Biochar Stimulated Actual Evapotranspiration and Wheat Productivity under Water Deficit Conditions in Sandy Soil Based on Non-Weighing Lysimeter
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Materials’ Source
2.2. The Non-Weighing Lysimeter
2.3. Biochar Preparation and Application
2.4. Agronomical Crop and Treatments Applied
2.5. Physicochemical Properties of the Soil
2.6. Plant Harvest
2.7. Water Use Efficiency Determination
2.8. Water Relations
2.8.1. Reference Evapotranspiration Computing
2.8.2. Crop Evapotranspiration Estimation
2.8.3. Determination of Irrigation Water Applied
2.8.4. Actual Evapotranspiration Estimation
2.9. Statistical Analysis
3. Results
3.1. Combined ANOVA
3.2. Biochar Soil Supplementation Altered the Actual Evapotranspiration of Stressed Wheat Plants
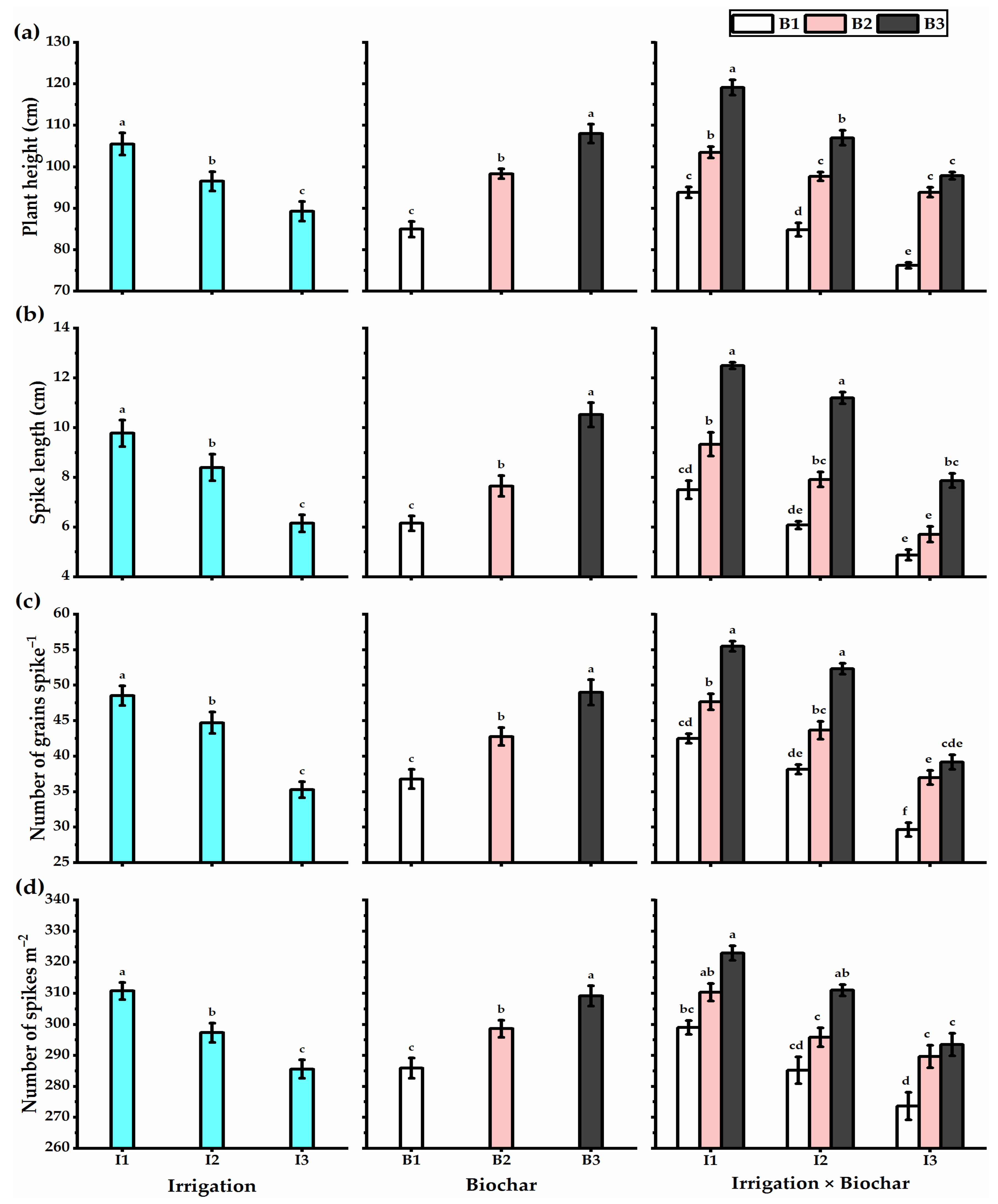
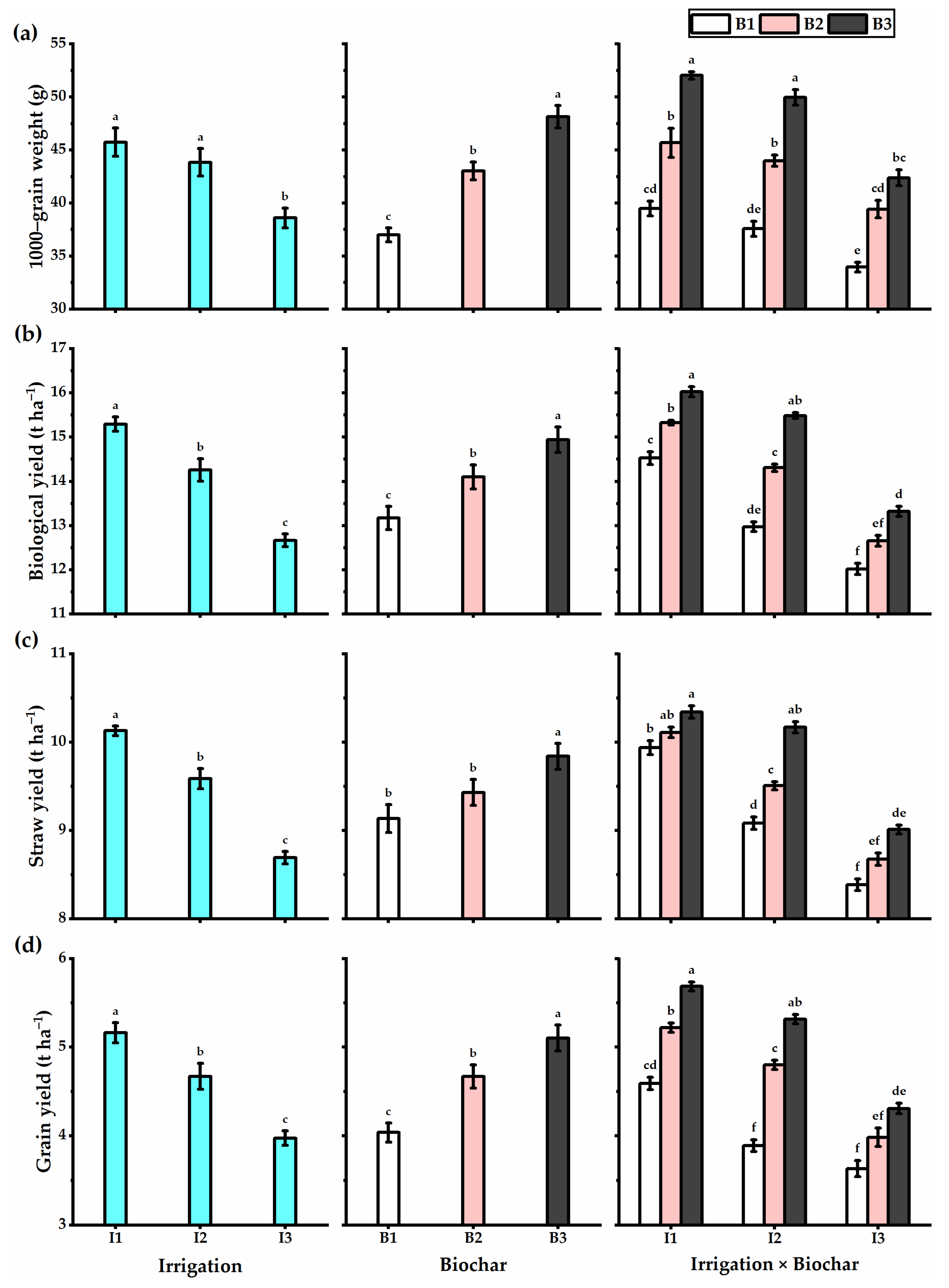
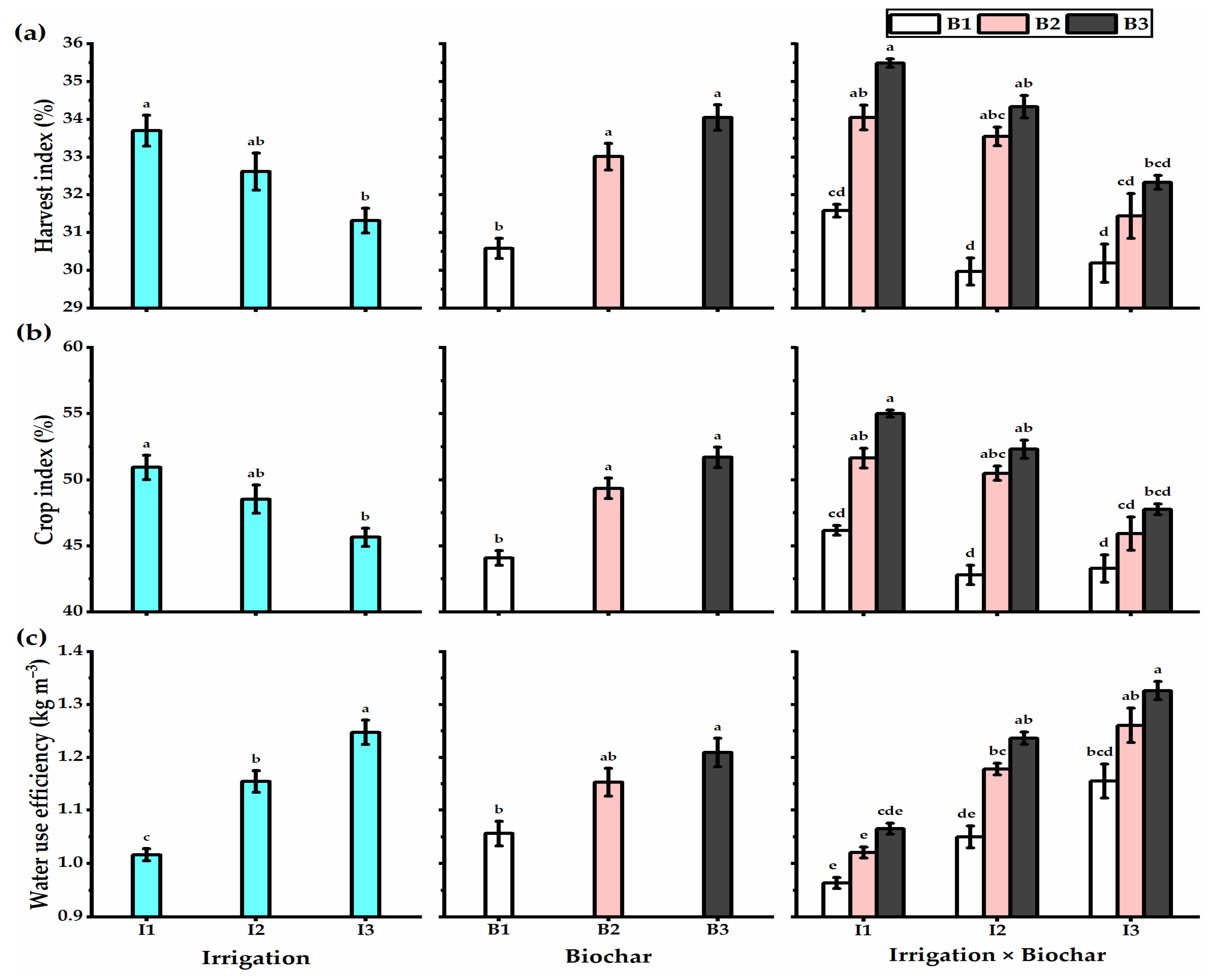
3.3. Biochar Soil Supplementation Stimulated the Yield Components and Biological Yield of Stressed Wheat Plants
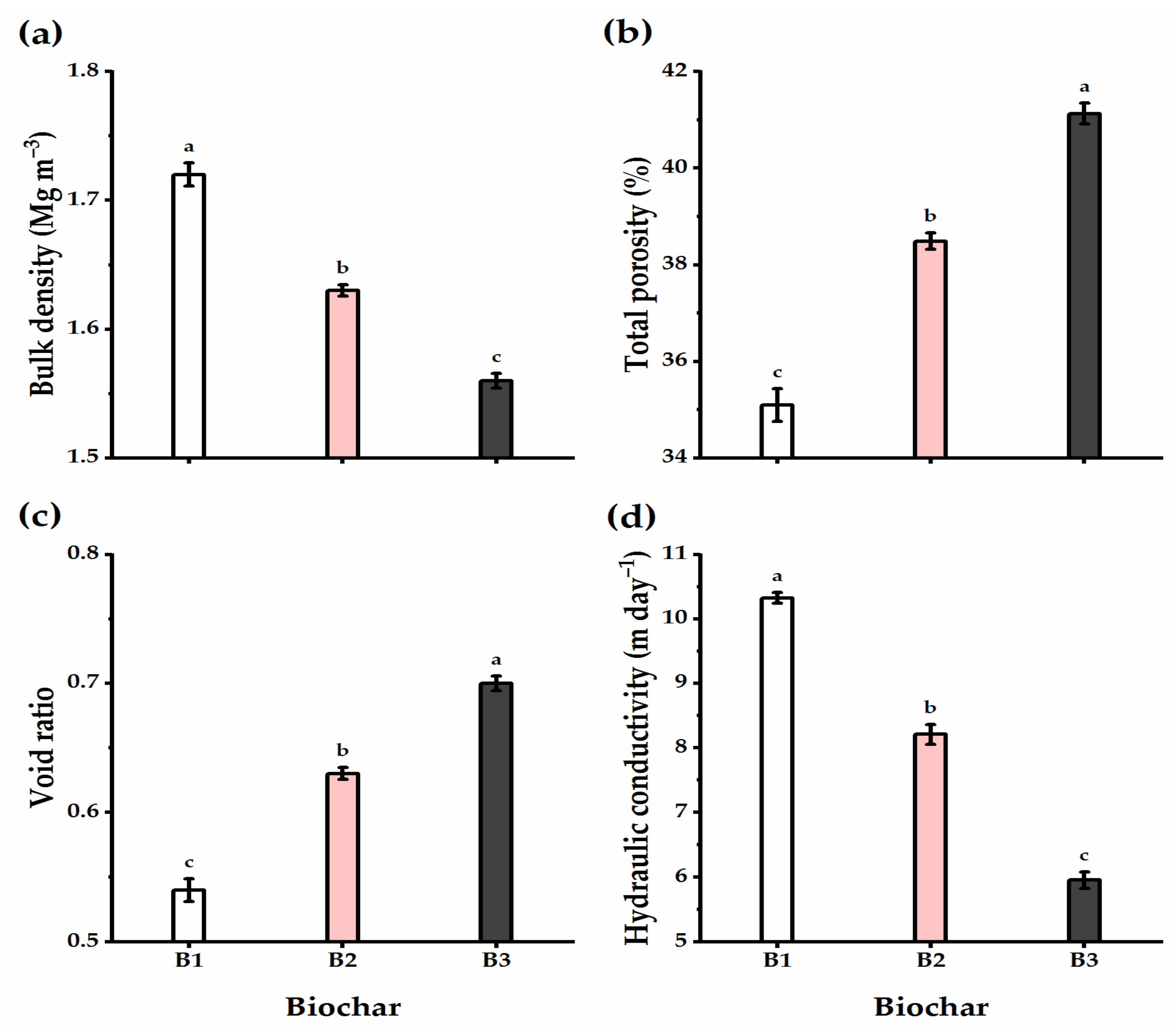
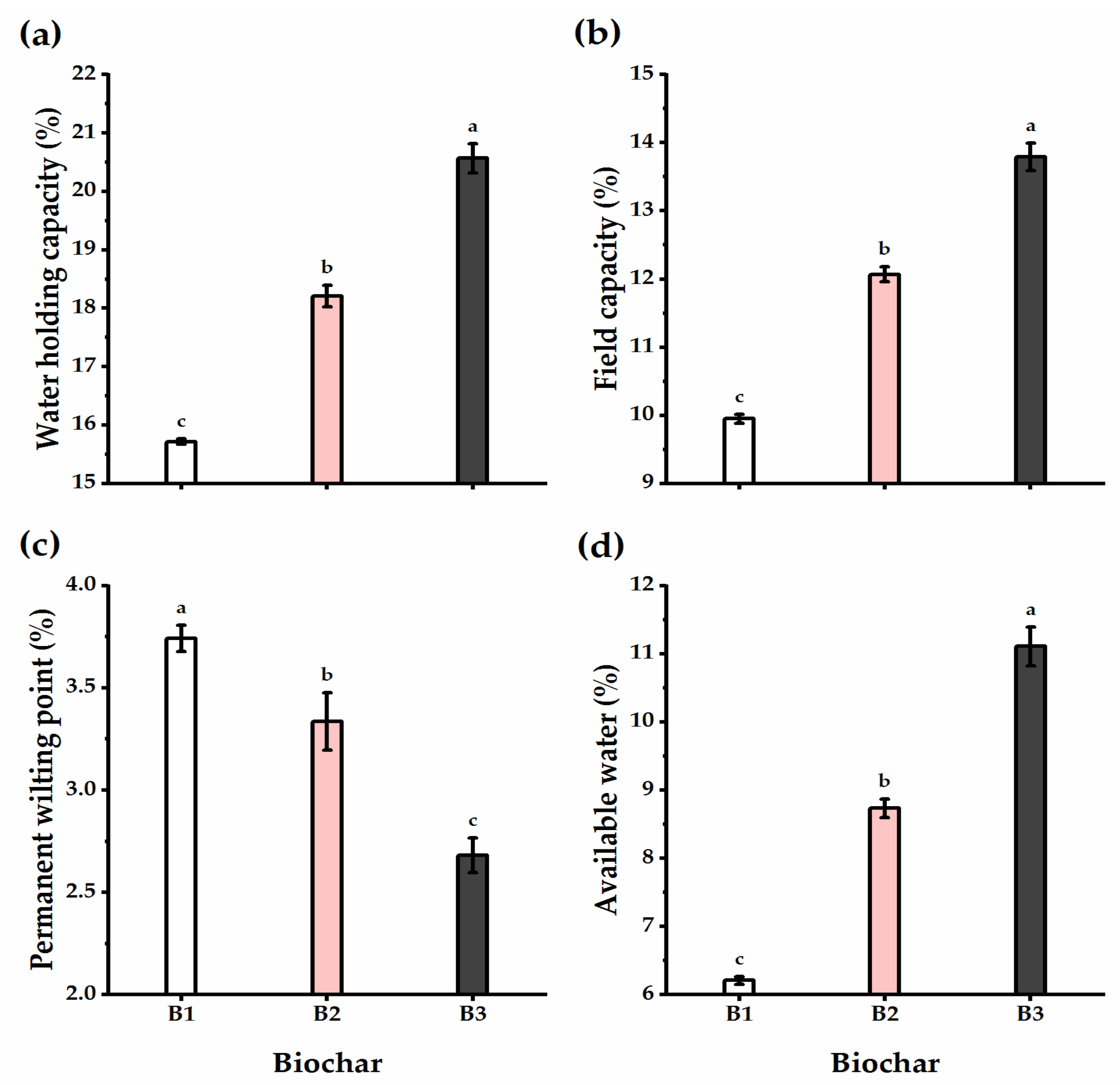
3.4. Biochar Soil Supplementation Altered the Soil’s Physical Traits
3.5. Correlation Analysis
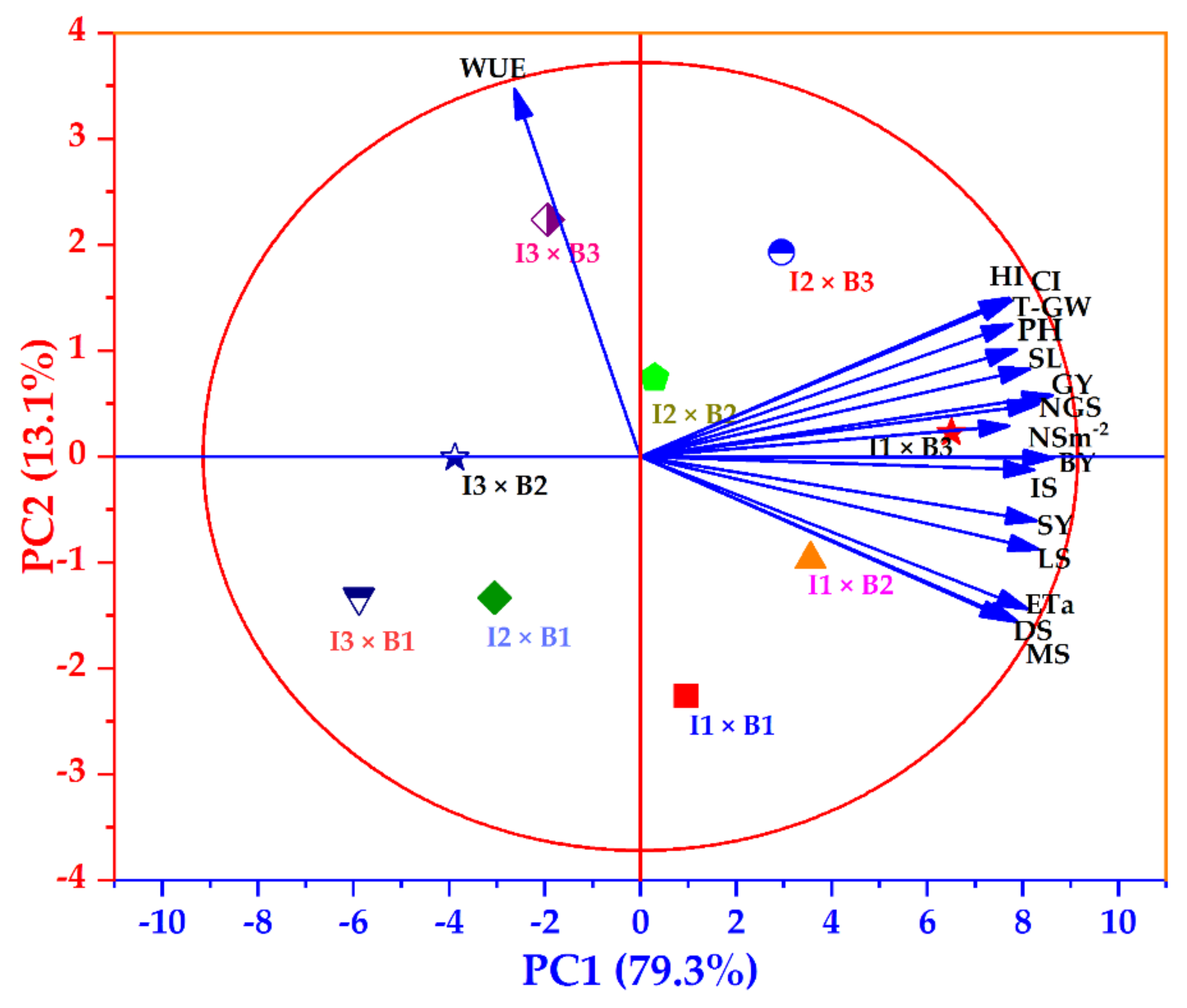
3.6. Principal Component Analysis
4. Discussion
4.1. Actual Evapotranspiration and Soil Characteristics
4.2. Wheat Productivity and Water Use Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pompeu, J.; Nolasco, C.L.; West, P.; Smith, P.; Gerage, J.; Ometto, J. Is domestic agricultural production sufficient to meet national food nutrient needs in Brazil? PLoS ONE 2021, 16, e0251778. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of abiotic stress factors on the antioxidant properties and polyphenols profile composition of green barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Atteya, A.K.G.; El-Serafy, R.S.; El-Zabalawy, K.M.; Elhakem, A.; Genaidy, E.A.E. Exogenously supplemented proline and phenylalanine improve growth, productivity, and oil composition of salted moringa by up-regulating osmoprotectants and stimulating antioxidant machinery. Plants 2022, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Sales, B.K.; Bryla, D.R.; Trippe, K.M.; Weiland, J.E.; Scagel, C.F.; Strik, B.C.; Sullivan, D.M. Amending sandy soil with biochar promotes plant growth and root colonization by mycorrhizal fungi in Highbush blueberry. HortScience 2020, 55, 353–361. [Google Scholar] [CrossRef]
- Prakongkep, N.; Gilkes, R.J.; Wisawapipat, W.; Leksungnoen, P.; Kerdchana, C.; Inboonchuay, T.; Delbos, E.; Strachan, L.; Ariyasakul, P.; Ketdan, C.; et al. Effects of biochar on properties of tropical sandy soils under organic agriculture. J. Agric. Sci. 2021, 13, 1–17. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.L.; Bastos, A.C.; Van Der Velde, M.; Diafas, I. Biochar Application to Soils: A Critical Scientific Review of Effects on Soil Properties, Processes and Functions, Environment; EUR 24099 EN; Office for the Official Publications of the European Communities: Luxembourg, Belgium, 2010. [Google Scholar]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Guo, M.; He, Z.; Uchimiya, S.M. Introduction to biochar as an agricultural and environmental amendment. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; Volume 63, pp. 1–14. [Google Scholar]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Lehmann, J.; Czimczik, C.; Laird, D.; Sohi, S. Stability of biochar in soil. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: Sterling, VA, USA, 2009; pp. 183–206. [Google Scholar]
- Sohi, S.P.; Yates, H.C.; Gaunt, J.L. Testing a practical indicator for changing soil organic matter. Soil Use Manag. 2010, 26, 108–117. [Google Scholar] [CrossRef]
- Laird, D.A. The charcoal vision: A win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Kim, S.; Kaplan, L.A.; Benner, R.; Hatcher, P.G. Hydrogen-deficient molecules in natural riverine water samples–evidence for the existence of black carbon in DOM. Mar. Chem. 2004, 92, 225–234. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Montanarella, L.; Bastos, A.C. Sustainability, certification, and regulation of biochar. Pesq. Agropec. Bras. 2012, 47, 649–653. [Google Scholar] [CrossRef]
- Reyes-Cabrera, J.; Leon, R.G.; Erickson, J.E.; Rowland, D.L.; Silveira, M.L.; Morgan, K.T. Differences in biomass and water dynamics between a cotton-peanut rotation and a sweet sorghum bioenergy crop with and without biochar and vinasse as soil amendments. Field Crop Res. 2017, 214, 123–130. [Google Scholar] [CrossRef]
- Kätterer, T.; Roobroeck, D.; Andrén, O.; Kimutai, G.; Karltun, E.; Kirchmann, H.; Nyberg, G.; Vanlauwe, B.; De Nowina, K.R. Biochar addition persistently increased soil fertility and yields in maize-soybean rotations over 10 years in sub-humid regions of Kenya. Field Crop Res. 2019, 235, 18–26. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Olayanju, A.; Ejue, W.S.; Adekanye, T.A.; Adenusi, T.T.; Ayeni, J.F. Effect of biochar on soil properties, soil loss, and cocoyam yield on a tropical sandy loam alfisol. Sci. World J. 2020, 2020, 9391630. [Google Scholar] [CrossRef]
- Khan, K.Y.; Ali, B.; Cui, X.; Feng, Y.; Yang, X.; Stoffella, P.J. Impact of different feedstocks derived biochar amendment with cadmium low uptake affinity cultivar of pak choi (Brassica Rapa ssb. chinensis L.) on phytoavoidation of Cd to reduce potential dietary toxicity. Ecotoxicol. Environ. Saf. 2017, 141, 129–138. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Rizwan, M.; Chaudhary, H.J.; Gurmani, A.R.; Munis, M.F.H. Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere 2020, 238, 124710. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Refay, Y.; Al-Suhaibani, N.; Al-Ashkar, I.; El-Hendawy, S.; Hafez, E.M. Integrative effects of rice-straw biochar and silicon on oil and seed quality, yield and physiological traits of Helianthus annuus L. grown under water deficit stress. Agronomy 2019, 9, 637. [Google Scholar] [CrossRef]
- Aghili, F.; Gamper, H.A.; Eikenberg, J.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Schulin, R.; Jansa, J.; Frossard, E. Green manure addition to soil increases grain zinc concentration in bread wheat. PLoS ONE 2014, 9, e101487. [Google Scholar] [CrossRef]
- Abd El-Azeim, M.M.; Menesi, A.M.; Abd El-Mageed, M.M.; Lemanowicz, J.; Haddad, S.A. Wheat Crop Yield and Changes in Soil Biological and Heavy Metals Status in a Sandy Soil Amended with Biochar and Irrigated with Drainage Water. Agriculture 2022, 12, 1723. [Google Scholar] [CrossRef]
- Camaille, M.; Fabre, N.; Clément, C.; Barka, E.A. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, M.E.; Ghobadi, M.; Zebarjadi, A. Effect of waterlogging at different growth stages on some morphological traits of wheat varieties. Int. J. Biometeorol. 2017, 61, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Mfarrej, M.F.B.; Wang, X.; Saleem, M.H.; Hussain, I.; Rasheed, R.; Ashraf, M.A.; Iqbal, M.; Chattha, M.S.; Alyemeni, M.N. Hydrogen sulphide and nitric oxide mitigate the negative impacts of waterlogging stress on wheat (Triticum aestivum L.). Plant Biol. 2022, 24, 670–683. [Google Scholar] [CrossRef] [PubMed]
- El-Nady, M.A.; Shalaby, A.A. Impact of irrigation intervals and fertilization on actual evapotranspiration and wheat production. Egypt. J. Soil Sci. 2014, 54, 305–318. [Google Scholar]
- Fernandes, B.C.C.; Mendes, K.F.; Júnior, A.F.D.; Caldeira, V.P.D.; Teófilo, T.M.D.; Silva, T.S.; Mendonça, V.; Souza, M.D.; Silva, D.V. Impact of pyrolysis temperature on the properties of eucalyptus wood-derived biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef]
- Dewis, J.; Freitas, F. Physical and Chemical Methods of Soil and Water Analysis; FAO soils Bulletin, 10; FAO: Rome, Italy, 1970. [Google Scholar]
- De-leenher, L.; De-Boodt, M. Soil Physics; International Training Center for Post Graduate Soil Scientists (ITC-Ghent): Gent, Belgium, 1965. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis Advanced Course; Department of Soils, University of Wisconsin: Madison, WI, USA, 1967. [Google Scholar]
- U.S. Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils; USDA Agric. Handbook No. 60; U.S. Salinity Laboratory Staff: Washington, DC, USA, 1954. [Google Scholar]
- Allison, L.E. Organic carbon. In Methods of Soil Analysis: Part 2–Chemical and Microbiological Properties; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1367–1378. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc./Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Klute, A. Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- James, L.G. Principles of Farm Irrigation System Design; John Willey and Sons Inc.: New York, NY, USA, 1988. [Google Scholar]
- Klute, A.; Direksen, C. Hydraulic conductivity and diffusivity: Laboratory methods. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; Klute, A., Ed.; SSA Book Series; Springer: Berlin/Heidelberg, Germany, 1986; Volume 5, pp. 687–734. [Google Scholar]
- Giriappa, S. Water Use Efficiency in Agriculture; Oxford & IBH: Oxford, UK, 1983. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotraspiration Guidelines for Computing Crop Water Requirements; FAO Irrigation & drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Doorenbos, J.; Pruitt, W.O. Crop Water Requirements; FAO Irrigation and Drainage Paper 24; FAO: Rome, Italy, 1977. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Huang, M.; Fan, L.; Chen, J.; Jiang, L.; Zou, Y. Continuous applications of biochar to rice: Effects on nitrogen uptake and utilization. Sci. Rep. 2018, 8, 11461. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, X.; Wang, S.; Pu, X. Benefits of organic manure combined with biochar amendments to cotton root growth and yield under continuous cropping systems in Xinjiang, China. Sci. Rep. 2020, 10, 4718. [Google Scholar] [CrossRef]
- Cen, Z.; Wei, L.; Muthukumarappan, K.; Sobhan, A.; McDaniel, R. Assessment of a biochar-based controlled release nitrogen fertilizer coated with polylactic acid. J. Soil Sci. Plant Nutr. 2021, 21, 2007–2019. [Google Scholar] [CrossRef]
- Kong, C.; Camps-Arbestain, M.; Clothier, B.; Bishop, P.; Vázquez, F.M. Use of either pumice or willow-based biochar amendments to decrease soil salinity under arid conditions. Environ. Technol. Innov. 2021, 24, 101849. [Google Scholar] [CrossRef]
- El-Agrodi, M.W.M.; Saeid, M.M.; Ahmed, G.L.; Khalifa, T.S.H. Effect of soil moisture depletion and nitrogen levels on wheat (Triticum aestivum L.). J. Soil Sci. Agric. Eng. Mansoura Univ. 2016, 7, 169–178. [Google Scholar] [CrossRef]
- Andrenelli, M.C.; Maienza, A.; Genesio, L.; Miglietta, F.; Pellegrini, S.; Vaccari, F.P.; Vignozzi, N. Field application of pelletized biochar: Short term effect on the hydrological properties of a silty clay loam soil. Agric. Water Manag. 2016, 163, 190–196. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Does biochar application alleviate soil compaction? Review and data synthesis. Geoderma 2021, 404, 115317. [Google Scholar] [CrossRef]
- Mendes, J.D.; Fernandes, J.D.; Chaves, L.H.G.; Guerra, H.O.C.; Tito, G.A.; Chaves, I.D. Chemical and physical changes of soil amended with biochar. Wat. Air Soil Poll. 2021, 232, 338. [Google Scholar] [CrossRef]
- Toková, L.; Igaz, D.; Horák, J.; Aydin, E. Effect of biochar application and re-application on soil bulk density, porosity, saturated hydraulic conductivity, water content and soil water availability in a silty loam Haplic Luvisol. Agronomy 2020, 10, 1005. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Barnes, R.T.; Gallagher, M.E.; Gonnermann, H. Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar–sand mixtures. J. Hydrol. 2016, 533, 461–472. [Google Scholar] [CrossRef]
- Keshavarz Afshar, R.; Hashemi, M.; DaCosta, M.; Spargo, J.; Sadeghpour, A. Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Rani, P.; Singh, A.P.; Rai, S. Effect of rice husk biochar and lime treated sludge on NPK concentration and uptake by rice crop. Environ. Ecol. 2015, 33, 1218–1224. [Google Scholar]
- Fischer, B.M.C.; Manzoni, S.; Morillas, L.; Garcia, M.; Johnson, M.S.; Lyon, S.W. Improving agricultural water use efficiency with biochar—A synthesis of biochar effects on water storage and fluxes across scales. Sci. Total. Environ. 2019, 657, 853–862. [Google Scholar] [CrossRef]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Bakry, A.B.; Abdelraouf, R.E.; Ahmed, M.A.; El Karamany, M.F. Effect of drought stress and ascorbic acid foliar application on productivity and irrigation water use efficiency of wheat under newly reclaimed sandy soil. J. Appl. Sci. Res. 2012, 8, 4552–4558. [Google Scholar]
- Moussa, A.M.; Abdel-Maksoud, H.H. Effect of soil moisture regime on yield and its components and water use efficiency for some wheat cultivars. Ann. Agric. Sci. Ain Shams Univ. 2004, 49, 515–530. [Google Scholar]
- Rodríguez-Vila, A.; Forján, R.; Guedes, R.S.; Covelo, E.F. Changes on the phytoavailability of nutrients in a mine soil reclaimed with compost and biochar. Water Air Soil Pollut. 2016, 227, 453. [Google Scholar] [CrossRef]
- Abbas, S.; Javed, M.T.; Ali, Q.; Chaudhary, H.J.; Rizwan, M. Alteration of plant physiology by the application of biochar for remediation of organic pollutants. In Handbook of Bioremediation: Physiological, Molecular and Biotechnological Interventions; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Academic Press/Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 475–492. [Google Scholar]
- Zhu, Q.; Kong, L.; Shan, Y.; Yao, X.; Zhang, H.; Xie, F.; Xue, A.O. Effect of biochar on grain yield and leaf photosynthetic physiology of soybean cultivars with different phosphorus efficiencies. J. Integr. Agric. 2019, 18, 2242–2254. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M.; et al. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; Van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 53001. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Karam, F.; Breidy, J.; Stephan, C.; Rouphael, J. Evapotranspiration, yield and water use efficiency of drip irrigated corn in the Bekaa Valley of Lebanon. Agric. Water Manag. 2003, 63, 125–137. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef]
- Ali, A.B.; Haofang, Y.; Hong, L.; You, W.Y.; Elshaikh, N.A.; Hussein, G.; Pandab, S.; Hassan, S. Enhancement of depleted loam soil as well as cucumber productivity utilizing biochar under water stress. Commun. Soil Sci. Plant Anal. 2019, 50, 49–64. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Li, X.; Liu, F. Effects of biochar amendment, CO2 elevation and drought on leaf gas exchange, biomass production and water use efficiency in maize. Pak. J. Bot. 2018, 50, 1347–1353. [Google Scholar]
- Telahigue, D.; Yahia, L.B.; Aljane, F.; Belhouchett, K.; Toumi, L. Grain yield, biomass productivity and water use efficiency in quinoa (Chenopodium quinoa Willd.) under drought stress. J. Sci. Agric. 2017, 1, 222–232. [Google Scholar] [CrossRef]
- Aslam, M.U.; Raza, M.A.S.; Saleem, M.F.; Waqas, M.; Iqbal, R.; Ahmad, S.; Haider, I. Improving strategic growth stage-based drought tolerance in quinoa by rhizobacterial inoculation. Commun. Soil Sci. Plant Anal. 2020, 51, 853–868. [Google Scholar] [CrossRef]
- Licht, J.; Smith, N. The influence of lignocellulose and hemicellulose biochar on photosynthesis and water use efficiency in seedlings from a Northeastern, U.S. pine-oak ecosystem. J. Sustain. For. 2018, 37, 25–37. [Google Scholar] [CrossRef]
- Haider, I.; Raza, M.A.S.; Iqbal, R.; Aslam, M.U.; Habib-ur-Rahman, M.; Raja, S.; Khan, M.T.; Aslam, M.M.; Waqas, M.; Ahmad, S. Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. J. Saudi Chem. Soc. 2020, 24, 974–981. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; Dongli, S.; Zhipeng, L.; Elshaikh, N.A.; Guangcheng, S.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Björklund, M. Be careful with your principal components. Evolution 2019, 73, 2151–2158. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A.; Abd El-Razek, U.A.; Abd El-Hakim, A.F.; Hasham, M.M.A.; Sami, R.; Khojah, E.; AlMushhin, A.A.M. Growth, yield, quality, and phytochemical behavior of three cultivars of quinoa in response to moringa and Azolla extracts under organic farming conditions. Agronomy 2021, 11, 2186. [Google Scholar] [CrossRef]



| Month | Temperature (°C) | Wind Speed (m s−1) | Relative Humidity (%) | Average Precipitation (mm day−1) | Surface Pressure (kPa) | ||
|---|---|---|---|---|---|---|---|
| Max | Min | Max | Min | ||||
| 2020/2021 | |||||||
| November | 24.64 | 13.66 | 5.15 | 1.87 | 63.38 | 0.36 | 100.10 |
| December | 22.57 | 10.47 | 5.08 | 1.97 | 60.63 | 0.02 | 100.14 |
| January | 21.52 | 8.33 | 5.82 | 2.34 | 59.04 | 0.07 | 100.26 |
| February | 21.78 | 8.29 | 5.26 | 2.05 | 61.52 | 0.72 | 100.26 |
| March | 23.27 | 9.20 | 6.11 | 2.09 | 62.38 | 3.36 | 100.06 |
| April | 29.43 | 11.71 | 6.54 | 2.63 | 50.20 | 0.13 | 99.98 |
| 2021/2022 | |||||||
| November | 27.71 | 15.14 | 4.81 | 1.96 | 61.68 | 0.67 | 100.02 |
| December | 19.37 | 8.96 | 5.84 | 2.17 | 68.45 | 0.34 | 100.19 |
| January | 16.76 | 5.40 | 5.51 | 2.14 | 67.08 | 1.08 | 100.30 |
| February | 19.48 | 6.57 | 5.67 | 2.20 | 66.60 | 0.39 | 100.22 |
| March | 21.82 | 7.46 | 6.87 | 2.36 | 54.20 | 0.06 | 100.21 |
| April | 32.22 | 14.14 | 6.82 | 2.73 | 38.93 | 0.02 | 99.61 |
| Property | 2020/2021 | 2021/2022 | Property | 2020/2021 | 2021/2022 |
|---|---|---|---|---|---|
| Particle size distribution | Field capacity (%) | 9.98 | 9.92 | ||
| Coarse sand (%) | 51.36 | 51.36 | Permanent wilting point (%) | 3.73 | 3.75 |
| Fine sand (%) | 37.75 | 37.75 | Available water (%) | 6.25 | 6.17 |
| Silt (%) | 4.96 | 4.96 | Water holding capacity (%) | 15.71 | 15.73 |
| Clay (%) | 5.93 | 5.93 | Bulk density (Mg m−3) | 1.73 | 1.71 |
| Textural class | Sandy | Sandy | Total porosity (%) | 34.72 | 35.47 |
| Ks (m day−1) | 10.17 | 10.17 | Void ratio | 0.53 | 0.55 |
| Pore size distribution | Soil pH (1:2.5) * | 8.47 | 8.45 | ||
| QDP (%) | 63.03 | 63.09 | ECe ** (dSm−1) | 0.82 | 0.79 |
| SDP (%) | 33.65 | 33.52 | Organic carbon (g kg−1) | 2.59 | 2.58 |
| WHP (%) | 1.96 | 2.05 | Organic matter (g kg−1) | 4.45 | 4.44 |
| FCP (%) | 1.35 | 1.34 | CaCO3 content (g kg−1) | 13.71 | 13.74 |
| Soluble cations | Soluble anions | ||||
| Ca+ + (mmolc L−1) | 1.86 | 2.02 | CO3− (mmolc L−1) | 0.00 | 0.00 |
| Mg+ + (mmolc L−1) | 3.25 | 3.57 | HCO3− (mmolc L−1) | 5.20 | 4.98 |
| Na+ (mmolc L−1) | 2.17 | 1.64 | Cl− (mmolc L−1) | 2.00 | 1.91 |
| K+ (mmolc L−1) | 0.90 | 0.70 | SO4− (mmolc L−1) | 0.98 | 1.04 |
| pH * | EC ** (dSm−1) | Organic Carbon (g kg−1) | Organic Matter (g kg−1) | CEC (cmolc kg−1) | C/N Ratio | Total N (g kg−1) | Total P (g kg−1) | Total K (g kg−1) |
|---|---|---|---|---|---|---|---|---|
| 8.11 | 1.92 | 213.10 | 366.53 | 56.21 | 20.1:1 | 10.60 | 2.50 | 3.60 |
| S.O.V | D.F | Bulk Density | Total Porosity | Void Ratio | Hydraulic Conductivity | ||
|---|---|---|---|---|---|---|---|
| (Mg m−3) | (%) | (m day−1) | |||||
| Year (Y) | 1 | 0.0004 | 0.507 | 0.0004 | 0.192 | ||
| R(Y) | 4 | 0.0001 | 0.159 | 0.0001 | 0.189 | ||
| Biochar (B) | 2 | 0.0386 * | 54.95 * | 0.0386 * | 28.68 ** | ||
| B × Y | 2 | 0.0004 | 0.607 | 0.0004 | 0.015 | ||
| Residual | 8 | 0.0003 | 0.421 | 0.0003 | 0.054 | ||
| S.O.V | D.F | Pore Size Distribution (%) | |||||
| QDP | SDP | WHP | FCP | ||||
| Year (Y) | 1 | 0.01 | 0.000 | 0.012 | 0.00002 | ||
| R(Y) | 4 | 0.08 | 0.322 | 0.092 | 0.03733 | ||
| Biochar (B) | 2 | 223.63 ** | 116.75 ** | 6.88 ** | 2.46 ** | ||
| B × Y | 2 | 0.02 | 0.020 | 0.004 | 0.0003 | ||
| Residual | 8 | 0.89 | 1.233 | 0.134 | 0.0213 | ||
| S.O.V | D.F | WHC | Field Capacity | Permanent Wilting Point | Available Water | ||
| (%) | |||||||
| Year (Y) | 1 | 0.009 | 0.001 | 0.0027 | 0.006 | ||
| R(Y) | 4 | 0.158 | 0.092 | 0.1616 | 0.393 | ||
| Biochar (B) | 2 | 35.32 ** | 22.20 ** | 1.72 ** | 36.05 ** | ||
| B × Y | 2 | 0.002 | 0.003 | 0.0004 | 0.002 | ||
| Residual | 8 | 0.289 | 0.166 | 0.0353 | 0.182 | ||
| S.O.V | D.F | IS | DS | MS | LS | ETa | |
| (m3 ha−1) | |||||||
| Year (Y) | 1 | 0.22 | 53.59 | 1781.22 | 78.34 | 1693.5 | |
| R (Y) | 4 | 675.23 | 175.63 | 171.40 | 47.81 | 2048.0 | |
| Irrigation (I) | 2 | 13,345.7 ** | 1,396,194.8 ** | 4,887,211.4 ** | 286,161 ** | 16,093,538.7 ** | |
| Y × I | 2 | 17.61 | 8.25 | 567.81 | 6.08 | 795.7 | |
| Biochar (B) | 2 | 6236.8 ** | 50,872.7 ** | 139,386.2 ** | 53,893.7 ** | 823,830.4 ** | |
| Y × B | 2 | 50.49 | 262.34 | 423.07 | 80.37 | 1507.0 | |
| I × B | 4 | 340.52 ** | 12,572.3 ** | 33,686.9 ** | 9352.3 ** | 125,769.8 ** | |
| Y × I × B | 4 | 16.95 | 119.63 | 493.48 | 16.85 | 832.8 | |
| Residual | 32 | 98.44 | 421.31 | 1064.92 | 288.74 | 2101.7 | |
| S.O.V | D.F | PH | SL | NGS | NS m−2 | T-GW | |
| (cm) | (cm) | (g) | |||||
| Year (Y) | 1 | 228.17 | 0.01 | 0.30 | 1380.17 | 10.73 | |
| R (Y) | 4 | 12.15 | 1.96 | 13.74 | 71.37 | 4.89 | |
| Irrigation (I) | 2 | 1188.96 * | 60.27 ** | 840.57 * | 2854.6 * | 246.86 * | |
| Y × I | 2 | 16.89 * | 0.28 | 8.91 | 58.50 | 4.04 | |
| Biochar (B) | 2 | 2412.80 ** | 88.85 ** | 672.30 ** | 2433.4 ** | 558.1 ** | |
| Y × B | 2 | 7.17 | 0.03 | 6.74 | 22.89 | 5.56 | |
| I × B | 4 | 54.30 * | 2.13 ** | 19.77 * | 53.35 * | 9.21 * | |
| Y × I × B | 4 | 5.06 | 0.04 | 1.60 | 7.39 | 1.13 | |
| Residual | 32 | 4.71 | 0.45 | 4.41 | 31.10 | 3.21 | |
| S.O.V | D.F | BY | SY | GY | HI | CI | WUE |
| (t ha−1) | (%) | (kg m−3) | |||||
| Year (Y) | 1 | 0.411 | 0.211 | 0.033 | 0.03 | 0.17 | 0.0033 |
| R (Y) | 4 | 0.101 | 0.029 | 0.060 | 1.41 | 6.82 | 0.0035 |
| Irrigation (I) | 2 | 31.582 ** | 9.484 ** | 6.459 ** | 25.68 ** | 125.9 ** | 0.243 ** |
| Y × I | 2 | 0.001 | 0.001 | 0.001 | 0.01 | 0.04 | 0.0001 |
| Biochar (B) | 2 | 14.161 ** | 2.253 ** | 5.198 ** | 57.17 ** | 273.8 ** | 0.108 ** |
| Y × B | 2 | 0.004 | 0.000 | 0.002 | 0.04 | 0.17 | 0.0005 |
| I × B | 4 | 0.640 ** | 0.187 ** | 0.232 ** | 2.87 * | 14.64 ** | 0.0034 * |
| Y × I × B | 4 | 0.002 | 0.002 | 0.005 | 0.19 | 0.90 | 0.0003 |
| Residual | 32 | 0.070 | 0.024 | 0.031 | 0.79 | 3.57 | 0.0026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanem, K.Z.; Hasham, M.M.A.; El-Sheshtawy, A.-N.A.; El-Serafy, R.S.; Sheta, M.H. Biochar Stimulated Actual Evapotranspiration and Wheat Productivity under Water Deficit Conditions in Sandy Soil Based on Non-Weighing Lysimeter. Plants 2022, 11, 3346. https://doi.org/10.3390/plants11233346
Ghanem KZ, Hasham MMA, El-Sheshtawy A-NA, El-Serafy RS, Sheta MH. Biochar Stimulated Actual Evapotranspiration and Wheat Productivity under Water Deficit Conditions in Sandy Soil Based on Non-Weighing Lysimeter. Plants. 2022; 11(23):3346. https://doi.org/10.3390/plants11233346
Chicago/Turabian StyleGhanem, Kholoud Z., Mostafa M. A. Hasham, Abdel-Nasser A. El-Sheshtawy, Rasha S. El-Serafy, and Mohamed H. Sheta. 2022. "Biochar Stimulated Actual Evapotranspiration and Wheat Productivity under Water Deficit Conditions in Sandy Soil Based on Non-Weighing Lysimeter" Plants 11, no. 23: 3346. https://doi.org/10.3390/plants11233346
APA StyleGhanem, K. Z., Hasham, M. M. A., El-Sheshtawy, A.-N. A., El-Serafy, R. S., & Sheta, M. H. (2022). Biochar Stimulated Actual Evapotranspiration and Wheat Productivity under Water Deficit Conditions in Sandy Soil Based on Non-Weighing Lysimeter. Plants, 11(23), 3346. https://doi.org/10.3390/plants11233346






