First Report of the Molecular Mechanism of Resistance to Tribenuron-Methyl in Silene conoidea L.
Abstract
1. Introduction
2. Results
2.1. Dose—Response Assay
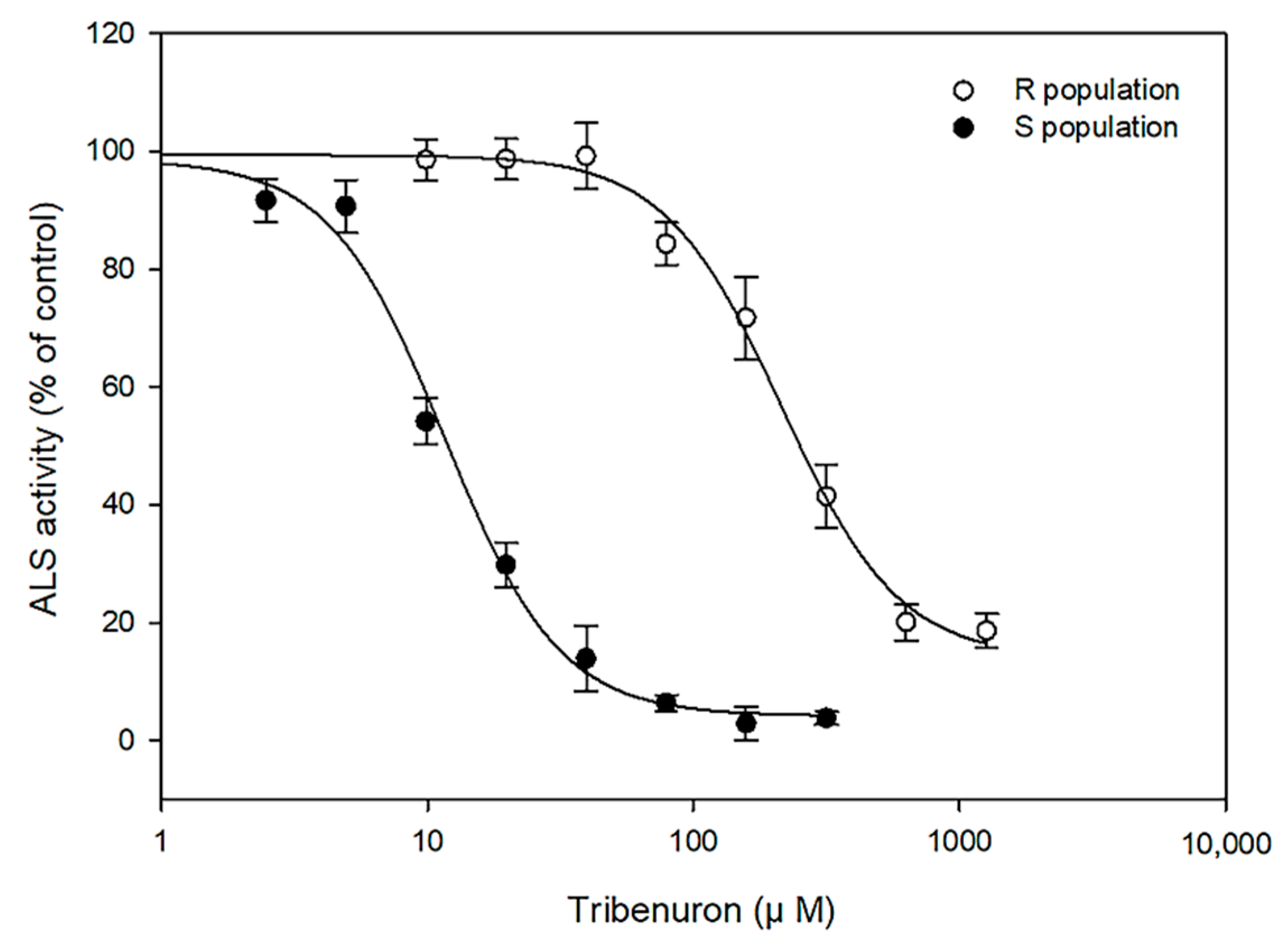
2.2. In Vitro ALS Activity Assay
2.3. Identification of ALS Mutations in S. conoidea
2.4. Effect of Malathion on Tribenuron-Methyl Resistance
2.5. Herbicide Cross-Resistance and Multiple Resistance
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Whole-Plant Dose Response
4.3. In Vitro Enzyme Activity Assay
4.4. Sequencing of the ALS Gene
4.5. Effect of Malathion on Tribenuron-Methyl Resistance
4.6. Sensitivity to Other ALS Inhibitors and Alternative Herbicides
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, F.; Ayaz, A.; Saqib, S.; Zaman, W.; Butt, M.A.; Ullah, A. Silene conoidea L.: A Review on Its Systematic, Ethnobotany and Phytochemical Profile. Plant Sci. Today 2019, 6, 373–382. [Google Scholar] [CrossRef]
- Liu, B. Investigation on ecological habits of Silene conoidea L. Chin. Wild Plant Resour. 2003, 22, 1. [Google Scholar] [CrossRef]
- Li, B.; Wang, G.; Wei, S.; Fan, C.; Huang, H.; Zhang, C. Characterization of weed community in winter wheat in Hebei Province. Acta Phytophylacica Sin. 2013, 40, 83–88. [Google Scholar]
- Liu, X.; Merchant, A.; Xiang, S.; Zong, T.; Zhou, X.; Bai, L. Managing Herbicide Resistance in China. Weed Sci. 2021, 69, 4–17. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS Inhibitor Herbicides: Current Understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Laforest, M.; Soufiane, B.; Simard, M.-J.; Obeid, K.; Page, E.; Nurse, R.E. Acetyl-CoA Carboxylase Overexpression in Herbicide-Resistant Large Crabgrass (Digitaria Sanguinalis): Herbicide-Resistant Digitaria. Pest. Manag. Sci 2017, 73, 2227–2235. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, Y.; Wang, H.; Bai, S.; Wang, Q.; Liu, W.; Wang, J. Acetolactate Synthase Overexpression in Mesosulfuron-Methyl-Resistant Shortawn Foxtail (Alopecurus Aequalis Sobol.): Reference Gene Selection and Herbicide Target Gene Expression Analysis. J. Agric. Food Chem. 2018, 66, 9624–9634. [Google Scholar] [CrossRef]
- Fang, J.; Yang, D.; Zhao, Z.; Chen, J.; Dong, L. A Novel Phe-206-Leu Mutation in Acetolactate Synthase Confers Resistance to Penoxsulam in Barnyardgrass (Echinochloa crus-galli (L.) P. Beauv). Pest Manag. Sci. 2022, 78, 2560–2570. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: http://www.weedscience.org/Home.aspx (accessed on 16 September 2022).
- Délye, C. Unravelling the Genetic Bases of Non-Target-Site-Based Resistance (NTSR) to Herbicides: A Major Challenge for Weed Science in the Forthcoming Decade: Unravelling the Genetic Bases of Non-Target-Site-Based Resistance to Herbicides. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S. Metabolism-Based Herbicide Resistance and Cross-Resistance in Crop Weeds: A Threat to Herbicide Sustainability and Global Crop Production. Plant Physiol. 2014, 166, 1106–1118. [Google Scholar] [CrossRef]
- Pan, L.; Yu, Q.; Han, H.; Mao, L.; Nyporko, A.; Fan, L.; Bai, L.; Powles, S. Aldo-Keto Reductase Metabolizes Glyphosate and Confers Glyphosate Resistance in Echinochloa Colona. Plant Physiol. 2019, 181, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of Evolved Herbicide Resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Délye, C.; Michel, S.; Bérard, A.; Chauvel, B.; Brunel, D.; Guillemin, J.; Dessaint, F.; Le Corre, V. Geographical Variation in Resistance to Acetyl-coenzyme A Carboxylase-inhibiting Herbicides across the Range of the Arable Weed Alopecurus Myosuroides (Black-grass). New Phytol. 2010, 186, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Bay, G.; Pernin, F.; Délye, C. Prevalence of Cross- or Multiple Resistance to the Acetyl-Coenzyme A Carboxylase Inhibitors Fenoxaprop, Clodinafop and Pinoxaden in Black-Grass (Alopecurus Myosuroides Huds.) in France: Cross- or Multiple Resistance to ACCase Inhibitors in A. Myosuroides. Pest Manag. Sci. 2010, 66, 168–177. [Google Scholar] [CrossRef]
- Preston, C.; Dolman, F.C.; Boutsalis, P. Multiple Resistance to Acetohydroxyacid Synthase–Inhibiting and Auxinic Herbicides in a Population of Oriental Mustard (Sisymbrium Orientale). Weed Sci. 2013, 61, 185–192. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R. Resistance of Weeds to ALS-Inhibiting Herbicides: What Have We Learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, R.; Zheng, X.; Ma, S.; Tan, W.; Li, M. Study on the Control of Broadleaf Weeds in Winter Wheat Field by 19%WP. Shandong Agric. Sci. 2006, 3, 68–69. [Google Scholar]
- Li, X.; Tang, T.; Wang, G.; Li, B.; Qi, S. Effect of 20% Tribenuron-Methyl on Weed Control in Winter Wheat Field. J. Anhui Agric. Sci. 2002, 30, 578–580. [Google Scholar]
- Deng, W.; Yang, Q.; Jiao, H.; Zhang, Y.; Li, X.; Zheng, M. Cross-Resistance Pattern to Four AHAS-Inhibiting Herbicides of Tribenuron-Methyl-Resistant Flixweed (Descurainia Sophia) Conferred by Asp-376-Glu Mutation in AHAS. J. Integr. Agric. 2016, 15, 2563–2570. [Google Scholar] [CrossRef][Green Version]
- Cui, H.L.; Li, X.; Wang, G.; Wang, J.; Wei, S.; Cao, H. Acetolactate Synthase Proline (197) Mutations Confer Tribenuron-Methyl Resistance in Capsella Bursa-Pastoris Populations from China. Pestic. Biochem. Physiol. 2012, 102, 229–232. [Google Scholar] [CrossRef]
- Liu, W.; Bi, Y.; Li, L.; Yuan, G.; Du, L.; Wang, J. Target-Site Basis for Resistance to Acetolactate Synthase Inhibitor in Water Chickweed (Myosoton aquaticum L.). Pestic. Biochem. Physiol. 2013, 107, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiang, S.; Zong, T.; Ma, G.; Wu, L.; Liu, K.; Zhou, X.; Bai, L. Herbicide Resistance in China: A Quantitative Review. Weed Sci. 2019, 67, 605–612. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, F.; Li, Z.; Wang, H.; Wang, Q.; Wang, J.; Liu, W.; Bai, L. Target-Site and Non-Target-Site-Based Resistance to Tribenuron-Methyl in Multiply-Resistant Myosoton aquaticum L. Pestic. Biochem. Physiol. 2019, 155, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tranel, P.J.; Wright, T.R.; Heap, I.M. Mutations in Herbicide-Resistant Weeds to Inhibition of Acetolactate Synthase. Available online: http://www.weedscience.com (accessed on 24 October 2022).
- Huang, Z.; Chen, J.; Zhang, C.; Huang, H.; Wei, S.; Zhou, X.; Chen, J.; Wang, X. Target-Site Basis for Resistance to Imazethapyr in Redroot Amaranth (Amaranthus retroflexus L.). Pestic. Biochem. Physiol. 2016, 128, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Panozzo, S.; Scarabel, L.; Tranel, P.J.; Sattin, M. Target-Site Resistance to ALS Inhibitors in the Polyploid Species Echinochloa Crus-Galli. Pestic. Biochem. Physiol. 2013, 105, 93–101. [Google Scholar] [CrossRef]
- Deng, W.; Yang, Q.; Zhang, Y.; Jiao, H.; Mei, Y.; Li, X.; Zheng, M. Cross-Resistance Patterns to Acetolactate Synthase (ALS)-Inhibiting Herbicides of Flixweed (Descurainia sophia L.) Conferred by Different Combinations of ALS Isozymes with a Pro-197-Thr Mutation or a Novel Trp-574-Leu Mutation. Pestic. Biochem. Physiol. 2017, 136, 41–45. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Duggleby, R.G.; McCourt, J.A.; Guddat, L.W. Structure and Mechanism of Inhibition of Plant Acetohydroxyacid Synthase. Plant Physiol. Biochem. 2008, 46, 309–324. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, Y.; Ge, L.; Zhu, B.; Liu, W.; Wang, J. Target Site Mutations and Cytochrome P450s Confer Resistance to Fenoxaprop-P-ethyl and Mesosulfuron-methyl in Alopecurus Aequalis. Pest Manag. Sci. 2019, 75, 204–214. [Google Scholar] [CrossRef]
- Iwakami, S.; Uchino, A.; Kataoka, Y.; Shibaike, H.; Watanabe, H.; Inamura, T. Cytochrome P450 genes induced by bispyribac-sodium treatment in a multiple-herbicide-resistant biotype of Echinochloa phyllopogon. Pest Manag. Sci. 2014, 70, 549–558. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, W.; Li, X.; Yu, Q.; Bai, L.; Zheng, M. Target-Site and Non-Target-Site Based Resistance to the Herbicide Tribenuron-Methyl in Flixweed (Descurainia sophia L.). BMC Genom. 2016, 17, 551. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Li, X.; Cui, H. RNA-Seq Transcriptome Analysis to Identify Candidate Genes Involved in Non-Target Site-Based Mesosulfuron-Methyl Resistance in Beckmannia Syzigachne. Pestic. Biochem. Physiol. 2021, 171, 104738. [Google Scholar] [CrossRef] [PubMed]
- Duhoux, A.; Carrère, S.; Gouzy, J.; Bonin, L.; Délye, C. RNA-Seq Analysis of Rye-Grass Transcriptomic Response to an Herbicide Inhibiting Acetolactate-Synthase Identifies Transcripts Linked to Non-Target-Site-Based Resistance. Plant Mol. Biol. 2015, 87, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Ozsolak, F.; Milos, P.M. RNA Sequencing: Advances, Challenges and Opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Jugulam; Shyam Non-Target-Site Resistance to Herbicides: Recent Developments. Plants 2019, 8, 417. [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yu, Q.; Shane Friesen, L.J.; Zhang, X.Q.; Powles, S.B. Tolerance to Acetolactate Synthase and Acetyl-Coenzyme A Carboxylase Inhibiting Herbicides in Vulpia Bromoides Is Conferred by Two Co-Existing Resistance Mechanisms. Pestic. Biochem. Physiol. 2004, 78, 21–30. [Google Scholar] [CrossRef]
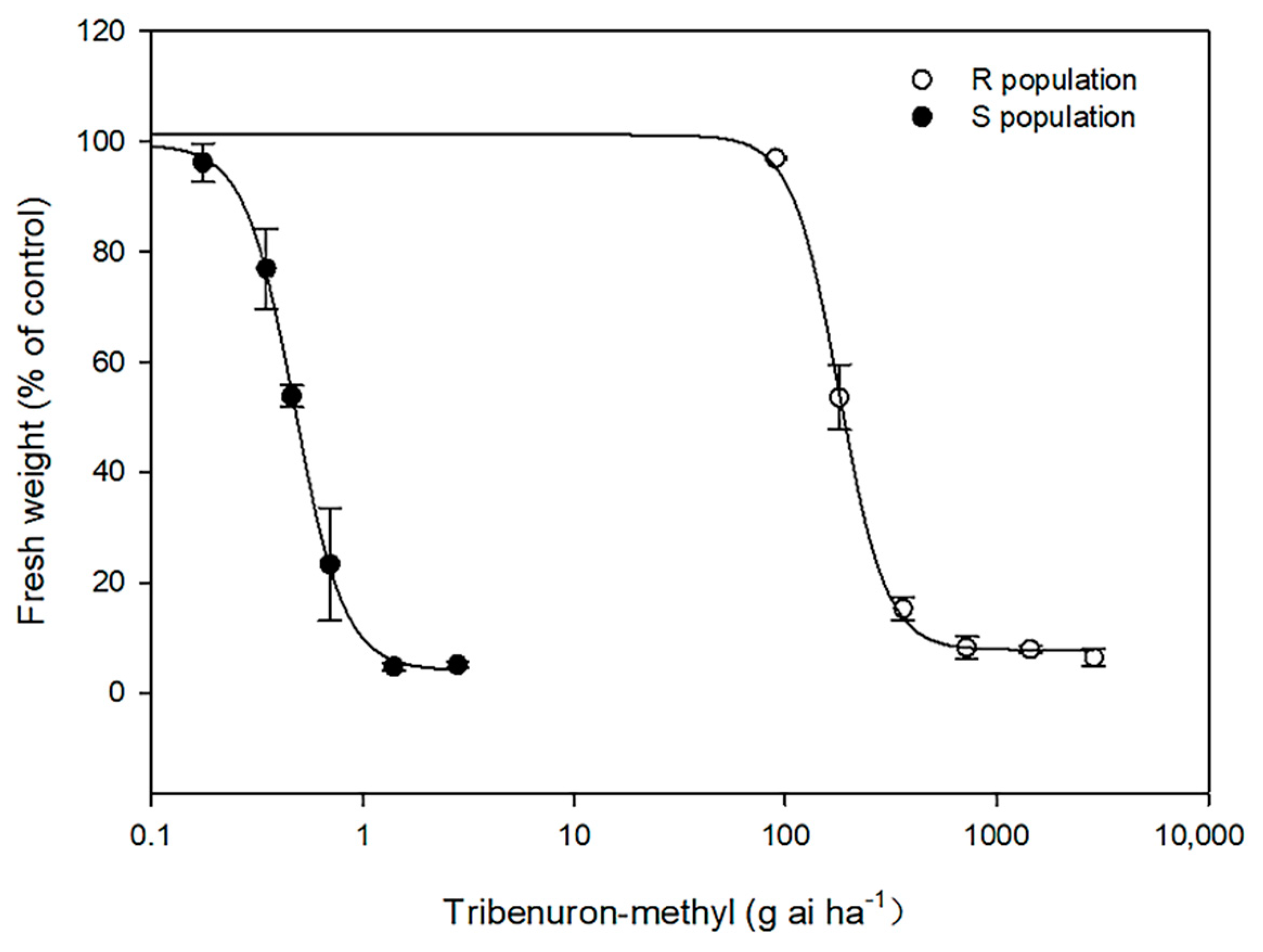

| Population a | GR50 (g a.i. ha−1) b | RI c |
|---|---|---|
| S | 0.47 ± 0.01 | - |
| R | 179.70 ± 4.01 | 382.3 |
| Population a | Total ALS Activity (nmol Acetoin mg−1 Protein min−1) | R/S b | I50 (μM) c | R/S b |
|---|---|---|---|---|
| S | 28.22 ± 2.37 | - | 11.61 ± 1.00 | - |
| R | 35.45 ± 1.72 | 1.3 | 214.95 ± 18.59 | 18.5 |
| Population a | Application Dose (g a.i. ha−1) | Fresh Weight Reduction (%) b | |
|---|---|---|---|
| Tribenuron-Methyl | Tribenuron-Methyl + Malathion | ||
| S | 0.35 | 76.9 | 74.5 |
| R | 90 | 94.6 | 25.4 |
| Herbicide | Population a | GR50 (g a.i. ha-1) b | RI c |
|---|---|---|---|
| Imazethapyr | S | 0.88 ± 0.06 | - |
| R | 832.53 ± 238.31 | 946.1 | |
| Bispyribac-sodium | S | 0.21 ± 0.01 | - |
| R | 16.97 ± 0.77 | 80.8 | |
| Florasulam | S | <0.04 | - |
| R | 71.48 ± 0.02 | >1787 |
| Primers | Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temperature (°C) | Amplification Point |
|---|---|---|---|---|
| ALS-1F | 5′-CGCCGCAAATACCAAAACCACTCCC-3′ | 649 | 58.5 | 122, 197 |
| ALS-1R | 5′-CCACCACCAACATACAGAACA-3′ | |||
| ALS-2F | 5′-CAAGTTCCGAGGCGAATGAT-3′ | 894 | 56 | 205, 206, 376, 377 |
| ALS-2R | 5′-CAAGCCCACTGGAGGTCA-3′ | |||
| ALS-3F | 5′-CGAGGGTGAGGAAGAGCA-3′ | 675 | 55 | 574, 653, 654 |
| ALS-3R | 5′-TCTTCCATCACCCTCGTTTA-3′ |
| Target a | Herbicide | Population b | Application Rate (g a.i. ha−1) |
|---|---|---|---|
| ALS | Imazethapyr | S | 0, 0.38, 0.76, 1.52, 3.05, 6.10, 12.18 |
| R | 0, 97.5, 195, 390, 780, 1560, 3120 | ||
| Bispyribac-sodium | S | 0, 2.81, 5.62, 11.25, 22.5, 45, 90 | |
| R | 0, 0.18, 0.35, 0.7, 1.4, 2.8, 5.6 | ||
| Florasulam | S | 0, 0.02, 0.04, 0.08, 0.16, 0.32, 0.64 | |
| R | 0, 4.5, 9, 18, 36, 72, 144 | ||
| Photosystem II | Bentazone | S | 1296, 2592 |
| R | |||
| Bromoxynil | S | 27, 54 | |
| R | |||
| Synthetic auxin | Fluroxypyr | S | 180, 360 |
| R | |||
| MCPA | S | 1260, 2520 | |
| R | |||
| PPO | Carfentrazone-ethyl | S | 562.5, 1125 |
| R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Han, Y.; Ma, H.; Wei, S.; Lan, Y.; Cao, Y.; Huang, H.; Huang, Z. First Report of the Molecular Mechanism of Resistance to Tribenuron-Methyl in Silene conoidea L. Plants 2022, 11, 3044. https://doi.org/10.3390/plants11223044
Sun Y, Han Y, Ma H, Wei S, Lan Y, Cao Y, Huang H, Huang Z. First Report of the Molecular Mechanism of Resistance to Tribenuron-Methyl in Silene conoidea L. Plants. 2022; 11(22):3044. https://doi.org/10.3390/plants11223044
Chicago/Turabian StyleSun, Ying, Yujun Han, Hong Ma, Shouhui Wei, Yuning Lan, Yi Cao, Hongjuan Huang, and Zhaofeng Huang. 2022. "First Report of the Molecular Mechanism of Resistance to Tribenuron-Methyl in Silene conoidea L." Plants 11, no. 22: 3044. https://doi.org/10.3390/plants11223044
APA StyleSun, Y., Han, Y., Ma, H., Wei, S., Lan, Y., Cao, Y., Huang, H., & Huang, Z. (2022). First Report of the Molecular Mechanism of Resistance to Tribenuron-Methyl in Silene conoidea L. Plants, 11(22), 3044. https://doi.org/10.3390/plants11223044






