Abstract
Soursop leaves are a source of phytochemical compounds, such as phenolic acids, flavonoids, hydrolyzable tannins, and acetogenins. These compounds can have several types of biological activities. Lactic acid bacteria can uptake phenolic compounds present in plants or fruits. The aim of the present work was to investigate the in vitro effect of hexane, acetone, methanolic, and aqueous extracts of soursop leaves (Annona muricata L.) on the growth, motility, and biofilm formation of Lactobacillus casei, and to determine compounds related to growth. The minimum concentration promoting growth, motility (swimming, swarming, and twitching), and biofilm-forming capacity (crystal violet) were evaluated. The results showed the growth-promoting capacity of acetone and aqueous extracts at low doses 25–50 mg/L, and an inhibition in the four extracts at higher doses of 100 mg/L. The L. casei growth is related to ellagic acid, quercetin rhamnoside, kaempferol dihexoside, quercetin hexoside, secoisolariciresinol, and kaempferol hexoside-rhamnoside. Hexane extract increased the three types of motility, while aqueous maintained swimming and twitching motility similar to control. The four extracts inhibited the biofilm formation capacity.
1. Introduction
The growing demand for functional foods and nutraceuticals has guided researchers to develop new products that meet the needs of consumers. An example of this is the use of beneficial bacteria, which can restore the balance of microbiota, stimulate the immune system, reduce digestive disorders, improve the absorption of nutrients, produce vitamins such as B and K, and prevent diseases [1,2]. Lactic acid bacteria (LAB) are a group of probiotic bacteria commonly used in the pharmaceutical and food industries [3], due to their low energy cost of production, production of compounds, such as lactic acid, flavorings, thickeners, and bacteriocins [4].
One of the most widely studied lactic acid bacteria, for its health-promoting properties, is Lactobacillus casei, a probiotic bacterium used to treat or prevent diverse diseases, so it is extensively used in the industry [5].
The demand for probiotics, such as L. casei, is increasing rapidly due to their impact on consumers’ health, so satisfying demand is a challenge [6]. Additionally, extracellular metabolic by-products of L. casei have been used as antagonist bacteria, bio-preservative, production of bacteriocin, and enzymes as clarification of juice [7,8,9,10].
Due to the above, recent research has focused on searching for new precursor resources for the growth of these microorganisms and the development of non-dairy products that preserve their viability and bioavailability [11,12].
Lactic acid bacteria can adapt to the characteristics of raw materials, such as plants or fruits, which are an abundant source of phenolic compounds [13]. Therefore, various interactions of medicinal plants and spices have been evaluated, such as extracts of oregano, pomegranate peel, and cloves, among others, which are rich in polyphenols. These plant materials have shown an antibacterial capacity against pathogenic bacteria at concentrations greater than 2500 µg/mL. However, they do not inhibit the growth of lactic acid bacteria (LAB) or probiotic bacteria [14]. Sutherland et al. [15] reported the growth of Lactobacillus reuteri by using aqueous extracts of garlic and black pepper. Furthermore, the aqueous extracts of banana, apple, and orange significantly increased the growth of L. reuteri, L. rhamnosus, and Bifidobacteria lactis. Park et al. [16] reported the production of lactic acid bacteria in fermented kimchi (cabbage, garlic, red pepper, and ginger).
Due to its wide nutraceutical and therapeutic use, a potential substrate for probiotics is soursop (Annona muricata L.) leaves [17]. It has bioactive properties, such as anti-inflammatory, anticarcinogenic, antidiabetic, antifungal, antibacterial, anthelmintic, and antiviral [18,19]. Furthermore, the soursop leaves are a source of antioxidant compounds and possess a wide diversity of phytochemical compounds, such as phenolic acids, flavonoids, hydrolyzable tannins, saponins, terpenoids, coumarins, annonaceous acetogenins, and cyclic hexapeptides [20,21]. The above compounds could be taken up by probiotic bacteria since their enzymes intervene in the glycosylation of the compounds, some oxidation processes, demethylation, and catabolism of small phenolic acids and aromatic compounds [22].
Therefore, this work aimed to investigate the in vitro effect of hexane, acetone, methanolic, and aqueous extracts of soursop leaves (Annona muricata L.) on the growth of Lactobacillus casei, and to determine compounds related to it. Further, we evaluate the effect of the extracts on the bacteria’s motility and biofilm-forming capacity.
2. Results
2.1. Effect of Extracts on Lactobacillus Casei Growth
Figure 1 shows the effect of the dosage of soursop leaf extracts, SHE (hexane extract), SAE (acetone extract), SME (methanolic extract), and SWE (aqueous extract), on the growth of L. casei. SAE and SWE treatments at 25 µg/L showed the highest growth (p < 0.05); they increased the growth 80% above the control. However, all extracts at 100 µg/mL decreased the bacterium’s growth percentage.
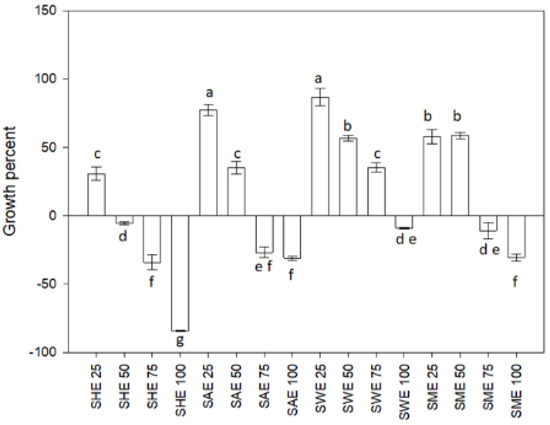
Figure 1.
Growth percent of Lactobacillus casei. SHE soursop hexane extract, SAE soursop acetone extract, SME soursop methanol extract, and SWE soursop aqueous extract. Different letters indicate significant differences according to the Fisher LSD test (p < 0.05).
2.2. Polyphenols in Soursop Leaf
Table 1 shows the content of polyphenolic compounds identified in the soursop leaf extracts. A total of 31 compounds were identified, including two flavanols, 12 flavonols, two hydroxybenzoic acids, 13 hydroxycinnamic acids, and two lignans. Of the identified compounds, 58% were found in the SAE, while SME presented 54% of the compounds. The polyphenols with the highest concentration were kaempferol dihexoside (286.01 µg/g) and kaempferol hexoside-rhamnoside (199.30 µg/g). Both compounds were found in SME.

Table 1.
Polyphenols profile of extracts from soursop (Annona muricata L.).
2.3. Effect of the Polyphenols on the Growth of L. casei
According to the PLS-DA model plot constructed with the profile of polyphenols and microbial growth (Figure 2), eight compounds from soursop leaf extracts were related to the L. casei growth (VIP > 0.8 and coefficients > 0.10). These compounds were identified as ellagic acid (27), quercetin rhamnoside (11), rhamnetin rhamnoside (8), coumaroyl hexoside (30), kaempferol dihexoside (5), quercetin hexoside (6), secoisolariciresinol (31), and kaempferol hexoside-rhamnoside (9). Quercetin hexoside (6), ellagic acid (27), coumaroyl hexoside (30), and secoisolariciresinol (31) were presented in the aqueous extract (SWE). These compounds are polar, so they have a greater affinity for water. Furthermore, quercetin hexoside was presented in acetone (SAE) and hexane (SHE) extracts, and secoisolariciresinol in hexane extract (SAE). Quercetin hexoside and secoisolariciresinol showed affinity for solvents of a range from polar to nonpolar, so they dissolve in water (polar), acetone (moderately polar), and, specifically, quercetin hexoside was soluble in hexane (nonpolar).
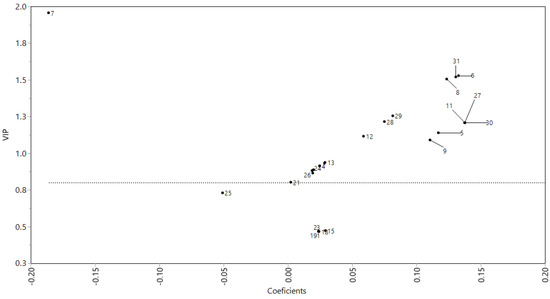
Figure 2.
Association of polyphenols profile of soursop (Annona muricata L.) leaf with their effect on the Lactobacillus casei growth capacity through a PLS-DA model. PLS-DA, partial least square-discriminant analysis.
2.4. Effect of Extracts on Motility and Biofilm-Forming Capacity
According to bacterial growth results, the dose of 25 µg/mL was used to evaluate motility and biofilm formation capacity. Figure 3A shows the swimming-type displacement of L. casei. Treatment SAE decreased the bacterium displacement, while SHE increased the displacement regarding control (p < 0.05). On the other hand, SME and SWE did not show significant differences regarding control (p > 0.05).
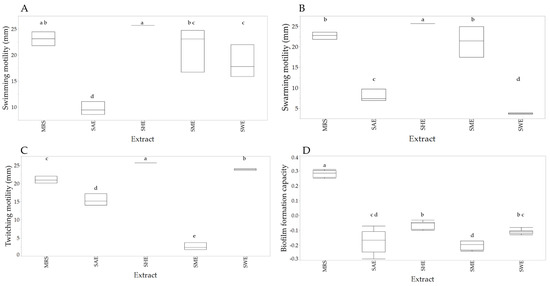
Figure 3.
Box-plot of the effect of the extracts on displacement swimming (A), swarming (B), twitching (C), and biofilm-forming capacity (D). Different letters indicate significant differences according to the Fisher LSD test (p < 0.05).
Figure 3B shows that treatments SAE and SWE decreased the displacement swarming-type regarding control, while SHE increased the displacement (p < 0.05). SME did not show significant differences concerning control (p > 0.05). The displacement twitching of L. casei is shown in Figure 3C. Treatments SHE and SWE significantly increased this type of displacement, while SME and SAE decreased it compared to the control (p < 0.05). All extracts decreased the biofilm-forming capacity (p < 0.05) of L. casei (Figure 3D).
3. Discussion
Different properties have been found in soursop (A. muricata L.), such as antimicrobial, anti-inflammatory, antiprotozoal, antioxidant, insecticidal, larvicidal, and cytotoxic activity to tumor cells. These properties have been related to the more than 200 chemical compounds found in this plant [23]. In this study, 31 compounds were identified. Secoisolariciresinol (Sccl) and medioresinol (Mdl) have not been previously reported in soursop leaves. Secoisolariciresinol has been reported in pulp and seed of another species of the same genera, such as Annona cherimola [24].
The highest concentration of phenolic compounds was found in the methanolic extract, followed by water. Previous studies have shown that methanol has a greater affinity for phenolic compounds [25,26]. Phenolic compounds such as quercetin and its derivates present several hydroxyl groups, which gives it the hydrophilic character; however, they can be both lipo- and hydrophilic, depending if the molecule shows O-methyl, C-methyl, and phenyl derivates, which are lipophilic, and they could be solubilized in acetone or hexane [27]. The affinity of the solvents for different compounds could cause the extracts to have different biological activities.
The ability to affect microbial growth is one of the biological properties of plant extracts, either by inhibiting or increasing microbial growth. In this sense, we found a differentiated response depending on the dose. The lower evaluated concentrations of acetonic, methanolic, and aqueous extracts increased bacterial growth regarding control. The acetonic and aqueous at 25 µg/mL highlight increased microbial growth. On the other hand, we also found that the extracts at a dose of 100 µg/mL decreased bacterial growth. Some authors have reported that soursop leaf extract inhibited the growth of Acanthamoeba triangularis, S. aureus, B. subtilis, E. coli, K. pneumonia, and Proteus vulgaris using doses between 500 and 1000 μg/mL [28,29,30,31]. This behavior related to dose-response could be due to the phenomenon of hormesis. According to toxicology, hormesis is the effect of non-nutritional substances, which may have beneficial or stimulatory effects at low doses and produce adverse effects at higher doses [32,33]. Furthermore, Laparra and Sanz [34] mention that phytochemicals may inhibit pathogenic bacteria while stimulating the growth of beneficial bacteria, exerting prebiotic-like effects.
The stimulatory effect of acetone and aqueous extracts on the L. casei growth could be related to extracts composition. The previous could be because this bacterium has been characterized as an auxotrophic microorganism; namely, it cannot synthesize all growth factors, so it is necessary to obtain them from the growth medium [35]. Lee and Paik [36] mention that Lactic Acid Bacteria (LAB), as L. casei, use polyphenols and polysaccharides as substrates in fermentations.
The PLS-DA model contructed with the polyphenols profile of soursop leaves extracts and the bacterial growth showed that the main polyphenols associated with L. casei growth were ellagic acid (27), quercetin rhamnoside (11), rhamnetin rhamnoside (8), coumaroyl hexoside (30), kaempferol dihexoside (5), quercetin hexoside (6), secoisolariciresinol (31), and kaempferol hexoside-rhamnoside (9). These compounds showed the higher values of VIP (>0.8) and coefficients (>0.10), as we observe in Figure 2, which indicate that those compounds can be considered as the principal compounds associated with L. casei growth. Some authors mention that Lactobacillus spp. may metabolize polyphenols using bacterial enzymes, such as β-glucosidase, α-rhamnosidase, and β-glucuronidase. These enzymes could hydrolyze conjugated polyphenols and release glycosides and aglycones (flavonols as kaempferol, quercetin, quercitrin, and rutin, which are identified as aglycones) [36,37,38].
LAB can uptake oligosaccharides from the conjugated polyphenols and assimilate them through the fermentative metabolism of hexoses and pentose. This process can release aglycones that possess bioactive capacities (biological activity) as an antioxidant, antibacterial, preservative, and chemoprotective, among other properties [39].
Kaempferol and glycosylated derivates can protect against reactive oxygen species in cells [40]. In addition, these flavonoids can protect cells from different insults that lead to mitochondria-mediated cell death and attenuate oxidative stress and mitochondrial dysfunction [40,41].
Valero-Cases et al. [42] mention that lactic acid bacteria can uptake ellagic acid and increase survival. Lactobacillus spp. Can metabolize ellagic acid and secoisolariciresinol and biotransform them into secondary metabolites like enterodiol and enterolactone, according to Bravo et al., which give them one of their probiotic features. Furthermore, ellagic acid has been shown to prevent possible modifications to the mitochondrial membrane, oxidative stress, and toxins’ effects on cell division [43,44,45].
Regarding quercetin and its glycosylated derivates, Curiel et al. [46] found that quercetin has effects dependent on pH and compound concentration. Moreover, quercetin increased the growth and sugar consumption of L. plantarum. Braga et al. [47] asseverate that quercetin could prevent lipid oxidation by its antioxidant properties. This could help to maintain the membrane integrity on L. casei. Besides, Herranz-López et al. [48] assert that quercetin and quercetin-3-O-glucuronide can restore the mitochondrial mass and biogenesis, postulating that they could act as agonists of specific proteins. Finally, Lacerda et al. [49] mentioned that substances like quercetin and kaempferol in Myrciaria jaboticaba fruit extracts stimulated the growth and metabolic activity of probiotics such as L. acidophilus, L. casei, and Bifidobacterium animalis subsp. Lactis.
Moreover, the flavonol rhamnetin and hydroxycinnamic acids as coumaroyl hexoside from Aloe arborescens and A. barbadensis allowed the growth of lactic acid bacteria such as Lactobacillus [50]. According to Hervert-Hernández et al. [51], LAB can metabolize hydroxycinnamic acids such as coumaroyl hexoside by reducing the side chain to produce the corresponding 2-hydroxyphenylpropionic acids, which can then be descarboxylated to p-ethylphenols, allowing these bacteria to grow and modulate the functional composition of gut microbiota [52].
Furthermore, in order to grow, bacteria require nutrients, so they have strategies to obtain them. Motility is one of the ways bacteria can take nutrients to reach new niches for colonization [53]. Lactobacillus casei’s motility is poorly characterized [54]. Our results showed displacement swimming and swarming of L. casei exposed to SHE and SME at low concentrations.
Swimming and swarming motility are related to flagellar motility, however, L. casei does not present flagella, so its motility is related to pilus. In addition, the twitching motility is related to pilus. Sengupta et al. [55] reported the presence of sortase-mediated pilus gene clusters in many strains of L. casei.
Ellagic acid has shown antiquorum sensing activity related to swimming motility and biofilm-forming capacity [56]. It is natural for microorganisms to adhere to biotic or abiotic surfaces, multiply and become embedded in a viscous matrix [57]. However, the extracts evaluated in this work decreased the biofilm-forming capacity. The food industry’s particular concern is the maintenance of the microbial planktonic lifestyle through an efficient cleaning and production process. The formation and growth of biofilms are influenced by several factors, such as bacterial strain, surface and environmental properties, pH, nutrient concentration, and temperature [58]. Even today, four strategies are known to combat biofilms. One of them is the biochemical strategy to disrupt the biofilm using hydrolytic enzymes that degrade extracellular matrix components and the use of compounds able to bind or block intracellular communication, decreasing its level and promoting biofilm dispersion. Although the mechanism of how polyphenols affect the motility, adhesion, and biofilm formation of bacteria is not yet clear and can be very complex, the interaction of these with the protein-membrane and the activity of the quorum sensing has been established [58,59,60].
Altogether, our results showed the capacity of soursop leaf extracts as a potential prebiotic. These extracts are constituted for non-digestible compounds that beneficial microorganisms can metabolize, allowing to improve the bioavailability and bioactivity of the compounds. These compounds can increase the growth of Lactobacillus casei, which is known as a probiotic. These results suggest a wide application of extracts improving the biomass production and viability of probiotic bacteria as L. casei, into the health, pharmaceutical, food, agricultural, veterinary, and zootechnical industries.
4. Materials and Methods
4.1. Biologic Material
Mature soursop leaves (completely developed) were collected from the community of Lima de Abajo, Compostela, Nayarit, Mexico (21°56′7.1808″ N 105°15′28.584″ W), in August, 2018. Leaves were washed, disinfected, and shade dried at 30 °C for 15 days. These were ground in a food processor (Nutribullet® NB-201, Ningbo, China) and stored in trilaminate bags at 25 °C until use. A commercial strain of Lactobacillus casei Shirota (Yakult ®, Guadalajara, Mexico) was used.
4.2. Preparation of Plant Extracts
The leaf powder was suspended in the extraction solvents (hexane, acetone, methanol, and water) at a ratio of 1:10. Subsequently, the suspensions were sonicated for 30 min at 42 Hz in an ultrasonic bath (BRANSON® 5510, Danbury, CT, USA) [61]. The extracts were filtered on Whatman No. 1 paper using vacuum filtration equipment (20 Torr). The filtrates were concentrated on a rotatory evaporator (BÜCHI Labortechnik AG, CH) at 40 °C and 40 Torr vacuum. Finally, a stream of nitrogen gas was circulated over the extracts to remove residual solvent. The extracts were stored in an amber flask until their analysis. The extracts were labeled as hexane extract (SHE), acetonic extract (SAE), methanolic extract (SME), and aqueous extract (SWE).
4.3. Effect of Extracts on the Growth of Lactobacillus casei
4.3.1. Inoculum Preparation
Lactobacillus casei was activated in 10 mL of Man, Ragosa, and Sharpe (MRS) medium for 24 h at 37 °C. Then, 1 mL aliquot was taken and added to 9 mL of MRS medium to prepare an inoculum; after, it was incubated for 16 h at 37 °C. Next, the inoculum was concentrated by centrifugation at 10,433× g for 10 min. Finally, a cellular suspension was prepared with an absorbance of 0.4 at a wavelength of 620 nm using a spectrophotometer (Thermo Fisher Scientific, Multiskan Go, Vantaa, Finland), equivalent to 108 cells/mL [62].
4.3.2. Determination of Minimum Growth-Promoting Concentration
The microdilution technique was used to determine the minimum growth-promoting concentration. Initially, a stock solution of the extracts was prepared in dimethyl sulfoxide (DMSO) at a concentration of 30,000 µg/mL. Then, four concentrations, 25, 50, 75, and 100 µg/mL, were prepared. Each treatment consisted of 175 µL of MRS medium, 20 µL of inoculum, and 5 µL of the extract [63]. The treatments were added to a sterile 96-well plate and incubated at 37 °C in a microplate reader (Thermo Fisher scientific, MultiSkan GO, Vantaa, Finland). The microplate was shaken every 5 min for 30 s. The OD at 620 nm was read after 24 h of incubation. The growth percentage was determined using the Equation (1).
% Growth = (OD initial − OD sample)/(OD control) × 100
4.4. Phytochemical Analysis
First, the polyphenols were extracted using the method described by Arranz et al. [64]. Then, 2 mL of the polyphenol extract (EPF) were concentrated in a 16,000× g vacuum centrifuge for 10 min at 4 °C. Next, the concentrate was resuspended in 200 µL of methanol and filtered using a PVDF syringe filter (13 mm, 0.45 µm) and stored in microvials until analysis.
The phytochemical profile was assessed in an Ultra-Performance Liquid Chromatograph (UPLC) coupled to a Diode Array Detector (DAD) and a Quadrupole Time-of-Flight (Q-ToF) mass spectrometer (MS) with an electrospray ionization (ESI) interphase (Vion IMS, Waters Co., Milford, MA, USA). Decoctions were filtered (0.45 mm) and directly injected into a BEH Acquity C18 column (2.1 × 100 mm, 1.7 mm) at 35 °C. For the chromatographic separation, water with 0.1% formic acid (A) and acetonitrile (B) were used as mobile phase at a flow of 0.5 mL/min. The gradient conditions were 0% B/0 min, 15% B/2.5 min, 21% B/10 min, 90% B/12 min, 95% B/13 min, 0% B/15 min, and 0% B/17 min. Absorbances were measured at 214, 280, 320, 360, 484, and 535 nm [65]. The following commercial standards were used to construct calibration curves and quantitate the different types of phenolic compounds: (+)-catechin (flavanols), naringenin (flavanones), quercetin (flavonols), p-hydroxybenzoic acid (hydroxybenzoic acids), and chlorogenic acid (hydroxycinnamic acids). Polyphenol results are expressed as µg/g of extract, whereas lignan and stilbene results are expressed as arbitrary units. The following MS conditions were used: capillary voltage, 2.0 kV; cone voltage, 40 eV; low collision energy, 6 V; high collision energy, 15–45 V; source temperature, 120 °C; cone gas flow, 50 L/h; and desolvation gas, N2 at 450 °C and 800 L/h. Data acquisition was carried out at negative ionization mode (ESI-) within a 100–1200 Da mass range. Leucine-enkephalin solution (50 pg/mL) was used for lock mass correction at 10 mL/min. Identification was carried out by analysis of the exact mass of the pseudomolecular ion (mass error < 5 µg/mL), isotope distribution, and fragmentation pattern.
4.5. Bacterial Motility
Bacterial motility (swimming, swarming, and twitching) was determined by measuring the displacement (mm) of the bacterium in MRS medium supplemented with agar. The medium was supplemented with 0.3% agar to swimming-type motility, 0.6% agar to swarming-type motility, and 1% agar to twitching, as Burrows [66] described. A total of 25 µg/mL of each soursop leaf extract was added to the culture media in each technique. The percentage displacement in each technique was determined using the Equation (2).
4.6. Biofilm Forming Capacity
The method proposed by Naves et al. [67] was followed with slight modifications. First, 200 µL of MRS medium at 50%, 10 µL of inoculum, and 25 µL of extract at 25 µg/mL were added to 96-well microplates. The cultures were incubated at 37 °C for 18 h. After that, OD at 620 nm was read in a microplate reader (Thermo Fisher scientific, MultiSkan GO, Vantaa, Finland). Then, the plate was washed with saline solution to remove unattached bacteria. Next, the plate was dried under airflow for 20 min. Wells were stained with 200 µL of crystal violet at 0.3%, washed with distilled water, and dried for 1 h. After, wells were filled with 200 µL of 95% ethyl alcohol, and absorbance was read at 540 nm. The biofilm-forming capacity (BFC) was calculated with Equation (3).
AB is the DO540 nm of the adhered bacterium to the microplate, CW is the DO540 of the control medium (MRS without bacteria), G is the DO620 nm of cell growth.
4.7. Statistical Analysis
Each extract was analysed by triplicate in three independent experiments. A completely randomized design was used. The data obtained in the microdilution technique to determine the bacterium minimum growth-promoting concentration of the bacterium were analysed by ANOVA with α = 0.05 to determine significant differences in the extracts. An LSD Fisher test (α = 0.05) was used when ANOVA showed statistical differences.
The association between polyphenolic compounds and the bacterial growth were analysed by plots means of variable importance in projection (VIP) vs. coefficient, constructed from partial least squares-discriminant analysis (PLS-DA) with centered and scaled data. A nonlinear intervention in partial least squares (NIPALS) was used. All analyses were carried out with JMP software 14.3 (Sytat Software, Inc., San José, CA, USA).
5. Conclusions
The acetone, methanolic, and aqueous extracts of soursop at 25 µg/mL increased the bacterial growth. However, all evaluated extracts inhibited the bacterial growth at 100 µg/mL. Hexane and methanolic extract of soursop leaf did not show differences with respect to control on swimming motility, while aqueous and acetonic decreased it. Hexane extract increased the swarming motility, methanolic extract maintained it, while acetone and aqueous extracts decreased this motility concerning control. Hexane and aqueous extracts increased twitching motility, while acetone and methanolic extracts decreased this type of motility. All extracts decreased the biofilm-forming capacity of Lactobacillus casei.
In order to obtain higher L. casei growth and to use the less toxic solvent, the best treatment was the aqueous extract at 25 µg/mL, which increased the bacterial growth and maintained the swimming and twitching motility without differences regarding control.
The L. casei growth was related to ellagic acid, quercetin rhamnoside, kaempferol dihexoside, quercetin hexoside, secoisolariciresinol, and kaempferol hexoside-rhamnoside.
The study suggests a broad range of applications to the aqueous extract of soursop leaf into the health, pharmaceutical, food, agricultural, veterinary, and zootechnical industries since it improves the biomass production and viability of probiotic bacteria as L. casei.
Author Contributions
Conceptualization, P.U.B.-R. and P.M.-S.; Methodology, P.U.B.-R., P.M.-S. and I.F.P.-R.; Formal Analysis, G.B.-V.; Investigation, N.N.M.-G. and I.F.P.-R.; writing-original draft preparation, N.N.M.-G.; writing-review and editing, N.N.M.-G.; visualization, G.G.L.-G.; supervision, R.B.-M.; project administration, R.B.-M. and P.U.B.-R.; funding acquisition R.B.-M. and P.U.B.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondo Sectorial de Investigación en materias agrícolas, pecuaria, acuacultura, agrobiotecnología y recursos fitogenéticos SADER-CONACyT, grant number 266891. “Aprovechamiento del germoplasma, desarrollo tecnológico e innovación en cadenas de valor de Anonáceas en México”.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors want to thank the National Council of Science and Technology (CONACyT) for the Ph.D. scholarship granted to Nimcy Noemí Meza-Gutiérrez (No. 718887).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mosso, A.L.; Jimenez, M.E.; Vignolo, G.; LeBlanc, J.G.; Samman, N.C. Increasing the folate content of tuber based foods using potentially probiotic Lactic Acid Bacteria. Food Res. Int. 2018, 109, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gu, Q.; Yang, L.; Yu, Y.; Wang, Y. Characterization of extracellular vitamin B12 producing Lactobacillus plantarum strains and assessment of the probiotic potentials. Food Chem. 2017, 234, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Heenan, C.N.; Adams, M.C.; Hosken, R.W.; Fleet, G.H. Growth medium for culturing probiotic bacteria for applications in vegetarian food products. LWT Food Sci. Technol. 2002, 35, 171–176. [Google Scholar] [CrossRef]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The efficient clade: Lactic Acid Bacteria for industrial chemical production. Trends Biotechnol. 2017, 35, 756–769. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Aditya, A.; Peng, M.; Young, A.; Biswas, D. Antagonistic mechanism of metabolites produced by Lactobacillus casei on lysis of enterohemorrhagic Escherichia coli. Front. Microbiol. 2020, 11, 574422. [Google Scholar] [CrossRef]
- Tian, J.X.; Kang, Y.H.; Chu, G.S.; Liu, H.J.; Kong, Y.D.; Zhao, L.H.; Kong, Y.X.; Shan, X.F.; Wang, G.Q. Oral administration of Lactobacillus casei expressing flagellin A protein confers effective protection against Aeromonas veronii in common carp, Cyprinus carpio. Int. J. Mol. Sci. 2019, 21, 33. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Yu, W.; Hou, J.; Han, X.; Shao, H.; Liu, Y. Characterization and production optimization of a broad-spectrum bacteriocin produced by Lactobacillus casei KLDS 1.0338 and its application in soybean milk Biopreservation. Int. J. Food Prop. 2020, 23, 677–692. [Google Scholar] [CrossRef] [Green Version]
- Olubode, T.P.; Oyelakin, A.O.; Olawale, B.R.; Bolaji, A.S.; Bada, T.V. Bio-preservative effect of Lactic Acid Bacteria isolated from fermented cow milk on nunu. Ind. J. Appl. Microbiol. 2019, 22, 7–18. [Google Scholar] [CrossRef]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Alves, N.N.; Messaoud, G.B.; Desobry, S.; Costa, J.M.C.; Rodrigues, S. Effect of drying technique and feed flow rate on bacterial survival and physicochemical properties of a non-dairy fermented probiotic juice powder. J. Food Eng. 2016, 189, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.L.; Gan, R.; Shah, N.P.; Corke, H. Enhancing antioxidant capacity of Lactobacillus acidophilus-fermented milk fortified with pomegranate peel extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Sutherland, J.; Miles, M.; Hedderley, D.; Li, J.; Devoy, S.; Sutton, K.; Lauren, D. In vitro effects of food extracts on selected probiotic and pathogenic bacteria. Int. J. Food Sci. Nutr. 2009, 60, 717–727. [Google Scholar] [CrossRef]
- Park, H.; Shin, H.; Lee, K.; Holzapfel, W. Autoinducer-2 properties of kimchi are associated with Lactic Acid Bacteria involved in its fermentation. Int. J. Food Microbiol. 2016, 225, 38–42. [Google Scholar] [CrossRef]
- Irrera, N.; Pallio, G.; Mannino, F.; Gugliotta, R.; Metro, D.; Altavilla, D.; Squadrito, F. Administration of a nutraceutical mixture composed by Aloe arborescens, Annona muricata, Morinda citrifolia, Beta rubra, Scutellaria baicalensis, and Vaccinium myrtillus reduces doxorubicin-induced side effects. Nutr. Cancer 2020, 72, 343–351. [Google Scholar] [CrossRef]
- Ferreira, L.E.; Castro, P.M.N.; Chagas, A.C.S.; França, S.C.; Beleboni, R.O. In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp. Parasitol. 2013, 134, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Rady, I.; Bloch, M.B.; Chamcheu, R.-C.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.-R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anticancer properties of graviola (Annona muricata): A comprehensive mechanistic review. Oxid. Med. Cell. Longev. 2018, 2018, 1826170. [Google Scholar] [CrossRef] [Green Version]
- Gavamukulya, Y.; Abou-Elella, F.; Wamunyokoli, F.; AEl-Shemy, H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Med. 2014, 7, S355–S363. [Google Scholar] [CrossRef] [Green Version]
- de-Moraes, I.V.M.; Ribeiro, P.R.V.; Schmidt, F.L.; Canuto, K.M.; Zocolo, G.J.; de Brito, E.S.; Luo, R.; Richards, K.M.; Tran, K.; Smith, R.E. UPLC–QTOF–MS and NMR analyses of graviola (Annona muricata) leaves. Rev. Bras. Farmacogn. 2016, 26, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef] [Green Version]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and quantification of phenolic and other polar compounds in the edible part of Annona cherimola and its by-products by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2015, 78, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Barros, R.G.C.; Rezende, Y.R.R.S.; Nogueira, J.P.; de Oliveira, C.S.; Gualberto, N.C.; Narain, N. Evaluation of bioactive compounds, phytochemicals profile and antioxidant potential of the aqueous and ethanolic extracts of some traditional fruit tree leaves used in brazilian folk medicine. Food Res. Int. 2021, 143, 110282. [Google Scholar] [CrossRef]
- Mousavi, L.; Salleh, R.M.; Murugaiyah, V. Phytochemical and bioactive compounds identification of ocimum tenuiflorum leaves of methanol extract and its fraction with an anti-diabetic potential. Int. J. Food Prop. 2018, 21, 2390–2399. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivates: Chemical structure and bioactivity—A review. Pol. J. Food. Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Mitsuwan, W.; Sin, C.; Keo, S.; Sangkanu, S.; de Lourdes Pereira, M.; Jimoh, T.O.; Salibay, C.C.; Nawaz, M.; Norouzi, R.; Siyadatpanah, A.; et al. Potential anti-acanthamoeba and anti-adhesion activities of Annona muricata and Combretum trifoliatum extracts and their synergistic effects in combination with chlorhexidine against Acanthamoeba triangularis trophozoites and cysts. Heliyon 2021, 7, e06976. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Wulanjati, M.P.; Windarsih, A.; Bhattacharjya, D.K.; Suzuki, T.; Katayama, T. In vitro studies of antioxidant, antidiabetic, and antibacterial activities of Theobroma cacao, Anonna muricata and Clitoria ternatea. Biocatal. Agric. Biotechnol. 2021, 33, 101995. [Google Scholar] [CrossRef]
- Oyedeji, O.; Taiwo, F.O.; Ajayi, O.S.; Oziegbe, M.; Oseghare, C.O. Biocidal and phytochemical analysis of leaf extracts of Annona muricata (Linn.). Int. J. Sci. 2015, 24, 12. [Google Scholar]
- Vijayameena, C.; Subhashini, G.; Loganayagi, M.; Ramesh, B. Phytochemical screening and assessment of antibacterial activity for the bioactive compounds in Annona muricata. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 1–8. [Google Scholar]
- Calabrese, E.; Blain, R. The Occurrence of Hormetic Dose Responses in the Toxicological Literature, the Hormesis Database: An Overview. Toxicol. Appl. Pharmacol. 2005, 202, 289–301. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly generalizable and beyond laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by Lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.-K.; Paik, H.-D. Bioconversion using Lactic Acid Bacteria: Ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, D.; Strahsburger, E.; Lopez de Lacey, A.M.; Bregola, V.; Marotti, I.; Aloisio, I.; Biavati, B.; Dinelli, G. Flavonoid bioconversion in Bifidobacterium pseudocatenulatum B7003: A potential probiotic strain for functional food development. J. Funct. Foods 2014, 7, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, B.; Qiao, Y.; Zhou, Q.; He, H.; He, M. Kaempferol protects mitochondria and alleviates damages against endotheliotoxicity induced by doxorubicin. Biomed. Pharmacother. 2020, 126, 110040. [Google Scholar] [CrossRef]
- Ulrih, N.P.; Maričić, M.; Ota, A.; Šentjurc, M.; Abram, V. Kaempferol and quercetin interactions with model lipid membranes. Food Res. Int. 2015, 71, 146–154. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of fermentation with different Lactic Acid Bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D.; Peirotén, Á.; Álvarez, I.; Landete, J.M. Phytoestrogen metabolism by Lactic Acid Bacteria: Enterolignan production by Lactobacillus salivarius and Lactobacillus gasseri strains. J. Funct. Foods 2017, 37, 373–378. [Google Scholar] [CrossRef]
- Alfredsson, C.F.; Ding, M.; Liang, Q.-L.; Sundström, B.E.; Nånberg, E. Ellagic acid induces a dose- and time-dependent depolarization of mitochondria and activation of caspase-9 and -3 in human neuroblastoma cells. Biomed. Pharmacother. 2014, 68, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Baeeri, M.; Momtaz, S.; Navaei-Nigjeh, M.; Niaz, K.; Rahimifard, M.; Ghasemi-Niri, S.F.; Sanadgol, N.; Hodjat, M.; Sharifzadeh, M.; Abdollahi, M. Molecular evidence on the protective effect of ellagic acid on phosalone-induced senescence in rat embryonic fibroblast cells. Food Chem. Toxicol. 2017, 100, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Curiel, J.A.; Muñoz, R.; López-de-Felipe, F. PH and dose-dependent effects of quercetin on the fermentation capacity of Lactobacillus plantarum. LWT Food Sci. Technol. 2010, 43, 926–933. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérez, L.M.; del Soazo, M.V.; Machado, F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of poly(vinyl chloride) films containing quercetin and silver nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Herranz-López, M.; Olivares-Vicente, M.; Rodríguez Gallego, E.; Encinar, J.A.; Pérez-Sánchez, A.; Ruiz-Torres, V.; Joven, J.; Roche, E.; Micol, V. Quercetin metabolites from Hibiscus sabdariffa contribute to alleviate glucolipotoxicity-induced metabolic stress in vitro. Food Chem. Toxicol. 2020, 144, 111606. [Google Scholar] [CrossRef]
- Lacerda-Massa, N.M.; Dantas-Duarte-Menezes, F.N.; de-Albuquerque, T.M.R.; de-Oliveira, S.P.A.; Lima, M.; dos-Santos-Lima, S.; Magnani, M.; de-Souza, E.L. Effects of digested jabuticaba (Myrciaria jaboticaba (Vell.) Berg) by-product on growth and metabolism of Lactobacillus and Bifidobacterium indicate prebiotic properties. LWT 2020, 131, 109766. [Google Scholar] [CrossRef]
- Chiodelli, G.; Pellizzoni, M.; Ruzickova, G.; Lucini, L. Effect of different Aloe fractions on the growth of Lactic Acid Bacteria: Aloe fractions and Lactic Acid Bacteria. J. Food Sci. 2017, 82, 219–224. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.J.; Lynch, S.M.; Harris, H.M.B.; McCann, A.; Lynch, D.B.; Neville, B.A.; Irisawa, T.; Okada, S.; Endo, A.; O’Toole, P.W. Detection and genomic characterization of motility in Lactobacillus curvatus: Confirmation of motility in a species outside the Lactobacillus salivarius Clade. Appl. Environ. Microbiol. 2015, 81, 1297–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediat. Inflamm. 2013, 2013, 237921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćirić, A.D.; Petrović, J.D.; Glamočlija, J.M.; Smiljković, M.S.; Nikolić, M.M.; Stojković, D.S.; Soković, M.D. Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends. S. Afr. J. Bot. 2019, 120, 65–80. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Volstatova, T.; Marsik, P.; Rada, V.; Geigerova, M.; Havlik, J. Effect of apple extracts and selective polyphenols on the adhesion of potential probiotic strains of Lactobacillus gasseri R and Lactobacillus casei FMP. J. Funct. Foods 2017, 35, 391–397. [Google Scholar] [CrossRef]
- Novik, G.; Savich, V. Beneficial microbiota. probiotics and pharmaceutical products in functional nutrition and medicine. Microbes Infect. 2020, 22, 8–18. [Google Scholar] [CrossRef]
- Ruiz-Montañez, G.; Burgos-Hernández, A.; Calderón-Santoyo, M.; López-Saiz, C.M.; Velázquez-Contreras, C.A.; Navarro-Ocaña, A.; Ragazzo-Sánchez, J.A. Screening antimutagenic and antiproliferative properties of extracts isolated from jackfruit pulp (Artocarpus heterophyllus Lam). Food Chem. 2015, 175, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, J.; Teixeira, A.; Allais, F.; Spinnler, H.-E.; Saulou-Bérion, C.; Clément, T. Wheat and sugar beet coproducts for the bioproduction of 3-hydroxypropionic acid by Lactobacillus reuteri DSM17938. Fermentation 2017, 3, 32. [Google Scholar] [CrossRef] [Green Version]
- Zgoda, J.R.; Porter, J.R. A Convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef]
- Parola-Contreras, I.; Guevara-González, R.G.; Feregrino-Pérez, A.A.; Reynoso-Camacho, R.; Pérez-Ramírez, I.F.; Ocampo-Velázquez, R.V.; Rojas-Molina, A.; Luna-Vazquez, F.; Tovar-Pérez, E.G. Phenolic compounds and antioxidant activity of methanolic extracts from leaves and flowers of chilcuague (Heliopsis longipes, Asteraceae). Bot. Sci. 2020, 99, 149–160. [Google Scholar] [CrossRef]
- Burrows, L.L. Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naves, P.; del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Rodrguez-Cerrato, V.; Ponte, M.C.; Soriano, F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J. Appl. Microbiol. 2008, 105, 585–590. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).