Abstract
The antifungal and antioxidant properties of essential oils (EOs) derived from four plants were assessed in vitro: Rosmarinus officinalis, Myrtus communis, Origanum compactum, and Eugenia aromatica. These plants are renowned for their diverse biological activities. Antioxidant activities were evaluated using DPPH, ABTS, and TAC tests. Antifungal activity was tested against four postharvest pathogens associated with chickpea in storage: Fusarium culmorum, Rhizopus oryzae, Penicillium italicum, and Aspergillus niger, using the broth microdilution technique. Additionally, the efficacy of several major compounds against fungi found in the EOs 1,8-cineole, carvacrol, and eugenol was evaluated. Furthermore, this study explored the potential synergy of combining eugenol and carvacrol in various ratios. Based on the results, E. aromatica EO exhibited the highest antioxidant activity, as evidenced by its lowest IC50 values for a DPPH of 0.006 mg/mL. This EO also demonstrated the best antifungal activity, with MIC values ranging from 0.098 to 0.13 μL/mL. The high concentration of eugenol in this oil was identified as a contributing factor to its potent antifungal effects. The individual application of eugenol displayed significant antifungal efficacy, which was further enhanced by incorporating carvacrol at a 1:3 ratio. This synergistic combination presents promising potential for the development of specific formulations aimed at optimizing grain protection during storage.
1. Introduction
The food industry is actively researching alternative preservation methods that are crucial for mitigating food waste and ensuring food security [1,2]. Seed storage is particularly vulnerable to oxidation and fungal infection during storage, resulting in significant quantity and quality losses. Free radical oxidative damage results in the degradation of lipids and proteins in seeds [3]. This oxidative process produces aldehydes or ketones, which cause irreversible alterations in the taste, flavor, color, and texture of food products, in addition to a decline in nutritional quality and seed viability [4,5]. Moreover, reactive radicals generated by oxidation can initiate a chain reaction in the human body, disrupting the structure and function of healthy cells [1,6]. Furthermore, fungal infection during post-harvest storage can result in substantial losses in seed quality and quantity. Grain storage fungi can produce a range of mycotoxins that adversely affect human health [3,7], including aflatoxins, trichothecenes, and malformin.
Exploring natural bioactive substances like essential oils (EOs) presents a promising approach for improving post-harvest quality without resorting to chemical methods, offering a sustainable and environmentally conscious alternative [4]. EOs are recognized for their wide array of biological activities, such as antioxidant, antibacterial, antiviral, insecticidal, and antifungal properties [5,8,9]. They offer potential substitutes for synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), which are associated with significant health risks and toxicity [10,11]. Additionally, EOs exhibit potent antifungal activities against various storage fungi, and their relatively harmless, biodegradable, and non-toxic nature makes them widely accepted by consumers [12,13,14]. EOs consist of complex mixtures of volatile organic compounds, including terpenoids and phenylpropanoids. Terpenoids, classified as monoterpene, sesquiterpene, or diterpene, consist of two, three, or four isoprene units [15]. The major elements of EOs have undergone extensive investigation in the pharmaceutical and cosmetic sectors [16,17] as well as for use as flavorings and preservatives in the food industry [18,19]. The combination of these key compounds offers a novel approach to enhancing their antimicrobial and antioxidant activities [20].
The increasing need to explore natural products with versatile functions for food preservation is evident [21]. These compounds can simultaneously serve as preservatives, antioxidants, and antimicrobial agents, fulfilling the demand for clean-label and environmentally friendly food products for consumers [22]. Extracts derived from plants containing polyphenols and EOs exemplify these adaptable natural products, providing diverse advantages for improving food safety and quality [23]. Accepting these compounds will pave the way for a safer, healthier, and more sustainable food industry, aligning with evolving consumer preferences [24,25].
This study aims to develop effective formulations to optimize the protection of grain storage by (1) evaluating in vitro the antioxidant and antifungal activities of EOs extracted from four aromatic and medicinal plant species, Rosmarinus officinalis, Myrtus communis, Origanum compactum, and Eugenia aromatica; (2) investigating the antifungal activity of some major components of the EOs cineol, carvacrol, and eugenol against storage fungi; and (3) assessing the potential synergy of combining eugenol and carvacrol in varying ratios to optimize the antifungal activity.
2. Results
2.1. Essential Oil Yields
The evaluation of EO quantities in relation to dry plant matter revealed a significant range of yield variability among distinct plant species (Table 1). The yields ranged from 0.4% to 15%, highlighting the diversity among species. Notably, E. aromatica demonstrated the highest yield, while the lowest yield (0.4%) was observed for M. communis.

Table 1.
EO yields extracted from four species of aromatic and medicinal plants.
2.2. Chemical Composition of Essential Oils
The results of the chemical composition analysis of the EOs are concisely summarized in Table 2. The EOs displayed a complex combination of monoterpene hydrocarbons, oxygenated monoterpenes, and sesquiterpene hydrocarbons. In R. officinalis, a substantial percentage of 1,8-cineole (48.22%) was identified, along with a notable quantity of camphor (22.58%). Similarly, M. communis exhibited significant content of 1,8-cineole (42.22%), along with α-pinene (17.56%) and other compounds listed in Table 2. The EO of E. aromatica was characterized by the notable presence of eugenol (96.29%). Remarkably, O. compactum exhibited a high level of carvacrol (79.20%). These findings emphasize the distinct chemical profiles of each botanical species, distinguished by specific compounds that likely play essential roles in their biological activities. The major constituents of these EOs, namely 1,8-cineole, carvacrol, and eugenol (Figure 1), were chosen to investigate their antifungal effectiveness against post-harvest fungi.

Table 2.
Relative percentages of the main components identified in EOs.
Figure 1.
Molecular structures of three monoterpenes using ChemDraw (version 12.0). (a) Eugenol; (b) carvacrol; (c) 1,8-Cineole.
2.3. Antioxidant Activity of Essential Oils
Among the four species tested, the EO of E. aromatica demonstrated significant antioxidant effectiveness, even surpassing that of ascorbic acid, as shown in Table 3. The IC50 values for the DPPH and ABTS assays were 0.006 mg/mL and 0.024 mg/mL, respectively. In contrast, O. compactum, R. officinalis, and M. communis exhibited relatively lower antioxidant activity (IC50 > 150 μg/mL) in both DPPH and ABTS assays. These results emphasize the notable ability of all analyzed EOs to scavenge DPPH and ABTS radicals. Additionally, the evaluation of reducing capacity further confirmed the strength of E. aromatica, demonstrating the highest reducing capacity, equivalent to 748.36 ascorbic acid units. In comparison, O. compactum, R. officinalis, and M. communis displayed moderate antioxidant capacity.

Table 3.
Evaluation of antioxidant activity of EOs using free radical DPPH, ABTS, and TAC assays.
2.4. Antifungal Activity
2.4.1. Antifungal Activity of Essential Oils
The antifungal activity of EOs extracted from four distinct plant species was assessed against four fungal strains (F. culmorum, R. oryzae, P. italicum, and A. niger) (Figure 2). Table 4 displays the values for the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC). All tested extracts demonstrated inhibitory effects on the fungal strains. E. aromatica EO showed the highest antifungal effectiveness, with MIC values ranging from 0.098 to 0.13 μL/mL. Interestingly, E. aromatica EO performed better than the commercial fungicide (Azoxystrobin) against most of the tested fungi. However, it is important to note that E. aromatica EO showed a fungistatic effect, limiting fungal growth without complete eradication. O. compactum EO also showed significant efficacy, with MIC values ranging from 0.781 to 3.125 μL/mL and with a fungicidal effect. In contrast, the extracts of R. officinalis and M. communis showed relatively modest antifungal activity, characterized by MIC values ranging from 6.25 to 25 μL/mL, indicating reduced effectiveness against the tested fungi.

Figure 2.
Cultures of selected fungal species on potato dextrose agar: (a) Fusarium culmorum, (b) Rhizopus oryzae, (c) Penicillium italicum, (d) Aspergillus niger.

Table 4.
Evaluation of MIC and MFC of EOs against four fungi species.
2.4.2. Antifungal Activity of the Main Components of EOs
The antifungal activity of the main components of the tested EOs (1,8-cineole, carvacrol, and eugenol) was evaluated against the four post-harvest fungi (Table 5). Notably, carvacrol demonstrated very high antifungal activity, with MIC values ranging from 0.098 to 0.13 μL/mL. Moreover, fungicidal effects were observed at concentrations ranging from 0.195 to 0.39 μL/mL. Similarly, eugenol showed high antifungal activity, displaying MIC values ranging from 0.195 to 0.781 μL/mL, along with fungicidal attributes. In contrast, 1,8-cineole exhibited restricted antifungal potential, characterized by higher MIC values. All tested compounds exhibited fungicidal properties against all fungal strains.

Table 5.
Evaluation of the MIC and MFC of the main components of EOs against four fungi.
2.4.3. Antifungal Activity of the Main Component Mixture
The antifungal activity of binary mixtures of eugenol and carvacrol against four post-harvest fungi was investigated (Table 6). The results indicate that mixture 2, composed of 25% eugenol and 75% carvacrol (in a ratio of 1:3), demonstrates greater efficacy than the other mixtures. The MIC value for this mixture was determined to be 0.098 μL/mL against all four fungi. Mixture 1, containing an equal proportion of the two components, showed a slightly reduced level of effectiveness.

Table 6.
Evaluation of MIC and MFC of the three mixtures of carvacrol and eugenol against four fungi.
Binary combination 2 demonstrated an additive effect, displaying fractional inhibitory concentration index (FICI) values ranging from 0.69 to 0.81 (0.5 ≤ FICI ≤ 1), while the other mixtures showed indifferent effects (1 ≤ FICI ≤ 4) (Table 7). The combination of eugenol and carvacrol resulted in enhanced antifungal effectiveness against the tested fungi.

Table 7.
Evaluation of the synergistic antifungal effect of eugenol–carvacrol mixtures.
2.5. Correlation Analysis between Antifungal and Antioxidant Activities
Pearson’s correlation analysis revealed a positive correlation between the antioxidant activity, quantified through the DPPH and ABTS assays, and the antifungal activity against the fungal strains (F. culmorum, R. oryzae, P. italicum, and A. niger) (Figure 3). The correlation coefficients was ranged from R2 = 0.73 to R2 = 0.99. The ABTS assay, in particular, showed a strong correlation with all fungal MICs, with coefficient values ranging from 0.9 to 1. Furthermore, a negative correlation was observed between TAC and fungal MICs of fungi, with correlation coefficients ranging from −0.59 to −0.46.
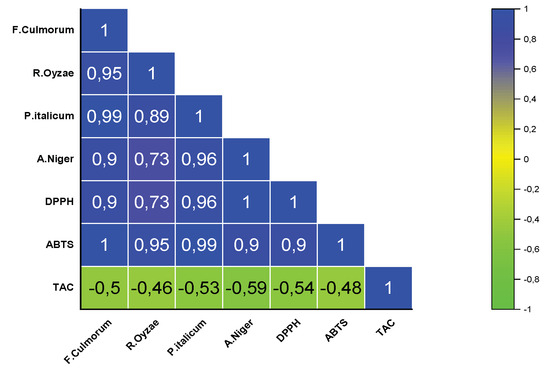
Figure 3.
Pearson’s correlation coefficient of the antifungal and antioxidant activities of EOs. Fusarium culmorum, Rhizopus oryzae, Penicillium italicum, Aspergillus niger; DPPH = 2.2−diphenyl−1−picrylhydrazyl; ABTS = 2.2’−azino−bis(3−ethylbenzothiazoline−6−sulfonic acid); TAC = total antioxidant capacity.
3. Discussion
The composition of essential oils (EOs) varies significantly due to factors such as plant species, harvest location, climate, and extraction techniques used [26]. EOs, rich in diverse chemical components, exhibit a range of biological activities and modes of action [27].
Among the EOs investigated in this study, E. aromatica EO demonstrated the highest antioxidant activity. This antioxidant potency, characterized by a low IC50 value of 6 µg/mL (<50 µg/mL), aligns with the findings of Saptarini et al., 2020 [28]. E. aromatica EO not only displayed a high scavenging capacity but also exhibited a notable reduction ability. Conversely, EOs of O. compactum, R. officinalis, and M. communis exhibited comparatively lower antioxidant effects, with R. officinalis and M. communis showing similar activities. Previous studies have highlighted clove oil’s excellent antioxidant properties, which corroborate our findings [29,30,31,32]. The antioxidant activity of clove oil might stem from its major component, eugenol, along with the complex interplay among its constituents, potentially involving synergistic and antagonistic effects [33]. The prominent presence of eugenol, constituting about 96% of E. aromatica EO’s composition, contributes to its antioxidant activity. Eugenol’s neuroprotective properties and its ability to inhibit lipid peroxidation are well documented [34,35]. Its structural capacity to stabilize phenoxy radicals through hydrogen atoms and neutralize damaged molecules underscores its role in preventing oxidative damage and carcinogenic mutations [36,37]. Notably, Jirovetz et al. (2006) revealed that clove EO exhibits DPPH radical scavenging activity at lower doses than eugenol alone, implying potential synergies within the EO’s components [38]. Additionally, Dahham et al. (2015) showed that caryophyllene, which composes 2% of E. aromatica, possesses significant antioxidant activity [39]. Eugenyl acetate, accounting for 0.7% of the EO, exhibited even greater antioxidant activity than clove EO, with IC50 values of 367.5 μg/mL and 283.9 μg/mL, respectively [40]. Therefore, E. aromatica EO’s antioxidant potential can be partially attributed to eugenol and the synergistic interactions among its phenolic compounds and secondary metabolites. Interestingly, carvacrol, comprising around 79% of O. compactum EO, displayed important antioxidant activity when tested individually [41]. Extensive studies corroborate carvacrol’s potent antioxidant properties [42,43]. Notably, oregano EO, which contains carvacrol, showcased higher antioxidant activity than carvacrol itself [44]. This suggests a synergistic action among O. compactum EO’s constituents, with carvacrol being the key contributor. M. communis and R. officinalis EOs, both featuring 1,8-cineole as a major compound (approximately 48% and 42% of composition, respectively), exhibited similar antioxidant effects. The synergistic effects of 1,8 cineole have been documented [45]. Phenolic and flavonoid compounds often account for EOs’ antioxidant attributes, acting as electron donors in free radical reactions [46,47], as also indicated by Noshad et al. (2021) [48].
Our study demonstrated significant inhibition of fungal strains by the tested EOs. Notably, E. aromatica EO exhibited the highest antifungal activity, even surpassing the efficacy of the fungicide azoxystrobin. O. compactum EO also showed pronounced fungicidal activity. In contrast, M. communis and R. officinalis EOs displayed lower antifungal effects. Consistent with previous research, clove and oregano oils are well-established for their antifungal activities [49,50,51,52,53]. E. aromatica EO’s strong inhibitory effect can be attributed to its major component, eugenol, or its synergetic interactions. Eugenol alone demonstrated substantial antifungal activity against the post-harvest fungal strains, although less effectively than E. aromatica EO. Eugenol’s minimum inhibitory concentration ranged from 0.195 to 0.781 μL/mL, while E. aromatica EO’s MIC values ranged from 0.098 to 0.13 μL/mL. Interestingly, eugenol displayed superior fungicidal properties compared to clove oil, aligning with Schmidt et al.’s findings (2007) [54]. This suggests that other components present in E. aromatica EO could enhance its antifungal activity synergistically. Carvacrol, the dominant compound in O. compactum EO (about 79%), exhibited the highest efficacy against fungal growth, with MIC values ranging from 0.098 to 0.13 μL/mL. Carvacrol outperformed O. compactum EO itself, which displayed MIC values ranging from 0.781 to 3.125 μL/mL. Our findings align with Schlösser et al.’s (2018) research [55], indicating carvacrol’s stronger antifungal effect compared to oregano oil. Multiple studies have highlighted carvacrol’s efficacy against various fungi, with low MIC values ranging from 0.1 to 0.2 μL/mL [56,57]. Hence, carvacrol appears to be the primary contributor to O. compactum EO’s antifungal activity. R. officinalis and M. communis EOs demonstrated similar antioxidant activities due to the presence of 1,8-cineole, their major compound. However, 1,8-cineole exhibited lower antifungal activity compared to the oils themselves. Our findings are in accordance with Dammak et al. (2019) [58], suggesting that 1,8-cineole’s antifungal potential is relatively modest compared to L. nobilis and L. dentate EOs. This indicates that other components in these EOs synergistically enhance their antifungal properties [59].
Our investigation extended to binary mixtures of eugenol and carvacrol to enhance their antifungal efficacy. A 25% eugenol to 75% carvacrol ratio exhibited the most promising results among the tested mixtures, with a MIC value of 0.098 µL/mL against the evaluated post-harvest fungi. The synergistic combination of eugenol and carvacrol demonstrated an additive effect, enhancing the overall antifungal efficacy. Previous research has highlighted the significant inhibitory effects of binary mixtures, such as carvacrol and thymol, major components of thyme oil, against various fungi [60,61]. Eugenol and citral, major constituents of citronella and lemongrass oils, have also shown potent fungal growth inhibition that surpasses their individual effects [62]. Our results are consistent with those of Schlosser et al. (2018) [55], which highlighted the additive antifungal effect of carvacrol and eugenol on foodborne mold fungi. Properly balanced mixtures can be valuable for food applications as long as sensory qualities are maintained [55]. Synergy among natural substances enhances stability and bioactivity [63]. Combining different major compounds can optimize absorption or penetration into fungal cells, producing an impact greater than the sum of its parts. Phenolic compounds and aromatic aldehydes enhance antifungal activity due to steric hindrance [64]. According to Nieto et al. (2013) [9], Alkyl groups added to the benzene ring can also enhance antifungal activity. Notably, EOs’ antimicrobial activity depends on their most common compounds’ composition and concentration [24]. The synergistic action of EO constituents can disrupt fungal growth by affecting different targets or stages of the fungal life cycle, often damaging cell membranes and altering permeability, leading to leakage of intracellular contents [59,65,66]. Therefore, the combined effect of eugenol and carvacrol optimizes antifungal properties, targeting multiple aspects of fungal growth.
In this study, a strong correlation emerged between antioxidant and antifungal activities, suggesting a potential synergistic relationship. This suggests the possibility of a dual-function natural food preservative. Monoterpenes in EOs can act as pro-oxidants in fungal cells, disrupting cycles and accumulating reactive oxygen species (ROS) [67]. This can prevent microbial adherence and biofilm formation [68]. Hence, exploring antioxidants as fungal infection treatments is promising. E. aromatica EO, with its dual antifungal and antioxidant actions or its major constituent, eugenol, in combination with carvacrol, holds potential applications.
While this initial study has revealed significant antioxidant and antifungal activities present in used essential oils (EOs) and their primary components, as demonstrated by in vitro analyses, further exploration through more comprehensive in vivo investigations is necessary. These investigations will assist in uncovering the most optimal approaches for utilizing their potential to effectively protect chickpea seeds.
4. Materials and Methods
4.1. Plant Materials and Extraction of Essential Oils
Four species of aromatic and medicinal plants, representing two different plant families (Lamiaceae and Myrtaceae), were harvested during the flowering season from their natural habitats at various locations in Morocco (Table 8).

Table 8.
Aromatic and medicinal plant species investigated in this study.
The essential oils (EOs) were extracted from the dried plant materials through hydrodistillation using a Clevenger apparatus following Guenther’s method [69]. In this process, 250 g of plant organs were placed in a 5000 mL flask filled with distilled water. The extraction proceeded for 3 h. This procedure was repeated iteratively to accumulate the required amount of EO for subsequent chemical and natural analyses. The extracted EOs were stored at 4 °C until use. The average EO yields were calculated based on dry plant material using Equation (1):
4.2. Gas Chromatography–Mass Spectrometry Analysis of Essential Oil
The chemical composition of the tested EOs was analyzed using gas chromatography coupled with mass spectrometry (GC–MS) in Rabat, Morocco. The identification of the different constituents of the essential oil was carried out using PerkinElmer Clarus 580 GC/Mass Spectrometer. A capillary column (30 m × 0.25 mm; 0.25 μm film thickness) was utilized for the separation of different compounds of the essential oils. The carrier gas used was helium, with a flow rate of 1 mL/min. The temperature program was fixed from 50 to 230 at 4 °C/min, with a final hold time of 5 min. The injector and detector were maintained at 235 to 240 °C. The injection volume was 0.02 μL with splitless mode. The quantification of individual components of essential oils was performed using a PerkinElmer autosystem XL gas chromatograph equipped with a flame ionization detector (GC-FID). The column used was a capillary DB5 (0.25 μm film thickness and 30 m × 0.25 mm i.d.).
4.3. Major Components of the EOs
Essential oil major components were of the highest purity available. Eugenol (≥99%), carvacrol (≥97%), and cineole (≥98%) were purchased from Fluka Chemica. These compounds were utilized to assess the antifungal activity against fungi strains. As a positive control, the commercial fungicide Azoxystrobin (250 g/L), obtained from Syngenta, was used.
4.4. Antioxidant Activity
The evaluation of the antioxidant activity of the EOs was performed using two scavenging methods: DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)). The determination of reducing capacity was carried out using the total antioxidant capacity (TAC) test. The ABTS and DPPH assays specifically target radicals, whereas the total antioxidant capacity assay offers a more comprehensive assessment of the overall antioxidant activity.
4.4.1. DPPH Radical Scavenging Activity
The DPPH assay was employed to determine the IC50 values, which represent the concentration of each EO required for 50% inhibition of the free radical. The methanolic DPPH solution was prepared with a concentration of 0.004% w/v and allowed to stabilize for one hour. Subsequently, 100 μL of each methanolic EO solution at varying concentrations (R. officinalis: 10–200 mg/mL; M. communis: 10–200 mg/mL; E. aromatica: 0.01–0.05 mg/mL; O. compactum: 1–10 mg/mL) was mixed with 750 μL of freshly prepared DPPH. The samples were then incubated in the dark at room temperature for 30 min. The change in color from purple to yellow was observed to assess the radical scavenging capacity of the volatiles relative to the negative control (DPPH solution only). As a positive control, a methanolic solution of ascorbic acid was prepared with concentrations ranging from 0.001 to 0.05 mg/mL. The absorbance of the samples was measured at 517 nm using a UV spectrophotometer [70]. The test was carried out in triplicate, with each experiment performing three repetitions. The antioxidant activity was calculated using Equation (2):
where AC and AS represent the absorbencies of the negative control and samples.
The antioxidant activity of each sample was quantified using IC50 values, which indicate the concentration (in mg/mL) needed to inhibit DPPH radical formation by 50%. The IC50 values, along with their corresponding 95% confidence intervals, were derived through linear regression analysis of the dose–response curve. In this analysis, the percentage of inhibition was plotted against the concentration.
4.4.2. ABTS Anion Radical Scavenging Activity
The ABTS solution was prepared by mixing 7 mM ABTS with 2.45 mM potassium persulfate in distilled water. The mixture was then incubated in the dark at room temperature for 16 h. The resultant ABTS+ solution was subsequently diluted with ethanol (1 mL per 1 mL) to attain an absorbance of 0.70 ± 0.02 at 734 nm [71]. For the experiment, 100 µL of each ethanolic solution of EO at varying concentrations was combined with 2 mL of fresh ABTS solution. The EO concentrations were as follows: R. officinalis (10–200 mg/mL), M. communis (10–200 mg/mL), E. aromatica (0.01–0.05 mg/mL), and O. compactum (1–8 mg/mL). After initial mixing, the absorbance was measured 6 min later at 734 nm. As a positive control, an ethanolic solution of ascorbic acid was prepared with concentrations ranging from 0.025 to 0.2 mg/mL. The test iwas conducted in three repetitions, and the entire experiment was repeated in triplicate. The percentage of inhibition was calculated using Equation (2), the same equation used for the DPPH-scavenging assay.
The antioxidant activity categories based on the IC50 values obtained from the scavenging activity of EOs are as follows: very strong when IC50 < 50 μg/mL, strong when 50 μg/mL < IC50 < 100 μg/mL, moderate effect when 100 μg/mL < IC50 < 150 μg/mL, and low when IC50 > 150 μg/mL [28].
4.4.3. Total Antioxidant Capacity
The determination of total antioxidant capacity was accomplished via the phosphomolybdenum assay [72]. The reagent solution was prepared by dissolving 4 mM ammonium molybdate, 28 mM sodium phosphate, and 0.6 M sulfuric acid in distilled water. For the assay, 25 µL of each methanolic EO solution at a concentration of 1 mg/mL was mixed with 1 mL of the reagent solution. The samples were then incubated in a water bath at 95 °C for 90 min. The reduction of the Mo complex was indicated by a color change in the samples from transparent to green. The optical density of the samples was measured at 695 nm using a UV spectrophotometer against a blank. The test was conducted in three repetitions, and the entire experiment was performed in triplicate. The antioxidant activity was expressed in terms of ascorbic acid equivalents (mg AAE/g of extract).
4.5. Antifungal Activity
4.5.1. Fungal Isolates and Inoculum Preparation
Four fungal species from distinct genera were employed in this assay: Fusarium (F. culmorum), Rhizopus (R. oryzae), Penicillium (P. italicum), and Aspergillus (A. niger). These post-harvest fungal species were isolated from chickpea seeds after storage. Identification of the isolates was conducted using standard identification keys based on their macroscopic and microscopic features [73,74,75]. These fungal species were chosen due to their prevalence within the seeds and their tendency to produce mycotoxins, such as zearalenone, B trichothecene, patulin, and ochratoxin A. The strains were maintained in a solution composed of 80% sabouraud dextrose broth (SDB) and 20% glycerol at −80 °C. Fungal strains were cultured on potato dextrose agar (PDA) for 7 to 10 days at a temperature of 22 ± 2 °C.
The inoculum suspension was prepared by rinsing the surface of the agar plates with 1 mL of sterile 0.9% saline water containing 0.1% Tween 20. Conidial suspensions were quantified using a hemocytometer and diluted to achieve a working suspension of 1.106 spores/mL.
4.5.2. Determination of the Minimum Inhibitory Concentration (MIC)
The antifungal activity of EOs and their main components was evaluated using the broth microdilution technique in sterile 96-well microplates [57]. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of oil or main component that completely inhibited visible fungal growth. Initially, each well of the microplates received 150 µL of SDB. Subsequently, 150 µL of a double-concentrated emulsion of each test product was introduced to the wells of the first row of the plate. Doubling dilution series were executed to establish a concentration range from 0.004% to 5%, with preparations in dimethyl sulfoxide (DMSO) attaining a final concentration of 5% (v/v). Following this, 15 µL of fungal suspension inoculum was introduced to all wells of the microplate, leading to a final concentration of 1.105 spores/mL. Wells containing inoculum but lacking treatment served as a negative control. A chemical treatment (azoxystrobin, 250 g/L) was utilized as the positive control. The plates were covered and incubated for 72 h at 22 ± 2 °C. Absorbance was measured at 492 nm using a microplate reader, with visual confirmation of outcomes under a microscope. Each test was replicated in triplicate, and the entire experiment was performed twice. The percentage of mycelium growth inhibition was computed using the subsequent Equation (3):
where AC is the absorbance of the inoculum suspension in the control at t = 0 h and t = 72 h, and AT is the absorbance of the treatment at t = 0 h and t = 72 h.
4.5.3. Determination of the Minimum Fungicidal Concentration (MFC)
The minimum fungicidal concentration (MFC) was determined to assess the fungicidal or fungistatic attributes of the EOs and their main components. MFC was defined as the lowest concentration leading to complete inhibition of mycelium growth on SDB plates, as indicated by subculturing wells showing the MIC. For MFC determination, 100 µL of contents from the MIC well and wells with concentrations surpassing the MIC were subcultured into a new microplate freshly supplemented with 150 µL of SDB [76]. These microplates were incubated under the same conditions as the MIC microplate, and growth was assessed visually through microscopy and absorbance measurement at 492 nm. The percentage of mycelium growth was calculated using Equation (3). Each test was executed in triplicate, with the experiment repeated twice to ensure accuracy and reproducibility. To determine the fungicidal or fungistatic impact of EOs, MFC/MIC ratios were computed. A ratio of MFC/MIC ≤ 4 denoted a fungicidal effect, while a ratio of MFC/MIC > 4 indicated a fungistatic effect [49].
4.5.4. Determination of the Fractional Inhibitory Concentration Index (FICI)
The fractional inhibitory concentration (FIC) was determined to reveal the synergistic antifungal effect of two main components, carvacrol and eugenol. FIC determination was accomplished via the checkerboard method within a 96-well microtiter plate, adhering to the same procedure for determining the MIC. The FIC index was ascertained using the two-fold dilution method based on the results of the MIC determination for each main component separately. Concentrations of 4 × MIC, 2 × MIC, 1 × MIC, 1/2 × MIC, 1/4 × MIC, 1/8 × MIC, and 1/16 × MIC were chosen for the test [62]. Three binary combinations of eugenol and carvacrol were examined using distinct volume proportions (Table 9). The test was performed in three repetitions, and the experiment was conducted in duplicate.

Table 9.
The three binary mixture ratios of eugenol and carvacrol used.
The synergistic effect was expressed according to the following expression:
FICI = Σ FIC = FIC (A) + FIC (B)
FIC (A) = MIC of the main compound (A) in combination/MIC of the main compound (A) alone.
FIC (B) = MIC of the main compound (B) in combination/MIC of the main compound (B) alone.
The values obtained for this index determined the combined effect of both compounds: FICI–FIC index values were interpreted as follows: FICI values ≤ 0.5 indicated synergy, 0.5 ≤ FICI ≤ 1 indicated an additive effect, 1 ≤ FICI ≤ 4 indicated an indifferent effect, and FICI values > 4 indicated an antagonistic effect [49].
4.6. Statistical Analysis
The results were presented as the mean ± standard deviation. Mean differences were assessed using analysis of variance (one-way ANOVA), followed by the Tukey post hoc test for multiple group comparisons. A significance level of p < 0.05 was considered to indicate statistical significance. The statistical analysis was performed using IBM SPSS v.22 software. Pearson’s correlation test was utilized to establish correlation coefficients between antifungal activity and antioxidant activity (DPPH, ABTS, and TAC) using Origin 2023 (10.0) software.
5. Conclusions
In conclusion, our study underscores the significant antioxidant and antifungal potential of E. aromatica among the tested EOs. The substantial presence of eugenol in this oil can contribute to its pronounced antifungal properties. Furthermore, the application of eugenol alone exhibited a notable antifungal effect, which was further potentiated when combined with carvacrol in a 1:3 ratio. This synergistic interaction suggests the possibility of developing customized formulations or treatments to enhance grain protection during storage. The combination of E. aromatica EO and carvacrol also presents a promising prospect for advancing natural preservation in diverse industries. Further investigation is required to optimize this combination and identify optimal application strategies.
Author Contributions
Conceptualization, S.K.B. and L.E.-t.; methodology, S.K.B., L.E.-t. and A.L.; formal analysis, L.E.-t., S.K.B., A.L., L.S. and M.E.; investigation.; L.E.-t. and S.K.B.; writing—original draft preparation, L.E.-t. and S.K.B.; writing—review and editing, S.K.B. and A.L.; visualization, S.K.B., A.L. and L.S.; supervision, S.K.B. and A.L.; funding acquisition, S.K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external funding. The journal’s article processing charges (APC) were covered by the National Institute of Agricultural Research (INRA, Morocco).
Acknowledgments
The authors express their gratitude to the Hassan II Institute of Agronomy & Veterinary Medicine—Rabat (IAV Hassan II) for their invaluable assistance in the analysis of essential oil chemical composition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ingram, J. A food systems approach to researching food security and its interactions with global environmental change. Food Sec. 2011, 3, 417–431. [Google Scholar] [CrossRef]
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercierb, S.; O’Donnelld, C.; Ktenioudakid, A. Global food security—Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Szymandera-Buszka, K.; Hęś, M. Effect of ethanolic fl ax (Linum usitatissimum L.) extracts on lipid oxidation and changes in nutritive value of frozen-stored meat products. Acta Sci. Pol. Technol. Aliment. 2014, 13, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiong, Y.L.; Kong, B.; Huang, X.; Li, J. Influence of storage temperature and duration on lipid and protein oxidation and flavour changes in frozen pork dumpling filler. Meat Sci. 2013, 95, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, R.I.; Singha, P.; Singh, S.K. A comprehensive review on impact of non-thermal processing on the structural changes of food components. Food Res. Int. 2021, 149, 110647. [Google Scholar] [CrossRef]
- Rasooli, I. Food Preservation—A Biopreservative Approach. Food 2007, 1, 111–136. [Google Scholar]
- Mannaa, M.; Deok Kim, K. Influence of Temperature and Water Activity on Deleterious Fungi and Mycotoxin Production during Grain Storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Manzur, M.; Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Citrus sinensis Essential Oils an Innovative Antioxidant and Antipathogenic Dual Strategy in Food Preservation against Spoliage Bacteria. Antioxidants 2023, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Jongberg, S.; Andersen, M.L.; Skibsted, L.H. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Sci. 2013, 95, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Ghamdi, F.L.A.; Bokhari, F.M.; Aly, M.M. Toxigenic fungi associated with dried Fruits and fruit-based products collected from Jeddah province. IOSR-JPBS 2019, 14, 10–20. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Karadayı, M.; Yıldırım, V.; Güllüce, M. Antimicrobial Activity and Other Biological Properties of Oregano Essential Oil and Carvacrol. Anatol. J. Biol. 2020, 17, 52–68. [Google Scholar]
- Ramzi, A.; Lalami, A.E.; Ez zoubi, Y.; Assouguem, A.; Almeer, R.; Najda, A.; Ullah, R.; Ercisli, S.; Farah, A. Insecticidal Effect of Wild-Grown Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Main Monoterpenes against Culex pipiens (Diptera: Culicidae). Plants 2022, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Kiki, M.J. In Vitro Antiviral Potential, Antioxidant, and Chemical Composition of Clove (Syzygium aromaticum) Essential Oil. Molecules 2023, 28, 2421. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Castanheira, I.; Fernando, A.L.; Loizzo, M.R.; Sanches Silva, A. A New Insight on Cardoon: Exploring New Uses besides Cheese Making with a View to Zero Waste. Foods 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, L.D.; Barbosa de Moraes, A.A.; da Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Leal Costa, C.M.; Cruz, J.N.; de Aguiar Andrade, E.H.; Guerreiro de Faria, L.J. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Almeida, L.Q.; Do Nascimento, L.D.; Da Costa, F.A.M.; da Costa, K.S.; de Aguiar Andrade, E.H. In Vitro Antibacterial Activity and in Silico Analysis of the Bioactivity of Major Compounds Obtained from the Essential Oil of Virola surinamensis Warb (Myristicaceae). J. Food Qual. 2022, 2022, 5275805. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2017, 45, 62–72. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; García-Gonzalo, D.; Pagán, R.; Laglaoui, A. Chemical composition and antioxidant properties of Laurus nobilis L. and Myrtus communis L. essential oils from Morocco and evaluation of their antimicrobial activity acting alone or in combined processes for food preservation. J. Sci. Food Agric. 2013, 94, 1197–1204. [Google Scholar] [CrossRef]
- Barberis, S.; Quiroga, H.G.; Barcia, C.; Talia, J.M.; Debattista, N. Natural Food Preservatives Against Microorganisms. In Food Safety and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 621–658. [Google Scholar] [CrossRef]
- Ogunnupebi, T.A.; Oluyori, A.P.; Dada, A.O.; Oladeji, O.S.; Inyinbor, A.A.; Egharevba, G.O. Promising Natural Products in Crop Protection and Food Preservation: Basis, Advances, and Future Prospects. Int. J. Agron. 2020, 2020, 8840046. [Google Scholar] [CrossRef]
- Du, W.X.; Avena-Bustillos, R.J.; Hua, S.S.T.; McHugh, T.H. Antimicrobial volatile essential oils in edible films for food safety. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 1124–1134. [Google Scholar]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [PubMed]
- Ghavam, M. Relationships of irrigation water and soil physical and chemical characteristics with yield, chemical composition and antimicrobial activity of Damask rose essential oil. PLoS ONE 2021, 16, e0249363. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Wardati, Y. Effect of Extraction Methods on Antioxidant Activity of Papery Skin Extracts and Fractions of Maja Cipanas Onion (Allium cepa L. var. ascalonicum). Sci. World J. 2020, 6, 3280534. [Google Scholar] [CrossRef]
- Abdelmoety, A.A.; Foda, F.F.A.; El-Hadary, A.E.; Abo-El-Seoud, M.A. Impact of Γ-Irradiation on Chemical Constituents, Antibacterial and Antioxidant Activity of Clove and Cinnamon Volatile Oils. J. Chem. Technol. Biotechnol. 2023, 14, 35–41. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Dashipour, A.; Khaksar, R.; Hosseini, H.; Shojaee-Aliabadi, S.; Ghanati, K. Physical, Antioxidant and Antimicrobial Characteristics of Carboxymethyl Cellulose Edible Film Cooperated with Clove Essential Oil. Zahedan J. Res. Med. Sci. 2014, 16, 34–42. [Google Scholar]
- Zengin, H.; Baysal, A.H. Antioxidant and Antimicrobial Activities of Thyme and Clove Essential Oils and Application in Minced Beef: Essential Oils and Application in Meat. J. Food Process. Preserv. 2014, 39, 1261–1271. [Google Scholar] [CrossRef]
- Guenane, H.; Gherib, A.; Carbonell-Barrachina, Á.; Cano-Lamadrid, M.; Krika, F.; Berrabah, M.; Maatallah, M.; Bakchiche, B. Minerals analysis, antioxidant and chemical composition of extracts of Laurus nobilis from southern Algeria. J. Mater. Environ. Sci. 2016, 7, 4253–4261. [Google Scholar]
- Das, M.; Roy, S.; Guha, C.; Saha, A.K.; Singh, M. In vitro evaluation of antioxidant and antibacterial properties of supercritical CO2 extracted essential oil from clove bud (Syzygium aromaticum). J. Plant Biochem. Biotechnol. 2020, 30, 387–391. [Google Scholar] [CrossRef]
- Kabuto, H.; Tada, M.; Kohno, M. Eugenol [2-Methoxy-4-(2-propenyl)phenol] Prevents 6-Hydroxydopamine-Induced Dopamine Depression and Lipid Peroxidation Inductivity in Mouse Striatum. Biol. Pharm. Bull. 2007, 30, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Vanin, A.B.; Orlando, T.; Piazza, S.P.; Puton, B.M.S.; Cansian, R.L.; Oliveira, D.; Paroul, N. Antimicrobial and Antioxidant Activities of Clove Essential Oil and Eugenyl Acetate Produced by Enzymatic Esterification. Appl. Biochem. Biotechnol. 2014, 174, 1286–1298. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Milos, M. Inhibition of lard oxidation by fractions of different essential oils. Grasas Y Aceites 2005, 56, 284–291. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, M.E.; Iriti, M.; Martorell, M.; Setzer, W.N.; Contreras, M.D.M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review: Carvacrol and Human Health. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Carvalho, F.O.; Silva, É.R.; Gomes, I.A.; Santana, H.S.R.; Santos, D.N.; Souza, G.P.O.; Silva, D.J.; Monteiro, J.C.M.; Júnior, R.L.C.A.; Araújo, A.A.S.; et al. Anti-inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta-analysis. Phytother. Res. 2020, 34, 2214–2229. [Google Scholar] [CrossRef] [PubMed]
- Özkan, A.; Erdoğan, A. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic components. Turk. J. Biol. 2011, 35, 735–742. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of Essential Oils Components and Polyphenols for Their Antioxidant Activity of Medicinal and Aromatic Plants Grown in Different Environmental Conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Asem, N.; Abdul Gapar, N.A.; Abd Hapit, N.H.; Omar, E.A. Correlation between total phenolic and flavonoid contents with antioxidant activity of Malaysian stingless bee propolis extract. J. Apic. Res. 2019, 59, 437–442. [Google Scholar] [CrossRef]
- Noshad, M.; Behbahani, B.A.; Jooyandeh, H.; Rahmati-Joneidabad, M.; Kaykha, M.E.H.; Sheikhjan, M.G. Utilization of Plantago major seed mucilage containing Citrus limon essential oil as an edible coating to improve shelf-life of buffalo meat under refrigeration conditions. Food Sci. Nutr. 2021, 9, 1625–1639. [Google Scholar] [CrossRef]
- Biernasiuk, A.; Baj, T.; Malm, A. Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions. Molecules 2022, 28, 215. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove Essential Oil as an Alternative Approach to Control Postharvest Blue Mold Caused by Penicillium italicum in Citrus Fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Ennouri, A.; Lamiri, A.; Essahli, M.; Bencheqroun, S.K. Chemical Composition of Essential Oils and Their Antifungal Activity in Controlling Ascochyta rabiei. J. Agr. Sci. Technol. 2020, 22, 1371–1381. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Bedoya-Serna, C.M.; Dacanal, G.C.; Fernandes, A.M.; Pinho, S.C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: In vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018, 49, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Jirovetz, L.; Wlcek, K.; Buchbauer, G.; Gochev, V.; Girova, T.; Stoyanova, A.; Geissler, M. Antifungal Activity of Eugenol and Various Eugenol-Containing Essential Oils against 38 Clinical Isolates of Candida albicans. J. Essent. Oil-Bear. Plants. 2007, 10, 421–429. [Google Scholar] [CrossRef]
- Schlösser, I.; Prange, A. Antifungal activity of selected natural preservatives against the foodborne molds Penicillium verrucosum and Aspergillus westerdijkiae. FEMS Microbiol. Lett. 2018, 365, 2018. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Markovic, T.; Chatzopoulou, P.; Šiljegović, J.; Nikolić, M.; Glamočlija, J.; Ćirić, A.; Soković, M. Chemical analysis and antimicrobial activities of the essential oils of Satureja thymbra L. and Thymbra spicata L. and their main components. Arch. Biol. Sci. 2011, 63, 457–464. [Google Scholar] [CrossRef]
- Dammak, I.; Hamdi, Z.; El Euch, S.K.; Zemni, H.; Mliki, A.; Hassouna, M.; Lasram, S. Evaluation of antifungal and anti-ochratoxigenic activities of Salvia officinalis, Lavandula dentata and Laurus nobilis essential oils and a major monoterpene constituent 1,8-cineole against Aspergillus carbonarius. Ind. Crops Prod. 2019, 128, 85–93. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S.N. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Vera-López, O.; Palou, E.; Avila-Sosa, R. Growth modeling to control (in vitro) Fusarium verticillioides and Rhizopus stolonifer with thymol and carvacrol. Rev. Argent. Microbiol. 2016, 50, 70–74. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Chen, Y.; Ji, L.; Yao, W. Synergistic properties of citral and eugenol for the inactivation of foodborne molds in vitro and on bread. LWT-Food Sci.Technol. 2020, 122, 109063. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Ochoa, L.R. Substitution de Solvants et Matières Actives de Synthèse par un Combiné «Solvant/Actif » d’Origine Végétale. Ph.D. Thesis, Autonomous University of Chihuahua, Mexico City, Mexico, 2005. [Google Scholar]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current anti-biofilm strategies and potential of antioxidants in biofilm control. Expert Rev. Anti-Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Otranto, D.; Cafarchia, C. Malassezia spp. Yeasts of Emerging Concern in Fungemia. Front. Cell. Infect. Microbiol. 2020, 10, 370. [Google Scholar] [CrossRef]
- Guenther, E. The Essential Oils; D. Van Nostrand Company, Inc.: New York, NY, USA, 1948; Volume 1, pp. 339–340. [Google Scholar]
- Hmamou, A.; Eloutassi, N.; Alshawwa, S.Z.; Al kamaly, O.; Kara, M.; Bendaoud, A.; El-Assri, E.; Tlemcani, S.; El Khomsi, M.; Lahkimi, A. Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver rhoeas L. Organ Extracts Growing in Taounate Region, Morocco. Molecules 2022, 27, 854. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Benziane Ouaritini, Z.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef]
- Ngombo-Nzokwani, A.; Manyi, M.M.; Mbuyi, A.K.; Kanyenga, A.L. Identification des champignons transmis par les semences biofortifiées au Kongo Central en République Démocratique du Congo. Afr. Sci. 2017, 13, 1–11. [Google Scholar]
- Tsedaley, B. Detection and identification of major storage fungal pathogens of maize (zea mays L.) in jimma, southwestern ethiopia. Eur. J. For. Res. 2016, 4, 38–49. [Google Scholar]
- Nyongesa, B.W.; Okoth, S.; Ayugi, V. Identification Key for Aspergillus Species Isolated from Maize and Soil of Nandi County, Kenya. J. Adv. Microbiol. 2015, 5, 205–229. [Google Scholar] [CrossRef]
- Sawadogo, I.; Paré, A.; Kaboré, D.; Montet, D.; Durand, N.; Bouajila, J.; Zida, E.P.; Sawadogo-Lingani, H.; Nikiéma, P.A.; Charles Nebié, R.H.; et al. Antifungal and Antiaflatoxinogenic Effects of Cymbopogon citratus, Cymbopogon nardus, and Cymbopogon schoenanthus Essential Oils Alone and in Combination. J. Fungi. 2022, 8, 117. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).