Maltodextrin-Coated Peppermint and Caraway Essential Oils Effects on Soil Microbiota
Abstract
1. Introduction
2. Results
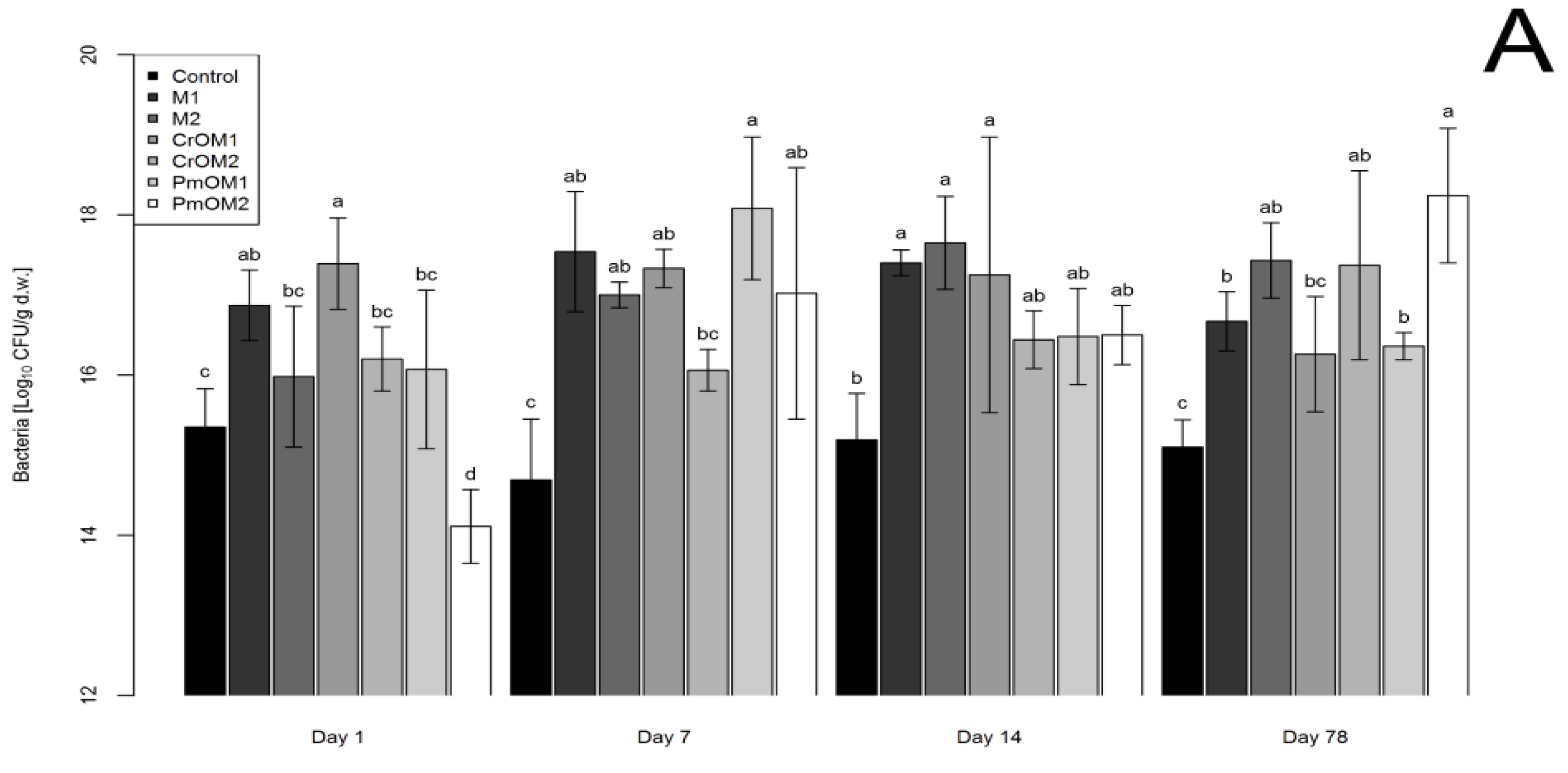
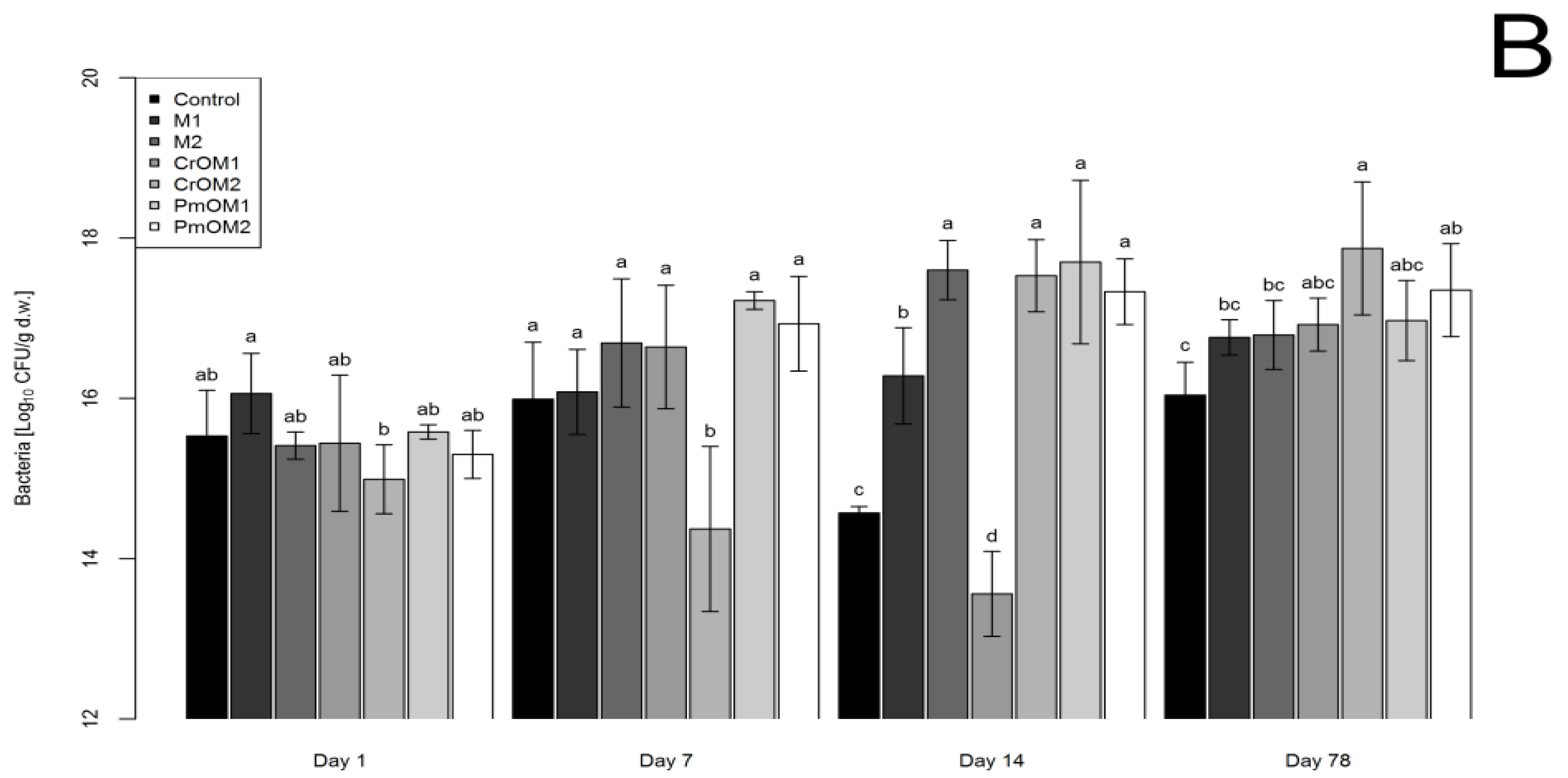
2.1. Effects on Bacteria
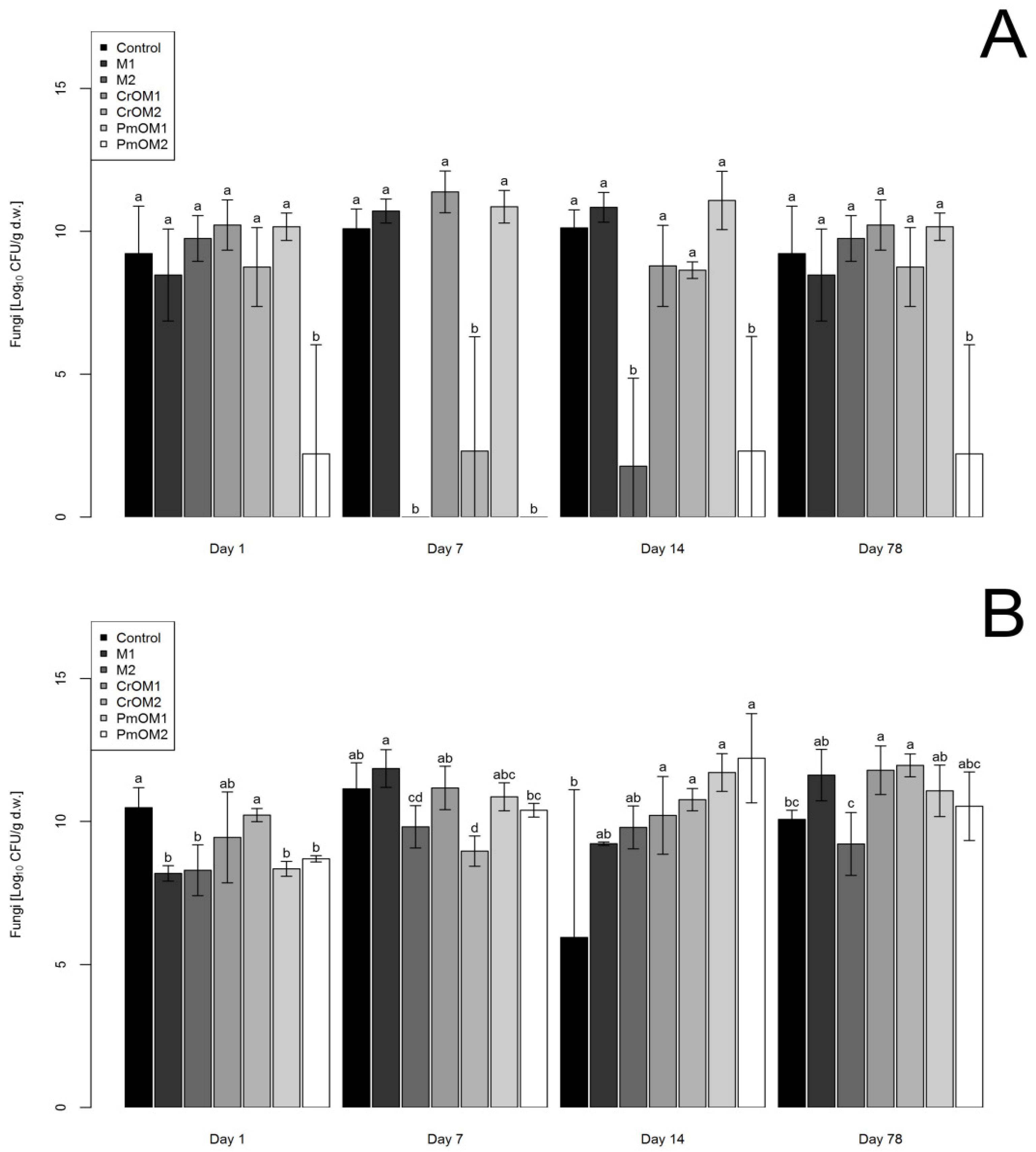
2.2. Effects on Fungi
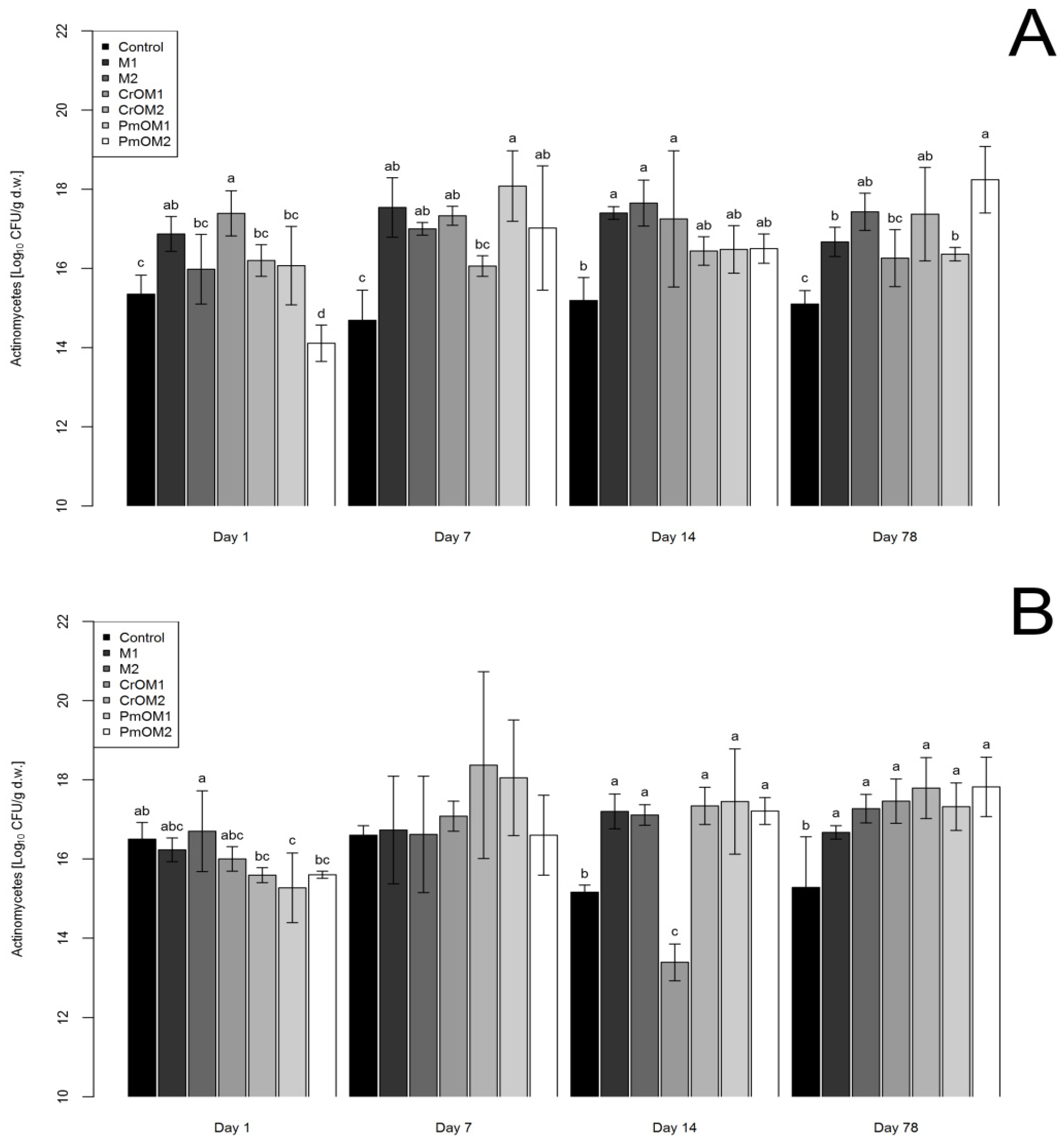
2.3. Effects on Actinomycetes
3. Discussion
4. Materials and Methods
Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Bian, Y.; Ali, B.; Jamil, A.; Majeed, U.; Khan, Q.; Iqbal, K.; Shoemaker, C.; Fang, Z. Essential oil encapsulations: Uses, procedures, and trends. RSC Adv. 2015, 5, 58449–58463. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, I.; Paunescu, A. The application of essential oils as a next-generation of pesticides: Recent developments and future perspectives. Z. Nat. 2020, 75, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Srivastava, S.; Nigam, N.; Singh, A.K.; Singh, S. Impact of essential oils of E. citriodora, O. basilicum and M. arvensis on three different weeds and soil microbial activities. Environ. Technol. Innov. 2019, 14, 100343. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- Enascuta, C.; Stepan, E.; Oprescui, E.; Radui, A.; Alexandrescu, E.; Stoica, R.; Epure, D.; Niculescu, M. Microencapsulation of Essential Oils. Rev. Chim. 2018, 69, 1612–1615. [Google Scholar] [CrossRef]
- Bakry, A.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Hofman, D.L.; Van Buul, V.J.; Brouns, F.J. Nutrition, health, and regulatory aspects of digestible maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release–a review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Sobolewska-Zielińska, J.; Fortuna, T. Retrogradation of starches and maltodextrins of various origin. Acta Sci. Pol. Technol. Aliment. 2010, 9, 71–81. [Google Scholar]
- Kalemba, D.; Synowiec, A. Agrobiological interactions of essential oils of two menthol mints: Mentha piperita and Mentha arvensis. Molecules 2019, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Deng, Z.W. Insecticidal activity of essential oil of Carumcarvi fruits from China and its main components against two grain storage insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef] [PubMed]
- Możdżeń, K.; Krajewska, A.; Bocianowski, J.; Jop, B.; Synowiec, A. Microencapsulated Caraway Essential Oil Affects Initial Growth of Maize Cultivars. Molecules 2021, 26, 5059. [Google Scholar] [CrossRef]
- Synowiec, A.; Lenart-Boroń, A.; Bocianowski, J.; Lepiarczyk, A.; Kalemba, D. How soil-applied maltodextrin with caraway (Carum carvi L.) oil affects weed and soil microbiological activity in maize (Zea mays L.) stands. Pol. J. Environ. Stud. 2019, 29, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Bocianowski, J.; Krajewska, A. The phytotoxicity of microencapsulated peppermint oil on maize (Zea mays L.) depending on the type of growth substrate and maize cultivar. Agronomy 2020, 10, 1302. [Google Scholar] [CrossRef]
- Synowiec, A.; Krajewska, A. Soil or vermiculite-applied microencapsulated peppermint oil effects on white mustard initial growth and performance. Plants 2020, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Rita, P.; Animesh, D.K. An updated overview on peppermint (Mentha piperita L.). Int. Res. J. Pharm. 2011, 2, 1–10. [Google Scholar]
- Loolaie, M.; Moasefi, N.; Rasouli, H.; Adibi, H. Peppermint and its functionality: A review. Arch. Clin. Microbiol. 2017, 8, 1–16. [Google Scholar]
- Singh, P.; Pandey, A.K. Prospective of Essential Oils of the Genus Mentha as Biopesticides: A Review. Front. Plant Sci. 2018, 9, 1295. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N. Chemical composition and antimicrobial activity of peppermint (Mentha piperita L.) Essential oil. Songklanakarin J. Sci. Technol. 2014, 36, 83–87. [Google Scholar]
- Laribi, B.; Bettaieb, I.; Kouki, K.; Sahli, A.; Mougou, A.; Marzouk, B. Water deficit effects on caraway (Carum carvi L.) growth, essential oil and fatty acid composition. Ind. Crops Prod. 2009, 30, 372–379. [Google Scholar] [CrossRef]
- Seidler-Łożykowska, K.; Kędzia, B.; Karpińska, E.; Bocianowski, J. Microbiological activity of caraway (Carum carvi L.) essential oil obtained from different origin. Acta Scientiarum. Agronomy 2013, 35, 495–500. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef]
- Simic, A.; Rančic, A.; Sokovic, M.D.; Ristic, M.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Essential oil composition of Cymbopogon winterianus. and Carum carvi. and their antimicrobial activities. Pharm. Biol. 2008, 46, 437–441. [Google Scholar] [CrossRef]
- Gibbons, S.M.; Scholz, M.; Hutchison, A.L.; Dinner, A.R.; Gilbert, J.A.; Coleman, M.L. Disturbance regimes predictably alter diversity in an ecologically complex bacterial system. mBio 2016, 7, 1620. [Google Scholar] [CrossRef]
- Konstantinou, S.; Monokrousos, N.; Kapagianni, P.; Menkissoglu-Spiroudi, U.; Gwynn-Jones, D.; Stamou, G.P.; Papatheodorou, E.M. Instantaneous responses of microbial communities to stress in soils pretreated with Mentha spicata essential oil and/or inoculated with arbuscular mycorrhizal fungus. Ecol. Res. 2019, 701–710. [Google Scholar] [CrossRef]
- Synowiec, A.; Hano, C. Phytotoxic and microbiological activities of soil-applied microencapsulated peppermint (Mentha x piperita L.) essential oil. LE STUDIUM Interdiscip. J. 2019, 3, 21–24. [Google Scholar]
- Weyman-Kaczmarkowa, W.; Pędziwilk, Z. The development of fungi as affected by pH and type of soil, in relation to the occurrence of bacteria and soil fungistatic activity. Microbiol. Res. 2000, 155, 107–112. [Google Scholar] [CrossRef]
- Sveshnikova, A.A.; Polyanskaya, L.M.; Lukin, S.M. The effect of tillage and mesorelief on the structure of soil microbial cenoses. Microbiology 2001, 70, 484–491. [Google Scholar] [CrossRef]
- Filip, Z. International approach to assessing soil quality by ecologically-related biological parameters. Agric. Ecosyt. Environ. 2002, 88, 169–174. [Google Scholar] [CrossRef]
- Rousk, J.; Nadkarni, N.M. Growth measurements of saprotrophic fungi and bacteria reveal differences between canopy and forest floor soils. Soil Biol. Biochem. 2009, 41, 862–865. [Google Scholar] [CrossRef]
- Vieira, F.C.S.; Nahas, E. Comparison of microbial numbers in soils by using various culture media and temperatures. Microbiol. Res. 2005, 160, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Procariote the unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Ainalidou, A.; Bouzoukla, F.; Menkissoglu-Spiroudi, U.; Vokou, D.; Karamanoli, K. Impacts of Decaying Aromatic Plants on the Soil Microbial Community and on Tomato Seedling Growth and Metabolism: Suppression or Stimulation. Plants 2021, 10, 1848. [Google Scholar] [CrossRef]
- Vokou, D.; Chalkos, D.; Karamanlidou, G.; Yiangou, M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J. Chem. Ecol. 2002, 28, 755–768. [Google Scholar] [CrossRef]
- Chalkos, D.; Karamanoli, K.; Vokou, D. Monoterpene Enrichments Have Positive Impacts on Soil Bacterial Communities and the Potential of Application in Bioremediation. Plants 2021, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Bail, S.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E.; Geissler, M. Antimicrobial Activities of Essential Oils of Mint and Peppermint as Well as Some of Their Main Compounds. J. Essent. Oil Res. 2009, 21, 363–366. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.; Vajs, V.; Van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hassani, A.; Ghosta, Y.; Javadi, T.; Meshkatalsadat, M.H. Essential oils as control agents of postharvest Alternaria and Penicillium rots on tomato fruits. J. Food Saf. 2010, 30, 341–352. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Mohammadi, S. Essential oils to control Botrytis cinerea in vitro and in vivo on plum fruits. J. Sci. Food Agric. 2012, 93, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.M.; Badeaa, R.I. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Kadoglidou, K.; Lagopodi, A.; Karamanoli, K.; Vokou, D.; Bardas, G.A.; Menexes, G.; Constantinidou, H.I.A. Inhibitory and stimulatory effects of essential oils and individual monoterpenoids on growth and sporulation of four soil-borne fungal isolates of Aspergillus terreus, Fusarium oxysporum, Penicillium expansum, and Verticillium dahliae. Eur. J. Plant Pathol. 2011, 130, 297–309. [Google Scholar] [CrossRef]
- Karamanoli, K.; Ainalidou, A.; Bouzoukla, F.; Vokou, D. Decomposition profiles of leaf essential oils in the soil environment. Ind. Crops Prod. 2018, 124, 397–401. [Google Scholar] [CrossRef]
- PN ISO 10390: 1997; Soil Quality-Determination of pH. Polish Committee for Standardization (PN): Warsaw, Poland, 1997.
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plant Properties. A Catalogue; Institute of Environmental Protection, National Research Institute: Warsaw, Poland, 1991. [Google Scholar]
- PN-ISO 11465; Soil quality-Determination of soil dry matter content and soil water based on soil dry matter weight-Weight method. Polish Committee for Standardization: Warsaw, Poland, 1999.
- Pepper, I.L.; Gerba, C.P. Environmental Microbiology. A Laboratoy Manual, 2nd ed.; Elsevier AP: Amsterdam, The Netherlands, 2004; p. 209. [Google Scholar]
- Atlas, R.M.; Parks, L.C. Handbook of Microbiological Media; CRC Press: Boca Raton, NJ, USA, 1997; p. 1706. [Google Scholar]
- Chmiel, M. Microbiological characteristics and sanitary evaluation of the natural environment at the Ojcowski National Park with particular emphasis on anthropogenic pressure. Zesz. Nauk. UR w Krakowie 2013, 382, 200. (In Polish) [Google Scholar]
- Bocianowski, J.; Kozak, M.; Liersch, A.; Bartkowiak-Broda, I. A heuristic method of searching for interesting markers in terms of quantitative traits. Euphytica 2011, 181, 89–100. [Google Scholar] [CrossRef]
| Group of Microorganisms | Sandy Soil | Loamy Soil |
|---|---|---|
| [CFU/g DW of Soil] | [CFU/g DW of Soil] | |
| Bacteria | 5.6 × 106 | 7 × 106 |
| Actinomycetes | 4 × 106 | 10 × 106 |
| Fungi | 2.7 × 104 | 3.9 × 104 |
| Bacteria | Fungi | Actinomycetes | |||||
|---|---|---|---|---|---|---|---|
| Additive | Soil | Model | R2 [in %] | Model | R2 [in %] | Model | R2 [in %] |
| Control | Loamy | y = 0.0051x2 − 0.3855x + 8.6848 | 16.7 | y = −0.0043x2 + 0.2546x + 30.27 | 0.75 | y = 0.0114x2 − 1.0501x + 18.686 | 52.13 |
| Control | Sandy | y = −0.0035x2 + 0.3265x + 3.0204 | 48.27 | y = 37.558x−0.258 | 19.6 | y = −0.233ln(x) + 4.5192 | 4.44 |
| M1 | Loamy | y = −0.0018x2 + 0.2652x + 9.4873 | 39.2 | y = −0.3391x2 + 28.238x + 1.0399 | 43.6 | y = −0.0235x2 + 1.8749x + 13.908 | 14.15 |
| M1 | Sandy | y = −0.023x2 + 1.9201x + 2.1614 | 45.62 | y = −0.0544x2 + 4.228x + 11.776 | 57.49 | y = −0.019x2 + 1.359x + 27.509 | 28.64 |
| M2 | Loamy | y = −0.0456x2 + 3.8267x − 0.2736 | 69.76 | y = 6.0015x0.1939 | 12.72 | y = 16.225x0.1436 | 7.88 |
| M2 | Sandy | y = −0.0581x2 + 5.1497x − 6.3094 | 50.13 | y = 0.0421x2 − 3.4406x + 33.904 | 42.77 | y = −0.0418x2 + 3.7111x + 4.9978 | 58.42 |
| CrOM1 | Loamy | y = 0.0087x2 − 0.5434x + 12.351 | 33.26 | y = −0.0096x2 + 2.3335x + 35.512 | 48.47 | y = 0.015x2 − 0.8572x + 17.551 | 50.44 |
| CrOM1 | Sandy | y = 8.4257x0.1483 | 7.28 | y = 0.0145x2 − 1.3838x + 55.557 | 2.65 | y = 44.896x−0.25 | 17.79 |
| CrOM2 | Loamy | y = 2.0117x0.75 | 50.81 | y = 0.0363x2 − 1.0267x + 23.899 | 86.11 | y = 11.176x0.4669 | 27.74 |
| CrOM2 | Sandy | y = −0.0328x2 + 2.8279x − 5.8971 | 50.64 | y = 0.002x2 − 0.0903x + 4.7247 | 24.94 | y = 0.0057x2 + 0.1382x + 10.328 | 34.8 |
| PmOM1 | Loamy | y = −0.0647x2 + 5.3902x − 1.1058 | 56.93 | y = 6.5295x0.598 | 52.54 | y = 8.9653x0.4534 | 25.79 |
| PmOM1 | Sandy | y = −0.0117x2 + 0.8046x + 15.638 | 11.44 | y = −0.093x2 + 7.5897x + 1.5533 | 50.91 | y = −0.0097x2 | 8.53 |
| PmOM2 | Loamy | y = 6.2719x0.4813 | 65.66 | y = −0.1445x2 + 12.252x − 19.2 | 57.06 | y = 6.2305x0.5203 | 69.06 |
| PmOM2 | Sandy | y = 2.2999x0.9165 | 85.53 | y = 0.0028x2 − 0.253x + 2.7545 | 58.62 | y = 1.8474x0.9122 | 71.4 |
| Characteristics | Peppermint Oil | Caraway oil |
|---|---|---|
| Content of oil (v/w %) | 9.6 | 6.5 |
| (−)-Menthone (%) | 20.6 | -- |
| (−)-Menthol (%) | 60.1 | -- |
| D-Limonene (%) | -- | 15.2 |
| S-(+)-Carvone (%) | -- | 79.9 |
| Characteristics | Sandy Soil | Loamy Soil |
|---|---|---|
| pH KCl | 6.8 | 6.5 |
| Ca (g kg−1) | 0.94 | 2.45 |
| K (g kg−1) | 0.49 | 1.65 |
| P (g kg−1) | 0.22 | 0.45 |
| Mg (g kg−1) | 0.36 | 1.68 |
| C organic (g kg−1) | 13.25 | 20.11 |
| Soil dry matter (%) | 87.9 | 87.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel, M.; Drzymała, G.; Bocianowski, J.; Komnenić, A.; Baran, A.; Synowiec, A. Maltodextrin-Coated Peppermint and Caraway Essential Oils Effects on Soil Microbiota. Plants 2022, 11, 3343. https://doi.org/10.3390/plants11233343
Chmiel M, Drzymała G, Bocianowski J, Komnenić A, Baran A, Synowiec A. Maltodextrin-Coated Peppermint and Caraway Essential Oils Effects on Soil Microbiota. Plants. 2022; 11(23):3343. https://doi.org/10.3390/plants11233343
Chicago/Turabian StyleChmiel, Maria, Gabriela Drzymała, Jan Bocianowski, Andreja Komnenić, Agnieszka Baran, and Agnieszka Synowiec. 2022. "Maltodextrin-Coated Peppermint and Caraway Essential Oils Effects on Soil Microbiota" Plants 11, no. 23: 3343. https://doi.org/10.3390/plants11233343
APA StyleChmiel, M., Drzymała, G., Bocianowski, J., Komnenić, A., Baran, A., & Synowiec, A. (2022). Maltodextrin-Coated Peppermint and Caraway Essential Oils Effects on Soil Microbiota. Plants, 11(23), 3343. https://doi.org/10.3390/plants11233343









