Use of Thermography to Evaluate Alternative Crops for Off-Season in the Cerrado Region
Abstract
1. Introduction
2. Results
2.1. Physiological Variables
2.2. Productivity of Dry Biomass and Grains
2.3. Average Canopy Temperature
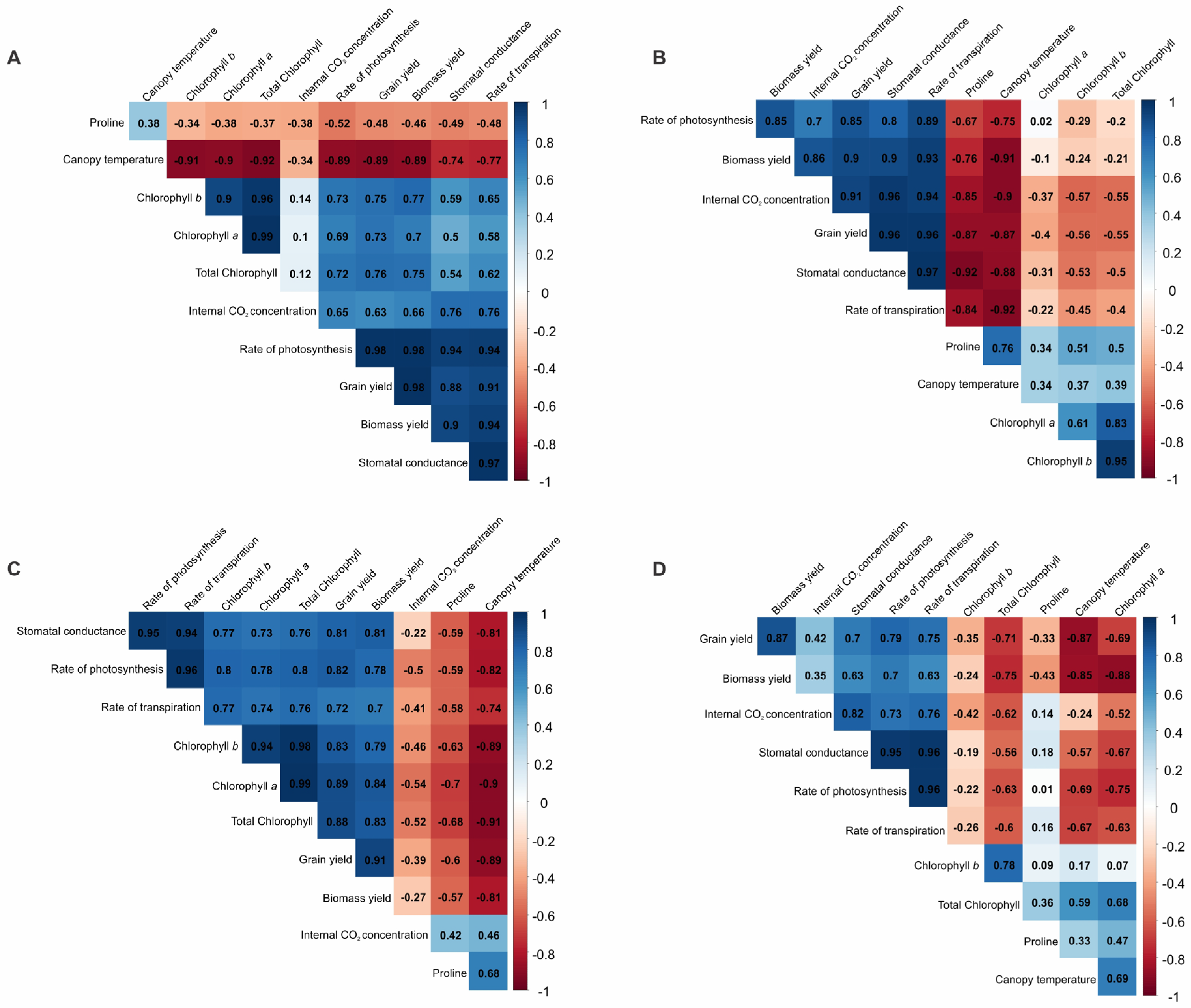
2.4. Pearson’s Correlation
3. Discussion
3.1. Physiological Variables
3.2. Production of Dry Matter and Grain
3.3. Average Canopy Temperature
4. Material and Methods
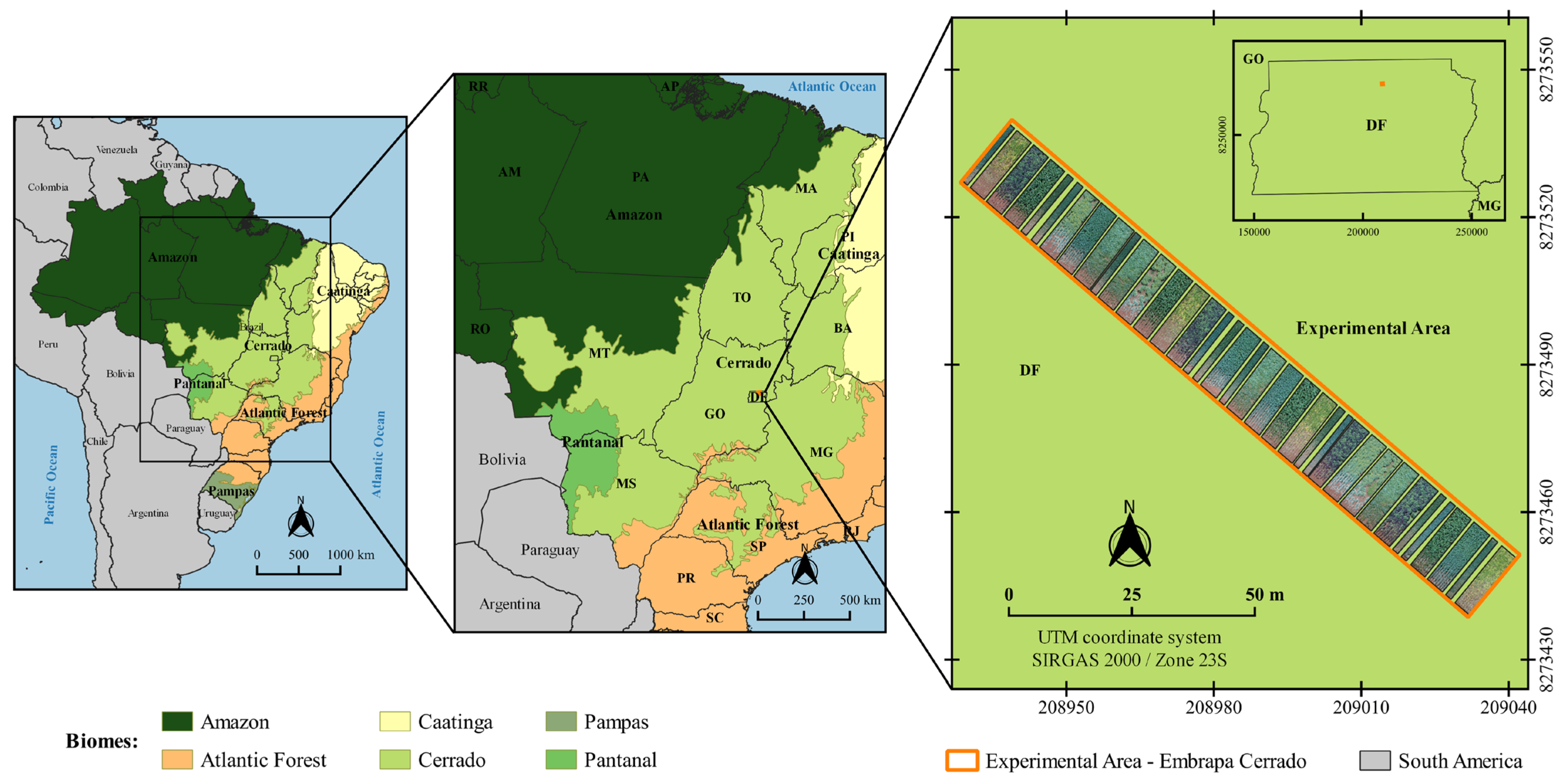
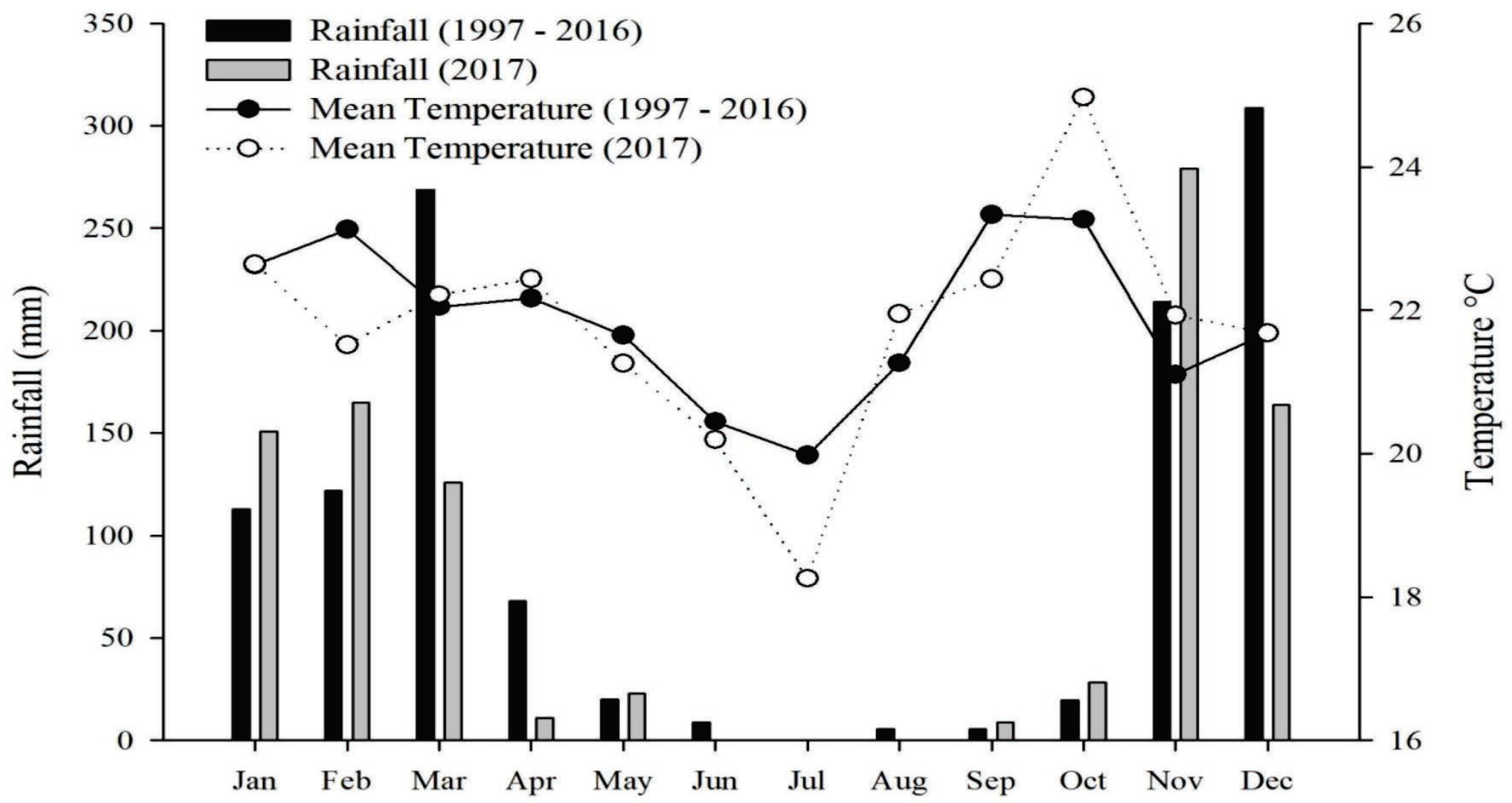
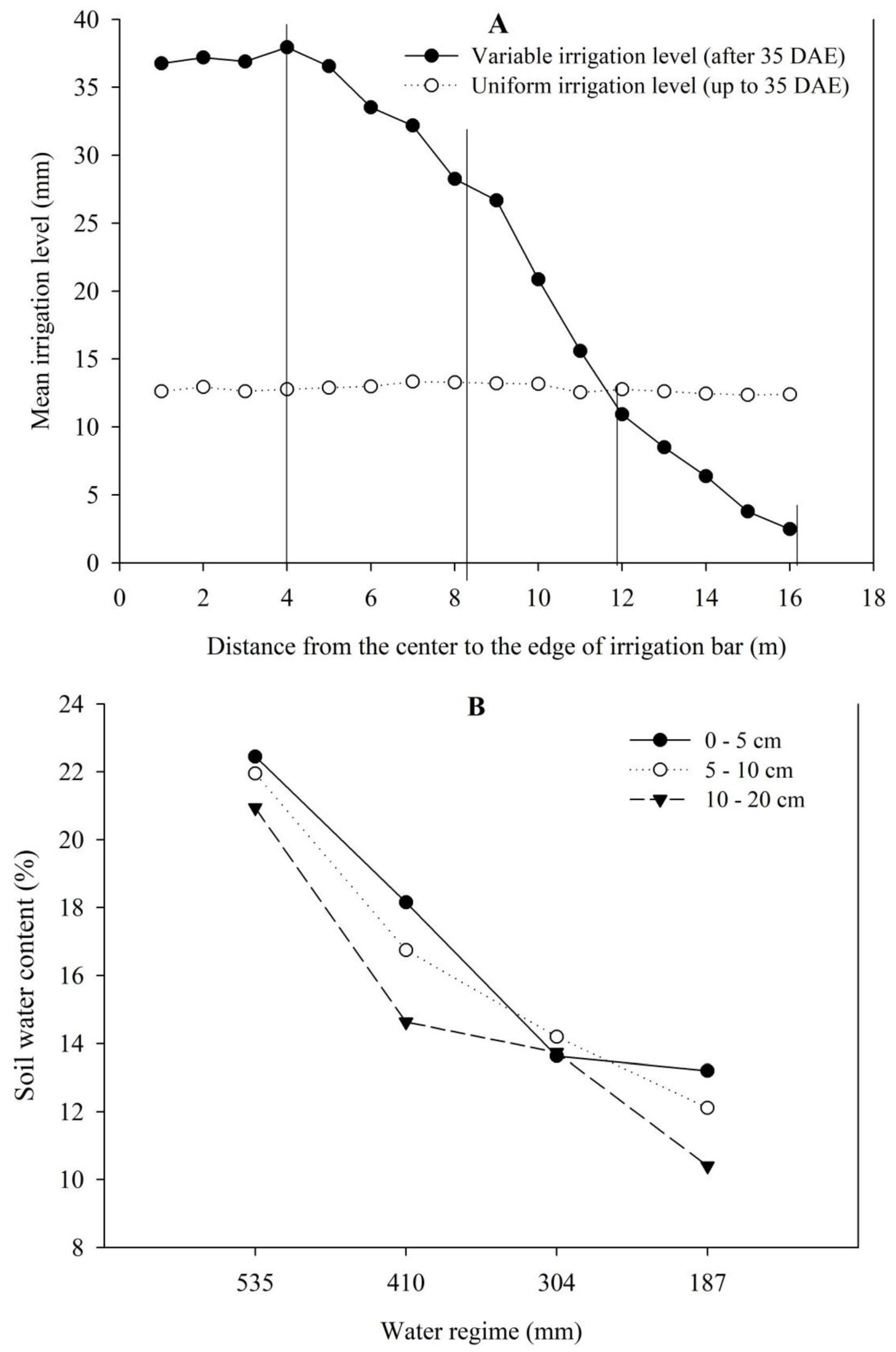
4.1. Experimental Design
4.2. Variables Analysed
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAB (Companhia Nacional de Abastecimento). Acompanhamento da Safra Brasileira de Grãos. v. 7—Safra 2019/20—N. 3—Terceiro Levantamento, Dezembro de 2019. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 11 January 2020).
- Silva, A.N.; Ramos, M.L.G.; Ribeiro, W.Q., Jr.; Alencar, E.R.; Silva, P.C.; Lima, C.A.; Vinson, C.C.; Silva, M.A.V. Water stress alters physical and chemical quality in grains of common bean, triticale and wheat. Agric. Water Manag. 2020, 231, 06023. [Google Scholar] [CrossRef]
- Nilahyane, A.; Islam, M.A.; Mesbah, A.O.; Herbert, S.K.; Garcia y Garcia, A. Growth, water productivity, nutritive value, and physiology responses of silage corn to water stress. Agron. J. 2020, 112, 1625–1635. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Molina-Montenegro, M.A. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Bastidas, E.G.; Roura, R.; Rizzolo, D.A.D.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd.), from nutritional value to potential health benefits: An integrative review. J. Food Sci. 2016, 6, 000497. [Google Scholar] [CrossRef]
- Reguera, M.; Conesa, C.M.; Gil-Gomez, A.; Haros, C.M.; Perez-Casas, M.A.; Briones-Labarca, V.; Bolanos, L.; Bonilla, L.; Alvarez, R.; Pinto, K.; et al. The impact of different agroecological conditions on the nutritional composition of C. quinoa seeds. PeerJ 2018, 6, e4442. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q.; Naeem, M.A.; He, X.; Zhang, Z.; Jacobsen, S.E. Biochar Mitigates Combined Effects of Drought and Salinity Stress in Quinoa. Agronomy 2020, 10, 912. [Google Scholar] [CrossRef]
- Peiretti, P.G. Amaranth in animal nutrition: A review. Livest. Res. Rural Dev. 2018, 30, 1–20. [Google Scholar]
- Sarker, U.; Oba, S.; Daramy, M.A. Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Sci. Rep. 2020, 10, 3892. [Google Scholar] [CrossRef]
- Debiage, R.R.; Regildo, M.Ã.; Porto, P.N.P.; Yoshihara, E.; de Mello Peixoto, E.C.T. Fagopyrum esculentum Moench: A crop with many purposes in agriculture and human nutrition. Afr. J. Agric. Res. 2016, 11, 983–989. [Google Scholar] [CrossRef]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wand, C.; Feng, B. Exogenous melatonin modulates the physiological and biochemical mechanisms of drought tolerance in Tartary Buckwheat (Fagopyrum tataricum). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hossain, M.S.; Ma, H.; Yang, Q.; Gong, X.; Yang, P.; Feng, B. Comparative metabolomics reveals differences in flavonoid metabolites among different colored buckwheat flowers. J. Food Compos. 2020, 85, 103335. [Google Scholar] [CrossRef]
- Hornyák, M.; Plazek, A.; Kopec, P.; Dziurka, M.; Pastuszak, J.; Szczerba, A.; Hura, T. Photosynthetic activity of common buckwheat (Fagopyrum esculentum Moench) exposed to thermal stress. Photosynthetica 2020, 58, 45–53. [Google Scholar] [CrossRef]
- Androcioli, L.G.; Zefta, D.M.; Alves, D.S.; Tomaz, J.P.; Cirino, V.M. Effect of water deficit on morphoagronomic and physiological traits of commom bean genotypes contrasting with drought tolerance. Water 2020, 12, 217. [Google Scholar] [CrossRef]
- Choudhary, S.; Guha, A.; Kholova, J.; Pandravada, A.; Messina, C.D.; Cooper, M.; Vadez, V. Maize, sorghum and pearl millet have highly contrasting species strategies to adapt to water stress and climate change-like conditions. Plant Sci. 2020, 295, 110297. [Google Scholar] [CrossRef]
- Ramos, M.L.G.; Parsons, R.; Sprent, J.I.; James, E.K. Effect of water stress on nitrogen fixation and nodule structure of common bean. Pesqui. Agropecuária Bras. 2003, 38, 339–347. [Google Scholar] [CrossRef]
- Nepomuceno, A.L. Drought tolerance in plants: Physiological and molecular mechanisms. Biotech. Sci. Dev. 2001, 4, 12–18. [Google Scholar]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves of Medicago truncatula plants. Free Radic. Biol. Med. 2013, 56, 172–183. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Al-Qurainy, F.; Foolad, M.R. Drought tolerance: Roles of organic osmolytes, growth regulators and mineral nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar] [CrossRef]
- Matysik, J.; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Soares, G.F.; Ribeiro, W.Q.; Pereira, L.F.; Lima, C.A.D.; Soares, D.D.S.; Muller, O.; Rashcer, U.; Ramos, M.L.G. Characterization of wheat genotypes for drought tolerance and water use efficiency. Sci. Agric. 2020, 78, e20190304. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.P.; Muller, O.; Albrecht, H.; Burkart, A.; Gatzke, S.; Janssen, B.; Keller, B.; Korber, N.; Rascher, U. Field Phenotyping: Concepts and Examples to Quantify Dynamic Plant Traits across Scales in the Field. In Terrestrial Ecosystem Research Infrastructures: Challenges and Opportunities; Chabbi, A., Loescher, H.W., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 53–79. [Google Scholar]
- Casari, R.A.C.N.; Paiva, D.S.; Silva, V.N.B.; Ferreira, T.M.M.; Souza, M.T.; Oliveira, N.G.; Kobayashi, A.; Molinari, H.B.C.; Santos, T.T.; Gomide, R.L.; et al. Using thermography to confirm genotypic variation for drought response in maize. Int. J. Mol. Sci. 2019, 20, 2273. [Google Scholar] [CrossRef]
- Vitek, P.; Veselá, V.; Klem, K. Spatial and Temporal Variability of Plant Leaf Responses Cascade after PSII Inhibition: Raman, Chlorophyll Fluorescence and Infrared Thermal Imaging. Sensors 2020, 20, 1015. [Google Scholar] [CrossRef]
- Blaya-Ros, P.J.; Blanco, V.; Domingo, R.; Soto-Valles, F.; Torres-Sanchez, R. Feasibility of Bow-Cost Thermal imaging for monitoring water stress in young and mature sweet cherry trees. Appl. Sci. 2020, 10, 5461. [Google Scholar] [CrossRef]
- Banerjee, K.; Krishnan, P. Normalized Sunlit Shaded Index (NSSI) for characterizing the moisture stress in wheat crop using classified thermal and visible images. Ecol. Indic. 2020, 110, 105947. [Google Scholar] [CrossRef]
- Knipper, K.R.; Kustas, W.P.; Anderson, M.C.; Nieto, H.; Alfieri, J.G.; Prueger, J.H.; Hain, C.R.; Gao, F.; Mckee, L.G.; Alsina, M.M.; et al. Using high-spatiotemporal thermal satellite ET retrievals to monitor water use over California vineyards of different climate, vine variety and trellis design. Agric. Water Manag. 2020, 241, 106361. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, Y.; Zhang, H.; Han, W.; Li, G.; Tang, J.; Peng, X. Maize canopy temperature extracted from UAV thermal and RGB imagery and its application in water stress monitoring. Front. Plant Sci. 2019, 10, 1270. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Identification of drought tolerant genotypes using physiological traits in soybean. Physiol. Mol. Biol. Plant 2019, 25, 697–711. [Google Scholar] [CrossRef]
- Sexton, T.M.; Steber, C.M.; Cousins, A.B. Leaf temperature impacts canopy water use efficiency independent of changes in leaf level water use efficiency. J. Plant Physiol. 2021, 258, 153357. [Google Scholar] [CrossRef]
- Jones, H.G. Application of Thermal Imaging and Infrared Sensing in Plant Physiology and Ecophysiology. In Advances in Botanical Research; Callow, J.A., Ed.; Elsevier Academic Press: San Diego, CA, USA; London, UK, 2004; Volume 41, pp. 107–163. [Google Scholar]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Chen, H.; Fan, T.; Huang, Y.; Yu, S.; Chen, S.; Hong, X. Rice flag leaf physiology, organ and canopy temperature in response to water stress. Plant Prod. Sci. 2012, 15, 92–99. [Google Scholar] [CrossRef]
- Sobejano-Paz, V.; Mikkelsen, T.N.; Baum, A.; Mo, X.; Liu, S.; Köppl, C.J.; Johnson, M.S.; Gulyas, L.; García, M. Hyperspectral and thermal sensing of stomatal conductance, transpiration, and photosynthesis for soybean and maize under drought. Remote Sens. 2020, 12, 3182. [Google Scholar] [CrossRef]
- Banerjee, K.; Krishnan, P.; Das, B. Thermal imaging and multivariate techniques for characterizing and screening wheat genotypes under water stress condition. Ecol. Indic. 2020, 119, 106829. [Google Scholar] [CrossRef]
- Ozkur, O.; Ozdenir, F.; Bor, M.; Turkan, I. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought Environ. Exp. Bot. 2009, 66, 487–492. [Google Scholar] [CrossRef]
- Gomes, P.; Oliva, M.A.; Mielke, M.S.; Almeida, A.F. Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Sci. Hortic. 2010, 126, 379–384. [Google Scholar] [CrossRef]
- Soureshjani, H.K.; Mezami, A.; Kafi, M.; Tadayon, M. Responses of two common bean genotypes to deficit irrigation. Agric. Water Manag. 2019, 213, 270–279. [Google Scholar] [CrossRef]
- Tardiel, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Haldimann, P.; Galle, A.; Feller, U. Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiol. 2008, 28, 785–795. [Google Scholar] [CrossRef]
- Valdayskikh, V.V.; Voronin, P.Y.; Artemyeva, E.P.; Rymar, V.P. Amaranth responses to experimental soil drought. AIP Conf. Proc. 2019, 2063, 030023. [Google Scholar] [CrossRef]
- Jensen, R.G. Photosynthesis: C3, C4. Mechanisms, and cellular and environmental regulation, of photosynthesis. Science 1983, 222, 1009. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosyntesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Achigan-Dako, E.G.; Sogbohossou, O.E.; Maundu, P. Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Silva, J.P.; Bianchini, A.; Costa, P.M.C.; Lobo, F.A.; Almeida, J.P.M.; Moraes, M.F. Amaranth responses to water stress. J. Exp. Agric. Int. 2019, 40, 1–9. [Google Scholar] [CrossRef]
- Fidelis, R.R.; de Sousa, S.A.; Martinez, R.A.S.; de Oliveira, T.C.; Ramos, D.P.; Tonello, L.P. Common beans genotypes behavior cultivated in cerrado soils (savannah) of southern Tocantins. Biosci. J. 2015, 31, 780–788. [Google Scholar] [CrossRef]
- Román-Avilés, B.; Snapp, S.S.; Kelly, J.D. Assessing root traits associated with root rot resistance in common bean. Field Crop Res. 2004, 86, 147–156. [Google Scholar] [CrossRef]
- Torabian, S.; Shakiba, M.R.; Orabian, S.; Shakiba, M.R.; Nasab, A.D.M.; Toorchi, M. Leaf gas exchange and grain yield of common bean exposed to spermidine under water stress. Photosynthetica 2018, 56, 1387–1397. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; del Mar Rubio-Wilhelmi, M.; Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Antioxidant response resides in the shoot in reciprocal grafts of drought-tolerant and drought-sensitive cultivars in tomato under water stress. Plant Sci. 2012, 188, 89–96. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Huang, J.; Hirji, R.; Adam, L.; Rozwadowski, K.L.; Hammerlindl, J.K.; Keller, W.A.; Selvaraj, G. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: Metabolic limitations. Plant Phisiol. 2020, 122, 747–756. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.G.; Taie, H.A.A.; Nassar, M.R.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological, biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. S. Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Durigon, A.; Evers, J.; Metselaar, K.; Lier, Q.I.V. Water stress permanently alters shoot architecture in common bean plants. Agronomy 2019, 9, 160. [Google Scholar] [CrossRef]
- Duan, H.; Chaszar, B.; Lewis, J.D.; Smith, R.A.; Huxman, T.E.; Tissue, D.T. CO2 and temperature effects on morphological and physiological traits affecting risk of drought-induced mortality. Tree Physiol. 2018, 38, 1138–1151. [Google Scholar] [CrossRef]
- Pandey, B.R.; Burton, W.A.; Salisbury, P.A.; Nicolas, M.E. Comparison of osmotic adjustment, leaf proline concentration, canopy temperature and root depth for yield of juncea canola under terminal drought. J. Agron. Crop Sci. 2017, 203, 397–405. [Google Scholar] [CrossRef]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. 2009, 103, 635–644. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Wheaton, A.; Cooley, N.; Moran, B. Automated canopy temperature estimation via infrared thermography: A first step towards automated plant water stress monitoring. Comput. Electron. Agric. 2010, 73, 74–83. [Google Scholar] [CrossRef]
- Padhi, J.; Misra, R.K.; Payero, J.O. Estimation of soil water deficit in an irrigated cotton field with infrared thermography. Field Crop Res. 2012, 126, 45–55. [Google Scholar] [CrossRef]
- Ballester, C.; Castel, J.; Jiménez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Thermographic measurement of canopy temperature is a useful tool for predicting water deficit effects on fruit weight in citrus trees. Agric. Water Manag. 2013, 122, 1–6. [Google Scholar] [CrossRef]
- Wijewardana, C.; Alsajri, F.A.; Irby, J.T.; Krutz, L.J.; Golden, B.; Henry, W.B.; Gao, W.; Reddy, K.R. Physiological assessment of water deficit in soybean using midday leaf water potential and spectral features. J. Plant Interact. 2019, 14, 533–543. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M. Modeling monthly mean air temperature for Brazil. Theor. Appl. Climatol. 2013, 113, 407–427. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Field and Laboratory Methods Manual; Soil survey investigations report No. 51, Version 2.0; Burt, R., Soil Survey Staff, Eds.; US Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Hanks, R.J.; Keller, J.; Rasmusse, V.P.; Wilson, G.D. Line source sprinkler for continuous variable irrigation crop production studies. Soil Sci. Soc. Am. J. 1976, 40, 426–429. [Google Scholar] [CrossRef]
- Jayme-Oliveira, A.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Ziviani, A.C.; Jakelaitis, A. Amaranth, quinoa, and millet growth and development under different water regimes in the Brazilian Cerrado. Pesqui. Agropecuária Bras. 2017, 52, 561–571. [Google Scholar] [CrossRef]
- EMBRAPA. Irrigation Monitoring Program; EMBRAPA: Brasília, Brazil, 2012. [Google Scholar]
- Nascimento, D.T.F.; Novais, G.T. Clima do Cerrado: Dinâmica atmosférica e características, variabilidades e tipologias climáticas. Rev. De Geogr. 2020, 9, 922021. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- ASAE—American Society of Agricultural Engineers. Moisture Measurement—Unground Grain and Seeds. In Standards; ASAE: St. Joseph, MO, USA, 2000; 563p. [Google Scholar]
- QGIS, Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project; 2015; Available online: http://qgis.osgeo.org (accessed on 17 September 2018).
- SAS/STAT. Guide for Personal Computers, version 8.2; SAS Institute: Cary, NC, USA, 2001. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.R-project.org/ (accessed on 17 September 2018).

| Water Regime | Amaranth | Common Bean | Quinoa | Buckwheat |
|---|---|---|---|---|
| Photosynthesis—A (µmol CO2 m−2 s−1) | ||||
| 535 | 43.86 Aab | 25.71 Ca | 35.53 Ba | 23.87 Ca |
| 410 | 43.80 Aab | 21.98 Ca | 35.13 Ba | 12.57 Db |
| 304 | 40.24 Aa | 8.47 Bb | 30.95 Aa | 7.66 Bb |
| 187 | 25.45 Ab | 4.77 Bb | 10.90 Bb | 4.32 Bb |
| Stomatal conductance—gs (µmol CO2 m−2 s−1) | ||||
| 535 | 0.30 Da | 0.56 Ba | 0.73 Aa | 0.40 Ca |
| 410 | 0.31 Ba | 0.34 Bb | 0.74 Aa | 0.10 Cb |
| 304 | 0.27 ABab | 0.06 CDc | 0.39 Ab | 0.05 Db |
| 187 | 0.18 Ab | 0.04 Cc | 0.07 Abc | 0.03 Cb |
| Internal CO2 concentration—Ci (µmol CO2 m−2 s−1) | ||||
| 535 | 120.64 Ba | 291.43 Aa | 283.64 Aa | 260.84 Aa |
| 410 | 128.31 Ba | 256.11 Aab | 280.72 Aa | 152.43 Bb |
| 304 | 116.55 Ba | 200.27 Bbc | 223.37 Aa | 108.91 Bb |
| 187 | 142.26 Aa | 135.76 BC | 124.42 Ab | 154.15 Ab |
| Transpiration (µmol H2O m−2 s−1) | ||||
| 535 | 8.01 Ca | 9.93 Ba | 12.10 Aa | 10.03 Ba |
| 410 | 7.58 Ba | 7.50 Bb | 11.89 Aa | 4.09 Cb |
| 304 | 7.56 Aab | 2.23 Cc | 8.73 Ab | 2.82 Cbc |
| 187 | 5.35 Ab | 1.30 Bc | 2.53 Bc | 1.79 Bc |
| Water use efficiency—WUE | ||||
| 535 | 5.80 Aa | 2.67 Ba | 4.30 Ba | 2.39 Ba |
| 410 | 5.43 Aa | 2.92 Ba | 3.58 Ba | 3.08 Ba |
| 304 | 5.31 Aab | 4.17 BCa | 2.94 Ca | 2.58 Ca |
| 187 | 4.73 Aab | 3.80 BCa | 2.92 BCa | 2.64 Ca |
| Water Regime | Amaranth | Common Bean | Quinoa | Buckwheat |
|---|---|---|---|---|
| Chlorophyll a | ||||
| 535 | 42.01 Aa | 39.47 Aa | 40.39 Aa | 39.77 Aa |
| 410 | 41.90 Aa | 39.90 Aa | 43.43 Aa | 40.89 Aa |
| 304 | 40.04 Aa | 39.21 Aa | 42.94 Aa | 41.69 Aa |
| 187 | 33.39 Bb | 34.77 Bb | 43.62 Aa | 44.18 Aa |
| Chlorophyll b | ||||
| 535 | 9.76 Ba | 9.63 Ba | 20.20 Aab | 12.91 Ba |
| 410 | 10.61 Ba | 9.67 Ba | 19.99 Ab | 10.91 Ba |
| 304 | 9.39 Cab | 9.20 Ca | 23.06 Aab | 15.75 Ba |
| 187 | 4.43 Cb | 6.40 Cb | 25.18 Aa | 13.78 Ba |
| Total Chlorophyll | ||||
| 535 | 120.64 Ba | 291.43 Aa | 283.64 Aa | 260.84 Aa |
| 410 | 128.31 Ba | 256.11 Aab | 280.72 Aa | 152.43 Bb |
| 304 | 116.55 Ba | 200.27 Bbc | 223.37 Aa | 108.91 Bb |
| 187 | 142.26 Aa | 135.76 BC | 124.42 Ab | 154.15 Ab |
| Proline (µmol g−1 FM) | ||||
| 535 | 0.128 Ab | 0.123 Ab | 0.106 Ab | 0.084 Aa |
| 410 | 0.155 Aab | 0.129 Ab | 0.116 Ab | 0.064 Aa |
| 304 | 0.196ABab | 0.185 Ba | 0.258 Aa | 0.058 Ca |
| 187 | 0.261 Aba | 0.189 Ba | 0.314 Aa | 0.097 Ca |
| Water Regime (mm) | Amaranth | Common Bean | Quinoa | Buckwheat |
|---|---|---|---|---|
| Biomass production (kg ha−1) | ||||
| 535 | 16,669.85 Aa | 6650.88 Ca | 14,756.53 Aa | 8513.8 Ba |
| 410 | 15,592.47 Aa | 6590.66 Ca | 18,289.1 Aa | 10,820.33 Ba |
| 304 | 9449.5 Bb | 4599.46 Bb | 15,881.52 Aa | 8289.08 Ba |
| 187 | 3490.91 Ac | 1162.09 Bc | 4878.83 Ab | 4533.17 Ab |
| Grain productivity (kg ha−1) | ||||
| 535 | 3450.01 Ca | 5295.47 Aa | 4084.69 Ba | 2387.43 Da |
| 410 | 3575.01 Ba | 4383.21 Aa | 3622.80 Ba | 2095.95 Ca |
| 304 | 2724.17 Ba | 1613.63 Cb | 3266.17 Aa | 1892.40 Cb |
| 187 | 567.58 Bb | 465.25 Bc | 541.02 Bb | 725.29 Ac |
| Productivity per unit of water applied (kg ha mm−1) | ||||
| 535 | 6.51 BCa | 9.89 Aab | 7.90 ABb | 4.46 Ca |
| 410 | 8.68 Aa | 10.69 Aa | 8.42 Aab | 5.11 Ba |
| 304 | 9.00 Aba | 7.83 BCb | 10.88 Aa | 6.22 Ca |
| 187 | 2.92 Ab | 3.99 Ac | 3.14 Ac | 3.87 Aa |
| Water Regime | Amaranth | Common Bean | Quinoa | Buckwheat |
|---|---|---|---|---|
| 535 | 27.05 Ab | 25.31 Abc | 25.01 Cb | 26.04 Bb |
| 410 | 26.90 Ab | 25.25 Bc | 24.92 Bb | 26.74 Ab |
| 304 | 27.29 Bb | 28.50 Ab | 25.66 Cb | 27.01 Bb |
| 187 | 31.89 Ba | 34.54 Aa | 29.79 Ca | 30.12 Ca |
| Harvest | Period | |
|---|---|---|
| Winter | Summer | |
| 2005/2006 | Fallow | Soybean |
| 2006/2007 | Fallow | Soybean |
| 2007/2008 | Fallow | Soybean |
| 2008/2009 | Fallow | Soybean |
| 2009/2010 | Fallow | Soybean |
| 2010/2011 | Fallow | Soybean |
| 2011/2012 | Soybean under different water regimes | Fallow |
| 2012/2013 | Wheat under different water regimes | Soybean |
| 2013/2014 | A. cruenthus, P. glaucum and C. quinoa under different water regimes | Crotalaria juncea |
| 2014/2015 | A. cruenthus, P. glaucum and C. quinoa under different water regimes | Zea mays |
| 2015/2016 | A. cruenthus, P. glaucum and C. quinoa under different water regimes | Crotalaria juncea |
| Culture | Features |
|---|---|
| Common bean (Carioca) | Determined growth habit (Type I), erect posture, emergence cycle to physiological maturation of approximately 67 days. It has an average yield of 1893 kg ha−1 in the water harvest, 2174 kg ha−1 in the dry season and 2269 kg ha−1 in the winter. It has a C3 photosynthesis mechanism. |
| Amaranth (BRS Alegria) | Average height of 1.8 m; period between emergence and physiological maturation is 90 days. The average grain yield is 2359 kg ha−1. It can be cultivated at any time of the year: for grain production off-season and winter, cultivation is recommended, whilst for forage production summer, sowing is ideal. Has a C4 photosynthesis mechanism. |
| Quinoa (BRS Piabiru) | Average height of 1.9 m; period between emergence and physiological maturity is 110 days; average productivity is 2800 kg ha−1. It can be cultivated at any time of the year: for grain production off-season and winter, cultivation is recommended, whilst for forage production, it can be sown at the beginning of the rainy season. It has a C3 photosynthesis mechanism. |
| Buckwheat (IPR 91) | Upright shrub growth habit; average height of 1.5 to 1.8 m; production of 3 to 6 tons per hectare of dry biomass and 15 to 25 tons per hectare of fresh biomass. For grain production, it can be planted from October to December (recommended) or January to March. It has a C3 photosynthesis mechanism. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.d.N.; Ramos, M.L.G.; Ribeiro Junior, W.Q.; da Silva, P.C.; Soares, G.F.; Casari, R.A.d.C.N.; de Sousa, C.A.F.; de Lima, C.A.; Santana, C.C.; Silva, A.M.M.; et al. Use of Thermography to Evaluate Alternative Crops for Off-Season in the Cerrado Region. Plants 2023, 12, 2081. https://doi.org/10.3390/plants12112081
Silva AdN, Ramos MLG, Ribeiro Junior WQ, da Silva PC, Soares GF, Casari RAdCN, de Sousa CAF, de Lima CA, Santana CC, Silva AMM, et al. Use of Thermography to Evaluate Alternative Crops for Off-Season in the Cerrado Region. Plants. 2023; 12(11):2081. https://doi.org/10.3390/plants12112081
Chicago/Turabian StyleSilva, Alberto do Nascimento, Maria Lucrecia Gerosa Ramos, Walter Quadros Ribeiro Junior, Patrícia Carvalho da Silva, Guilherme Filgueiras Soares, Raphael Augusto das Chagas Noqueli Casari, Carlos Antonio Ferreira de Sousa, Cristiane Andrea de Lima, Charles Cardoso Santana, Antonio Marcos Miranda Silva, and et al. 2023. "Use of Thermography to Evaluate Alternative Crops for Off-Season in the Cerrado Region" Plants 12, no. 11: 2081. https://doi.org/10.3390/plants12112081
APA StyleSilva, A. d. N., Ramos, M. L. G., Ribeiro Junior, W. Q., da Silva, P. C., Soares, G. F., Casari, R. A. d. C. N., de Sousa, C. A. F., de Lima, C. A., Santana, C. C., Silva, A. M. M., & Vinson, C. C. (2023). Use of Thermography to Evaluate Alternative Crops for Off-Season in the Cerrado Region. Plants, 12(11), 2081. https://doi.org/10.3390/plants12112081





