Abstract
To explore the effects of iminodisuccinic acid (a chelating agent) on maize (Zea mays L.) seed germination under lead (Pb) stress, we comparatively analyzed the effects of applying different concentrations of iminodisuccinic acid (0, 5, 20, and 100 mmol·dm−3) and combined an addition of exogenous substances regulating reactive oxygen species production on maize seed germination, seedling growth, H2O2 content, NADPH oxidase activity, and antioxidant enzyme activities under Pb-stressed and Pb-free conditions. Iminodisuccinic acid (100 mmol·dm−3) significantly delayed seed germination under normal germination conditions and alleviated the inhibitory effects of Pb stress (20 mmol·dm−3) on seed germination. Under normal conditions (without Pb stress), the iminodisuccinic acid-induced inhibition of seed germination was enhanced by treatment with dimethylthiourea (a specific scavenger of reactive oxygen species) or diphenyleneiodonium chloride (a specific inhibitor of NADPH oxidase), but diminished by treatment with H2O2, CaCl2, diethyldithiocarbamic acid (a specific inhibitor of superoxide dismutase), or aminotriazole (a specific inhibitor of catalase). Under Pb stress, iminodisuccinic acid partially eliminated the excessive H2O2 accumulation, improved superoxide dismutase and catalase activity, and weakened the high NADPH oxidase activity. In addition, Ca2+ chelation may be essential for maintaining the reactive oxygen species’ balance and improving seed germination and seedling growth by iminodisuccinic acid supplementation in maize under Pb stress. The proposed iminodisuccinic acid supplementation-based method improved maize seed germination in Pb-polluted soil.
1. Introduction
In recent decades, rapid increases in urbanization and industrialization have caused the excessive release of heavy metals in farmlands, which has led to ecosystem damage [1,2,3]. Heavy metal accumulation in soils is of great concern in agricultural production due to its adverse effects on food safety and marketability, crop growth due to phytotoxicity, and the environmental health of soil organisms [4,5,6,7,8]. Industrial wastewater and urban sewage have been increasingly used in urban and suburban agricultural irrigation systems to partially resolve the problem of wastewater treatment [9,10]. Although the concentration of heavy metals in industrial wastewater and urban sewage is usually low [11], the long-term usage of these wastewater sources to irrigate agricultural land culminates in the enrichment of and significant increase in heavy metals in soil [12]. Additionally, the global oil and gas exploitation industry plays an essential, indispensable, and dynamic role in the international energy matrix [13]. Drilling associated with oil and gas exploitation projects can generate significant quantities of waste [14] that are extremely difficult to manage due to the presence of several heavy metal pollutants [15,16]. These heavy metal pollutants also exert long-term ecological effects on populations and communities, and thus, pose a threat to biodiversity and agricultural ecosystem integrity [17,18].
Studies have shown that using high-accumulating crops (metal accumulators), such as nonfood products and dedicated bioenergy crops, may be a feasible strategy for the phytoremediation of heavy metal-polluted soils [19,20]. Among diverse crop plants, maize (Zea mays L.) exhibits the advantages of high biomass production and metal tolerant capacity; thus, it has been widely adopted for the phytomanagement of low- and medium-grade metal-contaminated soils [21,22,23]. Moreover, with the increasing global demand for maize due to population growth and industrial expansion, phytoremediation techniques that use maize to decontaminate contaminated environments represent cost-effective, environmentally friendly, and socially acceptable methods for remediating metal-contaminated soils [24,25,26,27]. However, seed germination and seedling growth are critical stages in maize plant development, and the presence of heavy metals may impair growth or even render plant formation non-viable [28]. Therefore, in a phytoremediation program, successful germination and seedling development (the initial stages of plant growth) are essential for obtaining maize plants capable of extracting heavy metal contaminants from the soil [29].
Lead (Pb) is a highly toxic and commonly occurring heavy metal contaminant in the environment [30,31]. Of the various heavy metals, Pb is considered to be the second most toxic after arsenic [32]. Pb toxicity inhibits seed germination and plant growth by negatively affecting the physiological traits of plants [33,34,35]. Studies have shown that reactive oxygen species (ROS) are central components of plant adaptations to biotic and abiotic stresses and function as signaling molecules to either positively or negatively regulate seed germination [36,37,38]. In seeds, ROS play roles in endosperm weakening, mobilization of seed reserves, protection against pathogens, and programmed cell death [39]. In addition, neither high nor low levels of ROS are conducive to seed germination [40]. Research has established that Pb induces ROS production and antioxidant defense responses. These responses include changes in the activities of ROS scavenging enzymes (e.g., superoxide dismutase (SOD) and catalase (CAT)) and NADPH oxidase (NOX; the key enzyme producing ROS) in plants [41,42,43,44,45].
Direct seeding technology is widely used in mechanized maize production [46,47]. Phytoremediation assisted by plant growth regulators is a new, promising technique for improving maize seed germination, seedling growth, and population formation. This technique also increases grain yield and improves the efficiency and applicability of phytoremediation practices [48,49]. Chelating agents, such as ethylenediaminetetraacetic acid (EDTA) and iminodisuccinic acid (IDS), have been widely used to enhance soil washing and metal phytoextraction. Different from the poor biodegradability of EDTA, a major proportion of IDS biodegrades after one week of treatment [50]. Moreover, IDS is characterized by its low environmental impact owing to its low toxicity [51]. However, the effects of IDS on maize growth under lead (Pb) stress remain unclear.
Hence, the objective of this study was twofold: to analyze the effects of IDS on seed germination and seedling growth under Pb-stressed and Pb-free conditions and to explore the regulatory mechanism underlying the effects of IDS on the seed germination process in terms of ROS balance to provide theoretical and technical support to facilitate the efficient implementation of phytoremediation projects for contaminated soil treatment.
2. Results
2.1. Effects of IDS and Pb on Maize Seed Germination
In the absence of heavy metal stress (Figure 1), an increase in the concentration of IDS gradually delayed the germination of maize seeds. Treatment with 100 mmol·dm−3 IDS significantly delayed seed germination. Compared with the germination rate (GR) of seeds in the distilled water (control) treatment group, the GR of seeds treated with 100 mmol·dm−3 IDS was approximately 58%, 62%, 52%, and 9% less at 1, 2, 3, and 4 d after sowing, respectively (Figure 1, p < 0.05). However, 5 d after sowing, significant differences were not observed in the final GR of seeds across the IDS treatment groups.
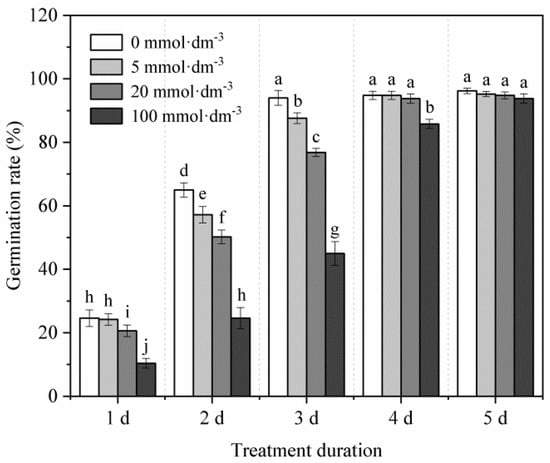
Figure 1.
Effects of IDS on maize seed germination. Maize seeds were treated with different concentrations (0, 5, 20, and 100 mmol·dm−3) of the chelating agent IDS. The number of germinated maize seeds was recorded at 1, 2, 3, 4, and 5 d after sowing. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different between treatments and time points (p < 0.05; Duncan’s new multiple range test). IDS, iminodisuccinic acid.
Although the final GR of seeds was reduced to approximately 18% under heavy metal stress (20 mmol·dm−3 PbCl2), the IDS treatment effectively alleviated the inhibitory effect of Pb stress on seed germination in maize (Figure 2). For instance, compared with the GR after the treatment with Pb alone, the GR after the treatment with 100 mmol·dm−3 IDS under conditions of Pb stress was approximately 2.03-, 1.85-, 1.86-, 1.92-, and 1.85-fold higher at 1, 2, 3, 4, and 5 d after sowing, respectively (Figure 2, p < 0.05). Similar changes were observed for maize seed germination under Pb stress in groups treated with 5 and 20 mmol·dm−3 IDS (Figure 2, p < 0.05).
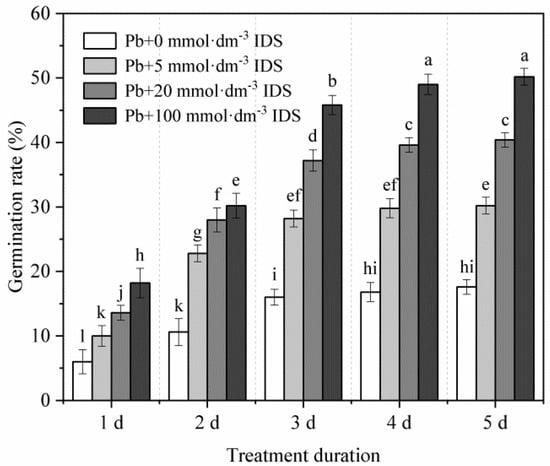
Figure 2.
Effects of IDS on maize seed germination under Pb stress. Maize seeds were treated with 20 mmol·dm−3 PbCl2 + 0 mmol·dm−3 IDS, 20 mmol·dm−3 PbCl2 + 5 mmol·dm−3 IDS, 20 mmol·dm−3 PbCl2 + 20 mmol·dm−3 IDS, or 20 mmol·dm−3 PbCl2 + 100 mmol·dm−3 IDS solutions. The number of germinated maize seeds was recorded at 1, 2, 3, 4, and 5 d after sowing. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different between treatments and time points (p < 0.05 level; Duncan’s new multiple range test). IDS, iminodisuccinic acid; Pb, lead.
2.2. Effects of Pb and IDS on Seedling Growth
Five days after sowing, we assessed the growth of maize seedlings treated with different concentrations of IDS under Pb-stressed and Pb-free conditions. Under normal incubation conditions, treatment with high concentrations of IDS slightly but significantly reduced the growth of maize seedlings. For instance, the seedling sprout length decreased by approximately 7% and 12% after treatment with 20 and 100 mmol·dm−3 IDS, respectively (Figure 3, p < 0.05). By contrast, Pb stress-inhibited seedling growth was significantly alleviated by IDS. Compared with the sprout length of seedlings under Pb stress alone, that of seedlings treated with 5, 20, and 100 mmol·dm−3 IDS while under Pb stress was approximately 20%, 50%, and 67% higher, respectively (Figure 3, p < 0.05).
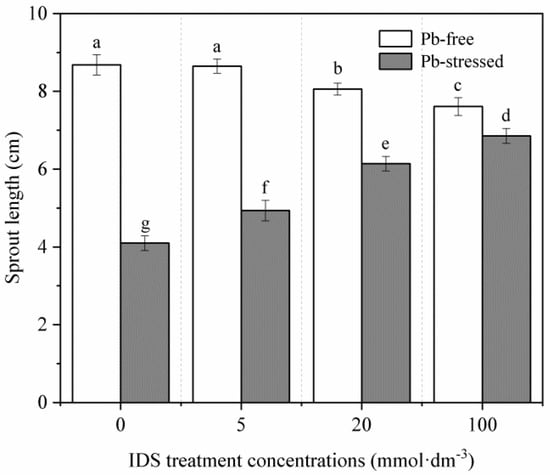
Figure 3.
Effects of IDS on the sprout length of maize seedlings under Pb-stressed and Pb-free conditions. Maize seeds treated with Pb (20 mmol·dm−3 PbCl2) and distilled water were further treated with different concentrations (0, 5, 20, and 100 mmol·dm−3) of IDS solutions. The sprout lengths of 5-day-old maize seedlings were measured. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different between treatments and time points (p < 0.05; Duncan’s new multiple range test). IDS, iminodisuccinic acid; Pb, lead.
2.3. Effects of ROS-Related Reagents on Seed Germination
The germination of maize seeds treated with Pb, IDS, or their combination was significantly affected by treatment with redox reagents, such as hydrogen peroxide (H2O2) and dimethylthiourea (DMTU) (Figure 4). After the maize seeds were incubated with Pb or Pb+IDS for 24 h, the GR of seeds treated with H2O2 was significantly reduced by approximately 60% and 27%, respectively, compared with seeds without H2O2 treatment (Figure 4, p < 0.05). A contrasting effect was observed in seeds treated with DMTU. For instance, in seeds incubated with Pb and Pb+IDS for 24 h, treatment with DMTU increased the GR by approximately 80% and 22%, respectively (Figure 4, p < 0.05). However, the GR of IDS-treated seeds increased by 46% and decreased by 42% after treatment with H2O2 and DMTU, respectively (Figure 4, p < 0.05).
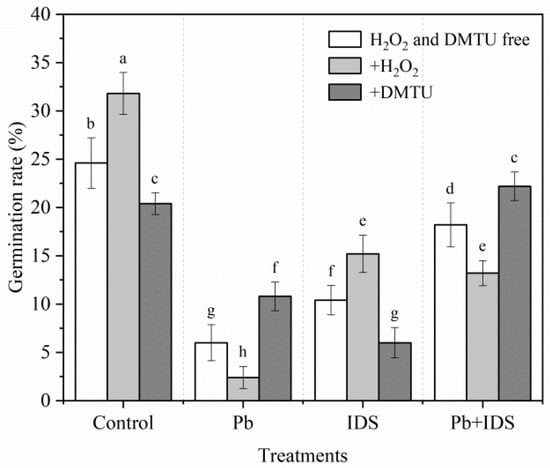
Figure 4.
Effects of DMTU and H2O2 on the germination of maize seeds treated with IDS, Pb, or their combination. Maize seeds treated with distilled water (control), Pb (20 mmol·dm−3 PbCl2), IDS (100 mmol·dm−3), or Pb+IDS were further treated with DMTU or H2O2 for 1 d. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different among treatments and time points (p < 0.05; Duncan’s new multiple range test). Pb, lead; IDS, iminodisuccinic acid; DMTU, dimethylthiourea.
We investigated the effects of diphenyleneiodonium chloride (DPI) (a specific inhibitor of NOX) and CaCl2 on maize seed germination in the distilled water (control), Pb, IDS, and Pb+IDS treatment groups during the first 24 h of incubation (Figure 5). In the control and IDS treatment groups, treatment with DPI reduced the GR of maize seeds by approximately 31% and 50%, respectively, compared with that in the DPI- and CaCl2-free conditions (Figure 5, p < 0.05). However, after the addition of DPI, the GR was approximately 120% and 26% higher in the Pb and Pb+IDS treatment groups, respectively, than in the corresponding DPI- and CaCl2-free condition (Figure 5, p < 0.05). Furthermore, compared with that in the DPI- and CaCl2-free conditions, the addition of CaCl2 increased the GR by approximately 21% and 25% in the control and IDS treatment groups, and reduced the GR by approximately 43% and 22% in the Pb and Pb+IDS treatment groups, respectively (Figure 5, p < 0.05).
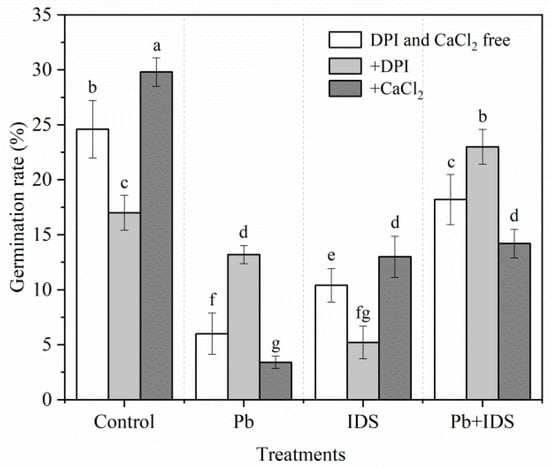
Figure 5.
Effects of DPI and CaCl2 on the germination of maize seeds treated with IDS, Pb, or Pb+IDS. Maize seeds treated with distilled water (control), Pb (20 mmol·dm−3 PbCl2), IDS (100 mmol·dm−3), or Pb+IDS were further treated with DPI or CaCl2 for 1 d. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different among treatments and time points (p < 0.05; Duncan’s new multiple range test). Pb, lead; IDS, iminodisuccinic acid; DPI, diphenyleneiodonium chloride.
Next, we investigated the effects of the antioxidant enzyme inhibitors diethyldithiocarbamic acid (DDC) (a specific inhibitor of SOD) and aminotriazole (ATZ) (a specific inhibitor of CAT) on the germination of maize seeds treated with Pb, IDS, or Pb+IDS. Twenty-four hours after the addition of DDC and ATZ, the GR of distilled water (control)- and IDS-treated maize seeds was significantly increased by approximately 16% and 33% (DDC) and by 20% and 44% (ATZ), respectively (Figure 6). However, treatment with DDC and ATZ enhanced the inhibitory effects of Pb and Pb+IDS on the germination of maize seeds. For instance, after treatment with DDC and ATZ, the GR of maize seeds was 30% and 24%, respectively, less than that of the DDC and ATZ-free seeds in the Pb+IDS-treated group (Figure 6, p < 0.05).
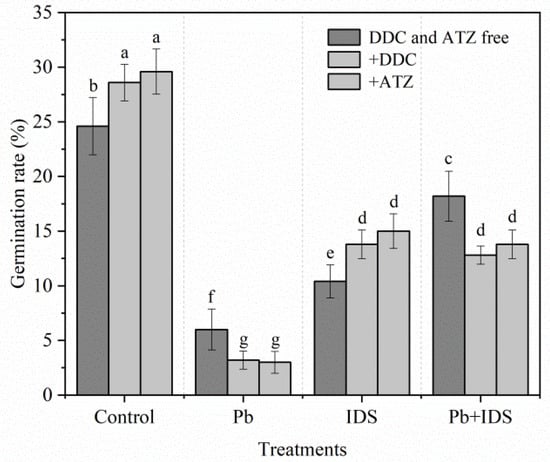
Figure 6.
Effects of DDC and ATZ on the germination of maize seeds treated with distilled water, IDS, Pb, or Pb+IDS. Maize seeds treated with distilled water (control), Pb (20 mmol·dm−3 PbCl2), IDS (100 mmol·dm−3), or Pb+IDS were further incubated with DDC or ATZ for 1 d. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different among treatments and time points (p < 0.05; Duncan’s new multiple range test). Pb, lead; IDS, iminodisuccinic acid; DDC, diethyldithiocarbamic acid; ATZ, aminotriazole.
2.4. Effects of Pb and IDS on H2O2 Content and NOX Activity
We determined the effects of Pb, IDS, and Pb+IDS on H2O2 accumulation in maize seeds after incubation for 1, 3, and 5 d (Figure 7). Throughout germination, the H2O2 content of maize seeds showed a significant increasing trend. In the control group treated with distilled water, the H2O2 content increased significantly by 53% and 94% in maize seeds after incubation for 3 and 5 d, respectively (Figure 7, p < 0.05), compared with that after incubation for 1 d. However, H2O2 accumulation was significantly affected by treatment with IDS and Pb. After incubation for 1 d, Pb and Pb+IDS increased the H2O2 content of germinating seeds by approximately 80% and 49%, respectively, compared with the distilled water (control) treatment (Figure 7, p < 0.05). After 1, 3, and 5 d of treatment with IDS, the H2O2 content of germinating seeds was approximately 29%, 22%, and 11% lower, respectively, than that in the distilled water (control) treatment group (Figure 7, p < 0.05).
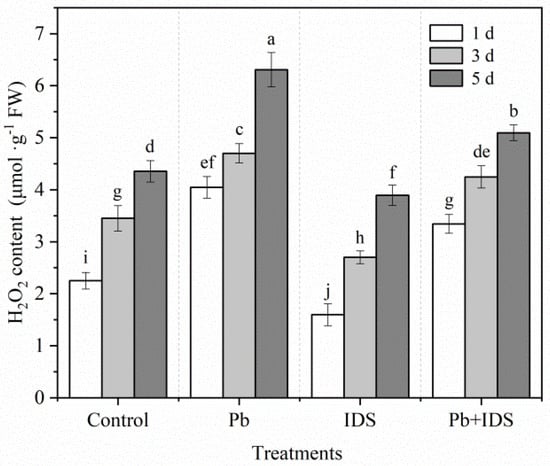
Figure 7.
Effects of Pb, IDS, and Pb+IDS on the H2O2 content of maize seeds. Maize seeds treated with distilled water (control), Pb (20 mmol·dm−3 PbCl2), IDS (100 mmol·dm−3), or Pb+IDS were used, and their H2O2 contents were measured 1, 3, and 5 d after sowing. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different among treatments and time points (p < 0.05; Duncan’s new multiple range test). Pb, lead; IDS, iminodisuccinic acid; FW, fresh weight.
Germination can significantly enhance NOX activity in maize seeds after incubation. Compared with the NOX activity after incubation for 1 d, germination increased the NOX activity by 47% and 79% after incubation for 3 and 5 d, respectively (Figure 8). After incubation for 3 d, Pb and Pb+IDS significantly enhanced NOX activity by approximately 85% and 46%, respectively, compared with that in maize seeds incubated with distilled water (control) (Figure 8, p < 0.05). However, IDS significantly decreased NOX activity by 33%, 25%, and 11% after germination for 1, 3, and 5 d, respectively, compared with that in the control group (Figure 8, p < 0.05).
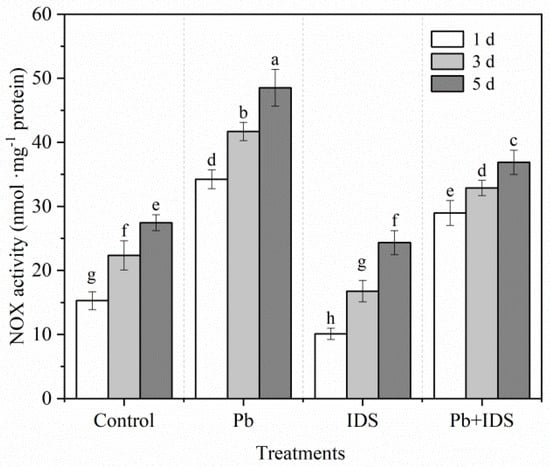
Figure 8.
Effects of Pb, IDS, and Pb+IDS on NOX activities of maize seeds. Maize seeds incubated with distilled water (control), Pb (20 mmol·dm−3 PbCl2), IDS (100 mmol·dm−3), or Pb+IDS were used, and NOX activities were measured 1, 3, and 5 d after sowing. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different among treatments and time points (p < 0.05; Duncan’s new multiple range test). Pb, lead; IDS, iminodisuccinic acid; NOX, NADPH oxidase.
2.5. Effects of Pb and IDS on Antioxidant Enzyme Activities
The effects of Pb, IDS, and Pb+IDS on the SOD and CAT activities in maize seeds after incubation for 1, 3, and 5 d are illustrated in Figure 9 and Figure 10. Germination of distilled water-treated maize seeds for 5 d enhanced the activities of SOD and CAT by approximately 53% and 72%, respectively, compared with that of seeds germinated for 1 d (Figure 9 and Figure 10, p < 0.05). After treatment with Pb and Pb+IDS, the SOD activity was increased and was approximately 20–30% and 40–56% higher, respectively, than that of the distilled water (control) treatment after incubation for 1–5 d (Figure 9, p < 0.05). Similar patterns of change were observed for CAT activity in maize seeds (Figure 10, p < 0.05). Compared with distilled water treatment, Pb and Pb+IDS treatment increased CAT activity in maize seeds incubated for 1–5 d, by approximately 10–17% and 18–41%, respectively (Figure 10, p < 0.05). However, IDS decreased SOD and CAT activities. Treatment with IDS reduced SOD and CAT activities by approximately 13–32% and 10–25%, respectively, in maize seeds after incubation for 1–5 d, compared with those in the control (Figure 9 and Figure 10, p < 0.05).
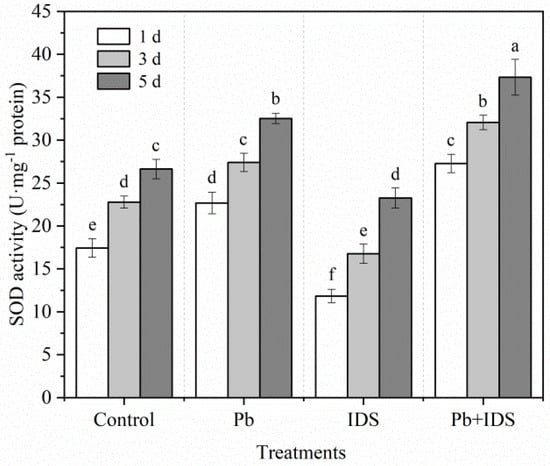
Figure 9.
Effects of Pb, IDS, and Pb+IDS on SOD activities of maize seeds. Maize seeds treated with distilled water (control), Pb (20 mmol·dm−3 PbCl2), IDS (100 mmol·dm−3), or Pb+IDS were used, and their SOD activities were measured 1, 3, and 5 d after sowing. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different among treatments and time points (p < 0.05; Duncan’s new multiple range test). Pb, lead; IDS, iminodisuccinic acid; SOD, superoxide dismutase.
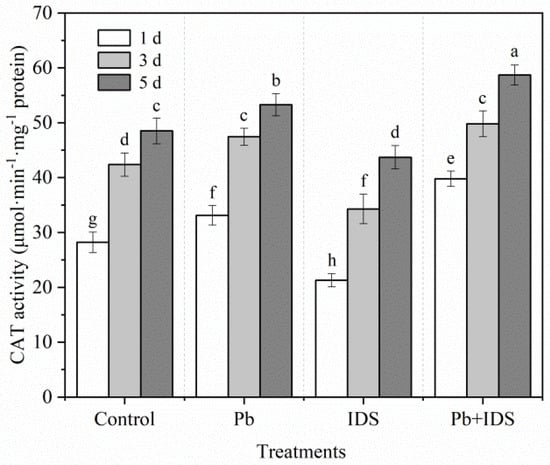
Figure 10.
Effects of Pb, IDS, and Pb+IDS on CAT activities of maize seeds. Maize seeds treated with distilled water (control), Pb (20 mmol·dm−3 PbCl2), IDS (100 mmol·dm−3), or Pb+IDS were used, and their CAT activities were measured 1, 3, and 5 d after sowing. Error bars represent the standard deviation of the mean (n = 5). Means associated with different letters are significantly different among treatments and time points (p < 0.05; Duncan’s new multiple range test). Pb, lead; IDS, iminodisuccinic acid; CAT, catalase.
3. Discussion
To counter the significant increase in the Pb content of cultivated soils near industrial areas [52,53], chelating agents, such as EDTA and IDS, are used for phytoremediation projects because these agents can remove heavy metals from contaminated soils [43,54]. Maize is considered a potential phytoremediation crop for heavy metal-polluted soil [19,21,22,23]. Seen as direct seeding technology is commonly used in maize cultivation [46,47], seed germination and subsequent seedling growth are sensitive to the environment, especially in heavy metal-contaminated soils [55,56]. However, few studies have investigated the effects of chelators on seed germination and subsequent seedling growth. Therefore, in this study, we investigated the effects of IDS on maize seed germination and subsequent seedling growth under Pb-stressed and Pb-free conditions.
Although significant differences were not observed in the final GR, treatment with 100 mmol·dm−3 IDS noticeably delayed the germination of maize seeds that were not subjected to Pb stress (Figure 1). Similarly, higher concentrations of IDS also delayed or slightly (but significantly) inhibited the growth of maize seedlings (Figure 3). One plausible explanation for this phenomenon is that these chelators can chelate essential elements (such as Ca, Fe, Cu, Mn, and Zn) that are indispensable for seed germination [57]. These findings suggest that IDS, at least at low concentrations, exhibits low levels of adverse effects on seed germination and subsequent seedling growth in maize. However, under conditions of Pb stress, treatment with 5−100 mmol·dm−3 IDS significantly improved seed germination and seedling growth to different extents (Figure 2 and Figure 3). This finding indicates that treatment with Pb+IDS substantially reduces the toxic effects of Pb on seed germination and seedling growth. Based on the “oxidative window for germination” theory, low or high ROS levels deter seed germination [58]. Therefore, we speculated that ROS levels may be associated with the IDS-mediated alteration of redox balance during seed germination in maize.
Studies have suggested that ROS levels can be regulated by ROS producers (e.g., NOX) and ROS-scavenging enzymes (e.g., SOD and CAT) [41]. In this study, we investigated the effects of IDS on the GR of maize seeds by adding ROS-related redox agents under Pb-stressed and Pb-free conditions. The Pb treatment inhibited seed germination more than the distilled water treatment in the control group. Moreover, these inhibitory effects were aggravated by H2O2 but alleviated by DMTU (a specific scavenger of ROS) [59] after incubation for 24 h (Figure 4). This result suggests that Pb can induce ROS overproduction, which inhibits germination in maize seeds after incubation. IDS-delayed seed germination was improved by H2O2 and aggravated by DMTU in Pb-free conditions, and the opposite results were observed in Pb-stressed conditions (Figure 4). Therefore, under favorable growth conditions, IDS may delay maize seed germination and inhibit seedling growth by limiting the production of H2O2. Although adding H2O2 to the Pb+IDS treatment group inhibited the germination process, adding DMTU promoted it. Taken together, these results demonstrate that IDS positively regulates seed germination in maize by reducing the overproduction of H2O2 under conditions of Pb stress.
We also investigated the effects of DPI (a specific inhibitor of NOX) [60] and Ca2+ (a non-specific activator of NOX) [61] on seed germination in maize (Figure 5). The groups treated with Pb and Pb+IDS had a lower GR than the control group; however, the GR of the Pb+IDS treatment group was significantly higher than that of the Pb treatment group. The inhibition of seed germination in the Pb and Pb+IDS treatment groups was partially decreased by the DPI treatment but aggravated further by the CaCl2 treatment. Under Pb-free conditions, however, the IDS-delayed seed germination was enhanced by DPI but reversed by CaCl2. This phenomenon suggests that NOX-mediated ROS play a key role in IDS-regulated seed germination under Pb-stressed or Pb-free conditions. In addition, treatment with DDC (an inhibitor of SOD) and ATZ (an inhibitor of CAT) [62] further reduced the GR in Pb- and Pb+IDS-treated maize seeds after incubation for 24 h (Figure 6). This reduction confirmed that IDS can attenuate Pb stress-induced ROS overproduction to a certain extent. In contrast, treatment with the inhibitors of SOD and CAT enhanced the GR in distilled water- and IDS-treated maize seeds (Figure 6). These results suggest that IDS reduces ROS production in germinating seeds and ROS-scavenging enzymes (e.g., SOD and CAT) and also play a notable role in IDS-regulated seed germination under favorable and Pb-stressed conditions.
To further investigate the IDS-regulated ROS balance during maize seed germination under Pb stress, we measured the H2O2 content of seeds after the incubation period. Pb stress induced H2O2 overproduction in germinating seeds. However, IDS significantly decreased the Pb-enhanced H2O2 levels and reduced H2O2 accumulation under Pb-free conditions (Figure 7). NOX, a major ROS-producing enzyme, plays a key role in regulating seed germination [60]. Our results show that IDS treatment slightly (but significantly) reduced NOX activity compared with that by distilled water treatment (Figure 8). In contrast, Pb stress significantly enhanced NOX activity. However, Pb-enhanced NOX activity was significantly attenuated by IDS (Figure 8). Thus, the results of the addition of ROS-related redox agents and ROS-related enzyme activity assays can be partially attributed to the chelating activity of IDS, that is, IDS chelates Ca2+, restricting the activation of NOX [61]. These results also suggest that NOX-produced ROS are required for IDS-regulated seed germination.
The activities of ROS-scavenging enzymes (SOD and CAT) increased gradually during seed germination in maize. However, compared with those in the control, SOD and CAT activities decreased significantly in the IDS-treated groups after incubation for 1, 3, and 5 d (Figure 9 and Figure 10). This can be partly attributed to the decrease in IDS-induced NOX activity, which resulted in decreased levels of ROS, such as O2− and H2O2. Pb stress increased SOD and CAT activities, which were further enhanced upon IDS treatment of the maize seeds after incubation. This finding indicates that IDS requires increased activities of ROS-scavenging enzymes (such as SOD and CAT) to improve seed germination under Pb stress. The single and combined treatment with IDS and Pb revealed that under Pb stress, IDS, as a chelating agent of metal ions, did not restrict the activities of metal-containing SOD and CAT by chelating metal ions, such as Fe, Cu, Mn, and Zn, which are required for the activation of these enzymes [41], but played a promoting role in comparison with the inhibitory effect of chelating Ca2+ on the activity of NOX. Therefore, further investigation is required to elucidate the mechanism underlying the regulation of antioxidant enzymes by IDS. However, protecting antioxidant enzymes from Pb-induced overproduction of ROS or free radicals, which can inactivate SOD and CAT [63,64], may be one of the crucial ways to promote seed germination and rescue seeds using IDS (Figure 2 and Figure 3).
To better understand the aforementioned findings, we propose a hypothetical model based on ROS levels to illustrate the mechanisms underlying IDS-regulated seed germination in Pb-free and Pb-stressed conditions (Figure 11). This model proposes that IDS-regulated seed germination in maize is closely associated with ROS levels. IDS reduces the activities of ROS-producing (e.g., NOX) and ROS-scavenging (e.g., SOD and CAT) enzymes in maize seeds incubated in normal environments. However, under conditions of Pb stress, IDS inhibits the activity of ROS-producing enzymes, increases the activities of ROS-scavenging enzymes, and promotes the germination of seeds in a Pb-stressed environment. In this above process, we suggest that Ca2+ chelation, which restricts NOX activity and protects and promotes SOD and CAT activity by inhibiting ROS overproduction, may be important for maintaining the ROS balance and improving seed germination and seedling growth under Pb stress resulting from IDS treatment in maize. In addition, IDS slightly interferes with the activity of SOD and CAT through the chelation of metal ions such as Fe, Zn, Cu, and Mn.
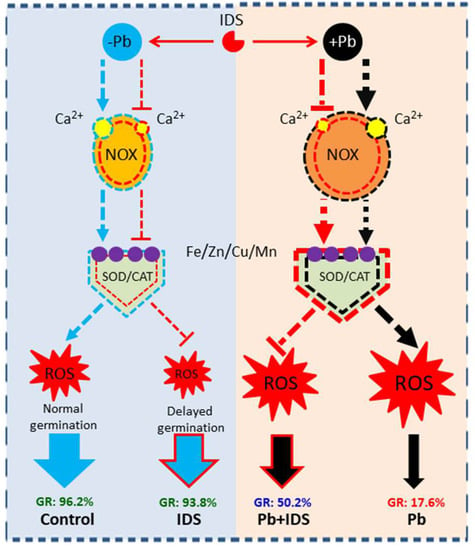
Figure 11.
Hypothetical model based on ROS scavenging and production illustrating IDS-regulated seed germination processes under Pb-free (−Pb) and Pb-stressed (+Pb) conditions. The blue dotted line indicates the control treatment (distilled water), the black dotted line indicates treatment with Pb stress, and the red dotted line indicates treatment with IDS. Pointed and blunt dashed arrows represent the positive and negative effects of these treatments, respectively. The thickness of the dotted line indicates the degree of influence of different treatments on relevant indicator values. Pb, lead; ROS, reactive oxygen species; IDS, iminodisuccinic acid; NOX, NADPH oxidase; SOD, superoxide dismutase; CAT, catalase; GR, germination rate.
4. Materials and Methods
4.1. Reagent Preparation
All chemical reagents used in this work were of analytical grade. DPI, DMTU, DDC, and ATZ were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
4.2. Seed Treatment Groups
The seeds of the hybrid maize variety ‘Zhengdan 958’ used in the experiments were purchased from DONEED Seed Industry Co., Ltd, Beijing, China. The seeds were harvested in October 2020 and used in this experimental study in April 2021. The seed purity was greater than 99%, and the GR was greater than 95%. Before sowing, the seeds were surface-sterilized with 0.1% NaClO for 5 min. Healthy and uniform seeds with an intact seed coat were selected and submerged in distilled water (25 °C) for 1 h. The swollen seeds were then sown in a plastic box containing quartz stones (diameter: approximately 2–3 mm) to a depth of 2 cm. The plastic box was covered with parafilm and placed in an incubator (KWB720, Binder GmbH, Tuttlingen, Germany). After incubation for 1, 3, and 5 d, the germinated seeds were collected and stored at −80 °C for use in further analysis.
The experiment included three treatment groups (all at 25 °C) as follows:
- Group 1: The swollen seeds were sown in a plastic box containing quartz stones moistened with 0, 5, 20, or 100 mmol·dm−3 IDS solution. The GR and seedling growth were monitored after sowing.
- Group 2: In accordance with the results of our previous study [45], we used 20 mmol·dm−3 PbCl2 as the Pb stress treatment condition. The swollen seeds were sown in a plastic box containing quartz stones moistened with 20 mmol·dm−3 PbCl2 + 0 mmol·dm−3 IDS, 20 mmol·dm−3 PbCl2 + 5 mmol·dm−3 IDS, 20 mmol·dm−3 PbCl2 + 20 mmol·dm−3 IDS, or 20 mmol·dm−3 PbCl2 + 100 mmol·dm−3 IDS solution. GR and seedling growth were monitored after sowing.
- Group 3: The swollen seeds were sown in a plastic box containing quartz stones moistened with distilled water (control), PbCl2 (20 mmol·dm−3), IDS (100 mmol·dm−3), or PbCl2 (20 mmol·dm−3) + IDS (100 mmol·dm−3) solution. Subsequently, DMTU (a specific scavenger of ROS, 10 mmol·dm−3), H2O2 (10 mmol·dm−3), DPI (a specific inhibitor of NOX, 0.1 mmol·dm−3), CaCl2 (0.1 mmol·dm−3), DDC (a specific inhibitor of SOD, 1 mmol·dm−3), and ATZ (a specific inhibitor of CAT, 1 mmol·dm−3) were applied separately. One milliliter of each ROS-related reagent solution was uniformly added to the quartz stones moistened with the distilled water, PbCl2, IDS, or PbCl2+IDS solutions, respectively.
All assays were repeated at least five times to minimize experimental error, and each replicate contained 100 seeds. The treatment concentration used in this study was based on the results of our preliminary experiments.
4.3. Determination of GR
The maize seeds were considered to have germinated when radicle emergence from the seeds was ≥1 mm. The number of germinated seeds was counted three times per day. The final GR was calculated as the percentage of seeds that had germinated 5 d after sowing [45].
4.4. Determination of Seedling Growth
Five-day-old maize seedlings (grown from seeds treated with PbCl2, IDS, or their combination) were washed with distilled water. Excess water was removed using bibulous paper, and the sprout length was measured using a vernier caliper.
4.5. Determination of H2O2 Content
The H2O2 contents of the maize seeds in different treatment groups (control, heavy metal-treated, and combination treatments) were determined using colorimetric measurements following the method described by Zhang et al. [65]. H2O2 was extracted by homogenizing germinating maize seeds (~0.5 g) in 3 mL of cold acetone. The samples were homogenized using a tissue homogenizer (Tissuelyser-24, Jingxin Industrial Development Co. Ltd., Shanghai, China) at 4 °C and immediately centrifuged at 12,000 rpm for 15 min. A 1 mL aliquot of the supernatant solution was mixed with 0.1 mL of 5% titanium sulfate in concentrated HCl, followed by the addition of 0.2 mL aqueous NH3 (25%) to precipitate the peroxide–titanium complex. The mixture was then centrifuged at 12,000 rpm for 15 min. The precipitate was solubilized in 3 mL of 2 mmol·dm−3 H2SO4, and the absorbance was measured at 415 nm. A standard response curve was prepared with a known concentration of H2O2 using the aforementioned method.
4.6. Determination of Antioxidant Enzyme Activities
Germinating maize seeds (~3 g) were ground to a powder in liquid nitrogen. The powder was then homogenized in 30 mL of an ice-cold extraction buffer, which consisted of 0.1 mol dm−3 Tris–HCl (pH 7.5), 0.23 mol dm−3 sucrose, 5% polyvinylpyrrolidone, 1 mmol·dm−3 EDTA, 10 mmol·dm−3 KCl, 10 mmol·dm−3 MgCl2, and 2.5 mmol·dm−3 ascorbic acid (added to the buffer just before use). After homogenization and centrifugation (12,000 rpm for 20 min), the supernatants were used for the enzyme assays.
To measure SOD activity, the reaction buffer (containing 13 mmol·dm−3 methionine, 75 μmol dm−3 nitro blue tetrazolium, 100 μmol dm−3 EDTA, and 2 μmol dm−3 riboflavin) was mixed with different volumes of enzyme extract in 50 mmol·dm−3 phosphate buffer (pH 7.8). The mixture was then exposed to light for 15 min. An increase in absorbance at 560 nm was observed due to formazan formation. One unit of SOD activity was defined as the amount of enzyme that inhibited nitro blue tetrazolium photoreduction by 50% [66].
To measure CAT activity, a reaction mixture was prepared in such way that 3 mL of it contained 150 mmol·dm−3 potassium phosphate buffer (pH 7.0), 15 mmol·dm−3 H2O2, and 50 μL enzyme extract. The reaction was initiated by adding 15 mmol·dm−3 H2O2. CAT activity was determined based on the consumption of H2O2 (E = 39.4 dm3·mmol−1·cm−1) at 240 nm for 3 min [67].
The soluble protein content was determined using the Bradford method [68], with bovine serum protein as a standard.
4.7. NADPH Oxidase Enzyme Assay
The NOX enzyme was extracted, and its activity was assayed using a Plant NOX assay kit (GMS50096.3 v.A, GenMed Scientifics Inc., Arlington, MA, USA), following the manufacturer’s instructions. The reaction was initiated by adding 0.2 mmol·dm−3 NADPH to a mixture containing 100 mmol·dm−3 Tris–HCl and 1 mmol·dm−3 EDTA. NOX activity was calculated by monitoring the decrease in absorbance at 340 nm, and the molar extinction coefficient used for NADPH was 6.22 × 103 dm3·mmol−1·cm−1 at pH 8.0 [69].
4.8. Data Analysis
All experiments included five replicates, and the values represent the mean ± standard deviation (SD) of the five replicates. Statistical analyses were performed using analysis of variance (ANOVA) with SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). The differences between treatments were determined by Duncan’s new multiple range test and considered statistically significant at p < 0.05.
5. Conclusions
Several significant conclusions can be drawn from the results of this study. First, IDS (100 mmol·dm−3) markedly delayed maize seed germination in the absence of Pb stress. Second, the Pb-mediated inhibition of seed germination and seedling growth was significantly alleviated by IDS. Third, IDS significantly inhibited the activity of ROS-producing enzymes, reduced the level of H2O2 in maize seeds under normal conditions, and markedly weakened the activities of ROS-producing enzymes under Pb stress. In contrast, the Pb stress-induced increase in the activities of ROS-scavenging enzymes was significantly enhanced by IDS. Under Pb stress, NOX (activated by Ca2+), SOD, and CAT were involved in maintaining the IDS-regulated ROS balance and promoting seed germination. Thus, Ca2+ chelation and protection of antioxidant enzymes may be essential for the regulatory roles of IDS. Overall, our results suggest that IDS can be used as an inexpensive, biodegradable, and readily available reagent for widespread application in agriculture to improve seed germination in Pb-polluted soils.
Author Contributions
Conceptualization, Writing and Editing, and Project Administration: Y.Z.; Visualization and Project Administration: Y.S., W.L. and Z.L.; Investigation, Validation, and Formal Analysis: J.L., R.X., J.D. and G.L.; Funding Acquisition and Supervision: K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Nature Scientific Foundation of Heilongjiang Province (Grant number: LH2019C051); the Postdoctoral Science Foundation Funded General Project of Heilongjiang Province (Grant number: LBH-Z19196); University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (Grant number: UNPYSCT-2020037); the Scientific Research Project for People Returned after Further Learn and Talent Introduction in Heilongjiang Bayi Agricultural University (Grant number: XYB2014-03); and the Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (Grant number: 2019-KYYWF-0546, TDJH201902).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We are grateful to Xingfen Miao for technical assistance and Song Yu for helpful discussion of data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M.; et al. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Yousaf, H.S.; Malik, A.; Abbas, Z.; Rizwan, M.; Abualreesh, M.H.; Alatawi, A.; Wang, X. Combined application of zinc and iron-lysine and its effects on morpho-physiological traits, antioxidant capacity and chromium uptake in rapeseed (Brassica napus L.). PLoS ONE 2022, 17, e0262140. [Google Scholar] [CrossRef] [PubMed]
- Afzal, J.; Wang, X.; Saleem, M.H.; Sun, X.; Hussain, S.; Khan, I.; Rana, M.S.; Ahmed, S.; Awan, S.A.; Fiaz, S.; et al. Application of ferrous sulfate alleviates negative impact of cadmium in rice (Oryza sativa L.). Biocell 2021, 45, 1631–1649. [Google Scholar] [CrossRef]
- Ghani, M.A.; Abbas, M.M.; Ali, B.; Aziz, R.; Qadri, R.W.K.; Noor, A.; Azam, M.; Bahzad, S.; Saleem, M.H.; Abualreesh, M.H.; et al. Alleviating role of gibberellic acid in enhancing plant growth and stimulating phenolic compounds in carrot (Daucus carota L.) under lead stress. Sustainability 2021, 13, 12329. [Google Scholar] [CrossRef]
- Saleem, M.H.; Wang, X.; Ali, S.; Zafar, S.; Nawaz, M.; Adnan, M.; Fahad, S.; Shah, A.; Alyemeni, M.N.; Hefft, D.I.; et al. Interactive effects of gibberellic acid and NPK on morpho-physio-biochemical traits and organic acid exudation pattern in coriander (Coriandrum sativum L.) grown in soil artificially spiked with boron. Plant Physiol. Biochem. 2021, 167, 884–900. [Google Scholar] [CrossRef]
- Kamal, A.; Saleem, M.H.; Alshaya, H.; Okla, M.K.; Chaudhary, H.J.; Munis, M.F.H. Ball-milled synthesis of maize biochar-ZnO nanocomposite (MB-ZnO) and estimation of its photocatalytic ability against different organic and inorganic pollutants. J. Saudi Chem. Soc. 2022, 26, 101445. [Google Scholar] [CrossRef]
- Tariq, M.; Ahmad, B.; Adnan, M.; Mian, I.A.; Khan, S.; Fahad, S.; Saleem, M.H.; Ali, M.; Mussarat, M.; Ahmad, M.; et al. Improving boron use efficiency via different application techniques for optimum production of good quality potato (Solanum tuberosum L.) in alkaline soil. PLoS ONE 2022, 17, e0259403. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Ashraf, M.A.; Ali, Q.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Zinc-lysine supplementation mitigates oxidative stress in rapeseed (Brassica napus L.) by preventing phytotoxicity of chromium, when irrigated with tannery wastewater. Plants 2020, 9, 1145. [Google Scholar] [CrossRef]
- Hussain, I.; Saleem, M.H.; Mumtaz, S.; Rasheed, R.; Ashraf, M.A.; Maqsood, F.; Rehman, M.; Yasmin, H.; Ahmed, S.; Ishtiaq, M.; et al. Choline chloride mediates chromium tolerance in spinach (Spinacia oleracea L.) by restricting its uptake in relation to morpho-physio-biochemical attributes. J. Plant Growth Regul. 2022, 41, 1594–1614. [Google Scholar] [CrossRef]
- Khan, A.A.; Gul, J.; Naqvi, S.R.; Ali, I.; Farooq, W.; Liaqat, R.; AlMohamadi, H.; Štěpanec, L.; Juchelková, D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.W.; Saroosh, S.; Mulk, S.; Hassan, M.N.; Yasmin, H.; Jabeen, Z.; Nosheen, A.; Shah, S.M.U.; Naz, R.; Hasnain, Z.; et al. Bacteria isolated from wastewater irrigated agricultural soils adapt to heavy metal toxicity while maintaining their plant growth promoting traits. Sustainability 2021, 13, 7792. [Google Scholar] [CrossRef]
- Kazamias, G.; Zorpas, A.A. Drill cuttings waste management from oil & gas exploitation industries through end-of-waste criteria in the framework of circular economy strategy. J. Clean. Prod. 2021, 322, 129098. [Google Scholar] [CrossRef]
- Sanzone, D.M.; Vinhaeiro, N.; Neff, J. Environmental Fates and Effects of Ocean Discharge of Drill Cuttings and Associated Drilling Fluids from Offshore Oil and Gas Operations [IOGP Report]; International Association of Oil & Gas Producers: London, UK, 2016; Volume 543. [Google Scholar]
- Leonard, S.A.; Stegemann, J.A. Stabilization/solidification of petroleum drill cuttings. J. Hazard. Mater. 2010, 174, 463–472. [Google Scholar] [CrossRef]
- De Almeida, P.C.; de Queiroz Fernandes Araújo, O.; de Medeiros, J.L. Managing offshore drill cuttings waste for improved sustainability. J. Clean. Prod. 2017, 165, 143–156. [Google Scholar] [CrossRef]
- Fernandes, I.; Pascoal, C.; Cássio, F. Intraspecific traits change biodiversity effects on ecosystem functioning under metal stress. Oecologia 2011, 166, 1019–1028. [Google Scholar] [CrossRef]
- Xu, T.; Wang, L.; Wang, X.; Li, T.; Zhan, X. Heavy metal pollution of oil-based drill cuttings at a shale gas drilling field in Chongqing, China: A human health risk assessment for the workers. Ecotoxicol. Environ. Saf. 2018, 165, 160–163. [Google Scholar] [CrossRef]
- Thewys, T.; Witters, N.; Van Slycken, S.; Ruttens, A.; Meers, E.; Tack, F.M.G.; Vangronsveld, J. Economic viability of phytoremediation of a cadmium contaminated agricultural area using energy maize. Part I: Effect on the farmer’s income. Int. J. Phytoremediation 2010, 12, 650–662. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.W.; Bashir, A.; Maqbool, A.; Tack, F.M.G.; Ok, Y.S. Cadmium phytoremediation potential of Brassica crop species: A review. Sci. Total Environ. 2018, 631–632, 1175–1191. [Google Scholar] [CrossRef]
- Van Slycken, S.; Witters, N.; Meers, E.; Peene, A.; Michels, E.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Wierinck, I.; et al. Safe use of metal-contaminated agricultural land by cultivation of energy maize (Zea mays). Environ. Pollut. 2013, 178, 375–380. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Zia-ur-Rehman, M.; Abbas, Z.; Hannan, F. Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: A critical review. Environ. Geochem. Health 2017, 39, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, Y.; Hu, N.; Shi, Y.; Li, T.; Zhao, Z. Differential responses of 23 maize cultivar seedlings to an arbuscular mycorrhizal fungus when grown in a metal-polluted soil. Sci. Total Environ. 2021, 789, 148015. [Google Scholar] [CrossRef] [PubMed]
- Meers, E.; Ruttens, A.; Hopgood, M.; Lesage, E.; Tack, F.M.G. Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 2005, 61, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Vigliotta, G.; Matrella, S.; Cicatelli, A.; Guarino, F.; Castiglione, S. Effects of heavy metals and chelants on phytoremediation capacity and on rhizobacterial communities of maize. J. Environ. Manag. 2016, 179, 93–102. [Google Scholar] [CrossRef]
- Wang, A.; Wang, M.; Liao, Q.; He, X. Characterization of Cd translocation and accumulation in 19 maize cultivars grown on Cd-contaminated soil: Implication of maize cultivar selection for minimal risk to human health and for phytoremediation. Environ. Sci. Pollut. Res. Int. 2016, 23, 5410–5419. [Google Scholar] [CrossRef]
- Maitra, S.; Singh, V. Invited review on ‘maize in the 21st century’ Emerging trends of maize biorefineries in the 21st century: Scientific and technological advancements in biofuel and bio-sustainable market. J. Cereal Sci. 2021, 101, 103272. [Google Scholar] [CrossRef]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, M.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Correction to: Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1635. [Google Scholar] [CrossRef]
- Soares, T.F.S.N.; dos Santos Dias, D.C.F.; Oliveira, A.M.S.; Ribeiro, D.M.; dos Santos Dias, L.A. Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar] [CrossRef]
- Watanabe, M.E. Phytoremediation on the brink of commericialization. Environ. Sci. Technol. 1997, 31, 182A–186A. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Nazli, F.; Mumtaz, M.Z.; Latif, M.; Al-Mosallam, M.S.; Alotaibi, F.S.; Dewidar, A.Z.; Mattar, M.A.; El-Shafei, A.A. Lead-Tolerant Bacillus Strains Promote Growth and Antioxidant Activities of Spinach (Spinacia oleracea) Treated with Sewage Water. Agronomy 2021, 11, 2482. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. 2022. Available online: https://www.atsdr.cdc.gov/ (accessed on 8 August 2021).
- Jayasri, M.A.; Suthindhiran, K. Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: Its potential role in phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef]
- Fatemi, H.; Pour, B.E.; Rizwan, M. Isolation and characterization of lead (Pb) resistant microbes and their combined use with silicon nanoparticles improved the growth, photosynthesis and antioxidant capacity of coriander (Coriandrum sativum L.) under Pb stress. Environ. Pollut. 2020, 266, 114982. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, F.; Li, Y.; Niu, W.; Li, J.; Yang, J.; Liu, K. Comparative analysis of the seed germination of pakchoi and its phytoremediation efficacy combined with chemical amendment in four polluted soils. Int. J. Phytoremediation 2020, 22, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef]
- Gomes, M.P.; Carneiro, M.M.L.C.; Nogueira, C.O.G.; Soares, A.M.; Garcia, Q.S. The system modulating ROS content in germinating seeds of two Brazilian savanna tree species exposed to As and Zn. Acta Physiol. Plant 2013, 35, 1011–1022. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Zularisam, A.W.; Sakinah, M.; Din, M.F.M. Lead induced oxidative stress and alteration in the activities of antioxidative enzymes in rice shoots. Biologia Plant. 2017, 61, 595–598. [Google Scholar] [CrossRef]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil washing with biodegradable chelating agents and EDTA: Effect on soil properties and plant growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef] [PubMed]
- Staszak, A.M.; Małecka, A.; Ciereszko, I.; Ratajczak, E. Differences in stress defence mechanisms in germinating seeds of Pinus sylvestris exposed to various lead chemical forms. PLoS ONE 2020, 15, e0238448. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, B.; Li, Z. Inhibition of NADPH oxidase increases defense enzyme activities and improves maize seed germination under Pb stress. Ecotoxicol. Environ. Saf. 2018, 158, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, P.; Zhao, Z.; Zhao, G.; Tian, B.; Wang, J.; Wang, G. Mapping QTL controlling maize deep-seeding tolerance-related traits and confirmation of a major QTL for mesocotyl length. Theor. Appl. Genet. 2012, 124, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Virk, S.S.; Fulton, J.P.; Porter, W.M.; Pate, G.L. Row-crop planter performance to support variable-rate seeding of maize. Precis. Agric. 2020, 21, 603–619. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef]
- Menhas, S.; Hayat, K.; Niazi, N.K.; Zhou, P.; Amna; Bundschuh, J.; Naeem, M.; Munis, M.F.H.; Yang, X.; Chaudhary, H.J. Microbe-EDTA mediated approach in the phytoremediation of lead-contaminated soils using maize (Zea mays L.) plants. Int. J. Phytoremediation 2021, 23, 585–596. [Google Scholar] [CrossRef]
- Kołodyńska, D. Application of a new generation of complexing agents in removal of heavy metal ions from different wastes. Environ. Sci. Pollut. Res. Int. 2013, 20, 5939–5949. [Google Scholar] [CrossRef]
- Kołodyńska, D. Iminodisuccinic acid as a new complexing agent for removal of heavy metal ions from industrial effluents. Chem. Eng. J. 2009, 152, 277–288. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Boussen, S.; Soubrand, M.; Bril, H.; Ouerfelli, K.; Abdeljaouad, S. Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 2013, 192, 227–236. [Google Scholar] [CrossRef]
- Guo, J.; Lv, X.; Jia, H.; Hua, L.; Ren, X.; Muhammad, H.; Wei, T.; Ding, Y. Effects of EDTA and plant growth-promoting rhizobacteria on plant growth and heavy metal uptake of hyperaccumulator Sedum alfredii Hance. J. Environ. Sci. 2020, 88, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Budelsky, R.A.; Galatowitsch, S.M. Effects of moisture, temperature, and time on seed germination of five wetland Carices: Implications for restoration. Restor. Ecol. 1999, 7, 86–97. [Google Scholar] [CrossRef]
- Jurado, E.; Flores, J. Is seed dormancy under environmental control or bound to plant traits? J. Veg. Sci. 2005, 16, 559–564. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Ziegelstein, R.C.; Zweier, J.L.; Mellits, E.D.; Younes, A.; Lakatta, E.G.; Stern, M.D.; Silverman, H.S. Dimethylthiourea, an oxygen radical scavenger, protects isolated cardiac myocytes from hypoxic injury by inhibition of Na(+)-Ca2+ exchange and not by its antioxidant effects. Circ. Res. 1992, 70, 804–811. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Tawaratsumida, T.; Zheng, S.; Yuasa, T.; Iwaya-Inoue, M. NADPH oxidases act as key enzyme on germination and seedling growth in barley (Hordeum vulgare L.). Plant Prod. Sci. 2010, 13, 45–52. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox. Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.M.; Park, J. Antioxidant enzyme inhibitors enhance singlet oxygen-induced cell death in HL-60 cells. Free Radic. Res. 2006, 40, 1190–1197. [Google Scholar] [CrossRef]
- Tabatabaie, T.; Floyd, R.A. Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Arch. Biochem. Biophys. 1994, 314, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.A.; Rubio, M.A.; Lissi, E.A. Sod and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 1996, 20, 285–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Xu, Z.; Shi, Z.; Chen, S.; Huang, X.; Chen, J.; Wang, X. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 2014, 65, 3189–3200. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996, 112, 1631–1640. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).