Abstract
Urginea maritima L. (squill) species is widely spread at the Mediterranean region as two main varieties, i.e., white squill (WS) and red squill (RS), that are recognized for several health potentials. The major secondary metabolite classes of the squill are cardiac glycosides, mainly, bufadienolides, flavonoids, and anthocyanins. Herein, a multiplex MS and NMR metabolomics approach targeting secondary and aroma compounds in WS and RS was employed for varieties classification. Solid-phase micro extraction-gas chromatography/mass spectroscopy (SPME-GC/MS), ultra-high-performance liquid chromatography/mass spectrometry (UPLC/MS), as well as nuclear magnetic resonance (NMR) provided fingerprinting and structural confirmation of the major metabolites for both types of the squill. For comparison of the different platforms’ classification potential, multivariate data analysis was employed. While Bufadienolides, viz. “hydroxy-scilliglaucosidin-O-rhamnoside, desacetylscillirosidin-O-rhamnoside and bufotalidin-O-hexoside” as well as oxylipids, were enriched in WS, flavonoids, i.e., dihydro-kaempferol-O-hexoside and its aglycon, taxifolin derivative, were predominant in RS. A cytotoxicity screening against three cancer cell lines, including breast adenocarcinoma (MCF-7), lung (A-549), and ovarian (SKOV-3) cell lines was conducted. Results revealed that WS was more effective on A-549 and SKOV-3 cell lines (WS IC50 0.11 and 0.4 µg/mL, respectively) owing to its abundance of bufadienolides, while RS recorded IC50 (MCF7 cell line) 0.17 µg/mL since is is rich inflavonoids.
1. Introduction
Urginea, closely related to the “Drimia” genera belonging to family Asparagaceaeis, is widely spread at the Mediterranean region, Africa and India [1]. In Arabic, it is known as Basal farion, Onsul, and Samm el-Far [2]. Other common names are termed squill and sea onion [3]. U. martima species comprises two varieties to include white and red, with both recognized for their bulb’s medicinal value and abundance of cardiac glycosides [4,5]. Stoll and coworkers isolated cardiac glycosides of U. maritima for the first time in structure similar to that of the Bufo genus termed bufadienolides, though it was less potent than digitalis cardiac glycosides [6]. U. maritima bulbs displayed a wide spectrum of pharmaceutical potentials. U. maritima bulbs enriched with “cardiac glycosides” Bufadienolides, viz. glucoscillarene A, proscillaridine A, scillarene A, scilliglaucoside and scilliphaeoside. Those bufadienolides involved in the cardiovascular system (CVS) via inhibiting Na+/K+ ATPase, which increases intracellular Ca+2, leading to enhancement of the contractility of cardiac muscles (positive inotropic effect) [6,7]. The methanolic extract and methylene chloride fraction of Urginea bulbs were reported as being antibacterial, anthelmintic, diuretic, and expectorant. Fresh bulbs were used for wound healing [7,8]. Besides its CVS effect, it possesses potential cytotoxic activity against lymphoma and breast cancer human cell lines in addition to its antirheumatic and antimicrobial actions [1,3,7]. The red variety of U. maritima differs from white squill mostly by the presence of red pigments, i.e., anthocyanins. Anthocyanins, such as cyanidin-3-monoglucoside and pelargonidin-3-monoglucoside, either free or acylated with caffeic or p-coumaric acid, were previously reported from the red bulbs of Spanish U. maritima. Taxifolin, taxifolin-4’-glucoside, quercetin-3-glucoside, C-flavone glycosides, and caffeic acid were isolated as major phenolic components [9]. Traces of fixed oil and volatile oil were also reported in addition to lignans and fatty acids [1,2,3,7,10,11,12]. Egyptian U. maritima showed more complex types of bufadienolides from its bulbs, which are from other Urginea species collected from Turkey, Greece and Tunisia [13]. Herein, we aimed to adopt a metabolomics approach for the classification of RS and WS, targeting their metabolome, including volatiles, as well as the secondary metabolites via multiple analytical platforms, viz. SPME/GC-MS, 1D, 2D NMR, and UPLC-MS, respectively. For data visualization and untargeted classification, multivariate data analyses, unsupervised learning, i.e., principal component analysis (PCA), and supervised learning, i.e., orthogonal projection to latent structures discriminant analysis (OPLS-DA) models, were applied. Both PCA and OPLS focus on identifying variances and similarities among specimens as extensively reported in our similar previous work using the same comparative MS and NMR platforms in metabolomics analysis of rosella [14,15,16].
2. Results and Discussion
2.1. Identification of Volatile Organic Compounds (VOCs)
The volatiles profiling of RS and WS was determined using headspace SPME coupled with GC-MS, as reported in the current study, for the first time. A total of 26 volatiles were identified belonging to monoterpenes (10), aliphatic hydrocarbons (6), oxygenated compounds (4), in addition to a heterocyclic, an aromatic, and a sesquiterpene, as listed in Table 1. Due to the higher percentiles of monoterpenes and aliphatic hydrocarbons in RS compared to WS (47.82% and 37.26% vs. 25.69% and 19.76%), respectively, RS had a stronger aroma profile than WS. Other classes found exclusively in WS included heterocyclics, oxygenated hydrocarbons, aromatics, sesquiterpenes, oxygenated monoterpenes, ca. 4.57%, 12.15%, 1.75%, 8.22% and 2.29%, respectively, as shown in Figure 1.

Table 1.
Volatiles percentile of WS and RS using SPME coupled with GC-MS (n = 1).
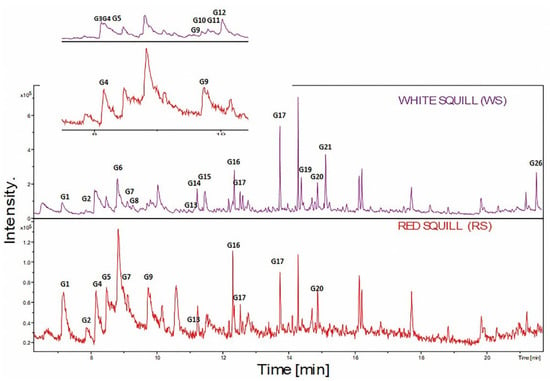
Figure 1.
SPME-GC/MS chromatogram of WS and RS headspace volatiles. The corresponding volatile names for each peak followed that listed in Table 1.
2.2. Secondary Metabolites Profiling via UHPLC/MS
UPLC-MS profiling was employed for secondary metabolites, and profiling in WS is denoted in pink color versus RS in red color. Figure 2 highlights the major differences between the two varieties. Diversity of secondary metabolite classes were identified in both squill, including mostly cardiac glycosides “bufadienolides” (50) followed by flavonoids (40) including“flavonols, flavanones and dihydroflavonols”. Minor classes of coumarins (1), anthocyanins (2), and phenolic acids (5) were detected, with full spectral data of identified peaks presented in Table 2.
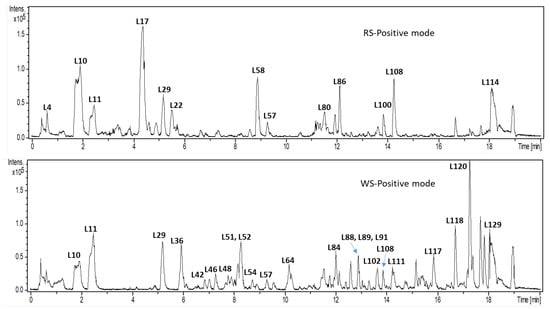
Figure 2.
UPLC/MS base peak chromatogram of both RS and WS squill varieties. Detections in “positive ionization mode” showing major secondary metabolites.

Table 2.
Secondary metabolites tentatively identified in two squill varieties (WS and RS) via UPLC/MS in positive (L) and negative (N) ionization modes.
The UV spectra of the major classes of secondary metabolites were observed at λ 280–299 nm. For bufadienolides glycosides, they were observed at 292–299 nm [17], while phenolics showed UV max at 280–283 and 296–340 nm [18]. Different classes have been annotated in both white and red squill, viz. bufadienolides, flavonoids, phenolic, amino, and fatty acids, as detailed in the next subsections. All identified secondary metabolites were listed in Table 2 with Supplementary Figures showing typical fragmentation pattern supporting their identification.
2.2.1. Bufadienolides
Bufadienolides are C-24 steroids with a pyranone ring at C-17β that are naturally present in plants and mammalian animals, specifically toads and snakes [19]. Bufadienolides were found more abundant in WS than RS as revealed from the inspection of the chromatograms of both WS and RS at retention time “Rt.” from 5 to 13 min. A series of different classes of bufadienolides were identified in WS and RS and included hydroxy-oxobufadienolide with a C-19 aldehyde group, i.e., bufotalidin, and hydroxybufaenolides with a C-19 methyl group, including scillarenin and scilliphosidin. Notably, identified bufadienolides structures were illustrated in Figure 3.
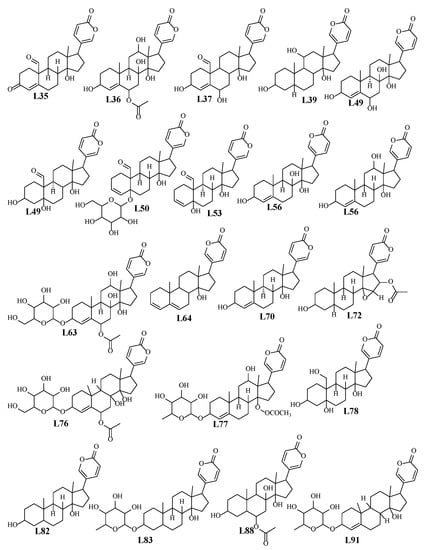
Figure 3.
Chemical structure of bufadienolides in both RS and WS detected using UPLC/MS.
Hydroxyoxobufaenolide
Hydroxyoxobufaenolides were detected in multiple peaks L53, L37, L49, while L36, L63, L67 and L68 were annotated as glycosides of hydroxyoxobufaenolides [20,21,22]. L36, L63, L67, and L68 were identified as hydroxyoxobufaenolide glycosides based on the loss of −162 or 146 amu. in L36 desacetylscillirosidin-O-thevetoside (m/z 577, (C31H44O10)+) (Figure S1A), L63 Hydroxyscilliroside (m/z 637, (C32H44O13)+) (Figure S1B) [23], L67 m/z 579 bufatalidin-O-hexoside, and L68 m/z 545 (C30H41O9)⁺ [M+H]+ scilliglaucosidin-O-rhamnoside (Figure S1C).
Identified hydroxybufaenolides are subdivided by the number of hydroxyl groups to mono, di, tri and tetra hydroxyl derivatives and were identified in both WS and RS as listed in Table 2.
The L64 peak m/z 367 (C24H31O3)+ [M+H]+ was detected in both WS and RS as monohydroxybufa-tetraenolide “Scillaridin A” [24,25,26] as an example of a monohydroxybufa-enolide, illustrated in Figure S1D.
Dihydroxybufaenolides were detected in several peaks including L82 m/z 387 (C24H35O4)+ [M+H]+ dihydroxybufadienolide “bufalin” [27] and L70 m/z 385 (C24H33O4)+ [M+H]+ dihydroxy-bufa-trienolide “Scillarenin” [28,29,30]. Glycosidic conjugates were observed in L91 m/z 531, showing a loss of rhamnose (−146 amu) (Figure S1E) and L86 m/z 693 (C36H53O13)+ [M+H]+ with a loss of hexose (−162 amu) (Figure S1F). L91 and L86 annotated as scillarenin-O-rhamnoside “Proscillaridin A” [11,31,32,33] and proscillaridin A-O-glucoside, respectively.
Acylated bufaenolides were detected in L90 at m/z 607 (C32H47O11)⁺ [M+H]+ and annotated as acetyl-scilliphaeoside. L39 at m/z 403 (C24H35O5)+ [M+H]+ annotated as trihydroxybufa-dienolide “gamabufotalin” [29,34,35] (Figure S1G).
Other acetylated tetrahydroxybufaenolide were detected in peak L54 at m/z 477, (C26H37O8)+ (M+2H)+ identified as hydroxyscillirosidin with a loss of acetyl group likely at C-16 [26,36,37] (Figure S1H).
2.2.2. Flavonoids
Compared to WS’s abundance of bufadienolides, flavonoids were more abundant in RS, with several being reported for the first time in this current study. Beside a positive mode, improved detection of flavonoids was observed in a negative mode, with MS2 fragments found to be characteristic of flavonoids, i.e., amu 151, 125 and 153 as well as neutral loss of amu −28 “CO”, −18 “H2O” and −44 “CO2” [38,39]. The next subsection shall summarize identification of the different flavonoid subclasses and distribution in Urginea species.
Identification of Flavanols/Flavonols
Identified flavanols in L22 and its methylated derivative in L24 at m/z 305 (C15H13O7) + [M+H] + and m/z 319 (C16H15O7)+ were annotated as dihydroquercetin (taxifolin) and dihydroisorhmentin (methyl taxifolin), respectively [40]. Flavonols were also detected in peak L40 at m/z 303 (C15H11O7)+ [M+H]+ identified as quercetin, as well as its glycosides L41 and L32 (Table 2).
Another major flavanol was detected in peak in RS L17 m/z 289 (C15H13O6)+ [M+H]+ and N13 m/z 287 (C15H11O6)− [M-H]− and were annotated as dihydrokaempferol [29,41,42] along with its glycoside in L16 (Figure S1I), N6 (Figure S1J), N7, N9 and N10. Furthermore, catechin and its hexoside belonging to flavanols, (C21H23O11)− and (C15H15O6)+, were detected in red squill as N3 and L14 (m/z 451 and 291), respectively [43].
Flavonols, such as N16 m/z 465 (C21H21O12)− and N24 m/z 447 (C21H19O11)− with loss of 180 amu (hexose + H2O) moieties and −162 hexose moiety, were identified as kaempferol-O-glucoside [44] (Table 2).
Identification of Flavones/Flavanones
Compared to the abundance of flavonols, the flavone subclass was detected in few peaks exemplified in L20 at m/z 271 (C15H11O5)+ [M+H]+ apigenin and its sugar glycoside in L28 and L33 (Table 2) [41]. L93 m/z 285 (C16H13O5)+ was interpreted as dihydroxymethoxyisoflavone [45].
L94 m/z 317 (C17H17O6)+, annotated as dihydroxy dimethoxy flavanone, represented an example of flavanone as well as N22 m/z 595 (C27H31O15)−, with MS2 m/z 271 attributed to the loss of two hexose units (−324 amu) and annotated as naringenin-O-dihexoside [46].
Finally, N12 m/z 479 (C22H23O12)− is annotated as noidesol A or B [47], and this is the first report of Noidesol in Urginea species (Figure S1K).
Compared to flavonols richness in red squill, anthocyanins were likewise identified exclusively in RS, accounting for its characteristic reddish color. Major anthocyanins included L3 at m/z 475 (C21H31O12)+, annotated as hydroxycinnamyl-O-dihexoside [9] (Figure S1L), and L13 at m/z 307 (C15H15O7)+ [M+H]+, identified as leucocyanidin [3] (Figure S1M).
Identification of Coumarins
The hydroxycoumarin class has been annotated in both squill types, represented as L29 and N15 m/z 163 (C9H7O3)+ and 161 (C9H5O3)−, respectively, and is in agreement with previous reports for coumarins in U. indica species [48,49].
2.2.3. Phenolic Acids
Phenolic acids are aromatic secondary plant metabolites found ubiquitously in plants and play a role in food quality and their organoleptic properties [50]. The primary detected phenolic acid was vanillic acid in L26 at m/z 169 (C8H9O4)+, and several glycosidic conjugates in peaks L25, L27, and N5, showing MS2 ion fragments of vanillic acid. L25 at m/z 447 (C20H29O13)+ vanillic-O-rhamnosyl-O-hexoside, L27 m/z 331 (C14H19O9)+ vanillic acid-O-hexoside [51], and N5 at m/z 491 (C20H27O14)− as vanillic acid-O-dihexoside with MS2 m/z 167 [26] in a negative ionization mode was detected. Other detected phenolic acids included in L105 at m/z 329 (C17H29O6)+ as spiculisporic acid and N14 at m/z 473 (C21H29O12)−, a glycoside of dimethoxyhydrocinnamic acid [52].
2.2.4. Amino Acids and Fatty Acids
Few amino acids were identified in both squills, including inL12 at m/z 205 (C11H13N2O2)⁺, L8 at m/z 182 (C9H12NO3)+, and L9 m/z 166 (C9H12NO2)+ for tryptophan, tyrosine, and phenyl alanine, respectively. Compared to amino acids showing earlier elution, fatty acids were observed at the end of the chromatogram, considering their lypophilic nature in both varieties. An example of major fatty acids includes L114 at m/z 279 (C18H31O2)+ [M+H]+ octadecatrienoic acid and L120 at m/z 281 (C18H33O2)+ linoleic acid. No difference in amino acids was observed among squill varieties compared to flavonoids; however, they later appear as stronger marker for variety type.
2.3. Multivariate Data Analysis of UPLC-MS Dataset
To aid in identifying further markers for each squill variety in an untargeted manner, unsupervised principle component analysis (PCA) and orthogonal projection to latent structures analysis (OPLS-DA) were attempted in both negative (Figure 4) and positive ionization modes (Figure 5). Complete segregation between the two squill varieties was observed, highlighting that RS was more rich in phenolics, whereas WS was abundant in bufadienolides.
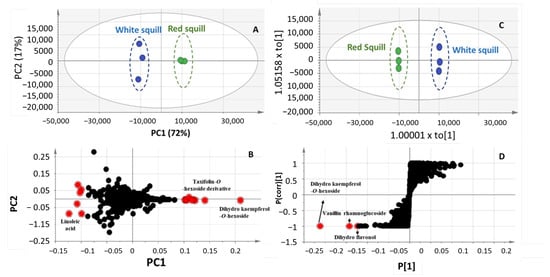
Figure 4.
PCA scoring plot (A), loading plot, (B) OPLS-DA scoring (C), and S-plot (D) models in negative ionization mode of RS and WS squill varieties.
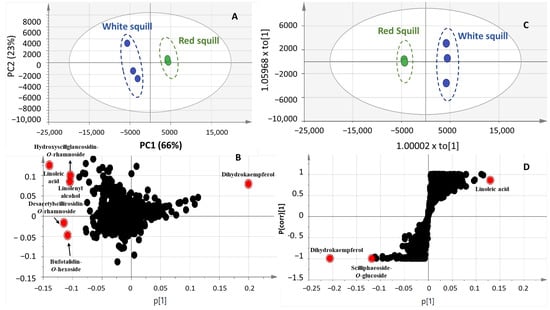
Figure 5.
PCA scoring plot (A), loading plot (B), OPLS-DA Scoring (C), and S-plot (D) models in positive ionization mode of RS and WS squill varieties.
A score plot model derived from the negative ionization mode of UHPLC/MS prescribed by PC1 and PC2 accounted for 72 and 17%, respectively, of the total variance (Figure 4A), with clear segregation of RS from WS. As for revealing the metabolites mediating RS and WS segregation, a PCA-loading plot (Figure 4B) revealed an abundance of flavanols, identified as dihydrokaempferol-O-hexoside and taxifolin-O-hexoside derivatives, in RS, which are likely to serve as precursors for anthocyanins solely found in RS variety. The OPLS-DA model (Figure 4C) further confirmed the PCA results from the S-plot (Figure 4D) revealing that dihydrokaempferol-O-hexoside, its aglycon, and vanillin rhamnoglucoside, were abundant in RS compared to WS.
The results from the UHPLC/MS-derived model in the positive ionisation mode were comparable to those in the negative mode (Figure 5A). The two squill varieties, RS and WS, were clearly separated in the PCA model, which was prescribed by PC1 66% and PC2 23%. Furthermore, according to Figure 5B, in accordance with the negative ionisation mode, RS was more abundant in dihydrokaempferol, which is a biosynthetic precursor for anthocyanins in RS [53]. On the other hand, WS had higher concentrations of bufadienolides, such as hydroxy-scilliglaucosidin-O-rhamnoside, desacetylscilliglaucosidin-O-rhamnoside, and bufotalidin-O-hexoside as well as oxylipids, such as linoleic acid and linoleyl alcohol. Supervised OPLS-DA model. Figure 5C,D confirmed linoleic acid enrichment in WS and identifying dihydro-kaempferol “aromandrin” and the bufadienolide “scillipheoside-O-glucoside” as markers for RS.
2.4. NMR Metabolites Fingerprinting
To provide a broader coverage of squill metabolome, NMR was employed to provide insight on both secondary and primary metabolites, especially with the later class not detected using LCMS. NMR offers also improved structural elucidation tool aided by its extensive 2D NMR experiments and quantitative determination of the major metabolites [54] for quality control purposes. Major classes detected in squill using NMR included sugars, flavonoids, bufadienolides, phenolics, and amino and fatty acids (Figure 6 and Table 3). Nevertheless, compared to MS, NMR suffers from low sensitivity and from signal overlap, especially in aliphatic regions. To overcome the problem of signal overlap, 2D NMR experiments were employed to allow for the resolution of overlapped signal along the second dimension, i.e., carbon in the case of HMBC [55,56]. 1H NMR spectra from WS showed the signals relative richness in both varieties (Figure 7 and Table S1).
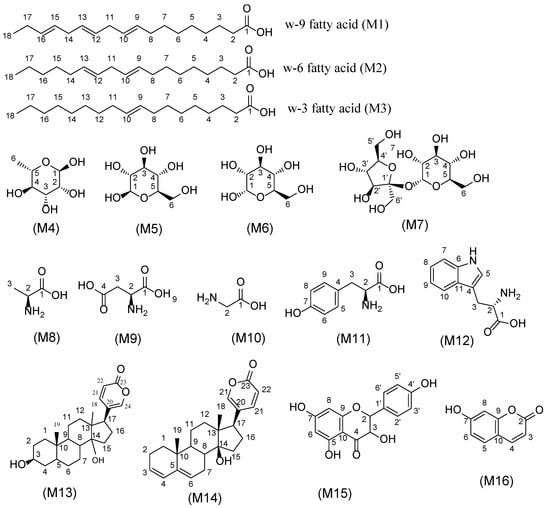
Figure 6.
Structure of the major primary and secondary metabolites detected in squill. Metabolite numbers follow those listed in Table 3 for metabolite identification using 1D and 2DNMR.

Table 3.
Assignment data from 1D and 2D NMR on WS and RS extracts.
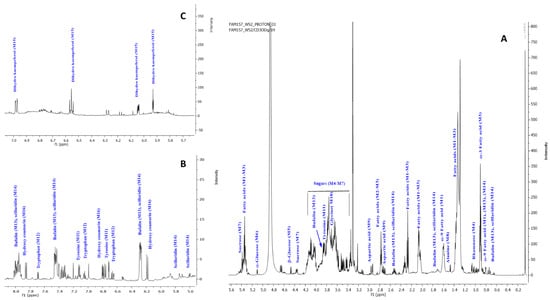
Figure 7.
1H-NMR of WS extract. (A) WS at δ 0–5.6 ppm (B) at δ 5.6–8.00 ppm. (C) RS at δ 5.7–7.0 ppm, prescribing characteristic signals for primary and secondary metabolites. Peaks annotated at the spectra labeled as follows: ω-9 fatty acid (M1), ω-6 fatty acid (M2), ω-3 fatty acid (M3), rhamnose (M4), β-glucose (M5), α-glucose (M6), sucrose (M7), alanine (M8), aspartic acid (M9), glycine (M10), tyrosine (M11), tryptophan (M12), bufalin (M13), scillaridin (M14), dihydro kaempferol (M15), and hydroxy coumarin (M16). The compounds spectral data were listed in Table 3.
2.4.1. Fatty Acids
A key feature of the assignment of unsaturated fatty acids (M1, M2 and M3) was based on the signals (-CH3) δH 0.89 ppm, a long chain of methylene groups (-CH2)n at δH 1.2 ppm, and olefinic bond (s) at δH 5.30–5.37 ppm, and HSQC cross-peak correlation with 13C showed signals at δ 15.7, 31.9, 128.8–132.1 ppm, respectively. Additionally, confirmation of unsaturation in fatty acids was based on allylic methylenes resonating at δ 2.05–2.10 ppm and correlated with 13C at δ 29.4 ppm. Bis allylic (-CH2) with two triplets at δ 2.76 and 2.78 with 13C at δ 26.2 and 27.8 ppm were correlated to ω-6 (M2) and ω-3 (M3) fatty acids, respectively. Furthermore, confirmation of these data was done using other 2D NMR experiments, as HSQC and HMBC displayed in Figures S2–S4.
2.4.2. Sugars
Rhamnose (M4), β-glucose (M5), α-glucose (M6), and sucrose (M7) were the major sugars detected in squill using NMR. Rhamnose (M4) was assigned based on its terminal CH3 at δH 1.22 and 13C at δ 13.8 ppm showing COSY correlation to anomeric proton at δ 3.34 and 13.51 ppm. Moreover, M5, M6, and M7 were identified based on the anomeric protons at δ 4.48 (d, J = 7.8 Hz), 5.10 (d, J = 3.7 Hz), and 5.39 (d, J = 3.8 Hz) ppm, respectively, alongside their respective 13C at δ 99.5, 95.2, and 94.8 ppm detected in HSQC (Figure S5).
2.4.3. Amino Acids
A total of five amino acids were identified in squill, including alanine (M8), aspartic acid (M9), glycine (M10), tyrosine (M11), tryptophan (M12). In details, alanine (M8) showed methyl group signals at δ 1.47 (d, J = 7.2 Hz) ppm. Moreover, M9, M10, and M11 showed methylene group signals at δ 2.95, 3.87, and 2.79 ppm. In Figure S2, the previously mentioned protons signals showed HSQC cross-peak correlation with 13C at δ 36.8, 45.1, and 37.3 ppm for M9 and M10, respectively. There were long-range HMBC correlations with carbons at δ 51.47 for alanine (M8) (Figure S3) while 52.6, 174.9, and 174.5 for aspartic acid (M9) (Figures S3 and S4) and 53.5 for tyrosine (M11) confirmed their assignments. Furthermore, a 1,4 di-substitution benzene ring appearing at δH 6.70 and 7.12 (d, J = 8.6 Hz) showed that HSQC correlations, alongside their 13C cross peaks at δ 118.0 ppm and 132.8 ppm were, assigned for tyrosine (M11) (Figure S6). Tryptophan (M12) was characterized from its indole moiety two triplet signals at δ 7.05 and 6.66 (t, J = 7.5 Hz), two doublets at δ 7.37 ppm and 7.69 ppm (d, J = 7.5 Hz), in addition to a singlet at δ 7.20 ppm, showing HSQC cross-peak correlation with 13C at δ 121.3, 117.4, 113.7, 120.5, and 126.5, respectively (Figure S6).
2.4.4. Bufadienolides
A key feature of bufadienolides assignment in 1H-NMR spectra are signals of α, β-unsaturated ketone of pyranone ring at δ 6.26, 7.93 ppm showing total correlation at δ 7.41 ppm, showing HSQC cross-peak with 13C at 116.7, 150.5 and 151.8 ppm, respectively. Quaternary carbons C-20 and C-23 at δ 125.9 and 164.8 ppm, respectively were identified from by HMBC distinct correlation and aiding in their assignment. A major bufadienolide “bufalin” (M13) was detected in both squill identified from CH signals at C-17 δ 2.54, C-5 2.16, and C-3 3.84 ppm showing HSQC cross-peak correlation with 13C at δ 42.5, 54.2 and 72.4 ppm, respectively (Figures S2, S5 and S6). Two methyl groups at δ 0.74 and 0.98 ppm with 13C at δ 18.5 and 23.2 ppm alongside a quaternary C-14 appearing at 13C δ 85.4 in HMBC spectrum (Figure S3), respectively. Furthermore, “scilliridin” (M14) another bufadienolide was recognized from two conjugated double bonds at δ 5.71, 5.75 and 5.88 ppm showing HSQC cross-peak correlation with 13C at 128.5, 129.3, and 129.8 ppm, respectively, (Figure S6). Further distinct HMBC cross peaks identified C-5 at δ 141.3 ppm (Figure S7).
2.4.5. Coumarins and Flavonoids
Hydroxy coumarin (M16) was identified based on α- and β-unsaturated ketone with signals at 6.18 and 7.85 ppm (d, J = 7.9 Hz), showing HSQC cross-peak correlations at 113.60 and 147.30 ppm, respectively (Figure S6). Moreover, an ABX benzene ring was revealed from signals at δ 6.71(d, J = 2.3 Hz), 6.80 (dd, J = 8.5, 2.3 Hz), and an overlapped peak at 7.4 ppm, showing HSQC cross-peak correlation at δ 104.63, 115.76, and 131.94 ppm, respectively (Figure S7).
Flavonoids were predicted primarily in RS and in accordance with UPLC-MS results are shown in Figure 2 and Table 2. An AABB system on chemical shift 6.98 (d, J = 8.5 Hz, 2H), 6.56 (d, J = 8.4 Hz, 2H), and chroman ring as AB system, m-position were elucidated from δH 6.04 (d, J = 1.8 Hz, 0H), and 6.04 (d, J = 1.8 Hz, H) was assigned to dihydrokaempferol (M15), (Figure S8).
2.5. Quantification of Major Metabolites via 1H-NMR
To aid in standardization of squill extract, 1H-NMR was further used to determine absolute levels of major metabolites in squill varieties via integration of their well-resolved signals in NMR spectra [57]. The concentration of metabolites was calculated as µg/mg dry powder, as shown in Supplementary Material Table S1.
With regard to primary metabolites, unsaturated fatty acids were detected at much higher levels in WS at 89.6 ± 25.3 versus 2.84 ± 0.5 µg/mL in RS. In contrast, a comparable amino acid level was detected in both varieties exemplified by aspartic acid as major form at 43.1 ± 7.2 and 38.3 ± 4.9 µg/mL in WS and RS, respectively. Other less abundant amino acids, including glycine, alanine, and tryptophan, were detected at 19.8 ± 3.5; 8.5 ± 1.6; and 2.2 ± 0.18 µg/mL in WS versus lower levels in RS at 7.2 ± 1.0; 0.3 ± 0.1; and 1.0 ± 0.1 µg/mL in RS, respectively. Sugars were found at comparable levels in both WS and RS at 42.5 ± 1.2 and 36.6 ± 0.5 µg/mL, respectively.
With regard to secondary metabolites to influence squill health effects, higher levels of total bufadeinolides distinguished by α and β-unsaturated ketone of pyranone ring (H-22) were measured in WS at 17.5 ± 7.5 µg/mL while in RS, dihydrokampferol was predominated at 43.6 ± 2.3 µg/mL. Finally, coumarins detected at almost equal levels in both WS and RS at ca. 5–6 µg/mg.
2.6. Cytotoxic Screening Activity
Squill is recognized for its anticancer effect against various cancer cells and for its antioxidant and cytotoxic properties [58]. Consequently, a comparative cytotoxic assay of both WS and RS was evaluated on the different cell lines to include breast adenocarcinoma (MCF-7), lung (A-549) and ovarian cancer (SKOV-3) cell lines using Sulforhodamine B assay (SRB). The results revealed the potential cytotoxic activity of both varieties, with WS found to be more active against both cell lines A-549 and SKOV-3 than RS, as evidenced by its lower IC50 values. The recorded IC50 values of WS against A-549 and SKOV-3 cell lines were at 0.108 ± 0.003 and 0.690 ± 0.018 µg/mL versus RS’s IC50 values at 0.271 ± 0.005 and 0.912 ± 0.021 µg/mL, respectively. In contrast, the RS extract showed a more potent effect than WS on the MCF-7 cell line, with IC50 values of 0.165 ± 0.007 and 0.326 ± 0.005 µg/mL, respectively. The doxorubicin “positive control” IC50 values recorded on MCF7, A-549, and SKOV-3 cancer cell lines were 0.2 ± 0.004, 0.56 ± 0.003 and 0.2 ± 0.01 µg/mL, respectively (Table 4 and Figure 8).

Table 4.
The IC50 values (mean ± SD) of RS and WS extract against different cancer cell lines measured by SRB assay versus dox. (doxorubicin) positive control.
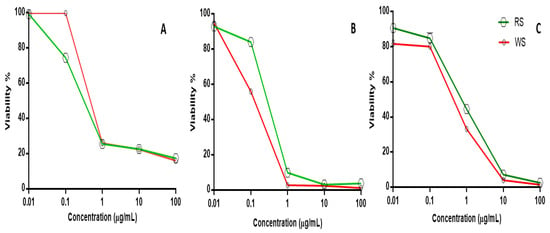
Figure 8.
Cytotoxicity screening viability percentile versus RS and WS concentration (µg/mL) on different cancer cell lines. (A) MCF7 breast adenocarcinoma, (B) A-549 lung, and (C) SKOV-3 ovarian cancer cell lines.
The potential cytotoxicity of WS compared to RS could be attributed to its abundance of bufadienolides, as revealed from both LC/MS and NMR reported for its cytotoxic action, and suggests that bufadieonolides are more determinant than flavonoids regarding cytotoxic action in squill, at least in case of A-549 and SKOV-3 cell lines.
Previous reports of hellebrigenin and bufatalin isolated from squill showed potential cytotoxic activity against leukemia, human colon carcinoma, human glioblastoma melanoma, and human liver carcinoma cells with IC50 values ranging from 0.0007 to 0.16 μM [59]. Moreover, bufatalin induced apoptosis in human leukemia cells [60]. Scillarenin exhibited stronger cytotoxic action in the nanomolar range in comparison with bufatalin [61].
Proscillaridin A, a cardiac glycoside isolated from U. maritima, was reported to exert an cytotoxic and/or antiproliferative effect against human breast cancer. proscillaridin A anticancer properties are mediated via its ability to block Na+/K+ ATPase, leading to increase in Ca2+ levels, activating the AMPK pathway. Interestingly, on the opposite, the Ca2+ level was reduced by ca. 30% after an 18 h administration of proscillaridin A to the normal lung fibroblast cell line CCD19-LU, which suggests differential action mechanisms against normal and cancer cells [62].
In a previous report, bufadienolides recorded effective cytotoxic action on human cancer cells [63]. Although in WS, the most abundant bufadienolide glycoside “scilliroside” is suggested to mediate the observed toxic action of Urginea sp. The lethal dose (LD50) of scilliroside was 0.7 and 0.43 mg/kg for male and female rats in vivo, respectively. Scilliroside and its aglycon, scillirosidin, exert more toxic effect than other bufadienolides, such as proscillaridin and desacetylscillirosidin. This would be attributed for the presence of an acetoxy group at the C-6 position of scilliroside [63].
3. Materials and Methods
3.1. Plant Material
Samples of U. maritima (Linn) Baker “Sea Squill” (RS and WS) were collected in summer 2018–2019 from the El-Arish desert, Sinai, Egypt. RS and WS were authenticated by Prof. Zaki Turki (Department of Botany, Faculty of Science, El-Menoufia University, Shebin El-Kom, Egypt) as U. maritima (L.) Baker, and voucher specimens Sp. No HRE139 have been deposited at the Herbarium of the Department of Botany, Faculty of Science, Menoufia University, Egypt.
3.2. Secondary Metabolites Extraction and Preparation of NMR and MS Analysis Sample
The extraction protocol for NMR and MS analysis followed that by Farag et al. [14]. Briefly, a freeze-dried squill sample was mixed with 5 mL methanol with umbelliferone “internal standard (10 μg/mL) for the quantification of metabolites using UPLC/MS”. The squill extract was vortexed and then centrifuged (3000× g) for half an hour to remove any plant wastes. NMR analysis was performed by 3 mL aliquot, then concentrated under “N2” stream. The dried squill extract was resuspended with CD3OD (700 μL) containing 0.94 mM HMDS, then centrifugation at 13,000× g for 1 min.
3.3. SPME/GC-MS
Solid phase micro extraction (SPME) technique was adopted for volatiles extraction as in Farag et al. [64]. Squill (5 g) was incubated at 50 °C for half an hour in a screw cap glass vial, through which the SPME fibers “stableflex fibers covered with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 50/30 μm), Supelco (Oakville, ON, Canada)” was placed for 15 min with the squill sample, then injected into the GC-MS injection port. The GC-MS specifications and the analysis method were previously explained in detail in a previous study by Farag et al. [64].
3.4. UHPLC/MS
The identification of secondary metabolites in the squill sample was following the specifications of the UPLC/MS as well as the procedure that was previously mentioned in Farag et al. [65]. Characterization of the secondary metabolites was carried by their UV-VIS spectra from 200–600 nm, the retention time (Rt.) relative to authentic, exact mass and upon comparing the mass spectra of those authentic, the natural products database dictionary (CRC), and the published literature [66].
Multivariate Data Analysis of UPLC-MS & GC-MS Dataset
Metabolites of U. maritima were identified using UHPLC-MS/MS-Orbitrap-HRMS. The quantification was followed what was mentioned in Farag et al. [65]. In brief, the quantification was done using XCMS analysis software downloaded from (http://137.131.20.83/download/, accessed on 14 March 2023) [67,68]. The data was then analyzed using both PCA and OPLS-DA (SIMCA-P 13.0 software package-Umetrics, Umea, Sweden).
3.5. Identification of Major Metabolites via NMR Analysis
All spectra were analyzed using VNMRS 600 NMR spectrometer. All the specifications were described in detail as mentioned in Farag et al. [69]. The 2D-NMR spectra were reported at 599.83 MHz frequency using CHEMPACK 6.2 pulse sequences as COSY, HSQC, and HMBC. The optimization of HSQC and HMBC experiments were documented previously in [68]. Similar to the previous work [15], WS was used as a reference to demonstrate the identification of U. maritima metabolites. Interpretation was achieved by chemical shifts of standards using 2D-NMR and 1H-1H-correlation spectroscopy COSY and TOCSY, 1H-13C-HSQC, and HMBC.
Quantification of Major Metabolites via 1H-NMR
16 metabolites were quantified by NMR spectroscopy Figure S1. The peak area of both target compounds and the internal standard (HMDS) specific protons were interpreted manually for both squill samples as described in [69].
3.6. Bioassays
3.6.1. Cell Culture
Different cancer cell lines have been tested, viz. breast adenocarcinoma (MCF-7), lung cancer (A-549), and ovarian cancer cells (SKOV-3). All cancer cells have been purchased from Nawah Scientific Inc., (Mokatam, Cairo, Egypt). Cells were kept in DMEM media supplemented with streptomycin (100 mg/mL), penicillin 100 (units/mL), and 10% of heat-inactivated fetal bovine serum in humid 5% (v/v) carbon dioxide at 37 °C.
3.6.2. Cytotoxic Screening Assay
The cell viability was conducted by SRB assay. A 100 μL of cell suspension aliquots (5 × 103 cells) was placed in 96-well plates, then incubated for 1 day. 100 μL media with squill extracts (0.01, 0.1, 1, 10, 100 µg/mL) were added to the cell suspension. After 3 days of treatment, the cells were fixed by changing the media with 150 μL (10% Trichloroacetic acid (TCA)) and incubated at 4 °C for 1 h. The cells were then washed with distilled water. SRB (70 μL) aliquots (0.4% w/v) were added and incubated in a dark place for 10 min. The plates were washed with acetic acid (1%) and allowed to dry overnight. Then, 150 μL of tris (hydroxymethyl) aminomethane (TRIS) (10 mM) was added, and absorbance was measured at 540 nm using a BMG LABTECH®-FLUOstar Omega microplate reader (Biotechnology company in Ortenberg, Germany).
3.6.3. Statistical Analysis
The cytotoxic screening results were represented as averages of 3 independent experiments with their standard deviation (mean ± SD). For statistical significance determination, results were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test to compare doxorubicin positive control with treatment groups of WS and RS extracts on different cancer cell lines tested in vitro using SRB assay. Graph pad prism version 5 was used where p was ≤ 0.05.
4. Conclusions
In this current study, the two squill varieties (red and white) were investigated using a metabolomic approach (PCA and OPLS-DA) coupled with different chromatographic (SPME-GC/MS and UPLC/MS) and spectroscopic techniques (1D and 2D-NMR) for the first time, where metabolites diversity was identified in the two varieties.
Volatiles assessment (SPME-GC/MS) resulted in identifying 27 volatiles in both red and white squills. Red squill was enriched with monoterpenes hydrocarbons (47.82%) than white one (25.90%), loading to more aroma profiling. The 1D and 2D-NMR spectroscopic technique was utilized to identify 16 major metabolites. The phenolic compounds were abundant in the red squill, whereas bufadienolides and fatty acids showed more intense peaks in the white one.
Secondary metabolites identification (UHPLC/MS) revealed 130 metabolites representing a myriad of classes. Bufadienolides class was the major one in white squill, whereas flavonoids were the major one in the red variety. The 1D and 2D-NMR spectroscopic technique was utilized to further identify 16 major metabolites. The phenolic compounds were abundant in the red squill, whereas bufadienolides and fatty acids showed more intense peaks in the white one.
Multivariate data analysis differentiated between both varieties and confirmed the abundance of flavonoids in red squill exemplified in dihydrokaempferol-O-hexoside, its aglycon, and taxifolin derivative, whereas fatty acids (oleic and linoleic acids) in addition to bufadienolides, viz. hydroxyscilliglaucosidin-O-rhamnoside, desacetylscillirosidin-O-rhamnoside, and bufotalidin-O-hexoside are more abundant in the white one. A cytotoxicity screening was implemented on both squills against different cell lines revealed the effectiveness of white squill over red one due to its enrichment with bufadienolides class, which has yet to be confirmed using isolated bufadienolides to be conclusive.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants12112078/s1, Figure S1: ESI-MS/MS spectrum of metabolites in the positive ion mode; Figure S2: Signal assignment of the 1H-NMR markers for fatty acids (M1–M3), rhamnose (M4), alanine (M8), aspartic acid (M9), glycine (M10), and bufadienolides (M13 & M14) using 1H-13C correlations observed in the HSQC spectrum of squill methanol extract; Figure S3: Signal assignment of the 1H-NMR markers for fatty acids (M1-M3), rhamnose (M4), alanine (M8), aspartic acid (M9), glycine (M10), and bufadienolides (M13&M14) using 1H-13C correlations observed in the HMBC spectrum of squill methanol extract; Figure S4: Signal assignment of the 1H-NMR markers for fatty acids (M1-M3), rhamnose (M4), β, α glucose (M5 & M6), sucrose (M7), aspartic acid (M9), glycine (M10), tryptophan (M12), and bufalin (M13) using 1H-13C correlations observed in the HMBC spectrum of squill methanol extract; Figure S5: Signal assignment of the 1H-NMR markers for rhamnose (M4), β, α glucose (M5& M6), sucrose (M7), aspartic acid (M9), glycine (M10), tryptophan (M12), and bufalin (M13) using 1H-13C correlations observed in the HSQC spectrum of squill methanol extract; Figure S6: Signal assignment of the 1H-NMR markers for tyrosine (M11), tryptophan (M12), bufalin (M13), scilliridin (M14), dihydro kaempferol (M15), and coumarin (M16); 1H-13C correlations observed in the HSQC spectrum of squill methanol extract; Figure S7: Signal assignment of the 1H-NMR markers for tyrosine (M11), tryptophan (M12), bufalin (M13), scilliridin (M14), dihydro kaempferol (M15), and coumarin (M16); 1H-13C correlations observed in the HMBC spectrum of squill methanol extract; Figure S8: Signal assignment of the proton markers for dihydro kaempferol (M15) observed in the 1H-NMR spectrum of RS methanol extract; Table S1: 1H-NMR quantification of most common primary and secondary metabolites detected in Urginea species, white squill (WS) and red squill (RS). Values are expressed as μg/mg dry powder ± S.D (n = 3). Chemical shifts used for metabolite quantification were determined in methanol-d6 and expressed as relative values to HMDS (0.94 mM final concentration); Table S2: RS and WS extract inhibition and viability values in different concentrations against different cancer cell lines measured by SRB assay.
Author Contributions
M.A.F. and H.R.E.-S.; conceptualization, O.M.K.; methodology, O.M.K., N.Y. and M.A.F.; software, D.M.E.-K. and M.A.F.; validation, M.A.F.; formal analysis, O.M.K. and D.M.E.-K.; investigation, H.R.E.-S.; resources, M.A.F.; data curation, O.M.K. and N.Y.; writing—original draft preparation, D.M.E.-K., H.R.E.-S. and M.A.F.; writing—review and editing, S.A.M.K. and M.A.F.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available from omarkhattab500@gmail.com upon request.
Acknowledgments
Mohamed A. Farag wishes to thank the Alexander von Humboldt foundation, Germany and Science and Technology Development Fund (STDF), Egypt grant number 47051.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bozorgi, M.; Amin, G.; Shekarchi, M.; Rahimi, R. Traditional medical uses of Drimia species in terms of phytochemistry, pharmacology and toxicology. J. Tradit. Chin. Med. 2017, 37, 124–139. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Burman, R.; Mansour, A.; Turki, Z.; Boulos, L.; Gullbo, J.; Goransson, U. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol. 2013, 145, 746–757. [Google Scholar] [CrossRef] [PubMed]
- BayazıT, V.; Konar, V. Analgesic Effects of Scilliroside, Proscillaridin-A and Taxifolin from Squill Bulb (Urginea maritima) on Pains. Dig. J. Nanomater. Biostruct. (DJNB) 2010, 5, 457–465. [Google Scholar]
- Dizaye, K.; Hamed, B. Cardiovascular studies of white squill (Urginea maritima) extract. Zanco J. Med. Sci. 2010, 14, 20–27. [Google Scholar]
- Leblanc, F.J.; Lee, C.O. A study of the toxic principles of red squill. J. Am. Pharm. Assoc. 1939, 28, 151–154. [Google Scholar]
- Santos, C.V.d.; Kerkhoff, J.; Tomazelli, C.A.; Wenceslau, C.F.; Sinhorin, A.P.; de Jesus Rodrigues, D.; Carneiro, F.S.; Bomfim, G.F. Vasoconstrictor and hemodynamic effects of a methanolic extract from Rhinella marina toad poison. Toxicon 2022, 218, 57–65. [Google Scholar] [CrossRef]
- Wang, H.-Y.L.; O’Doherty, G.A. Modulators of Na/K-ATPase: A patent review. Expert Opin. Ther. Pat. 2012, 22, 587–605. [Google Scholar] [CrossRef]
- Aswal, S.; Kumar, A.; Semwal, R.B.; Chauhan, A.; Kumar, A.; Lehmann, J.; Semwal, D.K. Drimia indica: A plant used in traditional medicine and its potential for clinical uses. Medicina 2019, 55, 255. [Google Scholar] [CrossRef]
- Wu, C.-H.; Tao, W.; Yamaguchi, Y.; Yue, C.; Han, L.-F.; Zhang, Y. A new phenylpropanol glycoside and its five known analogues from Boschniakia rossica. Chin. Herb. Med. 2013, 5, 5–8. [Google Scholar]
- Metin, M.; Bürün, B. Effects of the high doses of Urginea maritima (L.) baker extract on chromosomes. Caryologia 2010, 63, 367–375. [Google Scholar] [CrossRef]
- Bozorgi, M.; Amin, G.; Kasebzade, S.; Shekarchi, M. Development and validation of a HPLC-UV method for determination of Proscillaridin A in Drimia maritima. Res. J. Pharmacogn. 2016, 3, 1–7. [Google Scholar]
- Singh, V.; Soni, L.K.; Dobhal, S.; Jain, S.K.; Parasher, P.; Dobhal, M.P. Phytochemicals and Pharmacological Properties of Urginea Species. Chem. Sci. Rev. Lett. 2016, 5, 79–95. [Google Scholar]
- Krenn, L.; Kopp, B.; Steurer, S.; Schubert-Zsilavecz, M. 9-Hydroxyscilliphaeoside, a new bufadienolide from Urginea maritima. J. Nat. Prod. 1996, 59, 612–613. [Google Scholar] [CrossRef]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Okada, T.; Mochamad Afendi, F.; Altaf-Ul-Amin, M.; Takahashi, H.; Nakamura, K.; Kanaya, S. Metabolomics of medicinal plants: The importance of multivariate analysis of analytical chemistry data. Curr. Comput.-Aided Drug Des. 2010, 6, 179–196. [Google Scholar] [CrossRef]
- Jha, S. Bufadienolides. In Phytochemicals in Plant Cell Cultures; Elsevier: Amsterdam, The Netherlands, 1988; pp. 179–191. [Google Scholar]
- Feng, W.; Hao, Z.; Li, M. Isolation and Structure Identification of Flavonoids. In Flavonoids, from Biosynthesis to Human Health; Justino, G.C., Ed.; Intech Open: Rijeka, Croatia, 2017; pp. 17–43. [Google Scholar]
- Barrueto, F.; Kirrane, B.M.; Cotter, B.W.; Hoffman, R.S.; Nelson, L.S. Cardioactive steroid poisoning: A comparison of plant-and animal-derived compounds. J. Med. Toxicol. 2006, 2, 152–155. [Google Scholar] [CrossRef]
- Iizuka, M.; Warashina, T.; Noro, T. Bufadienolides and a new lignan from the bulbs of Urginea maritima. Chem. Pharm. Bull. 2001, 49, 282–286. [Google Scholar] [CrossRef]
- Crouch, N.R.; du Toit, K.; Mulholland, D.A.; Drewes, S.E. Bufadienolides from bulbs of Urginea lydenburgensis (Hyacinthaceae: Urgineoideae). Phytochemistry 2006, 67, 2140–2145. [Google Scholar] [CrossRef]
- Shimada, K.; Umezawa, E.; Nambara, T.; Kupchan, S.M. Isolation and characterization of cardiotonic steroids from the bulb of Urginea altissima Baker. Chem. Pharm. Bull. 1979, 27, 3111–3114. [Google Scholar] [CrossRef]
- Kopp, B.; Krenn, L.; Draxler, M.; Hoyer, A.; Terkola, R.; Vallaster, P.; Robien, W. Bufadienolides from Urginea maritima from Egypt. Phytochemistry 1996, 42, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, J.; Pan, H.; Wang, L. Chemical Profile and Multicomponent Quantitative Analysis for the Quality Evaluation of Toad Venom from Different Origins. Molecules 2019, 24, 3595. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; Shaala, L.A.; Alshali, K.Z.; Youssef, D.T.A. Urgineaglyceride A: A new monoacylglycerol from the Egyptian Drimia maritima bulbs. Nat. Prod. Res. 2014, 28, 1583–1590. [Google Scholar] [CrossRef]
- Koorbanally, N.A.; Koorbanally, C.; Harilal, A.; Mulholland, D.A.; Crouch, N.R. Bufadienolides from Drimia robusta and Urginea epigea (Hyacinthaceae). Phytochemistry 2004, 65, 3069–3073. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yu, Y.; Wang, X.; Yang, L.; Zhang, H.; Ji, H.; Li, Z.; Hou, J.; Wu, W.; Guo, D. Simultaneous Determination of Bufalin and Its Nine Metabolites in Rat Plasma for Characterization of Metabolic Profiles and Pharmacokinetic Study by LC–MS/MS. Molecules 2019, 24, 1662. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, E.; Kanakis, C.; Trigas, P.; Tarantilis, P.A. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: Study of their total phenolic content and antioxidant activity. Anal. Bioanal. Chem. 2019, 411, 3135–3150. [Google Scholar] [CrossRef]
- Knittel, D.N.; Stintzing, F.C.; Kammerer, D.R. Metabolic fate of cardiac glycosides and flavonoids upon fermentation of aqueous sea squill (Drimia maritima L.) extracts. J. Pharm. Biomed. Anal. 2015, 110, 100–109. [Google Scholar] [CrossRef]
- Bose, C.; Chakrabarty, A. 4,5-Dihydro-14-[beta]-Hydroxy Scilladienolide-3-O-[beta]-D-Glucopyranoside (AC-3) from the Stems of Milletia ovalifolia. Asian J. Chem. 2002, 14, 671. [Google Scholar]
- Fang, S.; Tao, H.; Xia, K.; Guo, W. Proscillaridin A induces apoptosis and inhibits the metastasis of osteosarcoma in vitro and in vivo. Biochem. Biophys. Res. Commun. 2019, 521, 880–886. [Google Scholar] [CrossRef]
- Triana-Martínez, F.; Picallos-Rabina, P.; Da Silva-Álvarez, S.; Pietrocola, F.; Llanos, S.; Rodilla, V.; Soprano, E.; Pedrosa, P.; Ferreirós, A.; Barradas, M. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat. Commun. 2019, 10, 4731. [Google Scholar] [CrossRef]
- Da Costa, E.M.; Armaos, G.; McInnes, G.; Beaudry, A.; Moquin-Beaudry, G.; Bertrand-Lehouillier, V.; Caron, M.; Richer, C.; St-Onge, P.; Johnson, J.R. Heart failure drug proscillaridin A targets MYC overexpressing leukemia through global loss of lysine acetylation. J. Exp. Clin. Cancer Res. 2019, 38, 251. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Shimada, R.; Xu, K.; Han, L.; Si, N.; Zhao, H.; Bian, B.; Hayashi, H.; Okazaki, M.; Takagi, N. Multiple cytotoxic effects of gamabufotalin against human glioblastoma cell line U-87. Chem.-Biol. Interact. 2019, 314, 108849. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ma, H.; Zhou, J.; Zhu, Z.; Lv, X.; Li, Q.; Wang, H.; Yan, Y.; Luo, N.; Di, L. High Resolution Mass Profile of Bufadienolides and Peptides Combing with Anti-Tumor Cell Screening and Multivariate Analysis for the Quality Evaluation of Bufonis Venenum. Molecules 2019, 24, 1943. [Google Scholar] [CrossRef] [PubMed]
- Pohl, T.; Koorbanally, C.; Crouch, N.R.; Mulholland, D.A. Bufadienolides from Drimia robusta and Urginea altissima (Hyacinthaceae). Phytochemistry 2001, 58, 557–561. [Google Scholar] [CrossRef]
- Krenn, L.; Stapf, V.; Kopp, B. Bufadienolides from Drimia robusta BAK. Sci. Pharm. 2000, 68, 421–427. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Justino, G.C. Structural analysis of flavonoids and related compounds—A review of spectroscopic applications. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 33–56. [Google Scholar]
- Ye, M.; Yang, W.-Z.; Liu, K.-D.; Qiao, X.; Li, B.-J.; Cheng, J.; Feng, J.; Guo, D.-A.; Zhao, Y.-Y. Characterization of flavonoids in Millettia nitida var. hirsutissima by HPLC/DAD/ESI-MSn. J. Pharm. Anal. 2012, 2, 35–42. [Google Scholar]
- Ragab, E.A.; Raafat, M. A new monoterpene glucoside and complete assignments of dihydroflavonols of Pulicaria jaubertii: Potential cytotoxic and blood pressure lowering activity. Nat. Prod. Res. 2016, 30, 1280–1288. [Google Scholar] [CrossRef]
- Knittel, D.N.; Stintzing, F.C.; Kammerer, D.R. Simultaneous determination of bufadienolides and phenolic compounds in sea squill (Drimia maritima (L.) Stearn) by HPLC-DAD-MS n as a means to differentiate individual plant parts and developmental stages. Anal. Bioanal. Chem. 2014, 406, 6035–6050. [Google Scholar] [CrossRef]
- Fernandez, M.; Vega, F.A.; Arrupe, T.; Renedo, J. Flavonoids of squill, Urginea maritima. Phytochemistry 1972, 11, 1534. [Google Scholar] [CrossRef]
- Belhaddad, O.E.; Charef, N.; Amamra, S.; Zerargui, F.; Baghiani, A.; Khennouf, S.; Arrar, L. Chromatographic fractionation, antioxidant and antibacterial activities of Urginea maritima methanolic extract. Pak. J. Pharm. Sci. 2017, 30, 127–134. [Google Scholar]
- March, R.E.; Lewars, E.G.; Stadey, C.J.; Miao, X.-S.; Zhao, X.; Metcalfe, C.D. A comparison of flavonoid glycosides by electrospray tandem mass spectrometry. Int. J. Mass Spectrom. 2006, 248, 61–85. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Kataeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana Species: Untargeted LC-MS Metabolic Profiling, Antioxidant and Digestive Enzyme Inhibiting Activity of Six Plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; He, Z.; Zhang, Y.; Fan, Q.; Feng, N. Naringenin Cocrystals Prepared by Solution Crystallization Method for Improving Bioavailability and Anti-hyperlipidemia Effects. AAPS PharmSciTech 2019, 20, 115. [Google Scholar] [CrossRef]
- Shimokawa, Y.; Akao, Y.; Hirasawa, Y.; Awang, K.; Hadi, A.H.A.; Sato, S.; Aoyama, C.; Takeo, J.; Shiro, M.; Morita, H. Gneyulins A and B, stilbene trimers, and noidesols A and B, dihydroflavonol-C-glucosides, from the bark of Gnetum gnemonoides. J. Nat. Prod. 2010, 73, 763–767. [Google Scholar] [CrossRef]
- Abbas, S.; Bashir, S.; Khan, A.; Mehmood, M.H.; Gilani, A.H. Gastrointestinal stimulant effect of Urginea indica Kunth. and involvement of muscarinic receptors. J. Phytother. Res. 2012, 26, 704–708. [Google Scholar] [CrossRef]
- Bashir, S.; Abbas, S.; Gilani, A.H.; Khan, A. Studies on bronchodilator and cardiac stimulant activities of Urginea indica. J. Bangladesh J. Pharmacol. 2013, 8, 249–254. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Méndez-Líter, J.A.; Tundidor, I.; Nieto-Domínguez, M.; de Toro, B.F.; Santana, A.G.; de Eugenio, L.I.; Prieto, A.; Asensio, J.L.; Sánchez, C.; Martínez, M.J. Transglycosylation products generated by Talaromyces amestolkiae GH3 β-glucosidases: Effect of hydroxytyrosol, vanillin and its glucosides on breast cancer cells. Microb. Cell Factories 2019, 18, 97. [Google Scholar] [CrossRef]
- Seong, Y.-A.; Hwang, D.; Kim, G.-D. The anti-inflammatory effect of Gnaphalium affine through inhibition of NF-κB and MAPK in lipopolysaccharide-stimulated RAW264.7 cells and analysis of its phytochemical components. Cell Biochem. Biophys. 2016, 74, 407–417. [Google Scholar] [CrossRef]
- Jaakola, L.; Määttä, K.; Pirttilä, A.M.; Törrönen, R.; Kärenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wu, H.; Tjeerdema, R.S.; Viant, M.R. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
- Khattab, A.R.; Rasheed, D.M.; El-Haddad, A.E.; Porzel, A.; Wessjohann, L.A.; Farag, M.A. Assessing phytoequivalency of four Zingiberaceae spices (galangals, turmeric and ginger) using a biochemometric approach: A case study. Ind. Crops Prod. 2022, 188, 115722. [Google Scholar] [CrossRef]
- Saket, K.; Afshari, J.T.; Saburi, E.; Yousefi, M.; Salari, R. Therapeutic aspects of Squill; an evidence-based review. Curr. Drug Discov. Technol. 2020, 17, 318–324. [Google Scholar] [CrossRef]
- Cunha-Filho, G.A.; Resck, I.S.; Cavalcanti, B.C.; Pessoa, C.Ó.; Moraes, M.O.; Ferreira, J.R.O.; Rodrigues, F.A.R.; dos Santos, M.L. Cytotoxic profile of natural and some modified bufadienolides from toad Rhinella schneideri parotoid gland secretion. Toxicon 2010, 56, 339–348. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pimenta, D.C.; Lebrun, I.; Sartorelli, P.; Taniwaki, N.N.; de Andrade Jr, H.F.; Antoniazzi, M.M.; Jared, C. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon 2008, 52, 13–21. [Google Scholar] [CrossRef]
- Mahringer, A.; Karamustafa, S.; Klotz, D.; Kahl, S.; Konkimalla, V.B.; Wang, Y.; Wang, J.; Liu, H.-Y.; Boechzelt, H.; Hao, X. Inhibition of P-glycoprotein at the blood–brain barrier by phytochemicals derived from traditional Chinese medicine. Cancer Genom. -Proteom. 2010, 7, 191–205. [Google Scholar]
- Li, R.-Z.; Fan, X.-X.; Duan, F.-G.; Jiang, Z.-B.; Pan, H.-D.; Luo, L.-X.; Zhou, Y.-L.; Li, Y.; Yao, Y.-J.; Yao, X.-J. Proscillaridin A induces apoptosis and suppresses non-small-cell lung cancer tumor growth via calcium-induced DR4 upregulation. Cell Death Dis. 2018, 9, 696. [Google Scholar] [CrossRef]
- Manganyi, M.C.; Tlatsana, G.S.; Mokoroane, G.T.; Senna, K.P.; Mohaswa, J.F.; Ntsayagae, K.; Fri, J.; Ateba, C.N. Bulbous Plants Drimia: “A Thin Line between Poisonous and Healing Compounds” with Biological Activities. Pharmaceutics 2021, 13, 1385. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, H.A.; Heiss, A.G.; Wessjohann, L.A. Metabolomics driven analysis of six Nigella species seeds via UPLC-qTOE-MS and GC-MS coupled to chemometrics. Food Chem. 2014, 151, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R. Nanoencapsulation of Jania rubens’ Phytochemicals: Antioxidant Properties for Food Applications. Ph.D. Thesis, American University in Cairo, New Cairo, Egypt, 2021. [Google Scholar]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Farag, M.A.; Khaled, S.E.; El Gingeehy, Z.; Shamma, S.N.; Zayed, A. Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC–MS, UV, and NMR Techniques. Metabolites 2022, 12, 614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).