Characterization of Bacillus velezensis RDA1 as a Biological Control Agent against White Root Rot Disease Caused by Rosellinia necatrix
Abstract
1. Introduction
2. Results
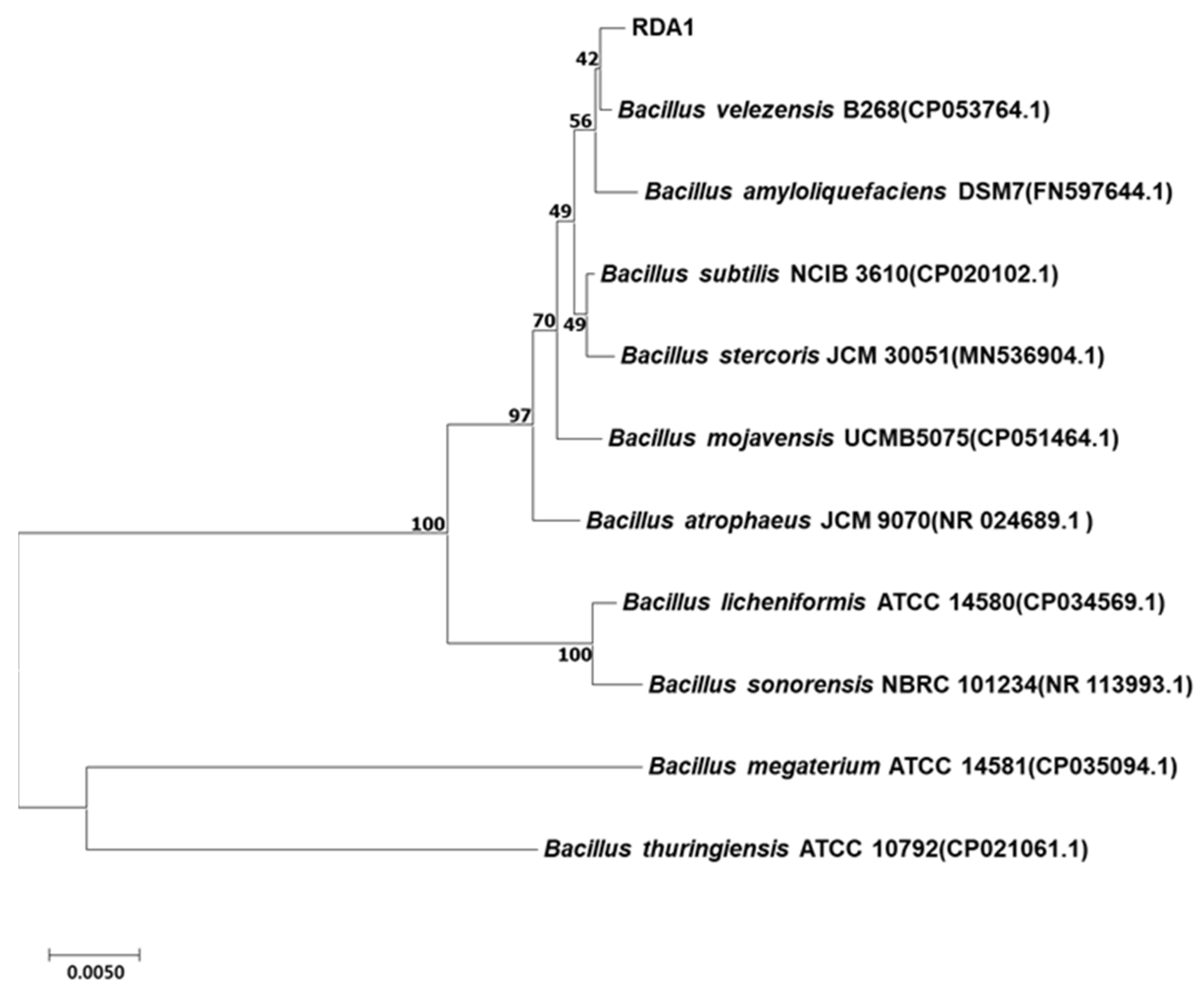
2.1. Isolation and Identification of Strain RDA1
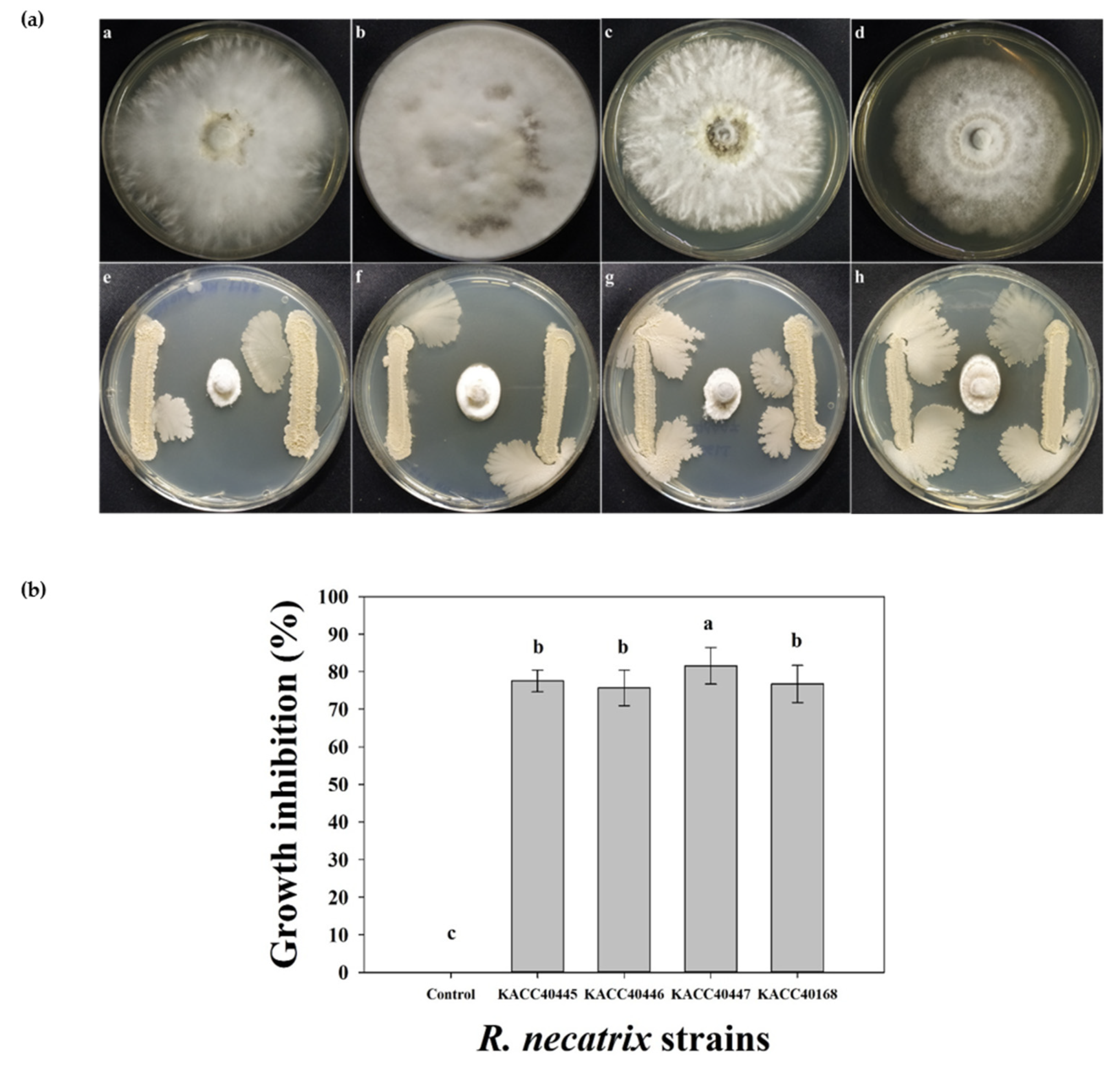
2.2. In Vitro Antifungal Efficacy of RDA1 against R. necatrix
2.3. Antifungal Efficacy of the RDA1 Cell-Free Supernatant (CFS)
2.4. Antifungal Activity of Bacterial Volatile Compounds (VOCs) against R. necatrix
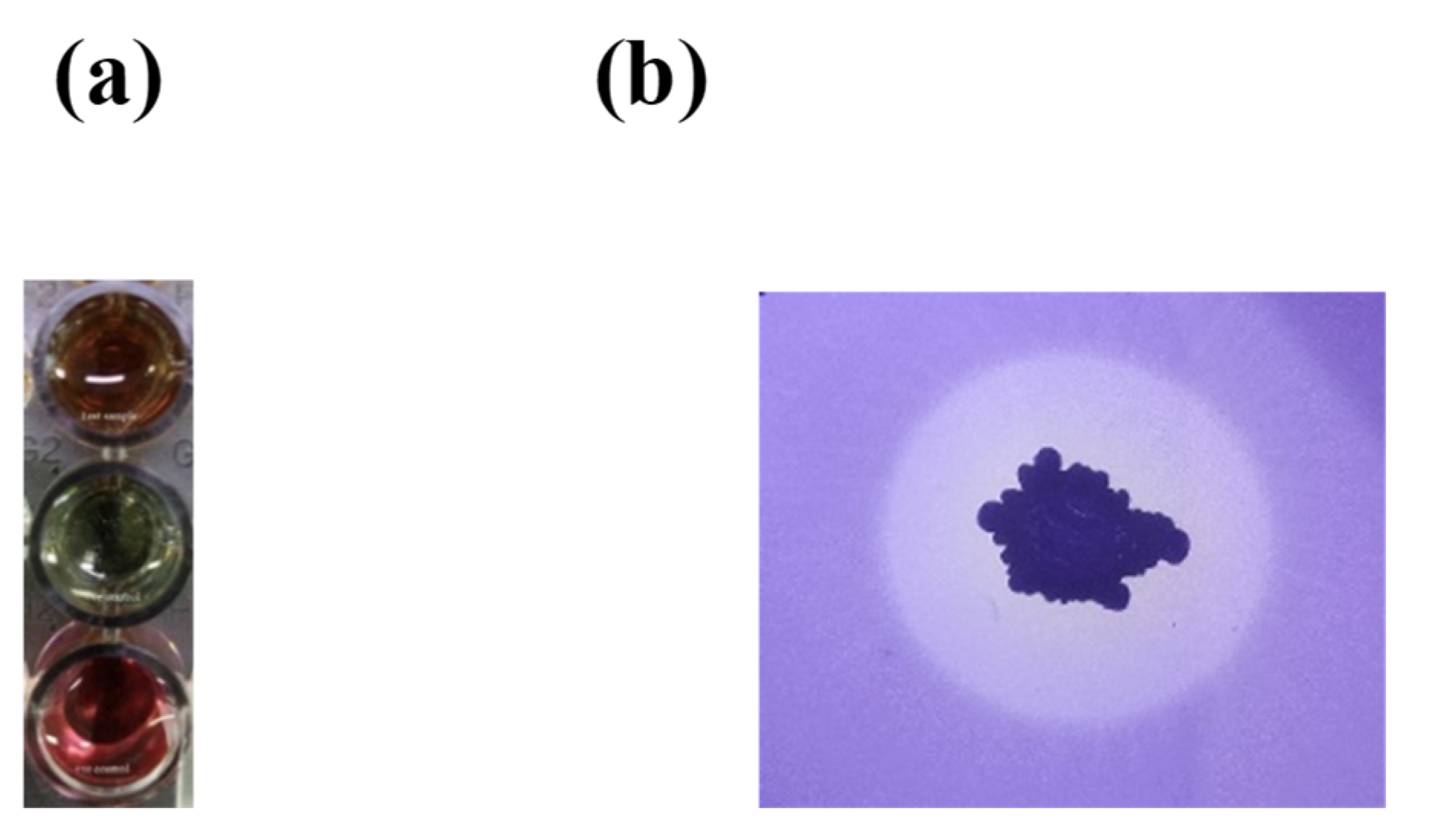
2.5. Plant Growth-Promoting Traits of RDA1
2.6. Detection of Antibiotic Biosynthesis Genes
3. Discussion
4. Materials and Methods
4.1. Pathogens
4.2. Soil Sampling
4.3. Isolation of Bacterial Strains
4.4. Screening and Selection of Antagonistic Bacteria
4.5. 16S rRNA Gene Analysis
4.6. Antifungal Activity Assays
4.7. Antagonistic Efficacy of the Cell-Free Supernatant (CFS)
4.8. Antagonistic Assay of Volatile Organic Compounds (VOCs) against Fungi
4.9. Measurement of PGP Activities of the RDA1 Isolate
4.10. Detection of Antibiotic Biosynthesis Genes
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arjona-López, J.M.; Capote, N.; Melero-Vara, J.M.; López-Herrera, C.J. Control of avocado white root rot by chemical treatments with fluazinam in avocado orchards. Crop Prot. 2020, 131, 105100. [Google Scholar] [CrossRef]
- Sawant, S.S.; Choi, E.D.; Song, J.; Seo, H.J. Current status and future prospects of white root rot management in pear orchards: A review. Res. Plant Dis. 2021, 27, 91–98. [Google Scholar] [CrossRef]
- Magagula, P.; Taylor, N.; Swart, V.; van den Berg, N. Efficacy of potential control agents against Rosellinia necatrix and their physiological impact on avocado. Plant Dis. 2021, 105, 3385–3396. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, Y.; Cheon, W.; Park, J.; Kwon, H.T.; Balaraju, K.; Kim, J.; Yoon, Y.J.; Jeon, Y. Characterization of Bacillus velezensis AK-0 as a biocontrol agent against apple bitter rot caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 626. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Duckett, S.B.; Bergström, E.T.; Noreen, S.; Odijk, R.; Lugtenberg, B.J.; Thomas-Oates, J.E.; Bloemberg, G.V. Biocontrol of avocado dematophora root rot by antagonistic Pseudomonas fluorescens PCL1606 correlates with the production of 2-hexyl 5-propyl resorcinol. Mol. Plant Microbe Interact. 2006, 19, 418–428. [Google Scholar] [CrossRef]
- Pliego, C.; Crespo-Gómez, J.I.; Pintado, A.; Pérez-Martínez, I.; de Vicente, A.; Cazorla, F.M.; Ramos, C. Response of the biocontrol agent Pseudomonas pseudoalcaligenes AVO110 to Rosellinia necatrix exudate. Appl. Environ. Microbiol. 2019, 85, e01741-18. [Google Scholar] [CrossRef]
- Ten Hoopen, G.M.; Krauss, U. Biology and control of Rosellinia bunodes, Rosellinia necatrix and Rosellinia pepo: A review. Crop Prot. 2006, 25, 89–107. [Google Scholar] [CrossRef]
- Gupta, V.K. Possible use of carbendazim in the control of Dematophora root rot of apple. Indian Phytopathol. 1977, 30, 527–531. [Google Scholar]
- López-Herrera, C.J.; Zea-Bonilla, T. Effects of benomyl, carbendazim, fluazinam and thiophanate methyl on white root rot of avocado. Crop Prot. 2007, 26, 1186–1192. [Google Scholar] [CrossRef]
- Hollensteiner, J.; Wemheuer, F.; Harting, R.; Kolarzyk, A.M.; Diaz Valerio, S.M.; Poehlein, A.; Brzuszkiewicz, E.B.; Nesemann, K.; Braus-Stromeyer, S.A.; Braus, G.H.; et al. Bacillus thuringiensis and Bacillus weihenstephanensis inhibit the growth of phytopathogenic Verticillium species. Front. Microbiol. 2017, 7, 2171. [Google Scholar] [CrossRef]
- Wang, L.T.; Lee, F.L.; Tai, C.J.; Kuo, H.P. Bacillus velezensis is a later heterotypic synonym of Bacillus amyloliquefaciens. Int. J. Syst. Evol. Microbiol. 2008, 58, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control. 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Liu, B.; Zhu, Y.; Xiao, R.; Yang, W.; Ge, C.; Chen, Z. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol. 2020, 20, 160. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, F.N.; Slama, H.B.; Bouket, A.C.; Cherif-Silini, H.; Silini, A.; Luptakova, L.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Bacillus velezensis: A treasure house of bioactive compounds of medicinal, biocontrol and environmental importance. Forests 2021, 12, 1714. [Google Scholar] [CrossRef]
- Jiang, C.H.; Liao, M.J.; Wang, H.K.; Zheng, M.Z.; Xu, J.J.; Guo, J.H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control. 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Mantell, S.H.; Wheeler, B.E.J. Rosellinia and white root rot of Narcissus in the Scilly isles. Trans. Br. Mycol. Soc. 1973, 60, 23–35, IN1. [Google Scholar] [CrossRef]
- Fernando, W.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F. Methods for the characterization of plant-growth promoting rhizobacteria. In Host-Pathogen Interactions; Medina, C., López-Baena, F., Eds.; Human Press: New York, NY, USA, 2017; Volume 1734, pp. 307–328. [Google Scholar] [CrossRef]
- Wu, B.; Wang, X.; Yang, L.; Yang, H.; Zeng, H.; Qiu, Y.; Wang, C.; Yu, J.; Li, J.; Xu, D.; et al. Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. Appl. Soil Ecol. 2016, 103, 1–12. [Google Scholar] [CrossRef]
- Al-Ali, A.; Deravel, J.; Krier, F.; Béchet, M.; Ongena, M.; Jacques, P. Biofilm formation is determinant in tomato rhizosphere colonization by Bacillus velezensis FZB42. Environ. Sci. Pollut. Res. Int. 2018, 25, 29910–29920. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Loots, D.T.; Babalola, O.O. Bacillus velezensis: Phylogeny, useful applications, and avenues for exploitation. Appl. Microbiol. Biotechnol. 2019, 103, 3669–3682. [Google Scholar] [CrossRef] [PubMed]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, H.Y.; Bae, W.; Cho, M.; Ryu, H. Functional genomic analysis of Bacillus thuringiensis C25 reveals the potential genes regulating antifungal activity against Rosellinia necatrix. Kor. J. Mycol. 2019, 47, 417–425. [Google Scholar]
- Sztejnberg, A.; Freeman, S.; Chet, I.; Katan, J. Control of Rosellinia necatrix in soil and in apple orchard by solarization and Trichoderma Harzianum. Plant Dis. 1987, 71, 365–369. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Romero, D.; Pérez-García, A.; Lugtenberg, B.J.J.; Vicente, A.D.; Bloemberg, G. Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J. Appl. Microbiol. 2007, 103, 1950–1959. [Google Scholar] [CrossRef]
- Freeman, S.; Sztejnberg, A.; Chet, I. Evaluation of Trichoderma as a biocontrol agent for Rosellinia necatrix. Plant Soil 1986, 94, 163–170. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; del Moral-Navarrete, L.; López-Herrera, C.J. Selection of Trichoderma spp. isolates antagonistic to Rosellinia necatrix. Span. J. Agric. Res. 2010, 4, 1084–1097. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Cazorla, F.M.; Bonilla, N.; Martín-Pérez, R.; De Vicente, A.; López-Herrera, C.J. Biological control of avocado white root rot with combined applications of Trichoderma spp. and rhizobacteria. Eur. J. Plant. Pathol. 2014, 138, 751–762. [Google Scholar] [CrossRef]
- Schroth, M.N.; Hancock, J.G. Disease-suppressive soil and root-colonizing bacteria. Science 1982, 216, 1376–1381. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Ye, M.; Tang, X.; Yang, R.; Zhang, H.; Li, F.; Tao, F.; Li, F.; Wang, Z. Characteristics and application of a novel species of Bacillus: Bacillus velezensis. ACS Chem. Biol. 2018, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Park, B.S.; Kim, H.Y.; Lee, K.H.; Kim, K.S. Antagonistic and plant growth-promoting effects of Bacillus velezensis BS1 isolated from rhizosphere soil in a pepper field. Plant Pathol. J. 2021, 37, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.J.; Jo, I.H.; Lee, J.; Bae, W.; Kim, H.; Won, K.; Hyun, T.K.; Ryu, H. Characterization of the Rosellinia necatrix transcriptome and genes related to pathogenesis by single-molecule mRNA sequencing. Plant Pathol. J. 2017, 33, 362–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohike, T.; Makuni, K.; Okanami, M.; Ano, T. Screening of endophytic bacteria against fungal plant pathogens. J. Environ. Sci. (China) 2013, 25, S122–S126. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Lv, Z.; Shi, Y.; Wang, Z. Antifungal activity and functional components of cell-free supernatant from Bacillus amyloliquefaciens LZN01 inhibit Fusarium oxysporum f. sp. niveum growth. Biotechnol. Biotechnol. Equip. 2019, 33, 1042–1052. [Google Scholar] [CrossRef]
- Cellini, A.; Spinelli, F.; Donati, I.; Ryu, C.M.; Kloepper, J.W. Bacterial volatile compound-based tools for crop management and quality. Trends Plant Sci. 2021, 26, 968–983. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Mo, M.H.; Zhou, J.P.; Zou, C.S.; Zhang, K.Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Farag, M.A.; Ryu, C.M.; Sumner, L.W.; Paré, P.W. GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef]
- Kulkarni, G.B.; Nayak, A.S.; Sajjan, S.S.; Oblesha, A.; Karegoudar, T.B. Indole-3-acetic acid biosynthetic pathway and aromatic amino acid aminotransferase activities in Pantoea dispersa strain GPK. Lett. Appl. Microbiol. 2013, 56, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Chagas Junior, A.F.; De Oliveira, A.G.; De Oliveira, L.A.; Dos Santos, G.R.; Chagas, L.F.; da Silva, A.L.; Costa, J.D. Production of indole-3-acetic acid by Bacillus isolated from different soils. Bulg. J. Agric. Sci. 2015, 21, 282–287. [Google Scholar]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Kesaulya, H.; Hasinu, J.V.; Tuhumury, G.N. Potential of Bacillus spp. produces siderophores in suppressing the wilt disease of banana plants. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012016. [Google Scholar] [CrossRef]
- Chen, X.H.; Vater, J.; Piel, J.; Franke, P.; Scholz, R.; Schneider, K.; Koumoutsi, A.; Hitzeroth, G.; Grammel, N.; Strittmatter, A.W.; et al. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J. Bacteriol. 2006, 188, 4024–4036. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Arguelles-Arias, A.; Ongena, M.; Halimi, B.; Lara, Y.; Brans, A.; Joris, B.; Fickers, P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Factories 2009, 8, 63. [Google Scholar] [CrossRef]
- Ramarathnam, R.; Bo, S.; Chen, Y.; Fernando, W.D.; Xuewen, G.; De Kievit, T. Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can. J. Microbiol. 2007, 53, 901–911. [Google Scholar] [CrossRef]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Antimicrobial peptide genes in Bacillus strains from plant environments. Int. Microbiol. 2011, 14, 213–223. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef]
- Mardanova, A.M.; Hadieva, G.F.; Lutfullin, M.T.; Khilyas, I.V.E.; Minnullina, L.F.; Gilyazeva, A.G.; Bogomolnaya, L.M.; Sharipova, M.R. Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agric. Sci. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Ben Abdallah, D.; Frikha-Gargouri, O.; Tounsi, S. Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. J. Appl. Microbiol. 2015, 119, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Tendulkar, S.R.; Saikumari, Y.K.; Patel, V.; Raghotama, S.; Munshi, T.K.; Balaram, P.; Chattoo, B.B. Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. J. Appl. Microbiol. 2007, 103, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015, 8, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Anith, K.N.; Nysanth, N.S.; Natarajan, C. Novel and rapid agar plate methods for in vitro assessment of bacterial biocontrol isolates’ antagonism against multiple fungal phytopathogens. Lett. Appl. Microbiol. 2021, 73, 229–236. [Google Scholar] [CrossRef]
- Ali, M.A.; Ren, H.; Ahmed, T.; Luo, J.; An, Q.; Qi, X.; Li, B. Antifungal effects of rhizospheric Bacillus species against bayberry twig blight pathogen Pestalotiopsis versicolor. Agronomy 2020, 10, 1811. [Google Scholar] [CrossRef]
- Kong, W.L.; Rui, L.; Ni, H.; Wu, X.Q. Antifungal effects of volatile organic compounds produced by Rahnella aquatilis JZ-GX1 against Colletotrichum gloeosporioides in Liriodendron chinense × tulipifera. Front. Microbiol. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Mehta, S.; Nautiyal, C.S. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol. 2001, 43, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Compaoré, C.S.; Nielsen, D.S.; Ouoba, L.I.; Berner, T.S.; Nielsen, K.F.; Sawadogo-Lingani, H.; Diawara, B.; Ouédraogo, G.A.; Jakobsen, M.; Thorsen, L. Co-production of surfactin and a novel bacteriocin by Bacillus subtilis subsp. subtilis H4 isolated from Bikalga, an African alkaline Hibiscus sabdariffa seed fermented condiment. Int. J. Food microbial. 2013, 162, 297–307. [Google Scholar] [CrossRef] [PubMed]



| PS | IAA Production in the Presence of Tryptophan | IAA Production in the Absence of Tryptophan | Siderophore (PSU) |
|---|---|---|---|
| +++ | 73.6 ± 4.9 | 23.2 ± 1.9 | 82.6 ± 4.3 |
| Lipopeptides | Gene | Primer | Sequence (5′-3′) | Annealing Temperature (°C) | PCR Product Size (bp) | Reference |
|---|---|---|---|---|---|---|
| Bacillomycin | bmyB | BMYB-F | GAATCCCGTTGTTCTCCAAA | 55 | 370 | [50] |
| BMYB-R | GCGGGTATTGAATGCTTGTT | |||||
| Fengycin | fend | FEND-F | GGCCCGTTCTCTAAATCCAT | 58 | 269 | [50] |
| FEND-R | GTCATGCTGACGAGAGCAAA | |||||
| Surfactin | srfAA | SRFA-F | TCGGGACAGGAAGACATCAT | 58 | 201 | [50] |
| SRFA-R | CCACTCAAACGGATAATCCTGA | |||||
| Macrolactin | mlnA | mlnA-F | CCGTGATCGGACTGGATGAG | 58 | 668 | [63] |
| mlnA-R | CATCGCACCTGCCAAATACG | |||||
| Bacillaene | baeA | baeA-F | ATGTCAGCTCAGTTTCCGCA | 59 | 688 | [63] |
| baeA-R | GATCGCCGTCTTCAATTGCC | |||||
| Difficidin | dfnA | dfnA-F | GGATTCAGGAGGCATACCG | 59 | 653 | [63] |
| dfnA-R | ATTGATTAAACGCGCCGAGC | |||||
| Bacilysin | BacA | BacA-F | CAGCTCATGGGAATGCTTTT | 58 | 498 | [50] |
| BacA-R | CTCGGTCCTGAAGGGACAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawant, S.S.; Song, J.; Seo, H.-J. Characterization of Bacillus velezensis RDA1 as a Biological Control Agent against White Root Rot Disease Caused by Rosellinia necatrix. Plants 2022, 11, 2486. https://doi.org/10.3390/plants11192486
Sawant SS, Song J, Seo H-J. Characterization of Bacillus velezensis RDA1 as a Biological Control Agent against White Root Rot Disease Caused by Rosellinia necatrix. Plants. 2022; 11(19):2486. https://doi.org/10.3390/plants11192486
Chicago/Turabian StyleSawant, Shailesh S., Janghoon Song, and Ho-Jin Seo. 2022. "Characterization of Bacillus velezensis RDA1 as a Biological Control Agent against White Root Rot Disease Caused by Rosellinia necatrix" Plants 11, no. 19: 2486. https://doi.org/10.3390/plants11192486
APA StyleSawant, S. S., Song, J., & Seo, H.-J. (2022). Characterization of Bacillus velezensis RDA1 as a Biological Control Agent against White Root Rot Disease Caused by Rosellinia necatrix. Plants, 11(19), 2486. https://doi.org/10.3390/plants11192486







