobial activity against different microorganisms and characterized for their phytochemical composition. Different concentrations of ethanolic, petroleum ether, steam-distilled and supercritical fluid extracts were tested using the well diffusion method to evaluate their antimicrobial activity. Selective activity against S. aureus and B. subtilis and the increase in extract concentration were correlated with an increase in the exhibited antimicrobial activity [82].
Ababutain et al. studied ethanolic, methanolic, and aqueous leaf extracts of V. agnus-castus for their antifungal activity, using the agar well diffusion method, against C. tropicalis, C. albicans, and C. ciferrii. Tested extracts showed selective antifungal activity against Candida species. The aqueous extract was the most active one against all species, followed by the methanolic and ethanolic extracts. These results showed significant zones of inhibition when compared with the nystatin control [138].
Essential oils of the leaf and fruit of V. agnus-castus were characterized for their chemical composition in a work conducted by Stojkovic et al. in 2011. Obtained phytochemical profiles showed that 1,8-cineole and α-pinene were major constituents present in all tested essential oils. An evaluation of the antibacterial activity of the essential oils obtained from different plant parts was conducted using the microdilution method, testing against a panel of Gram-positive and Gram-negative bacteria. Selective activity was observed against M. flavus, B. subtilis, S. typhimurium, S. aureus, and E. coli, with all oils showing Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) levels higher than the ones observed for the streptomycin control. The isolated compounds 1,8-cineole and α-pinene were also tested for antimicrobial activity. Both compounds exhibited significantly lower MICs and MBCs, not only compared to whole essential oils but also when compared to the streptomycin control, against all tested strains. The same behaviors were observed for all essential oils and isolated compounds against fungal pathogens like A. alternata, A. flavus, A. niger, A. ochraceus, F. tricinctum, P. ochrochloron, P. funiculosum, and T. viride. In this work, antifungal activity was also evaluated through an in vivo model of A. niger-induced rotting in apple fruits. Results showed that increasing concentrations of 1,8-cineole effectively reduced infectious disease incidence after 3 days of treatment [78].
It is well known that plant extracts are characterized by different natural products belonging to a wide variety of chemical classes of secondary metabolites. Phenolic compounds such as flavonoids constitute one of the most common chemical classes of secondary metabolites isolated from Vitex extracts and have previously been shown to exert antimicrobial activity through different mechanisms of action. For instance, apigenin, a very common flavonoid present in several plant species, can inhibit nucleic acid synthesis by binding to bacterial DNA gyrase. It can also induce bacterial cell lysis through membrane disruption (which leads to intracellular content leakage) and cell envelope synthesis inhibition, compromising structural integrity; apigenin also can inhibit biofilm formation and quorum sensing, two bacterial mechanisms with a high impact on infection prevalence and pathogenicity. Quercetin, also a very common phenolic compound, exhibits some similar behaviors. It can inhibit nucleic acid synthesis, which compromises bacterial metabolic viability. It is an active membrane disruptor and inhibitor of cell envelope synthesis. Quercetin can prevent efflux pump activity, reverting or preventing antibiotic resistance mechanisms, and can also directly inhibit bacterial toxins and enzymes [101].
Terpenoids and terpenoid derivatives also constitute a very common class of secondary metabolites, with known antimicrobial activity. Mechanistic pathways that lead to bacterial death or growth inhibition are yet to be determined, but research has shown that terpenoid compounds can inhibit oxygen uptake and oxidative phosphorylation, two metabolic processes crucial for bacterial survival. Monoterpenes carvacrol, thymol, menthol, and geraniol have exhibited antimicrobial activity against Gram-positive and Gram-negative bacteria. Geraniol can increase Enterococcus aerogenes susceptibility to antibiotics by inhibiting bacterial efflux pumps. Menthol and thymol are both active against E. coli and S. aureus. Carvacrol has been reported to inhibit the biofilm development of S. aureus and S. typhimurium. Oleanic acid, a triterpenoid, has shown antimicrobial activity against M. tuberculosis and a synergistic effect when administered with rifampicin, isoniazide, and ethambutol, significantly decreasing the MICs of these antibiotics [118].
The fact that most secondary metabolites isolated from Vitex plants are phenolic compounds or terpenoids falls in line with published literature focusing on the biological activity of these types of compounds and can be correlated with the exhibited antimicrobial activity. Most of the studies assessed in this review focused on methanolic and ethanolic extracts of the Vitex species’ leaf plant part. This may indicate that natural compounds present in this plant part are the main responsible ones for the antimicrobial activity exhibited and that these compounds must have high polarity rates since methanol and ethanol are polar solvents with high affinity for polar compounds. Regarding specific chemical compound isolation, studies conducted on Vitex species have identified diterpenoids and flavonoids. Since Vitex species have phytochemical profiles with high amounts of diterpenoids, these may be responsible for the exerted antimicrobial activity. However, further research should be conducted to better understand and characterize this activity.
The drug development of new antimicrobial agents is a major challenge for the pharmaceutical industry. There are limited mechanistic pathways that can be followed for antibacterial, antifungal, and antiviral activities, and some microorganisms can have intrinsic specific resistances that render them immune to some of these pathways. Moreover, the development of new drugs “from scratch” is a highly expensive and time-consuming process that often does not move from theory to practice due to limitations like synthesis yields, formulation compatibilities, bioavailabilities, and other technological aspects. Using natural products from plants with antimicrobial activities can circumvent some of these limitations since pharmaceutical engineering processes can use core molecular skeletons of these secondary metabolites to develop new therapeutic options with clinical significance. For example, amikacin, a semisynthetic aminoglycoside broad-spectrum antibiotic active against Pseudomonas aeruginosa and most Gram-negative aerobes, is derived from kanamycin A, which is a natural product isolated from Streptomyces kanamyceticus [137,138,139].
Numerous studies have explored the efficacy of different plant parts of Aloe vera, namely leaf extracts, against a range of bacterial and fungal species. Results showed high antibacterial activity against E. coli, with inhibition zones indicating significant activity against drug-resistant strains [140]. On the other hand, Artemisia has shown antibacterial activity against drug-resistant bacterial strains. For example, Artemisia absinthium L. leaf extracts exhibit antibacterial activity against S. aureus [141]. Given the results of our review, we believe that the limited nature of the direct evidence on the antimicrobial activities of Vitex species, when compared to other genera, is due to the fact that Vitex species have been less extensively studied for their antimicrobial properties, and more research is needed to understand their full potential.
5. Conclusions
This review summarizes the main traditional uses of plants belonging to the Vitex genus as anti-infective medicines in different traditional medicinal systems and the in vitro antimicrobial activity demonstrated by extracts made from these medicinal plants against a wide variety of bacterial, fungal, and protozoal strains. In vitro studies demonstrate that Vitex extracts very often exhibit antimicrobial activity against different bacterial, fungal, and protozoal species. Some of the natural compounds present in these extracts, most likely the main ones, can be responsible for this biological activity. The results analyzed contribute to legitimizing the traditional use of Vitex species in traditional medicinal systems. A better understanding of Vitex-genus medicinal plants and their natural compounds can constitute a valuable natural source for discovering antimicrobial drugs and helping fight and prevent infectious diseases.
Author Contributions
Conceptualization, O.S. and Z.I.; investigation, methodology, and data curation, O.S., Z.I. and G.I.C.; data analysis, O.S., Z.I., G.I.C., M.C. and N.I.; resources, O.S.; writing—original draft preparation, Z.I., G.I.C. and O.S.; writing—review and editing, O.S., Z.I., G.I.C., M.C. and N.I.; supervision, O.S.; project administration, O.S. and M.C.; funding acquisition, O.S. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology (FCT, Portugal) through national funds to iMed.ULisboa (UIDP/04138/2020 and UI/BD/153625/2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in Web of Science and Pubmed.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in Bacterial Resistance Mechanisms and the Potential Ways to Combat Them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Adu, F.; Boakye, Y.D.; Agyare, C.; Sam, G.H.; Boamah, V.E.; Osei, F.B. Antibacterial Resistance Modulatory Properties of Selected Medicinal Plants from Ghana. Afr. J. Pharm. Pharmacol. 2019, 13, 57–69. [Google Scholar] [CrossRef]
- Chambers, C.S.; Viktorová, J.; Řehořová, K.; Biedermann, D.; Turková, L.; Macek, T.; Křen, V.; Valentová, K. Defying Multidrug Resistance! Modulation of Related Transporters by Flavonoids and Flavonolignans. J. Agric. Food Chem. 2020, 68, 1763–1779. [Google Scholar] [CrossRef]
- Ganapaty, S.; Vidyadhar, K.N. Phytoconstituents and Biological Activities of Vitex—A Review. J. Nat. Remedies 2005, 5, 75–95. [Google Scholar]
- Bello, M.O.; Zaki, A.A.; Aloko, S.; Fasinu, P.S.; Bello, E.O.; Ajao, U.L.; Oguntoye, O.S. The Genus Vitex: An Overview of Iridoids as Chemotaxonomic Marker. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 414–419. [Google Scholar] [CrossRef]
- Li, B.; Cantino, P.D.; Olmstead, R.G.; Bramley, G.L.C.; Xiang, C.-L.; Ma, Z.-H.; Tan, Y.-H.; Zhang, D.-X. A Large-Scale Chloroplast Phylogeny of the Lamiaceae Sheds New Light on Its Subfamilial Classification. Sci. Rep. 2016, 6, 34343. [Google Scholar] [CrossRef] [PubMed]
- Whitten, T.; Soeriaatmadja, R.E.; Afiff, S.A. The Ecology of Indonesia Series Volume II: The Ecology of Java and Bali; Periplus: Hongkong, China, 1996. [Google Scholar]
- Chopra, R.N. 1882-Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: New Delhi, India, 1956. [Google Scholar]
- Katiraee, F.; Mahmoudi, R.; Tahapour, K.; Hamidian, G.; Emami, S.J. Biological Properties of Vitex agnus-castus Essential Oil (Phytochemical Component, Antioxidant and Antifungal Activity). Biotechnol. Health Sci. 2015, 2, 26797. [Google Scholar] [CrossRef]
- Abdolahi, F.; Azadbakht, M.; Shabankhani, B.; Rezaie Abhari, F.; Moslemizadeh, N. Effect of Aqueous Glycyrrhza Globra Extract on Menopausal Symptoms. J. Maz. Univ. Med. Sci. 2007, 16, 75–82. [Google Scholar]
- Odenthal, K.P. Vitex agnus-castusVitex agnus-castus L.,—Traditional Drug and Actual Indications. Phytother. Res. 1998, 12, S160–S161. [Google Scholar] [CrossRef]
- Amaning Danquah, C.; Minkah, P.A.B.; Osei Duah Junior, I.; Amankwah, K.B.; Somuah, S.O. Antimicrobial Compounds from Microorganisms. Antibiotics 2022, 11, 285. [Google Scholar] [CrossRef]
- Meena, A.K.; Niranjan, U.S.; Rao, M.M.; Padhi, M.M.; Babu, R. A Review of the Important Chemical Constituents and Medicinal Uses of Vitex Genus. Asian J. Tradit. Med. 2011, 6, 54–60. [Google Scholar]
- Meena, A.K.; Perumal, A.; Kumar, N.; Singh, R.; Ilavarasan, R.; Srikanth, N.; Dhiman, K.S. Studies on Physicochemical, Phytochemicals, Chromatographic Profiling & Estimation and in-Silico Study of Negundoside in Roots & Small Branches of Vitex Negundo Plant. Phytomed. Plus 2022, 2, 100205. [Google Scholar] [CrossRef]
- Das, N.; Salgueiro, A.C.F.; Choudhury, D.R.; Mandal, S.K.; Logesh, R.; Hassan, M.M.; Devkota, H.P. Traditional Uses, Phytochemistry, and Pharmacology of Genus Vitex (Lamiaceae). Phytother. Res. 2022, 36, 571–671. [Google Scholar] [CrossRef]
- Azadbakht, M.; Baheddini, A.; Shorideh, S.M.; Naserzadeh, A. Effect of Vitex Agnus—Castus L. Leaf and Fruit Flavonoidal Extracts on Serum Prolactin Concentration. J. Med. Plants 2005, 4, 56–61. [Google Scholar]
- Dugoua, J.-J.; Seely, D.; Perri, D.; Koren, G.; Mills, E. Safety and Efficacy of Chastetree (Vitex agnus-castus) During Pregnancy and Lactation. J. Popul. Ther. Clin. Pharmacol. 2008, 15, 74–79. [Google Scholar]
- Atmaca, M.; Kumru, S.; Tezcan, E. Fluoxetine versus Vitex agnus-castusVitex agnus-castus Extract in the Treatment of Premenstrual Dysphoric Disorder. Hum. Psychopharmacol. Clin. Exp. 2003, 18, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Saeed AL-Wajeeh, N.; Halabi, M.F.; Hajrezaie, M.; Dhiyaaldeen, S.M.; Abdulaziz Bardi, D.; Salama, S.M.; Rouhollahi, E.; Karimian, H.; Abdolmalaki, R.; Azizan, A.H.S.; et al. The Gastroprotective Effect of Vitex pubescens Leaf Extract against Ethanol-Provoked Gastric Mucosal Damage in Sprague-Dawley Rats. PLoS ONE 2016, 11, e0157431. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.; Ayres, V.F.; Carvalho, C.E.; Souza, M.G.; Guimaraes, A.C.; Correa, G.M.; Martins, C.H.; Takeara, R.; Silva, E.O.; Crotti, A.E. Chemical Composition and Antibacterial Activity of the Essential Oil of Vitex agnus-castus L. (Lamiaceae). An. Acad. Bras. Ciênc. 2017, 89, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.P.Y.; Basri, A.M.; Yasin, H.; Taha, H.; Ahmad, N. Ethnobotanical Review and Pharmacological Properties of Selected Medicinal Plants in Brunei Darussalam: Litsea elliptica, Dillenia suffruticosa, Dillenia excelsa, Aidia racemosa, Vitex pinnata and Senna alata. Asian Pac. J. Trop. Biomed. 2017, 7, 173–180. [Google Scholar] [CrossRef]
- Ladda, P.; Magdum, C. Vitex negundo Linn.: Ethnobotany, Phytochemistry and Pharmacology—A Review. Int. J. Adv. Pharm. Biol. Chem. 2012, 1, 111–120. [Google Scholar]
- Kilani, A.M. Antibacterial Assessment of Whole Stem Bark of Vitex doniana against Some Enterobactriaceae. Afr. J. Biotechnol. 2006, 5. [Google Scholar] [CrossRef]
- Vishwanathan, A.S.; Basavaraju, R. A Review on Vitex negundo L.—A Medicinally Important Plant. Eur. J. Biol. Sci. 2010, 3, 30–42. [Google Scholar]
- Chhabra, G.S.; Kulkarni, K.S. Vitex agnus-castus—An Overview. J. Nat. Remedies 2011, 11, 90–97. [Google Scholar] [CrossRef]
- Pereira, E.J.P.; do Vale, J.P.C.; da Silva, P.T.; Lima, J.d.R.; Alves, D.R.; Costa, P.S.; Rodrigues, T.H.S.; de Menezes, J.E.S.A.; de Morais, S.M.; Bandeira, P.N. Circadian Rhythm, and Antimicrobial and Anticholinesterase Activities of Essential Oils from Vitex gardneriana. Nat. Prod. Commun. 2018, 13, 1934578X1801300528. [Google Scholar] [CrossRef]
- Hernández, M.M.; Heraso, C.; Villarreal, M.L.; Vargas-Arispuro, I.; Aranda, E. Biological Activities of Crude Plant Extracts from Vitex trifolia L. (Verbenaceae). J. Ethnopharmacol. 1999, 67, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.R.; Khajuria, V.; Kapoor, B.; Kour, D.; Gupta, S. Hepatoprotective Activity of Vitex negundo Leaf Extract against Anti-Tubercular Drugs Induced Hepatotoxicity. Fitoterapia 2008, 79, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Tasduq, S.A.; Kaiser, P.J.; Gupta, B.D.; Gupta, V.K.; Johri, R.K. Negundoside, an Irridiod Glycoside from Leaves of Vitex negundo, Protects Human Liver Cells against Calcium-Mediated Toxicity Induced by Carbon Tetrachloride. World J. Gastroenterol. 2008, 14, 3693–3709. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Shareef, H.; Farrukh, U.; Kamil, A.; Rizwani, G.H. Antifungal Activities of Vitex negundo Linn. Pak. J. Bot. 2009, 41, 1941–1943. [Google Scholar]
- Sujanapal, P.; Sankaran, K.V. Common Plants of Maldives. Bangk. Food Agric. Organ. U. N. Kerala For. Res. Inst. 2016, 258, 9789251092958. [Google Scholar]
- Avadhoot, Y.; Rana, A.C. Hepatoprotective Effect of Vitex negundo against Carbon Tetrachloride-Induced Liver Damage. Arch. Pharm. Res. 1991, 14, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.R.; Joshi, K. Indigenous Knowledge and Uses of Medicinal Plants by Local Communities of the Kali Gandaki Watershed Area, Nepal. J. Ethnopharmacol. 2000, 73, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Au, D.T.; Wu, J.; Jiang, Z.; Chen, H.; Lu, G.; Zhao, Z. Ethnobotanical Study of Medicinal Plants Used by Hakka in Guangdong, China. J. Ethnopharmacol. 2008, 117, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rajadurai, M.; Vidhya, V.G.; Ramya, M.; Bhaskar, A. Ethno-Medicinal Plants Used by the Traditional Healers of Pachamalai Hills, Tamilnadu, India. Stud. Ethno Med. 2009, 3, 39–41. [Google Scholar] [CrossRef]
- Rana, S.; Rana, K.K. Review on Medicinal Usefulness of Vitex negundo Linn. OALib 2014, 1, 1–13. [Google Scholar] [CrossRef]
- Saikia, A.P.; Ryakala, V.K.; Sharma, P.; Goswami, P.; Bora, U. Ethnobotany of Medicinal Plants Used by Assamese People for Various Skin Ailments and Cosmetics. J. Ethnopharmacol. 2006, 106, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kirtikar, K.R. Indian Medicinal Plants: By K.R. Kirtikar, B.D. Basu, and An I.C.S. In 4 Volumes, 2nd ed.; Blatter, E., Caius, J.F., Mhaskar, K.S., Eds.; Lalit Mohan Basu: Allahabad, India, 1935. [Google Scholar]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants Used against Cancer—An Extension of the Work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Ullah, Z.; Ullah, R.; Shah, A.-H.A.; Ahmad, I.; Haider, S. Phytochemical and Biological Evaluation of Vitex negundo Linn: A Review. Int. J. Pharm. Sci. Res. 2012, 3, 2421. [Google Scholar]
- Naik, M.R.; Venugopalan, V.; Kumaravelayutham, P.; Krishnamurthy, Y.L. Ethnoveterinary Uses of Medicinal Plants among the Lambani Community in Chitradurga District, Karnataka, India. Asian Pac. J. Trop. Biomed. 2012, 2, S470–S476. [Google Scholar] [CrossRef]
- Chen, J.; Fan, C.-L.; Wang, Y.; Ye, W.-C. A New Triterpenoid Glycoside from Vitex negundo. Chin. J. Nat. Med. 2014, 12, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-L.; Fang, S.-M.; Liu, R.; Oppong, M.; Liu, E.-W.; Fan, G.-W.; Zhang, H. A Review on the Terpenes from Genus Vitex. Molecules 2016, 21, 1179. [Google Scholar] [CrossRef] [PubMed]
- Zabihullah, Q.; Rashid, A.; Akhtar, N. Ethnobotanical Survey of Kot Manzary Baba Valley, Malakand Agency, Pakistan’. Pak. J. Pl. Sci. 2006, 12, 115–121. [Google Scholar]
- Katare, A.K.; Singh, B.; Kumar, S.; Roy, S.; Gupta, A.P.; Kumar, A.; Singh, B.; Tabassum, A.; Sharma, A.K. Optimisation of Extraction Process for Negundoside and Agnuside from Vitex negundo L. Leaves Using Soxhlet Extraction, HPLC–MS/MS, and CCD-RSM Methods. Chem. Afr. 2022, 5, 907–915. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Bari, S.M.N.; Faruque, S.M. In Vitro and In Vivo Bactericidal Activity of Vitex negundo Leaf Extract against Diverse Multidrug Resistant Enteric Bacterial Pathogens. Asian Pac. J. Trop. Med. 2013, 6, 352–359. [Google Scholar] [CrossRef]
- Koirala, N.; Dhakal, C.; Munankarmi, N.N.; Ali, S.W.; Hameed, A.; Martins, N.; Sharifi-Rad, J.; Imran, M.; Arif, A.M.; Hanif, M.S.; et al. Vitex negundo Linn.: Phytochemical Composition, Nutritional Analysis, and Antioxidant and Antimicrobial Activity. Cell. Mol. Biol. 2020, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nyiligira, E.; Viljoen, A.M.; Van Heerden, F.R.; Van Zyl, R.L.; Van Vuuren, S.F.; Steenkamp, P.A. Phytochemistry and In Vitro Pharmacological Activities of South African Vitex (Verbenaceae) Species. J. Ethnopharmacol. 2008, 119, 680–685. [Google Scholar] [CrossRef]
- Rani, A.; Sharma, A. The Genus Vitex: A Review. Pharmacogn. Rev. 2013, 7, 188. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Kumpun, S.; Kirtikara, K.; Yingyongnarongkul, B.; Suksamrarn, S. Iridoids with Anti-Inflammatory Activity from Vitex peduncularis. Planta Med. 2002, 68, 72–73. [Google Scholar] [CrossRef]
- Vaughan, J.C.S. A Preliminary Note on the Use of Vitex peduncularis in Malarial Fever and in Blackwater Fever. Br. Med. J. 1921, 1, 186. [Google Scholar] [CrossRef]
- Batubara, I.; Mitsunaga, T.; Ohashi, H. Screening Antiacne Potency of Indonesian Medicinal Plants: Antibacterial, Lipase Inhibition, and Antioxidant Activities. J. Wood Sci. 2009, 55, 230–235. [Google Scholar] [CrossRef]
- Ata, A.; Mbong, N.; Iverson, C.D.; Samarasekera, R. Minor Chemical Constituents of Vitex pinnata. Nat. Prod. Commun. 2009, 4, 1934578X0900400. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Yoshimura, T. Antifungal and Antitermitic Activities of Wood Vinegar from Vitex pubescens Vahl. J. Wood Sci. 2013, 59, 344–350. [Google Scholar] [CrossRef]
- Anwar, L.; Efdi, M.; Ninomiya, M.; Ibrahim, S.; Putra, D.P.; Tanaka, K.; Koketsu, M. Labdane Diterpene Lactones of Vitex pubescens and Their Antileukemic Properties. Med. Chem. Res. 2017, 26, 2357–2362. [Google Scholar] [CrossRef]
- Leitão, S.G.; Delle Monache, F. 2″-O-Caffeoylorientin from Vitex polygama. Phytochemistry 1998, 49, 2167–2169. [Google Scholar] [CrossRef]
- Ahmadvand, H.; Hamadani, S.E.; Bagheri, S.; Moradi, H.; Khoramabadi, R.M.R.; Khosravi, P.; Cheraghi, A.; Cheraghi, R.A. Chemical Composition of Vitex pseudo-negundo Leavese Ssential Oil. J. Chem. Pharma. Res. 2014, 11, 300–304. [Google Scholar]
- Yan, C.-X.; Wei, Y.-W.; Li, H.; Xu, K.; Zhai, R.-X.; Meng, D.-C.; Fu, X.-J.; Ren, X. Vitex Rotundifolia L. f. and Vitex trifolia L.: A Review on Their Traditional Medicine, Phytochemistry, Pharmacology. J. Ethnopharmacol. 2023, 308, 116273. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Anis, M. Rapid In Vitro Propagation System through Shoot Tip Cultures of Vitex trifolia L.—An Important Multipurpose Plant of the Pacific Traditional Medicine. Physiol. Mol. Biol. Plants 2014, 20, 385–392. [Google Scholar] [CrossRef]
- Xavier, T.F.; Kannan, M.; Lija, L.; Auxillia, A.; Rose, A.K.F. Ethnobotanical Study of Kani Tribes in Thoduhills of Kerala, South India. J. Ethnopharmacol. 2014, 152, 78–90. [Google Scholar] [CrossRef]
- Ong, H.G.; Kim, Y.-D. Quantitative Ethnobotanical Study of the Medicinal Plants Used by the Ati Negrito Indigenous Group in Guimaras Island, Philippines. J. Ethnopharmacol. 2014, 157, 228–242. [Google Scholar] [CrossRef]
- Weiner, M.A. Secrets of Fijian Medicine; Ministry of Health: Wellington, New Zealand, 1984. [Google Scholar]
- Whistler, W.A. Polynesian Herbal Medicine; National Tropical Botanical Garden: Lawai, HI, USA, 1992. [Google Scholar]
- Boiteau, P.; Allorge, L. Plantes Médicinales de Madagascar; Lune Rouge: Ibiza, Spain, 1993. [Google Scholar]
- Panthong, A.; Kanjanapothi, D.; Taylor, W.C. Ethnobotanical Review of Medicinal Plants from Thai Traditional Books, Part I: Plants with Anti-Inflammatory, Anti-Asthmatic and Antihypertensive Properties. J. Ethnopharmacol. 1986, 18, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ghani, S.; Rafiee, B.; Bahrami, S.; Mokhtari, A.; Aghamiri, S.; Yarian, F. Green Synthesis of Silver Nanoparticles Using the Plant Extracts of Vitex agnus-castusVitex agnus-castus L: An Ecofriendly Approach to Overcome Antibiotic Resistance. Int. J. Prev. Med. 2022, 13, 13–133. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and Antimicrobial Activity of Essential Oils Isolated from Egyptian Plants against Plant Pathogenic Bacteria and Fungi. Ind. Crops Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
- Zhelev, I.; Petkova, Z.; Kostova, I.; Damyanova, S.; Stoyanova, A.; Dimitrova-Dyulgerova, I.; Antova, G.; Ercisli, S.; Assouguem, A.; Kara, M.; et al. Chemical Composition and Antimicrobial Activity of Essential Oil of Fruits from Vitex agnus-castus L., Growing in Two Regions in Bulgaria. Plants 2022, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Bakr, R.O.; Zaghloul, S.S.; Hassan, R.A.; Sonousi, A.; Wasfi, R.; Fayed, M.A. Antimicrobial Activity of Vitex agnus-castus Essential Oil and Molecular Docking Study of Its Major Constituents. J. Essent. Oil Bear. Plants 2020, 23, 184–193. [Google Scholar] [CrossRef]
- Balpınar, N.; Ökmen, G.; Vurkun, M. Antibacterial and Antioxidant Activities of Vitex agnus-castus L. Against Mastitis Pathogens. Fresenius Environ. Bull. 2019, 28, 278. [Google Scholar]
- Bayraktar, O.; Altıok, E.; Yılmazer, Ö.; Rusçuklu, D.; Büyüköz, M. Antioxidant, Antimicrobial and Cytotoxic Activities of Extracts from Some Selected Mediterranean Shrub Species (Maquis). Biointerface Res. Appl. Chem. 2016, 6, 1437–1444. [Google Scholar]
- Berrani, A.; Marmouzi, I.; Bouyahya, A.; Kharbach, M.; El Hamdani, M.; El Jemli, M.; Lrhorfi, A.; Zouarhi, M.; Faouzi, M.E.A.; Bengueddour, R. Phenolic Compound Analysis and Pharmacological Screening of Vitex agnus-castus Functional Parts. BioMed Res. Int. 2021, 2021, e6695311. [Google Scholar] [CrossRef]
- Eryigit, T.; Çig, A.; Okut, N.; Yildirim, B.; Ekici, K. Evaluation of Chemical Composition and Antimicrobial Activity of Vitex agnus-castusVitex agnus-castus L. Fruits’ Essential Oils from West Anatolia, Turkey. J. Essent. Oil Bear. Plants 2015, 18, 208–214. [Google Scholar] [CrossRef]
- Kadak, A.E.; Salem, M.O.A. Antibacterial Activity of Chitosan, Some Plant Seed Extracts and Oils against Pathogenic Organisms Escherichia coli and Staphylococcus aureus. Alinteri J. Agric. Sci. 2020, 35, 144–150. [Google Scholar] [CrossRef]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid. Based Complement. Alternat. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef]
- Stojković, D.; Soković, M.; Glamočlija, J.; Džamić, A.; Ćirić, A.; Ristić, M.; Grubišić, D. Chemical Composition and Antimicrobial Activity of Vitex agnus-castus L. Fruits and Leaves Essential Oils. Food Chem. 2011, 128, 1017–1022. [Google Scholar] [CrossRef]
- Tanhaeian, A.; Sekhavati, M.H.; Moghaddam, M. Antimicrobial Activity of Some Plant Essential Oils and an Antimicrobial-Peptide against Some Clinically Isolated Pathogens. Chem. Biol. Technol. Agric. 2020, 7, 13. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Çenet, M.; Öztürk, B.; Bozok, F.; Karabörklü, S.; Demirci, S.C. Chemical Characterization, Phytotoxic, Antimicrobial and Insecticidal Activities of Vitex agnus-castus’ Essential Oil from East Mediterranean Region. J. Essent. Oil Bear. Plants 2015, 18, 1500–1507. [Google Scholar] [CrossRef]
- Zazharskyi, V.V.; Davydenko, P.; Kulishenko, O.; Borovik, I.V.; Zazharska, N.M.; Brygadyrenko, V.V. Antibacterial and Fungicidal Activities of Ethanol Extracts of 38 Species of Plants. Biosyst. Divers. 2020, 28, 281–289. [Google Scholar] [CrossRef]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V. In Vitro Antibacterial Potential of Some Vitex Species against Human Pathogenic Bacteria. Asian Pac. J. Trop. Med. 2011, 4, 645–648. [Google Scholar] [CrossRef]
- Bunu, M.I.; Ndinteh, D.T.; Macdonald, J.R.; Langat, M.K.; Isyaka, S.M.; Sadgrove, N.J.; Melnikovova, I.; Fernandez-Cusimamani, E. Ecdysteroids from the Stem Bark of Vitex doniana sweet (Lamiaceae; Ex. Verbenaceae): A Geographically Variable African Medicinal Species. Antibiotics 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Sonibare, O.O.; Effiong, I.; Oladosu, I.A.; Ekundayo, O. Chemical Constituents and Antimicrobial Activity of the Essential Oil of Vitex doniana sweet (Verbernaceae). J. Essent. Oil Bear. Plants 2009, 12, 185–188. [Google Scholar] [CrossRef]
- Vale, J.P.C.d.; Ribeiro, L.H.d.F.; de Vasconcelos, M.A.; Sá-Firmino, N.C.; Pereira, A.L.; do Nascimento, M.F.; Rodrigues, T.H.S.; da Silva, P.T.; de Sousa, K.C.; da Silva, R.B.; et al. Chemical Composition, Antioxidant, Antimicrobial and Antibiofilm Activities of Vitex gardneriana schauer Leaves’s Essential Oil. Microb. Pathog. 2019, 135, 103608. [Google Scholar] [CrossRef]
- Montes-Avila, J.; López-Angulo, G.; Duarte-de-la-Peña, G.; Díaz-Camacho, S.P.; Osuna-Galindo, V.C.; López-Valenzuela, J.Á.; Delgado-Vargas, F. Antioxidant, Antibacterial, and Antiparasitary Activities of Green Nanoparticles Synthesized Using Water-Soluble Melanins of Fruits. BioNanoScience 2022, 12, 228–240. [Google Scholar] [CrossRef]
- Medina, M.F.E.; Alaba, P.A.; Estrada-Zuñiga, M.E.; Velázquez-Ordoñez, V.; Barbabosa-Pliego, A.; Salem, M.Z.M.; Alonso-Fresán, M.U.; Camacho-Díaz, L.M.; Salem, A.Z.M. Anti-Staphylococcal Properties of Four Plant Extracts against Sensitive and Multi-Resistant Bacterial Strains Isolated from Cattle and Rabbits. Microb. Pathog. 2017, 113, 286–294. [Google Scholar] [CrossRef]
- Uribe-Beltrán, M.d.J.; Ahumada-Santos, Y.P.; Díaz-Camacho, S.P.; Eslava-Campos, C.A.; Reyes-Valenzuela, J.E.; Báez-Flores, M.E.; Osuna-Ramírez, I.; Delgado-Vargas, F. High Prevalence of Multidrug-Resistant Escherichia coli Isolates from Children with and without Diarrhoea and Their Susceptibility to the Antibacterial Activity of Extracts/Fractions of Fruits Native to Mexico. J. Med. Microbiol. 2017, 66, 972–980. [Google Scholar] [CrossRef]
- Dogra, S.; Sharma, M.D.; Tabassum, S.; Mishra, P.; Bhatt, A.K.; Bhuyar, P. Green Biosynthesis of Silver Nanoparticles (Agnps) From Vitex negundo Plant Extract and Its Phytochemical Screening and Antimicrobial Assessment Next to Pathogenic Microbes. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5993. [Google Scholar] [CrossRef]
- Ahmad, N.; Khan, M.I.; Ahmed, S.; Javed, S.B.; Faisal, M.; Anis, M.; Rehman, S.; Umair, S.M. Change in Total Phenolic Content and Antibacterial Activity in Regenerants of Vitex negundo L. Acta Physiol. Plant. 2013, 35, 791–800. [Google Scholar] [CrossRef]
- Arivudainambi, U.S.E.; Anand, T.D.; Shanmugaiah, V.; Karunakaran, C.; Rajendran, A. Novel Bioactive Metabolites Producing Endophytic Fungus Colletotrichum Gloeosporioides against Multidrug-Resistant Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2011, 61, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Arumanayagam, S.; Arunmani, M. Antibacterial Activity of Vitex negundo Linn. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 829–834. [Google Scholar] [CrossRef]
- Balasubramani, S.; Rajendhiran, T.; Moola, A.K.; Diana, R.K.B. Development of Nanoemulsion from Vitex negundo L. Essential Oil and Their Efficacy of Antioxidant, Antimicrobial and Larvicidal Activities (Aedes aegypti L.). Environ. Sci. Pollut. Res. 2017, 24, 15125–15133. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Akhtar, M.; Parvez, S.; Alam, J.; Alam, F.M. Antitumor and Antibacterial Activity of a Crude Methanol Leaf Extract of Vitex negundo L. Arch. Biol. Sci. 2013, 65, 229–238. [Google Scholar]
- Perumal Samy, R.; Ignacimuthu, S.; Sen, A. Screening of 34 Indian Medicinal Plants for Antibacterial Properties. J. Ethnopharmacol. 1998, 62, 173–182. [Google Scholar] [CrossRef]
- Sichaem, J.; Nguyen, H.-H.; Nguyen, V.-H.; Mac, D.-H.; Mai, D.-T.; Nguyen, H.-C.; Tran, T.-N.-M.; Pham, N.-K.-T.; Nguyen, H.-H.; Niamnont, N.; et al. A New Labdane-Type Diterpenoid from the Leaves of Vitex negundo L. Nat. Prod. Res. 2021, 35, 2329–2334. [Google Scholar] [CrossRef]
- Mamatha, G.; Sowmya, P.; Madhuri, D.; Mohan Babu, N.; Suresh Kumar, D.; Vijaya Charan, G.; Varaprasad, K.; Madhukar, K. Antimicrobial Cellulose Nanocomposite Films with In Situ Generations of Bimetallic (Ag and Cu) Nanoparticles Using Vitex negundo Leaves Extract. J. Inorg. Organomet. Polym. Mater. 2021, 31, 802–815. [Google Scholar] [CrossRef]
- Khan, A.M.; Qureshi, R.A.; Gillani, S.A.; Ullah, F. Antimicrobial Activity of Selected Medicinal Plants of Margalla Hills, Islamabad, Pakistan. J. Med. Plant Res. 2011, 5, 4665–4670. [Google Scholar]
- Kumar, S.; Singh, A.K.; Verma, S.K.; Misra, R.; Seniya, C. Antibacterial and Phyto-Chemical Analysis of Some Medicinal Plants and Their Efficacy on Multidrug Resistant Bacteria. J. Pure Appl. Microbiol. 2013, 7, 2191–2204. [Google Scholar]
- Mishra, S.; Mekap, S.K.; Patra, S.; Dhal, N.K.; Sahoo, S. Antioxidant and Anti Infective Potential of Oleanolic Acid Acetate Vis-à-Vis Vitex negundo Linn. and Oroxylum Indicum Vent. against Human Pathogens Causing Infections of UT, GIT and Skin. Orient. Pharm. Exp. Med. 2015, 15, 73–82. [Google Scholar] [CrossRef]
- Nagarsekar, K.S.; Nagarsenker, M.S.; Kulkarni, S.R. Evaluation of Composition and Antimicrobial Activity of Supercritical Fluid Extract of Leaves of Vitex negundo. Indian J. Pharm. Sci. 2010, 72, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Naidu, G.K.; Sujatha, B. Screening of Preliminary Phytochemical Analysis and In-Vitro Antimicrobial Activity of Stem Bark Extracts of Vitex negundo L. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1001–1006. [Google Scholar]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.-H.; Mohanta, T.K.; Bae, H. Large Scale Screening of Ethnomedicinal Plants for Identification of Potential Antibacterial Compounds. Molecules 2016, 21, 293. [Google Scholar] [CrossRef]
- Panda, S.K.; Thatoi, H.N.; Dutta, S.K. Antibacterial Activity and Phytochemical Screening of Leaf and Bark Extracts of Vitex negundo L. from Similipal Biosphere Reserve, Orissa. J. Med. Plant Res. 2009, 3, 294–300. [Google Scholar]
- Prabhu, N.; Raj, D.T.; Yamuna, G.K.; Ayisha, S.S.; Joseph Puspha, I.D. Synthesis of Silver Phyto Nanoparticles and Their Antibacterial Efficacy. Dig. J. Nanomater. Biostructures DJNB 2010, 5, 185–189. [Google Scholar]
- Prakash, S.; Ramasubburayan, R.; Ramkumar, V.S.; Kannapiran, E.; Palavesam, A.; Immanuel, G. In Vitro—Scientific Evaluation on Antimicrobial, Antioxidant, Cytotoxic Properties and Phytochemical Constituents of Traditional Coastal Medicinal Plants. Biomed. Pharmacother. 2016, 83, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Sathiamoorthy, B.; Gupta, P.; Kumar, M.; Chaturvedi, A.K.; Shukla, P.K.; Maurya, R. New Antifungal Flavonoid Glycoside from Vitex negundo. Bioorg. Med. Chem. Lett. 2007, 17, 239–242. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, S.; Nag, R.; Chaturvedi, A.; Nag, T.N. Antimicrobial Activity and Cellular Toxicity of Flavonoid Extracts from Pongamia pinnata and Vitex negundo. Rom. Biotechnol. Lett. 2011, 16, 6396–6400. [Google Scholar]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic Antioxidant and Antimicrobial Activities of Essential Oils of Some Selected Medicinal Plants in Combination and with Synthetic Compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Sumathi, P.; Parvathi, A. Antimicrobial Activity of Some Traditional Medicinal Plants. J. Med. Plants Res. 2010, 4, 316–321. [Google Scholar]
- Zargar, M.; Hamid, A.A.; Bakar, F.A.; Shamsudin, M.N.; Shameli, K.; Jahanshiri, F.; Farahani, F. Green Synthesis and Antibacterial Effect of Silver Nanoparticles Using Vitex negundo L. Molecules 2011, 16, 6667–6676. [Google Scholar] [CrossRef] [PubMed]
- Nuraskin, C.A.; Marlina, M.; Idroes, R.; Soraya, C.; Djufri, D. Antibacterial Activity Tests of N-Hexane, Ethyl Acetate, and Methanol Leaves (Vitex) Extract (Pinnata) against Streptococcus mutans. Open Access Maced. J. Med. Sci. 2020, 8, 181–184. [Google Scholar] [CrossRef]
- Shafie, N.A.; Suhaili, N.A.; Taha, H.; Ahmad, N. Evaluation of Antioxidant, Antibacterial and Wound Healing Activities of Vitex pinnata. F1000Research 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Nyiligira, E.; Viljoen, A.M.; Başer, K.H.C.; Õzek, T.; van Vuuren, S.F.; Houghton, P.J. Essential Oil Composition and In Vitro Antimicrobial and Anti-Inflammatory Activity of South African Vitex Species. South Afr. J. Bot. 2004, 70, 611–617. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Kamani, M.H.; Amani, H.; Mousavi Khaneghah, A. Voltage and NaCl Concentration on Extraction of Essential Oil from Vitex pseudo negundo Using Ohmic-Hydrodistillation. Ind. Crops Prod. 2019, 141, 111734. [Google Scholar] [CrossRef]
- Geetha, V.; Doss, A.; Doss, A.P.A. Antimicrobial Potential of Vitex trifolia Linn. Anc. Sci. Life 2004, 23, 30. [Google Scholar]
- Ali, A.M.; El-Sharhawy, S.H.; Hamid, J.A.; Ismail, N.H.; Lajis, N.H. Antimicrobial Activity of Selected Malaysian Plants. Pertanika J. Trop. Agric. Sci. 1995, 18, 57–62. [Google Scholar]
- Ababutain, I.M.; Alghamdi, A.I. In Vitro Anticandidal Activity and Gas Chromatography-Mass Spectrometry (GC-MS) Screening of Vitex agnus-castus Leaf Extracts. PeerJ 2021, 9, e10561. [Google Scholar] [CrossRef] [PubMed]
- Keikha, N.; Shafaghat, M.; Mousavi, S.M.; Moudi, M.; Keshavarzi, F. Antifungal Effects of Ethanolic and Aqueous Extracts of Vitex agnus-castus against Vaginal Isolates of Candida Albicans. Curr. Med. Mycol. 2018, 4, 30186986. [Google Scholar] [CrossRef] [PubMed]
- Abu-Tahon, M.A.; Mogazy, A.M.; Isaac, G.S. Resistance Assessment and Enzymatic Responses of Common Bean(Phaseolus vulgaris L) against Rhizoctonia solani Damping-off in Response to Seed Presoaking in Vitex agnus-castus L. Oils and Foliar Spray with Zinc Oxide Nanoparticles. South Afr. J. Bot. 2022, 146, 77–89. [Google Scholar] [CrossRef]
- Maltaş, E.; Uysal, A.; Yildiz, S.; Durak, Y. Evaluation of Antioxidant and Antimicrobial Activity of Vitex agnus-castus L. Fresen Env. Bull 2010, 19, 3094–3099. [Google Scholar]
- Mansour, M.M.A.; EL-Hefny, M.; Salem, M.Z.M.; Ali, H.M. The Biofungicide Activity of Some Plant Essential Oils for the Cleaner Production of Model Linen Fibers Similar to Those Used in Ancient Egyptian Mummification. Processes 2020, 8, 79. [Google Scholar] [CrossRef]
- López-Velázquez, J.G.; Delgado-Vargas, F.; Ayón-Reyna, L.E.; López-Angulo, G.; Bautista-Baños, S.; Uriarte-Gastelum, Y.G.; López-López, M.E.; Vega-García, M.O. Postharvest Application of Partitioned Plant Extracts from Sinaloa, Mexico for Controlling Papaya Pathogenic Fungus Colletotrichum Gloeosporioides. J. Plant Pathol. 2021, 103, 831–842. [Google Scholar] [CrossRef]
- Aiyaz, M.; Divakara, S.T.; Chandranayaka, S.; Niranjana, S.R. Efficacy of Seed Hydropriming with Phytoextracts on Plant Growth Promotion and Antifungal Activity in Maize. Int. J. Pest Manag. 2015, 61, 153–160. [Google Scholar] [CrossRef]
- Guleria, S.; Kumar, A. Antifungal Activity of Some Himalayan Medicinal Plants Using Direct Bioautography. J. Cell Mol. Biol. 2006, 5, 95–98. [Google Scholar]
- Panda, S.K.; Behera, B.; Dutta, S.K. Anti-Candidal Activity of Vitex negundo L.: An Ethnomedicinal Plant. J. Pure Appl. Microbiol. 2009, 3, 777–784. [Google Scholar]
- Bello, O.M.; Zaki, A.A.; Khan, S.I.; Fasinu, P.S.; Ali, Z.; Khan, I.A.; Usman, L.A.; Oguntoye, O.S. Assessment of Selected Medicinal Plants Indigenous to West Africa for Antiprotozoal Activity. South Afr. J. Bot. 2017, 113, 200–211. [Google Scholar] [CrossRef]
- Pan, W.; Liu, K.; Guan, Y.; Tan, G.T.; Hung, N.V.; Cuong, N.M.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S.; Zhang, H. Bioactive Compounds from Vitex leptobotrys. J. Nat. Prod. 2014, 77, 663–667. [Google Scholar] [CrossRef]
- Ban, N.K.; Thoa, N.T.K.; Linh, T.M.; Trang, D.T.; Van Kiem, P.; Nhiem, N.X.; Tai, B.H.; Van Minh, C.; Song, J.-H.; Ko, H.-J.; et al. Labdane-Type Diterpenoids from Vitex limonifolia and Their Antivirus Activities. J. Nat. Med. 2018, 72, 290–297. [Google Scholar] [CrossRef]
- Kannan, M.; Rajendran, P.; Vedha, V.; Ashok, G.; Anushka, S.; Chandran, P.; Nair, R. HIV-1 Reverse Transcriptase Inhibition by Vitex negundo L. Leaf Extract and Quantification of Flavonoids in Relation to Anti-HIV Activity. J. Cell. Mol. Biol. 2012, 10, 53–59. [Google Scholar]
- Gonçalves, J.L.S.; Leitão, S.G.; Monache, F.D.; Miranda, M.M.F.S.; Santos, M.G.M.; Romanos, M.T.V.; Wigg, M.D. In Vitro Antiviral Effect of Flavonoid-Rich Extracts of Vitex polygama (Verbenaceae) against Acyclovir-Resistant Herpes Simplex Virus Type 1. Phytomedicine 2001, 8, 477–480. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Ignacimuthu, S.; Hudson, J.B. Medicinal Plants of Tamil Nadu (Southern India) Are a Rich Source of Antiviral Activities. Pharm. Biol. 2009, 47, 422–429. [Google Scholar] [CrossRef]
- Kuruüzüm-Uz, A.; Ströch, K.; Demirezer, L.Ö.; Zeeck, A. Glucosides from Vitex agnus-castus. Phytochemistry 2003, 63, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Lan, X.-P.; Wang, Y.; Huang, B.-K.; Han, T.; Zhang, Q.-Y.; Qin, L.-P. A New Labdane Diterpene from Vitex negundo. Pharm. Biol. 2012, 50, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Gautam, L.N.; Shrestha, S.; Wagle, P.; Tamrakar, B. Chemical Constituents from Vitex negundo Linn. of Nepalese Origin. Sci. World 2010, 6, 27–32. [Google Scholar] [CrossRef]
- Rudrapaul, P.; Sarma, I.S.; Das, N.; De, U.C.; Bhattacharjee, S.; Dinda, B. New Flavonol Methyl Ether from the Leaves of Vitex peduncularis Exhibits Potential Inhibitory Activity against Leishmania Donovani through Activation of iNOS Expression. Eur. J. Med. Chem. 2014, 87, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Amikacin. In Meyler’s Side Effects of Drugs, 6th ed.; Aronson, J.K., Ed.; Elsevier: Oxford, UK, 2016; pp. 207–209. ISBN 978-0-444-53716-4. [Google Scholar]
- Mrugała, B.; Miłaczewska, A.; Porebski, P.J.; Niedzialkowska, E.; Guzik, M.; Minor, W.; Borowski, T. A Study on the Structure, Mechanism, and Biochemistry of Kanamycin B Dioxygenase (KanJ)—An Enzyme with a Broad Range of Substrates. FEBS J. 2021, 288, 1366–1386. [Google Scholar] [CrossRef] [PubMed]
- Scholar, E. Amikacin. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–5. ISBN 978-0-08-055232-3. [Google Scholar]
- Danish, P.; Ali, Q.; Hafeez, M.M.; Malik, A. Antifungal and Antibacterial Activity of Aloe vera Plant Extract. Biol. Clin. Sci. Res. J. 2020, 4, 1. [Google Scholar] [CrossRef]
- Bordean, M.-E.; Ungur, R.A.; Toc, D.A.; Borda, I.M.; Marțiș, G.S.; Pop, C.R.; Filip, M.; Vlassa, M.; Nasui, B.A.; Pop, A.; et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants 2023, 12, 596. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).