Abstract
The environmental and health risks associated with synthetic pesticides have increased the demand for botanical insecticides as safer and biodegradable alternatives to control insect pests in agriculture. Hence in this study, five Meliaceae species were evaluated for their insecticidal activities against the Spodoptera frugiperda and the Plutella xylostella larvae, as well as their chemical constituents. Repellence, feeding deterrence, and topical application bioassays were employed to evaluate their insecticidal activities. GC-MS analysis was performed to identify chemical compounds present in each plant. The repellence bioassay indicated that Melia azedarach extracts exhibited the highest repellence percentage against S. frugiperda (95%) and P. xylostella (90%). The feeding deterrence bioassay showed that M. azedarach and Trichilia dregeana extracts displayed excellent antifeeding activity against the S. frugiperda (deterrent coefficient, 83.95) and P. xylostella (deterrent coefficient, 112.25), respectively. The topical application bioassay demonstrated that Ekebergia capensis extracts had the highest larval mortality against S. frugiperda (LD50 0.14 mg/kg). Conversely, M. azedarach extracts showed the highest larval mortality against P. xylostella (LD50 0.14 mg/kg). GC-MS analysis revealed that all plant extracts had compounds belonging to the two noteworthy groups (phenols and terpenes), which possess insecticidal properties. Overall, this study lends scientific credence to the folkloric use of Meliaceae species as potential biocontrol agents against insect pests.
1. Introduction
The agricultural sector has always been faced with challenges due to insect pests and will continue to do so in the future [1]. These pests damage crops during the growing period, and they may also subsequently cause damage to the harvested products stored in storehouses [2]. Controlling insect pests remains a problem as the insects keep building resistance to common pesticides while, on the other hand, toxic pesticides are being removed from the markets [1]. Synthetic pesticides have been commonly used and are considered a highly effective means of controlling plant damage caused by insects [3], which leads to remarkable improvements in plant yield productivity [4]. However, the indiscriminate and haphazard usage of synthetic pesticides has adversely affected human health and the ecosystem as a whole [5].
The presence of pesticide residues in foods, fruits, vegetables, and even in breast-feeding mothers’ milk creates a threat to human health. In developing countries, nearly 3 million farmworkers experience severe pesticide poisoning, resulting in about 18,000 deaths, while 25 million workers suffer from mild pesticide poisoning each year [6]. The use of synthetic pesticides also raises several environmental concerns because over 5% of the sprayed synthetic pesticides do not reach their target insect pests; instead, they can be found in air, soil, and water streams [7]. As a result of these devastating occupational synthetic pesticide poisoning cases, research to find alternative methods that are environmentally friendly and cost-effective in controlling insect pests has increased [1].
Based on recent studies to find ways to mitigate problems caused by synthetic pesticides, natural bio-insecticides from medicinal plants can be an excellent alternative strategy to overcome pest resistance and environmental contamination [8]. This possibility is not surprising as plants are rich sources of bioactive chemicals, and botanical insecticides have been reported to have fewer adverse effects on the environment or human health [1].
Meliaceae is one of two flowering plant families that have gained considerable attention, whereby systematic investigations of its members for their insecticidal potential have been undertaken [9,10]. Chemicals extracted from members of the Meliaceae have received attention recently from applied entomologists due to their excellent properties as control agents for insects [11]. This knowledge has prompted the interest to assess other family members for their insecticidal and antifeedant properties in this study.
Plutella xylostella (L.) (Lepidoptera: Plutellidae), commonly known as the diamondback moth (DBM) or the cabbage moth, is an economically important pest of cruciferous plants globally [12]. The Diamondback moth is an oligophagous insect that mainly feeds on cole crops, including broccoli, brussels sprouts, canola, cauliflower, and cabbage, which are of essential economic value [13]. The insect is important in agriculture as causes yield losses of as much as 100% [14]. In the 1970s, there was a major outbreak of DBM, mainly due to the development of resistance to synthetic insecticides [13]. It has been estimated that the yield losses and control associated with diamondback moth globally ranged between 4–5 US billion dollars yearly [14]. In sub-Saharan Africa, crop losses due to diamondback moths have been reported to be between 8–22% in the field [15].
Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), commonly known as the fall armyworm (FAW), is a polyphagous insect that is important in agriculture as it is difficult to control and, as a result, causes a lot of damage [16]. This migratory insect also causes enormous economic losses, mainly attacking crops that form part of the primary staple food [17], including rice, maize, forage grasses, sorghum, alfalfa, vegetable crops, and many others [16]. The first case to be reported in Africa of the fall armyworm was in late 2016, when it attacked most West African farms and subsequently spread throughout the continent rapidly and is now found in 44 African countries [18]. Environmentally friendly and effective methods to control fall armyworms are crucial as these insects are heavy foliage feeders [17] and can result in the total loss of crops. In sub-Saharan Africa, maize, rice, sorghum, and sugarcane crop damage is estimated to cause up to USD 13 billion yearly [19].
Ever since plant-derived products have gained increased attention from researchers to assess their insecticidal properties, more than 2000 plant species have been recorded to be used traditionally as insecticides [20]. However, many studies that attempted to validate these properties scientifically are incomplete; the bioassays procedures used were usually inappropriate or inadequate [21]. As a result, biological compounds that are potentially useful remain uninvestigated, undiscovered, underutilized, or undeveloped from this reservoir of unstudied plant materials [22]. Hence, in this study, four Meliaceae species that had previously not been evaluated extensively for their insecticidal and antifeedant properties against the test insects S. frugiperda and P. xylostella were selected. Melia azedarach was chosen as a positive control as it is a well-known bioinsecticide plant. Water extracts were selected for extraction in this study because it is one of the simplest and safest (non-toxicity) solvents. In addition, aqueous plant extracts are traditionally used to control insect pests. Using aqueous extracts is fitting because the main purpose of this study is to identify safer, cost-effective, and renewable alternative methods to synthetic pesticides. Slightly polar acetone and ethanol extracts were selected because the main targeted compounds, limonoids (terpenes), were reported to have a higher solubility in polar solvents and alcohol [23].
2. Results
2.1. Antifeedant and Insecticidal Analysis
2.1.1. Repellence Test
Repellence Bioassay against S. frugiperda Larvae
Table 1 indicates the results of the repellence bioassay test of the five selected Meliaceae species against the S. frugiperda. Positive average percentage repulsion values exhibit repellence, and negative average percentage repulsion values exhibit attractancy. Plant extracts that are ranked in higher classes (i.e., III, IV, and V) are considered to have a high repellence against the larvae, and those that are ranked to lower classes (I and II) have partial repellence against the larvae. Aqueous and ethanolic extracts of Melia azedarach L. and Trichilia dregeana Harv. & Sond. acetone extracts were found to have strongly repelled the S. frugiperda larvae with repellence of 95%, 65%, and 71%, and they belonged to class V, IV, and IV, respectively. It is followed by aqueous extracts of Turraea floribunda Hochst. (49%) belonging to class III. Aqueous and ethanol extracts of T. dregeana and ethanolic extracts of Turraea obtusifolia Hochst. moderately repelled the S. frugiperda with repellence of 40%, 30%, and 30%, respectively. Aqueous and acetone extracts of T. obtusifolia were recorded to have the lowest repellency (3% and 5%) and were all assigned to the lowest class (I). Ethanolic extracts of T. floribunda (−55%) indicated S. frugiperda larvae stimulation.

Table 1.
Average repellence of five Meliaceae species leaf extracts against Spodoptera frugiperda larvae using the treated filter paper test.
Repellence Bioassay against P. xylostella Larvae
Table 2 indicates the average percentage repulsion for the five Meliaceae species screened for their repellence activity against P. xylostella larvae. The overall highest percentage repulsion against the P. xylostella larvae was recorded for the aqueous (90%) and ethanol (80%) extracts of Melia azedarach, meaning that they exhibited excellent repellent activity, hence they were assigned to classes V and IV, respectively. Good repellent activity against P. xylostella was also recorded for acetone (65%) and ethanol (65%) extracts of T. dregeana, and they were assigned to class IV. Extracts of E. capensis moderately repelled P. xylostella larvae with repellence of 60% (aqueous), 50% (acetone), and 50% (ethanol), assigned to class III. All extracts of T. obtusifolia, i.e., aqueous (15%), acetone (30%), and ethanol (31%), recorded the lowest repellent activities against P. xylostella. Ethanolic extracts of T. floribunda (−10%) indicated the P. xylostella larvae stimulation.

Table 2.
Average repellence of five Meliaceae species leaf extracts against Plutella xylostella larvae using the treated filter paper test.
2.1.2. Feeding Deterrence Test
Feeding Deterrence Activity of S. frugiperda Larvae
Table 3 indicates the feeding deterrent activity coefficients of Meliaceae species against the fall armyworm larvae. All extracts exhibited feeding activity against the larvae to a certain extent, except for the ethanolic extracts of T. floribunda, which were found to have inert antifeedant compounds against the S. frugiperda larvae with a feeding deterrent coefficient of −12.89. Of all the tested extracts, aqueous extracts of M. azedarach (83.92) and aqueous (68.44) and ethanol (67.29) extracts T. obtusifolia recorded the highest coefficient of deterrence, indicating a good feeding deterrence activity. Aqueous extracts of T. floribunda and aqueous extracts of T. dregeana moderately caused larvae fertility, with feeding deterrence coefficients of 66.96 and 62.02, respectively, ranked ++. Furthermore, ethanolic extracts of E. capensis and acetone extracts of T. floribunda were the least effective feeding deterrents against the S. frugiperda larvae.

Table 3.
Feeding deterrent activity coefficient of five Meliaceae species leaf extracts against Spodoptera frugiperda.
Feeding Deterrence Activity of P. xylostella Larvae
Table 4 indicates the feeding deterrent activities of the studied Meliaceae species against the diamondback moth larvae. All plant extracts exhibited noteworthy deterrence against the P. xylostella larvae. All extracts of T. dregeana showed exceptionally high feeding deterrent activities, with acetone recording a 112.25 deterrence coefficient ranked +++, ethanol (99.39, ++), and aqueous (98.77, ++). Aqueous extracts of T. obtusifolia and ethanolic extracts of E. capensis moderately caused feeding deterrence of the larvae, with feeding coefficients of 86.74 and 85.79, respectively, both ranked ++. Meanwhile, aqueous (13.95, +) and acetone (25.93, +) extracts of T. floribunda were the least effective feeding deterrents against P. xylostella larvae.

Table 4.
Feeding deterrent activity coefficient of five Meliaceae species leaves extracts against Plutella xylostella larvae.
2.1.3. Topical Application Test
Contact Toxicity against S. frugiperda Larvae
Table 5 shows the direct contact toxicity of the Meliaceae plant extracts to the S. frugiperda larvae using different concentrations. Aqueous extracts of T. dregeana and M. azedarach exhibited a positive correlation, where the least concentrated extracts [0.5] showed less toxicity than the more concentrated extracts [1.0]. At [0.5], extracts recorded a 20% mortality rate, while [1.0] recorded an 80% mortality rate. The negative correlation between the concentration of extracts and the rate of mortality observed was recorded for E. capensis (acetone), M. azedarach (acetone and ethanol), and T. floribunda (acetone), where at [0.5] 20%, the mortality rate and at [1.0] mortality rate was 80%. Extracts that did not show any correlation and had constant mortality rates were aqueous extracts of E. capensis where, at [0.5] and [1.0], 80% of the larvae died, aqueous extracts of T. floribunda at [0.5] and [1.0] caused 60% mortality, and ethanolic extracts of T. floribunda at [0.5] and [1.0] caused 20% larval mortality. Probability unit (Probit) analysis showed that aqueous extracts of E. capensis (LD50 value of 0.14 mg/kg) and T. floribunda (LD50 value of 0.56 mg/kg) were more toxic to the S. frugiperda larvae. Probit analysis also indicated that ethanolic extracts of E. capensis were the least toxic to the fall armyworm, with LD50 values of 851.14 mg/kg.

Table 5.
Toxicity of five Meliaceae species leaf extracts applied topically to Spodoptera frugiperda larvae.
Contact Toxicity against P. xylostella Larvae
Results of the direct contact toxicity of the Meliaceae plant extracts to the P. xylostella larvae using different concentrations are outlined in Table 6. All extracts of M. azedarach at 500 ppm and 1000 ppm concentrations showed excellent results, as they killed 80% of the P. xylostella larvae. The Probit analysis further supported this and indicated that all three different extracts of M. azedarach were the most toxic to P. xylostella, with LD50 of 0.14 mg/kg. Results for acetone extracts of E. capensis and ethanolic extracts of T. floribunda showed a positive correlation between the concentration of extracts and mortality rates recorded. At [0.5], E. capensis and T. floribunda recorded a mortality of 20%, and at [1.0], they recorded an 80% mortality rate. The negative correlation between the concentration of extracts and the rate of mortality observed was recorded for acetone extracts of T. dregeana and aqueous extracts of T. floribunda. At [0.5], both extracts killed 80% of the larvae; at [1.0], T. dregeana killed 20%, while T. floribunda killed 40% of the larvae. Extracts that did not show any correlation and had a constant mortality rate were aqueous extracts of T. obtusifolia because, at [0.5] and [1.0], the extracts killed 40% of the P. xylostella larvae. Probit analysis indicated that only the aqueous extract of T. obtusifolia was the second most toxic to the P. xylostella larvae, with an LD50 value of 1.78 mg/kg. Meanwhile, all other plant extracts displayed insignificant toxicity to the P. xylostella, with acetone and ethanol extracts of T. obtusifolia recording the highest LD50 value of 1318.26 mg/kg.

Table 6.
Toxicity of five Meliaceae species leaf extracts applied topically to Plutella xylostella larvae.
2.2. GC-HRT-MS Analyses
The presence of chemical compounds in plants is important as they may be responsible for their biological activities, antifeedant and insecticidal properties. Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13 and Table 14 indicate active compounds present in each Meliaceae species using GC-MS analyses, with their retention time (RT), observed mass to charge ion ratio (m/z), molecular formula (MF), metabolite class (MC), and fold change (FC, the average of the peak area values obtained at the different injections of the same compound). In E. capensis acetone extracts, thirty-three compounds were identified (Table 7), most of which are triterpenoids (five), alkanes (three), esters (three), sesquiterpenoids (three), diterpenoids (two), methyl esters (two), and two compounds were unclassified. Ethanolic extracts of E. capensis in Table 8 identified fifty compounds, of which most are sesquiterpenoids (eight), fatty acids (five), diterpenoids (three), methyl esters (three), triterpenoids (three), benzofurans (two), esters (two), fatty amides (two), and one compound was unclassified.

Table 7.
Compounds identified in leaf acetone extracts of Ekebergia capensis.

Table 8.
Compounds identified in leaf ethanol extracts of Ekebergia capensis.

Table 9.
Compounds identified in leaf acetone extracts of Melia azedarach.

Table 10.
Compounds identified in leaf ethanol extracts of Melia azedarach.

Table 11.
Compounds identified in leaf acetone extracts of Trichilia dregeana.

Table 12.
Compounds identified in leaf ethanol extracts of Trichilia dregeana.

Table 13.
Compounds identified in leaf acetone extracts of Turraea floribunda.

Table 14.
Compounds identified in leaf ethanol extracts of Turraea floribunda.
Table 9 shows the results of acetone extracts of M. azedarach; twenty-six compounds were identified. Of these, two are classified as esters, alkanes (three), diterpenoids (two), methyl esters (two), non-metal compounds (two), organosilicons (two), and triterpenoid (two). Thirty-three compounds were identified in ethanolic extracts of M. azedarach, shown in Table 10. Four compounds are classified as esters, diterpenoids (three), fatty acids (three), fatty amides (three), hydrocarbons (two), ketones (two), steroids (two), triterpenoids (two), and three compounds were unclassified.
Thirty-nine compounds were identified in acetone extracts of T. dregeana (Table 11), most of which are diterpenoids (six), esters (four), triterpenoids (four), alkanes (three), fatty acids (two), non-metal compounds (two), steroids (two), and one compound was unclassified. Forty-three compounds were identified in ethanolic extracts of T. dregeana (Table 12), with most of the compounds in the classes: diterpenoids (seven), sesquiterpenoids (five), fatty acids (four), triterpenoids (four), steroids (three), alcohols (two), esters (two), fatty amides (two), and one compound was unclassified.
Acetone leaf extracts of T. floribunda (Table 13) had sixty compounds, of which seven are diterpenoids (seven), sesquiterpenoids (seven), alkanes (four), methyl esters (four), triterpenoids (four), non-metal compounds (three), esters (three), fatty acids (two), fatty alcohols (two), hydrocarbons (two), and three compounds were unclassified. Table 14 shows thirty compounds identified in ethanol extracts of T. floribunda; most of the chemical compounds belong to the chemical classes: fatty acids (four), diterpenoids (three), esters (two), methyl esters (two), non-metal compounds (two), and one compound was unclassified.
Table 15 shows forty-four compounds identified in acetone extracts of T. obtusifolia; chemical classes with the most chemical compounds are: fatty acids (six), sesquiterpenoids (five), alkanes (three), diterpenoids (three), triterpenoids (three), esters (two), non-metal compounds (two), steroids (two), and two compounds were unclassified. There were forty-six compounds identified in ethanolic extracts of T. obtusifolia (Table 16), chemical classes with the most chemical compounds: sesquiterpenoids (seven), diterpenoids (five), fatty acids (five), methyl esters (three), steroids (three), fatty alcohols (two), resorcinols (two), and five compounds were unclassified.

Table 15.
Compounds identified in leaf acetone extracts of Turraea obtusifolia.

Table 16.
Compounds identified in leaf ethanol extracts of Turraea obtusifolia.
3. Discussion
Most of the botanical extracts tested for their insecticidal activities proved to be effective repellents, feeding deterrents, and contact toxic against the S. frugiperda and P. xylostella larvae. Repellence, feeding deterrence, and contact toxicity of E. capensis, T. dregeana, Turraea floribunda, and T. obtusifolia extracts are recorded for the first time in this study. Melia azedarach was used as a positive control in this study as it is a well-known insecticidal plant in the Meliaceae family. The species has been proven to be an excellent insecticide against S. frugiperda [24,25,26,27,28,29,30,31]. In addition, M. azedarach extracts were found to be an effective botanical insecticide against P. xylostella in studies by Charleston et al. [32], Charleston et al. [12], Chen et al. [33], Chen et al. [34], Defagó et al. [35], Dilawari et al. [36], Dilawaxi et al. [37], Kumar et al. [38], Patil and Goud [39], Qiu et al. [40], Rani et al. [41], Sharma et al. [42], and Singh et al. [43].
Plant extracts with repellent activities are those with compounds that have irritating effects, causing insects to move away from them [44]. All plant extracts evaluated had repellence against the S. frugiperda and P. xylostella larvae, except for the ethanolic extracts of T. floribunda that had attractancy against the two tested larvae. However, there were interspecific differences as the botanical extracts were more susceptible as repellents to the P. xylostella larvae than to the S. frugiperda larvae (Table 1 and Table 2). Accordingly, seven extracts displayed repellency against P. xylostella larvae as follows: one in class V, three in IV, and five in III. In comparison, extracts displayed repellency against S. frugiperda according to the following level of activity: one in class V, two in IV, and one in III. It is not surprising that M. azedarach extracts were found to have the highest repellent activity against both S. frugiperda and P. xylostella larvae in this study, as this plant is known to have excellent repellent and insecticidal properties against several insect pests in several studies. Interestingly, T. dregeana recorded the same repellence activity as M. azedarach against both S. frugiperda and P. xylostella larvae. Against S. frugiperda, acetone extracts repelled 71% of the larvae (Table 1); meanwhile, against P. xylostella, acetone and ethanol extracts repelled 65% of the larvae (Table 2). Trichilia dregeana extracts in the study by Adinew [45] were found to have a highly positive protectant ability against Sitophilus zeamais Motschulsky (Maize weevil), which is also a major pest of maize similar to S. frugiperda. Extracts of T. floribunda moderately repelled the S. frugiperda larvae, while E. capensis and T. obtusifolia extracts recorded the lowest repellence activity.
Plants with antifeeding activities have compounds that, once consumed, cause the insects to stop feeding and eventually die due to starvation [44]. A study by Farag et al. [46] suggested that the plant extracts with feeding deterrence activity act as a stomach poison when ingested by insects. These feeding deterrent compounds could help reduce crop damage [18]. Melia azedarach aqueous extracts, followed by T. obtusifolia (aqueous and ethanol) and T. floribunda aqueous extracts, recorded the highest feeding deterrence activity against the S. frugiperda larvae. It does not come as a surprise that Turraea species recorded high feeding deterrence activity as in the study by Chimbe and Galley [47], another species of the genus, Turraea nilotica Kotschy & Peyr., was found to be effective against Sitophilus oryzae (rice weevil) larvae. Several studies also evaluated other species of the genus Trichilia for antifeedant properties. Trichilia elegans A.Juss. [48], T. pallens C.DC. [49], T. pallida Sw. [49], and T. roka (Forssk.) Chiov. [50] were found to have antifeedant activities against S. frugiperda larvae. Surprisingly, all T. dregeana extracts used in this study inhibited more P. xylostella larval feeding than M. azedarach extracts. This is contrary to the studies by Charleston et al. [32] and Dilawari et al. [36], where M. azedarach extracts recorded the highest antifeedant properties against the P. xylostella larvae. In this study, aqueous extracts of T. obtusifolia and ethanolic extracts of E. capensis also recorded high feeding deterrence activity against the P. xylostella larvae, with feeding deterrence coefficients of 86.74 and 85.79, respectively. The repellence activity of E. capensis coincides with that in the study by Champagne [51], in which the extracts acted as growth inhibitors and were toxic to Peridroma saucia Hübner (variegated cutworm) larvae. Trichilia silvatica C.DC. extracts were reported as good antifeedants against P. xylostella larvae [52].
Aqueous extracts of E. capensis and T. floribunda caused the highest S. frugiperda larval mortality, with recorded LC50 values of 0.14 mg/kg and 0.56 mg/kg, respectively. In the current study, all extracts of M. azedarach were less toxic to the S. frugiperda larvae (with an LC50 of 707.95 mg/kg). These results are contrary to the results obtained in the study by Bullangpoti et al. [26], where M. azedarach ethanolic extracts caused high mortality against the S. frugiperda with a recorded a lower LC50 value of 1.4 g L−1. Ekebergia capensis and T. dregeana extracts caused the least mortality to the larvae. However, in the study by Rioba and Stevenson [53], two members of the genus Trichilia, T. pallens C.DC., and T. pallida Sw., were found to cause high larval mortality against the S. frugiperda larvae. Trichilia trijuga Vell. extracts were found to be toxic to the Crocidolomia binotalis (cabbage cluster caterpillar) larvae [54] and T. americana (Sessé & Moc.) T.D.Penn. was toxic to the Trichoplusia ni (cabbage looper) and Pseudaletia unipuncta (armyworm moth) larvae [10]. On the other hand, all three extracts of M. azedarach and aqueous extracts of T. obtusifolia were more lethal to the P. xylostella larvae, with LC50 values of 0.14 mg/kg and 1.78 mg/kg, respectively. Ekebergia capensis, T. dregeana, and T. floribunda extracts caused insignificant toxicity against the P. xylostella larvae. This is contrary to this present study, as higher levels (LC50 value of 691.83 and 707.95 mg/kg) of the extracts of T. dregeana were needed to kill 50% of the larvae. Trichilia emetica methanol extracts resulted in high P. xylostella larval mortality with a recorded LC50 value of 0.94 mg.m−1 in the study by Munyemana and Alberto [55]. There may be a relationship between the toxicity against the P. xylostella of Turraea species screened in this study with the study of Essoung et al. [56] and Essoung et al. [57], where T. floribunda and other Turraea species T. abyssinica Hochst., T. nilotica Kotschy & Peyr., and T. wakefieldii Oliv. extracts were toxic to Tuta obsoluta (tomato leafminer) larvae. Growth inhibitory and toxicity activity of eight Trichilia species T. americana (Sesse & Mocino) Pennington, T. connaroides (Wright & Am.), T. glabra L., T. havanensis Jacq., T. hirta L., T. martiana C.DC, T. pleeana (A. Juss.) C.DC, and T. quadrijuga subsp. cinerascens (C.DC) Pennington extracts were evaluated on Peridroma saucia (variegated cutworm) and Spodoptera litura (cotton leafworm).
In the present work, ethanol extracts yielded the highest number of chemical compounds except for T. floribunda, where acetone extracts yielded 60 compounds, whereas ethanol yielded 30 compounds. This coincides with the antifeedant results, as ethanol extracts had better repellence, feeding deterrence, and contact toxicity than acetone extracts. Four chemical compounds were present in acetone and ethanol extracts of all five Meliaceae species studied: methyl alcohol, neophytadiene, phytol, and β-sitosterol. The tridecanoic acid methyl ester was present in all plant extracts except in ethanolic extracts of T. dregeana. The terpene derivatives phytol (present in all plant extracts in the current study) have been reported to have insecticidal [58] and pesticidal activities [59]. After all the chemical compounds identified in GC-MS analysis of plant extracts were classified, it was found that eight classes were common in all ethanol extracts. These were alcohols, diterpenoids, esters, fatty acids, fatty aldehydes, methyl esters, steroids, and triterpenoids. The five species’ most common classes in acetone extracts were alcohol, alkane, diterpenoid, ester, non-metal compound, organosilicon, and triterpenoid. All five plant species evaluated (either aqueous, acetone, or ethanol extracts) had repellence, feeding deterrence, and contact toxicity activity against S. frugiperda and P. xylostella larvae to some extent. The GC-MS analysis results strongly support these results as the two most well-known groups, phenols, and terpenes, known to have insecticidal and antifeedant properties, were present in all the plant extracts. Trichilia dregeana extracts exhibited excellent repellence activity and feeding deterrence against the two test larvae as the positive control, M. azedarach extracts. GC-MS analysis revealed that ethanol extracts of T. dregeana contained a high number of chemical classes that are terpenes (i.e., diterpenoid, sesquiterpenoid, triterpenoid, and steroid) and phenols (i.e., gingerdione). In acetone extracts of T. dregeana, four terpenes were identified (i.e., diterpenoid, sesquiterpenoid, triterpenoid, and steroid), as well as four phenols (i.e., 7-hydroxycoumarin, alkylbenzene, gingerdione, and resorcinol). Chemical compound phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl- identified in ethanolic extracts of T. dregeana was reported to have repellent, larvicidal, adulticidal, and oviposition deterrence activities against insects in a study by Chen et al. [60]. In studies by Curcino-Vieira et al. [61] and Tan and Luo [62], chemical compounds such as coumarins, diterpenes, flavonoids, glycosylated lignans, limonoids, monoterpenes, sesquiterpenes, steroids, and triterpenes isolated from the genus Trichilia were found to have insect feeding activities [63,64,65], and they may be toxic to insects [66,67]. Ekebergia capensis extracts exhibited good repellence, feeding deterrence, and contact toxicity against the test insects. GC-MS analysis revealed that ethanol extracts of E. capensis contained 50 compounds, of which 16 are terpenes belonging to diterpenoid, ergosterol, sesquiterpenoid, and steroid chemical classes. Conversely, acetone extracts identified thirty-three compounds, of which nine are terpenes (belonging to classes diterpenoid, sesquiterpenoid, and triterpenoid), and one is a phenol (cholesterol). The two members of the genus Turraea, T. floribunda, and T. obtusifolia, recorded minor activities in the antifeedant testing against the test insects. The presence of different chemical classes of compounds such as diterpenoids, flavonoids, limonoids, and terpenoids in some Turraea spp. have been associated with insecticidal activities in previous studies by Essoung et al. [56]; Ndung’u et al. [68]; Udenigwe et al. [69]; Yuan et al. [70]; Xu et al. [71]; and Zanin et al. [72]. Chemical groups other than phenols and terpenes, which have been recorded to have insecticidal, antifeedant, and insect repellent activities, have also been identified in the current study. For example, 9,12-otadecadienoic acid (Z,Z)- present in all T. obtusifolia extracts was reported to have insect-repellent properties in the study by Paulpriya et al. [59].
4. Materials and Methods
4.1. Antifeedant and Insecticidal Analysis
4.1.1. Sample Preparations
Leaves were dried under shade at room temperature (25 °C), then ground into a fine powder using an electric grinder. Extraction was carried out according to the procedures of Warthen et al. [73], with some slight modifications. Ten grams of each powdered sample were extracted in 100 mL of water, acetone, and ethanol separately for 72 h at room temperature. After extraction, the solutions were filtered through Whatman No.40 filter paper, and the solvents were removed using a rotary evaporator. Methanol was used to dissolve the organic residues, where 0.5% and 1.0% solutions were prepared for each sample.
4.1.2. Insects Selection and Rearing
S. frugiperda and P. xylostella second instar larvae strains (between 3 to 7 days old) were obtained from the Agricultural Research Council- Vegetable and Ornamental Plants (ARC- VOPI) in Pretoria, where they were reared, and the information on their age was also obtained.
4.1.3. Repellence Bioassay
The repellence bioassay of the plant samples was assessed using Standard Method Number 3, described by McDonald et al. [74], with some modifications. Repellence tests were conducted using Whatman No.40 filter papers as opposed to the strips of aluminum foil laminated to 40 lb. kaft paper, as described in the study by McDonald et al. [74]. The substrata were prepared by cutting a filter paper in half and placing it in 0.5% and 1.0% solutions of the plant extracts for 1 min, and then allowing it to air dry at room temperature overnight. Each half of the treated disk was attached lengthwise, edge to edge, to an untreated half-disk of the filter paper with cellulose tape and placed in a petri dish (Figure 1A,B). To avoid cannibalism in a petri dish, five larvae of each insect were placed in the middle of each filter paper circle and covered. For five hours, at hourly intervals, individuals that settled on each half of the filter paper disk were counted, and the experimental design was run once. The average of the counts was converted to express the percentage repulsion (PR) as follows:
where C is the percentage of insects on the untreated half of the disk. Positive percentage repulsion values expressed repellence, and negative percentage repulsion values expressed attractancy. The averages of the percentage repulsion were then assigned different classes using the scale as follows [75] (Table 17):
PR = 2 × (C − 50)
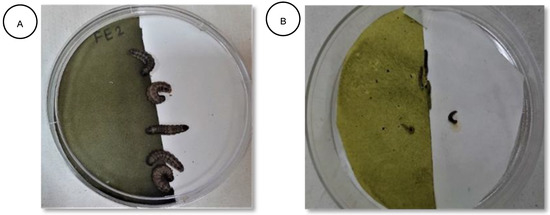
Figure 1.
(A) Treated half and untreated half of filter paper with S. frugiperda larvae. (B) Treated half and untreated half of filter paper with P. xylostella larvae.

Table 17.
Scale used to assign different classes of percentage repulsion values [75].
4.1.4. Feeding Deterrence Test
The potency of the feeding deterrence effect of plant leaf extracts against S. frugiperda and P. xylostella was determined by using the leaf disk bioassay. Maize and cabbage leaves were used as the test food for S. frugiperda and P. xylostella, respectively. The leaves were soaked in either water only (control leaf disks K) or in a 1% plant extract solution of aqueous, acetone, and ethanol separately (treated leaf disks E). The leaf disks (Figure 2A,B) were allowed to air dry at room temperature for about 30 min and weighed before they were presented to the larvae in petri dishes for 24 h, during which they were serving as the sole food source. The feeding behaviour of the larvae was recorded under three different conditions: (1) pure food, which comprised two control leaves (KK) (control test); (2) food with one control leaf (K) and one treated leaf (E) (choice test); and (3) food with two treated leaves (EE) (no choice test). After 24 h, the remaining leaves were reweighed, and mean percentages of feeding deterrence (FD) were calculated for each plant extract based on the weight of leaves before and after the tests. FD was calculated as follows:
FD = (C − T/C + T) × 100
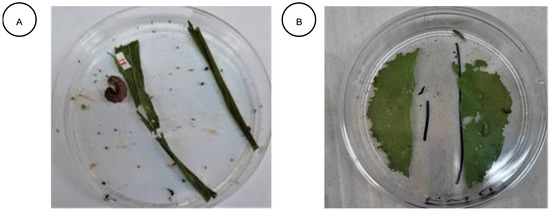
Figure 2.
(A) Maize leaf disk for the feeding deterrence assay against S. frugiperda after 24 h. (B) Cabbage leaf disk for the feeding deterrence assay against P. xylostella after 24 h.
C = weight of control leaves; T = weight of treated leaves.
After the FD values were calculated, three coefficients for the feeding deterrent activity from all three tests for each plant extract were calculated as follows [75]:
- 1.
- Absolute deterrence coefficient
A = (KK − EE/KK + EE) × 100
- 2.
- Relative deterrence coefficient
R = (K − E/K + E) × 100
- 3.
- Total deterrence coefficient
T = A + R
Values of the total deterrence coefficient (A) served as an index of the feeding deterrence activity which was expressed on a scale between 0 and 200. Plant extracts with a total deterrence coefficient of between 150–200 were marked ++++; 100–150, +++; 50–100, ++, and 0–50 + [75].
4.1.5. Topical Application Bioassay
The topical treatment assay tested the direct contact toxicity of the plant extracts, using Standard Method Number 1 described by McDonald et al. [74] with some modifications. Plant extract solutions of 0.5% and 1% were used for this test. Larvae were chilled for 10 min instead of being anesthetized with carbon dioxide in a Buchner funnel for about 5 min, as described in the study by McDonald et al. [74]. The immobilized larvae were picked up individually with forceps. Ten microliters of each plant extract solution were applied to the dorsum of each larva. Five larvae were treated at each dose and then transferred to a petri dish. After 24 h, the larvae were examined, and those that did not respond to gentle touch were considered dead. The number of dead larvae was recorded, and corrected mortality rates were calculated using the formula:
Percent larval mortality = (number of dead larvae/total number of treated larvae) × 100
Probit analysis [76] was used to analyse concentration-mortality data.
4.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was used to identify chemical compounds present in all five selected Meliaceae species. The patterns of the mass spectra fragmentation and their retention indices were compared with the ones stored in the computer library to identify the chemical components found in the plant extracts [77].
4.2.1. Sample Preparation
One gram of powdered samples was extracted in acetone and ethanol for 24 h at room temperature. The extracts were centrifuged at 13,000× g rpm for 10 min at 10 °C. Whatman No.1 filter paper was used to filter the solutions, and a rotary evaporator was used to evaporate or concentrate the solvents. One milliliter of methanol was used to dissolve the organic residues. The solutions were transferred into dark amber vials using syringe filters.
4.2.2. Gas Chromatography-High-Resolution-Time-of-Flight Mass Spectrometry (GC-HRTOF- MS) Analyses
The samples were analysed on the GC-HRTOF- MS system equipped with an Agilent 7890A gas chromatograph (Agilent Technologies, Inc., Wilmington, DE, USA). This system operates in high-resolution, equipped with a Gerstel MPS multipurpose autosampler (Gerstel Inc., Germany) and capillary column (Rxi- 5 ms- 30 m × 0.25 mm ID × 0.25 µm). For each plant extract, a volume of 1 µL was injected in a spitless mode. The program was started at 70 °C, held for 0.5 min, ramped at 10 °C/min to 150 °C, held for 2 min, ramped at 10 °C/min to 330 °C, and held for 3 min for the column to bake out. The samples were analysed at an MS data acquisition rate of 13 spectra/s, m/z range of 30–1000, electron ionization at 70 eV, ion source temperature was set at 250 °C, and the system extraction frequency was set at 1.25 kHz. Solvent blanks were also used to observe for contamination and impurities. Compounds were identified by matching the generated spectra with the NIST, Mainlib, and Feihn reference library databases on ChromaTOF-HRT® (LECO Corporation, St. Joseph, MI, USA). Subsequent retention time alignment, matched filtration, peak picking, detection, and matching were conducted on a data station equipped with the ChromaTOF-HRT® software (LECO Corporation, St. Joseph, MI, USA). Parameters adopted for processing included a signal-to-noise ratio (S/N) of 100, a similarity match above 70%, and data presented in Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15 and Table 16 representing only compounds occurring at least twice in triplicate injections. The collected GC-HRTOF-MS dataset was converted to mzML format using the LECO ChromaTOF-HRT software and then processed (peak picking and alignment) on the XCMS open-source tool.
5. Conclusions
Meliaceae species are abundant large tree species, so they would be suitable to supply very large-scale production of botanical insecticides; thus, their potential use in controlling insect pests is promising. This study provides potential evidence that further confirms the findings of many previous reports that Meliaceae members can be used as repellents, insecticides, and antifeedants to control S. frugiperda and P. xylostella insects, two of the most important agricultural pests that mostly attack crops which form part of the primary staple food. All extracts of the five evaluated species indicated repellence to the S. frugiperda and P. xylostella larvae, except for the ethanolic extracts of T. floribunda, which showed attraction to both the larvae. All extracts evaluated exhibited feeding deterrence to the S. frugiperda and P. xylostella larvae to some extent, except for the ethanol extracts of T. floribunda, which had inert antifeeding compounds. Aqueous extracts of E. capensis and T. floribunda were more toxic to the S. frugiperda larvae, and all extracts of M. azedarach were more toxic to the P. xylostella larvae. The GC-MS analysis results strongly support the insecticidal activities of the evaluated extracts as the two most well-known groups, phenols and terpenes, known to have insecticidal and antifeedant properties, were present in all the plant extracts. Therefore, this further corroborates the recorded traditional uses of these plants as insecticides and antifeedants. Plants that have indicated the most promising results are E. capensis, T. floribunda, and T. obtusifolia. These plants should be subjected to further quantitative phytochemical studies focusing on isolating and identifying active compounds rather than simply screening the plant extracts for insecticidal and antifeedant activity, as plant extracts may contain many compounds along with those that may cause negative side effects and toxicity. Further research should also be conducted regarding their safe use and non-target effects, and to determine if they can maintain yield at comparable levels to synthetic pesticides. Field trial evaluations of insecticidal and antifeedant plant extracts may also need to be undertaken to assess their impact on crop yield and damage and evaluate insect resistance issues in comparison to synthetic pesticides. This study’s results are significant as they will generate new and alternative natural products that can help improve biological effectiveness, lower residuals, increase nontoxic agricultural products, and decrease their presence in foods.
Author Contributions
Conceptualization, A.N.M. and K.C.S.; writing—original draft preparation, K.C.S.; writing, review, and editing, A.N.M. and K.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation (NRF), grant number 114686.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the University of Johannesburg for the logistical and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, S.; Taning, C.N.T.; Bonneure, E.; Mangelinckx, S.; Smagghe, G.; Shah, M.M. Insecticidal activity of plant-derived extracts against different economically important pest insects. Phytoparasitica 2017, 45, 113–124. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal plants in Africa: A global vision of new biological control products from local uses. Ind. Crops Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Kalpana, P.; Kaur, V.; Gahlyan, S.; Jangra, A.; Pradeep, A.; Rani, M.; Maken, S. Exploring the phytochemicals of Delphinium ajacis and their applications in biocontrol activity against some plant pathogens. J. Chem. Pharm. Res. 2016, 8, 11–18. [Google Scholar]
- Kabdwal, B.C.; Sharma, R.; Tewari, R.; Tewari, A.K.; Singh, R.P.; Dandona, J.K. Field efficacy of different combinations of Trichoderma harzianum, Pseudomonas fluorescens, and arbuscular mycorrhiza fungus against the major diseases of tomato in Uttarakhand (India). Egypt. J. Biol. Pest Control. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Thapa, C.B. Survey of Integrated Pest Management (IPM) Practice in Vegetable Crops of Rupandehi District, Western Nepal. Int. J. Appl. Sci. Biotechnol. 2017, 5, 237–242. [Google Scholar] [CrossRef]
- Karunamoorthi, K. Medicinal and Aromatic Plants: A Major Source of Green Pesticides/Risk-Reduced Pesticides. Med. Aromat. Plants 2012, 1, 2164-0412. [Google Scholar] [CrossRef]
- Gunnell, D.; Eddleston, M.; Phillips, M.R.; Konradsen, F. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health 2007, 7, 357. [Google Scholar] [CrossRef]
- Rangiah, K.; Varalaxmi, B.A.; Gowda, M. UHPLC-MS/SRM method for quantification of neem metabolites from leaf extracts of Meliaceae family plants. Anal. Methods 2016, 8, 2020–2031. [Google Scholar] [CrossRef]
- Isman, M.; Arnason, J.; Towers, G. Chemistry and Biological Activity of Ingredients of Other Species of Meliaceae. The Neem Tree: Azadichta indica A. Juss and Other Meliaceous Plants: Sources of Unique Natural Products for Integrated Pest Management, Medicine, Industry and Other Purposes; VCH: Weinheim, Germany, 1995; pp. 652–666. [Google Scholar]
- Akhtar, Y.; Yeoung, Y.R.; Isman, M.B. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta. Phytochem. Rev. 2007, 7, 77–88. [Google Scholar] [CrossRef]
- Senthil, N. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 2013, 4, 359. [Google Scholar]
- Charleston, D.S.; Kfir, R.; Dicke, M.; Vet, L.E. Impact of botanical extracts derived from Melia azedarach and Azadirachta indica on populations of Plutella xylostella and its natural enemies: A field test of laboratory findings. Biol. Control. 2006, 39, 105–114. [Google Scholar] [CrossRef]
- Rattan, R.S.; Sharma, A. Plant Secondary Metabolites in the Sustainable Diamondback Moth Plutella xylostella L. Management. Indian J. Fundam. Appl. Life Sci. 2011, 1, 295–309. [Google Scholar]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Nicol, H.I.; Munyakazi, L.; Stevenson, P.C. Tri-Trophic Insecticidal Effects of African Plants against Cabbage Pests. PLoS ONE 2013, 8, e78651. [Google Scholar] [CrossRef]
- Machekano, H.; Mvumi, B.; Nyamukondiwa, C. Diamondback Moth, Plutella xylostella (L.) in Southern Africa: Research Trends, Challenges and Insights on Sustainable Management Options. Sustainability 2017, 9, 91. [Google Scholar] [CrossRef]
- Risco, G.V.S.; Idrogo, C.R.; Kato, M.J.; Díaz, J.S.; Armando, J., Jr.; Paredes, G.E.D. Larvicidal activity of Piper tuberculatum on Spodoptera frugiperda (Lepidoptera: Noctuidae) under laboratory conditions. Rev. Colomb. Entomol. 2012, 38, 35–41. [Google Scholar] [CrossRef]
- Sisay, B.; Tefera, T.; Wakgari, M.; Ayalew, G.; Mendesil, E. The Efficacy of Selected Synthetic Insecticides and Botanicals against Fall Armyworm, Spodoptera frugiperda, in Maize. Insects 2019, 10, 45. [Google Scholar] [CrossRef]
- Phambala, K.; Tembo, Y.; Kasambala, T.; Kabambe, V.H.; Stevenson, P.C.; Belmain, S.R. Bioactivity of Common Pesticidal Plants on Fall Armyworm Larvae (Spodoptera frugiperda). Plants 2020, 9, 112. [Google Scholar] [CrossRef]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Adeniyi, S.A.; Orjiekwe, C.L.; Ehiagbonare, J.E.; Arimah, B.D. Preliminary phytochemical analysis and insecticidal activity of ethanolic extracts of four tropical plants (Vernonia amygdalina, Sida acuta, Ocimum gratissimum and Telfaria occidentalis) against beans weevil (Acanthscelides obtectus). Int. J. Phys. Sci. 2010, 5, 753–762. [Google Scholar]
- Pino, O.; Sánchez, Y.; Rojas, M.M. Plant secondary metabolites as an alternative in pest management. I: Background, research approaches and trends. Rev. Protección Veg. 2013, 28, 81. [Google Scholar]
- Fischer, D.; Imholt, C.; Pelz, H.J.; Wink, M.; Prokop, A.; Jacob, J. The repelling effect of plant secondary metabolites on water voles, Arvicola amphibius. Pest Manag. Sci. 2013, 69, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Kanwar, R.K.; Sehgal, A.; Cahill, D.M.; Barrow, C.J.; Sehgal, R.; Kanwar, J.R. Progress on Azadirachta indica Based Biopesticides in Replacing Synthetic Toxic Pesticides. Front. Plant Sci. 2017, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Berlitz, D.L.; Azambuja, A.O.D.; Sebben, A.; Oliveira, J.V.D.; Fiuza, L.M. Mortality of Oryzophagus oryzae (Costa Lima, 1936) (Coleoptera: Curculionidae) and Spodoptera frugiperda (J E Smith, 1797) (Lepidoptera: Noctuidae) Larvae Exposed to Bacillus thuringiensis and Extracts of Melia azedarach. Braz. Arch. Biol. Technol. 2012, 55, 725–731. [Google Scholar] [CrossRef]
- Breuer, M.; Schmidt, G. Studies on the effect of Melia azedarach extracts on Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Mitt. Dtsch. Ges. Allg. Angew. Entomol. 1990, 7, 419–429. [Google Scholar]
- Bullangpoti, V.; Wajnberg, E.; Audant, P.; Feyereisen, R. Antifeedant activity of Jatropha gossypifolia and Melia azedarach senescent leaf extracts on Spodoptera frugiperda (Lepidoptera: Noctuidae) and their potential use as synergists. Pest Manag. Sci. 2012, 68, 1255–1264. [Google Scholar] [CrossRef]
- Hernandez, C.; Vendramim, J. Bioactivity evaluation of aqueous extracts of Meliaceae to Spodoptera frugiperda (JE Smith). Rev. Agric. 1997, 72, 305–318. [Google Scholar]
- Jiménez-Durán, A.; Barrera-Cortés, J.; Lina-García, L.P.; Santillan, R.; Soto-Hernández, R.M.; Ramos-Valdivia, A.C.; Ponce-Noyola, T.; Ríos-Leal, E. Biological Activity of Phytochemicals from Agricultural Wastes and Weeds on Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sustainability 2021, 13, 13896. [Google Scholar] [CrossRef]
- Maroneze, D.M.; Gallegos, D.M.N. Effect of Melia azedarach aqueous extract on the development of immature and reproductive stages of Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae). Semin. Ciências Agrárias 2009, 30, 537–549. [Google Scholar]
- Scapinello, J.; Oliveira, J.V.; Ribeiros, M.L.; Tomazelli, O., Jr.; Chiaradia, L.A.; Dal Magro, J. Effects of supercritical CO2 extracts of Melia azedarach L. on the control of fall armyworm (Spodoptera frugiperda). J. Supercrit. Fluids 2014, 93, 20–26. [Google Scholar] [CrossRef]
- Scapinello, J.; Oliveira, J.V.D.; Chiaradia, L.A.; Tomazelli, O., Jr.; Niero, R.; Dal Magro, J. Insecticidal and growth inhibiting action of the supercritical extracts of Melia azedarach on Spodoptera frugiperda. Rev. Bras. Eng. Agrícola Ambient. 2014, 18, 866–872. [Google Scholar] [CrossRef]
- Charleston, D.S.; Kfir, R.; Vet, L.E.M.; Dicke, M. Behavioural responses of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) to extracts derived from Melia azedarach and Azadirachta indica. Bull. Entomol. Res. 2005, 95, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chang, S.J.; Cheng, L.L.; Hou, R.F. Effects of chinaberry fruit extract on feeding, growth and fecundity of the diamondback moth, Plutella xylostella L.(Lep.; Yponomeutidae). J. Appl. Entomol. 1996, 120, 341–345. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, S.J.; Hou, R.F.; Cheng, L.L. Deterrent effect of the chinaberry extract on oviposition of the diamondback moth, Plutella xylostella (L.)(Lep.; Yponomeutidae). J. Appl. Entomol. 1996, 120, 165–169. [Google Scholar] [CrossRef]
- Defagó, M.T.; Dumón, A.; Avalos, D.S.; Palacios, S.M.; Valladares, G. Effects of Melia azedarach extract on Cotesia ayerza, parasitoid of the alfalfa defoliator Colias lesbia. Biol. Control. 2011, 57, 75–78. [Google Scholar] [CrossRef]
- Dilawari, V.; Singh, K.; Dhaliwal, G. Effects of Melia azedarach L. on oviposition and feeding of Plutella xylostella L. Int. J. Trop. Insect Sci. 1994, 15, 203–205. [Google Scholar] [CrossRef]
- Dilawaxi, V.; Singh, K.; Dhaliwal, G. Sensitivity of Diamondback Moth, Plutella xylostella L. to Melia azedarach L. Pestic. Res. J. 1994, 6, 71–74. [Google Scholar]
- Kumar, R.; Sharma, K.; Kumar, D. Studies on ovicidal effects of some plant extracts against the diamondback moth, Plutella xylostella (L.) infesting cauliflower cro. Biol. Forum Int. J. 2009, 1, 47–50. [Google Scholar]
- Patil, R.S.; Goud, K.B. Efficacy of methanolic plant extracts as ovipositional repellents against diamondback moth, Plutella xylostella (L.). J. Entomol. Res. 2003, 27, 13–18. [Google Scholar]
- Qiu, Y.-T.; Loon, J.J.A.; Roessingh, P. Chemoreception of oviposition inhibiting terpenoids in the diamondback moth Plutella xylostella. Entomol. Exp. Appl. 1998, 87, 143–155. [Google Scholar] [CrossRef]
- Rani, M.; Suhag, P.; Kumar, R.; Singh, R.; Kalidhar, S.B. Chemical components and biological efficacy of Melia azedarach Stems. J. Med. Aromat. Plant Sci. 1999, 21, 1043–1047. [Google Scholar]
- Sharma, A.; Kaushal, P.; Sharma, K.C.; Kumar, R. Bioefficacy of some plant products against Diamond back moth Plutella xylostella L. (Lepidoptera: Yponomeutidae). J. Entomol. Res. 2006, 30, 213–217. [Google Scholar]
- Singh, G.; Kaur, V.; Singh, D. Lethal and sublethal effects of different ecotypes of Melia azedarach against Plutella xylostella (Lepidoptera: Plutellidae). Int. J. Trop. Insect Sci. 2006, 26, 92–100. [Google Scholar] [CrossRef]
- Castillo, L.E.; Jiménez, J.; Delgado, M. Secondary metabolites of the Annonaceae, Solanaceae and Meliaceae families used as biological control of insects. Trop. Subtrop. Agroecosystems 2010, 12, 445–462. [Google Scholar]
- Adinew, B. Comparative Efficacy of Jatropha Curcas and Trichilia Dregeana Seed Oil on Sitophilus Zeamais in Stored Maize Grain. Elixir Entomol. 2017, 111, 48802–48806. [Google Scholar]
- Farag, M.; Ahmed, M.H.; Yousef, H.; Abdel-Rahman, A.H. Repellent and Insecticidal Activities of Melia azedarach L. against Cotton Leafworm, Spodoptera littoralis (Boisd.). Z. Für Nat. C 2011, 66, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Chimbe, C.; Galley, D. Evaluation of material from plants of medicinal importance in Malawi as protectants of stored grain against insects. Crop Prot. 1996, 15, 289–294. [Google Scholar] [CrossRef]
- Longhini, R.; Lonni, A.A.; Sereia, A.L.; Krzyzaniak, L.M.; Lopes, G.C.; Mello, J.C.P.D. Trichilia catigua: Therapeutic and cosmetic values. Rev. Bras. De Farmacogn. 2017, 27, 254–271. [Google Scholar] [CrossRef]
- Bogorni, P.C.; Vendramim, J.D. Sublethal effect of aqueous extracts of Trichilia sp on Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) development on maize. Neotrop. Entomol. 2005, 34, 311–317. [Google Scholar] [CrossRef]
- Kubo, I.; Klocke, J. An insect growth inhibitor fromTrichilia roka (Meliaceae). Experientia 1982, 38, 639–640. [Google Scholar] [CrossRef]
- Champagne, D.E.; Isman, M.B.; Downum, K.R.; Towers, G.H. Insecticidal and growth-reducing activity of foliar extracts from Meliaceae. Chemoecology 1993, 4, 165–173. [Google Scholar] [CrossRef]
- Couto, I.F.; Fuchs, M.L.; Pereira, F.F.; Mauad, M.; Scalon, S.P.; Dresch, D.M.; Mussury, R.M. Feeding preference of Plutella xylostella for leaves treated with plant extracts. An. Acad. Bras. Ciências 2016, 88, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Rioba, N.B.; Stevenson, P.C. Opportunities and scope for botanical extracts and products for the management of fall armyworm (spodoptera frugiperda) for smallholders in africa. Plants 2020, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Prijono, D. Insecticidal activity of meliaceous seed extracts against Crocidolomia binotalis Zeller (Lepidoptera: Pyralidae). Bul. HPT 1998, 10, 1–7. [Google Scholar]
- Munyemana, F.; Alberto, A.L. Evaluation of larvicidal activity of selected plant extracts against Plutella xylostella (Lepidoptera: Plutellidae) larvae on cabbage. Adv. Med. Plant Res. 2017, 5, 11–20. [Google Scholar] [CrossRef]
- Essoung, F.; Chhabra, S.C.; Mba'ning, B.M.; Mohamed, S.A.; Lwande, W.; Lenta, B.N.; Ngouela, S.A.; Tsamo, E.; Hassanali, A. Larvicidal activities of limonoids from Turraea abyssinica (Meliaceae) on Tuta absoluta (Meyrick). J. Appl. Entomol. 2018, 142, 397–405. [Google Scholar] [CrossRef]
- Essoung Ehawa, F.R.; Mohamed, S.A.; Hassanali, A.; Chhabra, S.C. Bioassay-Guided Isolation of Active Phytochemicals Against Tuta absoluta (Meyrick) from Turraea floribunda and Caesalpinia welwitschiana. In Sustainable Management of Invasive Pests in Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 11–29. [Google Scholar]
- Cruz-Estrada, A.; Gamboa-Angulo, M.; Borges-Argáez, R.; Ruiz-Sánchez, E. Insecticidal effects of plant extracts on immature whitefly Bemisia tabaci Genn.(Hemiptera: Aleyroideae). Electron. J. Biotechnol. 2013, 16, 6. [Google Scholar]
- Paulpriya, K.; Tresina, P.; Mohan, V. Assessment of bioactive constituents by GC-MS of Crotalaria longipes Wight & Arn.: An endemic plant. Int. J. Pharmacogn. Phytochem. Res. 2014, 15, 4. [Google Scholar]
- Chen, Y.; Dai, G. Acaricidal, repellent, and oviposition-deterrent activities of 2, 4-di-tert-butylphenol and ethyl oleate against the carmine spider mite Tetranychus cinnabarinus. J. Pest Sci. 2015, 88, 645–655. [Google Scholar] [CrossRef]
- Curcino Vieira, I.J.; da Silva Terra, W.; dos Santos Gonçalves, M.; Braz-Filho, R. Secondary Metabolites of the Genus Trichilia: Contribution to the Chemistry of Meliaceae Family. Am. J. Anal. Chem. 2014, 5, 91–121. [Google Scholar] [CrossRef][Green Version]
- Tan, Q.-G.; Luo, X.-D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Li, X. Recent studies on insecticidal activities of limonoids from meliaceous plants. Insect Sci. 1999, 6, 283–288. [Google Scholar] [CrossRef]
- Ramĺrez, M.; Toscano, R.A.; Arnason, J.; Omar, S.; Cerda-Garcĺa-Rojas, C.M.; Mata, R. Structure, conformation and absolute configuration of new antifeedant dolabellanes from Trichilia trifolia. Tetrahedron 2000, 56, 5085–5091. [Google Scholar] [CrossRef]
- Simmonds, M.S.; Stevenson, P.C.; Porter, E.A.; Veitch, N.C. Insect Antifeedant Activity of Three New Tetranortriterpenoids from Trichilia pallida. J. Nat. Prod. 2001, 64, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-B.; Chen, H.Q.; Feng, G.; Guo, Z.K.; Cai, C.H.; Wang, J.; Mei, W.L.; Dai, H.F. A new insecticidal havanensin-type limonoid from the roots of Trichilia sinensis Bentv. Nat. Prod. Res. 2018, 32, 2797–2802. [Google Scholar] [CrossRef]
- Matos, A.P.; Nebo, L.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.D.G.F.D.; Rodrigues, R.R. Constituintes químicos e atividade inseticida dos extratos de frutos de Trichilia elegans e T. catigua (Meliaceae). Química Nova 2009, 32, 1553–1556. [Google Scholar] [CrossRef]
- Ndung’u, M.W.; Kaoneka, B.; Hassanali, A.; Lwande, W.; Hooper, A.M.; Tayman, F.; Zerbe, O.; Torto, B. New mosquito larvicidal tetranortriterpenoids from Turraea wakefieldii and Turraea floribunda. J. Agric. Food Chem. 2004, 52, 5027–5031. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Ata, A.; Samarasekera, R. Glutathione S-Transferase Inhibiting Chemical Constituents of Caesalpinia bonduc. Chem. Pharm. Bull. 2007, 55, 442–445. [Google Scholar] [CrossRef]
- Yuan, C.-M.; Tang, G.H.; Wang, X.Y.; Zhang, Y.; Cao, M.M.; Li, X.H.; Li, Y.; Li, S.L.; Di, Y.T.; He, H.P.; et al. New steroids and sesquiterpene from Turraea pubescens. Fitoterapia 2013, 90, 119–125. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, J.; Zhou, X.; Li, W.; Zhu, N.; Wu, H.; Li, P.; Sun, Z.; Yang, J.; Ma, G. Cassane diterpenes with oxygen bridge from the seeds of Caesalpinia sappan. Fitoterapia 2016, 112, 205–210. [Google Scholar] [CrossRef]
- Zanin, J.L.B.; De Carvalho, B.A.; Salles Martineli, P.; Dos Santos, M.H.; Lago, J.H.G.; Sartorelli, P.; Viegas, C., Jr.; Soares, M.G. The genus Caesalpinia L.(Caesalpiniaceae): Phytochemical and pharmacological characteristics. Molecules 2012, 17, 7887–7902. [Google Scholar] [CrossRef]
- Warthen, J.D.; Stokes, J.B.; Jacobson, M.; Kozempel, M.F. Estimation of Azadirachtin Content in Neem Extracts and Formulations. J. Liq. Chromatogr. 1984, 7, 591–598. [Google Scholar] [CrossRef]
- McDonald, L.L.; Guy, R.H.; Speirs, R.D. Preliminary Evaluation of New Candidate Materials as Toxicants, Repellents, and Attractants Against Stored-Product Insects; US Agricultural Research Service: Washington, DC, USA, 1970.
- Talukder, F.A.; Howse, P.E. Deterrent and insecticidal effects of extracts of pithraj, Aphanamixis polystachya (Meliaceae), against Tribolium castaneum in storage. J. Chem. Ecol. 1993, 19, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Al-Marzoqi, A.H.; Hameed, I.H.; Idan, S.A. Analysis of bioactive chemical components of two medicinal plants (Coriandrum sativum and Melia azedarach) leaves using gas chromatography-mass spectrometry (GC-MS). Afr. J. Biotechnol. 2015, 14, 2812–2830. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).